Abstract
The vascular organ of the lamina terminalis, subfornical organ (SFO), and area postrema comprise the sensory circumventricular organs (CVO) which are central structures that lie outside the blood brain barrier and are thought to provide an interface between peripherally circulating signals and the brain through their projections to central autonomic structures. The SFO expresses mRNA for the G protein-coupled apelin receptor (APJ, gene name aplnr) and exogenous microinjection of the neuropeptide apelin (apln) to the SFO elicits a depressor effect. Here we investigated the expression and cellular distribution of aplnr, apln and the recently described ligand apela (apela) in the CVOs and investigated whether differences in the levels of expression of apelinergic gene transcripts in these regions might underlie the chronic elevated blood pressure seen in hypertension. We carried out multiplex in situ hybridization histochemistry on CVO tissue sections from spontaneously hypertensive rats (SHR) and normotensive Wistar Kyoto (WKY) controls. Confocal immunofluorescent images indicated strong aplnr expression, with lower levels of apln and modest apela expression, in the CVOs of both WKY rats and SHRs, in both neurons and glia. The expression level of aplnr transcripts was increased in the SFO of SHRs compared to WKY rats. Our data may highlight a potential dysfunction in the communication between CVOs and downstream signalling pathways in SHRs, which may contribute to its different phenotype/s.
Introduction
Apelin, a 36 amino acid peptide, originally isolated from bovine stomach extracts, exists in numerous isoforms in vivo, all of which bind to, and activate, the G protein-coupled apelin receptor (APJ; gene name aplnr) [1, 2, 3]. Apelin (gene name apln) and APJ are ubiquitously expressed in peripheral cells and tissues, including vascular endothelial cells [4], cardiac myocytes [5], and kidney [6], as well as in discrete central regions such as forebrain (e.g. hypothalamic supraoptic (SON) and paraventricular (PVN) nuclei) and lower brainstem structures (e.g. nucleus of the solitary tract (NTS), area postrema (AP)) [7], thus allowing for multiple roles in the central regulation of physiological systems such as cardiovascular control [8, 9], angiogenesis [10], autonomic signalling [11–13], neuroprotection [14], fluid homeostasis [15], and modulation of hypothalamic-pituitary-adrenal axis activity [16, 17]. In some brain structures, e.g. cortex, APJ immunoreactivity (ir) is present in both neuronal and supporting cell populations such as glia [16]. Recently, an additional functional ligand for APJ has been identified, termed apela (gene name apela) or elabela [18–20], that is expressed in the rodent and human heart, kidney and placenta (e.g., see ncbi.nlm.nih.gov/gene/100506013; GEO Profiles). Apela is vital for normal cardiovascular development in embryonic zebrafish [18] and mice [21] and has been implicated in fluid homeostasis in adult rats through an action in the kidney [22]. Injection of apela into the cerebroventricular system is anorexigenic [23] although, to date, no endogenous central expression of apela has been reported [22].
A complex picture has emerged in the role that the apelinergic system plays in the modulation of blood pressure (BP), depending on the route of administration (systemic or central) and the brain region to which apelin is applied. The overall effect of systemic treatment with apelin-13 and [Pyr1]apelin-13, which may be acting at peripheral and/or central sites, is a depressor response in both anaesthetized rats [24–26] and conscious humans [27, 28] through increased vasodilatation and decreased vascular resistance. Additionally, intravenously administered apela normalized hypertension in pregnant apela knockout mice [21]. In contrast, central administration of apelin-13 and [Pyr1]apelin-13 directly to the ventricles [29] or to autonomic centres, such as the PVN [8] and rostral ventrolateral medulla (RVLM) [9, 30, 31], increases mean arterial BP via an increase in sympathetic nerve activity. Apelin may also act at other central sites to mediate cardiovascular responses. Interestingly, microinjection of apelin-13 to the subfornical organ (SFO) decreases BP and heart rate in anaesthetized rats [32], a response similar to that observed after systemic injection of apelin. The SFO, vascular organ of the lamina terminalis (OVLT), and the AP are collectively known as the sensory circumventricular organs (CVOs) and are midline, central structures that lie outside the blood brain barrier (BBB)—they have been identified as a possible interface between systemically circulating signals, such as ghrelin [33], leptin [34], vasopressin [35] and adrenomedullin [36], and the brain [37]. Efferent neurons from the CVOs project to brain regions involved in cardiovascular control such as the PVN, OVLT, RVLM, and NTS [38–40]. Aplnr expression is found in the SFO in transcriptomic studies (e.g. microarray analysis and RT-PCR [32]) while apelin immunoreactive fibres are observed in the SFO, OVLT, and AP [7]. The precise anatomical location of apelinergic gene expression in the CVOs has not been reported and to our knowledge no role as been assigned to these apelinergic neurones. The levels of apelinergic gene expression in some tissues however are altered in rodent models of hypertension, e.g. the cardiac apelinergic system is downregulated in the Dahl salt-sensitive rat [41], and decreased aplnr and apln levels are seen in the heart and aorta of spontaneously hypertensive rats (SHR) compared to normotensive Wistar Kyoto (WKY) rats [42], while APJ and apelin mRNA and protein are upregulated in the SHR PVN [8].
We hypothesized that changes in the levels of APJ, or its ligands, within the CVOs may lead to aberrant activity in the afferent arm and central circuitry of the autonomic nervous system manifesting in chronic elevated BP, or hypertension [43, 44]. Additionally, local apela gene expression in distinct brain regions involved in autonomic cardiovascular control would lend an intriguing layer of complexity to the role of the apelinergic system in the brain. Therefore, this study aimed to determine the anatomical localization of aplnr, apln and apela in distinct cell types in the SFO, OVLT, and AP, in normotensive rats using multiplex in situ hybridization histochemistry (ISHH), and to determine whether the hypertensive phenotype of SHRs is associated with differences in the levels of apelinergic gene expression in these regions.
Materials and methods
Animals
Adult male (approx 250g) Wistar Kyoto (WKY, Envigo, UK; n = 4) and spontaneously hypertensive rats (SHR, Animal Services Unit, University of Bristol, UK; n = 4) were used in this study. Animals were housed under constant temperature (21±2°C), light (lights on from 0700 to 1900h) and humidity (45–50%) regimens with food and water ad libitum. Animal care and maintenance were performed in accordance with the Animal Scientific Procedures Act (1986) United Kingdom and approved by the Bristol University Animal Welfare and Ethical Review body.
Branched chain in situ hybridization histochemistry (ISHH)
Naïve adult WKY rats and SHRs were humanely killed between 0900–1100 using concussion to the cranium and immediately decapitated with a small animal guillotine. Brains were removed, frozen on powdered dry ice and stored desiccated at -80°C prior to processing. Brains were then sectioned (CM3050 S, Leica Microsystems) and the OVLT (Fig 28 Bregma +0.60mm), SFO (Fig 42 Bregma -1.08mm) and AP (Fig 148 Bregma -13.80mm) were identified with reference to the Rat Brain Atlas [45]. Twenty consecutive sections (16μm) were collected from the central portion of each structure. Sections were thaw-mounted onto SuperFrost Plus slides (Fisher Scientific, UK) and every fifth section was stained with toluidine blue (0.1%) as a histological reference. Sections were stored at -80°C for at least a week before use.
