Abstract
Introduction
Chronic obstructive pulmonary disease (COPD) may, in some patients, be characterized by recurring acute exacerbations. Often these exacerbations are associated with airway infections. As immunoglobulins (Ig) are important parts of the immune defence against airway infections, the aim of this study was to relate the levels of circulating immunoglobulins to clinical features in unselected patients with COPD included in a Norwegian multicenter study.
Methods
Clinical and biological data, including circulating levels of immunoglobulins, were assessed in 262 prospectively included patients with COPD GOLD stage II–IV at five hospitals in south-eastern Norway. A revisit was done after one year, and survival was assessed after five years. Clinical features and survival of those with immunoglobulin levels below reference values were compared to those with normal levels.
Results
In total, 11.5% of all COPD patients and 18.5% of those with GOLD stage IV had IgG concentrations below reference values. These patients were more likely to use inhaled or oral steroids, had lower BMI, and lower FEV1%. Moreover, they had significantly more COPD-related hospital admissions (2.8 vs 0.6), number of prednisolone courses (3.9 vs 1.2), and antibiotic treatments (3.7 vs 1.5) in the preceding year. Importantly, hypogammaglobulinemia was significantly associated with reduced survival in a log-rank analysis. In multivariate regression analysis, we found that the higher risk for acute exacerbations in these patients was independent of other risk factors and was associated with impaired survival.
Conclusion
In conclusion, our study suggests that hypogammaglobulinemia may be involved in poor outcome in COPD and may thus be a feasible therapeutic target for interventional studies in COPD.
Keywords: COPD, immunodeficiency, IGG deficiency
Introduction
Chronic obstructive pulmonary disease (COPD) is considered the third leading cause of death worldwide.1 Traditionally, the disease is staged by spirometry results defined by the Global Initiative for Chronic Obstructive Lung Disease (GOLD) classification of severity of airflow limitation.2 In the 2011 revision, a model for staging based on intensity of symptoms and the frequency of exacerbations was introduced,3 and it was shown that the new classification was better at predicting exacerbations in COPD patients.4 Aiming to better predict prognosis and to direct targeted therapy, a new classification has recently been issued, further integrating the role of airflow obstruction and disease manifestations in COPD in order to better predict prognosis and treatment response.5
With the same aim, such predictions have also been made using clinical criteria to define groups of patients, so called phenotypes.6 The classically defined phenotypes of COPD are chronic bronchitis and emphysema.7 In a review article from 2012 three different phenotypes were suggested: 1) overlap or mixed COPD-asthma, 2) exacerbator (two or more exacerbations annually), and 3) emphysema-hyperinflation.8 Specifically, frequent exacerbations are an important factor in disease development, affecting lung function decline, and also quality of life.9 It has been shown that although those with frequent exacerbations may be relatively few, they account for more than half of the exacerbation-related hospitalizations, which are associated with a three-fold increase in mortality.10 Identifying the exacerbator-phenotype, therefore, may be of clinical importance.
An exacerbation of COPD may have several causes, and COPD exacerbations have been classified into four groups termed: bacteria-predominant, virus-predominant, eosinophil-predominant, and pauci-inflammatory. Interestingly, patients tend to repeat the same kind of exacerbation,11 suggesting certain individual qualities in patients that lead to exacerbation, such as eosinophilia, microbial colonization, or immunodeficiency. Regarding the latter, immunoglobulin (Ig) G is the most predominant immunoglobulin in plasma, and represents about 75% of total Ig.12 Immunoglobulin deficiency, known as hypogammaglobulinemia, is characterized by recurrent airway infections, particularly by encapsulated bacteria. It is treated using intravenous or subcutaneous immunoglobulin replacement therapy.
Despite the similarities between airway infections in hypogammaglobulinemia and the infections in some individuals with COPD, only a few studies have explored the correlation between the manifestations of COPD and Ig levels. In a reassessment of patients included in two previous trials, Leitao Filho et al found that 18–20% of the patients had one or more IgG subclass deficiencies, and that reduced levels of IgG1 and IgG2 were associated with increased risk of acute exacerbations and hospitalizations.13 Finally, two smaller observational studies found that COPD patients who were on Ig-replacement treatment had fewer acute exacerbations, further suggesting a link between hypogammaglobulinemia and acute exacerbations of COPD.14,15 The aim of this study was to determine the prevalence of hypogammaglobulinemia in a cohort of stable COPD patients and to relate Ig levels to manifestations of COPD, such as lung function, frequency of exacerbations and self-reported symptoms, and to survival, with the ultimate purpose of facilitating future interventional studies using gammaglobulin replacement therapy in COPD.
Methods
This study was part of a larger study, termed “Symptom Clusters and Immune Markers in Patients with COPD”.16 Patients with stable COPD were consecutively included at three outpatient clinics and one referral hospital in the South Eastern region of Norway, and clinical and biological data were registered. Patients were included if they were >18 years of age, were diagnosed with stage II–IV disease using the GOLD criteria,2 were able to read and understand Norwegian, and had no cognitive impairment. Patients who had pulmonary infection, acute exacerbation, or cancer at the time of evaluation were excluded. Written informed consent was obtained from all patients.
