Abstract
The Drosophila ventral nerve cord (VNC) receives and processes descending signals from the brain to produce a variety of coordinated locomotor outputs. It also integrates sensory information from the periphery and sends ascending signals to the brain. We used single-cell transcriptomics to generate an unbiased classification of cellular diversity in the VNC of five-day old adult flies. We produced an atlas of 26,000 high-quality cells, representing more than 100 transcriptionally distinct cell types. The predominant gene signatures defining neuronal cell types reflect shared developmental histories based on the neuroblast from which cells were derived, as well as their birth order. The relative position of cells along the anterior-posterior axis could also be assigned using adult Hox gene expression. This single-cell transcriptional atlas of the adult fly VNC will be a valuable resource for future studies of neurodevelopment and behavior.
Research organism: D. melanogaster
Introduction
The adult Drosophila central nervous system (CNS) consists of the brain in the head capsule and the ventral nerve cord (VNC; also known as ventral nervous system) in the thorax (Court et al., 2017; Ito et al., 2014). The VNC receives and integrates sensory input from the periphery and sends this information to the brain in ascending neurons through the cervical connective (Tsubouchi et al., 2017). The brain, in turn, sends sensory-motor signals to the VNC via descending neurons (Namiki et al., 2018). The VNC transforms these signals into locomotor actions (Harris et al., 2015). It controls muscles in the thorax in unique ways, depending on whether it is steering the wings during flight or generating acoustic communication signals during both reproductive and agonistic behaviors (Clyne and Miesenböck, 2008; Jonsson et al., 2011; Shirangi et al., 2013; von Philipsborn et al., 2011). It coordinates muscles in the legs to walk, jump, groom, reach, touch, and taste (Bidaye et al., 2014; Card and Dickinson, 2008; Chen et al., 2018; Gowda et al., 2018; Harris et al., 2015; Howard et al., 2019; Kim et al., 2017; Mamiya et al., 2018; Mendes et al., 2013; Mendes et al., 2014; Seeds et al., 2014; Tuthill and Wilson, 2016; Wosnitza et al., 2013). The VNC also controls musculature in the abdomen relevant to copulation and reproduction including abdominal bending, attachment, and ejaculation in the male (Crickmore and Vosshall, 2013; Jois et al., 2018; Pan et al., 2011; Pavlou et al., 2016; Tayler et al., 2012), and sperm storage and oviposition in the female (Kimura et al., 2015; Lee et al., 2015).
These different functions are orchestrated by anatomically discrete segments of the VNC. Whereas the thoracic neuromeres control the legs and wings, the abdominal neuromere controls the abdominal muscles, gastric system, and reproductive organs. Much of the developmental mechanisms that generate and assemble the VNC have been characterized (Venkatasubramanian and Mann, 2019). Like other holometabolous insects, Drosophila undergo two stages of neurogenesis building two distinct nervous systems. Embryonic neurogenesis gives rise to the larval nervous system, and post-embryonic neurogenesis produces adult specific neurons. The structure of the adult nervous system is established in the embryo where pioneering neurons setup networks of tracts, which later developing neurons follow (Hartenstein, 2018). The adult VNC is comprised of 4 primary neuromeres, three thoracic (one per each pair of legs: the prothoracic neuromere, ProNm; mesothoracic neuromere, MesoNm and metathoracic neuromere, MetaNm) and one fused abdominal neuromere (ANm) (Court et al., 2017; Niven et al., 2008). The neuromeres, composed of one or more fused neuropils, are segment-specific parts of the CNS which process sensory signals and control movements of their specific segments. The thoracic neuromeres are homologous structures and are thus morphologically similar to each other (Smarandache-Wellmann, 2016). The neurons in these segments are derived from a set of repeating type I neuroblasts. Cells derived from a given neuroblast produce a lineage, which can be split into Notch+ and Notch- hemilineages (Truman et al., 2010). The formation of tagmatic boundaries, which group these segments into morphological units along the body axis, is established through differential expression of Hox family transcription factors (TFs) (Angelini and Kaufman, 2005). Cell types within each neuromere are genetically encoded by developmental programs. However, it is unclear whether the mature terminal identity of an individual neuron can be determined from its adult transcriptome.
Although a large body of work has investigated how the adult Drosophila CNS is established from that of the embryo, many outstanding questions remain. It is for example unknown whether the TFs involved in establishing the cellular diversity of the nervous system also play a role in the form and function of these same neurons in the adult. Although certain features of individual neurons can be plastic, the identity of a terminally differentiated neuron is likely to remain stable throughout the animal’s life. It is therefore important to understand gene expression and regulation that permit mature neurons to preserve their subtype identity, morphology and connectivity, and maintain neural circuit function throughout adulthood. The VNC of the genetically tractable vinegar fly, Drosophila melanogaster, is ideally suited for these studies.
Here we used single-cell RNA-sequencing to characterize the transcriptomes of individual cells in a 5 day old adult Drosophila VNC. We generated an atlas of the VNC with 26,768 single-cell transcriptional profiles that define more than 100 cell types. This analysis reveals that the VNC has a roughly equal number of inhibitory GABAergic neurons and excitatory cholinergic neurons, and that genes encoding preproneuropeptides are amongst the most highly expressed. The segmentally repeating nature and developmental history of the VNC is born out in the cellular transcriptomes, as maintained expression of Hox and several other neuronal lineage marker genes persist. In addition, these profiles provide many novel markers for known and new cell types. This single-cell atlas of the adult Drosophila VNC provides a useful resource to help connect cellular identity to behaviorally relevant neural circuit function.
Results
Single-cell transcriptomic atlas of the adult VNC
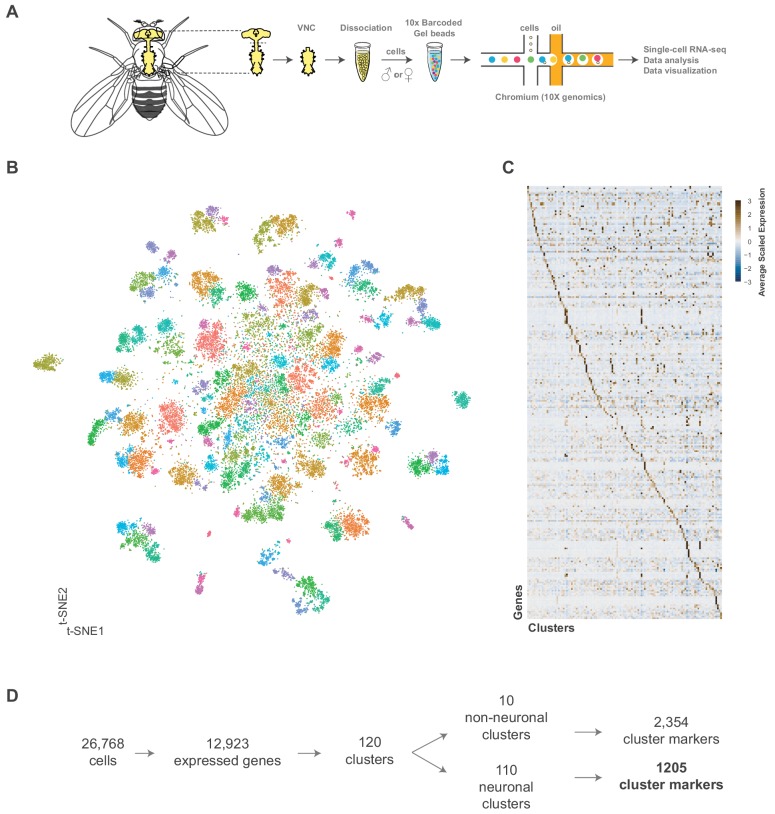
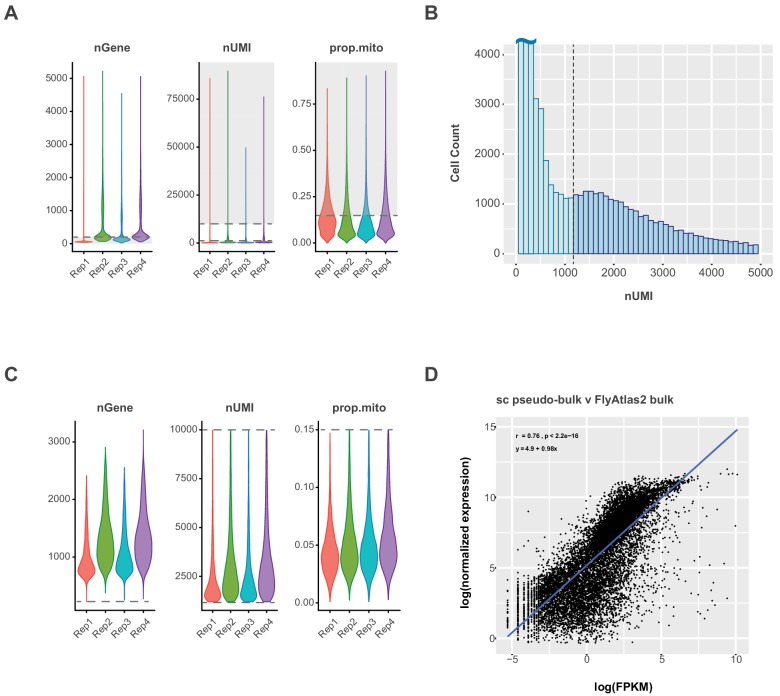
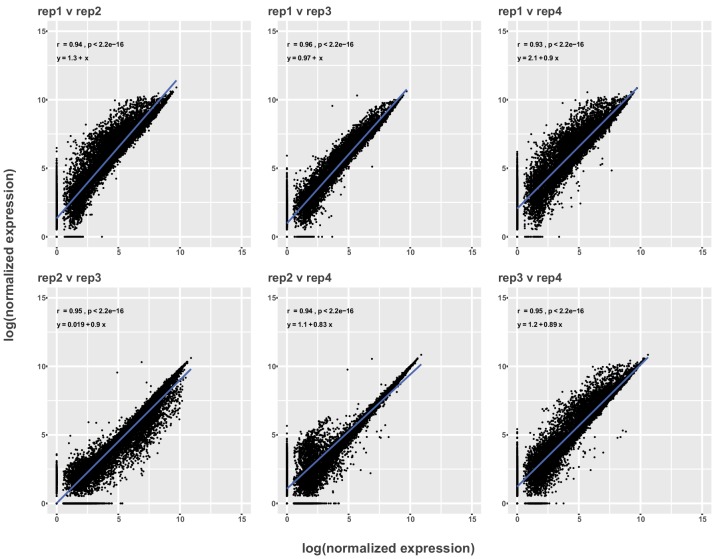
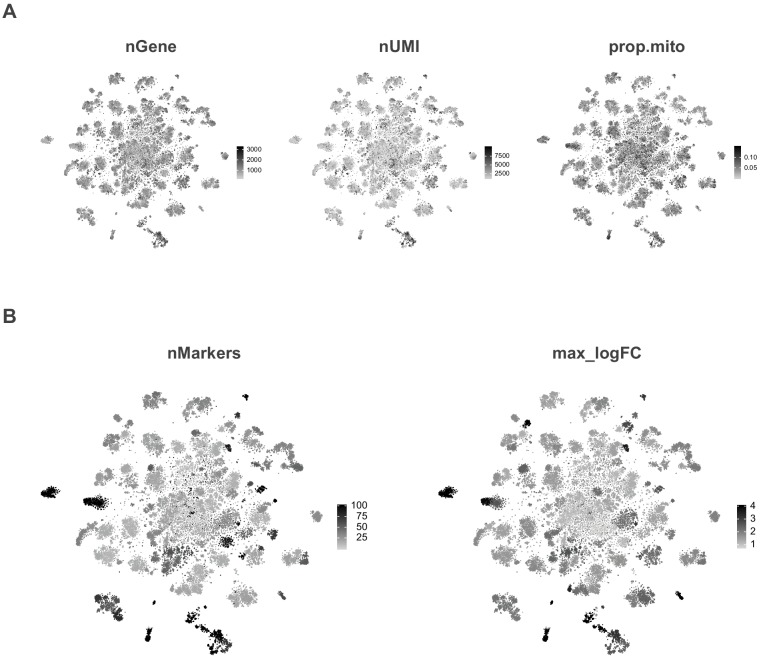
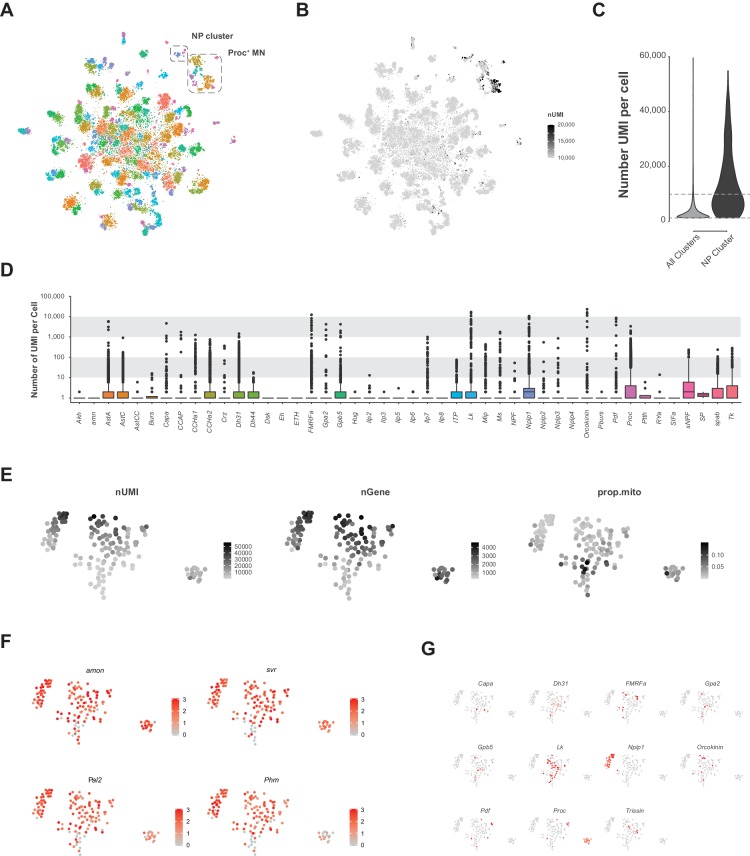
We created a single-cell transcriptome atlas of the VNC using single-cell RNA-sequencing with 10x Chromium chemistry. We processed 80 VNCs, 20 per independent replicate (Figure 1A; see Materials and methods). The data were filtered according to the number of genes (nGene), the number of unique molecule identifiers (nUMI), and the proportion of mitochondrial genes (prop.mito) (Figure 1—figure supplement 1A,B; see Materials and methods). A similar number of expressed genes, and UMIs were retrieved in each replicate (Figure 1—figure supplement 1C). Pooling the data, we recovered 26,768 high quality cells with a median of 1170 genes and 2497 transcripts per cell. We compared the expression levels observed in this single-cell data set with those reported from bulk sequencing, to evaluate the extent to which the tissue dissociation and the 10x procedure effect gene expression. The filtered single-cell data showed high correlation (r = 0.76) to FlyAtlas’ bulk VNC sequencing data (Figure 1—figure supplement 1D; Leader et al., 2018). Comparisons of the pseudo-bulk expression levels between replicates of the filtered cells yielded correlations coefficients from 0.93 to 0.95 (Figure 1—figure supplement 2).