A branched chain in situ hybridization histochemistry (ISHH) assay (RNAscope Multiplex Fluorescence Assay kit; Bio-Techne) was used as described previously [6]. Briefly, sections were post-fixed in 4% PFA (pH 7.4), dehydrated in increasing concentrations of ethanol and treated for 30 minutes with RNAscope Protease IV solution. Sections were then incubated for 2 hours with proprietary probes, designed by Bio-Techne, for the following rat sequences: apela (GenBank Accession XM_008772035 (LOC100912649); bp147-1053; 12 Z probe pairs), apln (apelin; NM_031612; bp2-996; 20 Z probe pairs), aplnr (APJ; NM_031349; bp147-1053; 20 Z probe pairs), rbfox (NeuN; NM_00134498.2; bp16-1116; 15 Z probe pairs) and gfap (Gfap; NM_017009.2, bp1539-2534; 20 Z probe pairs). The specificity of the aplnr, apln and apela probes have been validated previously [6]. A combination of 3 probes at ratio 50:1:1 was used on any one section, as determined by the channel (C1-3) designated to each probe. The fluorescent colour for each probe (Alexa 488 (green), Atto 550 (orange) and Atto 647 (far red)) was dependent on the fluorescent colour module (Amp4 AltA-C) and channels (C1-3) used. Sections were counterstained with DAPI as per manufacturer’s instructions. Positive (rat polymerase (RNA) II (DNA directed) polypeptide A, transcript variant 1 (POLR2A), peptidylprolyl isomerase (PPIB) and ubiquitin C (UBC); 3-plex probe set (ACD#320891) and negative (DapB (of Bacillus subtilis strain)) (ACD#320871)) probes purchased from Bio-Techne were included in all experiments. Experiments were repeated twice on different slide sets.
Image capture and statistical analysis
Figures for RNAscope are representative of sections from four animals. Experimental groups of 4 were used based on past experiments detecting a statistically significant difference (p<0.05) between groups. This was confirmed by statistical power calculations. The fluorescence signal in the OVLT, SFO and AP was visualized using a x40 oil lens on a Leica SPE single channel confocal laser scanning microscope attached to a Leica DMi8 inverted epifluorescence microscope equipped with a Lecia DFC365FX monochrome digital camera and LAS X workstation (Wolfson Bioimaging Centre, University of Bristol). Images were captured using the LAS X software and Z stacks (Z step size = 0.5μm) were taken for all images. Raw image files were processed to generate composite images using the open access image analysis software, FIJI [46]. Automated image analysis (for dots/DAPI nuclei) was carried out using a custom plugin for FIJI provided by the Wolfson Bioimaging Facility at the University of Bristol. To obtain the percentage of positive cells, DAPI-labelled nuclei were segmented and numbers of round dots over DAPI nuclei were manually counted independently by two observers. Statistical analysis was carried out using GraphPad Prism software v7 (GraphPad). Data was tested for normality using a Kolmogorov-Smirnov test. Non-parametric variables are expressed as medians with interquartile ranges (IQR) and statistical significance was testing using the Mann-Whitney test. p<0.05 was considered statistically significant between groups.
Results
Apelinergic distribution in rat circumventricular organs (CVOs) of WKY rats and SHRs
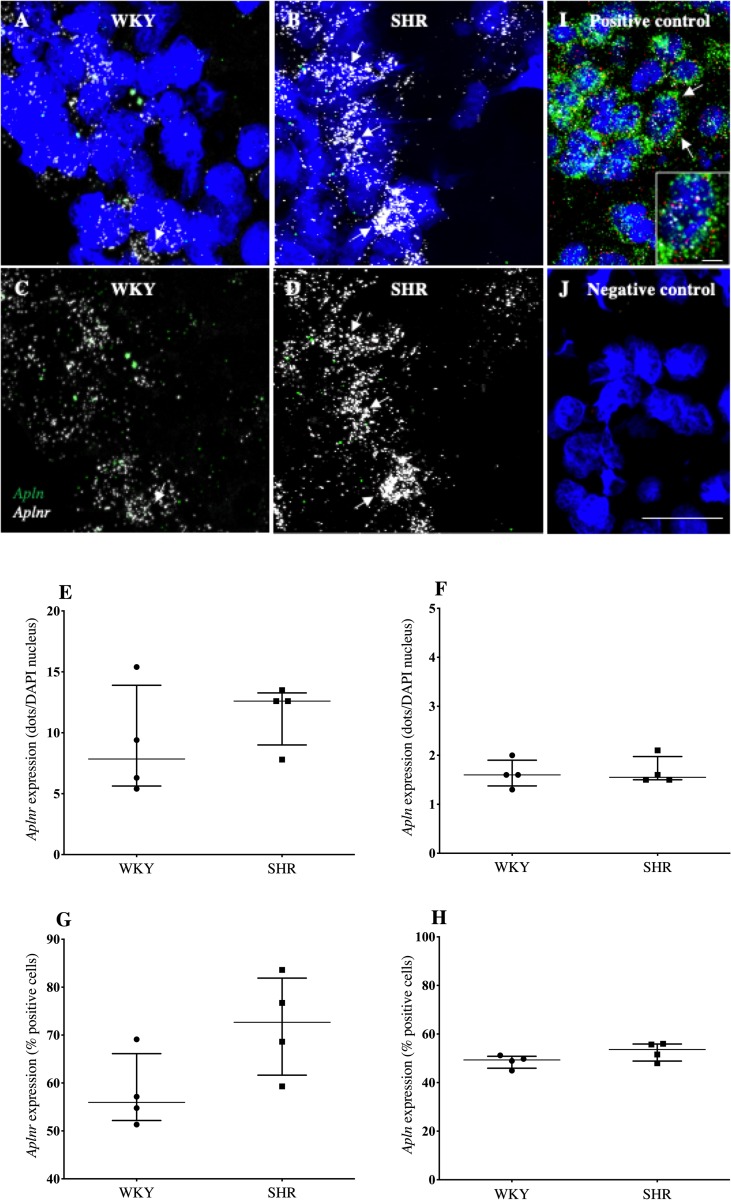
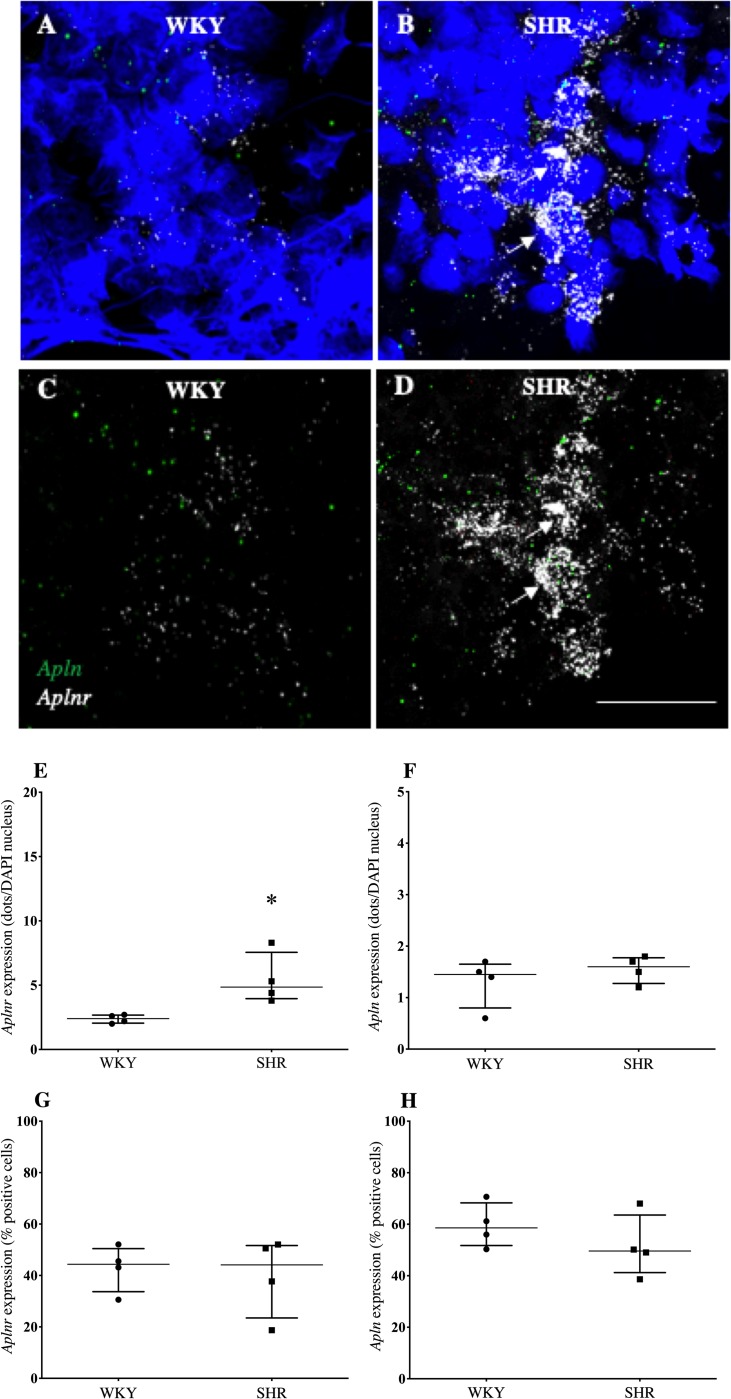
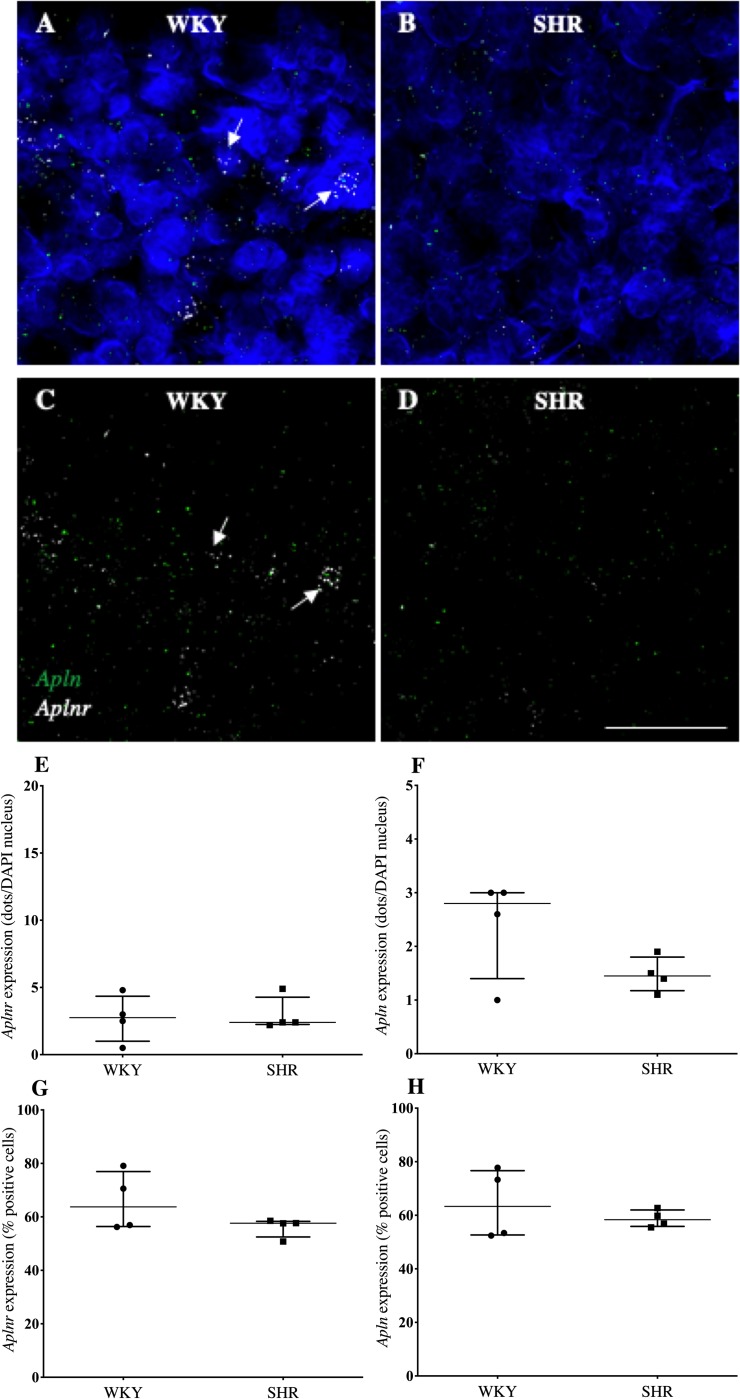