At enrollment, patients were asked to complete study questionnaires regarding symptoms, demographics, and comorbidities.17 The St. George’s Respiratory Questionnaire (SGRQ) was used to measure quality of life. A change in the total score of four was regarded a clinically meaningful.18 Body mass index, number of years smoking, and number of years since diagnosis of COPD were registered and medical records were reviewed for disease and treatment information. The modified Medical Research Council (mMRC) Dyspnea Scale was used to assess dyspnea severity.19 At enrollment, all patients underwent pulmonary function tests (PFTs), such as forced expiratory volume in one second (FEV1) and forced vital capacity (FVC), with predicted values calculated according to the guidelines of the European Respiratory Society.20 Number of hospital admissions, prednisolone courses, and antibiotic treatments in the previous year were registered, but precise time of these signs of exacerbation was not registered. Disease severity was classified using the GOLD criteria.2,3 For this calculation, the number of prednisolone courses (self-reported) in the last 12 months was used as a measure of the number of exacerbations. Six minute walk test (6MWT) was performed according to standard procedures. Blood gas analyses, plasma immunoglobulin analyses, and a chest X-ray were performed at inclusion. In the first 180 consecutively included patients, pneumococcal antibody titers and IgG subclass concentrations were measured. IgG, IgA, and IgM were quantified by turbidimetry on a Roche Modular P instrument (Roche, Switzerland) with reagents from Roche (Oslo University Hospital) or on a similar instrument from Abott (Østfold Hospital) or a Dimension Vista instrument from Siemens Healthcare (Bærum Hospital). IgG subclasses were measured by immunonephelometry on a ProSpec instrument (Siemens Healthcare Diagnostics, Munich, Germany) with reagent kits from Siemens. Patients with IgG levels below reference values (6.1–14.9 g/L) were termed hypogamma-COPD. Pneumococcal antibody levels given as arbitrary units (U/mL) to a mix of 23 serotypes were measured using enzyme linked immunosorbent assay (ELISA) after CWPS adsorption of sera.21
Patients who participated in the study were summoned to a one-year follow-up, and 185 patients attended. All tests and questionnaires were repeated at follow-up, including blood tests and X-rays. For survival assessment, data from the civic registration system in Norway were obtained five years after inclusion of the last patient (September 1, 2017), but cause of death was not available.
The Regional Committees for Medical and Health Research Ethics, the Norwegian Directorate of Health and the privacy ombudsman at Oslo University Hospital approved this study (approval no. S-09102a 2009). The study was registered at ClinicalTrials.gov with the identifier NCT01016587. The study was conducted according to the Declaration of Helsinki.
Independent Student’s t-tests, Mann–Whitney U-tests, and Chi-squared tests were used to evaluate differences between the groups. To explore independent predictors of two or more COPD exacerbations per year, a logistic regression model was fitted using backwards elimination retaining variables with a significance level above 10%. The following factors were included in the multivariate regression analysis: age, gender, COPD grade, current smoking, BMI, use of inhaled steroids, FEV1% predicted, years since diagnosis, hyperinflation on X-ray, daily cough, bowel and rheumatic disease. P-values of <0.05 were considered statistically significant. PASW Statistics 22 (SPSS Inc., Chicago, IL, USA) was used to perform statistical analysis. For the multivariate regression analysis, STATA (StataCorp LP, College Station, TX, USA) was used. Latent class analysis (LCA) has previously been used to identify subgroups of patients included in this study based on physical and psychological symptoms.16 For comparisons of transplant-free survival between groups log rank test was applied using STATA. Anonymized raw data may be accessed by contacting the corresponding author.
Results
In total, 267 patients were included, and plasma levels of IgG were available in 262. The five patients without known IgG values were excluded from further analyses. Severity of COPD disease according to the GOLD criteria was available in all cases.
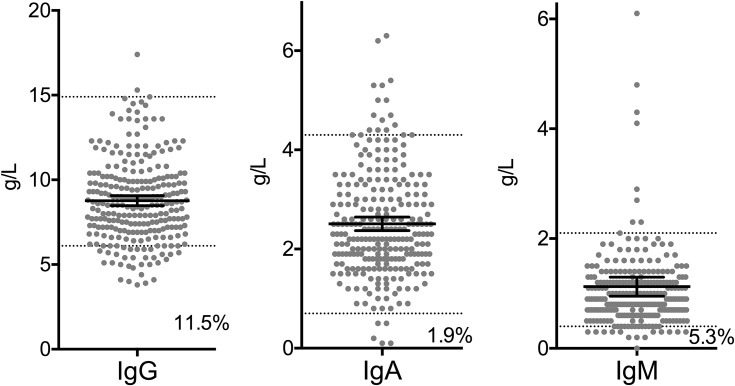
Thirty patients (11.5%) had IgG levels below reference values. These patients are hereafter termed the hypogamma-COPD group. Moreover, 14 patients (5.3%) had an IgM value below reference values, while five patients (1.9%) had low IgA (Figure 1). Only one patient had low IgG and low IgA, while four patients had low IgG and low IgM.
Figure 1.
Plasma concentration of immunoglobulins in 262 patients with COPD stage II–IV. Black lines indicate mean and 95% CI, dotted line indicates upper and lower reference values. Numbers in graph indicate percentage of patients with values below reference.
There was no significant difference between hypogamma-COPD patients and the others regarding gender, smoking history or comorbidities such as heart disease, rheumatic disease, osteoporosis, diabetes or kidney disease. Notably, the patients with hypogamma-COPD had lower BMI (21.1 vs 24.4, p<0.001) and a higher St. George total score (67.4 vs 54.7, p<0.001, Table 1). There were no significant differences in inflammatory parameters, such as CRP (mean 5.2 mg/mL vs 7.5 mg/mL, NS), leukocytes (mean 9.0 x 10 9/L vs 8.3 x 10 9/L, NS), and eosinophils (mean 0.2 vs 0.2, NS) between hypogamma-COPD and non-hypogamma-COPD. There was no difference in blood albumin (not shown).