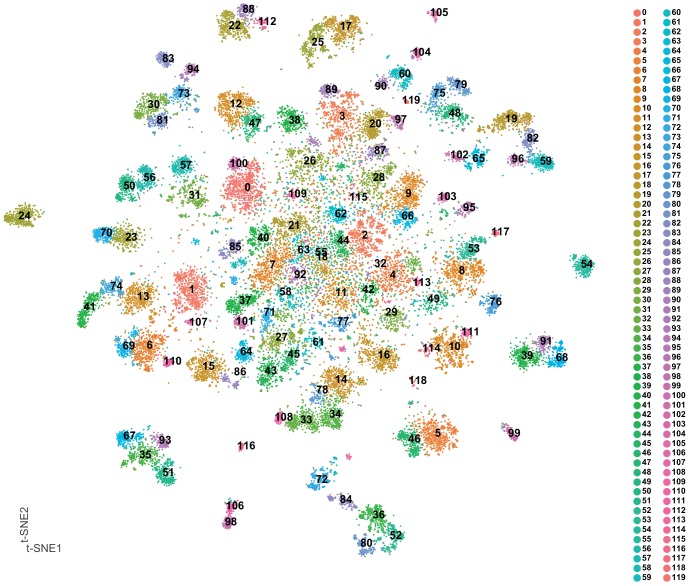
Figure 1. Single cell sequencing of 5 day old adult Drosophila VNC.
(A) Schematic workflow showing single cell sequencing data generation. Male and female Drosophila VNCs were dissected and dissociated prior to droplet encapsulation of individual cells with barcoded beads, forming gel beads in emulsion (GEMs). Following barcode incorporation, molecular amplification and sequencing the transcriptional profiles of individual cells were determined. (B) Two-dimensional representation (t-SNE) of 26,768 Drosophila VNC cells grouped into 120 clusters. Clusters were assigned using the Louvain algorithm, using 45 CCA dimensions with a cluster resolution of 12. Each dot is a cell colored by cluster identity. (C) Heatmap showing scaled, log-normalized expression of top 5 cluster discriminative genes per cluster. (D) Flow diagram representing neuronal and non-neuronal cluster identification, including total number of genes identified as cluster markers.
Figure 1—figure supplement 1. Cut-offs and correlation to bulk sequencing.
Figure 1—figure supplement 2. Pseudo-bulk comparisons between replicates of filtered VNC data.
Figure 1—figure supplement 3. Cluster resolution analysis of adult VNC dataset.
Figure 1—figure supplement 4. t-SNE plot of 5 day old adult VNC with cluster numbers labeled.
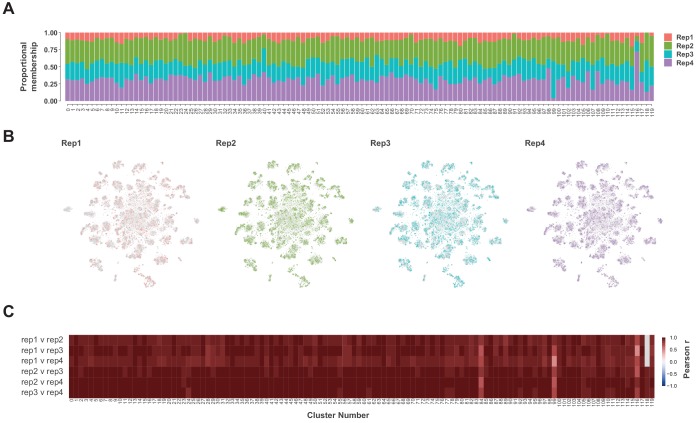
Figure 1—figure supplement 5. Replicate contributions.
Figure 1—figure supplement 6. t-SNE spatial distributions.
Figure 1—figure supplement 7. Identification of neuronal and glia clusters.
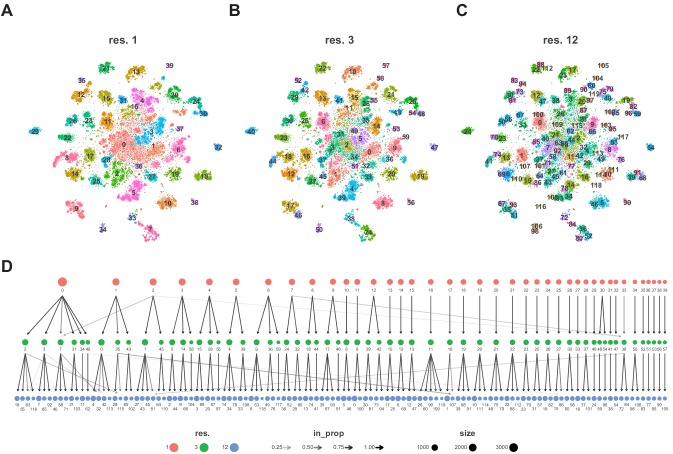
We performed a Canonical-Correlation Analysis (CCA) on the transcriptomes and reduced the first 45 dimensions into two t-SNE dimensions (van der Maaten, 2014; Figure 1B). We evaluated the clustering resolution with a clustering tree, comparing the relationship between clusters at different resolutions (Figure 1—figure supplement 3). A cluster resolution of 12 was chosen as it best resolved established substructures within our data (as described below). This resulted in 120 distinct clusters (Figure 1—figure supplement 4). Cells from independent experimental replicates were proportionally distributed across the clusters with an alignment metric of 94.6% (Figure 1—figure supplement 5A,B). All replicates contributed to all clusters (with one exception) and showed high correlation of cluster level expression (Figure 1—figure supplement 1C). Replicate one did not have any constituents in cluster 118, which had only 22 cells total. The number of genes and transcripts showed a uniform distribution across the t-SNE (Figure 1—figure supplement 6A).
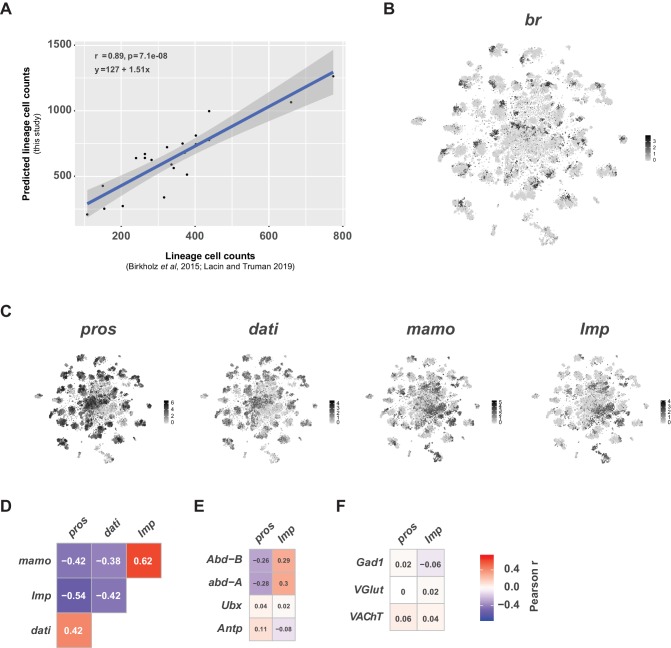
These 26,768 cells represent an approximate 1.5x coverage of the adult VNC. Lineage based counts of the adult specific post-embryonic neurons in the VNC range from 10,000 to 11,000 cells (Birkholz et al., 2015; Lacin et al., 2019), with a total number of cells in the VNC forecasted to be ~16,000 (Lacin et al., 2019). Connectomic analyses of the adult VNC using electron microscopy estimated a larger total number of ~20,000 cells (Bates et al., 2019). Based on these prior numbers, our cell atlas has 1.3–1.7x coverage of the VNC.
Cluster-defining marker genes
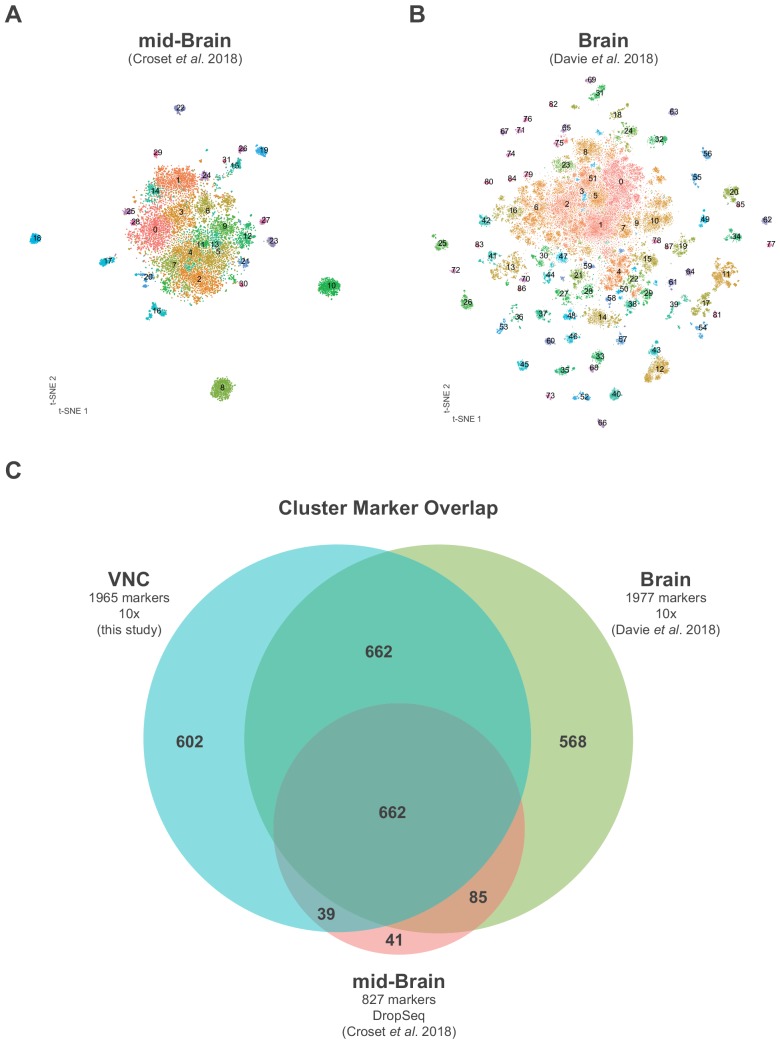
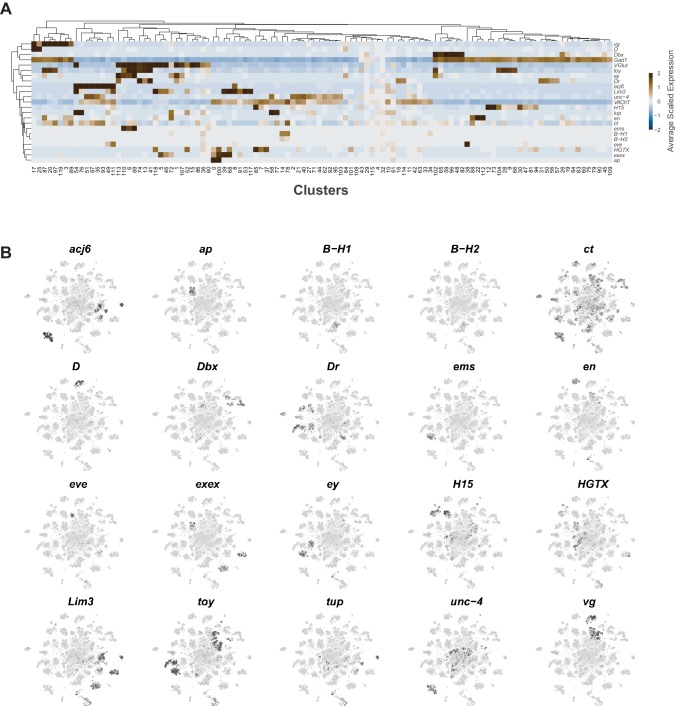
The 120 t-SNE clusters are defined by unique combinations of significantly enriched genes that we refer to as cluster markers (Figure 1C, Figure 1—source data 1). The number of significantly enriched genes and the maximum observed log fold-change varies by cluster (Figure 1—figure supplement 6B; Figure 1—source data 2). These cluster markers are likely to be important for the development and/or maintenance of the cell types represented by the clusters. We compared the significantly enriched cluster markers from this data set (excluding the salivary and sperm clusters, see below) to the cluster markers of re-analyzed mid-brain Drop-seq (Croset et al., 2018) and whole brain 10x (Davie et al., 2018) data sets (Figure 2—figure supplement 1; Figure 2—figure supplement 1—source data 1; Figure 2—figure supplement 1—source data 2). 85% of the mid-brain cluster markers were also found in our VNC cluster markers, while 67% of the whole brain markers overlapped. This suggests that the majority of the genes that define neuronal and glial identity in the brain also define neuronal and glial identity in the VNC.