Confocal immunofluorescent images indicated aplnr was more abundantly expressed than apln in the OVLT (Fig 1), SFO (Fig 2) and AP (Fig 3) of both WKY rats (Figs 3A and 3C, 4A and 4C, 5A and 5C) and SHRs. (Figs 1B and 1D, 2B and 2D, 3B and 3D). Aplnr was relatively strongly expressed in all CVOs with a patchy distribution, in contrast to the level of apln expression which was low and more uniform. In the SFO and OVLT, aplnr expression was seen lateral of the main portion, and in the ventral portion of the lateral zone, respectively. Gene expression was quantified as a function of the number of dots/DAPI nuclei for each gene in both WKY rats and SHRs. The SFO from SHRs showed a significantly greater number of aplnr RNAscope dots/cell than normotensive WKY rats (4.9 [4.0–7.6] dots/cell vs 2.4 [2.0–2.7] dots/cell respectively; p<0.05, Fig 2E). No difference was seen in the number of aplnr RNAscope dots/cell in the OVLT or AP of SHRs (12.6 [9.0–13.2] dots/cell, Fig 1E; 2.4 [2.3–4.3] dots/cell, Fig 3E respectively) compared to WKY rats (7.9 [5.6–13.9] dots/cell, Fig 1E; 2.8 [1.0–4.4] dots/cell, Fig 3E respectively). Additionally, no difference was seen in the number of apln RNAscope dots/cell in the OVLT (1.6 [1.5–2.0] dots/cell, Fig 1G), SFO (1.6 [1.3–1.8] dots/cell, Fig 2G) or AP (1.5 [1.2–1.8] dots/cell, Fig 3G) of SHRs compared to WKY (1.6 [1.4–1.9] dots/cell, Fig 1G; 1.5 [0.8–1.7] dots/cell, Fig 2G; 2.8 [1.4–3.0] dots/cell, Fig 3G respectively) rats. The percentage of cells in each region positive for aplnr or apln gene expression was also calculated. There were no differences in the percentage of cells expressing aplnr (73 [62–82]%, Fig 1F) or apln (54 [–56]%, Fig 1H) in the SHR OVLT compared to WKY rat OVLT (56 [52–66]%, Fig 1F; 49 [46–51]% Fig 1H, respectively). Similarly there were no differences in the percentage of cells expressing aplnr or apln in the SHR SFO compared to WKY rats (44 [33–50]%, Fig 2F; 50 [41–64]% Fig 2H, respectively vs 44 [33–50]%, Fig 2F; 59 [52–68]%, Fig 2H respectively) or in the SHR AP compared to WKY rats (58 [56–62]%, Fig 3F; 58 [52–58]%, Fig 3H respectively vs 64 [57–77]%, Fig 3F; 63 [53–77]%, Fig 3H respectively). The increase in aplnr expression in SHR SFO appears to be in the amount of mRNA expressed per cell and not in the absolute number of cells expressing the gene.
Fig 1. Aplnr and apln expression in the OVLT of WKY rats and SHRs.
(A-D) Representative fluorescent images showing expression of aplnr and apln in the OVLT of WKY rats (A and C) and SHRs (B and D). DAPI: blue, aplnr: white, apln: green. DAPI removed from C and D to highlight distribution of mRNA. Arrows point to representative cells with relatively high levels of aplnr expression. (E-H) Graphs showing gene expression as a function of dots/DAPI nuclei or percentage positive cells for aplnr (E and F) and apln (G and H) expression. n = 4/group. Data is median [IQR]. Sections were also analysed with positive (rat polymerase (RNA) II (DNA directed) polypeptide A, transcript variant 1 (POLR2A;white dots), peptidylprolyl isomerase (PPIB; red dots) and ubiquitin C (UBC; green dots)) (I) and negative (DapB (of Bacillus subtilis strain)) (J) with nuclei counterstained with DAPI (blue). The inset shows a high magnification image of the cell depicted by white arrows. Scale bar = 50μm and 10μm for the inset image.
Fig 2. Aplnr and apln expression in the SFO of WKY rats and SHRs.