Table 1.
Patient Descriptives
| Hypogamma-COPD (n=30) | Non-Hypogamma-COPD (n=232) | P-value | |
|---|---|---|---|
| Female n (%) | 19 (63.3) | 118 (50.9) | P=0.198 |
| Age | 60.2 (7.73) | 63.5 (8.89) | P=0.058 |
| BMI | 21.1 (3.06) | 24.4 (4.66) | P<0.001 |
| Currently smoking n (%) | 3 (10.0) | 58 (25.0) | P=0.067 |
| Tobacco use (years) | 38.0 (8.16) | 40.0 (11.54) | P=0.249 |
| Alpha 1-antitrypsin deficiency n (%) | 4 (14.8) | 21 (9.9) | P=0.432 |
| Heart disease n (%) | 5 (16.7) | 59 (25.4) | P=0.293 |
| Rheumatic disease n (%) | 0 | 19 (8.2%) | P=0.104 |
| Osteoporosis n (%) | 3 (10.0) | 20 (8.6) | P=0.802 |
| Kidney disease n (%) | 1 (3.3) | 6 (2.6) | P=0.811 |
| Diabetes n (%) | 2 (6.7) | 16 (6.9) | P=0.963 |
| Bowel disease n (%) | 3 (10.0) | 7 (3.0) | P=0.060 |
| Cancer n (%) | 1 (3.3) | 9 (3.9) | P=0.883 |
Among patients with hypogamma-COPD, 76.7% had GOLD stage IV while among patients with non-hypogamma-COPD only 43.1% had GOLD stage IV (p=0.001). Of all patients with COPD grade IV, 18.7% had hypogamma-COPD. Correspondingly, only 6.7% of the patients with hypogamma-COPD had GOLD stage II while 33.6% of the non-hypogamma-COPD patients had GOLD stage II (p=0.002). Applying the 2011 GOLD criteria, we found that 86.2% of the hypogamma-COPD patients were in group D (high risk – more symptoms) while among the non-hypogamma-COPD 57.5% were in group D (p=0.003). There was no significant difference between those with or without hypogamma-COPD regarding the distribution between groups A, B, and C (not shown).
A higher proportion of patients with hypogamma-COPD had dyspnea equivalent to an MMRC grade 4 (62.1% vs 30.1%, p=0.001, Table 2), and they also had shorter walking distance in the 6MWT (310.7 vs 387.6 meters, p=0.007). There were no differences between the patients with hypogamma-COPD and the others in the occurrence of chronic bronchitis (daily coughing). There was a trend toward higher occurrence of hyperinflation on chest X-ray among hypogamma-COPD patients (89.3% vs 73.3%, p=0.063, Table 3).
Table 2.
MMRC Scale
| Hypogamma-COPD n=30 |
Non-Hypogamma-COPD n=232 |
P-value | |
|---|---|---|---|
| Grade 0 n (%) | 1 (3.4) | 18 (8.0) | P=0.383 |
| Grade 1 n (%) | 3 (10.3) | 54 (23.9) | P=0.099 |
| Grade 2 n (%) | 1 (3.4) | 45 (19.9) | P=0.030 |
| Grade 3 n (%) | 6 (20.7) | 41 (18.1) | P=0.739 |
| Grade 4 n (%) | 18 (62.1) | 68 (30.1) | P=0.001 |
Table 3.
Clinical Presentation and Treatment of COPD
| Hypogamma-COPD (n=30) | Non-Hypogamma-COPD (n=232) | P-value | |
|---|---|---|---|
| Years since COPD diagnosis | 10.3 (6.69) | 7.4 (6.11) | P=0.019 |
| Cough daily for last three months n (%) | 9 (31.0) | 83 (38.1) | P=0.461 |
| Hyperinflation on X-ray n (%) | 25 (89.3) | 140 (73.3) | P=0.067 |
| Numbers of COPD admissions last year median (IQR) | 1.5 (0.75–3.25) | 0.0 (0.0–1.0) | P<0.0001(MW) |
| Number of prednisolone treatments last year median (IQR) | 3.0 (1.25–5.0) | 0.0 (0.0–2.0) | P<0.0001(MW) |
| Number of antibiotic treatments last year median (IQR) | 2.0 (1.0–5.0) | 1.0 (0.0–2.0) | P=0.0001(MW) |
| Use of steroids n (%)a | 30 (100) | 170 (76.2) | P=0.003 |
| Use of inhaled beta 2 agonist n (%) | 27 (90.0) | 152 (68.2) | P=0.014 |
| Use of inhaled anticholinergics n (%) | 30 (100) | 186 (81.9) | P=0.011 |
| Use of leukotriene antagonist n (%) | 7 (25.0) | 12 (5.3) | P<0.001 |
| Use of theophylline n (%) | 14 (46.7) | 26 (11.4) | P<0.001 |
| Prednisolone treatment n (%) | 13 (46.4) | 31 (13.7) | P<0.001 |
| Oxygen therapy n (%) | 18 (60.0) | 63 (27.2) | P<0.001 |
| FEV1% predicted | 26.7 (14.46) | 40.0 (19.29) | P<0.001 |
| FVC (L) | 1.9 (0.71) | 2.4 (0.89) | P=0.002 |
| DLCO (mmol/(min × kPa)) | 3.5 (1.98) | 4.4 (3.50) | P=0.198 |
| RV (L) | 4.5 (2.05) | 5.0 (6.09) | P=0.794 |
| TLC (L) | 7.5 (1.73) | 7.0 (1.89) | P=0.416 |
| 6 minute walk test (m) | 311 (123.7) | 388 (130.5) | P=0.007 |
| SGRQ score total | 67.4 (15.01) | 54.7 (17.99) | P<0.001 |
Notes: aEither steroid in combined inhaler or alone. All categorical data shown as number and percentage, P-value calculated using Chi-squared test. All continuous data stated as mean (SD) and P-values calculated using Student's t-test unless otherwise stated.