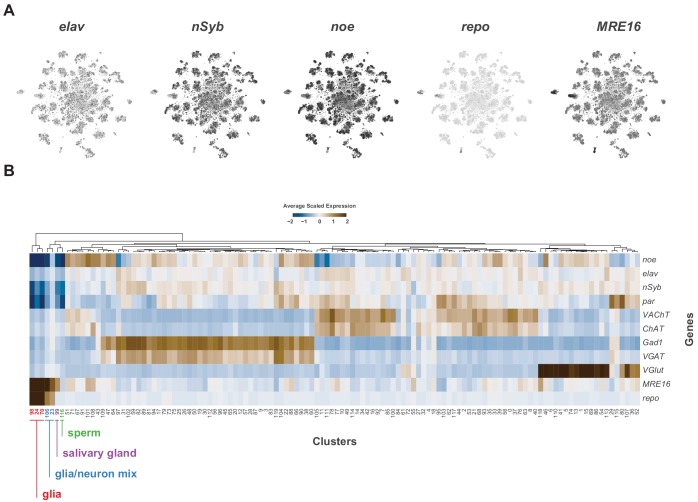
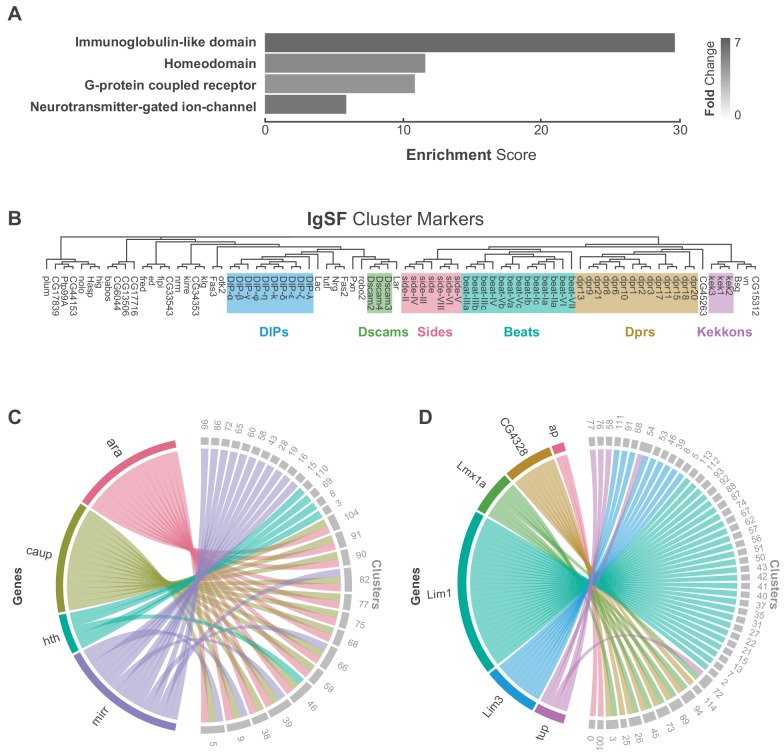
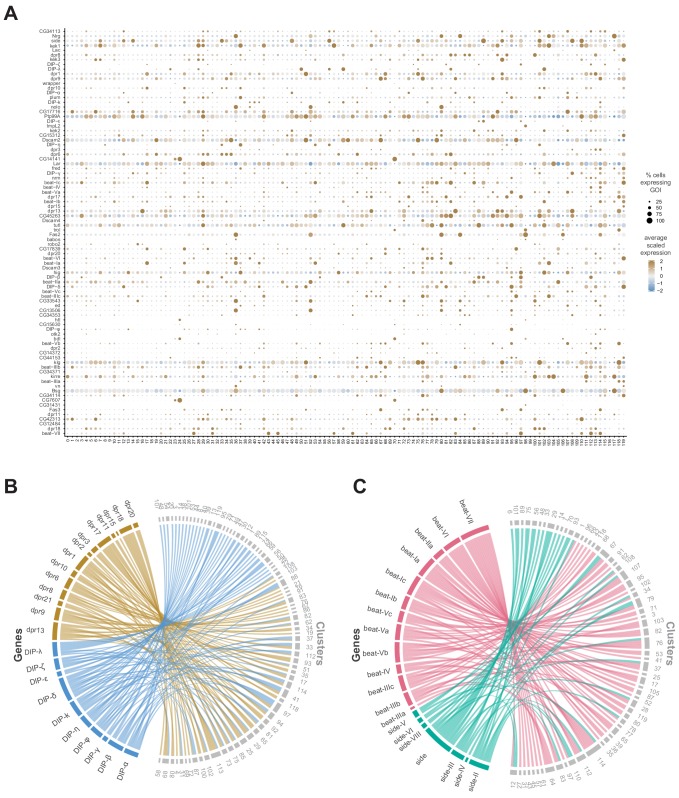
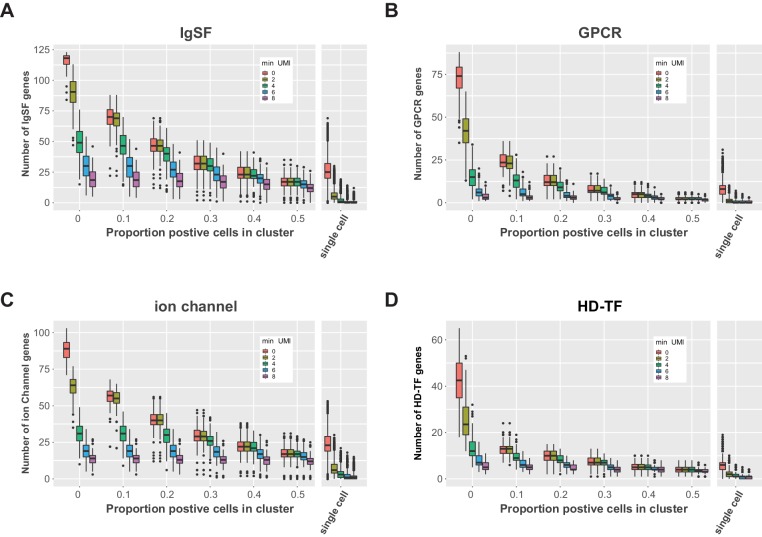
We defined 110 neuronal clusters using expression of known neuron-specific and neuron-enriched genes (Figure 1—figure supplement 7; see methods), such as embryonic lethal abnormal vision (elav), neuronal Synaptobrevin (nSyb) and the long non-coding (lnc) RNA noe (noe) (Davie et al., 2018; DiAntonio et al., 1993; Kim et al., 1998; Robinow et al., 1988). These neuronal clusters can be distinguished from each other by differential expression of 1205 additional cluster markers (Figure 1D, Figure 1—source data 1). Functional enrichment analysis of these cluster-defining marker genes identified those encoding immunoglobulin (Ig)-like domains as most significantly enriched (Figure 2A), including many cell adhesion molecules that are known to establish neuronal connectivity during development (Özkan et al., 2013). Specifically, many members of the Immunoglobulin superfamily (IgSF) continue to be expressed in the adult and contribute to neuronal cluster identity (Figure 2B; Figure 2—figure supplement 2A). The defective proboscis extension response (Dpr) subfamily, and their binding partners, the Dpr-interacting protein (DIP) subfamily, are markers for over 50% of neuronal clusters identified in the adult VNC (Figure 2—figure supplement 2B). Dpr and DIP genes provide a complex interaction network regulating neural circuit assembly and have been proposed to act as neuronal ‘identification tags’ during development. Distinct combinations of Dpr and DIP proteins are expressed by different neuronal classes in the developing optic lobe, the antennal lobe, and the VNC (Carrillo et al., 2015; Özkan et al., 2013). Specific combinations of Beat and Side IgSF proteins play an important role in the sculpting the nervous system (Özkan et al., 2013; Pipes et al., 2001). Almost all Beat and Side family members were found as cluster markers in the VNC (Figure 2B), and their enrichment was largely mutually exclusive, with 80% of the identified clusters enriched for either Beats or Sides (Figure 2—figure supplement 2C). The enrichment of IgSFs in the adult VNC supports their importance in establishing cell type specificity and suggests an ongoing role in maintenance of neuronal connectivity in the mature nervous system (Figure 2B).
Figure 2. Characterization of neuronal cluster markers.
(A) Functional analysis of neuronal cluster markers (DAVID) showing representative functional terms (>5 fold-change) and their corresponding enrichment scores (representing mean p-values). (B) Phylogenetic tree of IgSF neuronal cluster markers. IgSF subfamily members are highlighted in color. (C) Chord diagram comparing the relationship between Tale Homeobox TF cluster markers (left) and the clusters in which they are significantly enriched (right). (D) Chord diagram comparing the relationship between LIM Homeobox TF cluster markers (left) and the clusters in which they are significantly enriched (right). (B–D) Gene were classified based on Flybase Gene Group annotations (www.flybase.org). (C,D) Chord diagrams are visual aids based on data available in Figure 1—source data 1.
Figure 2—figure supplement 1. Comparison of central nervous system single-cell data sets.
Figure 2—figure supplement 2. Relationships between IgSF subfamilies in defining cluster identity.
Figure 2—figure supplement 3. GPCR neuronal cluster markers.
Figure 2—figure supplement 4. Co-expression of gene family members in the VNC.
Figure 2—figure supplement 5. Ion channel neuronal cluster markers.
Figure 2—figure supplement 6. TF neuronal cluster markers.
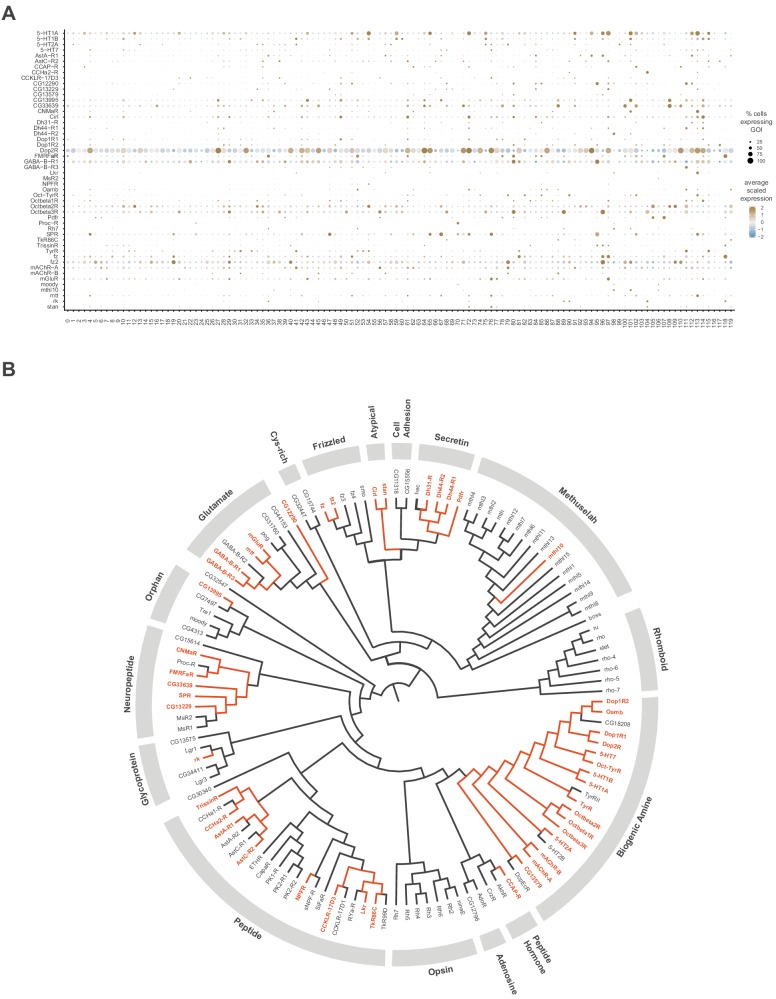
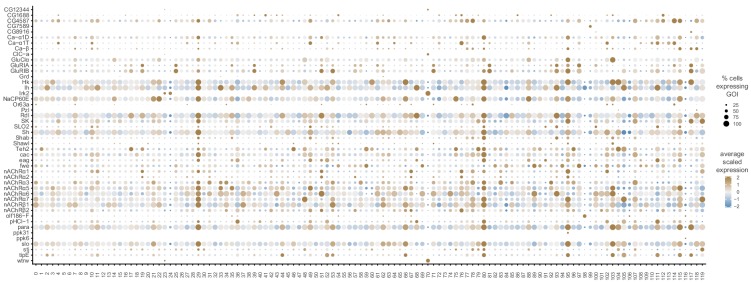
G-protein coupled receptors (GPCRs) were also significantly over-represented as cluster markers (Figure 2A, Figure 2—figure supplement 3A). GPCRs play a critical role in intercellular communication by interacting with a diverse group of extracellular ligands such as neurotransmitters, modulatory neuropeptides and biogenic amines, peptide hormones, and gases (Hanlon and Andrew, 2015). We observed a median of 8 GPCRs expressed per cell and a median of 22 transmembrane receptors in total per cell. Due to the potential for drop-out events (a gene is expressed in a cell, but not detected) these values may be an underestimate. We saw a median of 12 GPCRs when we consider the expression at the cluster level (with a threshold of 20% positive cells per cluster; Figure 2—figure supplement 4B). Although many GPCRs are significantly enriched in the individual clusters, they were, in general, expressed at low levels, in few cells per cluster, and across many clusters (Figure 2—figure supplement 3A). The neuropeptide receptor family members had more inter-cluster variation in expression levels than the other GPCR subtypes, suggesting that they confer an added level of specialization. We also saw that 80% of biogenic amine receptor family members, a diverse group of neurotransmitter receptors sensitive to biogenic amine neurotransmitters including dopamine, octopamine, serotonin and tyramine, are found as cluster markers (Figure 2—figure supplement 3B). Odorant receptors, gustatory receptors, ionotropic receptors, and pickpocket sodium channels are predominantly found in first-order sensory neurons (Joseph and Carlson, 2015) and in general, were not expressed in the VNC, as expected. There were exceptions, however, as Or63a, Gr28b, Ir76a, ppk31 are all expressed in the VNC, albeit at low levels and in few cells. Neurotransmitter-gated ion-channels were also highly enriched as neuronal cluster markers (Figure 2A, Figure 2—figure supplement 5, Figure 1—source data 1), including 8 of the 10 nicotinic acetylcholine receptor subunits (Littleton and Ganetzky, 2000). Expression of transmembrane receptors that provide fast- and slow-acting responses to neurotransmitters, therefore, contribute to cellular identity.
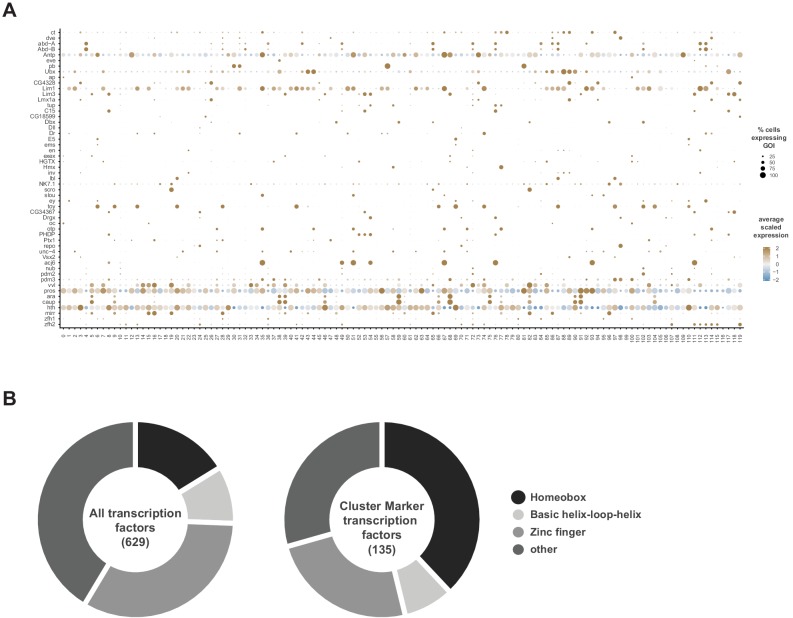
Finally, over 20% of all Drosophila TFs were cluster markers in the adult VNC, the most enriched class of which was the homeodomain (HD) family (Figure 2A, Figure 2—figure supplement 6). Homeodomain TFs play central roles in establishing regional-, tissue- and cell-specific fates (Bürglin and Affolter, 2016). We observed a median of 6 HD TFs per cell, and a median of 52 TFs in total per cell. We saw a median of 10 HD TFs when we consider the expression at the cluster level (20% positive cells per cluster threshold; Figure 2—figure supplement 4D). We detected high variance in HD TF expression levels across clusters (Figure 2—figure supplement 6A). Four members of the three amino acid loop extension (TALE) class of HD TFs mark specific clusters. This included all the three members of the evolutionarily conserved Iroquois gene complex (Iro-C) araucan (ara), caupolican (caup), and mirror (mirr) (Cavodeassi et al., 2001). The Iro-C arose through two duplication events, one ancient event in arthropods led to independent ara/caup and mirr genes, followed by a more recent event in dipterans which gave rise to the caup and ara genes (Kerner et al., 2009). Cluster expression of Iro-C genes appears to reflect this evolutionary history. Whereas caup and ara overlap as cluster markers, mirr expression is only partially overlapping and mirr is an independent marker for 11 additional clusters, suggesting specialization (Figure 2C). In addition, we found largely mutually exclusive enrichment of individual members of the LIM class of HD TFs defining cell clusters in the VNC (Figure 2D). LIM TFs are known to specify distinct neuronal identities in the embryo (Thor et al., 1999). In the VNC only Lmx1a and CG4328 appear to be co-expressed which given their genomic linkage likely represents a relatively recent tandem duplication event. These findings suggest that aspects of the TF-code that establishes cellular identity during development actively maintains neuronal identity throughout the life of the animal (Deneris and Hobert, 2014).
Hox genes define neuromere identity
Hox genes encode homeodomain proteins that confer positional identities along the antero-posterior axis in all bilaterian animals (McGinnis and Krumlauf, 1992). In both vertebrates and invertebrates, the Hox family of transcription factors are known to govern key aspects of nervous system development, notably the formation of neuromuscular networks (Philippidou and Dasen, 2013). The neuromeres of the VNC show distinct segment-specific properties under Hox gene control (Baek et al., 2013; Suska et al., 2011).