(A-D) Representative fluorescent images showing expression of aplnr and apln in the SFO of WKY rats (A and C) and SHRs (B and D). DAPI: blue, aplnr: white, apln: green. DAPI removed from C and D to highlight distribution of mRNA. Arrows point to representative cells with relatively high levels of aplnr expression. Scale bar = 50μm. (E-H) Graphs showing gene expression as a function of dots/DAPI nuclei or percentage positive cells for aplnr (E and F) and apln (G and H) expression. n = 4/group. Data is median [IQR]. *p<0.05.
Fig 3. Aplnr and apln expression in the AP of WKY rats and SHRs.
(A-D) Representative fluorescent images showing expression of aplnr and apln in the AP of WKY rats (A and C) and SHRs (B and D). DAPI: blue, aplnr: white, apln: green. DAPI removed from C and D to highlight distribution of mRNA. Cells with a high relative abundance of aplnr are arrowed. Scale bar = 50μm. (E-H) Graphs showing gene expression as a function of dots/DAPI nuclei or percentage positive cells for aplnr (E and F) and apln (G and H) mRNA. n = 4/group. Data is median [IQR].
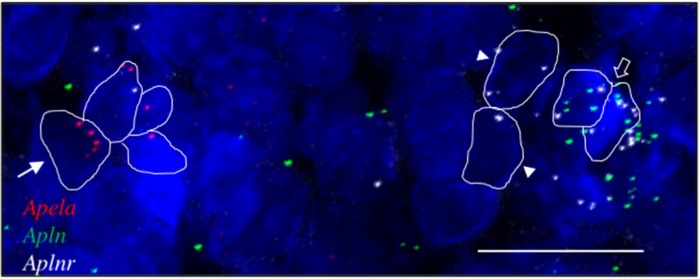
Fig 4. Aplnr, apln and apela transcripts in the SHR AP.
Representative fluorescent images showing cells expressing apela (red dots; arrowed), aplnr (white dots, arrowheads) and apln (green dots). Cells co-expressing aplnr and apln are indicated (open arrow). Scale bar = 50μm. n = 4.
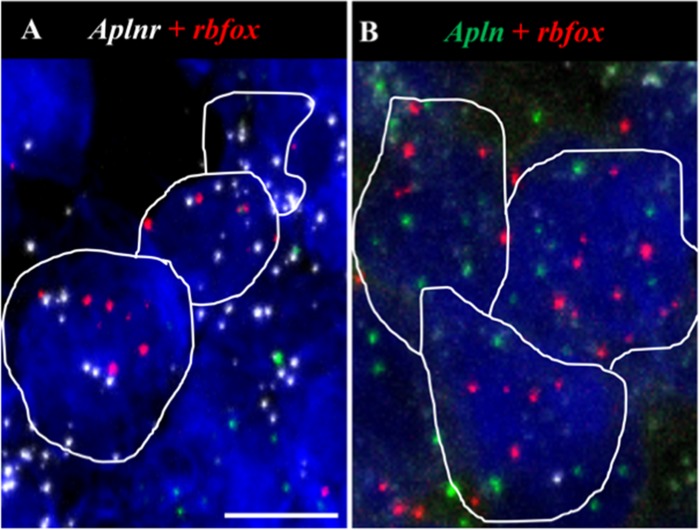
Fig 5. Aplnr and apln transcripts co-localise with rbfox in the AP.
(A,B) Representative fluorescent images showing colocalization of aplnr (white dots, A) and apln (green dots, B) with rbfox (red) in the AP. Scale bar = 10μm. n = 4.
Positive control probe sets detected mRNA throughout all sections (see Fig 1I) and gave the expected relative levels of gene expression with UBC (green) more abundant than PPIB (red) and POLR2A (white). The negative control probe set (DapB) generated only rare isolated signal (mainly in the far-red channel) (e.g. signal detected in one out of 89 cells; Fig 1J).
In a small follow up study, we investigated the expression of apela in the CVOs. Apela was much less abundantly expressed than apln (or aplnr) in all three CVOs, evident predominantly in the AP. Up to ~9% of cells in the AP expressed apela (9.4% (28/298 cells) and 5.5% (17/308 cells) of cells were apela-positive in two images of AP from different SHR rats (see Fig 4); 5.8% (16/276 cells) of cells were apela-positive in one image of AP from a WKY rat).
In contrast, in the SFO and the OVLT apela was rarely expressed and was seen in fewer than 1% of cells in either SHRs or WKY rats. Due to the low abundance of apela expression, we did not systematically determine whether apela was expressed in rbfox- or gfap-positive cells or differed between CVOs of SHRs or WKY rats.
Localization of aplnr and apln to OVLT, SFO and AP neurons and/or glia in normotensive and SHR CVOs
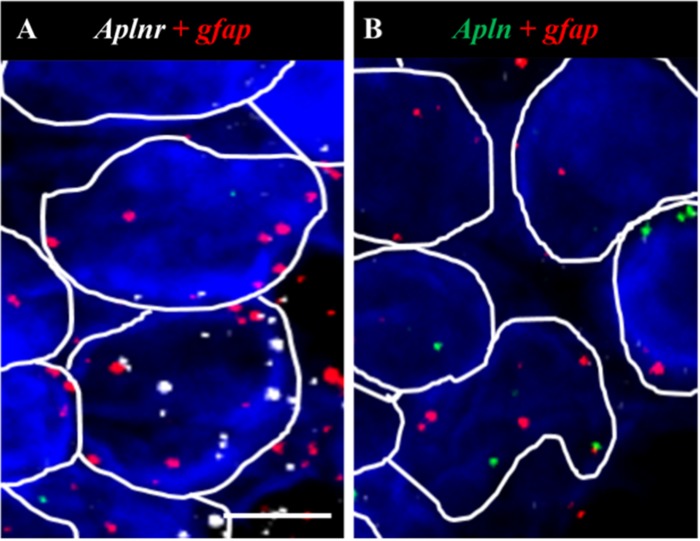
To examine the cell types expressing aplnr and apln, aplnr- or apln-positive cells were identified and the number of cells double-positive for either the neuronal marker rbfox (NeuN) or the prototypical glial-astrocyte marker gfap (Gfap) were quantified (4 brain slices/probe set). Aplnr and apln were expressed in both neurons and glial cells in all three CVOs in WKY rats and SHRs as evidenced by their colocalization with rbfox (e.g., Fig 5A and 5B respectively) or gfap (e.g., Fig 6A and 6B respectively).
Fig 6. Aplnr and apln transcripts colocalise with gfap in the AP.
(A, B) Representative fluorescent images showing colocalization of aplnr (white dots, A) and apln (green dots, B) with gfap (red) in the AP. Scale bar = 20μm. n = 4.
The colocalization of both aplnr or apln with rbfox was decreased in the SHR AP (11 [8–13]% and 26 [24–27]% respectively, Table 1) compared to WKY rats (35 [29–50]% and 56 [48–71]% respectively (Table 1, p<0.05) while the co-localization of both aplnr or apln with gfap was increased in the SHR OVLT (76 [74–78]% and 49 [43–69]% respectively, Table 2) compared to WKY rats (51 [49–55]% and 29 [26–36]% respectively, Table 2, p<0.05). No other change in the co-localization of aplnr or apln with rbfox or gfap in the three CVOs was seen between SHRs and WKY rats (Tables 1 and 2 respectively).
Table 1. The percentage of rbfox positive cells co-localizing with aplnr or apln in the OVLT, SFO and AP of WKY rats and SHRs.
| % rbfox Positive cells | ||||||
|---|---|---|---|---|---|---|
| OVLT | SFO | AP | ||||
| Aplnr | Apln | Aplnr | Apln | Aplnr | Apln | |
| WKY | 18[11–36] | 39[30–45] | 17[10–26] | 53[39–75] | 35[29–50] | 56[48–71] |
| SHR | 28[22–48] | 38[24–60] | 11[10–16] | 43[23–58] | 11[8–13]* | 26[24–27]* |
n = 4/group. Data is median [IQR].
*: p<0.05.
Table 2. The percentage of gfap positive cells co-localizing with aplnr or apln in the OVLT, SFO and AP of WKY rats and SHRs.
| % gfap Positive Cells | ||||||
|---|---|---|---|---|---|---|
| OVLT | SFO | AP | ||||
| Aplnr | Apln | Aplnr | Apln | Aplnr | Apln | |
| WKY | 51[49–55] | 29[26–36] | 50[39–71] | 44[38–58] | 27[19–40] | 31[22–39] |
| SHR | 76[74–78]* | 49[43–69]* | 53[48–68] | 34[27–49] | 38[25–52] | 43[30–51] |
n = 4/group. Data is median [IQR].
*: p<0.05.
Co-localization of aplnr and apln in normotensive and SHR CVOs
ISHH to detect aplnr and apln in the same sections revealed that a proportion (16–42%) of aplnr-positive cells in the three CVOs also expressed apln, however a number of cells also expressed either aplnr or apln alone. There was a significant decrease in the proportion of cells expressing both aplnr and apln in the AP of SHRs in comparison to WKY rats (24 [18–39]% vs 41 [32–47]% respectively, p<0.05). No change in the percentage co-localization of aplnr with apln was seen in the OVLT or SFO of SHRs in comparison to WKY rats (36 [29–40]% vs 31 [16–39]% and 15 [12–20]% vs 20 [17–26]% respectively, p>0.05).
Discussion
The present study confirms that the apelinergic system in the sensory CVOs has a potential role in mediating cardiovascular responses. We employed highly specific RNAscope multiplex ISHH to demonstrate expression of aplnr, apln and apela transcripts in all three CVOs in both WKY rats and SHRs. Additionally, we report aplnr and apln were expressed in both neurons and glial cells in the three CVOs in these rat strains. Importantly aplnr levels within the SFO of SHRs were significantly higher compared with normotensive WKY rats, suggesting that this receptor within the SFO may be associated with the pathogenesis of hypertension.
APJ/apelin/apela have important roles in the regulation of cardiovascular function and control of BP as evidenced in numerous studies [8, 9, 20, 24, 29–31, 47–58]. Some of these central effects may be mediated by APJ in the SFO, OVLT and/or AP. The SFO is a mid-line sensory CVO that has been shown to project to the preoptic-hypothalamic circuit, OVLT, and brainstem cardiovascular centers such as the RVLM, while the OVLT itself projects to the PVN (a source of presympathetic neurons regulating sympathetic outflow via descending fibres) and SON; and the AP directly projects to the NTS, dorsal motor nucleus of the vagus, ventrolateral medulla and the lateral division of the parabrachial nucleus [59]. All three CVOs are known to be involved in the regulation of the cardiovascular system, fluid balance and energy homeostasis (see [59] for review).
Central structures, including the SFO, OVLT and brainstem structures involved in the modulation of BP, contain apelinergic ir-fibres [56] and express aplnr [17]—anatomical observations that support roles for apelin and APJ in the control of the cardiovascular system. By contrast apela expression has been found in the zebrafish and rodent cardiovascular system [6, 18, 19], however while expression has been demonstrated in developing and adult human and mouse brain samples in transcriptomic repositories (such as Ensembl; GEO Profiles), no anatomical distribution of apela in the CNS has been described in the literature. We report the expression of apln, aplnr and apela within the three CVOs in SHRs and WKY rats. Since apelin presumably does not cross the BBB (although this has been disputed [60], and may change in hypertension [61]), the CVOs are likely important conduits allowing circulating apelin access to the brain. These sites also incorporate a high degree of neural interconnectivity and reciprocal communication, which may thus transduce peripheral apelin signals in critical cardiovascular control centers that regulate sympathetic activity to maintain BP. The presence of apln in the CVOs indicates that apelin may be synthesised locally within these brain regions. While the major site of apelin/APJ expression in the brain appears to be the hypothalamus, apelin is detected in neuronal cell bodies and fibres throughout the entire brain [56]. Apelin is also found in the systemic circulation, possibly secreted from adipocytes or endothelial cells [62, 63]. Both systemic [64] and central [8, 30] administration of the APJ antagonist F13A abolishes apelin-mediated MABP effects. The co-existence of aplnr and apln (and to a lesser extent apela) in the CVOs, outside the BBB, indicates an intimate connection between the central and peripheral apelinergic systems and suggests that APJ within these organs may be activated by locally synthesised apelin (suggesting autocrine/paracrine actions within the CVOs) and/or by circulating levels of apelin—the presence of aplnr in the choroid plexus [24] is consistent with the latter premise. Additionally, the presence of apela expression within the CVOs raises questions as to which APJ ligand (and perhaps their proteoforms) is functionally active, on the same or different cells, during development and in response to which physiological perturbations.
Levels of peripheral apelinergic gene expression are downregulated during hypertension [41, 42, 58]. However, within the brain, we and others have shown APJ and apelin gene transcripts and protein are upregulated in the SHR PVN [8] and RVLM [9, 65] by mechanisms that are not yet understood. Here we report that aplnr levels within the SFO of SHR were significantly higher compared with normotensive WKY rats, highlighting a potential association of SFO aplnr with hypertension in SHRs, while there was no significant difference in aplnr levels in OVLT or AP, or in apln levels in any of the CVOs, between the two rat strains. The consistently increased aplnr expression observed in the SFO of hypertensive animals appears to reflect the amount of gene transcript expressed per cell rather than the absolute number of cells expressing the aplnr gene. We do not know the extent to which changes in aplnr expression are mirrored at the protein, and thus functional, level however in previous studies where we have silenced aplnr expression in the RVLM we have seen a corresponding loss of APJ functionality [65]. Our data provide a foundation upon which to address the role of APJ in the sensory CVOs, and in the SFO in particular, in cardiovascular control. This finding is in accord with our previous study where we have shown attenuated c-fos mRNA expression in the SFO of aplnr-KO mice after osmotic stress [66], suggesting reduced neuronal activity in this region in the absence of APJ, and reflecting a role for APJ in the SFO in fluid regulation. We postulate that circulating (and/or local) apelin activates APJ neurons in the SFO, and this increased neuronal activity is relayed downstream directly or indirectly (perhaps via another receptor system such as vasopressin V1a receptors, as shown in the RVLM [30]) to primary SFO projection regions, namely the PVN, RVLM and/or NTS. How the apelinergic system in the CVOs coordinates cardiovascular activity between each CVO, and in line with apelinergic activity in the RVLM/PVN, is not known.
APJ-ir has been localised to both neuronal and glial cell populations within the spinal cord and cortex [16] but no study has reported the APJ-expressing cellular phenotype in the sensory CVOs. In the SFO, OVLT, and AP, aplnr and apln were expressed in both neurons and glial cells in both SHRs and WKY rats. Aplnr and apln were not expressed in every neuron or glia, raising the possibility that components of the apelinergic system are present in distinct cell subpopulations. Defining the expression of genes such as those encoding GPCRs and their peptide ligands by transcriptomics may help characterise neuronal and glial cell diversity in CVOs, as has been shown in other brain regions (e.g., [67]). In addition, we report that the expression of neuronal aplnr and apln is decreased in the AP of SHRs, with an accompanying decrease in the co-localization of aplnr with apln, in comparison to WKY rats, while the glial expression of receptor and ligand genes is increased in the OVLT of SHRs. As the composition of the CVO neuronal and glial population does not appear to differ between SHRs and WKY rats, changes seen in the expression pattern of apln and/or aplnr in these CVO cell types may reflect a functional change in activity within the CVOs, indicating a potential dysfunction in the communication between CVOs and downstream signalling pathways in the SHR that may be responsible, at least in part, for the hypertensive phenotype. Our results suggest that the mechanisms by which apelin mediates its hypertensive actions may involve an interaction between the activation of APJ on both neurons and glia within individual CVOs to impact neurophysiology.
