Abstract
Objective
To evaluate the survival outcomes of combined liver resection (LR) and radiofrequency ablation (RFA) on multi-focal hepatocellular carcinoma (HCC) in patients with Barcelona clinic liver cancer (BCLC) stage B.
Methods
A total of 210 cases of HCC were included in this study. In 42 cases, patients were treated with combination therapy using LR and RFA (LRCRFA). In 84 cases, patients underwent transarterial chemoembolization (TACE), and in another 84 cases, patients underwent LR; both the TACE and LR groups served as controls. It both categorized as BCLC stage B for LRCRFA and TACE groups but as BCLC stage A for LR group.
Results
The overall survival (OS) rate of the LRCRFA group was significantly higher than that of the TACE group (P<0.001) but was not significantly different when compared with the LR group (P=0.544). The disease-free survival (DFS) rate of the LRCRFA group was significantly lower than that of the LR group (P=0.029). Patients with ≤4 tumors or those with ≤5 tumors no larger than 6 cm in diameter experienced better long-term outcomes than other patients in the same LRCRFA group. The OS rates and DFS rates were not significantly different from those of patients in the LR group (P>0.05). Having more than 2 existing tumors was an independent risk factor for OS rate.
Conclusion
Combination therapy using LR and RFA can more effectively improve the prognosis of these patients than TACE. Patients with BCLC stage B HCC with ≤4 tumors or ≤5 tumors smaller than 6 cm in diameter are the ideal candidates for the application of LRCRFA.
Keywords: hepatocellular carcinoma, surgical resection, radiofrequency ablation, transarterial chemoembolization, survival, Barcelona clinic liver cancer stage
Introduction
Hepatocellular carcinoma (HCC) is the most common malignancy of the liver and the fourth leading cause of cancer death worldwide.1 Liver resection (LR), liver transplantation (LT) and radiofrequency ablation (RFA) are radical treatment options for HCC.2 However, only 20% of clinically-diagnosed HCC can be removed surgically.3
The Barcelona clinic liver cancer (BCLC) cancer staging system was published in 1999 and is currently the most widely used staging system.1,4 The main guidelines for the treatment of HCC were developed in accordance with the BCLC guidelines.5,6 Approximately 20% of HCC patients, when first diagnosed, present with multiple intrahepatic tumors and are in BCLC stage B.7 The treatment for multifocal HCC is still controversial. These cases have already advanced beyond the point where radical resection can be beneficial. Surgical treatment for these patients is not recommended according to the guidelines in Europe and the US. Instead, the only recommended treatment option is palliative transarterial chemoembolization (TACE). However, the long-term survival outcomes of patients managed with this technique do not appear fully satisfactory for patients in BCLC stage B.8 Some research showed that there was not enough evidence to suggest that TACE was beneficial for this type of patients.9–11 The prognoses of these patients were extremely poor.5,6
As we all know, the removal of lesions is the most important task in the treatment of solid malignancies. LR is the most common and established approach. However, more than 90% of HCC patients have complications with cirrhosis.12 Surgical removal of all tumors is not only difficult to perform but can also cause significant liver damage for HCC patients in BCLC stage B. In particular, the removal of small lesions buried deep in the liver parenchyma often results in the loss of large amounts of liver tissue. This drastically increases both the trauma caused by the surgical procedure and the risk of post-surgical liver failure in cirrhosis.13,14 It is generally acknowledged that the effects of RFA treatment on small HCC lesions are comparable to those of surgical resection, and in such cases, RFA can be used as a first-line treatment.1,5,15 RFA can produce satisfactory effects and preserve liver tissues for patients with small lesions located deep in the liver. Whether these two curative methods can be combined to improve the outcome of these HCC patients in BCLC stage B is unclear. The aim of this study was to compare the long term outcomes of LR combined with RFA (LRCRFA) and TACE for HCC patients in BCLC stage B.
Patients and Methods
Patients
The patients were diagnosed with HCC based on pathological evidence from resected specimens or, in the absence of biopsy results, based on the diagnostic criteria for HCC used by the AASLD.16 Table 1 shows the inclusion criteria for each group.
Table 1.
Inclusion Criteria for Each Group
| Factors | LRCRFA | TACE | LR |
|---|---|---|---|
| Confirmed diagnosis of HCC | Pathological | Clinical | Pathological |
| No anti-tumor treatments performed before treatment | Yes | Yes | Yes |
| Child–Pugh score | A or B | A or B | A or B |
| Extrahepatic metastasis | No | No | No |
| Adjacent organ invasion | No | No | No |
| Detectable violation of intrahepatic vessels or bile duct with image | No | No | No |
| BCLC stage | B | B | A |
Study Design
The primary end point of the study was the 5-year OS rate. The secondary end point was the DFS rate. Postoperative complication rates, mortality, operating time, blood loss, and length of hospital stay were also compared. The stage of all patients was determined according to the BCLC staging system. TNM stage was determined according to guidelines published by the Union for International Cancer Control (UICC), version 7. The diameters of the tumors were measured using preoperative computed tomography (CT). Localization and number of the tumors were evaluated and determined using the combined results of imaging examination, intraoperative probing, and post-operative dissection. The amount of bleeding during surgery, time of surgery and the time of Pringle maneuver were recorded from surgery and anesthesia logs. Data were collected and stored in the cancer database management system by a designated clinical study center assistant chosen by the Research Ethics Committee.