Abbreviations: BMI, body mass index; MW, Mann–Whitney test; SD, standard deviation; IQR, interquartile range; FEV1, forced expiratory flow in one second; FVC, forced vital capacity; DLCO, diffusion capacity for carbon monoxide; RV, residual volume; TLC, total lung capacity.
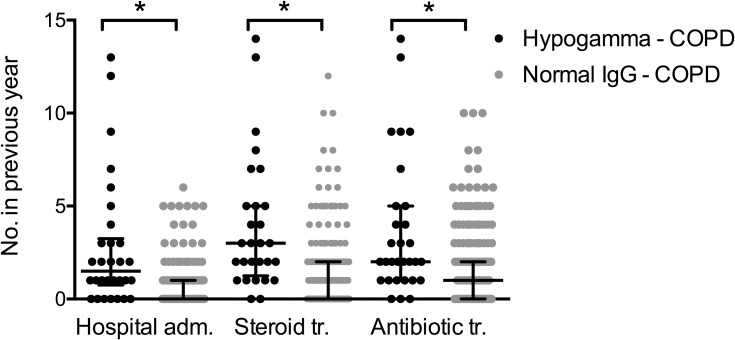
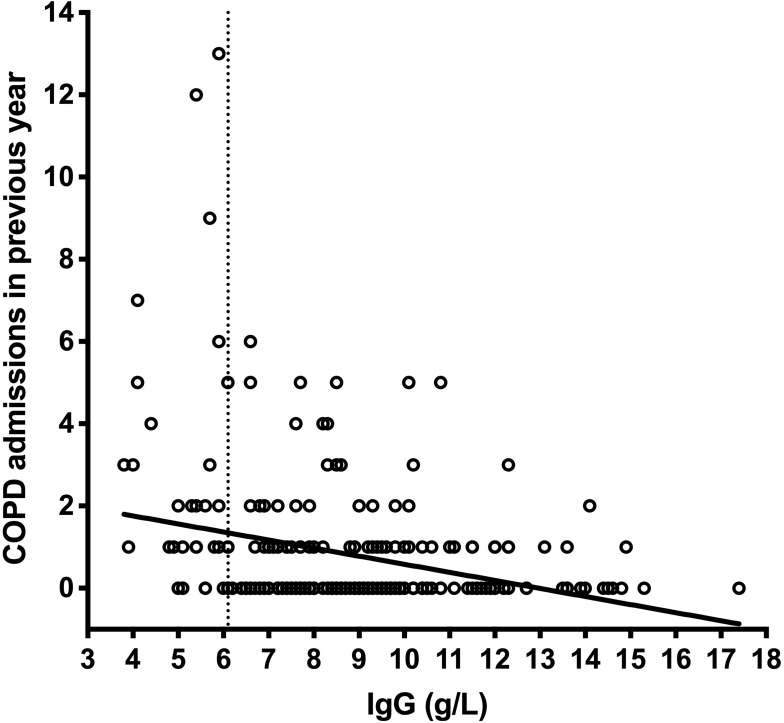
Importantly, patients with hypogamma-COPD had significantly more COPD-related hospital admissions (2.8 vs 0.6, p=0.002), number of prednisolone tapers (3.9 vs 1.2, p<0.001), and antibiotic treatments (3.7 vs 1.5, p=0.003) in the year preceding inclusion (Figure 2). Patients with hypogamma-COPD also had lower FEV1% predicted (26.7 vs 40.0, p<0.001). A higher proportion used inhaled steroids (100% vs 76.2%, p=0.003), inhaled beta2-agonists (90% vs 68.2%, p=0.014), and oral prednisolone at inclusion (46.4% vs 13.7%, p<0.001, Table 3). There was a significant non-parametric correlation between serum levels of IgG and number of hospital admissions (Figure 3).
Figure 2.
Acute exacerbations of COPD in hypogamma-COPD vs normal-IgG-COPD. Black dots: COPD patients with IgG <6.1 g/L, grey dots: COPD patients with normal IgG levels. Black lines indicate median and interquartile range. *Indicates significant difference (p<0.0001, Mann–Whitney test). Hospital adm., hospital admissions; Steroid tr., oral steroid treatments; Antibiotic tr., antibiotic treatments.
Figure 3.
COPD admissions in previous year by serum IgG levels. There was a significant non-parametric correlation between number of COPD admissions in the previous year (the year preceding inclusion) and the serum levels of IgG measured upon inclusion (Spearman's test p<0.0001).
When analyzing only patients with GOLD stage IV, there was still a higher number of COPD admissions (3.4 vs 0.9, p=0.004), prednisolone treatments (4.5 vs 1.8, p=0.004), and antibiotic treatments (4.3 vs 2.1, p=0.015) in the preceding year in the patients with hypogamma-COPD compared to those with non-hypogamma-COPD. The use of oral prednisolone at inclusion was also higher (52.4% vs 20.4%, p=0.002), while differences in use of inhaled steroids did not reach statistical significance (100% vs 86.7%, p=0.064).