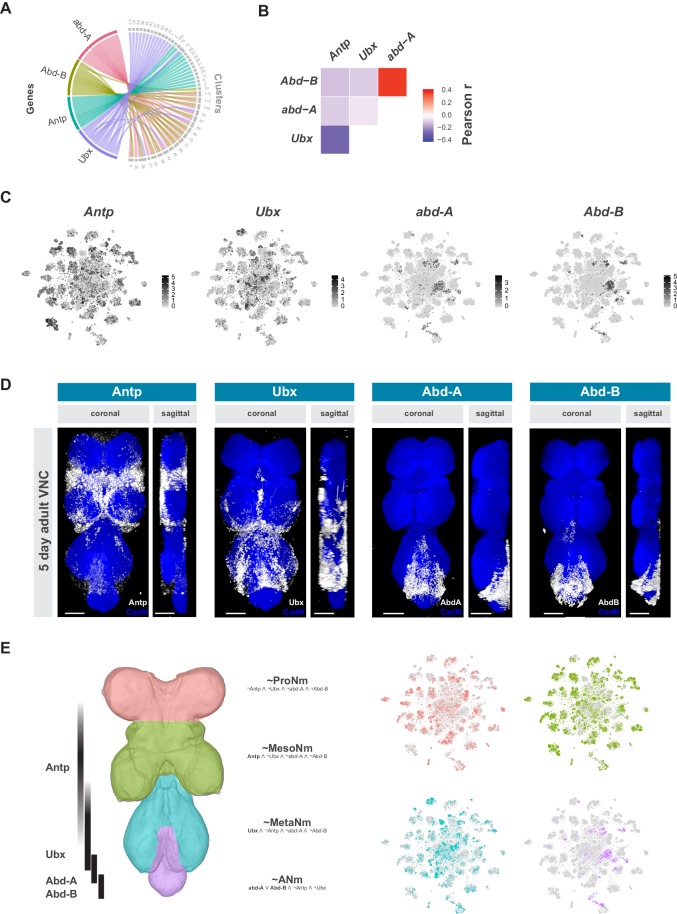
The Hox genes Antennapedia (Antp), Ultrabithorax (Ubx), abdominal-A (abd-A), and Abdominal-B (Abd-B), which specify the thoracic, and abdominal segments of the fly VNC, define specific clusters in the single-cell atlas (Figure 3A). Hox gene expression patterns were significantly anti-correlated at the individual cell level, except for abd-A and Abd-B (Figure 3B). Similar patterns were seen with the correlation of cluster-level expression, and the patterns were consistent between replicates. Antp and Ubx are both expressed throughout the t-SNE plot, with many clusters expressing both, albeit in mutually exclusive cells, while abd-A and Abd-B expression is more restricted and overlapping (Figure 3C). Immunostaining the adult VNC with antibodies against these Hox proteins showed belts of expression along the anterior-posterior axis (Figure 3D). These patterns of expression are different to those in the larva but are consistent with those observed in the mid-pupal stage (Baek et al., 2013). Antp protein is most highly expressed in the mesothoracic neuromere (MesoNm), whereas Ubx expression is highest in the metathoracic neuromere (MetaNm). Abd-A and Abd-B protein showed overlapping expression restricted to the abdominal neuromere (ANm), with Abd-A expressed more anteriorly than Abd-B. By combining the neuromere enriched expression revealed by antibody staining, with the high level of anti-correlation in the single-cell data, we can separate the single-cell clusters into approximations for each neuromere (Figure 3E).
Figure 3. Neuromeres are defined by Hox gene expression in the adult VNC.
(A) Chord diagram comparing the relationship between Hox genes found as cluster markers (left) and the clusters in which they are significantly enriched (right), based on data available in Figure 1—source data 1. (B) Heatmap of Pearson correlation coefficient for Hox gene expression in all single cells. We saw consistent patterns of correlation at the cluster-level expression, and between replicates. (C) t-SNE plot of Hox gene expression distribution. Expression shown in black, intensity is proportional to the log-normalized expression levels. (D) Visualization of Hox protein expression in the 5 day old adult VNC. Coronal and sagittal views of anti-Antp, -Ubx, -Abd-A, and -Abd-B (white). Neuropil counterstained with anti-Cad-N (blue). Scale bars = 50 μm. (E) Schematic representing bands of Hox expression along the anterior-posterior axis of the VNC (left). Cells assigned to approximate neuromeres in VNC t-SNE plots (right) based on differential Hox gene expression: ProNm (pink), MesoNm (green), MetaNm (blue) and ANm (purple). Expressed genes used in defining each neuromere are in bold. (symbols represent the following: ‘¬’ not, ‘∧’ and, ‘∨’ or, ‘~’ approximate).
Neuroblast lineage identity
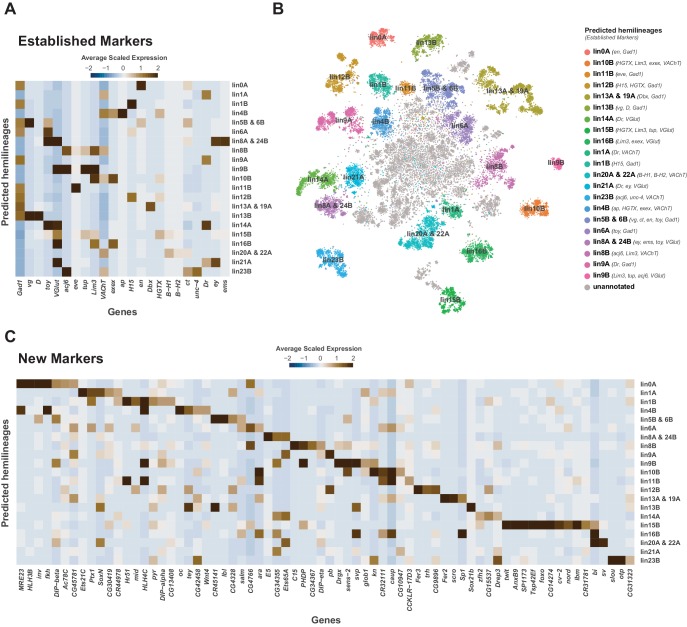
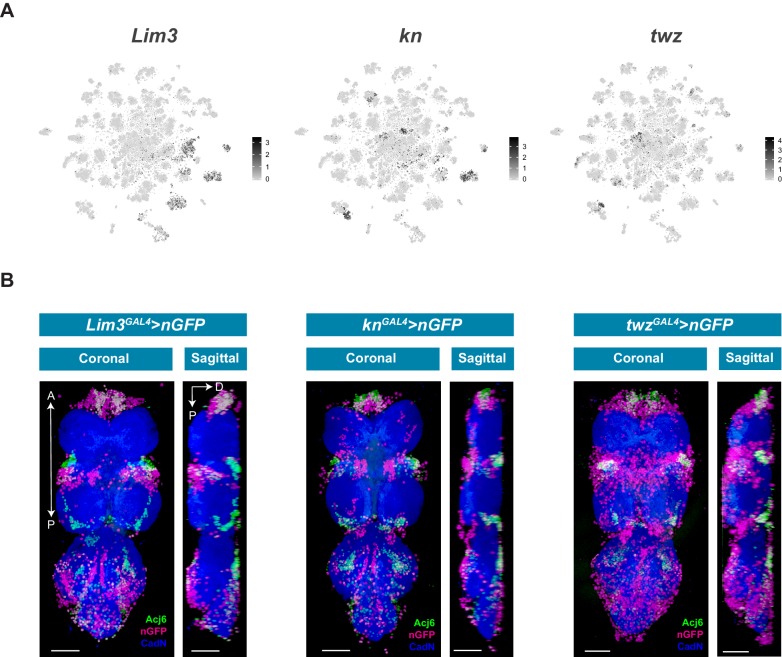
Each neuromere contains a repeating set of neuroblast lineages. We used prior knowledge of biomarkers defining the development of post-embryonic lineages (Bossing et al., 1996; Schmid et al., 1999) to further annotate the 5 day old adult VNC single-cell atlas. All known lineage biomarkers showed continued expression in the adult VNC, and most were found as significantly enriched cluster markers (Figure 4A and B; Figure 4—figure supplement 1; Figure 1—source data 1). These established markers therefore allowed us to assign potential hemilineage identities to many of the cell clusters (Figure 4B; Figure 1—source data 2). We also used fast-acting neurotransmitter (FAN) identity as an additional marker to specify potential hemilineages, since FAN usage is acquired in a lineage-dependent manner in post-embryonic neurons in the VNC (Lacin et al., 2019). For example, cluster 54 has enriched expression of lineage markers Lim3, tailup (tup), abnormal chemosensory jump 6 (acj6), and the glutamatergic neuron marker Vesicular glutamate transporter (VGlut), which collectively suggests this cluster is hemilineage 9B. The correspondence between cluster markers and hemilineage markers shows that cell cluster identity is closely linked to hemilineage identity. We observed a strong concordance between the number of cells within our predicted hemilineages and previous cell counts (Figure 4—figure supplement 2A; Birkholz et al., 2015; Lacin et al., 2019). This correlation of cell counts further supports our atlas having an approximately 1.5x coverage of the VNC.
Figure 4. Cellular identities defined by developmental lineages.
(A) Heatmap of the mean scaled log-normalized expression of established lineage markers genes (bottom) within predicated neuroblast hemilineage (right). (B) t-SNE plot of neuronal cells highlighting predicted hemilineages based on the expression of established hemilineage marker genes (shown beside list of predicted hemilineages on the right). (C) Heatmap of the mean scaled log-normalized expression of potential new hemilineage markers genes (bottom) within predicated neuroblast hemilineage (right). Full list of predicted hemilineage markers can be found in Figure 4—source data 1.
Figure 4—figure supplement 1. Neuroblast lineage marker gene expression in the VNC.
Figure 4—figure supplement 2. Neurodevelopment gene expression.
Many of the established lineage markers are expressed at low levels and in few cells (e.g., B-H1/2, exex, tup), while others are robustly expressed (e.g., acj6, toy, Lim3) (Figure 4—figure supplement 1). Nevertheless, the restricted cluster-specific expression of these established markers and co-expression with more robust novel markers (Figure 4C; Figure 4—source data 1) allowed us to assign cells to putative hemilineages. At present some hemilineages cannot be defined with certainty due to the lack of specific sets of established markers. For instance, there is no marker for hemilineage 2A, other than VGlut, and hemilineage 3B’s Dbx expression does not persist past the pupal stage (Lacin and Truman, 2016; Lacin et al., 2019). Similarly, unc-4 is currently the sole established marker for many cholinergic hemilineages, including 7B, 12A, 18B and 19B. We observe multiple cholinergic clusters enriched for unc-4 (Figure 1—source data 1; Figure 4—figure supplement 1). To assign these clusters to known hemilineages, additional cluster specific enriched genes can be investigated by immunohistochemistry. Conversely, clusters 6, 69, and 110 express markers for both hemilineages 8A (ems, ey, VGlut) and 24B (ems, toy, VGlut), suggesting that these lineages may exhibit very similar transcriptional profiles and resolving them will likely require more cells (Figure 4C).
We have identified several novel potential hemilineage marker genes (Figure 4—figure supplement 1). Of the 59 most highly enriched hemilineage-defining genes, shown in Figure 4C, 34 are TFs, approximately half of which contain homeodomains. Interestingly, 5 of these novel marker genes encode lncRNAs. lncRNAs have been shown to have complex spatiotemporal regulation throughout the embryo (Wilk et al., 2016; Karaiskos et al., 2017) and specifically during embryonic neurogenesis (Scruggs et al., 2015), suggesting their functional importance during development. Two of these lncRNAs, MRE23 and CR45141, are adjacent to and transcribed divergently to their nearest neighbour protein coding genes, fork head (fkh) and vestigial (vg), respectively. These gene pairs show clear positive correlations of expression within the hemilineages (Figure 4C), suggesting they share a cis-regulatory landscape. Such divergent transcription has been shown to enhance transcriptional output (Scruggs et al., 2015; Schor et al., 2018). As the roles of many of these novel marker genes have yet to be studied either during development or in the adult nervous system, our VNC analysis provides a valuable resource for future investigation of hemilineage cell types.
Many predicted hemilineages contain multiple clusters (Figure 4B; Figure 1—source data 2). We propose that these clusters represent distinct variations within a hemilineage, partly informed by birth order and partly related to thoracic vs. abdominal anatomical position. Consistent with a subdivision based on birth order, many of our predicted hemilineages contain a distinct subgroup of broad (br) expressing cells (Figure 4—figure supplement 2B). A specific isoform of br continues to be expressed in the adult and marks cells born around L2-L3 ecdysis (Zhou et al., 2009). Other hemilineage sub-division was evident when visualizing expression of neurodevelopmental genes such as prospero (pros), datilografo (dati), maternal gene required for meiosis (mamo), and IGF-II mRNA-binding protein (Imp) (Figure 4—figure supplement 2C). Expression of pros and Imp in the VNC were negatively correlated (Figure 4—figure supplement 2D), as previously observed in the adult brain (Davie et al., 2018). In contrast, expression of pros was highly correlated with that of dati, while Imp and mamo were highly correlated, suggesting these pairs of genes may be co-regulated in the adult (Figure 4—figure supplement 2D). pros and Imp also showed an anatomical bias, with pros being negatively correlated with abdominally expressed abd-A and Abd-B, while Imp is positively correlated with these markers (Figure 4—figure supplement 2E). However, we did not observe a striking physiological bias, as neither pros nor Imp showed correlation with fast-acting neurotransmitter markers (Figure 4—figure supplement 2F). We saw consistent patterns of correlation at the cluster-level expression, and between replicates. It has previously been shown that pros labels late-born motor neurons from hemilineage 15B (Baek et al., 2013), and that a trade-off of Imp and pros expression is essential for cell cycle exit of post-embryonic type I neuroblasts in the VNC (Maurange et al., 2008; Yang et al., 2017). Studies investigating the transcriptomes of mature (embryonic) vs. immature (post-embryonic) neurons in the larval VNC have shown that Imp is enriched in embryonic neurons, and pros is enriched in post-embryonic neurons (Etheredge, 2017). We propose that the continued expression of Imp in the adult VNC labels early-born and embryonic neurons while pros labels late-born post-embryonic neurons.
Gene expression defines neuronal subtypes within a hemilineage
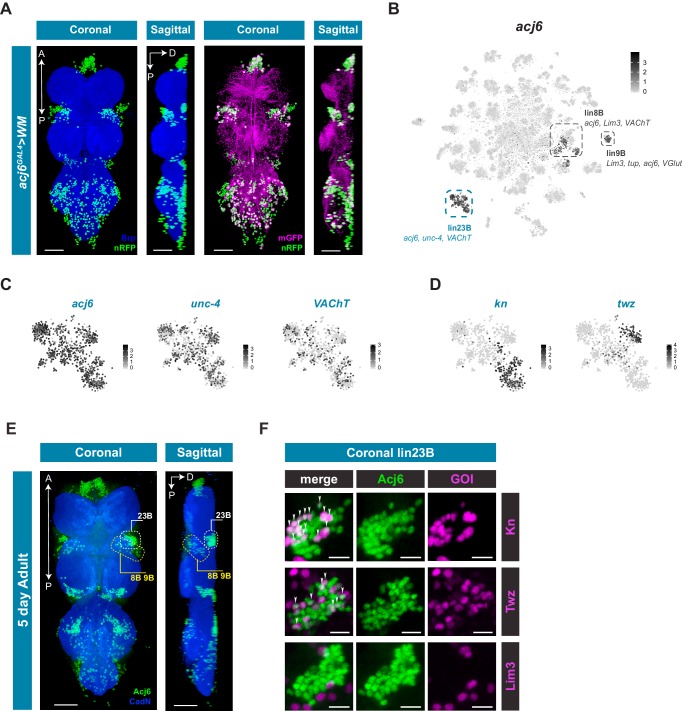
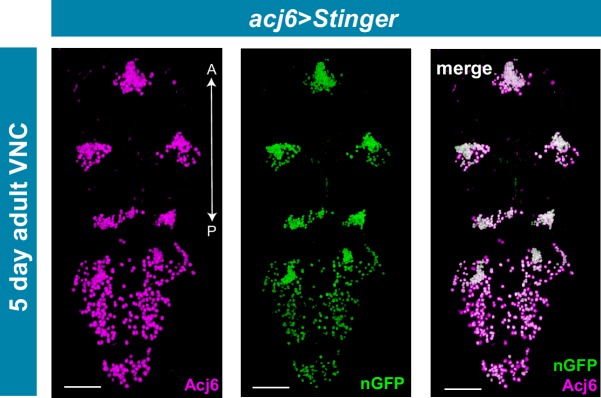
Hemilineage 23B marked by acj6, unc-4, and VAChT has four distinct clusters that can only be partially explained by potential birth order and neuromere identity (Figure 3; Figure 4). acj6 is also a marker for the cholinergic hemilineage 8B and the glutamatergic hemilineage 9B (Figure 4A; Lacin et al., 2019). We examined acj6 expressing cells in more detail by visualizing the anatomical distribution of acj6 expressing cell bodies and neuronal projections in the VNC using acj6GAL4 to express the GAL4-responsive dual reporter UAS-Watermelon (WM), which simultaneously marks the plasma membrane with GFP and the cell nucleus with mCherry (Figure 5A; Lee et al., 2018). We also validated the accuracy of acj6GAL4 using an anti-Acj6 antibody (Clyne et al., 1999; Figure 5—figure supplement 1). Consistent with previous reports, acj6GAL4 labeled distinct clusters of cells in the three thoracic neuromeres of the VNC, which predominantly innervate the leg neuropils. (Figure 5A; Harris et al., 2015; Shepherd et al., 2019). The acj6 expressing neurons were confirmed as hemilineages 8B, 9B, and 23B (D Shepherd, pers. comm.) on the basis of their primary neurite projections (Shepherd et al., 2016).