The present study complies with the Declaration of Helsinki and was approved by the medical ethics committee of the Southwest Hospital, Third Military Medical University. Written informed consent was obtained from all patients in the study.
Sample Size
Since more HCC patients classified as BCLC stage B tend to receive TACE because it is minimally invasive, the ratio of LRCRFA cases to TACE cases was set at 1:2. Therefore, 84 cases were included as an external control group (LR group). In this group, all patients were BCLC stage A, with a single tumor in the liver. The surgical procedure involved LR alone. All cases in the TACE and LR groups were randomly selected from all patients who met the inclusion criteria using a random number table.
Surgical Methods
All surgeries were performed by 3 hepatobiliary surgeons with over 20 years of experience; RFAs were conducted by surgeons who had over 2 years of experience and had finished over 100 RFA procedures. In all cases, intraoperative ultrasound (IOUS) was used to probe the liver to determine the location and size of all lesions and to determine whether any lesions had been missed during preoperative examinations.
Liver Resection
Incisions were made below the right costal margin with the patient under general anesthesia. All tumor removal involved non-anatomic partial hepatectomy. First, IOUS was used to verify that the cutting edge was at least 1 cm away from the tumor. For liver parenchymal transection, the forceps crush clamping method was adopted. Intrahepatic ductules (<2 mm) were coagulated using bipolar coagulation (120 w), and thick ductules were ligated or sutured. The Pringle maneuver was used to prevent massive bleeding during the resection, and clamping periods of 15 minutes were separated by 5-minute periods of declamping. After surgery, when liver function had recovered to normal levels and the complications had been treated, the patients were discharged from the hospital.
RFA
RFA was performed immediately after removal of the primary tumor during surgery. A LDRF-120S radio frequency ablation device (Lead Electron Corporation, Mianyang, China) was used. Under the guidance of IOUS, RFA electrodes were inserted into the deepest part of the tumor. The open electrode bundle covered a range of at least 1 cm outside the boundaries of the tumor in order to ensure complete ablation. The initial power was set at 50 W. The power was increased by 10 W every 2 min until it reached the maximum at 95 W. The power was kept at 95 W until the impedance increased to maximum and automatically stopped. To clear residual cancer cells, after the electrode bundle was closed, it was turned by 15 degrees and another treatment cycle was performed. This procedure was repeated to treat each lesion. Ultrasound or contrast-enhanced ultrasound (CEUS) was used 1 week after surgery (CEUS was used after 2005). If any residual lesion was detected, percutaneous RFA guided by ultrasound/CEUS was repeated.
TACE
TACE were performed by an interventional radiologist with over 20 years of experience. To identify the feeding arteries of the tumor, angiography of celiac, hepatic, superior mesenteric, left gastric, and inferior phrenic arteries was performed. A micro catheter was inserted into the target artery. A mixture of iodized oil (5–20 mL), epirubicin (30–50 mg) or Oxaliplatin (50–100 mg) and fluorouracil glycosides (500–1000 mg) was infused under fluoroscopic monitoring. The infusing rate was 0.5–1 mL/min until stasis flow in tumor vascularity was achieved. Finally, a gelatin sponge was used to embolize the feeding artery of tumor.
Follow-Up Examinations
Regular follow-up examinations were conducted in all cases. Enhanced CT (accompanied by plain scanning of the lung) and alpha-fetoprotein (AFP) blood testing were performed every 3 months for 2 years after surgery and then every 6 months thereafter.17 To evaluate the effect of the treatment, the above procedures were performed within 1 month of primary treatment in the TACE group. All follow up ended on May 30, 2015. Data collection was conducted and well preserved independently by the clinical study center of the hospital.
Treatment After Relapse for LRCRFA Group and LR Group
Relapse of Intrahepatic Tumors
Patients with ≤3 tumors and tumors ≤3 cm in diameter were treated with RFA. Patients with a single tumor larger than 3 cm were treated with repeated surgical resection. Patients with tumors >3 cm in diameter and >3 in number were treated with TACE. Patients with lung or lymph node metastasis were treated with CT-guided I125 seed implantation (fewer than 5 lesions). Patients with bone metastasis were treated with radiotherapy.