All included patients were invited to a follow-up after one year. We found that there was good agreement between reported number of exacerbations in the year preceding inclusion and in the prospective registration in the following year (R=0.68, p<0.0001). Importantly, the hypogamma-COPD patients again reported to have had significantly more hospital admissions (1.7 vs 0.4, p=0.026), prednisolone treatments (2.5 vs 1.1, p=0.006), and antibiotic treatments (2.7 vs 1.3, p=0.029) in the year following inclusion compared to the non-hypogamma COPD patients, confirming the observations made at inclusion.
Pneumococcal antibody levels were measured in 204 patients. Patients with hypogamma-COPD had significantly lower pneumococcal antibody levels (11.04 U/mL vs 15.28 U/mL, p=0.04). Of note, only 44.2% of the included patients reported to have received pneumococcal vaccine despite current vaccine recommendations. There was a higher trend toward positive vaccine status among the patients with hypogamma-COPD (73.9% vs 52.9%, p=0.057).
In the 180 patients where IgG subclasses were measured, we found that 29 (16.1%) had low IgG1, while 40 (22.2%) had low IgG2. Similar to the pattern seen when considering total IgG levels, we found that compared to patients with high or normal IgG2, the patients with low IgG2 had a significantly higher number of COPD admissions in the preceding year (1.9 vs 0.7, p<0.001), as well as number of antibiotic treatments (2.9 vs 1.7, p=0.01) and prednisolone treatments (3.0 vs 1.5, p=0.002).
An earlier study of this patient cohort found three latent classes regarding physical and psychological symptoms. Of those with hypogamma-COPD, 3.3% were in the class reporting a low score on all symptoms, while 46.7% of the hypogamma-COPD patients reported a low score on psychological symptoms but high on physical symptoms, and finally 50.0% reported a high score on psychological and physical symptoms. There was no significant difference in the distribution of latent class groups when comparing hypogamma-COPD patients to those with non-hypogamma COPD.
In the multivariate regression analysis, we found that hypogamma-COPD was an independent predictor for number of prednisolone treatments (OR = 4.3, p=0.003) and COPD admissions (OR = 5.1, p=0.001).
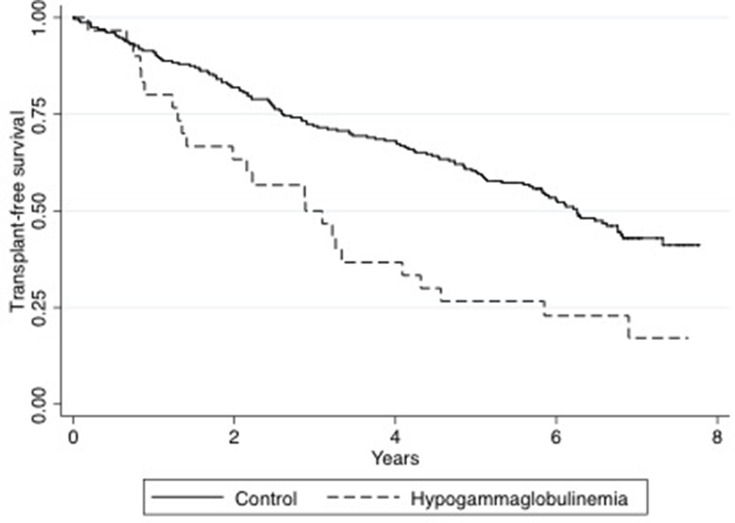
Survival status (transplant-free survival) for all included patients was assessed five years after inclusion of the last patient, ie, in 2017. Of all patients included, 27.3% were dead five years after inclusion. Notably, we found that those with hypogamma-COPD had significantly poorer transplant-free survival than the controls using Log Rank analysis (p=0.0003) (Figure 4).
Figure 4.
Survival after inclusion in hypogamma-COPD vs normal-IgG-COPD. The Kaplan–Meyer plot shows transplant-free survival of all included patients from time of inclusion to five years after inclusion of the last patient. Black line: normal-IgG-COPD. Dotted line: hypogamma-COPD. Survival compared using Log-rank test (p=0.0003).
Discussion
In this study of patients with stable COPD, 30 patients (11.5%) had IgG below reference values. Compared to the other COPD patients, subjects with hypogamma-COPD had lower FEV1% predicted and thus a higher proportion had GOLD stage IV. They had significantly more COPD admissions, antibiotic treatments, and prednisolone treatments in the preceding year. Also, a larger fraction of the hypogamma-COPD patients used medication such as inhaled steroids and oral prednisolone at inclusion compared to the other COPD patients, and, importantly, they had lower survival.
The prevalence of low IgG in COPD patients has not previously been studied in a large cohort. In the patient cohorts analyzed by Leitao Filho et al, 18–20% had reduced levels of one or more subclasses.13 In a study of 40 patients waiting for lung transplantation, pre-transplant values showed that six of 13 patients with COPD had mild hypogammaglobulinemia.22 In another study of 15 patients with corticosteroid-dependent COPD, five COPD patients had low IgG.23 Why such a high percentage of the COPD population have hypogammaglobulinemia is not clear. As discussed by others, it could be secondary, either as a side effect of medications or through some unknown disease mechanism in COPD.24,25 Alternatively, it could be a manifestation of an immune dysfunction not secondary to COPD, leading to more frequent exacerbations, possibly related to infections, and consequently a more rapid disease progression.
The data obtained in this study do not allow conclusions about whether hypogammaglobulinemia is the cause or the consequence of having an aggressive, exacerbating COPD phenotype. It is possible that the hypogammaglobulinemia observed is related to a high degree of systemic inflammation in the more severely ill patients, independently of treatment given, and it is known that frequent exacerbations may be caused by chronic inflammation.26 However, in our study we found no difference in CRP or white blood cell counts at inclusion when comparing patients with hypogamma-COPD and those with normal IgG levels.
In this study, ¾ of the hypogamma-COPD patients had GOLD stage IV. Earlier studies have shown a relation between frequent exacerbations and faster decline in FEV1, as well as mortality.27,28 Although we found that the association between hypogammaglobulinemia and having frequent exacerbations was independent of the current FEV1% predicted value, it is conceivable that previous exacerbations may have caused the increased bronchial obstruction in the hypogamma-COPD patients. Conversely, it is also possible that having a rapidly progressive type of COPD with frequent exacerbations can lead to an increased use of medication, such as steroids in various forms, which may enhance the tendency toward hypogammaglobulinemia.29 The relationship between inhaled corticosteroids, either alone or combined, and exacerbations is unclear.30,31 In a study of 100 patients with asthma and either inhaled corticosteroid alone, or in combination with an oral corticosteroid, no patients with inhaled corticosteroids alone had hypogammaglobulinemia. In contrast, patients with oral corticosteroids >12.5 mg/day for at least one year had reduced levels of serum IgG.32 Moreover, in a study of COPD patients with or without steroid therapy, it was observed that significantly lower levels of IgG were found in the patients on steroid therapy, although oral or inhaled steroids were not distinguished.33 In the present study, all of the hypogamma-COPD patients used ICS and significantly more patients with hypogamma-COPD used oral prednisolone at inclusion. This suggests that steroid use may be associated with hypogammaglobulinemia in COPD, but the causal relationship is still unclear.
Another known factor related to hypogammaglobulinemia is smoking. Lower plasma levels of IgG have been found in smokers compared to non-smokers but no correlation between low IgG and recurrent exacerbations could be demonstrated.34–36 In the present study we found no relation between smoking status and hypogamma-COPD.
The observation that the patients with hypogamma-COPD have a higher rate of infections may indicate that preventive measures are warranted. However, we found that although the patients with hypogamma-COPD tended to report a higher pneumococcal vaccination rate, their titers of pneumococcal antibodies were lower. Whether this is because of a reduced immunological response to vaccines or some other mechanisms is unclear, but this observation may be of importance when designing preventive measures in COPD, and should be subjected to further studies.
When following the included patients for at least five years, we found that the hypogamma-COPD patients had significantly lower transplant-free survival. Whatever the causal relationship between severe COPD and hypogammaglobulinemia may be, this observation may suggest that immunoglobulin replacement therapy may be a feasible target for interventional studies in patients with COPD who have low levels of IgG, particularly considering the poor prognosis and the scarcity of other effective treatment options in this common disease.24,25
The study has some limitations. First, of the total study population, for five individuals (1.8%) blood tests were missing, including IgG. These patients came from three of the five study hospitals, and had GOLD stage II or III. Second, it might be argued that some unreliability is associated with the self-reporting of data by the patients, as data regarding COPD admissions, antibiotics, and prednisolone cures were patient-reported through questionnaires. However, it has previously been demonstrated that the agreement between self-reported medical information and data assessed from medical charts is good in patients with diabetes and other well-known chronic diseases.37,38 Moreover, in the present study there was good agreement between reported number of exacerbations in the year preceding inclusion and number of exacerbations registered prospectively in the year following inclusion. Unfortunately, the precise time of exacerbation was not registered, and the temporal relationship between IgG measurements and exacerbation could therefore not be calculated. Also, the analyses were limited by the absence of information about cause of death. Finally, some of the included patients received a lung transplant. In the survival analyses, this was treated as a competing risk, but we do not know how these patients would have affected the results had they not received a transplant. There is, however, a consensus not to perform transplant unless a survival of less than two years may be assumed.39
In conclusion, we found that 11.5% of the COPD patients included in this study had reduced serum levels of IgG, and that the occurrence of hypogammaglobulinemia in those with COPD GOLD stage IV approached 20%. The occurrence of hypogammaglobulinemia was related to use of inhaled and oral steroids, but independently of these factors, we found that hypogammaglobulinemia predicted an increased number of exacerbations and hospitalizations and that hypogammaglobulinemia is associated with impaired long-term survival. Our observations suggest that both the function of specific elements of the immune system, such as B-cells, and the clinical consequences and effects of hypogammaglobulinemia on survival in patients with COPD should be studied. Furthermore, our observations suggest that the possibility of interventional studies using intravenous immunoglobulins to prevent exacerbations in selected COPD patients should be considered.
Acknowledgments
This study was part of a study funded by the South-Eastern Norway Regional Health Authority (2009055) and also received a research grant from The Norwegian Society of Pulmonary Medicine. The IgG subclass analyses were partly sponsored by Siemens Healthcare AS, Norway. We are grateful to all the patients and clinicians who contributed to this study, especially the research nurses Gunilla Solbakk at Østfold Hospital and Mari-Ann Øvreseth and Britt Drægni at Bærum Hospital, Norway.
Funding Statement
The study was funded by the South-Eastern Norway Regional Health Authority (id.no. 2009055) and also received a research grant from The Respiratory Society in 2015.
Author Contributions
Are M Holm: principal investigator, designed study, patient inclusion, collected data, analyzed data, wrote manuscript.
Siw L Andreassen: data analysis and interpretation, contributed to writing manuscript.
Vivi Lycke Christensen: patient inclusion, collected data, analyzed data, contributed to writing of manuscript.
Tone Rustøen: designed study, analyzed data, contributed to writing of manuscript.
Johny Kongerud: designed study, analyzed data, contributed to writing of manuscript.
Øystein Almås: patient inclusion, collected data, analyzed data, contributed to writing of manuscript.