Figure 5. acj6 expression, hemilineage identity, and novel sub-lineage marker co-expression in the VNC.
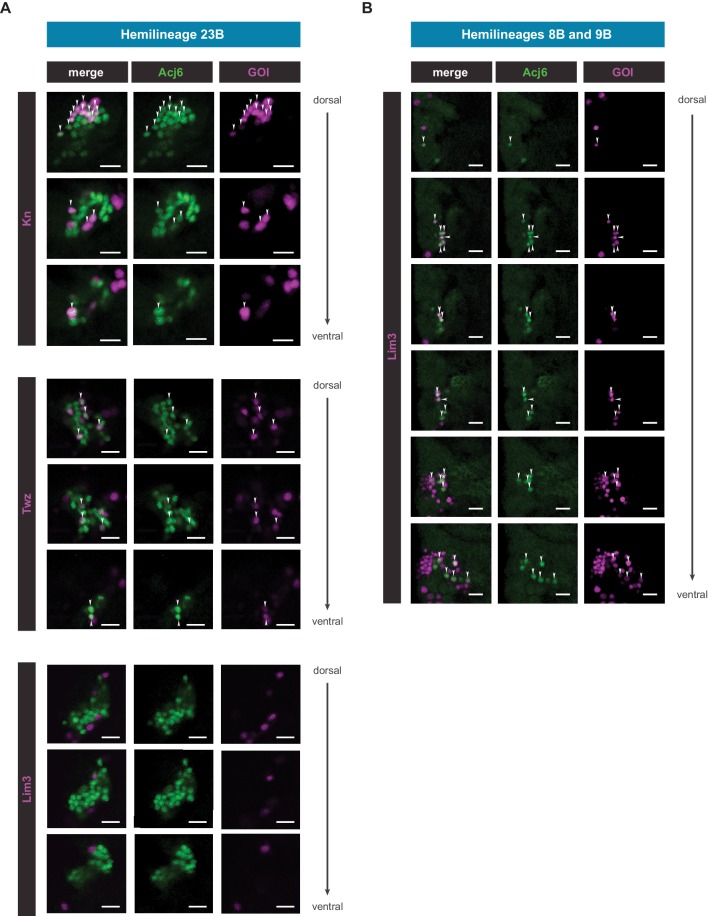
(A) Visualization of acj6 expressing cells in the 5 day old adult VNC. Maximal coronal projection, and hemi-maximal sagittal projectionof acj6GAL4 driving expression of UAS-WM, enabling the visualization of cell nuclei (nRFP; green) and neuronal morphology (mGFP; magenta). Neuropil is counterstained (Brp; blue). A, Anterior, P, posterior, D, dorsal; scale bars = 50 μm. (B) t-SNE plot of acj6 expression in the VNC. Predicted hemilineage 8B, 9B, and 23B, based on known markers (shown below) are highlighted. (C) t-SNE plots of predicted hemilineage 23B, showing expression of markers acj6, unc-4, and VAChT. (D) t-SNE plots of predicted hemilineage 23B, showing expression of novel sub-type markers kn and twz. (B–D) Expression shown in black, intensity is proportional to the log-normalized expression levels. (E) Maximal coronal view (left) and hemi-maximal sagittal view (right) of Acj6 expression (green) in the 5 day old adult VNC. The posterior prothoracic hemilineages 23B (dashed white box) and the anterior mesothoracic 8B and 9B (dashed yellow box) are marked. A, Anterior, P, posterior, D, dorsal; Neuropil is counterstained (Cad-N; blue) scale bars = 50 μm. (F) Close-up maximal coronal views of dorsolateral hemilineage 23B showing the co-expression of knGAL4 and twzGAL4 driven UAS-Stinger expression (nGFP; magenta) with Acj6 (green). Co-positive cells (white) are indicated with arrow heads. Lim3GAL4 driven expression did not colocalize with Acj6 in hemilineage 23B. GOI = gene of interest, scale bars = 10 μm.
Figure 5—figure supplement 1. acj6GAL4 recapitulates Acj6 protein expression in the adult VNC.
Figure 5—figure supplement 2. Novel sub-lineage marker expression in the VNC.
Figure 5—figure supplement 3. Single slices of novel sub-lineage marker co-expression in the VNC.
The acj6 expressing clusters in the single-cell data set are highlighted in Figure 5B. Based on co-expression of additional markers, we could assign acj6-expressing hemilineages within the VNC data; lin8B expresses acj6, Lim3, and VAChT; lin9B acj6, Lim3, VGlut, and tup; Lin23B acj6, unc-4, and VAChT (Figure 5C). Of the 4 distinct clusters within predicted hemilineage 23B, the genes knot (kn/collier) and tiwaz (twz) (Crozatier et al., 1996; Williams et al., 2014) are significantly enriched in two independent clusters; 51 and 93, respectively (Figure 5D; Figure 1—source data 1). We validated the co-expression evident in the single-cell data using GAL4 lines that represent kn and twz expression, and co-stained these VNCs with anti-Acj6 antibody (Figure 5E and F; Figure 5—figure supplement 2). We also used a reporter for the established marker Lim3, which is co-expressed with acj6 in hemilineages 8B and 9B, but not 23B (Figure 5B; Lacin and Truman, 2016). Hemilineage 23B is located dorsolaterally at the posterior edge of each of the three thoracic neuromeres, whereas 8B and 9B are both located laterally at the anterior side of each thoracic neuromere with 8B located near the ventral surface and 9B located more dorsally (Figure 5E; Lacin et al., 2019; Shepherd et al., 2019). Focusing on the ProNm-MesoNm border, which encompasses prothoracic 23B and mesothoracic 8B and 9B, we found that a subset of 23B co-expressed kn and a subset co-expressed twz (Figure 5F; Video 1; Video 2). Of the 40 dorsolateral Acj6+ 23B cells, 14 were kn+ and 9 were twz+ (Figure 5—source data 1; Figure 5—figure supplement 3). These proportions (34% and 21%) are roughly consistent with that seen in the single-cell data (31% and 18% for kn and twz, respectively). Lim3 expression was restricted to 8B and 9B, with 90% of Acj6+ 8B and 9B cells expressing Lim3, and 0% co-expression in hemilineage 23B (Figure 5F; Figure 5—source data 1; Video 3; Figure 5—figure supplement 3). kn was also expressed in a subset of predicted hemilineage 9B in the single cell data (Figure 5—figure supplement 2), and co-expression was seen between knGAL4 and Acj6 in the 8B/9B cluster in vivo (Figure 5—figure supplement 3; Figure 5—source data 1). Full expression patterns in the VNC are shown in Figure 5—figure supplement 2. These findings highlight the predictive power of the single-cell transcriptome to identify new markers that refine our understanding of hemilineage subtypes which contribute to neuronal cell diversity in post-embryonic lineages.
Video 1. Video of 3D volume showing the co-expression of Acj6 (anti-Acj6; green) with knGAL4 driven expression of UAS-Stinger (nGFP; magenta) in the 5 day adult VNC, with neuropil counterstained (CadN; blue).
Video 2. Video of 3D volume showing the co-expression of Acj6 (anti-Acj6; green) with twzGAL4 driven expression of UAS-Stinger (nGFP; magenta) in the 5 day adult VNC, with neuropil counterstained (CadN; blue).
Video 3. Video of 3D volume showing the co-expression of Acj6 (anti-Acj6; green) with Lim3GAL4 driven expression of UAS-Stinger (nGFP; magenta) in the 5 day adult VNC, with neuropil counterstained (CadN; blue).
Classification using fast-acting neurotransmitters
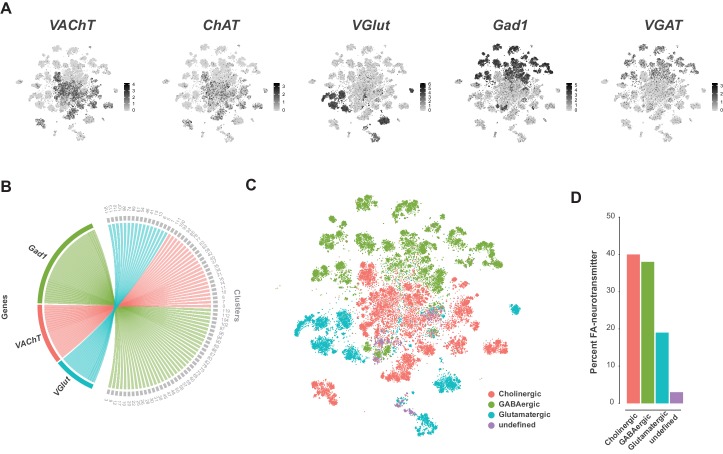
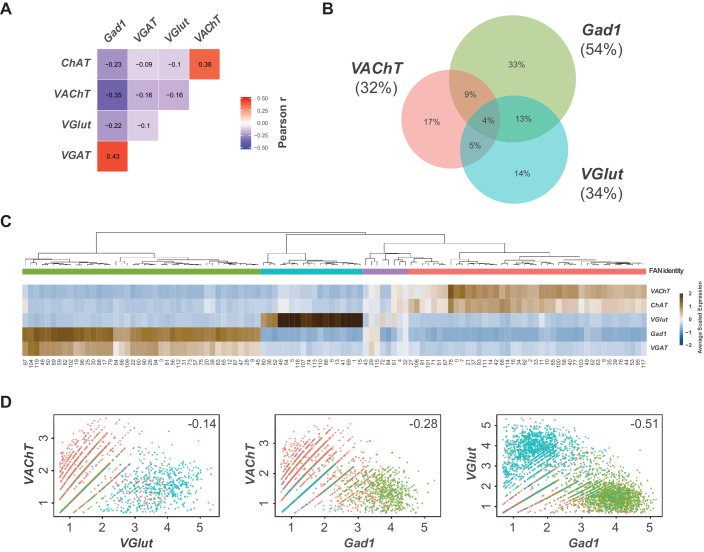
A recent comprehensive map of fast-acting neurotransmitter (FAN) usage across the VNC found that neurons within a post-embryonic hemilineage use the same neurotransmitter; either acetylcholine, gamma-aminobutyric acid (GABA), or glutamate, unifying their shared developmental and functional identities (Lacin et al., 2019). We examined FAN usage in the VNC using expression of established biosynthetic and vesicular loading markers: Choline acetyltransferase (ChAT) and Vesicular acetylcholine transporter (VAChT) for acetylcholine, VGlut for glutamate, and Glutamic acid decarboxylase 1 (Gad1) and Vesicular GABA Transporter (VGAT) for GABA (Figure 6A). Clusters showed mutually exclusive enrichment of VAChT, VGlut, and Gad1 (Figure 6B). However, this exclusivity partially breaks down at the cell-by-cell level. Although expression levels of VAChT, VGlut, and Gad1 were negatively correlated (Figure 6—figure supplement 1A), we also observed significant levels of co-expression, with 31% of cells expressing at least two of these markers (Figure 6—figure supplement 1B). A similar pattern of co-expression was observed in single-cell data from the adult brain (Croset et al., 2018; Davie et al., 2018). However, immunostaining of the intact adult VNC suggested that cytoplasmic co-expression of these markers does not occur (Lacin et al., 2019). Nuclear transcriptomic profiles of neuronal subtypes from both the visual system and the mushroom body found no evidence for FAN co-release (Davis et al., 2020; Shih et al., 2019). Co-expression in the VNC may therefore represent contamination due to ambient RNA present in the cell suspension (discussed below). Since the cells cluster due to hemilineage identity and hemilineages are restricted to a single FAN, we assigned FAN identity by comparing the average expression of these FAN markers at the cluster level (Figure 6—figure supplement 1C; Figure 1—source data 2). Amongst the co-expressing cells, the expression of FAN makers is negatively correlated, and cells assigned to one FAN identity tended to show higher expression of the corresponding marker (Figure 6—figure supplement 1D). This is, however, not always the case, presumably due to the much higher expression levels seen for VGlut and Gad1 (Figure 6A; Figure 6—figure supplement 1D). With these criteria (see methods), we estimate that the VNC is 40% cholinergic, 38% GABAergic, and 18% glutamatergic. The remaining 4% of neurons did not show a strong signature for any FAN (Figure 6C and D). These proportions differ to those in the adult brain (Croset et al., 2018; Davie et al., 2018), with inhibitory GABAergic neurons being much more prominent in the VNC (38% vs 15% in the adult midbrain). The inhibitory glutamate receptor, GluClα (Cully et al., 1996), was broadly expressed in these data and is particularly enriched in cluster 103 (see Figure 1—source data 1), suggesting that a portion of glutamatergic signaling is also inhibitory. We expect this relative abundance of inhibitory signaling reflects the importance of inhibition in the initiation, maintenance, and termination of complex motor programs (Burrows, 1992; Grillner, 2006), especially in coordination between bilateral and intersegmental circuits (Gowda et al., 2018).
Figure 6. Fast-acting neurotransmitter usage in the VNC.
(A) t-SNE plots showing distribution of cells expressing biomarkers for the fact-acting neurotransmitters acetylcholine (VAChT, ChAT), glutamate (VGlut) and GABA (VGAT, Gad1). Expression shown in black, intensity is proportional to the log-normalized expression levels. (B) Chord diagram comparing the relationship between fast-acting neurotransmitter cluster markers (left, genes) and the clusters in which they are significantly enriched (right, clusters). (C) t-SNE plot colored according to fast-acting neurotransmitter usage based on assigned cluster identity. (D) Percentage of cells in the VNC assigned as releasing distinct fast-acting neurotransmitters.
Figure 6—figure supplement 1. Expression of fast-acting neurotransmitter biomarkers in the VNC.
Monoaminergic neurons
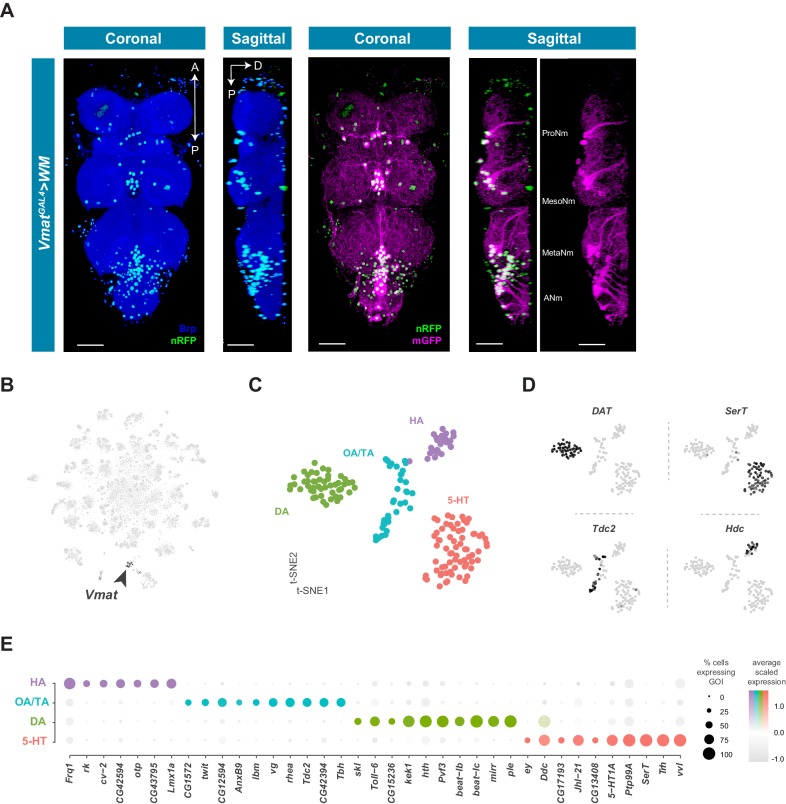
Cells that produce and likely release monoamines in the VNC can be identified based on expression of the Vesicular monoamine transporter (Vmat) gene (Greer et al., 2005). To examine the anatomical distribution of Vmat expressing nuclei and projections in the VNC we combined VmatGAL4 with UAS-Watermelon (WM) (Figure 7A). VmatGAL4 labels 115 ± 6.3 neurons distributed across every neuromere of the VNC (n = 9, data not shown). Each neuromere has a main cluster of cells located on the ventral surface, near the midline, consistent with known developmental origins of monoaminergic neurons. Serotonin- and dopamine-producing neurons are born from the ventromedially located paired neuroblast 7–3, and octopamine-producing neurons are born from the ventral midline unpaired median neuroblast (Bossing et al., 1996; Schmid et al., 1999). Projections of Vmat-labeled neurons formed thick fascicles of fibers projecting from each cluster to the dorsal surface of the VNC (Figure 7A, sagittal view). Each thoracic neuromere has one main fiber tract, whereas the ANm has 8 parallel tracts, one for each abdominal segment. Despite the relatively small number of Vmat expressing cells, they project throughout the VNC and densely innervate the entire neuropil. Additional Vmat nuclei associated with the VNC are consistent with its reported expression in perineural surface glia (DeSalvo et al., 2014). Surface glial Vmat expressing cells can be seen in cluster 98 of our primary t-SNE plot (Figure 1—figure supplement 4; Figure 1—source data 1).