Statistical Methods
The statistical analyses were performed by a statistician who was “blinded” to the patients’ actual treatment categories. SPSS 22.0 was used for statistical analysis. Inter-group comparisons of qualitative data were performed using χ2 testing. For quantitative data, t-testing was performed. Kaplan–Meier survival analysis was adopted to calculate the OS rate and the DFS rate of the groups. The log-rank method was used for comparison of survival rates between the groups. Univariate and multivariate Cox proportional hazards regression models were used to determine independent risk factors affecting the OS rate. Differences were considered significant at P< 0.05.
Results
Between 2000 and 2011, 4223 HCC patients were admitted to our hospital for treatment. Among those patients, 1293 underwent LR and 559 patients were treated with TACE. A total of 210 cases were included in this study according to the inclusion criteria.
In the LRCRFA group, 42 cases were included in the present study between February 2000 and May 2011. The total number of tumors was 112, while 47 primary lesions were removed: 18 from the left liver and 29 from the right liver. There were 65 small lesions ablated using RFA (34 tumors in the left hepatic lobe and 31 in the right lobe). In the TACE group, 84 HCC patients treated at the hospital’s Department of Interventional Radiology between March 2005 and October 2009 were included. The total number of tumors was 211. All the lesions were treated with TACE only (mean 2.93±1.59, 2–9 times in each case). In the LR group, 84 cases with patients who underwent LR at the Institute of Hepatobiliary Surgery between February 2005 and May 2009 were included.
The median time of follow-up for the LRCRFA group, TACE group and LR groups were 35.7 ± 18.1 months (3.0–71.3 months), 14.2 ± 17.8 months (2.0–83.3 months) and 39.3 ± 21.6 months (2.3–76.0 months), respectively. In the LRCRFA group, no patients withdrew from follow-up, but 4 and 3 patients withdrew from the TACE group and LR group, respectively.
Clinicopathological Data
There were no significant differences between the LRCRFA group and the TACE group (P>0.05). The TNM stage, number of tumors and maximum tumor diameter of patients in the LRCRFA group were significantly higher than those in the LR group. (P<0.001, P<0.001 and P<0.001, respectively). There were no significant differences between the two groups with respect to other clinicopathological data (P>0.05). See Table 2.
Table 2.
Clinicopathological Data for Each Group
| Factors | LRCRFA | TACE | LR | P value |
|---|---|---|---|---|
| Sex (male/female) | 34/8 | 76/8 | 75/9 | 0.139 a/0.152 b |
| Age (median range) | 49.4 (29–76) | 52.1 (31–90) | 48.5 (24–80) | 0.260 a/0.810 b |
| HBV infected | 42/42 | 84/84 | 84/84 | NA |
| AFP (ng/mL) # (median range) | 4411.6 (2.1–112,989) | 14,355.6 (3.0–338,897) | 988.1 (1.17–7790) | 0.612 a/0.451 b |
| Cirrhosis | 35/42 | 59/84 | 62/84 | 0.105 a/0.151 b |
| Child–Pugh (A/B) | 31/11 | 55/29 | 57/27 | 0.326 a/0.180 b |
| Degree of differentiation (L/M/H) | 5/33/4 | NA | 10/62/12 | NA a/0.808 b |
| TNM stage (I/II/IIIa) | 0/17/25 | 0/29/55 | 84/0/0 | 0.322 a/0.000 b * |
| Number of tumors # (median range) | 2.7 (2–5) | 2.5 (2–5) | 1 | 0.404 a/0.000 b * |
| Tumor distribution (L/R/L&R) | 1/7/34 | 4/60/20 | 33/51/0 | NA |
| Size of the primary tumor (cm) (mean, SD) (range) | 6.0±2.9 (3.2–16.0) | 6.7±3.0 (3.3–14.0) | 4.0±1.8 (3.1–14.0) | 0.306 a/0.000 b * |
Notes: aBetween the LRCRFA and the TACE groups. bBetween the LRCRFA and the LR groups. #Non-normal distribution data analyzed using the Wilcoxon rank sum test. *Between-group comparison, P<0.05.
Treatment Conditions and Complications
Treatment time, bleeding and duration of hospitalization were significantly higher in the LRCRFA group than in the TACE group. (P<0.001, P<0.001 and P<0.001, respectively). There were no significant differences in surgery time, Pringle maneuver time, bleeding and duration of hospitalization between the LRCRFA group and the LR group (P>0.05). The average time spent in RFA during surgery in the LRCRFA group was 16.8±6.2 min. In the LRCRFA group, no thermal damage to surrounding organs or bile ducts occurred. After surgery, there was 1 case of biliary fistula and 1 case of incision infection, and both patients recovered after treatment. One week after surgery, ultrasound/CEUS suggested a single residual lesion in two cases, and percutaneous RFA guided by ultrasound/CEUS was administered again. There were no cases with obvious complications in the TACE group. In the LR group, after surgery, there were 2 cases of biliary fistula and 2 cases of pneumonia. These patients were treated with drainage and anti-inflammatory treatment, respectively, and all 4 patients recovered. There was no treatment-related hospital mortality in either group.
Relapse and Treatment for LRCRFA Group and LR Group
Intrahepatic relapses occurred in 24 and 44 cases in the LRCRFA group and LR group, respectively. Extrahepatic relapses occurred in 5 cases in the LRCRFA group (2 in both liver and lung, 1 in bone, 2 in Lymph nodes) and in 7 cases in the LR group (4 in both liver and lung, 1 in lung, 2 in Lymph nodes). There were no significant differences between the two groups (P>0.05). Relapses were treated as described in the methods section. In the LRCRFA and LR groups, there were 22 cases (46 times) and 31 cases (49 times) respectively in which patients underwent RFA for intrahepatic lesions, 6 cases (14 times) and 7 cases (15 times) respectively in which patients received TACE, and 4 cases (7 times) and 7 cases (9 times) respectively in which patients received 125 I seed implantation. In 1 case, radiotherapy was administered for a relapsed bone lesion in the LRCRFA group and in 6 LR cases patients received a second LR for single-tumor relapses in the liver.
Survival
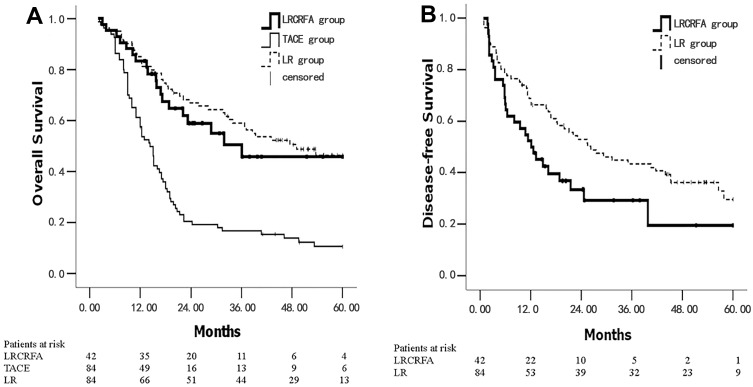
The 1, 3, and 5 year OS rates were 83.3%, 50.4%, 45.8%, 61.3%, 16.6%, 10.5% and 85.0%, 57.6%, 46.3% for the LRCRFA, TACE and LR groups, respectively. The median OS times of the three groups were 36.1 months (95% CI: 9.3–66.0), 14.3 months (95% CI: 12.0–16.6) and 49.1 months (95% CI: 28.3–69.9), respectively. The OS rate of the LRCRFA group was significantly higher than that of the TACE group (P<0.001). There was no significant difference between the LRCRFA group and the LR group with respect to OS rate (P=0.544) (Figure 1A). The 1, 3, and 5 year DFS rates in the LRCRFA group were 52.4%, 29.3%, and 19.5%, respectively, while the LR group was 65.3%, 51.8%, and 41.2%. The median DFS times of the LRCRFA and LR groups were 12.1 months (95% CI: 6.6–17.6) and 26.3 months (95% CI: 15.8–36.7), respectively. The DFS rate of the LRCRFA group was significantly lower than that of the LR group (P=0.029). See Figure 1B. Univariate analysis and multivariate analysis Cox regression analysis in LRCRFA group showed that number of tumors ≥2 was an independent risk factor for OS rate. See Tables 3 and 4.
Figure 1.
Kaplan–Meier survival curves for the LRCRFA, TACE and LR groups. (A) The OS curves were plotted in LRCRFA, TACE and LR groups; (B) the DFS curves were plotted in LRCRFA and LR groups. The number of survival and total patients in each group is presented.
Table 3.
Univariate Analysis of Overall Survival Prognostic Factors in LRCRFA Group
| Factors | n | HR (95% CI) | χ2 | P |
|---|---|---|---|---|
| Gender | ||||
| Female | 8 | |||
| Male | 34 | 1.289(0.510–3.258) | 0.287 | 0.349 |
| Age (years) | ||||
| <60 | 23 | |||
| ≥60 | 19 | 2.070(0.857–5.002) | 2.613 | 0.704 |
| AFP (ng/mL) | ||||
| <20 | 9 | |||
| ≥20 | 33 | 1.404(0.627–3.147) | 0.680 | 0.720 |
| Major Tumor Size (cm) | ||||
| <5 | 28 | |||
| ≥5 | 14 | 3.906(1.957–9.134) | 10.354 | 0.000 |
| Tumor Number | ||||
| ≤2 | 4 | |||
| >2 | 38 | 3.260(1.462–5.973) | 8.972 | 0.000 |
| Tumor Location | ||||
| Mono-lobe | 16 | |||
| Double-lobe | 26 | 1.690(0.699–4.087) | 1.358 | 0.108 |
| Tumor Differentiation | ||||
| Well | 37 | |||
| Poorly | 5 | 0.549(0.242–1.379) | 2.257 | 0.849 |
| Cirrhosis | ||||
| No | 7 | |||
| Yes | 35 | 1.297(0.530–3.170) | 0.325 | 0.578 |
| Child–Pugh | ||||
| A | 31 | |||
| B | 11 | 0.687(0.266–1.572) | 1.721 | 0.678 |
Table 4.