Henrik Auråen: statistical analysis, contributed to writing of manuscript.
Anne H Henriksen: contributed to writing of manuscript.
Ingeborg S Aaberge: responsible for laboratory methodology and analysis of data regarding pneumococcal antibodies, contributed to writing of manuscript.
Olav Klingenberg: responsible for laboratory methodology and analysis of data regarding immunoglobulin measurements, contributed to writing of manuscript.
All authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that they have no conflicts of interest or competing interests regarding any of the contents of this document or the work related to it.
References
- 1.Lozano R, Naghavi M, Foreman K, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2095–2128. doi: 10.1016/S0140-6736(12)61728-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rabe KF, Hurd S, Anzueto A, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2007;176(6):532–555. doi: 10.1164/rccm.200703-456SO [DOI] [PubMed] [Google Scholar]
- 3.Vestbo J, Hurd SS, Agusti AG, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2013;187(4):347–365. doi: 10.1164/rccm.201204-0596PP [DOI] [PubMed] [Google Scholar]
- 4.Lange P, Marott JL, Vestbo J, et al. Prediction of the clinical course of chronic obstructive pulmonary disease, using the new GOLD classification: a study of the general population. Am J Respir Crit Care Med. 2012;186(10):975–981. doi: 10.1164/rccm.201207-1299OC [DOI] [PubMed] [Google Scholar]
- 5.Vogelmeier CF, Criner GJ, Martinez FJ, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease 2017 report: GOLD executive summary. Eur Respir J. 2017;49(3):3. doi: 10.1183/13993003.00214-2017 [DOI] [PubMed] [Google Scholar]
- 6.Han MK, Agusti A, Calverley PM, et al. Chronic obstructive pulmonary disease phenotypes: the future of COPD. Am J Respir Crit Care Med. 2010;182(5):598–604. doi: 10.1164/rccm.200912-1843CC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Snider GL. Chronic obstructive pulmonary disease: a definition and implications of structural determinants of airflow obstruction for epidemiology. Am Rev Respir Dis. 1989;140(3 Pt 2):S3–8. doi: 10.1164/ajrccm/140.3_Pt_2.S3 [DOI] [PubMed] [Google Scholar]
- 8.Miravitlles M, Soler-Cataluna JJ, Calle M, et al. Treatment of COPD by clinical phenotypes: putting old evidence into clinical practice. Eur Respir J. 2013;41(6):1252–1256. doi: 10.1183/09031936.00118912 [DOI] [PubMed] [Google Scholar]
- 9.Hurst JR, Vestbo J, Anzueto A, et al. Susceptibility to exacerbation in chronic obstructive pulmonary disease. N Engl J Med. 2010;363(12):1128–1138. doi: 10.1056/NEJMoa0909883 [DOI] [PubMed] [Google Scholar]
- 10.Beeh KM, Glaab T, Stowasser S, et al. Characterisation of exacerbation risk and exacerbator phenotypes in the POET-COPD trial. Respir Res. 2013;14(1):116. doi: 10.1186/1465-9921-14-116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bafadhel M, McKenna S, Terry S, et al. Acute exacerbations of chronic obstructive pulmonary disease: identification of biologic clusters and their biomarkers. Am J Respir Crit Care Med. 2011;184(6):662–671. doi: 10.1164/rccm.201104-0597OC [DOI] [PubMed] [Google Scholar]
- 12.Schroeder HW Jr., Cavacini L. Structure and function of immunoglobulins. J Allergy Clin Immunol. 2010;125(2 Suppl 2):S41–52. doi: 10.1016/j.jaci.2009.09.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leitao Filho FS, Won Ra S, Mattman A, et al. Serum IgG and risk of exacerbations and hospitalizations in chronic obstructive pulmonary disease. J Allergy Clin Immunol. 2017;140(4):1164–67 e6. doi: 10.1016/j.jaci.2017.01.046 [DOI] [PubMed] [Google Scholar]
- 14.McCullagh BN, Comellas AP, Ballas ZK, et al. Antibody deficiency in patients with frequent exacerbations of Chronic Obstructive Pulmonary Disease (COPD). PLoS One. 2017;12(2):e0172437. doi: 10.1371/journal.pone.0172437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cowan J, Gaudet L, Mulpuru S, et al. A retrospective longitudinal within-subject risk interval analysis of immunoglobulin treatment for recurrent acute exacerbation of chronic obstructive pulmonary disease. PLoS One. 2015;10(11):e0142205. doi: 10.1371/journal.pone.0142205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Christensen VL, Rustoen T, Cooper BA, et al. Distinct symptom experiences in subgroups of patients with COPD. Int J Chron Obstruct Pulmon Dis. 2016;11:1801–1809. doi: 10.2147/COPD.S105299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sangha O, Stucki G, Liang MH, et al. The self-administered comorbidity questionnaire: a new method to assess comorbidity for clinical and health services research. Arthritis Rheum. 2003;49(2):156–163. doi: 10.1002/art.10993 [DOI] [PubMed] [Google Scholar]
- 18.Jones PW. Interpreting thresholds for a clinically significant change in health status in asthma and COPD. Eur Respir J. 2002;19(3):398–404. doi: 10.1183/09031936.02.00063702 [DOI] [PubMed] [Google Scholar]