Figure 7. Identification and characterization of monoaminergic cell sub-types.
(A) Visualization of Vmat expressing cells in the 5 day old adult VNC. Coronal and sagittal views of VmatGAL4 driving expression of UAS-WM, enabling the visualization of cell nuclei (nRFP; magenta) and neuronal morphology (mGFP; green). Neuropil is counterstained (Brp; blue). A, Anterior, P, posterior, D, dorsal; scale bars = 50 μm. (B) t-SNE plot of Vmat expression in black, intensity is proportional to the scaled log-normalized expression level. Vmat enriched Cluster 72 is highlighted with arrowhead. (C) t-SNE plot showing sub-clustering analysis of Vmat+ cells from clusters 72 and 84. Four sub-clusters are identified representing dopaminergic (DA), octopaminergic/tyraminergic (OA/TA), histaminergic (HA), and serotonergic neurons (5-HT). (D) Expression of established monoaminergic subtype-specific biomarkers used to determine cluster identity. Histidine decarboxylase (Hdc) labels Histamine (HA) neurons, Tyrosine decarboxylase 2 (Tdc-2) labels Tyramine (TA) and Octopamine (OA) neurons, Dopamine transporter (DAT) labels Dopamine (DA) neurons and Serotonin transporter (SerT) labels Serotonin (5-HT) neurons. (E) Dot plot of the top genes in each monoaminergic sub-type based on fold-enrichment (Figure 8—source data 1). Size of dots represent percent of cells in cluster expressing gene of interest (GOI); intensity of color reflects average scaled expression. Figure 7—source data 1 contain the full list of significantly enriched genes.
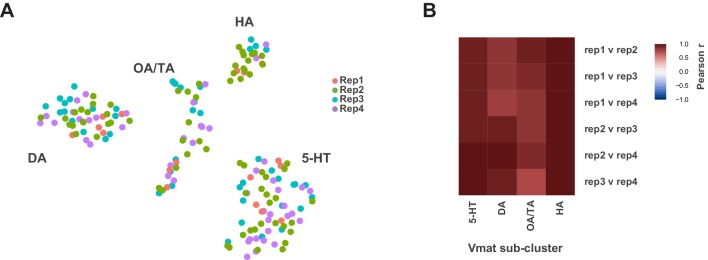
Figure 7—figure supplement 1. Individual replicate contribution to Vmat+ sub-clusters.
Neurons that express Vmat co-cluster in the single-cell data (Clusters 72 and 84, Figure 1—figure supplement 4; Figure 7B). To define the specific monoaminergic identity of Vmat expressing neurons, we sub-clustered these Vmat+ cells from clusters 72 and 84 (Figure 7C) and identified distinct groups synthesizing specific monoamines, as defined by expression of known biosynthetic and transporter markers (Figure 7D; Martin and Krantz, 2014). All replicates contributed to all clusters and showed high correlation of cluster level expression (Figure 7—figure supplement 1).
Histaminergic neurons
Histamine (HA) is well established as the primary fast neurotransmitter of adult photoreceptors in many insects, including Drosophila (Hardie, 1987; Nässel et al., 1988). The VNC contains 18 ventral HA-immunoreactive neurons with extensive axons and projections, six in the thoracic neuromeres and twelve in the abdominal neuromere (Buchner et al., 1993; Nässel et al., 1990). Potential new markers for HA neurons, distinguishing them from other monoaminergic neurons, include Frequenin 1 (Frq1), a Ca2+-binding protein that regulates neurotransmitter release (Dason et al., 2009), and CG43795; an uncharacterized GPCR predicted to have Glutamate/GABA receptor activity (Agrawal et al., 2013).
Tyraminergic and octopaminergic neurons
Tyramine (TA) and Octopamine (OA) neurons innervate and modulate many tissues throughout the fly, including female and male reproductive systems, skeletal muscles, and sensory organs (Pauls et al., 2018; Rezával et al., 2014). Tdc2 encoded tyrosine decarboxylase catalyzes the synthesis of TA (Cole et al., 2005), which can also be converted to OA by Tbh encoded Tyramine beta-hydroxylase (Monastirioti et al., 1996). All Tdc2 expressing neurons in the VNC also express Tbh suggesting that they are likely to be octopaminergic, consistent with a previous report using Tdc2GAL4 in the VNC (Pauls et al., 2018). Prior work in the larval VNC suggested that the TA:OA ratio might be altered in a state-dependent manner (Schützler et al., 2019).
Dopaminergic neurons
Dopamine (DA) is a critical neuromodulator controlling learning and state-dependent plasticity in the fly brain (Cognigni et al., 2018). Dopamine and DA neurons in the VNC have been implicated in motor behaviors such as grooming and copulation (Crickmore and Vosshall, 2013; Yellman et al., 1997). Novel DA markers in the VNC include beat-Ib and beat-Ic, a likely recent duplication in the beat family of genes which act as heterophilic cell-cell adhesion molecules (Pipes et al., 2001). VNC DA neurons overexpress PDGF- and VEGF-related growth factor (Pvf3) and kekkon 1 (kek1) which encodes a transmembrane protein that binds the EGF receptor and controls the activity of this pathway. Adult midbrain DA neurons also overexpress these genes (Croset et al., 2018), suggesting a possible common role for Pvf3 and kek1 in DA neuron function. The Toll-6 neurotrophin-like receptor implicated in neuronal survival and motor-axon targeting (McIlroy et al., 2013), appears specifically enriched in VNC DA neurons.
Serotonergic neurons
Serotonin (5-hydroxytryptamine, 5-HT) releasing neurons within the thoracic neuromeres of the VNC modulate walking speed in a context-independent manner as well as in response to startling stimuli (Howard et al., 2019). In the abdominal ganglion, two clusters of sexually dimorphic neurons expressing 5-HT and fruitless (approximately ten cells per cluster in males) innervate the internal reproductive organs. These abdominal clusters control transfer of sperm and seminal fluid during copulation (Billeter et al., 2006; Lee and Hall, 2001; Lee et al., 2001). 5-HT neurons in the VNC express the 5-HT receptor 5-HT1A, suggesting auto-regulatory/autocrine control of 5-HT neurons. VNC 5-HT neurons also express the amino acid transporter encoded by juvenile hormone inducible 21 (JhI-21) which is required for 5-HT-dependent evaluation of dietary protein (Ro et al., 2016). Finding JhI-21 expression in 5-HT neurons in the VNC, therefore suggests that some 5-HT neurons may directly sense circulating amino acids.
A transcription factor code for monoaminergic neurons
The top group of genes defining each subclass of monoaminergic neurons types also included unique expression of Homeobox-containing TFs (Bürglin and Affolter, 2016; Figure 7E). orthopedia (otp) and the LIM homeobox transcription factor 1 alpha (Lmx1a) define the HA cluster. DA neurons are enriched for the Hox co-factor homothorax (hth) as well as the Iroquois homeobox TF mirror (mirr). Lastly, 5-HT neurons express the homeobox TFs ventral veins lacking (vvl), eyeless (ey) and Lim3. Although these different combinations of homeobox genes likely act in these cells to specify position-specific patterning decisions and wiring specificity during development, it is also possible that they contribute to the function of mature monoaminergic neurons.
Peptidergic neurons
Neuropeptides are the largest family of signaling molecules in the nervous system and act as important regulators of development, physiology, and behavior (Nässel and Zandawala, 2019). We analyzed neuropeptide expression in the adult VNC by looking at the expression of genes encoding neuropeptide precursors, from which active neuropeptides are derived. We identified 28 neuropeptides that are expressed in at least one cell, at a level of 10 or more transcripts per cell (Figure 8A). Some neuropeptides are known to be co-expressed with FANs, where they serve to increase signaling flexibility within neural networks (Croset et al., 2018; Nässel, 2018; Nusbaum et al., 2017). We found that in the VNC some neuropeptide genes are predominantly co-expressed with particular FANs, e.g. sNPF and spab in cholinergic neurons, MIP and CCHa2 in GABAergic neurons, and Ilp7 and Proc in glutamatergic neurons (Figure 8A; Figure 6). A few neuropeptides were also co-expressed in the same cells, e.g. AstC and CCHa2 in GABAergic cluster 94. Some of these associations are similar to what was observed previously in mid-brain single-cell data (Croset et al., 2018), e.g. sNPF and spab in cholinergic neurons, but others are strikingly different. AstC and CCHa2 are robustly expressed in GABAergic neurons in the VNC but are notably absent from Gad1 expressing neurons in the mid-brain.
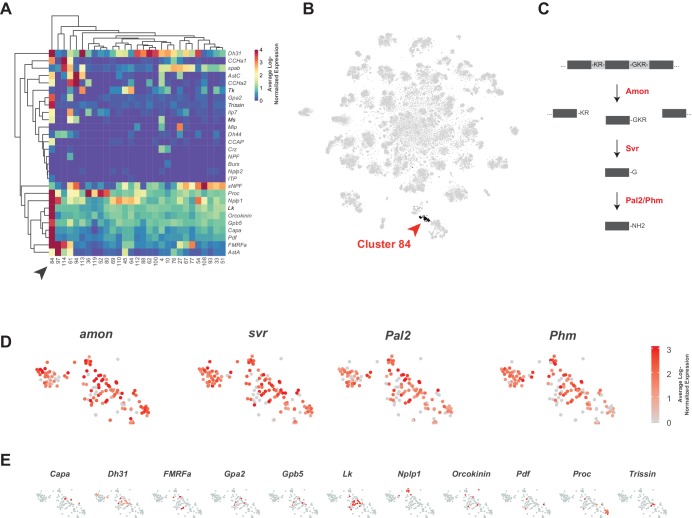
Figure 8. Identification of neuropeptide expressing cells.
(A) Heatmap of the mean log-normalized expression (0–4), by cluster (bottom), of neuropeptide genes (right). Only clusters with at least one neuropeptide gene expressing in excess of mean log-normalized value of 10 are shown. Only neuropeptide genes that were expressed in at least one cell at a level >10 transcripts per cell are shown. Arrowhead highlights cluster 84. Dendrograms represent hierarchical clustering. (B) t-SNE plot highlighting neuropeptide expressing cluster 84. (C) Schematic of neuropeptide processing steps highlighting enzymes (in red) identified as enriched markers for cluster 84. Propeptides are cleaved by the pro-hormone convertase Amontillado (Amon); the carboxypeptidase Silver (Svr) then removes the C-terminal cleavage sequence. C-terminal amidation occurs through the combined actions of Peptidyl-α-hydroxyglycine-α-amidating lyase 2 (Pal2) and Peptidylglycin-α-hydroxylating monooxygenase (Phm) (reviewed in Pauls et al., 2014). (D, E). Expression of neuropeptide processing enzymes (D) and multiple neuropeptide genes (E) in t-SNE cluster 84. Intensity of red is proportional to the log-normalized expression level.
Figure 8—figure supplement 1. Neuropeptide gene expression levels.
Figure 8—figure supplement 2. Analysis of VNC data removing upper limit on transcript number (UMI) per cell.
Figure 8—figure supplement 3. Orcokinin expression analysis.
Some neuropeptides are also expressed in ‘neurosecretory’ neurons that lack FANs. Peptides released from these cells into circulation provide neuroendocrine signals, whereas those released into the nervous system have neuromodulatory function. Cells in cluster 84 exhibit several characteristics of neurosecretory cells. The TF dimmed (dimm) is required for the differentiation of neurosecretory cells (Hamanaka et al., 2010; Hewes et al., 2003; Park et al., 2008). Although dimm is detected at very low levels and in few cells in our dataset, it showed a bias to cluster 84 (10.7% of cells express dimm vs. 0.03% elsewhere). Cluster 84 is also particularly enriched for neuropeptide expression (Figure 8A and B) and lacking strong expression of FAN or monoaminergic neuron markers (Figure 1—figure supplement 7; Figure 6C; Figure 7B). They also express several genes involved in neuropeptide processing (see Figure 8C and D; Figure 1—source data 1). Most neuropeptides are expressed in non-overlapping subsets of the cluster (Figure 8E), suggesting that a particular neuropeptide does not drive the unifying identity of the cluster, but rather that it is generically peptidergic. The Glycoprotein hormone alpha 2 (Gpa2) and Glycoprotein hormone beta 5 (Gpb5) genes, whose peptides form heterodimers, are co-expressed in the same cells in this peptidergic cluster (Sellami et al., 2011).
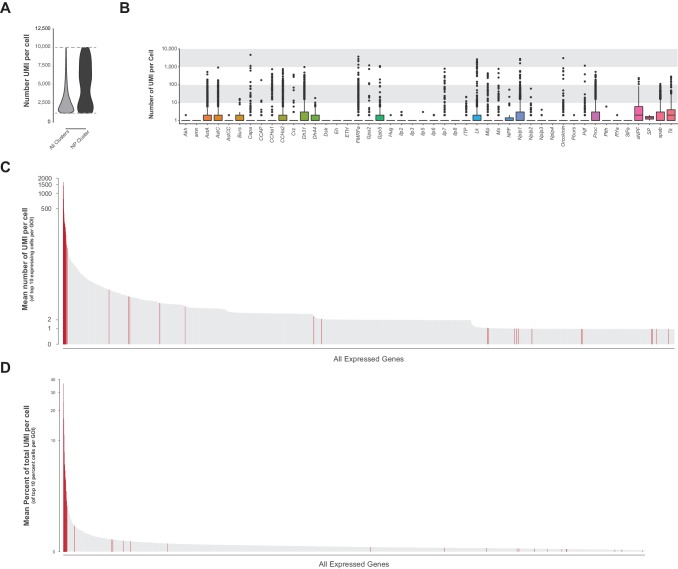
High neuropeptide gene expression reveals important technical considerations
When investigating neuropeptide expressing cluster 84 (NP cluster) we noticed that the median number of transcripts in the cluster was 5277, which was far greater than that observed in the data set as a whole, 2497 (Figure 8—figure supplement 1A). This large number of transcripts is due, in part, to high expression levels of the neuropeptide genes themselves. For example, the maximum expression level of Leucokinin (Lk) was 2629 transcripts in a single cell (Figure 8—figure supplement 1B), whereas the maximum expression level of an average gene was 11 transcripts per cell. Amongst neuropeptide genes, Lk is not an anomaly, as 18 of the top 40 most highly expressed genes per cell encode neuropeptides (Figure 8—figure supplement 1C). All 28 of the expressed neuropeptide genes had a maximum observed expression above the 93rd percentile (see methods), with 23 of the 28 above the 99th percentile (Figure 8—source data 1). In some cases, these genes represented 40% of a cell's captured transcriptional output (Figure 8—figure supplement 1D). Similar patterns of high neuropeptide precursor gene expression have also been seen in the mouse cortex (Smith et al., 2019).