Along with its expression in neurons and glia, it is possible that aplnr is localised in endothelial cells in the CVOs, which are highly vascularized [68]. Ir-APJ is found in vascular and tissue endothelial cells (ECs) [69, 70] and aplnr and apln transcripts are present in brain and peripheral tissue ECs [71]. In the brain aplnr is present at low levels in normal human meningeal blood vessels [72], and both apln and ir-apelin, and aplnr expression have been found in microvascular proliferations in human glioblastoma specimens [72, 73]. Aplnr is expressed in the ECs of some vessels in the rodent brain [74], and apelin promotes angiogenesis and endothelial function after brain insults in mice [75, 76], and stimulates the proliferation of mouse brain microvasculature-derived ECs [77]. The potential expression of components of the apelinergic system in brain ECs, e.g. using an EC-specific marker such as CD31, warrants investigation in future studies.
In conclusion our study supports the potential physiological importance of APJ and apelin in the sensory CVOs by showing that (1) the SFO, OVLT, and AP, which are linked to the central networks that modulate central cardiovascular responses, express components of the apelinergic system, and (2) the expression of aplnr in the SFO is altered in SHRs indicating CVO apelinergic dysfunction in this model of hypertension.
Data Availability
All relevant data are within the paper.
Funding Statement
A-MO'C, SJL, JFRP This work was supported by the British Heart Foundation (BHF; www.bhf.org.uk) (PG/15/14/31311). The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Kawamata Y, Habata Y, Fukusumi S, Hosoya M, Fujii R, Hinuma S, et al. Molecular properties of apelin: tissue distribution and receptor binding. Biochim Biophys Acta. 2001; 1538:162–171. 10.1016/s0167-4889(00)00143-9 [DOI] [PubMed] [Google Scholar]
- 2.Maguire JJ, Kleinz MJ, Pitkin SL, Davenport AP. [Pyr1]apelin-13 identified as the predominant apelin isoform in the human heart: vasoactive mechanisms and inotropic action in disease. Hypertension 2009, 54:598–604. 10.1161/HYPERTENSIONAHA.109.134619 [DOI] [PubMed] [Google Scholar]
- 3.Tatemoto K, Hosoya M, Habata Y, Fujii R, Kakegawa T, Zou MX, et al. Isolation and characterization of a novel endogenous peptide ligand for the human APJ receptor. Biochim Biophys Res Commun. 1998; 251:471–476. 10.1006/bbrc.1998.9489 [DOI] [PubMed] [Google Scholar]
- 4.Liu Q, Hu T, He L, Huang X, Tian X, Zhang H, et al. Genetic targeting of sprouting angiogenesis using Apln-CreER. Nat Commun. 2015; 6:6020 10.1038/ncomms7020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Szokodi I, Tavi P, Földes G, Voutilainen-Myllyla S, Ilves M, Tokola H, et al. Apelin, the novel endogenous ligand of the orphan receptor APJ, regulates cardiac contractility. Circ Res. 2002; 91:434–440. 10.1161/01.res.0000033522.37861.69 [DOI] [PubMed] [Google Scholar]
- 6.O'Carroll A-M, Salih S, Griffiths PR, Bijabhai A, Knepper MA, Lolait SJ. Expression and functional implications of the renal apelinergic system in rodents. PLoS One 2017; 12:e0183094 10.1371/journal.pone.0183094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reaux A, Gallatz K, Palkovits M, Llorens-Cortes C. Distribution of apelin-synthesizing neurons in the adult rat brain. Neuroscience. 2002; 113:653–662. 10.1016/s0306-4522(02)00192-6 [DOI] [PubMed] [Google Scholar]
- 8.Zhang F, Sun H-J, Xiong X-Q, Chen Q, Li Y-H, Kang Y-M et al. Apelin-13 and APJ in paraventricular nucleus contribute to hypertension via sympathetic activation and vasopressin release in SHR. Acta Physiol. 2014; 212:17–27. 10.1111/apha.12342 [DOI] [PubMed] [Google Scholar]
- 9.Zhang Q, Yao F, Raizada MK, O'Rourke ST, Sun C. Apelin gene transfer into the rostral ventrolateral medulla induces chronic blood pressure elevation in normotensive rats. Circ Res. 2009; 104:1421–1428. 10.1161/CIRCRESAHA.108.192302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kidoya H, Takakura N. Biology of the apelin-APJ axis in vascular formation. J Biochem. 2012; 152:125–131. 10.1093/jb/mvs071 [DOI] [PubMed] [Google Scholar]
- 11.Bülbül M, Sinen O. Dual inhibitory action of central apelin on gastric motor functions in rats. Autonom Neurosci. 2018; 212:17–22. 10.1016/j.autneu.2018.03.005 [DOI] [PubMed] [Google Scholar]
- 12.Masaki T, Yasuda T, Yoshimatsu H. Apelin-13 microinjection into the paraventricular nucleus increased sympathetic nerve activity innervating brown adipose tissue in rats. Brain Res Bull. 2012; 87:540–543. 10.1016/j.brainresbull.2012.02.004 [DOI] [PubMed] [Google Scholar]
- 13.Bülbül M, Sinen O, Gök M, Travagli RA. Apelin-13 inhibits gastric motility through vagal cholinergic pathway in rats. Am J Physiol Gastrointest Liver Physiol. 2018; 314:G201–G210. 10.1152/ajpgi.00223.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lv S-Y, Chen W-D, Wang Y-D. The apelin/APJ system in psychosis and neuropathy. Front Pharmacol. 2020. 10.3389/fphar.2020.00320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.O'Carroll A-M, Lolait SJ. Regulation of rat APJ receptor messenger ribonucleic acid expression in magnocellular neurones of the paraventricular and supraoptic nuclei by osmotic stimuli. J Neuroendocrinol. 2003; 15:661–666. 10.1046/j.1365-2826.2003.01044.x [DOI] [PubMed] [Google Scholar]
- 16.Medhurst AD, Jennings CA, Robbins MJ, Davis RP, Ellis C, Winborn KY, et al. Pharmacological and immunohistochemical characterization of the APJ receptor and its endogenous ligand apelin. J Neurochem. 2003; 84:1162–1172. 10.1046/j.1471-4159.2003.01587.x [DOI] [PubMed] [Google Scholar]
- 17.O'Carroll A-M, Lolait SJ, Harris LE, Pope GR. The apelin receptor APJ: journey from an orphan to a multifaceted regulator of homeostasis. J Endocrinol. 2013; 219:R13–35. 10.1530/JOE-13-0227 [DOI] [PubMed] [Google Scholar]
- 18.Chng SC, Ho L, Tian J, Reversade B. Elabela: a hormone essential for heart development signals via the apelin receptor. Dev Cell. 2013; 27:672–680. 10.1016/j.devcel.2013.11.002 [DOI] [PubMed] [Google Scholar]
- 19.Pauli A, Norris ML, Valen E, Chew GL, Gagnon JA, Zimmerman S, et al. Toddler: an embryonic signal that promotes cell movement via apelin receptors. Science 2014; 343:1248636 10.1126/science.1248636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang Z, Yu D, Wang M, Wang Q, Kouznetsova J, Yang R, et al. Elabela-apelin receptor signaling pathway is functional in mammalian systems. Sci Rep. 2015; 5: 8170 10.1038/srep08170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ho L, van Dijk M, Chye STJ, Messerschmidt DM, Chng SC, Ong S, et al. ELABELA deficiency promotes preeclampsia and cardiovascular malformations in mice. Science 2017; 357:707–713. 10.1126/science.aam6607 [DOI] [PubMed] [Google Scholar]
- 22.Deng C, Chen H, Yang N, Feng Y, Hsueh AJ. Apela regulates fluid homeostasis by binding to the APJ receptor to activate Gi signaling. J Biol Chem. 2015; 290: 18261–8268. 10.1074/jbc.M115.648238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Santoso P, Maejima Y, Kumamoto K, Takenoshita S, Shimomura K. Central action of elabela reduces food intake and activates arginine vasopressin and corticotropin-releasing hormone neurons in the hypothalamic paraventricular nucleus. Neuroreport. 2015; 26:820–826. 10.1097/WNR.0000000000000431 [DOI] [PubMed] [Google Scholar]
- 24.Lee DK, Cheng R, Nguyen T, Fan T, Kariyawasam AP, Liu Y, et al. Characterization of apelin, the ligand for the APJ receptor. J Neurochem. 2000; 74:34–41. 10.1046/j.1471-4159.2000.0740034.x [DOI] [PubMed] [Google Scholar]
- 25.Najafipour H, Soltani Hekmat A, Nekooian AA, Esmaeili-Mahani S. Apelin receptor expression in ischemic and non- ischemic kidneys and cardiovascular responses to apelin in chronic two-kidney-one-clip hypertension in rats. Regul Pept. 2012; 178:43–50. 10.1016/j.regpep.2012.06.006 [DOI] [PubMed] [Google Scholar]
- 26.Pang H, Han B, Yu T, Zong Z. Effect of apelin on the cardiac hemodynamics in hypertensive rats with heart failure. Int J Mol Med. 2014; 34:756–764. 10.3892/ijmm.2014.1829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Barnes GD, Alam S, Carter G, Pedersen CM, Lee KM, Hubbard TJ, et al. Sustained cardiovascular actions of APJ agonism during renin-angiotensin system activation and in patients with heart failure. Circ Heart Fail. 2013; 6:482–491. 10.1161/CIRCHEARTFAILURE.111.000077 [DOI] [PubMed] [Google Scholar]
- 28.Japp AG, Cruden NL, Barnes G, van Gemeren N, Mathews J, Adamson J, et al. Acute cardiovascular effects of apelin in humans: potential role in patients with chronic heart failure. Circulation 2010; 121:1818–1827. 10.1161/CIRCULATIONAHA.109.911339 [DOI] [PubMed] [Google Scholar]
- 29.Kagiyama S, Fukuhara M, Matsumura K, Lin Y, Fujii K, Iida M. Central and peripheral cardiovascular actions of apelin in conscious rats. Regul Pept. 2005; 125:55–59. 10.1016/j.regpep.2004.07.033 [DOI] [PubMed] [Google Scholar]
- 30.Griffiths PR, Lolait SJ, Harris LE, Paton JFR, O’Carroll A-M. Vasopressin V1a receptors mediate the hypertensive effects of [Pyr1]apelin-13 in the rat rostral ventrolateral medulla. J Physiol. 2017; 595: 3303–3318. 10.1113/JP274178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Seyedabadi M, Goodchild AK, Pilowsky PM. Site-specific effects of apelin-13 in the rat medulla oblongata on arterial pressure and respiration. Auton Neurosci. 2002; 101:32–38. 10.1016/s1566-0702(02)00178-9 [DOI] [PubMed] [Google Scholar]
- 32.Dai L, Smith PM, Kuksis M, Ferguson AV. Apelin acts in the subfornical organ to influence neuronal excitability and cardiovascular function. J Physiol. 2013; 591:3421–3432. 10.1113/jphysiol.2013.254144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pulman KJ, Fry WM, Cottrell GT, Ferguson AV. The subfornical organ: a central target for circulating feeding signals. J Neurosci. 2006; 26:2022–2030. 10.1523/JNEUROSCI.3218-05.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Smith PM, Chambers AP, Price CJ, Ho W, Hopf C, Sharkey KA, et al. The subfornical organ: a central nervous system site for actions of circulating leptin. Am J Physiol Regul Integr Comp Physiol. 2009; 296:R512–R520. 10.1152/ajpregu.90858.2008 [DOI] [PubMed] [Google Scholar]
- 35.Smith PM, Ferguson AV. Vasopressin acts in the subfornical organ to decrease blood pressure. Neuroendocrinology 1997; 66:130–135. 10.1159/000127230 [DOI] [PubMed] [Google Scholar]
- 36.Allen MA, Smith PM, Ferguson AV. Adrenomedullin microinjection into the area postrema increases blood pressure. Am J Physiol. 1997; 272:R1698–1703. 10.1152/ajpregu.1997.272.6.R1698 [DOI] [PubMed] [Google Scholar]
- 37.Johnson AK, Gross PM. Sensory circumventricular organs and brain homeostatic pathways. FASEB J. 1993; 7:678–686. 10.1096/fasebj.7.8.8500693 [DOI] [PubMed] [Google Scholar]
- 38.Larsen PJ, Mikkelsen JD. Functional identification of central afferent projections conveying information of acute "stress" to the hypothalamic paraventricular nucleus. J Neurosci. 1995; 15:2609–2627. 10.1523/JNEUROSCI.15-04-02609.1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Prager-Khoutorsky M, Bourque CW. Anatomical organization of the rat organum vasculosum lamina terminalis. Am J Physiol Regul Integr Comp Physiol. 2015; 309:R324–337. 10.1152/ajpregu.00134.2015 [DOI] [PubMed] [Google Scholar]
- 40.Shapiro RE, Miselis RR. The central neural connections of the area postrema of the rat. J Comp Neurol. 1985; 234:344–364. 10.1002/cne.902340306 [DOI] [PubMed] [Google Scholar]
- 41.Iwanaga Y, Kihara Y, Takenaka H, Kita T. Down-regulation of cardiac apelin system in hypertrophied and failing hearts: possible role of angiotensin II-angiotensin type 1 receptor system. J Mol Cell Cardiol. 2006; 41:798–806. 10.1016/j.yjmcc.2006.07.004 [DOI] [PubMed] [Google Scholar]
- 42.Zhong JC, Huang DY, Liu GF, Jin HY, Yang YM, Li YF, et al. Effects of all-trans retinoic acid on orphan receptor APJ signaling in spontaneously hypertensive rats. Cardiovas Res. 2005; 65:743–750. 10.1016/j.cardiores.2004.10.020 [DOI] [PubMed] [Google Scholar]
- 43.Fisher JP, Paton JF. The sympathetic nervous system and blood pressure in humans: implications for hypertension. J Hum Hypertens. 2012; 26:463–475. 10.1038/jhh.2011.66 [DOI] [PubMed] [Google Scholar]
- 44.Guyenet PG. The sympathetic control of blood pressure. Nat Rev Neurosci. 2006; 7:335–346. 10.1038/nrn1902 [DOI] [PubMed] [Google Scholar]
- 45.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Academic Press, San Diego, CA: 1998. [Google Scholar]
- 46.Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, et al. Fiji: an open-source platform for biological-image analysis. Nat Methods 2012; 9:676–682. 10.1038/nmeth.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Barnes G, Japp AG, Newby DE. Translational promise of the apelin-APJ system. Heart 2010; 96:1011–1016. 10.1136/hrt.2009.191122 [DOI] [PubMed] [Google Scholar]
- 48.Chandra SM, Razavi H, Kim J, Agrawal R, Kundu RK, de Jesus Perez V, et al. Disruption of the apelin-APJ system worsens hypoxia-induced pulmonary hypertension. Arterioscler Thromb Vasc Biol. 2011; 31:814–20. 10.1161/ATVBAHA.110.219980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ishida J, Hashimoto T, Hashimoto Y, Nishiwaki S, Iguchi T, Harada S, et al. Regulatory roles for APJ, a seven-transmembrane receptor related to angiotensin-type 1 receptor in blood pressure in vivo. J Biol Chem. 2004; 279:26274–26279. 10.1074/jbc.M404149200 [DOI] [PubMed] [Google Scholar]
- 50.Jia YX, Lu ZF, Zhang J, Pan CS, Yang JH, Zhao J, et al. Apelin activates L-arginine/nitric oxide synthase/nitric oxide pathway in rat aortas. Peptides 2007; 28:2023–2029. 10.1016/j.peptides.2007.07.016 [DOI] [PubMed] [Google Scholar]
- 51.Kang Y, Kim J, Anderson JP, Wu J, Gleim SR, Kundu RK, et al. Apelin-APJ signaling is a critical regulator of endothelial MEF2 activation in cardiovascular development. Circ Res. 2013; 113:22–31. 10.1161/CIRCRESAHA.113.301324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Katugampola SD, Maguire JJ, Matthewson SR, Davenport AP. [(125)I]-(Pyr(1))Apelin-13 is a novel radioligand for localizing the APJ orphan receptor in human and rat tissues with evidence for a vasoconstrictor role in man. J Pharmacol. 2001; 132:1255–1260. 10.1038/sj.bjp.0703939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kuba K, Zhang L, Imai Y, Arab S, Chen M, Maekawa Y, et al. Impaired heart contractility in apelin gene-deficient mice associated with aging and pressure overload. Circ Res. 2007; 101:e32–42. 10.1161/CIRCRESAHA.107.158659 [DOI] [PubMed] [Google Scholar]