- 19.Bestall JC, Paul EA, Garrod R, et al. Usefulness of the Medical Research Council (MRC) dyspnoea scale as a measure of disability in patients with chronic obstructive pulmonary disease. Thorax. 1999;54(7):581–586. doi: 10.1136/thx.54.7.581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Quanjer PH, Tammeling GJ, Cotes JE, et al. Lung volumes and forced ventilatory flows. Report working party standardization of lung function tests, European Community for steel and coal. Official statement of the European Respiratory Society. Eur Respir J Suppl. 1993;16(Suppl 16):5–40. doi: 10.1183/09041950.005s1693 [DOI] [PubMed] [Google Scholar]
- 21.Aaberge IS, Steinsvik TE, Groeng EC, et al. Human antibody response to a pneumococcal vaccine in SCID-PBL-hu mice and simultaneously vaccinated human cell donors. Clin Exp Immunol. 1996;105(1):12–17. doi: 10.1046/j.1365-2249.1996.d01-728.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yip NH, Lederer DJ, Kawut SM, et al. Immunoglobulin G levels before and after lung transplantation. Am J Respir Crit Care Med. 2006;173(8):917–921. doi: 10.1164/rccm.200510-1609OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Klaustermeyer WB, Gianos ME, Kurohara ML, et al. IgG subclass deficiency associated with corticosteroids in obstructive lung disease. Chest. 1992;102(4):1137–1142. doi: 10.1378/chest.102.4.1137 [DOI] [PubMed] [Google Scholar]
- 24.Cowan J, Mulpuru S, Alvarez G, et al. Chronic obstructive pulmonary disease exacerbation frequency and serum IgG levels. J Allergy Clin Immunol. 2018;141(2):830–831. doi: 10.1016/j.jaci.2017.09.036 [DOI] [PubMed] [Google Scholar]
- 25.Leitao Filho FS, Ra SW, Mattman A, et al. Reply. J Allergy Clin Immunol. 2018;141(2):831. doi: 10.1016/j.jaci.2017.10.013 [DOI] [PubMed] [Google Scholar]
- 26.Papi A, Luppi F, Franco F, et al. Pathophysiology of exacerbations of chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2006;3(3):245–251. doi: 10.1513/pats.200512-125SF [DOI] [PubMed] [Google Scholar]
- 27.Donaldson GC, Seemungal TA, Bhowmik A, et al. Relationship between exacerbation frequency and lung function decline in chronic obstructive pulmonary disease. Thorax. 2002;57(10):847–852. doi: 10.1136/thorax.57.10.847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hoogendoorn M, Hoogenveen RT, Rutten-van Molken MP, et al. Case fatality of COPD exacerbations: a meta-analysis and statistical modelling approach. Eur Respir J. 2011;37(3):508–515. doi: 10.1183/09031936.00043710 [DOI] [PubMed] [Google Scholar]
- 29.Settipane GA, Pudupakkam RK, McGowan JH. Corticosteroid effect on immunoglobulins. J Allergy Clin Immunol. 1978;62(3):162–166. doi: 10.1016/0091-6749(78)90101-X [DOI] [PubMed] [Google Scholar]
- 30.Calverley PM, Anderson JA, Celli B, et al. Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease. N Engl J Med. 2007;356(8):775–789. doi: 10.1056/NEJMoa063070 [DOI] [PubMed] [Google Scholar]
- 31.Crim C, Calverley PM, Anderson JA, et al. Pneumonia risk in COPD patients receiving inhaled corticosteroids alone or in combination: TORCH study results. Eur Respir J. 2009;34(3):641–647. doi: 10.1183/09031936.00193908 [DOI] [PubMed] [Google Scholar]
- 32.Kawano T, Matsuse H, Obase Y, et al. Hypogammaglobulinemia in steroid-dependent asthmatics correlates with the daily dose of oral prednisolone. Int Arch Allergy Immunol. 2002;128(3):240–243. doi: 10.1159/000064258 [DOI] [PubMed] [Google Scholar]
- 33.O’Keeffe S, Gzel A, Drury R, et al. Immunoglobulin G subclasses and spirometry in patients with chronic obstructive pulmonary disease. Eur Respir J. 1991;4(8):932–936. [PubMed] [Google Scholar]
- 34.Bridges RB, Chow CK, Rehm SR. Micronutrient status and immune function in smokers. Ann N Y Acad Sci. 1990;587:218–231. doi: 10.1111/j.1749-6632.1990.tb00149.x [DOI] [PubMed] [Google Scholar]
- 35.Gulsvik A, Fagerhoi MK. Smoking and immunoglobulin levels. Lancet. 1979;1(8113):449. doi: 10.1016/S0140-6736(79)90934-6 [DOI] [PubMed] [Google Scholar]
- 36.Qvarfordt I, Riise GC, Andersson BA, et al. IgG subclasses in smokers with chronic bronchitis and recurrent exacerbations. Thorax. 2001;56(6):445–449. doi: 10.1136/thorax.56.6.445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Haapanen N, Miilunpalo S, Pasanen M, et al. Agreement between questionnaire data and medical records of chronic diseases in middle-aged and elderly Finnish men and women. Am J Epidemiol. 1997;145(8):762–769. doi: 10.1093/aje/145.8.762 [DOI] [PubMed] [Google Scholar]
- 38.Lovaas KF, Cooper JG, Sandberg S, et al. Feasibility of using self-reported patient data in a national diabetes register. BMC Health Serv Res. 2015;15(1):553. doi: 10.1186/s12913-015-1226-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Weill D, Benden C, Corris PA, et al. A consensus document for the selection of lung transplant candidates: 2014–an update from the pulmonary transplantation council of the international society for heart and lung transplantation. J Heart Lung Transplant. 2015;34(1):1–15. doi: 10.1016/j.healun.2014.06.014 [DOI] [PubMed] [Google Scholar]