For all previous data analyses, we used an upper limit of 10,000 total transcripts (nUMI) per cell to remove potential outliers. Given the high expression seen for neuropeptide genes, we repeated our analysis without this cut-off, revealing that many cells in the neuropeptide (NP) and Proctolin+ motor neuron (Proc+ MN) clusters express more than 10,000 transcripts (Figure 8—figure supplement 2). It is worth considering the filtering cut-off when studying cells with high transcriptional output, and the genes expressed therein, whose expression can exceed the threshold. For example, the maximum observed expression of Orcokinin was 23,092 transcripts.
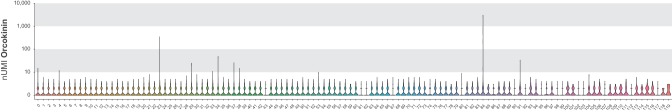
Another important consideration when investigating cells expressing genes at very high levels, as many neuropeptide cells do, is the consequence of these cells rupturing during the dissociation process. Once ruptured, large numbers of neuropeptide transcripts could be present in the ambient solution, leading to background levels of neuropeptide transcripts being ‘picked up’ with non-expressing cells. Our data for the neuropeptide Orcokinin illustrates this point (Figure 8—figure supplement 3). Orcokinin is expressed in just 5 neurons in the adult VNC, two pairs of neurons in thoracic neuromeres, with additional expression in one unpaired neuron in the abdominal neuromere (Chen et al., 2015). In our data, 5 cells in the NP cluster show Orcokinin expression at a level of more than 100 transcripts (Figure 8—figure supplement 3). However, almost 8000 cells, across all clusters, also appear to express Orcokinin at a level of just 1–10 transcripts (Figure 8—figure supplement 3). We speculate that the low-level expression outside the NP cluster reflects background levels, due to rupture of Orcokinin expressing cells, while the expression above 100 transcripts within the NP cluster is bona fide.
Non-neuronal cell types: Glia
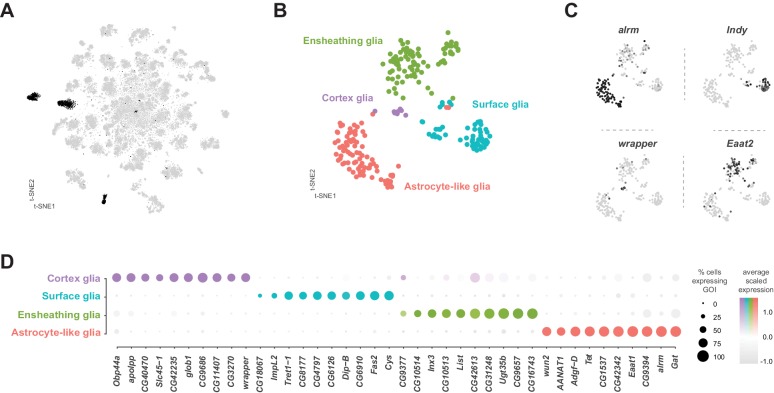
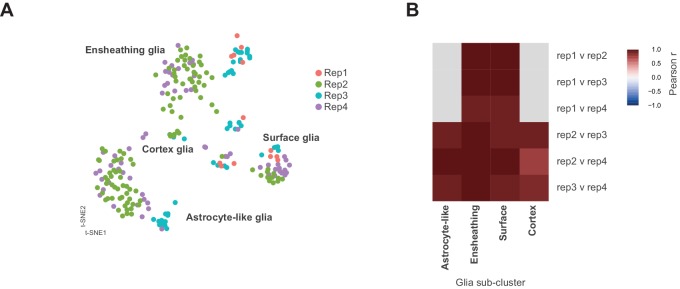
Glia are key regulators of nervous system physiology maintaining the concentration of chemicals in the extracellular environment. We identified glia cells in our VNC data using the established glial markers reversed polarity (repo) (Xiong et al., 1994) and the long non-coding RNA MRE16 (Davie et al., 2018; Figure 1—figure supplement 7). Five clusters, representing 3.6% of the total cells, were highly enriched for repo and MRE16. In addition, these clusters lacked expression of neuronal markers, such as elav, nSyb, and noe (Figure 1—figure supplement 7). Two of these clusters contained cells that were positive for both glial markers and cells positive for neuronal markers, suggesting mixed populations. To define specific glial cell types, we performed a sub-clustering analysis of the five glial clusters (Figure 9A), while removing any cell with a neuronal signature (see methods). This sub-clustering revealed four distinct glial subpopulations that could be classified based on enriched expression of established markers (Figure 9B): astrocytic leucine-rich repeat molecule (alrm) for astrocytes (Doherty et al., 2009), I'm not dead yet (Indy) for surface glia (Knauf et al., 2002), wrapper for cortex glia (Noordermeer et al., 1998), and Excitatory amino acid transporter 2 (Eaat2) for ensheathing glia (Stahl et al., 2018).
Figure 9. Identification and characterization of glial cell sub-types.
(A) t-SNE plot highlighting glial cell clusters. Clusters 23, 24, 70, 98, and 106 are shown in black, as they were determined to be glia based on differential expression of established glial and neuronal biomarkers (Figure 1—figure supplement 7). (B) t-SNE plot showing sub-clustering analysis of glial cells. Distinct clusters are color-coded and named. (C) Expression of established glial subtype-specific biomarkers used to determine cluster identity. astrocytic leucine-rich repeat molecule (alrm), for astrocytes; I’m not dead yet (Indy) for surface glia; wrapper for cortex glia; Excitatory amino acid transporter 2 (Eaat2) for ensheathing glia. Expression shown in black, intensity is proportional to the log-normalized expression levels. (D) Dot plot of the top 10 genes in each glial sub-type based on fold-enrichment (Figure 9—source data 1). Size of dots represent percent of cells in cluster expressing gene of interest (GOI); intensity of color reflects average scaled expression. Figure 9—source data 1 contain the full list of significantly enriched genes.
Figure 9—figure supplement 1. Identification of neuronal and glia clusters.
Surface glia
Consistent with the established metabolic role of glia in the brain, many solute carrier (SLC) membrane transporters are enriched in glial cell types (Figure 9D; Figure 9—source data 1). Surface glia, which form the blood-brain barrier (BBB), metabolically insulate the nervous system from the hemolymph. The BBB also subserves the considerable energetic demands of the nervous system (Laughlin et al., 1998), by efficiently transporting sugars, ions, and metabolites between the hemolymph and brain (Limmer et al., 2014). Surface glia strongly express the previously described surface glia marker SLC2 Trehalose transporter 1–1 (Tret1-1), which as the name suggests transports trehalose, the main carbohydrate in the hemolymph, into the nervous system (Volkenhoff et al., 2015). Surface glia also contain high levels of a putative SLC2 sugar transporter CG4797, previously reported to be expressed in the perineural glia of the optic lobe (Figure 9D; Konstantinides et al., 2018). Both of these sugar transporters are proton-dependent and therefore, efficient sugar transport requires glial cells to have lower H+ concentration than the hemolymph (Kikuta et al., 2012). We found enriched expression of multiple V-type ATPase H+ pumping genes (p-value=3.9×10−9 based on DAVID analysis; Figure 9—source data 1; Chintapalli et al., 2013) in surface glia. Expression of the SLC bicarbonate transporter CG8177, which has been shown to reduce extracellular pH (Overend et al., 2016), and the Ecdysone-inducible gene L2 (ImpL2) were also enriched in surface glia (Figure 9D). ImpL2 antagonizes insulin-like peptide 2 (Dilp2) and inhibits insulin/insulin-like growth factor signaling (Honegger et al., 2008) suggesting that the BBB is responsive to the metabolic demands of the fly.
Astrocytes and ensheathing glia
Over 70% of the VNC astrocyte cell-specific markers are shared with those in astrocytes in the midbrain (Croset et al., 2018). This similarity suggests that there is minimal regional specialization of astrocyte identity and function within the CNS. All astrocytes and ensheathing glia in the thoracic VNC are derived from the same lineages as leg motor neurons (Enriquez et al., 2018). However, we did not find evidence of a lineage-specific transcriptional code in these glial cell types (Figure 9—source data 1). Astrocytes instead, strongly express the paired-like homeobox TF CG34367, previously reported to be expressed in astrocytes throughout development (Huang et al., 2015). In contrast, ensheathing glia express the POU homeobox TF ventral veins lacking (vvl) (Anderson et al., 1995). These TFs can, therefore, be considered as novel markers for these adult VNC cell types. The rarity of TF markers is in accord with the finding that the morphological specificity of motor neurons depends on a unique TF code, whereas astrocytes and ensheathing glia show plasticity in their morphologies dependent on their position, rather than a distinct TF code (Enriquez et al., 2018).
Non-neural ‘contamination’
We identified two clusters of cells that represent contamination during the dissection process (Figure 1—figure supplement 7B). Cluster 99 was determined to be salivary gland tissue, since the cluster markers for cluster 99 contains 8 of the top 10 genes enriched in salivary gland tissue in the FlyAtlas 2 dataset (Figure 1—source data 1; Leader et al., 2018). Cluster 116 appears to be sperm as many of the markers for this cluster are unique to the testis and sperm (Figure 1—source data 1; Witt et al., 2019).
Discussion
As the field tries to categorize nervous systems at higher resolution, the question arises what precisely constitutes a cell type? The goal itself seems straightforward in principle, to find a way to define different groups of cells that carry out distinct tasks. Single-cell mRNA sequencing techniques provide a possible route to answering this question by allowing the neuronal transcriptome of thousands of individual cells within a complex nervous system to be collected in parallel. To this end, we generated a single-cell transcriptional atlas that reveals extensive cellular diversity in the adult Drosophila ventral nerve cord. In combination with previous single-cell data from the antennal lobe, optic lobe, and brain (Croset et al., 2018; Davie et al., 2018; Konstantinides et al., 2018; Li et al., 2017), our data contribute towards a comprehensive cell atlas representative of the entire adult central nervous system.
Distinguishing between some cell types such as neurons and glia is relatively straightforward, but the extent of neuronal diversity provides a real challenge. Different neuronal types transmit particular neurotransmitters, neuropeptides and monoamines, and they also respond to a variety of these signals using their complement of cell-surface receptors. Specific neurons might also express unique ion-channels and cell-signaling cascades that provide the cell with a range of electrophysiological characteristics, and potential mechanisms of plasticity. In principle, we can also define neuronal cell types by characterizing their neuroanatomy - where the neurons are located and to which neurons they are pre- and post-synaptically connected. Since neurons acquire their anatomy through developmental programs, it was not known whether this information would remain accessible in any form in the snapshot of the transcriptome of mature fully differentiated adult VNC. However, one of the most evident cluster-defining features of our VNC data is the abundance of transcription factors and cell-adhesion molecules that are classically thought of as being developmental.
The cluster-defining TFs are particularly useful for annotating the cell types in the VNC. Decades of work has investigated the development and structural organization of the VNC and has described roles for these genes in developmental specification of hemilineages (Baek and Mann, 2009; Birkholz et al., 2015; Bossing et al., 1996; Lacin et al., 2019; Lacin and Truman, 2016; Prokop and Technau, 1991; Schmid et al., 1999; Shepherd et al., 2016; Shepherd et al., 2019; Truman and Bate, 1988; Truman et al., 2004). Hemilineages have been proposed to be the functional units of the VNC, representing fundamental organizational principles for connectivity (Harris et al., 2015). Our data are entirely consistent with the hemilineages as functional units with each hemilineage made up of a population of neurons that share morphological, transcriptional, and neurochemical features. They represent a familial unit and our data clearly in an unbiased way, pulls them out as separate and identifiable genetic units, reinforcing the idea that the hemilineages are functional groups that share molecular/genetic identity as well as morphology and function. Moreover, we can see that hemilineages are not all homogenous in their composition; there are distinct subtypes evident from the single-cell data. For example, we documented that kn and twz are expressed in distinct subsets of hemilineage 23B. The fact that subtypes are seen in our data, as well as the number of subtypes for a given hemilineage, is consistent with what others have observed anatomically (D. Shepherd, pers. comm).
We also found continued expression in hemilineages of neurodevelopment genes, such as br, Imp, and pros, which may signify cell birth order and therefore further distinguish cell types. Expression levels of pros indicate the birth order of post-embryonic leg motor neurons belonging to hemilineage 15B, that precedes muscle-specific innervation (Baek et al., 2013; Baek and Mann, 2009). Relative levels of Imp and pros in our predicted hemilineage 15B, can therefore be used to infer muscle-specific innervation along the leg. Imp and pros expression may also distinguish between embryonic born and post-embryonic born neurons. Mature (embryonic) neurons of the larval VNC show elevated Imp expression, whereas immature (post-embryonic) neurons have higher levels of pros, relative to each other (Etheredge, 2017). Dopaminergic neurons in the PAM1 cluster in the larva are the only dopaminergic neurons to be born post-embryonically (Hartenstein et al., 2017). So, in some instances, embryonic vs. post-embryonic identity can also be used to identify neurons within a given class.
Fast-acting neurotransmitter identity is acquired in a hemilineage dependent manner, amongst post-embryonic neurons (Lacin et al., 2019), but the underlying transcriptional programs appear to be complex (Estacio-Gómez et al., 2019). We did not identify single TFs that globally defined FAN identity. Different neurons have been reported to use different TFs to define the same FAN identity in the adult fly optic lobes (Konstantinides et al., 2018) and in C. elegans (Hobert and Kratsios, 2019). In addition, a recent gene-profiling study of cholinergic, glutamatergic, and GABAergic neurons across development found that many FAN-specific TFs are transiently expressed at particular times, and only a few remain constant (Estacio-Gómez et al., 2019). As noted previously (Lacin et al., 2019), we found some TFs that are restricted to individual FAN-specific clusters. Some GABAergic clusters express Dbx, vg, D; some cholinergic neurons express unc-4 and some glutamatergic neurons express ems. We also found Lim3 to be expressed in cholinergic and glutamatergic neurons, but not in GABAergic neurons (Lacin et al., 2019). This is in contrast to what is seen in the optic lobes, where Lim3 specifically regulates GABAergic cellular identity (Konstantinides et al., 2018). Yet, consistent with the optic lobes, Lim3 is only expressed in Notchoff hemilineages in the VNC (Li et al., 2017). Therefore, the TF code specifying FAN identity may change across development and also differ depending on the regional context.
Hox gene expression (Antp, Ubx, abd-A, and Abd-B) developmentally define the neuromeres of the VNC. Finding that established neuromere- and hemilineage-enriched developmental markers are expressed in the 5 day old adult, suggests they may continue to act in these differentiated cells to maintain the distinct transcriptional profile underlying neuronal identity and function (Deneris and Hobert, 2014). It will therefore be interesting to investigate functional consequences of disrupting these potential maintenance programs.
Not all cells group by their developmental lineage, for example, monoaminergic cells formed a distinct cluster, despite originating from multiple different neuroblasts (Schmid et al., 1999). We also identified a cluster of neurosecretory cells that includes cells with different developmental origins (Park et al., 2008). High expression levels of neuropeptides in this cluster is consistent with features of neurosecretory biology. Neuropeptides are often produced by small numbers of cells, yet they can be broadly released and by volume transmission and/or secretion into the hemolymph act at a distance to modulate disparate neural circuits, physiological functions and behaviors (Nässel, 2018). The extraordinarily high-level expression of pre-pro-neuropeptide genes in these cells suggests that producing these molecules presents a considerable burden. The TF dimmed (dimm), which marks neurosecretory cells of this type, has been shown to promote neurosecretory identity and suppress FAN identity (Hewes et al., 2003; Park et al., 2008).
In this study, we have demonstrated the predictive power of the single-cell atlas of the VNC. Despite the caveats associated with this technique, such as sparse sampling of a cell’s transcriptome and ambient RNA contamination, cellular identity signals, especially developmental programs, are surprisingly robust. Defining cell types based on clustering should be viewed as an exploratory rather than a confirmatory process (Crow and Gillis, 2019). It is worth noting that the genes identified using this technique that most robustly define cell clusters do not necessarily reflect their importance for cell type function, however many will no doubt be useful for the generation of tools to study and ultimately define cell phenotypes. Our results suggest many new directions for further investigation. For example, understanding the correspondence and potential causal relationships between transcriptomic signatures and specific anatomical, physiological and functional properties, and how these relationships change depending on cell state.
Materials and methods
Fly strains and husbandry
The fly strain used for VNC analysis (+/w*; UAS-Stinger, 13XLexAop2-IVS-tdTomato.nls/+; dsxGAL4/+) was a genetic cross between w*; UAS-Stinger, 13XLexAop2-IVS-tdTomato.nls males and +; +; dsxGAL4 virgin females (Rideout et al., 2010). All flies were reared at 25°C in a 12:12 hr light:dark cycle on standard food at 40–50% relative humidity. Virgin males and females were collected and stored individually. Flies were aged 5 days post-eclosion at 25°C prior to dissection. Additional fly strains used in this study include: (1) wild-type Canton-S (2) y1w*; Mi{Trojan-GAL4.0}Vmat[MI07680-TG4.0] (BDSC:66806) (3) y1w*; Mi{Trojan-GAL4.1}Lim3[MI03817-TG4.1]/SM6a (BDSC:67450) (4) y1w*; {Mi{Trojan-GAL4.2}kn[MI15480-TG4.2]/SM6a (BDSC:67516) (5) y1w*; Mi{Trojan-GAL4.2}twz[MI14153-TG4.2]/SM6a (BDSC:76758) (6) y1w* Mi{Trojan-GAL4.0}acj6[MI07818-TG4.0]/FM7c (BDSC:77788) (7) w*; {UAS-Stinger} denoted as UAS-nGFP (Barolo et al., 2000) (8) w*; UAS-myr-GFP-V5-P2A-H2B-mCherry-HA/TM3, Ser (aka UAS-WM; Chang et al., 2019).
VNC single-cell sample preparation
The VNC dissociation protocol was carried out as described previously (Croset et al., 2018). 40 Male and 40 female VNCs (20 per sexed replicate) were individually dissected in toxin-supplemented ice-cold calcium- and magnesium-free DPBS (Gibco, 14190–086 + 50 µM D(−)−2-Amino-5-phosphonovaleric acid, 20 µM 6,7-dinitroquinoxaline-2,3-dione and 0.1 µM tetrodotoxin). Each replicate was then washed in 1 mL ice-cold toxin-supplemented Schneider’s medium (tSM: Gibco, 21720–001 + toxins, as above). VNCs were then incubated for 30 min in 0.5 mL of tSM containing 1 mg/mL papain (Sigma, P4762) and 1 mg/mL collagenase I (Sigma, C2674). VNCs were washed once more with tSM and subsequently triturated with flame-rounded 200 µL pipette tips. Dissociated VNCs were resuspended into 1 mL PBS + 0.01% BSA and filtered through a 10 µm CellTrix strainer (Sysmex, 04-0042-2314).
Data processing
Libraries were made using the Chromium Single Cell 3ʹ v2 kit from 10x Genomics. Cells were loaded in accordance with 10x Genomics documentation, with the aim of 5000–8000 cells per sample. The samples were sequenced with 8 lanes of Illumina HiSeq4000 by Oxford Genomics Centre. We obtained a mean of 20,550 reads per cell and mean sequence saturation of 71%. The fastq data were processed with Drop-seq_tools v2.1.0 (Macosko et al., 2015) and aligned to the D. melanogaster genome (R6.13) to generate digital expression matrices for each sample.
Data analysis with seurat
The digital expression matrices were analyzed with the Seurat 2.3.4 R package (Satija et al., 2015). Genes expressed in fewer than 3 GEMs (Gel Bead-In EMulsions) were removed. GEMs with more than 10,000 UMI (1.4% of GEMs, 3.6 standard deviations away from the median) were removed as outliers (unless otherwise mentioned). GEMs with fewer than 1,200 UMI were removed. The lower limit was determined using a histogram of the number of UMI per GEM. This distribution has a local minimum between 1000 and 1,200 UMI. GEMs with fewer than 200 genes, or more than 15% mitochondrial derived UMI were removed. This resulted in 26,768 cells in total and 2590, 9060, 6522, 8596 cells for the four replicates, respectively. Sex-specific replicates were merged with the ‘MergeSeurat’ function and normalized with the ‘NormalizeData’ function, using default parameters. The merged sex-specific groups were then scaled with the ‘ScaleData’ function while regressing out variation due to replicate, nUMI, and proportion mitochondrial transcript. Low expression level, and low dispersion cut-offs of 0.001 were used to identify variable expressed genes in each sex. The intersection of these genes was used to perform a canonical correlation analysis (CCA) with the first 45 dimensions. t-distributed stochastic neighbor embedding (t-SNE) was performed, with perplexity of 30, theta of 0.05, and 20,000 iterations, on the data to reduce the dimensionality to 2 for visualization. Clusters were defined using the ‘FindClusters’ function with the default Louvain algorithm and using 45 dimensions and a cluster resolution of 12 (unless otherwise mentioned). Comparison of different cluster resolutions was evaluated with the ‘clustree’ package (Zappia and Oshlack, 2018). Cluster resolutions above 12 yielded few new clusters and increased the between cluster exchanges. Positively enriched cluster markers were identified using the ‘FindAllMarkers’ function with a negative binomial distribution test and fold enrichment of at least 0.5 and a Bonferroni adjusted p-value of less than 0.05. Neuronal identity was determined by having an average scaled expression (calculated with the ‘AverageExpression’ function in ‘Seurat’) of at least 0.15 for any of the following neuronal markers (elav, nSyb, para, VAChT, ChAT, Gad1, VGAT, VGlut, noe). All other clusters were deemed to be non-neuronal, with the exception of neuropeptide-expressing cluster 84. Glial identity was determined in a similar manner with the following glial markers (repo, alrm, wrapper, Indy). Functionally related gene enrichment analysis on neuronal cluster markers was performed using DAVID (Huang et al., 2009).
Comparisons to bulk sequence data and between replicates
FlyAtlas 2 bulk RNA-seq data of the adult VNC was obtained from http://flyatlas.gla.ac.uk/FlyAtlas2/index.html (Leader et al., 2018). The convert IDs tool from Flybase was used to convert gene symbols and Flybase ID between release versions (http://flybase.org/convert/id). Pseudo-bulk normalized expression from the filtered single-cell VNC data was then compared to the pooled female and male FPKM from the FlyAtlas 2 data set. Pearson correlation coefficients were calculated with the ‘stat_cor’ function from the ‘ggpubr’ package. Pseudo-bulk normalized expression from each replicate were compared to each other, and correlations were calculated with ‘stat_cor’. Alignment of the replicates was assessed with the ‘CalcAlignmentMetric’ function in ‘Seurat’. The ‘AverageExpression’ function in ‘Seurat’ along with the ‘correlate’ function in ‘corrr’ were used to determine the correlations of gene expression between the replicates at the cluster level.
Comparison with brain Single-Cell data
Loom files for the mid-brain (Croset et al., 2018) and the whole (Davie et al., 2018) were downloaded from ‘scope.aertslab.org’. Digital expression matrices of filtered cells were extracted from the loom files and reanalyzed with similar parameters to those used for the VNC. The data were normalized with the ‘NormalizeData’ function with default parameters. The data were scaled with the ‘ScaleData’ function while regressing out variation due to nUMI and proportion mitochondrial expression. Variably expressed genes were identified with the ‘FindVariableGenes’ function with the following cutoffs: x.low.cutoff = 0.001, x.high.cutoff = Inf, y.cutoff = 0.001. A principal component analysis was performed using the identified variable genes. Clusters were identified with the ‘FindClusters’ function using the default Louvain algorithm. A cluster resolution of 2.5 and the first 50 PCAs were used for the mid-brain data (Croset et al., 2018), and a cluster resolution of 2 and the first 82 PCAs were used for the brain data (Davie et al., 2018). t-SNE was performed with perplexity of 30, theta of 0.1, and 20,000 iterations, on the data to reduce the dimensionality to two for visualization. Significantly enriched genes for each cluster were identified using the ‘FindAllMarkers’ function using the negative binomial test with a log fold-change threshold of 0.5 while repressing out variation due to replicate, and a Bonferroni adjusted p-value of less than 0.05. The resulting cluster markers for the mid-brain and brain data sets were compared to the VNC data cluster markers (excluding the salivary gland cluster and the sperm cluster). The overlap of markers was visualized with an Euler plot using the ‘eulerr’ package in R.
Predicting hemilineage identity
The average expression of previously established hemilineage markers (Lacin et al., 2019; Venkatasubramanian and Mann, 2019) across all neuronal clusters was calculated with the ‘AverageExpression’ function in ‘Seurat’. Predicted hemilineage identities were deduced from these expression patterns. With these assignments, we used the ‘FindAllMarkers’ function (with the above settings) to identify novel biomarkers for each predicted hemilineage. Hemilineage specific cell counts (inferred from Birkholz et al., 2015 and Lacin et al., 2019) were compared to the cell counts from our predicted labeled hemilineages. To approximate post-embryonic thoracic cells, only pros+, abd-A-, Abd-B- cells were considered.
Assigning fast-acting neurotransmitter identity
The following genes were used to assign fast-acting neurotransmitter (FAN) identity; Vesicular acetylcholine transporter (VAChT) and Choline acetyltransferase (ChAT) for acetylcholine, Vesicular glutamate transporter (VGlut) for glutamate, and Vesicular GABA Transporter (VGAT) and Glutamic acid decarboxylase 1 (Gad1) for GABA. Cells from clusters with average scaled expression above 0.5 for Gad1 or VGAT, and average scaled expression less than 0 for the other markers were all assigned to be GABAergic. Glutamatergic and cholinergic cells were assigned similarly. For clusters with an average expression less than 0.5 for all markers, cells were assigned FAN identity at a cell-by-cell level. For these remaining cells, we established different criteria for assigning cholinergic vs. glutamatergic or GABAergic since the cholinergic markers were expressed at much lower levels. If both VAChT and ChAT had a log-normalized expression greater than 0, then the cells were assigned to be cholinergic. Additionally, if either VAChT or ChAT had a log-normalized expression greater than 2, the cells were assigned to be cholinergic. Otherwise, if VGlut had a log-normalized expression greater than 2 and greater than either VAChT or Gad1, then the cells were assigned to be glutamatergic. Otherwise, if Gad1 had a log-normalized expression greater than 2 and greater than either VAChT or VGlut, the cells were assigned to be GABAergic. All remaining cells were labelled undefined.
Sub-clustering of monoaminergic cells
Vmat expressing cells from clusters 72 and 84 were sub-clustered. Identification of variable genes and canonical correlation analysis were performed as above. t-SNE was performed using the first 7 dimensions and a cluster resolution of 1.2 was used to identify clusters. Positively enriched cluster markers were identified as above.
Sub-clustering of glial cells
Clusters 23, 24, 70, 98, and 106 were identified as being predominantly glial cells. Cells from these clusters were sub-clustered. Any cell expressing elav, nSyb, VAChT, VGlut, or Gad1 was removed. Identification of variable genes and canonical correlation analysis were performed as above. t-SNE was performed using the first 6 dimensions and a cluster resolution of 0.9 was used to identify clusters. Positively enriched cluster markers were identified as above.
R packages and plotting
Expression heatmaps were drawn using the ‘pheatmap’ package, correlation heatmaps were drawn using the ‘ggcorplot’ package. Chord diagrams were drawn using the ‘circlize’ package (Gu et al., 2014), and represent the relationship between cluster markers and the clusters in which they are significantly enriched, the data is taken from Figure 1—source data 1. No weighted relationships are inferred in chord diagrams. Scatter plots and bar charts were drawn using ggplot2 in R. A full list of packages used, with their version numbers can be found at https://github.com/aaron-allen/VNC_scRNAseq/blob/master/sessionInfo.txt. All plots were edited in Adobe Illustrator.
Phylogeny
The multiple sequence alignment (not shown) and the phylogenetic tree were created with Clustal Omega (Sievers and Higgins, 2018) and FigTreeV1.4.4 (http://tree.bio.ed.ac.uk/software/figtree/), respectively.
Immunohistochemistry
Flies were reared at 25°C and aged for 5 days prior to dissection and staining as per Rideout et al. (2010) with the following modifications: VNC samples were fixed in 4% PFA (40 mins at room temperature) immediately following dissection, to maintain tissue integrity and minimize cell loss. Samples were pre-incubated in 5% NGS overnight at 4°C. Samples were then incubated with primary antibodies for 3 days at 4°C, followed by an overnight incubation in secondary antibodies at 4°C. Primary antibodies used were: mouse mAb nC82 (1:50), mouse anti-AbdA (1:100, C-11), mouse anti-AbdB (1:10, 1A2E9-s), mouse anti-Acj6-s (1:20), mouse anti-AntP (1:20, 8C11-s), mouse anti-Ubx (1:20, FP3.38-s), rat anti-CadN (1:30, DN-Ex #8;) from DSHB, Univ. of Iowa. Additional primary antibodies used were chicken anti-GFP (1:1200, Abcam) and anti-AbdA (1:100, C-11 Santa Cruz Biotechnology). Secondary antibodies used included: anti-chicken Alexa Fluor488, anti-mouse Alexa Fluor633, anti-rat Alexa Fluor546, (1:300, Invitrogen Molecular Probes, Carlsbad, CA). Samples were left in 70% Glycerol/30% PBT overnight at 4°C prior to mounting with Vectashield (Vector Labs) and imaged with a Leica SP5 Microscope. Stacks of optical sections were generated at 0.5 µm intervals. Images were processed in Imaris 8.2.1 (Bitplane Scientific, AG, Zürich).
Data and code availability
Raw sequencing files (fastq) and digital expression matrices from each replicate are available from the Gene Expression Omnibus (GSE141807). Code used in this analysis is available from GitHub (https://github.com/aaron-allen/VNC_scRNAseq; Allen, 2020; copy archived at https://github.com/elifesciences-publications/VNC_scRNAseq).
Acknowledgements
We thank Tetsuya Nojima and Annika Rings for dissection assistance. We thank David Shepherd, Devika Agarwal, Deniz Erezyilmaz, Julian Dow, and members of the Goodwin lab for helpful discussions and critical reading of the manuscript. We thank the Bloomington Stock Center for flies. We thank Josh Dubnau for the UAS-WM line. Sequencing was carried out by The Oxford Genomics Centre. We thank Deborah J Andrew and Caitlin D Hanlon for assistance generating a Figure of the GPCRs, and Andreas Prokop for the adult Drosophila illustration. SW is supported by a Wellcome Principal Research Fellowship (200846/Z/16/Z) and ERC Advanced Grant (789274). This work was funded by a Wellcome Investigator Award (106189/Z/14/Z) to SFG, and a Wellcome Collaborative Award (209235/Z/17/Z) to SFG and SW.
Funding Statement
The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
Contributor Information
Aaron M Allen, Email: aaron.allen@dpag.ox.ac.uk.
Stephen F Goodwin, Email: stephen.goodwin@dpag.ox.ac.uk.
Richard S Mann, Columbia University, United States.
K VijayRaghavan, National Centre for Biological Sciences, Tata Institute of Fundamental Research, India.
Funding Information
This paper was supported by the following grants:
Wellcome Trust Wellcome Trust Investigator Award (106189/Z/14/Z) to Aaron M Allen, Megan C Neville, Stephen F Goodwin.
ERC Advanced Grant 789274 to Vincent Croset, