Abstract
Gliomas are the most common tumor of the central nervous system. However, the presence of the brain barrier blocks the effective delivery of drugs and leads to the treatment failure of various drugs. The development of a nanoparticle drug delivery system (NDDS) can solve this problem. In this review, we summarized the brain barrier (including blood–brain barrier (BBB), blood–brain tumor barriers (BBTB), brain–cerebrospinal fluid barrier (BCB), and nose-to-brain barrier), NDDS of glioma (such as passive targeting systems, active targeting systems, and environmental responsive targeting systems), and NDDS efficacy improvement strategies and deficiencies. The research prospect of drug-targeted delivery systems for glioma is also discussed.
Keywords: glioma, brain barrier, nanoparticle drug delivery system, efficacy improvement strategies, deficiencies of NDDS
Introduction
Cancer remains the most threatening disease to human life and health. The cancer mortality rate remains alarming despite recent advances.1 In 2016, there were 330,000 new cases and 227,000 deaths from central nervous system (CNS) tumors.2 Glioma is the most common CNS tumor (40–50%) with an annual incidence of 3–8 cases/100,000 people.3 The World Health Organization (WHO) classifies glioma into four grades: WHO grade I and II tumors are low-grade gliomas including astrocytomas, oligodendrogliomas, dysembryoplastic neuroepithelial tumor, gangliogliomas, and mixed glioma. WHO III and IV are high-grade gliomas (malignant gliomas, MG) including anaplastic astromassa, oligodendroglioma, ependymoma, glioblastoma, and gliosarcoma. MG is more aggressive and worse prognosis. The 5-year survival rate in grade IV patients was less than 5%.4,5 Three conventional methods are usually used in clinical treatment: surgical treatment, radiotherapy, and chemotherapy.6 However, it is difficult to carry out an accurate resection due to the infiltrative growth of glioma.7,8 Furthermore, radiotherapy and chemotherapy greatly and negatively impact the quality of life.9–11
Nano-targeted agents are drug delivery systems that integrate drugs into different nanocarriers to concentrate them in targeted tissues/organs.12 This strategy is also called a nanoparticle drug delivery system (NDDS) and offers high drug stability, sustained release potential, and low drug toxicity to solve these issues.12–15 NDDS can increase the blood-drug concentration, prolong the half-life, and reduce the drug delivery frequency by improving solubility, stability, and bioavailability of hydrophobic drugs. Thus, NDDS is an active area of research. However, the diagnosis and treatment of glioma remain difficult because the brain barrier restricts drug transport to the brain.
In this review, we summarize the biological characteristics of various brain barriers and other neural barriers that limit the delivery of brain-targeted drugs as well as the shortcomings of existing work. The latest progress in targeted drug delivery in glioma was discussed (Figure 1). The characteristics and advantages of NDDS are introduced, and strategies for enhancing brain targeting drug delivery are summarized. Finally, possible development directions are predicted.
Figure 1.
Schematic diagram of this review.
Research Difficulties or Challenges
Brain Barriers
Brain barriers are structures that prevent certain harmful substances from entering the brain. They are composed of blood–brain barrier (BBB), blood–cerebrospinal fluid barrier, and brain–cerebrospinal fluid barrier (BCB).16 In addition, there are blood-brain tumor barriers (BBTB) in brain tumor tissues.17
BBB
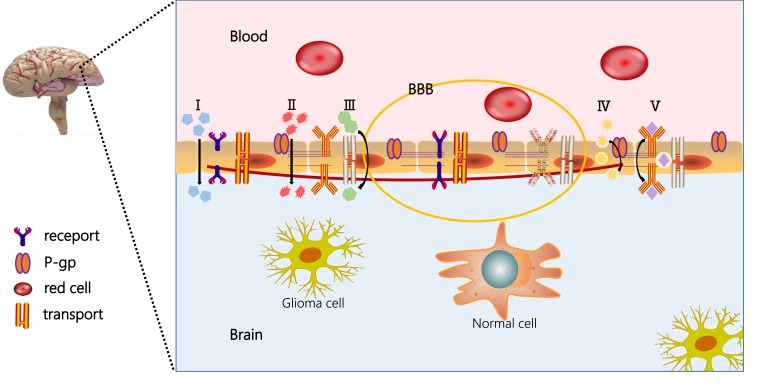
Ehrlich18 first identified BBB in 1885 when he discovered that intravenous dyes could stain most organs except the brain. The BBB excludes almost all macromolecular drugs and more than 98% of small molecules.18,19 The BBB is mainly composed of brain capillary endothelial cells (BCECs), which can differentiate into pericytes, astrocytes, and neurons.20 Most importantly, the BCECs prevent cross-cell transport of compounds from blood to brain, which greatly restricts the passive diffusion of compounds.21,22 Therefore, many studies have worked to develop targeted-NPs that can overcome BBB to improve the treatment and diagnosis of glioma—examples include liposomes, nanomicelles, microspheres, or nanoemulsions.23 The structure and drug transport route of the BBB is shown in Figure 2.
Figure 2.
Structure and drug transport route of BBB. (I) penetrating through the tight junctions; (II) passive diffusion across the endothelial cells; (III) carrier-mediated transport; (IV) adsorption-mediated transcytosis or endocytosis; and (V) receptor-mediated transcytosis.
BBTB
The vascular permeability increased when the BBB was destroyed,24 but some nourishing blood vessels of the tumor retained the characteristics of the BBB, which forms the BBTB.17,25 Similar to the BBB, the BBTB is composed of specific endothelial cells located between tumor cells and tumor blood vessels.26 Some receptors are highly expressed in the blood vessels of brain tumors and thus facilitate ligand-dependent drug transport to provide convenience for targeting drugs in tumor tissues.16,17,24 The BBTB contains three distinct microvascular groups: continuous, nonporous capillaries (similar to normal blood vessels), continuous, porous capillaries, and capillaries with gaps between endothelial cells.17 Although the BBTB is more permeable than the BBB, its presence still prevents the use of drugs in cancer therapy.27 Therefore, finding a way to facilitate drugs to cross the BBTB is crucial for drug treatment of glioma.
BCB
In the ventricular system, a ventricular tube separates the cerebral spinal fluid and brain tissue. The BCB28 is composed of ependymal epithelial cells and astrocytes, and its high permeability makes it easy for substances in cerebrospinal fluid to enter brain tissue through ependymal. Therefore, drugs that do not easily cross the BBB can be injected directly into the cerebrospinal fluid in clinical practice so that it can enter the brain tissue quickly.29 After intrathecal injection, drugs can pass through the subarachnoid space and reach the cerebrospinal fluid to directly reach the brain tissue without passing the BBB. It has rapid effects and is suitable for the prevention and treatment of meningopathy or malignant tumor metastasis.30
Nose to Brain Barrier
Nasal administration can also allow more drugs to reach the brain than peripheral intravenous administration. There are two parts of the nasal cavity (the breathing area and the olfactory area)—these are responsible for absorbing drugs into the brain or blood.31 Some drugs are absorbed into blood circulation through the mucous membrane of the respiratory department and then enter the brain tissue through the BBB. Other drugs bypass the BBB and enter the brain tissue directly through the olfactory mucous membrane or nerve. Of these pathways, the olfactory mucous pathway is the fastest and main route—the drugs travel from the nasal cavity to the brain. Indeed, humans only receive tiny amounts of drugs (25–200 µL) from the 3–10% mucous membrane, which limits the concentration of drugs delivered to the brain. Moreover, the clearance of nasal cilia can shorten the drug absorption time. Drug metabolism and secretion can also inhibit drug access to the brain. Furthermore, long-term nasal administration can produce toxic and side effects on the nasal mucosa and cilia.32
Security Issues
Anti-tumor drugs can have off-target effects including neurotoxicity to normal tissues due to low selectivity. Furthermore, drug resistance and dose toxicity after long-term chemotherapeutic drug use are also serious problems.10 Second, drug carriers generally have poor biodegradability and long-term safety concerns.33 Peptides and antibodies are exogenous substances used for targeting but are often not selective to the brain and are highly unstable and immunogenic. Third, carrier-mediated active targeting and endocytosis are highly dependent on the corresponding receptor expression density of glioma cells. Individual differences are common among patients. Thus, it is quite likely to activate the rejection mechanism of the immune system (reticuloendothelial system and mononuclear phagocytic system).16,22,34
Molecular Weight of Drug Carriers
PEG or other polymers are commonly used as drug carriers in NDDS. However, the molecular weights (MW) of polymers range from a few thousand to tens of thousands. Polymers with appropriate MW can reduce the adsorption of serum protein and the recognition ability of the immune system. PEG2000, PEG3000, and PEG3400 are often used, but there are few studies that carefully evaluate the most appropriate MW in glioma therapy. Different MW polymers should be used for NPs with different targeted functions. For the passive targeting drug delivery system (PTDDS) and the environmental responsive targeting drug delivery systems (ERTDDS), polymers with a large MW can lead to sufficient residence time to act on the EPR effect and promote NPs to enter the target site. For the active targeting drug delivery systems (ATDDS), polymers with a small MW can minimize interference between BBB-targeted ligands and BBB interactions—this is more conducive to vector-mediated endocytosis.35
Properties of NPs
In addition, the size and morphology of NPs can greatly impact the efficiency of glioma treatment. The size only influences the tumor retention and penetration and also affects the BBB targeting and penetrating efficiency. Size changes are an enhancement strategy for glioma therapy and have been described in detail in “Reduction of the particle size of drug delivery system”. The shape of nanoparticles also affects endocytosis between BBB and cell membranes. Rod/chain-like targeted NPs have higher brain accumulation than spherical NPs.36 Other NP features include surface charge, ligand density, ratio of dual-targeting systems, and water solubility.
Application of NDDS
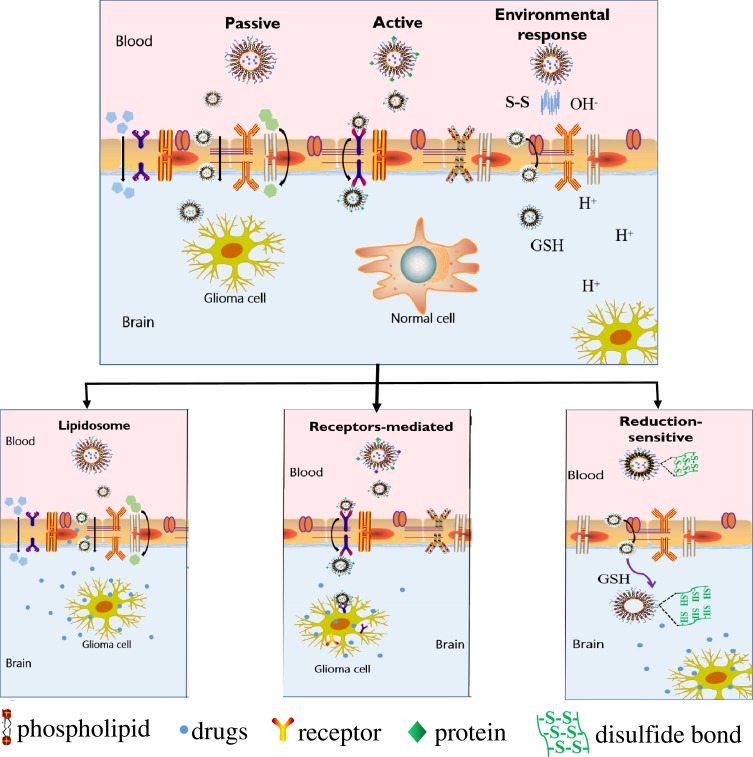
NDDS can be classified into passive targeting systems, active targeting systems, and environmental responsive targeting systems according to their different modes of action.37,38 Part of the glioma drug delivery system and the relative mechanisms are shown in Figure 3 and Table 1. Nano-carriers are mainly used in passive targeting systems to encapsulate drugs such as nanoparticles, liposomes, and microspheres.39 Drug-containing nano-carriers are naturally engulfed through the physiological process of cell endocytosis to achieve targeted drug distribution. Besides, various targeted molecules act as “missiles” to modify drug-carrying nano-carriers such as proteins, antibodies, small molecules, or nucleic acid aptamers. These are common in active-targeting systems and deliver drugs through a directional role to target sites.40 Moreover, the physicochemical targeting system could realize the targeted distribution of drug-carrying nanocarriers in the body through physical and chemical effects such as magnetism, heat, sound, light, electricity, and pH.41
Figure 3.
Schematic diagram of the classification and transport mechanism of a targeted drug delivery system.
Table 1.