Multivariate Analysis of Overall Survival Prognostic Factors in LRCRFA Group
| Factors | β | SE | Wald χ2 | HR (95% CI) | P |
|---|---|---|---|---|---|
| Gender | 0.430 | 0.544 | 0.626 | 1.538(0.529–4.468) | 0.429 |
| Age | 0.280 | 0.734 | 0.146 | 2.774(1.186–6.487) | 0.703 |
| AFP | 0.019 | 0.596 | 0.003 | 1.219(0.317–3.280) | 0.903 |
| Major tumor size | 0.960 | 0.541 | 3.152 | 2.612(0.905–7.538) | 0.088 |
| Tumor number | 1.169 | 0.475 | 5.761 | 3.219(1.269–8.166) | 0.001 |
| Tumor location | 0.096 | 0.372 | 0.067 | 1.101(0.531–2.281) | 0.796 |
| Tumor differentiation | 0.506 | 0.293 | 2.143 | 1.745(0.790–3.312) | 0.208 |
| Cirrhosis | 0.346 | 0.264 | 2.419 | 1.407(0.705–3.041) | 0.212 |
| Child–Pugh | 0.110 | 0.874 | 0.016 | 1.116(0.201–6.188) | 0.870 |
Ideal Candidates for LRCRFA
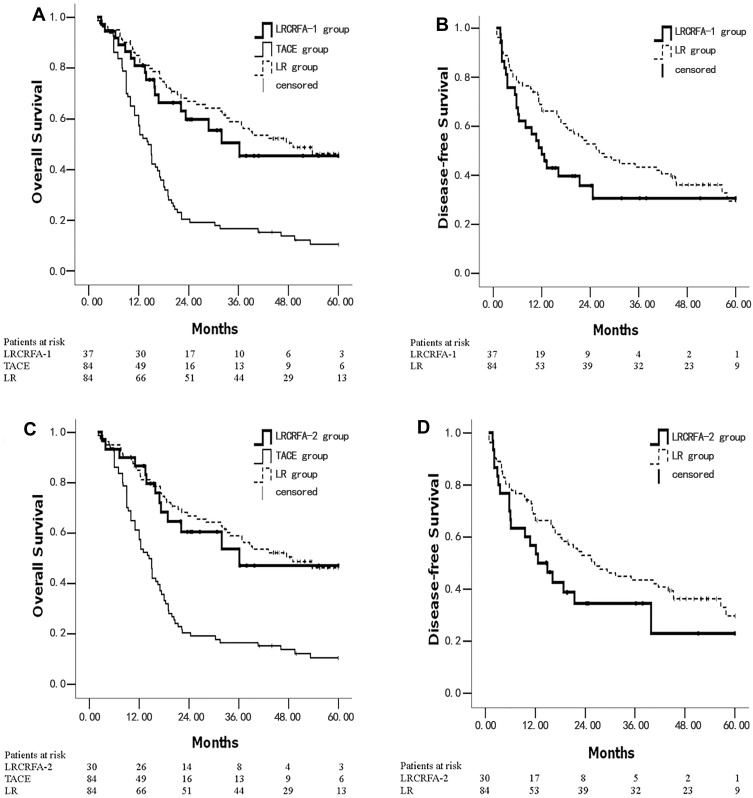
We analyzed the survival rate of patients in the LRCRFA group who had no more than 4 lesions (LRCRFA-1 group) (37/42). The 1, 3, and 5 year OS rates were 81.1%, 50.5%, and 45.4%, respectively, and the 1, 3, and 5 year DFS rates were 51.4%, 30.7%, and 30.7% respectively. Median OS and DFS times were 36.1 months (95% CI: 8.7–61.8) and 12.1 months (95% CI: 7.6–16.6), respectively. These were not significantly different from the corresponding values in the LR group (χ2=0.434, P=0.510, χ2=3.069, P=0.080) for OS and DFS rates. The OS rate of the LRCRFA-1 group was significantly higher than that of the TACE group (P<0.001). See Figure 2A and B. The survival of patients with 5 tumors or fewer and primary tumors ≤6 cm in diameter (LRCRFA-2 group) (30/42) were as follows: the 1, 3, and 5 year OS rates were 86.7%, 53.8%, and 47.1%, respectively; the 1, 3, and 5 year DFS rates were 56.7%, 34.4%, and 22.9%, respectively. Median OS and DFS times were 36.1 months (95% CI: 9.8–69.5) and 12.6 months (95% CI: 5.7–19.6), respectively. These were not significantly different from the corresponding values in the LR group (χ2=0.150, P=0.698, χ2=2.538, P=0.111) for OS and DFS rates. The OS rate of the LRCRFA-2 group was significantly higher than that of the TACE group (P<0.001). See Figure 2C and D.
Figure 2.
Kaplan–Meier survival curves of ideal candidates for LRCRFA. (A) The OS curves were plotted in LRCRFA-1, TACE and LR groups; (B) the DFS curves were plotted in LRCRFA-1 and LR groups; (C) the OS curves were plotted in LRCRFA-2, TACE and LR groups; (D) the DFS curves were plotted in LRCRFA-2 and LR groups. The number of survival and total patients in each group is presented.
Discussion
To the best of our knowledge, this is the first paper to compare the treatment results of patients with BCLC stage B HCC who underwent LRCRFA or TACE.
In this study, the OS rate of BCLC stage B HCC patients who underwent LRCRFA treatment was significantly higher than that of the TACE group but was no less than the OS rate of HCC patients with single tumors who underwent resection. The DFS rate of the LRCRFA group was significantly lower than that of the LR group. However, close post-surgical follow-up examinations and active treatment of recurring lesions could still help promote satisfactory survival for these patients. In the LRCRFA group, the following two characteristics were associated with favorable long-term effects: (1) number of tumors ≤4; and (2) number of tumors ≤5 and primary tumor diameter ≤6 cm. OS rate and DFS rate were not significantly different between the LRCRFA and LR-only groups. We believe that of BCLC stage B HCC patients, these individuals are the ideal candidates for LRCRFA treatment.
Currently, there is no effective surgical treatment strategy for patients with BCLC stage B HCC. The prognoses of these patients were extremely poor with palliative TACE according to the guidelines.5,6,18 The results of this study may provide an effective treatment method for HCC patients with BCLC stage B cancer. LR combined with RFA can improve the prognosis of these patients. For recurring tumors after surgery, a second treatment is usually necessary.19 HCC relapses are mostly intrahepatic.20,21 Early detection and early treatment can improve the prognosis. In the treatment of repeatedly recurring intrahepatic tumors, RFA has the advantages of being safe, effective, repeatable and minimally invasive.22,23 The application of IOUS must be emphasized. Preoperative imaging techniques may miss small tumors in the liver.24 IOUS can detect small lesions that may have been missed before. One study showed that such detections caused doctors to change treatment strategies in favor of surgical resection in 16.5% of HCC patients.25 In the present study, IOUS detected small lesions that had been missed preoperatively in 6 out of 42 patients in the LRCRFA group (14.3%).
Chang showed reported that among 304 patients with BCLC stage B HCC, the 5-year survival rate was 46.5% after resection.26 This was similar to the results observed in the LRCRFA group. Some studies showed that RFA can achieve similar survival rate compared to SR for tumour < 5 cm.27 Pawlik research has shown combination of LR and RFA treatment is benefit for advanced hepatic malignancies.28 Sandonato reported that, for 1 HCC patient who had multiple intrahepatic lesions, DFS time reached 3 years after LRCRFA.29 Eisele performed LRCRFA on 10 HCC patients with multifocal tumors and reported that the 3-year survival rate of these patients was 50%.30 Choi performed LRCRFA on 53 HCC patients with multifocal tumors, and the treatment was found to be effective: the 1, 3, and 5 year survival rates reached 87%, 80%, and 55%, respectively.31 However, none of these reports included controls, and some of the cases included patients with metastatic tumors.
Our study has several limitations. First, there have been no reports on prospective randomized controlled studies using combination therapy with resection and RFA for HCC. This was a retrospective study, so we used a random method to select cases for TACE and LR groups to avoid selection bias as much as possible. LRCRFA patients are by definition ultra-selected because the majority of BCLC B patients were still treated with TACE, which made randomization difficult, so we included all 42 cases in the LRCRFA group. Second, the implementation of LRCRFA surgery not only requires the patient to have a good general condition, the appropriate number, size and location of the ablated tumors, but also a skilled medical team. Finally, the small lesions detected with IOUS were treated directly with intraoperative RFA and were not biopsied in all cases. The IOUS is completed by experienced ultrasound physician. Based on the typical liver cancer image of ultrasound and postoperative follow-up data, especially the imaging data of relapsed patients, we have great evidence that these 6 lesions are liver malignant tumor. We believed that intraoperative RFA did not cause extra pain in the patients, but not treating these suspected lesions can lead to poor prognosis. In addition, frequent biopsy may increase the risks of metastasis caused by needle track and false negative results.32
Conclusion
In conclusion, for patients with BCLC stage B HCC and fewer than 5 lesions, active surgical intervention is necessary. LR combined with RFA can improve the prognosis of these patients. Patients with BCLC stage B HCC and ≤4 tumors or ≤5 tumors (with largest diameter ≤6 cm) are the ideal candidates for the application of LRCRFA. Our results may change the current treatment strategies for patients with BCLC stage B HCC and we will conduct a prospective random controlled study in the future.
Acknowledgments
We thank all the patients who consented to the study and the anonymous reviewers for their helpful comments. We thank Li Liu for data management.
Funding Statement
The work was supported by the National Science and Technology Major Project of the Ministry of Science and Technology of China (Grant No.2017ZX100203205) and the General Program of Natural Science Foundation of China (81372272).
Author Contributions
All authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that they have no competing interests.
References
- 1.Villanueva A. Hepatocellular carcinoma. N Engl J Med. 2019;380(15):1450–1462. [DOI] [PubMed] [Google Scholar]
- 2.de Lope CR, Tremosini S, Forner A, Reig M, Bruix J. Management of HCC. J Hepatol. 2012;56(Suppl 1):S75–S87. doi: 10.1016/S0168-8278(12)60009-9 [DOI] [PubMed] [Google Scholar]
- 3.Lai EC, Lau WY. The continuing challenge of hepatic cancer in Asia. Surgeon. 2005;3(3):210–215. doi: 10.1016/S1479-666X(05)80043-5 [DOI] [PubMed] [Google Scholar]
- 4.Llovet JM, Bru C, Bruix J. Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin Liver Dis. 1999;19(3):329–338. doi: 10.1055/s-2007-1007122 [DOI] [PubMed] [Google Scholar]
- 5.European Association for the Study of the Liver. Electronic address eee and European Association for the Study of the L. EASL clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2018;69(1):182–236. doi: 10.1016/j.jhep.2018.03.019 [DOI] [PubMed] [Google Scholar]
- 6.Marrero JA, Kulik LM, Sirlin CB, et al. Diagnosis, staging, and management of hepatocellular carcinoma: 2018 practice guidance by the American Association for the Study of Liver Diseases. Hepatology. 2018;68(2):723–750. doi: 10.1002/hep.29913 [DOI] [PubMed] [Google Scholar]
- 7.European Association For The Study Of The L, European Organisation For R and Treatment Of C. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2012;56(4):908–943. doi: 10.1016/j.jhep.2011.12.001 [DOI] [PubMed] [Google Scholar]
- 8.Dufour JF, Bargellini I, De Maria N, De Simone P, Goulis I, Marinho RT. Intermediate hepatocellular carcinoma: current treatments and future perspectives. Ann Oncol. 2013;24(Suppl 2):ii24–ii29. doi: 10.1093/annonc/mdt054 [DOI] [PubMed] [Google Scholar]
- 9.Forner A, Llovet JM, Bruix J. Chemoembolization for intermediate HCC: is there proof of survival benefit? J Hepatol. 2012;56(4):984–986. doi: 10.1016/j.jhep.2011.08.017 [DOI] [PubMed] [Google Scholar]
- 10.Hsu CY, Hsia CY, Huang YH, et al. Comparison of surgical resection and transarterial chemoembolization for hepatocellular carcinoma beyond the Milan criteria: a propensity score analysis. Ann Surg Oncol. 2012;19(3):842–849. doi: 10.1245/s10434-011-2060-1 [DOI] [PubMed] [Google Scholar]
- 11.Yin L, Li H, Li AJ, et al. Partial hepatectomy vs. transcatheter arterial chemoembolization for resectable multiple hepatocellular carcinoma beyond Milan criteria: a RCT. J Hepatol. 2014;61(1):82–88. doi: 10.1016/j.jhep.2014.03.012 [DOI] [PubMed] [Google Scholar]
- 12.Bolondi L, Gramantieri L. From liver cirrhosis to HCC. Intern Emerg Med. 2011;6(Suppl 1):93–98. doi: 10.1007/s11739-011-0682-8 [DOI] [PubMed] [Google Scholar]
- 13.Poon RT, Fan ST, Lo CM, Liu CL, Ng IO, Wong J. Long-term prognosis after resection of hepatocellular carcinoma associated with hepatitis B-related cirrhosis. J Clin Oncol. 2000;18(5):1094–1101. doi: 10.1200/JCO.2000.18.5.1094 [DOI] [PubMed] [Google Scholar]
- 14.Xie ZB, Zhu SL, Peng YC, et al. Postoperative hepatitis B virus reactivation and surgery-induced immunosuppression in patients with hepatitis B-related hepatocellular carcinoma. J Surg Oncol. 2015;112(6):634–642. doi: 10.1002/jso.24044 [DOI] [PubMed] [Google Scholar]
- 15.Lau WY, Lai EC. The current role of radiofrequency ablation in the management of hepatocellular carcinoma: a systematic review. Ann Surg. 2009;249(1):20–25. doi: 10.1097/SLA.0b013e31818eec29 [DOI] [PubMed] [Google Scholar]
- 16.Bruix J, Sherman M, Practice Guidelines Committee AAftSoLD. Management of hepatocellular carcinoma. Hepatology. 2005;42(5):1208–1236. doi: 10.1002/hep.20933 [DOI] [PubMed] [Google Scholar]
- 17.Mok TS, Yeo W, Yu S, et al. An intensive surveillance program detected a high incidence of hepatocellular carcinoma among hepatitis B virus carriers with abnormal alpha-fetoprotein levels or abdominal ultrasonography results. J Clin Oncol. 2005;23(31):8041–8047. doi: 10.1200/JCO.2005.01.9927 [DOI] [PubMed] [Google Scholar]
- 18.Lin CT, Hsu KF, Chen TW, et al. Comparing hepatic resection and transarterial chemoembolization for Barcelona Clinic Liver Cancer (BCLC) stage B hepatocellular carcinoma: change for treatment of choice? World J Surg. 2010;34(9):2155–2161. doi: 10.1007/s00268-010-0598-x [DOI] [PubMed] [Google Scholar]
- 19.Tranchart H, Chirica M, Sepulveda A, et al. Long-term outcomes following aggressive management of recurrent hepatocellular carcinoma after upfront liver resection. World J Surg. 2012;36(11):2684–2691. [DOI] [PubMed] [Google Scholar]
- 20.Imamura H, Matsuyama Y, Tanaka E, et al. Risk factors contributing to early and late phase intrahepatic recurrence of hepatocellular carcinoma after hepatectomy. J Hepatol. 2003;38(2):200–207. doi: 10.1016/S0168-8278(02)00360-4 [DOI] [PubMed] [Google Scholar]
- 21.Kobayashi A, Miyagawa S, Miwa S, Nakata T. Prognostic impact of anatomical resection on early and late intrahepatic recurrence in patients with hepatocellular carcinoma. J Hepatobiliary Pancreat Surg. 2008;15(5):515–521. doi: 10.1007/s00534-007-1293-7 [DOI] [PubMed] [Google Scholar]
- 22.Rossi S, Ravetta V, Rosa L, et al. Repeated radiofrequency ablation for management of patients with cirrhosis with small hepatocellular carcinomas: a long-term cohort study. Hepatology. 2011;53(1):136–147. doi: 10.1002/hep.23965 [DOI] [PubMed] [Google Scholar]
- 23.Cho E, Cho HA, Jun CH, Kim HJ, Cho SB, Choi SK. A review of hepatocellular carcinoma in elderly patients focused on management and outcomes. In Vivo. 2019;33(5):1411–1420. doi: 10.21873/invivo.11618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yu NC, Chaudhari V, Raman SS, et al. CT and MRI improve detection of hepatocellular carcinoma, compared with ultrasound alone, in patients with cirrhosis. Clin Gastroenterol Hepatol. 2011;9(2):161–167. doi: 10.1016/j.cgh.2010.09.017 [DOI] [PubMed] [Google Scholar]
- 25.D’Hondt M, Vandenbroucke-Menu F, Preville-Ratelle S, et al. Is intra-operative ultrasound still useful for the detection of a hepatic tumour in the era of modern pre-operative imaging? HPB. 2011;13(9):665–669. doi: 10.1111/j.1477-2574.2011.00349.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chang WT, Kao WY, Chau GY, et al. Hepatic resection can provide long-term survival of patients with non-early-stage hepatocellular carcinoma: extending the indication for resection? Surgery. 2012;152(5):809–820. doi: 10.1016/j.surg.2012.03.024 [DOI] [PubMed] [Google Scholar]
- 27.Zhang W, Jiang L, Yan L, et al. Radiofrequency ablation for HCC patients with multifocal tumours meeting the Milan criteria: a single-centre experience. Dig Liver Dis. 2016;48(12):1485–1491. doi: 10.1016/j.dld.2016.07.018 [DOI] [PubMed] [Google Scholar]
- 28.Pawlik TM, Izzo F, Cohen DS, Morris JS, Curley SA. Combined resection and radiofrequency ablation for advanced hepatic malignancies: results in 172 patients. Ann Surg Oncol. 2003;10(9):1059–1069. doi: 10.1245/ASO.2003.03.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sandonato L, Cipolla C, Soresi M, et al. Combined hepatectomy and radiofrequency ablation for multifocal hepatocellular carcinoma: a case report. Cases J. 2009;2:7987. doi: 10.4076/1757-1626-2-7987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Eisele RM, Zhukowa J, Chopra S, et al. Results of liver resection in combination with radiofrequency ablation for hepatic malignancies. Eur J Surg Oncol. 2010;36(3):269–274. doi: 10.1016/j.ejso.2009.07.188 [DOI] [PubMed] [Google Scholar]
- 31.Choi D, Lim HK, Joh JW, et al. Combined hepatectomy and radiofrequency ablation for multifocal hepatocellular carcinomas: long-term follow-up results and prognostic factors. Ann Surg Oncol. 2007;14(12):3510–3518. [DOI] [PubMed] [Google Scholar]
- 32.Germani G, Pleguezuelo M, Stigliano R, Burroughs AK. Risk of seeding is reduced by associating diagnostic biopsy with percutaneous ablation for hepatocellular carcinoma. Gut. 2009;58(5):734–735. [PubMed] [Google Scholar]