- 54.Li Y, Yang X, Ouyang S, He J, Yu B, Lin X, et al. Declined circulating Elabela levels in patients with essential hypertension and its association with impaired vascular function: A preliminary study. Clin Exp Hypertens. 2019; 43:239–243. 10.1080/10641963.2019.1619756 [DOI] [PubMed] [Google Scholar]
- 55.Mitra A, Katovich MJ, Mecca A, Rowland NE. Effects of central and peripheral injections of apelin on fluid intake and cardiovascular parameters in rats. Physiol Behav. 2006; 89:221–225. 10.1016/j.physbeh.2006.06.006 [DOI] [PubMed] [Google Scholar]
- 56.Reaux A, De Mota N, Skultetyova I, Lenkei Z, El Messari S, Gallatz K, et al. Physiological role of a novel neuropeptide, apelin, and its receptor in the rat brain. J Neurochem. 2001; 77:1085–1096. 10.1046/j.1471-4159.2001.00320.x [DOI] [PubMed] [Google Scholar]
- 57.Yang P, Read C, Kuc RE, Buonincontri G, Southwood M, Torella R, et al. Elabela/Toddler is an endogenous agonist of the apelin APJ receptor in the adult cardiovascular system, and exogenous administration of the peptide compensates for the downregulation of its expression in pulmonary arterial hypertension. Circulation 2017; 135:1160–1173. 10.1161/CIRCULATIONAHA.116.023218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhang J, Ren CX, Qi YF, Lou LX, Chen L, Zhang LK, et al. Exercise training promotes expression of apelin and APJ of cardiovascular tissues in spontaneously hypertensive rats. Life Sci. 2006; 79:1153–1159. 10.1016/j.lfs.2006.03.040 [DOI] [PubMed] [Google Scholar]
- 59.McKinley MJ, McAllen RM, Davern P, Giles ME, Penschow J, Sunn N, et al. The sensory circumventricular organs of the mammalian brain Adv Anat Embryol Cell Biol, edited by Beck F, Christ B, Kriz W, Kummer W, Marani E, Putz R, Sano Y, Schiebler TH, Zilles K: Springer-Verlag Berlin Heidelberg, 2003;1–127. [DOI] [PubMed] [Google Scholar]
- 60.Higuchi K, Masaki T, Gotoh K, Chiba S, Katsuragi I, Tanaka K, et al. Apelin, an APJ receptor ligand, regulates body adiposity and favors the messenger ribonucleic acid expression of uncoupling proteins in mice. Endocrinology 2007; 148:2690–2697. 10.1210/en.2006-1270 [DOI] [PubMed] [Google Scholar]
- 61.Biancardi VC, Son SJ, Ahmadi S, Filosa JA, Stern JE. Circulating angiotensin II gains access to the hypothalamus and brain stem during hypertension via breakdown of the blood-brain barrier. Hypertension 2014; 63: 572–579. 10.1161/HYPERTENSIONAHA.113.01743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Boucher J, Masri B, Daviaud D, Gesta S, Guigné C, Mazzucotelli A, et al. Apelin, a newly identified adipokine up-regulated by insulin and obesity. Endocrinology 2005; 146: 1764–1771. 10.1210/en.2004-1427 [DOI] [PubMed] [Google Scholar]
- 63.Kälin RE, Kretz MP, Meyer AM, Kispert A, Heppner FL, Brändli AW. Paracrine and autocrine mechanisms of apelin signaling govern embryonic and tumor angiogenesis. Dev Biol. 2007; 305:599–614. 10.1016/j.ydbio.2007.03.004 [DOI] [PubMed] [Google Scholar]
- 64.Lee DK, George SR, O'Dowd BF. Unravelling the roles of the apelin system: prospective therapeutic applications in heart failure and obesity. Trends Pharmacol Sci. 2006; 27:190–104. 10.1016/j.tips.2006.02.006 [DOI] [PubMed] [Google Scholar]
- 65.Griffiths PR, Lolait SJ, Pearce LE, McBryde FD, Paton JFR, O’Carroll A-M. Blockade of rostral ventrolateral medulla apelin receptors does not attenuate arterial pressure in SHR and L-NAME-induced hypertensive rats. Front Physiol. 2018; 9:1488 10.3389/fphys.2018.01488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Roberts EM, Newson MJ, Pope GR, Landgraf R, Lolait SJ, O'Carroll A-M. Abnormal fluid homeostasis in apelin receptor knockout mice. J Endocrinol. 2009; 202:453–462. 10.1677/JOE-09-0134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hodge RD, Bakken TE, Miller JA, Smith KA, Barkan ER, Graybuck LT, et al. Conserved cell types with divergent features in human versus mouse cortex. Nature 2019; 573: 61–68. 10.1038/s41586-019-1506-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Duvernoy HM, Risold P-Y. The circumventricular organs: an atlas of comparative anatomy and vascularization. Brain Res Rev. 2007; 56:119–147. 10.1016/j.brainresrev.2007.06.002 [DOI] [PubMed] [Google Scholar]
- 69.Kleinz MJ, Skepper JN, Davenport AP. Immunocytochemical localisation of the apelin receptor, APJ, to human cardiomyocytes, vascular smooth muscle and endothelial cells. Regul Pept. 2006; 126:233–240. 10.1016/j.regpep.2004.10.019. [DOI] [PubMed] [Google Scholar]
- 70.Read C, Nyimanu D, Williams TL, Huggins DJ, Sulentic P, Macrae RGC, et al. International Union of Basic and Clinical Pharmacology. CVII. Structure and pharmacology of the apelin receptor with a recommendation that elabela/toddler is a second endogenous ligand. Pharm Rev. 2019; 71:467–502. 10.1124/pr.119.017533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Munji RN, Soung AL, Weiner GA, Sohet F, Semple BD, Trivedi A, et al. Profiling the mouse brain endothelial transcriptome in health and disease models reveals a core blood-brain-barrier dysfunction module. Nat Neurosci. 2019; 22:1892–1902. 10.1038/s41593-019-0497-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kälin RE, Kretz MP, Meyer AM, Kispert A, Heppner FL, Brändli AW. Paracrine and autocrine mechanisms of apelin signaling govern embryonic and tumour angiogenesis. Dev Biol. 2007; 30:599–614. 10.1016/j.ydbio.2007.03.004 [DOI] [PubMed] [Google Scholar]
- 73.Harford-Wright E, Andre-Gregoire G, Jacobs KA, Treps L, Le Gonidec S, Leclair HM, et al. Pharmacological targeting of apelin impairs glioblastoma growth. Brain 2017; 140:2939–2954. 10.1093/brain/awx253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lathen CL, Zhang Yu, Chow J, Singh M, Lin G, Nigam V, et al. ERG-APLNR axis controls pulmonary venule endothelial proliferation in pulmonary veno-occlusive disease. Circulation 2014; 130:1179–1191. 10.1161/CIRCULATIONAHA.113.007822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chen D, Lee J, Gu X, Wei L, Yu SP. Intranasal delivery of apelin-13 is neuroprotective and promotes angiogenesis after ischemic stroke in mice. ASN Neuro. 2015; 7:1–15. 10.1177/1759091415605114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chu H, Yang X, Gao Z, Tang Y, Dong Q. Apelin-13 protects against ischemic blood-brain-barrier damage through the effects of aquaporin-4. Cerebrovasc Dis. 2017; 44:10–25. 10.1159/000460261 [DOI] [PubMed] [Google Scholar]
- 77.Cox CM, D’Agostino SL, Miller MK, Heimark RL, Krieg PA. Apelin, the ligand for the endothelial G protein-coupled receptor, APJ, is a potent angiogenic factor required for normal vascular development of the frog embryo. Dev Biol. 2006; 296:177–189. 10.1016/j.ydbio.2006.04.452 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.