Application and Mechanism of Glioma Drug Delivery Systems
| NDDS | Types | Mechanisms | Ref. |
|---|---|---|---|
| PTDDS | |||
| Liposomes | Phospholipid bilayer | EPR effect/across the endothelial cells of BBB | 46,47 |
| Microemulsions | P-gp/endocytosis | EPR effect/inhibition of P-gp efflux/across the endothelial cells of BBB | 49,51 |
| Niosome | P-gp | EPR effect/inhibition of P-gp efflux | 87,88 |
| Nanoparticles | Endothelial cells | EPR effect/transcytosis or endocytosis | 57,59 |
| ATDDS | |||
| Receptor-mediated | Insulin | Endocytosis/insulin receptor | 89 |
| Transferrin | Endocytosis/transferrin receptor | 71 | |
| Lactoferrin | Endocytosis/lactoferrin receptor | 73,74 | |
| Endothelial growth factors | Endocytosis/endothelial growth factors receptor | 90 | |
| Amino acids | Endocytosis/amino acids receptor | 91 | |
| Apolipoproteins/ angiopep-2 | Endocytosis/low density lipoprotein receptor | 92,93 | |
| H-ferritin | Endocytosis/HFn receptor (transferrin receptor 1) | 72 | |
| Peptide-mediated | iRGD | Endocytosis/αvβ3/αvβ5 | 78 |
| RDP | Endocytosis/nerve-penetrating properties | 82,83 | |
| Chlorotoxin | Endocytosis/ matrix metalloproteinase-2 | 54 | |
| T7 | Endocytosis/transferrin receptor | 94 | |
| CDX peptide | Nicotinic acetylcholine receptors | 95 | |
| F3 | Bind to nucleolin | 96 | |
| CendR motif | Neuropilin-1/across the endothelial cells | 97 | |
| Small molecule-mediated | Folic acid | Folate receptor | 85 |
| Apt-mediated | AS1411/FB4 | Recognize proteins/phospholipids/ nucleic acids | 98,99 |
| Cytokine-mediated | Interleukin-13 | IL-13Rα2 receptor | 100 |
| Matrix metalloproteinases-mediated | MMP-9 | Key modulators of tumor invasion/ metastasis | 101 |
| Cell-mediated | Mesenchymal stem cells | Endocytosis/across the endothelial cells of BBB | 102 |
| Macrophages | Endocytosis/across the endothelial cells of BBB | 103 | |
| Dual targeting-mediated | Transferrin-folic acid | Promote drug across BBB/targeting tumor cells | 66 |
| Ang-2-VEGF receptors | Endothelial growth factors receptor/low density lipoprotein receptor | 104 | |
| T7-DA7R-LS | Transferrin receptor/VEGF receptor 2 | 105 | |
| Thermosensitive-P1NS | Winding-contraction phase transition /nerve-penetrating properties | 106 | |
| Transferrin-pH | Promote drug across BBB/pH difference | 107 | |
| pH-reduction | pH difference and glutathione/dithiothreitol | 108 | |
| ERTDDS | |||
| Photo-sensitive | Photosensitizer/photothermal agent | ROS/singlet oxygen/local hyperthermia | 109–111 |
| pH-sensitive | Acidic microenvironment | pH difference | 112 |
| Reduction-sensitive | Disulfide bond | Glutathione/dithiothreitol | 113,114 |
| Magnetic sensitive | Magnetic resonance | The magnetic field | 115 |
| Ultrasonic sensitive | Ultrasonic | Ultrasonic cavitation effect | 116 |
| Thermo-sensitive | Hydrogen bonding | Winding-contraction phase transition | 106 |
Passive Targeting Drug Delivery System
The PTDDS mainly delivers drugs to tumor tissues based on the “enhanced permeability and retention effect” (EPR effect) in tumor tissues instead of normal tissues.42 Interestingly, in the late stage of brain tumors, passive targeting nanoparticles can even enter the brain tumors through gaps in the endothelium due to the weak difference of EPR effect between tumor tissues and peripheral tissues. The most common passive-targeting drug delivery systems include liposomes, microemulsions, and nanoparticles.
Liposomes
Liposomes are spherical vesicles formed by phospholipid bilayer membranes that have attracted wide interest in biocompatibility and targeting with BBB. They are a common carrier for glioma PTDDS.
The elemene liposome (EL) injection was approved by the China Food and Drug Administration (CFDA) in 1994. More than 20 years of clinical studies have shown that EL is a non-cytotoxic anti-cancer drug with a high content of anti-cancer active ingredients (β-elemene, 85%).43,44 It can suppress varieties of cancer cells and improve the immune system. It has obvious effects on improving patients’ quality of life, prolonging the survival period, resisting metastasis and recurrence, and reversing multiple-drug resistance (MDR), etc. EL has benefited more than 7.07 million cancer patients in southeast Asia, Hong Kong, Japan, Korea, Europe, and the US.45 Zhu et al46 found that EL (48 h) significantly accelerated the apoptosis initiation time of C6 glioma cells versus the normal elemene group (72 h). Moreover, Gao et al47 formulated the temozolomide-liposome (TL) that could be distributed in the brain. The t1/2, mean residence time (MRT), Cmax, and AUC values of TL were several-fold higher than the solution, which showed that TL could improve the therapeutic effect of the brain, reduce heart-lung toxicity, and prolong the circulation time of TZM in vivo.
Microemulsions
Microemulsions are drug delivery systems formed spontaneously in the water phase and oil phase with surfactant and cosurfactants. Oil phase components can increase the affinity of drugs to BBB endothelial cells, and surfactants/cosurfactants can inhibit or reduce P-gp efflux.45,48
Li et al49 constructed a microemulsion containing linoleic acid conjugated with paclitaxel (CLA-PTX) against brain tumors. The CLA-PTX microemulsion had a lower IC50 and a higher tumor inhibition rate than CLA-PTX solution and free PTX, which is possibly because of the higher affinity of CLA-PTX to BBB endothelial cells and the enhanced permeability and retention effect for passive targeting. Xie et al50,51 prepared a hyaluronic acid chitosan-based microemulsion (HAC-ME), which significantly increased the BBTB permeability of glioma cells after 0.25 h of administration. The material down-regulated the expression level of the tight junction protein claudin-5. Carboplatin combined with HAC-ME can promote the apoptosis of glioma cells versus free carboplatin.
Nanoparticles
Nanoparticles are widely used in the treatment of glioma.52–54 An et al55 designed realgar nanoparticles (RN) that could significantly increase the proportion of C6 cells in S and G2/M phases. This decreased the proportion of C6 cells in the G0/G1 phase, downregulated Bcl-2 expression, and substantially upregulated Bax expression versus free realgar and control. Jain et al56 fabricated surface-modified PLA nanoparticles loaded with TZM. Such PLA-NP nanoparticles can significantly increase the accumulation of the macromolecular dye coumarin-6 in the brain and reduce the accumulation in other organs. These results indicated that the nanoparticles could enhance the permeability of drug transport across the BBB into the brain. Thus, nanoparticles have potential value in glioma treatment and may reduce drug toxicity to other organs.
Many other nanomaterials have been used to treat glioma including chitosan that can effectively prolong the time of drug action and increase circulation.57 Dendritic polymers can improve drug release and biocompatibility.58 Macromolecular block copolymer micelles have a long cycle capacity and high stability.59
Active Targeting Drug Delivery Systems
However, the PTDDS has unfortunately not shown clinical value due to a lack of selectivity and affinity—this can lead to toxicity and side effects in normal tissues.34,40,60 Therefore, many researchers are working on ATDDS to achieve selective treatment of glioma.61 ATDDS are designed to exploit differences in receptors or antigen expression on the surface of tumor cells and normal cells. By virtue of the high affinity between ligand and receptors, ATDDS can achieve higher cross-BBB transport ability or tumor penetration. These features promote selective drug targeting with anti-tumor activity. Common active targeting vectors include small molecules, proteins, peptides, and aptamers; examples include folic acid, transferrin, and the RGD peptide.62–69
Receptors-Mediated ATDDS
Many receptors are expressed in the BBB including receptors of insulin, transferrin, endothelial growth factors, and amino acids. Receptor-mediated endocytosis is one of the main mechanisms by which drugs cross the BBB into the brain.70 Herein, researchers have modified nano-carriers with proteins to improve the ability of transporting across BBB and to improve the effectiveness of glioma drugs.
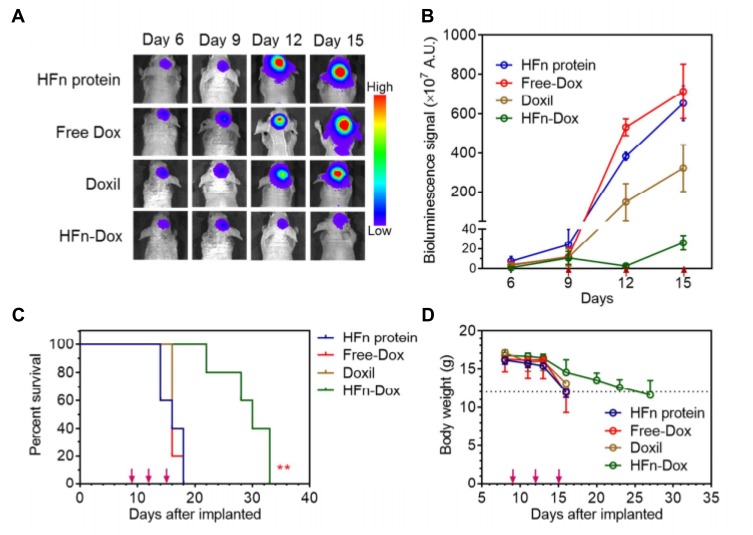
Sun et al71 fabricated TMZ/transferrin (Tf)-nanoparticles (PAMAM-PEG-Tf/TMZ) that successfully crossed the BBB and killed glioma tumor cells. These particles lead to a significantly longer MST in mice with gliomas. Fan et al72 prepared a human H-ferritin (HFn) nanocarrier. The main entry point is HFn receptor 1, which is overexpressed in BCECs and glioma cells. In contrast, when specifically targeted and loaded into glioma cells, nearly all HFn accumulates in lysosomes leading to the death of glioma tumor cells—the surrounding healthy brain tissues do not have HFn aggregation. Such anti-glioma tumor activity is shown in Figure 4.
Figure 4.
HFn-encapsulated Dox effectively improves anti-glioma tumor activity. (A) In vivo BLI images of GBM tumor cells in orthotopic mice that were intravenously injected with different formulations, ie, HFn-Dox, Doxil, free Dox, and HFn protein. (B) Quantitative analysis (n=5) of the BLI signals of (A). The red arrows indicate the time points of administration. (C) Animal survival curves in different groups. Asterisks indicate that the difference between HFn-Dox and free Dox or Doxil was statistically significant (Kaplan–Meier, p=0.0019 and 0.0023, respectively). (D) The effect of different treatments on mouse body weight (mean±SD, n=5). Reprinted with permission from Fan K, X Jia, M Zhou, et al Ferritin Nanocarrier Traverses the Blood Brain Barrier and Kills Glioma. ACS Nano. 2018; 12(5): 4105–4115. Copyright (2018) American Chemical Society.72
Similarly, Chen and Li et al73,74 used a lactoferrin-modified PEG-poly(D,L-lactide-co-glycolide) (PLGA) nanoparticles loaded with shikonin and procationic liposomes loaded with doxorubicin (DOX) for glioma therapy. The drug delivery system modified by lactoferrin can promote the drug-carrying nanoparticles across BBB so that the drugs can be enriched in the brain and achieve better efficacy.
Peptides-Mediated ATDDS
Polypeptides are small molecules composed of 2–50 amino acids with non-advanced structure. When ligand peptides bind to receptors, nano-carriers linked to peptides generally enter cells through receptor-mediated endocytosis. For example, RGD peptide including cyclic RGD peptide (cRGD) or internalizing RGD peptide (iRGD).75–78 Cell-penetrating peptides (CPPs) such as rabies-derived peptide (RDP) are also of interest because they can transport exogenous molecules to tumor tissues.79–81
Gu et al78 modified PEG-PLA nanoparticles containing PTX with MT1-AF7p peptide (MT1-NP-PTX). This material has obvious anti-proliferation activity on C6 glioma cells with IC50 values of 2.81- and 2.47-fold lower than free PTX and NP-PTX, respectively. The percentage apoptosis of MT1-NP-PTX was the highest versus PTX and NP-PTX, which suggested more PTXs were delivered when combined with iRGD (high-affinity for cancer-related integrins such as αvβ3 and αvβ5). Zhao et al82 established RDP-conjugated curcumin-coated nanoliposomes (RCL). Expectedly, the growth inhibition efficiency of RCL was 3.33-fold higher than curcumin liposomes (CL) in U251MG. The results of anti-glioma activity showed the survival time of glioma model mouse in the RCL group was longer than model and CL. In addition, RDP-p53 could efficiently and selectively enter and inhibit SH-SY5Y cells; the total percentage of apoptotic cells of RDP-p53 significantly improved versus the PBS and p53.83 All of the above showed that RDP-mediated nano-carriers were promising options for glioma therapy.
Small Molecule-Mediated ATDDS
Small molecules, such as folic acid (FA), biotin, and bisphosphate, are often used as targeted ligands in ATDDS for tumors. They offer good safety, no immunogenicity, and easy modification. FA is the most widely used because the folate receptor (FR) is highly expressed on many cancers but not in normal tissues.84
Xu and Li et al85,86 proposed a nano-targeted delivery of DOX for glioma based on PAMAM G5 dendrimer. The therapeutic effect of glioma was significantly improved after FA modification. The tumor growth inhibition of FA-PAMAM/DOX was increased up to 57.44%, while free DOX was 17.70% only. Meanwhile, FA-PAMAM/DOX could significantly reduce tumor volume in mice. Moreover, the data show that FA-PAMAM/DOX could significantly prolong the half-life time and improve DOX accumulation in brain tumor versus DOX. The MST of xenograft rats of FA-PAMAM/DOX was obviously longer than free DOX. This novel strategy of targeting nano-carriers modified with FA offers a promising way to increase the accumulation of drugs at tumor sites.
Aptamer-Mediated ATDDS
Aptamers (Apt) are oligonucleotides that can be synthesized chemically to promote targeted drug delivery. Compared with other targeted molecules, Apt attracted more and more attention due to their specific binding ability, high affinity, and low immunogenicity. Apt could easily penetrate tumor tissues because they can specifically identify target molecules such as proteins, phospholipids, sugars, and nucleic acids.117 Of these, AS1411 has extensive application value and potential development ability in clinical treatment of tumor—it could inhibit the growth of a variety of tumor cells without affecting normal cells.118,119
Luo et al98 established a nanoparticle with AS1411-functionalized poly (l-γ-glutamyl-glutamine)-PTX (AS1411-PGG-PTX). The percentage of apoptosis of S1411-PGG-PTX was significantly increased versus PGG-PTX. In addition, the MST of mice in the AS1411-PGG-PTX group was significantly longer than the PTX group and the saline group. This might be due to the good stability in blood, long circulation time, and more accumulation of PTX in glioblastoma tissues. Mu et al99 combined FB4—a segment of RNA that can specifically bind to transferrin receptor—with micelles loaded with flurbiprofen and conducted in vitro experiments with bEND5 cells (brain microvascular endothelial cells) in mice. Compared with untargeted micelles, the drug concentration in the micelles with FB4 ligand increased by 1.67-fold.
Cytokine-Mediated ATDDS
Human interleukin-13 (IL-13) is a cytokine secreted by activated T cells and has two receptors (IL-13/4R and IL-13Rα2). It can induce pro-inflammatory and anti-inflammatory immune responses.120,121 IL-13/4R is present in normal cells and binds to IL-4, whereas IL-13Rα2 is associated with glioblastoma but not expressed in normal tissues. A recent study showed that IL-13Rα2 receptor is overexpressed in the most common hair cell astrocytomas in children.121–123 Therefore, it is a promising target for cytotoxic drugs in various brain tumors.
For example, Madhankumar et al124,125 prepared a DOX liposome modified with IL-13 to guide the liposome to the tumor site by binding to the over-expressed IL-13Rα2 receptor in glioblastoma. Compared with free DOX, liposomes modified with IL-13 enhanced cytotoxicity and increased the accumulation/retention of DOX in glioma cells. After 7 weeks of i.v. injection, the average tumor volume in IL-13-modified DOX liposome group had a 5-fold reduction compared with the non-targeted DOX liposome group. These data show that IL-13 targeted nanovesicles are a viable option for the treatment of brain tumors.
Stem Cell-Mediated ATDDS
Recent studies showed that the application of cell-modified nanoparticles for drug delivery system is an effective method to encourage the drugs to pass through for drug delivery. These drugs have an affinity for brain tumors and can be used as a drug carrier for the treatment of glioma such as mesenchymal stem cells (MSCs).126–128
Wang et al102 manufactured MSCs loaded with PTX-encapsulated PLGA NPs to demonstrate the therapy for orthotopic glioma in rats. The PTX-migration rate of MSC-loaded PTX-PLGA NPs increased by 44.4±5.4%, and MSC-loaded PTX-PLGA NPs had enhanced sustained PTX release to induce glioma cell death compared with PTX-PLGA NPs. Survival was significantly longer in control MSC-loaded PTX-PLGA NPs than those injected with PTX-PLGA NPs alone. These data suggest that MSC-loaded PTX-PLGA NPs are effective for glioma treatment and are a promising strategy for tumor-targeted therapy.
Dual-Targeting-Mediated ATDDS
Compared with PTDDS, ATDDS enhances the efficacy of glioma treatment via the two following aspects: (1) They promote drug delivery systems for transport across the BBB; (2) They selectively target glioma cells while increasing the distribution of drugs in glioma and reduce their distribution in normal brain tissues.129 Currently, most ATDDS have only one feature that allows drugs to be non-selectively distributed throughout the brain after passing through the BBB. This low selectivity can cause some serious neurological side effects. Thus, an ideal glioma TDDS should have the above two characteristics at the same time, ie, dual-targeting-mediated ATDDS. Dual-targeting-mediated ATDDS usually refers to the modification of two functional ligands on the PTDDS. One is targeted to the BBB to promote the cross-BBB transport of the drug delivery system, and the other is targeted to the glioma tissue and further concentrates the drug in the glioma. A ligand, targeting both BBB and glioma, is connected to the PTDDS—this can also be called a dual-targeting-mediated ATDDS.32
Gao et al66 prepared DOX liposomes modified by transferrin and folate (Tf/F-DOX-Lip). A near-infrared fluorescent probe showed that the fluorescence of DOX solution and DOX-Lip was mainly in the cardiac region, but Tf/F-DOX-Lip was mainly distributed in brain—these data illustrated that liposomes modified by transferrin and folate could promote the migration of liposome to the brain. The tumor inhibition rate of Tf/F-DOX-Lip was six-fold higher than DOX-Lip after i.v. administration. The data show that Tf/F-DOX-Lip could not only promote DOX for transport across the BBB but also increase the distribution of DOX in glioma.
Environmental Responsive Targeting Drug Delivery Systems
Traditional drug delivery systems are not ideal for maintaining system stability and promoting drug release at target sites.130 Therefore, the design of ERTDDS has gradually become a research hotspot in the field of pharmacy. To understand the physiological differences between tumors and normal tissues, ERTDDS131 can specifically respond to endogenous or exogenous stimuli including physical (such as photosensitivity109,132,133), chemical (as pH sensitivity134–137 and reduction sensitivity113,138), and biological (as matrix metalloproteinases139) features. ERTDDS only responds to internal and external environmental conditions when it is close to the drug action site—these conditions then trigger the release of drugs in tumor tissues while remaining stable in other tissues. Thus, it can improve the anti-tumor efficacy and reduce the toxicity and side effects.
Photo-Sensitive TDDS
Photosensitive TDDS is a tumor therapy method based on photodynamic therapy (PDT) and photothermal therapy (PTT).109,140-142 It is based on light conditions reaching the photosensitive TDDS in the tumor site. Photosensitizer (PS) or photothermal agent (PA) will absorb the light, and reactive oxygen species (ROS), singlet oxygen, or local hyperthermia will be released. While preserving normal tissues, the nanoparticles selectively and efficiently produce cytotoxic activity and kill tumor cells.
Xu et al109 constructed a therapeutic nano-platform (ICG-SFNPs) with silk fibroin (SF) to use indocyanine green (ICG) for PTT of glioblastoma. Meanwhile, the photothermal effect of ICG-SFNPS is more stable than free ICG under near-infrared radiation (NIR). After local NIR, the temperature of ICG-SFNPS rapidly increased by 33.9°C within 10 min and remained high for a long time to kill tumor cells. The growth of glioblastoma cells was completely inhibited by ICG-SFNPS.
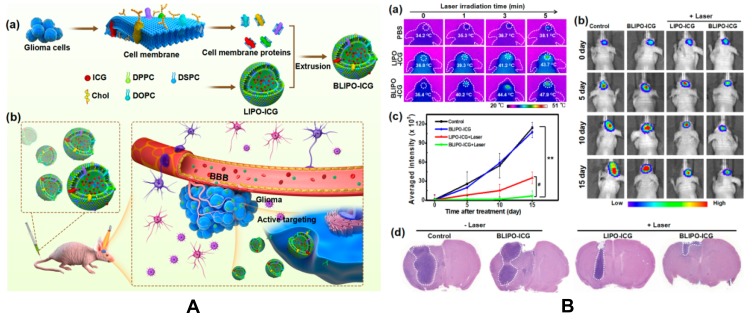
Jia et al142 developed a biomimetic proteolipid nanoparticles (NPs) with ICG for phototherapy of orthotopic glioma in mice. Biomimetic ICG-loaded liposome (BLIPO-ICG) NPs were obtained by embedding the glioma cell membrane protein into NPs. Due to the good homologous targeting and immune escape characteristics, BLIPO-ICG NPs can pass through BBB and specifically bind to glioma cells and reach glioma in early stages. At 12 h post-injection, the signal-to-background ratio in the brain tumor reached 8.4 demonstrating good accumulation. In addition, after NIR (1 W/cm2, 5 min), the photothermal effect of BLIPO-ICG NPs effectively inhibited the proliferation of glioma cells with an inhibition rate of 94.2%. No photothermal damage of normal brain tissue or other side effects of treatment were observed. These results indicate that the bionic protein lipid NP is a promising phototheranostic nanoplatform for brain tumor-specific treatment. The mechanism and anti-glioma tumor activity are shown in Figure 5.
Figure 5.
(A) Schematic illustration of biomimetic proteolipid BLIPO-ICG for crossing the BBB and active targeting delivery of orthotopic glioma. (a) Preparation process of BLIPO-ICG. (b) Schematic of BLIPO-ICG for crossing BBB and active targeting imaging. (B) In vivo PTT of BLIPO-ICG in orthotopic glioma-bearing mice. (a) Representative in vivo infrared thermal images of the brain region before and after 808 nm laser irradiation (1 W/cm2, 5 min). CLIPO-ICG = CBLIPO-ICG = ICG 1 mg/kg. (b) Representative bioluminescent images of C6-Luc glioma-bearing mice in different groups. (c) Semiquantitative bioluminescent signal intensity in the brain. **p < 0.01 versus control. #p < 0.05 versus LIPO-ICG+laser. (d) H&E staining of brain sections of orthotopic glioma-bearing mice in all groups. Scale bar = 200 μm. Reprinted with permission from Jia Y, X Wang, D Hu, et al Phototheranostics: Active Targeting of Orthotopic Glioma Using Biomimetic Proteolipid Nanoparticles. ACS Nano. 2019; 13(1): 386–398. Copyright (2019) American Chemical Society.142
pH-Sensitive TDDS
The normal pH of human blood and interstitial fluid is usually alkaline (pH 7.4), while the tumor microenvironment is acidic (approximately 5.6).143,144 The slightly acidic environment is mainly caused by the rapid proliferation of tumor cells and excessive accumulation of lactic acid. In addition, most tumor cells are characterized by insufficient blood supply and slow excretion, which further acidify the tumor microenvironment. Using pH differences between tumor tissues and normal tissues, numerous researchers have constructed drug delivery systems via pH-responsive materials.112,145-148 These delivery systems can change their physicochemical properties such as swelling and increasing solubility during the transition from weakly alkaline to slightly acidic environment. Thus, it can further trigger the release of drug molecules in the package and play a targeted role in tumor therapy.
Yin et al112 developed a pH-sensitive prodrug (Lf-HA-DOX) by combining hyaluronic acid (HA) with DOX. The release of DOX in Lf-HA-DOX was 45%, 35%, and 12% at pH 5.0, 6.0, and 7.4 at 24 h, respectively. Furthermore, the geometric mean fluorescence intensity (GMFI) of DOX in the free DOX group was almost unchanged when the concentration of DOX changed; it increased in the Lf-HA-DOX group. Similarly, Li et al107 synthesized a pH-sensitive dual-targeting drug carrier (G4-DOX-PEG-Tf-TAM) with conjugated Tf and Tamoxifen (TAM) of the PAMAM dendrimers for enhancing the BBB transportation and improving the drug accumulation in the glioma cells. They found that the DOX release was 32% at pH 4.5 and 6% at pH 7.4 indicating that the drug release is relatively fast at weak acidic conditions and stable in normal physiological environments.
Reduction-Sensitive TDDS
Reduction-sensitive TDDS refers to the particle delivery system connected by a disulfide bond (S-S) between the carrier and the drug. These particles self-assemble in solvent to form a nanostructure.149 Disulfide bonds are a special yet common chemical bond that is stable in normal physiology. However, they are broken in tumor tissues (reductive environment) with high expression of dithiothreitol and glutathione (GSH) to release the drug and achieve targeted release.113,150-153 Indeed, reduction-sensitive TDDS are reduction-sensitive and biodegradable and are an ideal platform for targeted tumor chemotherapy.
Zhu et al113 showed a biodegradable PEG-SS-PCL micelle functionalized by cRGD (cRGD/PEG-SS-PCL). These materials can enhance DOX delivery in a reductive environment (10 mM GSH) to significantly improve tumor inhibition and decrease toxicity upon comparison with non-targeting agents and reduction-insensitive cRGD/PEG-PCL. Su et al150 proposed a novel copolymer mPEG-PAsp (MEA)–CA. The DOX was only slightly released in the absence of GSH. However, there was 37% DOX released after only 2 h when 10 mM GSH was added indicating that GSH broke the S-S through a thiol-disulfide exchange reaction to promote the release of DOX.
ERTDDS are also thermosensitive,106 magnetic sensitive,115,154 and ultrasonic sensitive.155 They are a research focus in recent years and can also specifically target the treatment of glioma under different conditions without affecting normal tissues.
Strategies to Improve the Drug Targeting to Glioma
Reduce the Particle Size of the Drug Delivery System
A smaller (200–800 nm) particle can usually be easily endocytosed by phagocytes. However, when the particle size is less than 200 nm, the curvature of local areas on the surface of the carrier will also decrease as the particle size decreases. Therefore, the particles could avoid being adsorbed by receptors on the surface of the phagocytes—they can escape phagocytosis and be cleared by phagocytes.156,157
Wan et al158 placed tartaric acid vinorelbine liposomes with particle size of 200 nm and 800 nm in medium containing mouse mononuclear macrophage RAW264.7 and luciferin, respectively. After 12 h, the probability of devouring liposomes with particle size of 200 nm was 13%, while that of 800 nm was only 8%. Bi et al159 injected curcumin nano-suspension with the size of 70 nm (A) and 200 nm (B) into the body. The AUC0–60min of A was 2.58 times higher than B in the brain. When the size of the curcumin decreased from 200 nm to 70 nm, the AUC0–60min of liver drug also decreased from 8491 ng·min·g−1 to 2300 ng·min·g−1. These results indicate that the size decrease can not only promote drug delivery to the brain but also reduce drug uptake by the liver. This is critical for the treatment of brain diseases and the reduction of drug toxicity and liver side effects.
Structural Modification
Studies have shown that small lipophilic drug molecules with a molecular weight of less than 500 Da can cross the BBB by passive diffusion. Therefore, small-molecule drugs can have improved properties by modifying their polar groups such as esterification and alkylation of hydroxyl or carboxyl groups. Non-polar groups such as methyl and benzene rings can also be introduced to increase lipophilicity and improve BBB transmittance.160,161
Chen et al162 designed and synthesized a series of novel derivatives to improve the anticancer efficacy of natural β-elemene. Most derivatives exhibited significant anti-proliferative activities against the three cancer cell lines (SGC-7901, HeLa, and U87) versus natural β-elemene. Interestingly, these compounds displayed excellent sensitivity to glioma cells. Moreover, further mechanistic studies revealed that 11a caused the G2 phase arrest of the cell cycle and induced apoptosis of glioma cells by preventing the activation of the PI3K/Akt pathway. In addition, 11a significantly suppressed the tumor growth in cancer xenograft mouse model, which was superior to β-elemene at the same condition. In conclusion, the remarkable biological properties of these new β-elemene derivatives may make them promising candidates for glioma treatment.
Combination with Resuscitative CHMs
Resuscitative CHMs163,164 are traditional Chinese medicines considered “orifice-opening” agents including musk, borneol, acorus gramineus, and benzoin. With spicy and fragrant attributes, Resuscitative CHMs can better enter the brain tissue through the BBB. The active ingredients have strong lipid solubility and minimal molecular weights. In addition, resuscitative CHMs can also induce other drugs upward into the brain, ie, they can increase the permeability of BBB, promote drugs to pass through the BBB, and improve the efficacy of drugs. The cell structure is basically complete after this action. Pharmacological studies have found that resuscitative CHMs can increase the content of Evans blue (EB) in the brain tissue of normal mice confirming that Resuscitative CHMs can open the BBB in normal mice.
Borneol
The mechanism of borneol passing through BBB may be inhibition of transporters to increase the vasodilatory neurotransmitters and inhibit active transport of ion channels.165,166 P-glycoprotein (P-gp) is an efferent protein on the BBB. It can easily bind with a variety of substrates and expel them from brain tissues to reduce the therapeutic drug effects. Borneol inhibited the expression of P-gp to allow drugs to enter brain tissue. For instance, tanshinol has been used to treat epilepsy and other brain diseases, but it will be excreted by P-gp within a short time after entering the brain. When borneol was introduced into tanshinol to form an esterification complex, it could easily travel through the BBB into the brain for a longer retention time. Moreover, the inhibitory effect on P-gp of borneol made the higher concentration of tanshinol play a role in brain tissue.167 The effect of borneol on aprotinase-modified nanoparticles can significantly increase the absorption of nanoparticles by capillary endothelial cells in the brain of Alzheimer’s disease and can improve memory.168 These data provide a new idea for targeted glioma drug delivery.169
α-Asarone
Wu et al170 evaluated the “orifice-opening” effect of α-asarone (a main active ingredient of acorus gramineus) on the improvement of brain delivery of Puerarin (PUE) and tetramethylpyrazine (TMP). They then investigated whether the enhancing effects were associated with adenosine receptors (ARs)-mediated trans-BBB pathway. Their results indicated that α-asarone significantly increased the accumulation of permeated PUE and TMP, and the enhancing effects could be counteracted by AR inhibitors. Indeed, α-asarone decreased the expression of ZO-1 (an important BBB junction protein) and increased the expression of A1AR and A2AAR. Pharmacokinetic studies showed that oral administration of α-asarone significantly increased AUCbrain 1.34-fold and 1.79-fold for PUE and TMP. These results suggested that α-asarone is an effective adjuvant agent for the delivery of PUE and TMP to the brain.
Application of Physical Technology
During ultrasonic processing, liquids undergo a series of dynamic processes such as vibration, growth, and rupture under the action of sound waves. When physical effects are applied to the cell interface of the surrounding tissues, tight junctions between cells become loose and reversible pores appear. This improves cell endocytosis. In addition, ultrasonic vibration can improve the permeability of cell membrane and promote drug uptake.171,172
EB can bind with plasma albumin to form an EB-albumin complex, which has a large molecular weight and cannot penetrate the complete BBB. The gap between BBB cells becomes larger under the action of ultrasound, which may promote the passage of the complex through the BBB. Based on this hypothesis, Chen et al173 performed ultrasound treatment on the brains of rats successfully transplanted with tumor cells. The results showed that the compound could pass through BBB after i.v. injection. After injection of lipid microbubble solution and saline, the brain tumor sites of the two groups of rats were first treated with ultrasound (1.0 MHz). The EB solution was injected immediately, and glioma tumor tissues were extracted after 72 h. Under confocal laser microscope, only sparse red spots of fluorescence were observed in the control group, while many areas of red fluorescence were observed in the experimental group. This confirms that ultrasound can promote EB through BBB into glioma tissue.
Currently, ultrasound-mediated targeted therapy174 is still in the experimental stage. The appropriate ultrasound parameters for optimal drug delivery must be identified with studies for potential adverse reactions including bleeding, brain injury, and inflammation after the destruction of ultrasound BBB. Nevertheless, this technology is expected to lead to breakthroughs in targeted drug delivery to the brain and cancer therapy after the development of new ultrasound targeted therapy and continuous optimization of ultrasound parameters.
Alternative Routes of Administration
Nasal Drug Delivery
After nasal administration (NA), some drugs bypass the BBB and enter brain tissue directly through the olfactory mucosa or olfactory nerves. Compared with peripheral vein administration, NA allows increased delivery and faster transfer of drugs into the brain.175 Zhang et al176 administrated a β-asarone microemulsion through the nose and tail vein injection on rats. The results showed that β-asarum microemulsion was absorbed into the blood and brain tissues quickly and could be detected at 2 min after NA. However, the ratio of AUCbrain(0–2 min)/AUCplasma(0–2 min) was 1.49, and more drugs entered the brain with a nearly 50% higher amount of drug in brain tissues than in plasma. Meanwhile, a lower amount of drug could enter into the brain after caudal vein administration. Compared with NA, the concentration of β-asarum in the brain after i.v. administration was only 56.85% of the NA. This suggests that NA has unique advantages in promoting drug delivery to the brain and may be one of the pre-selected methods for glioma treatment.
Intrathecal Injection
Intrathecal injection (i.t.) refers to the injection of drugs into the spinal canal. The delivery of the cerebrospinal fluid plays a key role after delivery through the subarachnoid space. This approach is suitable for the prevention and/or treatment of meningeal lesions, malignant tumor metastasis, and other diseases.177 The i.t. offers low dosages and targeted drug delivery. It can be administered by a single puncture or multiple rounds of delivery after the sheath is implanted. After i.t., the drug can directly reach the target site through BCB without crossing the BBB for quick action.
Etminanet et al30 used nimodipine microspheres for intrathecal injection, and the results showed that nimodipine microspheres could prolong drug release time, enhance healing effect, and reduce drug toxicity. While drugs act more directly when given i.t., they can cause more damage. Strategies for enhancing glioma treatment are shown in Table 2.
Table 2.
Strategies for Enhancing the Treatment of Glioma
| Types | Strategies | Mechanisms | Enhancements | Ref. | |
|---|---|---|---|---|---|
| Before | Behind | ||||
| Use new drug delivery system | Free realgar | Realgar nanoparticles | EPR effect/endocytosis | C6 cells apoptosis rate was increased by 5.50 times | 55 |
| Free TMZ | PAMAM-PEG-Tf/TMZ | Endocytosis/Tf receptor | MST of mice bearing gliomas was extended by 22.9 days | 71 | |
| Free TMZ | TMZ-Lf/NPs | Endocytosis/Lf receptor | The concentration of TMZ in brain was higher 3 times; IST increased 1.4 times | 178 | |
| Free DOX | Lf-HA-DOX | Endocytosis/pH difference/Lf receptor | The GMFI of DOX was increased 8.71 times | 112 | |
| Reduce the particle size | 200nm | 70nm | Promote drug delivery to the brain; reduce drug uptake by the liver; avoid being adsorbed by receptors on the surface of phagocytes, escaping phagocytosis and clearance by phagocytes | After 5 minutes of administration, the concentration in the brain improved 2-fold; after 20 min of administration, the concentration in the brain improved 3-fold; AUC0–60 min in the brain was 2.58 times | 159 |
| Structural modification | β-elemene | Derivatives-11a | Caused the G2 phase arrest of the cell cycle; induced apoptosis of glioma cells by preventing the activation of the PI3K/Akt pathway | Tumor inhibitory ratio increased from 49.6% to 64.8% | 162 |
| Combination with resuscitative CHMs | Tanshinol | Tanshinol-borneol esterification complex | Inhibit the expression of P-gp; reduce P-gp efflux | It was easy to travel through BBB to the brain with the higher concentration of tanshinol; had a longer retention time | 167 |
| PUE and TMP | Combination with α-asarone | Decrease expression of ZO-1, while increase the expression of A1AR and A2AAR | The accumulation of permeated PUE and TMP was increased; AUCbrain for PUE and TMP was increased 1.34-fold and 1.79-fold | 170 | |
| Application of physical technology | EB-albumin complex | Performed ultrasound treatment | The tight junctions between cells become loose and reversible pores appear; improved endocytosis of cells | Fluorescence intensity increased significantly | 173 |
| Change route of administration | Oral/intravenous | Nasal drug delivery/ intrathecal injection |
Directly through the BCB without crossing BBB | i.v. administration was only 56.85% of the nasal administration/ prolong drug release time; enhanced healing and reduced drug toxicity | 30,176 |
Perspectives
Due to the protective effect of the brain barrier on the brain, the clinical treatment of glioma still remains difficult. Brain-targeted drug delivery has attracted wide attention because of its slow release and controllable and targeted safety. Regardless of the target, drug targeting is based on the interaction between the dosage form characteristics of the drug delivery system and the specific physiological/pathological structure of the body. The development of biomimetic drugs/materials with homologous binding and immune escape functions has been regarded as the most ideal alternative. These drugs/materials use autologous tissues/cells/proteins from the human body as carriers to quickly reach the target site after entering the human body.
For example, NPs wrapped in glioma cell membrane or glioma cell membrane proteins were loaded on NPs. Under certain conditions, tumor cells are killed by various mechanisms such as starvation therapy and photodynamic/photothermal therapy.132,142 In addition, as endogenous substances, it is difficult for the immune system to recognize the drug-carrying system in membrane disguise. Thus, these systems can escape rejection by the immune system for long therapy cycles. The carriers are gradually degraded or absorbed without accumulation in the body. Such bionic materials can be applied independently without chemotherapy drugs. Thus, they do not cause serious adverse reactions and might be safer.
Accordingly, the following four suggestions are proposed for further research on targeted agents for glioma. (1) While studying the brain-targeted delivery of drugs, researchers should also pay attention to the latest progress in basic research including structural characteristics, composition, and permeability of BBB and glioma. It is important to improve the efficiency of brain-targeted delivery of drugs. (2) Researchers should identify and evaluate the factors that affect the behavior of the glioma NDDS in vivo, which is the basis of the effectiveness. (3) Considering the perspective of biosafety, biodegradable material without immunogenicity or biotoxicity that can be removed from the brain should be utilized in glioma NDDS. (4) A unified preparation method should be developed to make NPs more uniform, stable, and controllable—this will ensure clinical efficacy.
Acknowledgments
This work was supported by Zhejiang Provincial Natural Science Foundation of China (Grant No.LY18H180009), National Natural Science Foundation of China (Grant No. 61801160, 81730108), Key Project of Zhejiang Project Ministry of Science and Technology (2015C03055), and Key Project of Hangzhou Ministry of Science and Technology (20162013A07). We thank LetPub (www.letpub.com) for its linguistic assistance during the preparation of this manuscript.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136(5):E359–E386. doi: 10.1002/ijc.29210 [DOI] [PubMed] [Google Scholar]
- 2.Patel AP, Fisher JL, Nichols E, et al. Global, regional, and national burden of brain and other CNScancer, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019;18(4):376–393. doi: 10.1016/S1474-4422(18)30468-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Norden AD, Drappatz J, Wen PY. Malignant Gliomas in Adults. Blue Books Neurol. 2010;36(10):99–120. [Google Scholar]
- 4.Cowan AJ, Allen C, Barac A, et al. Global burden of multiple myeloma: a systematic analysis for the Global Burden of Disease Study 2016. JAMA Oncol. 2018;4(9):1221–1227. doi: 10.1001/jamaoncol.2018.2128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Claus EB, Walsh KM, Wiencke JK, et al. Survival and low-grade glioma: the emergence of genetic information. Neurosurg Focus. 2015;38(1):E6. doi: 10.3171/2014.10.FOCUS12367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Talibi SS, Talibi SS, Aweid B, et al. Prospective therapies for high-grade glial tumours: a literature review. Ann Med Surg. 2014;3(3):55–59. doi: 10.1016/j.amsu.2014.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Krivosheya D, Prabhu SS, Weinberg JS, et al. Technical principles in glioma surgery and preoperative considerations. J Neurooncol. 2016;130(2):243–252. [DOI] [PubMed] [Google Scholar]
- 8.Flouty O, Reddy C, Holland M, et al. Precision surgery of rolandic glioma and insights from extended functional mapping. Clin Neurol Neurosurg. 2017;163:60–66. doi: 10.1016/j.clineuro.2017.10.008 [DOI] [PubMed] [Google Scholar]
- 9.Hanna A, Birla R, Iosif C, et al. Benefits and Disadvantages of Neoadjuvant Radiochemotherapy (RCT) in the Multimodal Therapy of Squamous Esophageal Cancer (ESC). Chirurgia. 2016;111(1):12–25. [PubMed] [Google Scholar]
- 10.Park SB, David G, Krishnan AV, et al. Chemotherapy-induced peripheral neurotoxicity: a critical analysis. Ca a Cancer J Clin. 2013;63(6):419–437. doi: 10.3322/caac.21204 [DOI] [PubMed] [Google Scholar]
- 11.Bar-Zeev M, Livney YD, Assaraf YG. Targeted nanomedicine for cancer therapeutics: towards precision medicine overcoming drug resistance. Drug Resist Updat. 2017;31:15–30. doi: 10.1016/j.drup.2017.05.002 [DOI] [PubMed] [Google Scholar]
- 12.Allen TM, Cullis PR. Liposomal drug delivery systems: from concept to clinical applications ☆. Adv Drug Deliv Rev. 2013;65(1):36–48. doi: 10.1016/j.addr.2012.09.037 [DOI] [PubMed] [Google Scholar]
- 13.Kumari P, Ghosh B, Biswas S. Nanocarriers for cancer-targeted drug delivery. J Drug Target. 2015;24(3):179. doi: 10.3109/1061186X.2015.1051049 [DOI] [PubMed] [Google Scholar]
- 14.Ashfaq UA, Riaz M, Yasmeen E, et al. Recent advances in nanoparticle-based targeted drug-delivery systems against cancer and role of tumor microenvironment. Crit Rev Ther Drug Carrier Syst. 2017;34(4):36. doi: 10.1615/CritRevTherDrugCarrierSyst.2017017845 [DOI] [PubMed] [Google Scholar]
- 15.Ostrom QT, Gittleman H, Farah P, et al. CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in. Neuro-Oncology. 2006–2010;15(suppl2):ii1–ii56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pardridge WM. CSF, blood-brain barrier, and brain drug delivery. Expert Opin Drug Deliv. 2016;13(7):963–975. doi: 10.1517/17425247.2016.1171315 [DOI] [PubMed] [Google Scholar]
- 17.van Tellingen O, Yetkin-Arik B, de Gooijer MC, et al. Overcoming the blood–brain tumor barrier for effective glioblastoma treatment. Drug Resist Updates. 2015;19:1–12. doi: 10.1016/j.drup.2015.02.002 [DOI] [PubMed] [Google Scholar]
- 18.Obermeier B, Verma A, Ransohoff RM. The blood-brain barrier. Handb Clin Neurol. 2016;133(8246):39–59. [DOI] [PubMed] [Google Scholar]
- 19.Banks WA. From blood-brain barrier to blood-brain interface: new opportunities for CNS drug delivery. Nat Rev Drug Discovery. 2016;15(4):275–292. doi: 10.1038/nrd.2015.21 [DOI] [PubMed] [Google Scholar]
- 20.Chaves C, Remião F, Cisterninoa S, et al. Opioids and the blood-brain barrier: a dynamic interaction with consequences on drug disposition in brain. Curr Neuropharmacol. 2017;15(8):1156. doi: 10.2174/1570159X15666170504095823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Matsumoto J, Stewart T, Banks WA, et al. The transport mechanism of extracellular vesicles at the blood-brain barrier. Curr Pharm Des. 2017;23(40):6206–6214. doi: 10.2174/1381612823666170913164738 [DOI] [PubMed] [Google Scholar]
- 22.Bhowmik A, Khan R, Ghosh MK. Blood brain barrier: a challenge for effectual therapy of brain tumors. Biomed Res Int. 2015;2015:1–20. doi: 10.1155/2015/320941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Posadas I, Monteagudo S, Ceña V. Nanoparticles for brain-specific drug and genetic material delivery, imaging and diagnosis. Nanomedicine. 2016;11(7):833–849. doi: 10.2217/nnm.16.15 [DOI] [PubMed] [Google Scholar]
- 24.Berghoff AS, Preusser M. Role of the blood-brain barrier in metastatic disease of the central nervous system. Handb Clin Neurol. 2018;149:57–66. [DOI] [PubMed] [Google Scholar]
- 25.Emily K. Development of a Three-Dimensional, All-Human in vitro Model of the Blood-Brain Barrier for Cancer Metastasis Studies. University of Portsmouth; 2010. [Google Scholar]
- 26.Yin L, Li H, Liu W, et al. A highly potent CDK4/6 inhibitor was rationally designed to overcome blood brain barrier in glioblastoma therapy. Eur J Med Chem. 2017;144:1–28. doi: 10.1016/j.ejmech.2017.12.003 [DOI] [PubMed] [Google Scholar]
- 27.Weidle UH, Niewöhner J, Tiefenthaler G. The blood-brain barrier challenge for the treatment of brain cancer, secondary brain metastases, and neurological diseases. Cancer Genomics Proteomics. 2015;12(4):167. [PubMed] [Google Scholar]
- 28.Garcia MA, Carrasco M, Godoy A, et al. Elevated expression of glucose transporter-1 in hypothalamic ependymal cells not involved in the formation of the brain-cerebrospinal fluid barrier. J Cell Biochem. 2015;80(4):491–503. doi: [DOI] [PubMed] [Google Scholar]
- 29.Zhou J, Kong H, Hua X, et al. Altered blood-brain barrier integrity in adult aquaporin-4 knockout mice. Neuroreport. 2008;19(1):1–5. doi: 10.1097/WNR.0b013e3282f2b4eb [DOI] [PubMed] [Google Scholar]
- 30.Etminan N, Macdonald RL, Davis C, et al. Intrathecal application of the nimodipine slow-release microparticle system EG-1962 for prevention of delayed cerebral ischemia and improvement of outcome after aneurysmal subarachnoid hemorrhage. Acta Neurochirurgica Suppl. 2015;120:281–286. doi: 10.1007/978-3-319-04981-6_47 [DOI] [PubMed] [Google Scholar]
- 31.Hongbing W, Kaili H, Xinguo J. From nose to brain: understanding transport capacity and transport rate of drugs. Expert Opin Drug Deliv. 2008;5(10):1159–1168. doi: 10.1517/17425247.5.10.1159 [DOI] [PubMed] [Google Scholar]
- 32.Huang Meng-yao YX, Jin-feng XING, Zhen-ping WEI. Strategies for enhancing nanoscale brain targeting drug delivery. Acta Pharm Sin. 2019;4:629–637. [Google Scholar]
- 33.Prieto A. To be, or not to be biodegradable … that is the question for the bio-based plastics. Microb Biotechnol. 2016;9(5):652–657. doi: 10.1111/1751-7915.12393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Alavi M, Hamidi M. Passive and active targeting in cancer therapy by liposomes and lipid nanoparticles. Drug Metab Pers Ther. 2019;34(1):424–436. doi: 10.1515/dmpt-2018-0032 [DOI] [PubMed] [Google Scholar]
- 35.Gao H. Perspectives on dual targeting delivery systems for brain tumors. J Neuroimmune Pharmacol. 2016;12(1):6–16. doi: 10.1007/s11481-016-9687-4 [DOI] [PubMed] [Google Scholar]
- 36.Richards DM, Endres RG. Target shape dependence in a simple model of receptor-mediated endocytosis and phagocytosis. Proc Natl Acad Sci U S A. 2016;113(22):6113. doi: 10.1073/pnas.1521974113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Unsoy G, Gunduz U. Smart drug delivery systems in cancer therapy. Curr Drug Targets. 2018;19(3):202–212. [DOI] [PubMed] [Google Scholar]
- 38.Jain KK. Current status and future prospects of drug delivery systems. Methods Mol Biol. 2014;1141(5):1. [DOI] [PubMed] [Google Scholar]
- 39.Torchilin VP. Passive and active drug targeting: drug delivery to tumors as an example. Handb Exp Pharmacol. 2010;197(197):3. [DOI] [PubMed] [Google Scholar]
- 40.Yu S, Kazuaki K, Hiroto H, et al. Advances in an active and passive targeting to tumor and adipose tissues. Expert Opin Drug Deliv. 2015;12(1):41–52. doi: 10.1517/17425247.2015.955847 [DOI] [PubMed] [Google Scholar]
- 41.Müller J, Martins A, Csábi J, et al. BBB penetration-targeting physicochemical lead selection: ecdysteroids as chemo-sensitizers against CNS tumors. Eur J Pharm Sci. 2017;96:571–577. doi: 10.1016/j.ejps.2016.10.034 [DOI] [PubMed] [Google Scholar]
- 42.Maeda H, Sawa TMY, Hori K, et al. Tumor vascular permeability and the EPR effect in macromolecular therapeutics: a review. J Controlled Release. 2000;65(1):271–284. doi: 10.1016/S0168-3659(99)00248-5 [DOI] [PubMed] [Google Scholar]
- 43.T CL X, Wang SL, Zeng ZW, Wang F, Zhao R. Advances in the research of Elemene Liposome series targeted anticancer natural drugs. Chin J Integr Trad West Med. 2014;34(4):507–512. [PubMed] [Google Scholar]
- 44.Chen MW, Zhong ZF, Wang SP, WANG YT. Research progress in anticancer activity and novel delivery system of β-elemene. Chin J New Drugs. 2012;21(12):1358–1361. [Google Scholar]
- 45.Zhai B, Zeng Y, Zeng Z, et al. Drug delivery systems for elemene, its main active ingredient β-elemene, and its derivatives in cancer therapy. <![CDATA[International Journal of Nanomedicine]]>. 2018;13:6279–6296. doi: 10.2147/IJN.S174527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhu YJ, Jue HU, Dong-Hang XU. Effect of nano-liposome sustained elemene in inducing cell apoptosis of C6 glioma. Chin J Integr Traditional West Med. 2008;28(7):637–639. [PubMed] [Google Scholar]
- 47.Gao J, Wang Z, Liu H, et al. Liposome encapsulated of temozolomide for the treatment of glioma tumor: preparation, characterization and evaluation. Drug Discov Ther. 2015;9(3):205–212. doi: 10.5582/ddt.2015.01016 [DOI] [PubMed] [Google Scholar]
- 48.Xavier-Junior FH, Vauthier C, Morais AR, et al. Microemulsion systems containing bioactive natural oils: an overview on the state of the art. Drug Dev Commun. 2017;43(5):700–714. [DOI] [PubMed] [Google Scholar]
- 49.Li D, Yang K, Li JS, et al. Antitumor efficacy of a novel CLA-PTX microemulsion against brain tumors: in vitro and in vivo findings. Int J Nanomedicine. 2012;7:6105–6114. doi: 10.2147/IJN.S38927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Xie H, Wei-Cheng LU, Ding XH, et al. Study of hyaluronic acid chitosan based microemulsion on the permeability of blood tumor barrier in rat. J China Med Univ;2014. [Google Scholar]
- 51.Lu W, Xie H, Ding X, Qi R, Xing H, Wu H. Therapeutic effect of hyaluronic acid chitosan-based microemulsion and carboplatin on glioma in rats. J Shenyang Med Coll. 2016;18(3):149–150. [Google Scholar]
- 52.Maryam R, Brian H, Nathan K, et al. The future of glioma treatment: stem cells, nanotechnology and personalized medicine. Future Oncol. 2012;8(9):1149–1156. doi: 10.2217/fon.12.111 [DOI] [PubMed] [Google Scholar]
- 53.Minghui L, Hongbing D, Haisheng P, et al. Functional nanoparticles in targeting glioma diagnosis and therapies. J Nanosci Nanotechnol. 2014;14(1):415–432. doi: 10.1166/jnn.2014.8757 [DOI] [PubMed] [Google Scholar]
- 54.Zhao L, Shi X, Zhao J. Chlorotoxin-conjugated nanoparticles for targeted imaging and therapy of glioma. Curr Top Med Chem. 2015;15(13):1196–1208. doi: 10.2174/1568026615666150330110822 [DOI] [PubMed] [Google Scholar]
- 55.An YL, Nie F, Wang ZY, et al. Preparation and characterization of realgar nanoparticles and their inhibitory effect on rat glioma cells. Int J Nanomedicine. 2011;6(6):3187–3194. doi: 10.2147/IJN.S26237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jain D, Bajaj A, Athawale R, et al. Surface-coated PLA nanoparticles loaded with temozolomide for improved brain deposition and potential treatment of gliomas: development, characterization and in vivo studies. Drug Deliv. 2014;395(1):1–18. [DOI] [PubMed] [Google Scholar]
- 57.Wang J, Wang X, Cao Y, et al. Therapeutic potential of hyaluronic acid/chitosan nanoparticles for the delivery of curcuminoid in knee osteoarthritis and an in vitro evaluation in chondrocytes. Int J Mol Med. 2018;42(5):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Li M, Wang B, Wu Z, et al. Treatment of Dutch rat models of glioma using EphrinA1-PE38/GM-CSF chitosan nanoparticles by in situ activation of dendritic cells. Tumor Biol. 2015;36(10):7961–7966. [DOI] [PubMed] [Google Scholar]
- 59.Gao X, Yu T, Xu G, et al. Enhancing the anti-glioma therapy of doxorubicin by honokiol with biodegradable self-assembling micelles through multiple evaluations. Sci Rep. 2017;7:43501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mukherjee A, Kumar B, Hatano K, et al. Development and application of a novel model system to study ‘active’ and ‘passive’ tumor targeting. Mol Cancer Ther. 2016;15(10):2541. doi: 10.1158/1535-7163.MCT-16-0051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yinan Z, Fenghua M, Chao D, et al. Ligand-directed active tumor-targeting polymeric nanoparticles for cancer chemotherapy. Biomacromolecules. 2014;15(6):1955–1969. doi: 10.1021/bm5003009 [DOI] [PubMed] [Google Scholar]
- 62.Aigner A, Kãgel D. Nanoparticle/siRNA-based therapy strategies in glioma: which nanoparticles, which siRNAs? Nanomedicine. 2018;13(1):89–103. doi: 10.2217/nnm-2017-0230 [DOI] [PubMed] [Google Scholar]
- 63.Liu C, Yao S, Li X, et al. iRGD-mediated core-shell nanoparticles loading carmustine and O6-benzylguanine for glioma therapy. J Drug Target. 2016;25(3):235–246. doi: 10.1080/1061186X.2016.1238091 [DOI] [PubMed] [Google Scholar]
- 64.Chi Y, Zhu S, Wang C, et al. Glioma homing peptide-modified PEG-PCL nanoparticles for enhanced anti-glioma therapy. J Drug Target. 2015;24(3):224. [DOI] [PubMed] [Google Scholar]
- 65.Feng X, Yao J, Gao X, et al. Multi-targeting peptide-functionalized nanoparticles recognized vasculogenic mimicry, tumor neovasculature and glioma cells for enhanced anti-glioma therapy. ACS Appl Mater Interfaces. 2015;7(50):acsami.5b09934. doi: 10.1021/acsami.5b09934 [DOI] [PubMed] [Google Scholar]
- 66.Gao JQ, Lv Q, Li LM, et al. Glioma targeting and blood-brain barrier penetration by dual-targeting doxorubincin liposomes. Biomaterials. 2013;34(22):5628–5639. doi: 10.1016/j.biomaterials.2013.03.097 [DOI] [PubMed] [Google Scholar]
- 67.Madhankumar AB, Mrowczynski O, Patel S, et al. Interleukin-13 conjugated quantum dots for identification of glioma initiating cells and their extracellular vesicles. Acta Biomater. 2017;58:205–213. doi: 10.1016/j.actbio.2017.06.002 [DOI] [PubMed] [Google Scholar]
- 68.Gao H, Zhang S, Cao S, et al. Angiopep-2 and activatable cell-penetrating peptide dual-functionalized nanoparticles for systemic glioma-targeting delivery. Mol Pharm. 2014;11(8):2755–2763. doi: 10.1021/mp500113p [DOI] [PubMed] [Google Scholar]
- 69.Zhu X, Zhou H, Liu Y, et al. Transferrin/aptamer conjugated mesoporous ruthenium nanosystem for redox-controlled and targeted chemo-photodynamic therapy of glioma. Acta Biomater. 2018;82:143–157. doi: 10.1016/j.actbio.2018.10.012 [DOI] [PubMed] [Google Scholar]
- 70.Smith MW, Mark G. Endocytosis at the blood-brain barrier: from basic understanding to drug delivery strategies. J Drug Target. 2006;14(4):191–214. doi: 10.1080/10611860600650086 [DOI] [PubMed] [Google Scholar]
- 71.Sun T, Wu H, Li Y, et al. Targeting transferrin receptor delivery of temozolomide for a potential glioma stem cell-mediated therapy. Oncotarget. 2017;8(43):74451–74465. doi: 10.18632/oncotarget.20165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fan K, Jia X, Zhou M, et al. Ferritin nanocarrier traverses the blood brain barrier and kills glioma. ACS Nano. 2018;12(5):4105–4115. doi: 10.1021/acsnano.7b06969 [DOI] [PubMed] [Google Scholar]
- 73.Chen H, Qin Y, Zhang Q, et al. Lactoferrin modified doxorubicin-loaded procationic liposomes for the treatment of gliomas. Eur J Pharm Sci. 2011;44(1):164–173. doi: 10.1016/j.ejps.2011.07.007 [DOI] [PubMed] [Google Scholar]
- 74.Li H, Tong Y, Bai L, et al. Lactoferrin functionalized PEG-PLGA nanoparticles of shikonin for brain targeting therapy of glioma. Int J Biol Macromol. 2018;107(Pt A):204–211. doi: 10.1016/j.ijbiomac.2017.08.155 [DOI] [PubMed] [Google Scholar]
- 75.Gandioso A, Cano M, Massaguer A, et al. A green light-triggerable RGD peptide for photocontrolled targeted drug delivery: synthesis and photolysis studies. J Org Chem. 2016;81(23):11556. doi: 10.1021/acs.joc.6b02415 [DOI] [PubMed] [Google Scholar]
- 76.Ma H, Hao P, Zhang L, et al. A new cyclic RGD peptide dimer for integrin αvβ3 imaging. Eur Rev Med Pharmacol Sci. 2016;20(4):613. [PubMed] [Google Scholar]
- 77.Wang K, Zhang X, Liu Y, et al. Tumor penetrability and anti-angiogenesis using iRGD-mediated delivery of doxorubicin-polymer conjugates. Biomaterials. 2014;35(30):8735–8747. doi: 10.1016/j.biomaterials.2014.06.042 [DOI] [PubMed] [Google Scholar]
- 78.Gu G, Gao X, Hu Q, et al. The influence of the penetrating peptide iRGD on the effect of paclitaxel-loaded MT1-AF7p-conjugated nanoparticles on glioma cells. Biomaterials. 2013;34(21):5138–5148. doi: 10.1016/j.biomaterials.2013.03.036 [DOI] [PubMed] [Google Scholar]
- 79.Gestin M, Dowaidar M, Langel Ü. Uptake mechanism of cell-penetrating peptides. Adv Exp Med Biol. 2017;1030:255–264. [DOI] [PubMed] [Google Scholar]
- 80.Ramsey JD, Flynn NH. Cell-penetrating peptides transport therapeutics into cells. Pharmacol Ther. 2015;154:78–86. doi: 10.1016/j.pharmthera.2015.07.003 [DOI] [PubMed] [Google Scholar]
- 81.Ruoslahti E. Tumor penetrating peptides for improved drug delivery. Adv Drug Deliv Rev. 2016;110–111:3–12. doi: 10.1016/j.addr.2016.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhao M, Zhao M, Fu C, et al. Targeted therapy of intracranial glioma model mice with curcumin nanoliposomes. Int J Nanomedicine. 2018;13:1601–1610. doi: 10.2147/IJN.S157019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wu J, Zhang E, Fu A. A novel cell-permeable RDP-p53 fusion protein for specific inhibition on the growth of cancerous neural cells. Drug Deliv. 2015;23(7):1. doi: 10.3109/10717544.2015.1013199 [DOI] [PubMed] [Google Scholar]
- 84.Parker N, Turk MJ, Westrick E, et al. Folate receptor expression in carcinomas and normal tissues determined by a quantitative radioligand binding assay. Anal Biochem. 2005;338(2):284–293. doi: 10.1016/j.ab.2004.12.026 [DOI] [PubMed] [Google Scholar]
- 85.Xu XL, Li JJ, Han SP, et al. A novel doxorubicin loaded folic acid conjugated PAMAM modified with borneol, a nature dual-functional product of reducing PAMAM toxicity and boosting BBB penetration. Eur J Pharm Sci. 2016;88:178–190. doi: 10.1016/j.ejps.2016.02.015 [DOI] [PubMed] [Google Scholar]
- 86.Jing-Jing LI, Guo MM, Han SP, et al. Preparation and in vitro evaluation of borneol and folic acid co-modified doxorubicin loaded PAMAM drug delivery system. Acta Pharm Sin. 2015;50(7):899. [PubMed] [Google Scholar]
- 87.Khatoon M, Shah KU, Din FU, et al. Proniosomes derived niosomes: recent advancements in drug delivery and targeting. Drug Deliv. 2017;24(sup1):56–69. doi: 10.1080/10717544.2017.1384520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bartelds R, Nematollahi MH, Pols T. Niosomes, an alternative for liposomal delivery. PLoS One. 2018;13(4):e0194179. doi: 10.1371/journal.pone.0194179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Almiron Bonnin DA, Ran C, Havrda MC, et al. Insulin-mediated signaling facilitates resistance to PDGFR inhibition in proneural hPDGFB-driven gliomas. Mol Cancer Ther. 2017;16(4):705. doi: 10.1158/1535-7163.MCT-16-0616 [DOI] [PubMed] [Google Scholar]
- 90.You T, Bi Y, Li J, et al. IL-17 induces reactive astrocytes and up-regulation of vascular endothelial growth factor (VEGF) through JAK/STAT signaling. Sci Rep. 2017;7:41779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bhunia S, Vangala V, Bhattacharya D, et al. Large amino acid transporter 1 selective liposomes of l-DOPA functionalized amphiphile for combating glioblastoma. Mol Pharm. 2017;14(11):3834–3847. doi: 10.1021/acs.molpharmaceut.7b00569 [DOI] [PubMed] [Google Scholar]
- 92.Maleklou N, Allameh A, Kazemi B. Preparation, characterization and in vitro-targeted delivery of novel Apolipoprotein E-based nanoparticles to C6 glioma with controlled size and loading efficiency. J Drug Target. 2016;24(4):348–358. doi: 10.3109/1061186X.2015.1077849 [DOI] [PubMed] [Google Scholar]
- 93.Lu F, Pang Z, Zhao J, et al. Angiopep-2-conjugated poly(ethylene glycol)-co- poly(ε-caprolactone) polymersomes for dual-targeting drug delivery to glioma in rats. Int J Nanomedicine. 2017;12:2117–2127. doi: 10.2147/IJN.S123422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Liang M, Gao C, Wang Y, et al. Enhanced blood–brain barrier penetration and glioma therapy mediated by T7 peptide-modified low-density lipoprotein particles. Drug Deliv. 2018;25(1):1652–1663. doi: 10.1080/10717544.2018.1494223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Chai Z, Hu X, Wei X, et al. A facile approach to functionalizing cell membrane-coated nanoparticles with neurotoxin-derived peptide for brain-targeted drug delivery. J Controlled Release. 2017;264:102–111. doi: 10.1016/j.jconrel.2017.08.027 [DOI] [PubMed] [Google Scholar]
- 96.Hu Q, Gu G, Liu Z, et al. F3 peptide-functionalized PEG-PLA nanoparticles co-administrated with tLyp-1 peptide for anti-glioma drug delivery. Biomaterials. 2013;34(4):1135–1145. doi: 10.1016/j.biomaterials.2012.10.048 [DOI] [PubMed] [Google Scholar]
- 97.Liu Y, Mei L, Xu C, et al. Dual receptor recognizing cell penetrating peptide for selective targeting, efficient intratumoral diffusion and synthesized anti-glioma therapy. Theranostics. 2016;12(2):500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Luo Z, Yan Z, Jin K, et al. Precise glioblastoma targeting by AS1411 aptamer-functionalized poly (l-gamma-glutamylglutamine)-paclitaxel nanoconjugates. J Colloid Interface Sci. 2017;490:783–796. doi: 10.1016/j.jcis.2016.12.004 [DOI] [PubMed] [Google Scholar]
- 99.Mu c, Nimita D, Jing H, et al. Solubilization of flurbiprofen into aptamer-modified PEG-PLA micelles for targeted delivery to brain-derived endothelial cells in vitro. J Microencapsul. 2013;30(7):701–708. doi: 10.3109/02652048.2013.778907 [DOI] [PubMed] [Google Scholar]
- 100.Tinghui L, Susan M, Boris K, et al. A new interleukin-13 amino-coated gadolinium metallofullerene nanoparticle for targeted MRI detection of glioblastoma tumor cells. J Am Chem Soc. 2015;137(24):7881–7888. doi: 10.1021/jacs.5b03991 [DOI] [PubMed] [Google Scholar]
- 101.Lee W, Nam JH, Cho HJ, et al. Epimedium koreanum Nakai inhibits PMA-induced cancer cell migration and invasion by modulating NF-κB/MMP-9 signaling in monomorphic malignant human glioma cells. Oncol Rep. 2017;38(6):3619–3631. doi: 10.3892/or.2017.6043 [DOI] [PubMed] [Google Scholar]
- 102.Wang X, Gao J, Ouyang X, et al. Mesenchymal stem cells loaded with paclitaxel-poly(lactic-co-glycolic acid) nanoparticles for glioma-targeting therapy. Int J Nanomedicine. 2018;13:5231–5248. doi: 10.2147/IJN.S167142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zou L, Tao Y, Payne G, et al. Targeted delivery of nano-PTX to the brain tumor-associated macrophages. Oncotarget. 2016;8(4):6564–6578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Peterson TE, Kirkpatrick ND, Huang Y, et al. Dual inhibition of Ang-2 and VEGF receptors normalizes tumor vasculature and prolongs survival in glioblastoma by altering macrophages. Proc Natl Acad Sci U S A. 2016;113(16):4470–4475. doi: 10.1073/pnas.1525349113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Zhang Y, Zhai M, Chen Z, et al. Dual-modified liposome codelivery of doxorubicin and vincristine improve targeting and therapeutic efficacy of glioma. Drug Deliv. 2017;24(1):1045–1055. doi: 10.1080/10717544.2017.1344334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Shi D, Mi G, Shen Y, Webster TJ. Glioma-targeted dual functionalized thermosensitive Ferri-liposomes for drug delivery through an in vitro blood-brain barrier. Nanoscale. 2019;11(32):15057–15071. doi: 10.1039/C9NR03931G [DOI] [PubMed] [Google Scholar]
- 107.Li Y, He H, Jia X, Lu W-L, Lou J, Wei Y. A dual-targeting nanocarrier based on poly(amidoamine) dendrimers conjugated with transferrin and tamoxifen for treating brain gliomas. Biomaterials. 2012;33:3899–3908. [DOI] [PubMed] [Google Scholar]
- 108.Zhou T, Xu J, Zhao X, et al. Facile preparation of pH/reduction dual-responsive prodrug nanohydrogels for tumor-specific intracellular triggered release with enhanced anticancer efficiency. J Mater Chem B. 2017;5(15):2840–2848. doi: 10.1039/C7TB00433H [DOI] [PubMed] [Google Scholar]
- 109.Xu HL, Zhuge DL, Chen PP, et al. Silk fibroin nanoparticles dyeing indocyanine green for imaging-guided photo-thermal therapy of glioblastoma. Drug Deliv. 2018;25(1):364–375. doi: 10.1080/10717544.2018.1428244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Bœuf-Muraille G, Rigaux G, Callewaert M, et al. Evaluation of mTHPC-loaded PLGA nanoparticles for in vitro photodynamic therapy on C6 glioma cell line. Photodiagnosis Photodyn Ther. 2019;25:448–455. doi: 10.1016/j.pdpdt.2019.01.026 [DOI] [PubMed] [Google Scholar]
- 111.Shibata S, Shinozaki N, Suganami A, et al. Photo-immune therapy with liposomally formulated phospholipid-conjugated indocyanine green induces specific antitumor responses with heat shock protein-70 expression in a glioblastoma model. Oncotarget. 2019;10(2):175–183. doi: 10.18632/oncotarget.26544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Yin Y, Fu C, Li M, et al. A pH-sensitive hyaluronic acid prodrug modified with lactoferrin for glioma dual-targeted treatment. Mater Sci Eng C. 2016;67:159–169. doi: 10.1016/j.msec.2016.05.012 [DOI] [PubMed] [Google Scholar]
- 113.Zhu Y, Zhang J, Meng F, et al. cRGD-functionalized reduction-sensitive shell-sheddable biodegradable micelles mediate enhanced doxorubicin delivery to human glioma xenografts in vivo. J Controlled Release. 2016;233:29–38. doi: 10.1016/j.jconrel.2016.05.014 [DOI] [PubMed] [Google Scholar]
- 114.Guo H, Kim JC. Reduction-sensitive poly(ethylenimine) nanogel bearing dithiodipropionic acid. Chem Pharm Bull (Tokyo). 2017;65(8):718. doi: 10.1248/cpb.c17-00029 [DOI] [PubMed] [Google Scholar]
- 115.van Dijken BRJ, van Laar PJ, Holtman GA, et al. Diagnostic accuracy of magnetic resonance imaging techniques for treatment response evaluation in patients with high-grade glioma, a systematic review and meta-analysis. Eur Radiol. 2017;27(10):4129–4144. doi: 10.1007/s00330-017-4789-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Li-Juan C, Cui-Tao L, Ying-Zheng Z, et al. Ultrasonic microbubbles for glioma-targeted drug delivery. Yao Xue Xue Bao = Acta Pharmaceutica Sinica. 2015;50(1):99–103. [PubMed] [Google Scholar]
- 117.Delač M, Motaln H, Ulrich H, et al. Aptamer for imaging and therapeutic targeting of brain tumor glioblastoma. Cytometry Part A. 2015;87(9):806–816. doi: 10.1002/cyto.a.22715 [DOI] [PubMed] [Google Scholar]
- 118.Reyes-Reyes EM, Yun T, Bates PJ. A new paradigm for aptamer therapeutic AS1411 action: uptake by macropinocytosis and its stimulation by a nucleolin-dependent mechanism. Cancer Res. 2010;70(21):8617–8629. doi: 10.1158/0008-5472.CAN-10-0920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Bates PJ, Reyes-Reyes EM, Malik MT, et al. G-quadruplex oligonucleotide AS1411 as a cancer-targeting agent: uses and mechanisms ☆. Biochim Biophys Acta. 2016;1861(5 Pt B):1414. doi: 10.1016/j.bbagen.2016.12.015 [DOI] [PubMed] [Google Scholar]
- 120.Minty A, Chalon P, Derocq JM, et al. lnterleukin-13 is a new human lymphokine regulating inflammatory and immune responses. Nature. 1993;362(6417):248–250. doi: 10.1038/362248a0 [DOI] [PubMed] [Google Scholar]
- 121.Debinski W, Slagle B, Gibo DM, et al. Expression of a restrictive receptor for interleukin 13 is associated with glial transformation. J Neurooncol. 2000;48(2):103. doi: 10.1023/A:1006446426611 [DOI] [PubMed] [Google Scholar]
- 122.Debinski W, Gibo DM. Molecular expression analysis of restrictive receptor for interleukin 13, a brain tumor-associated cancer/testis antigen. Mol Med. 2000;6(5):440–449. doi: 10.1007/BF03401786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Joshi BH, Puri RA, Leland P, et al. Identification of interleukin-13 receptor α2 chain overexpression in situ in high-grade diffusely infiltrative pediatric brainstem glioma. Neuro-Oncology. 2008;10(3):265–274. doi: 10.1215/15228517-2007-066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Madhankumar AB, Slaglewebb B, Mintz A, et al. Interleukin-13 receptor–targeted nanovesicles are a potential therapy for glioblastoma multiforme. Mol Cancer Ther. 2006;5(12):3162. doi: 10.1158/1535-7163.MCT-06-0480 [DOI] [PubMed] [Google Scholar]
- 125.Madhankumar AB, Slagle-Webb B, Wang X, et al. Efficacy of interleukin-13 receptor-targeted liposomal doxorubicin in the intracranial brain tumor model. Mol Cancer Ther. 2009;8(3):648–654. doi: 10.1158/1535-7163.MCT-08-0853 [DOI] [PubMed] [Google Scholar]
- 126.Roger M, Clavreul A, Venier-Julienne MC, et al. Mesenchymal stem cells as cellular vehicles for delivery of nanoparticles to brain tumors. Biomaterials. 2010;31(32):8393–8401. doi: 10.1016/j.biomaterials.2010.07.048 [DOI] [PubMed] [Google Scholar]
- 127.Zhang X, Yao S, Liu C, et al. Tumor tropic delivery of doxorubicin-polymer conjugates using mesenchymal stem cells for glioma therapy. Biomaterials. 2015;39:269–281. doi: 10.1016/j.biomaterials.2014.11.003 [DOI] [PubMed] [Google Scholar]
- 128.Wang W, Liu F, Xiang B, et al. Stem cells as cellular vehicles for gene therapy against glioblastoma. Int J Clin Exp Med. 2015;8(10):17102. [PMC free article] [PubMed] [Google Scholar]
- 129.Gao H. Progress and perspectives on targeting nanoparticles for brain drug delivery. Acta Pharm Sin B. 2016;6(4):268–286. doi: 10.1016/j.apsb.2016.05.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Felice B, Prabhakaran MP, Rodríguez AP, et al. Drug delivery vehicles on a nano-engineering perspective. Mater Sci Eng C. 2014;41:178–195. doi: 10.1016/j.msec.2014.04.049 [DOI] [PubMed] [Google Scholar]
- 131.Bruschi ML, Borghi FB, Junqueira MV, et al. Environmentally responsive systems for drug delivery. Recent Pat Drug Deliv Formul. 2017;11(2):89–100. doi: 10.2174/1872211311666170328151455 [DOI] [PubMed] [Google Scholar]
- 132.Li SY, Cheng H, Xie BR, et al. Cancer cell membrane camouflaged cascade bioreactor for cancer targeted starvation and photodynamic therapy. ACS Nano. 2017;11(7):7006–7018. doi: 10.1021/acsnano.7b02533 [DOI] [PubMed] [Google Scholar]
- 133.Pollack IF, Kawecki, S. The effect of calphostin C, a potent photodependent protein kinase C inhibitor, on the proliferation of glioma cells in vitro. J Neurooncol. 1997;31(3):255–266. doi: 10.1023/A:1005729626354 [DOI] [PubMed] [Google Scholar]
- 134.Zhang J, Li J, Shi Z, et al. pH-sensitive polymeric nanoparticles for co-delivery of doxorubicin and curcumin to treat cancer via enhanced pro-apoptotic and anti-angiogenic activities. Acta Biomater. 2017;58:349. doi: 10.1016/j.actbio.2017.04.029 [DOI] [PubMed] [Google Scholar]
- 135.Zhang J, Chen R, Fang X, Chen F, Wang Y, Chen M. Nucleolin targeting AS1411 aptamer modified pH-sensitive micelles for enhanced delivery and antitumor efficacy of paclitaxel. Nano Res. 2015;8(1):201–218. doi: 10.1007/s12274-014-0619-4 [DOI] [Google Scholar]
- 136.Fang XB, Zhang JM, Xie X, et al. pH-sensitive micelles based on acid-labile pluronic F68–curcumin conjugates for improved tumor intracellular drug delivery. Int J Pharm. 2016;502(1–2):28–37. doi: 10.1016/j.ijpharm.2016.01.029 [DOI] [PubMed] [Google Scholar]
- 137.Li M, Shi K, Tang X, et al. pH-sensitive folic acid and dNP2 peptide dual-modified liposome for enhanced targeted chemotherapy of glioma. Eur J Pharm Sci. 2018;124:124240–124248. doi: 10.1016/j.ejps.2018.07.055 [DOI] [PubMed] [Google Scholar]
- 138.SS P, Mr R. Redox sensitive cationic pullulan for efficient gene transfection and drug retention in C6 glioma cells. Int J Pharm. 2017;530(1–2):401–414. doi: 10.1016/j.ijpharm.2017.08.004 [DOI] [PubMed] [Google Scholar]
- 139.Yang X, Lv S, Liu Y, et al. The clinical utility of matrix metalloproteinase 9 in evaluating pathological grade and prognosis of glioma patients: a meta-analysis. Mol Neurobiol. 2015;52(1):38–44. doi: 10.1007/s12035-014-8850-2 [DOI] [PubMed] [Google Scholar]
- 140.Horne TK, Cronjé MJ. Mechanistics and photo-energetics of macrocycles and photodynamic therapy: an overview of aspects to consider for research. Chem Biol Drug Des. 2017;89(2):221–242. doi: 10.1111/cbdd.12761 [DOI] [PubMed] [Google Scholar]
- 141.Monteiro AF, Rato M, Martins C. Drug-induced photosensitivity: photoallergic and phototoxic reactions. Clin Dermatol. 2016;34(5):571–581. doi: 10.1016/j.clindermatol.2016.05.006 [DOI] [PubMed] [Google Scholar]
- 142.Jia Y, Wang X, Hu D, et al. Phototheranostics: active targeting of orthotopic glioma using biomimetic proteolipid nanoparticles. ACS Nano. 2019;13(1):386–398. doi: 10.1021/acsnano.8b06556 [DOI] [PubMed] [Google Scholar]
- 143.Johung T, Monje M. Neuronal activity in the glioma microenvironment. Curr Opin Neurobiol. 2017;47:156–161. doi: 10.1016/j.conb.2017.10.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Wu T, Dai Y. Tumor microenvironment and therapeutic response. Cancer Lett. 2016;387:61–68. doi: 10.1016/j.canlet.2016.01.043 [DOI] [PubMed] [Google Scholar]
- 145.Zheng XC, Ren W, Zhang S, et al. The theranostic efficiency of tumor-specific, pH-responsive, peptide-modified, liposome-containing paclitaxel and superparamagnetic iron oxide nanoparticles. Int J Nanomedicine. 2018;13:1495–1504. doi: 10.2147/IJN.S157082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Zhao Y, Ren W, Zhong T, et al. Tumor-specific pH-responsive peptide-modified pH-sensitive liposomes containing doxorubicin for enhancing glioma targeting and anti-tumor activity. J Controlled Release. 2016;222:56–66. doi: 10.1016/j.jconrel.2015.12.006 [DOI] [PubMed] [Google Scholar]
- 147.Stephen ZR, Gebhart RN, Jeon M, et al. pH-sensitive O6-benzylguanosine polymer modified magnetic nanoparticles for treatment of glioblastomas. Bioconjug Chem. 2016;28(1):194–202. doi: 10.1021/acs.bioconjchem.6b00545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.He-Lin X, Zi-Liang F, De-Li Z, et al. Ratiometric delivery of two therapeutic candidates with inherently dissimilar physicochemical property through pH-sensitive core–shell nanoparticles targeting the heterogeneous tumor cells of glioma. Drug Deliv. 2018;25(1):1302–1318. doi: 10.1080/10717544.2018.1474974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Sun H, Zhang Y, Zhong Z. Reduction-sensitive polymeric nanomedicines: an emerging multifunctional platform for targeted cancer therapy. Adv Drug Deliv Rev. 2018;132:16–32. doi: 10.1016/j.addr.2018.05.007 [DOI] [PubMed] [Google Scholar]
- 150.Su Z, Xu Y, Wang Y, et al. A pH and reduction dual-sensitive polymeric nanomicelle for tumor microenvironment triggered cellular uptake and controlled intracellular drug release. Biomater Sci. 2019;7(9):3821–3831. doi: 10.1039/C9BM00825J [DOI] [PubMed] [Google Scholar]
- 151.Chen F, Zhang J, He Y, et al. Glycyrrhetinic acid-decorated and reduction-sensitive micelles to enhance the bioavailability and anti-hepatocellular carcinoma efficacy of tanshinone IIA. Biomater Sci. 2016;4(1):167–182. doi: 10.1039/C5BM00224A [DOI] [PubMed] [Google Scholar]
- 152.Chen F, Zhang J, Wang L, et al. Tumor pH(e)-triggered charge-reversal and redox-responsive nanoparticles for docetaxel delivery in hepatocellular carcinoma treatment. Nanoscale. 2015;7(38):15763–15779. doi: 10.1039/C5NR04612B [DOI] [PubMed] [Google Scholar]
- 153.Chen FQ, Zhang JM, Fang XF, et al. Reversal of paclitaxel resistance in human ovarian cancer cells with redox-responsive micelles consisting of alpha-tocopheryl succinate-based polyphosphoester copolymers. Acta Pharmacol Sin. 2017;38(6):859–873. doi: 10.1038/aps.2016.150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Kim MM, Lawrence TS, Yue C. Advances in magnetic resonance and positron emission tomography imaging: assessing response in the treatment of low-grade glioma. Semin Radiat Oncol. 2015;25(3):172–180. doi: 10.1016/j.semradonc.2015.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Chen L, Cong D, Li Y, et al. Combination of sonodynamic with temozolomide inhibits C6 glioma migration and promotes mitochondrial pathway apoptosis via suppressing NHE-1 expression. Ultrason Sonochem. 2017;39:654. doi: 10.1016/j.ultsonch.2017.05.013 [DOI] [PubMed] [Google Scholar]
- 156.Guarnieri D, Melone P, Moglianetti M, et al. Particle size affects the cytosolic delivery of membranotropic peptide-functionalized platinum nanozymes. Nanoscale. 2017;9(31):11288. doi: 10.1039/C7NR02350B [DOI] [PubMed] [Google Scholar]
- 157.Wang T, Wang L, Li X, et al. Size-dependent regulation of intracellular trafficking of polystyrene nanoparticle-based drug delivery carriers. ACS Appl Mater Interfaces. 2017;9(22):18619. doi: 10.1021/acsami.7b05383 [DOI] [PubMed] [Google Scholar]
- 158.Wan F, You J, Sun Y, et al. Studies on PEG-modified SLNs loading vinorelbine bitartrate (I): preparation and evaluation in vitro. Int J Pharm. 2008;359(1):104–110. doi: 10.1016/j.ijpharm.2008.03.030 [DOI] [PubMed] [Google Scholar]
- 159.Bi C, Miao XQ, Chow SF, et al. Particle size effect of curcumin nanosuspensions on cytotoxicity, cellular internalization, in vivo pharmacokinetics and biodistribution. Nanomed Nanotechnol Biol Med. 2016;13(3):943–953. doi: 10.1016/j.nano.2016.11.004 [DOI] [PubMed] [Google Scholar]
- 160.Patel MM, Goyal BR, Bhadada SV, Bhatt JS, Amin AF. Getting into the brain: approaches to enhance brain drug delivery. CNS Drugs. 2009;23(1):35–58. doi: 10.2165/0023210-200923010-00003 [DOI] [PubMed] [Google Scholar]
- 161.Zhang F, Xu CL, Liu CM. Drug delivery strategies to enhance the permeability of the blood–brain barrier for treatment of glioma. Drug Des Devel Ther. 2015;9:2089. doi: 10.2147/DDDT.S79592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Chen J, Wang T, Xu S, et al. Discovery of novel antitumor nitric oxide-donating beta-elemene hybrids through inhibiting the PI3K/Akt pathway. Eur J Med Chem. 2017;135:414–423. doi: 10.1016/j.ejmech.2017.04.045 [DOI] [PubMed] [Google Scholar]
- 163.Hong-jin W, Jun-jie W, Qiang X, et al. Effect factors and mechanisms of borneol’s bidirectional regulation on blood-brain barrier permeability. China J Chin Mater Med. 2017;42(11):2200–2207. doi: 10.19540/j.cnki.cjcmm.2017.0100 [DOI] [PubMed] [Google Scholar]
- 164.Li-ping W, Jian-fang F, Kai-li H. Progress in regulation effect of aromatic refreshing traditional Chinese medicine on BBB permeability and its mechanism. China J Chin Mater Med. 2014;39(6):949–954. [PubMed] [Google Scholar]
- 165.Zheng Q, Chen ZX, Xu MB, et al. Borneol, a messenger agent, improves central nervous system drug delivery through enhancing blood–brain barrier permeability: a preclinical systematic review and meta-analysis. Drug Deliv. 2018;25(1):1617–1633. doi: 10.1080/10717544.2018.1486471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Zhang QL, Fu BM, Zhang ZJ. Borneol, a novel agent that improves central nervous system drug delivery by enhancing blood–brain barrier permeability. Drug Deliv. 2017;24(1):1037. doi: 10.1080/10717544.2017.1346002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Yuan X, Fei F, Sun H, et al. Tanshinol borneol ester on nanostructured lipid carriers has longer brain and systemic effector retention and better antioxidant activity in vivo. Int J Nanomedicine. 2018;13:2265–2274. doi: 10.2147/IJN.S159789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Lin Z, Limei H, Jing Q, et al. The use of borneol as an enhancer for targeting aprotinin-conjugated PEG-PLGA nanoparticles to the brain. Pharm Res. 2013;30(10):2560–2572. doi: 10.1007/s11095-013-1055-y [DOI] [PubMed] [Google Scholar]
- 169.Yin Y, Cao L, Ge H, et al. L-Borneol induces transient opening of the blood–brain barrier and enhances the therapeutic effect of cisplatin. Neuroreport. 2017;28(9):506. doi: 10.1097/WNR.0000000000000792 [DOI] [PubMed] [Google Scholar]
- 170.Wu JY, Li YJ, Yang L, et al. Borneol and Α-asarone as adjuvant agents for improving blood–brain barrier permeability of puerarin and tetramethylpyrazine by activating adenosine receptors. Drug Deliv. 2018;25(1):1858–1864. doi: 10.1080/10717544.2018.1516005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Dgr E, Salvador H, Chang VY, et al. Cancer and central nervous system tumor surveillance in pediatric neurofibromatosis 2 and related disorders. Clin Cancer Res off J Am Assoc Cancer Res. 2017;23(12):e54. doi: 10.1158/1078-0432.CCR-17-0590 [DOI] [PubMed] [Google Scholar]
- 172.Yoon YI, Yoon TJ, Lee HJ. Optimization of ultrasound parameters for microbubble-nanoliposome complex-mediated delivery. Ultrasonography. 2015;34(4):297–303. doi: 10.14366/usg.15009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Chen LJ, Cui-Tao LU, Zhao YZ, et al. Ultrasonic microbubbles for glioma-targeted drug delivery. Yao Xue Xue Bao = Acta Pharmaceutica Sinica. 2015;50(1):99–103. [PubMed] [Google Scholar]
- 174.Sennoga CA, Kanbar E, Auboire L, et al. Microbubble-mediated ultrasound drug-delivery and therapeutic monitoring. Expert Opin Drug Deliv. 2017;14(9):1031–1043. doi: 10.1080/17425247.2017.1266328 [DOI] [PubMed] [Google Scholar]
- 175.Marcel Menon M, Bleier BS. The blood-brain barrier and nasal drug delivery to the central nervous system. Am J Rhinol Allergy. 2015;29(2):124–127. doi: 10.2500/ajra.2015.29.4149 [DOI] [PubMed] [Google Scholar]
- 176.Zhang LK, Ri-Xin XU, Jiang M, et al. Evaluation of brain-targeting of β-asarone microemulsion by intranasal administration. Chin Traditional Herbal Drugs. 2014;45(1):86–89. [Google Scholar]
- 177.Nair A. Implications of intrathecal chemotherapy for anaesthesiologists: a brief review. Scientifica,2016,(2016-3-31). 2016;2016(5):1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Kumari S, Ahsan SM, Kumar JM, et al. Overcoming blood brain barrier with a dual purpose Temozolomide loaded Lactoferrin nanoparticles for combating glioma (SERP-17-12433). Sci Rep. 2017;7(1):6602. doi: 10.1038/s41598-017-06888-4 [DOI] [PMC free article] [PubMed] [Google Scholar]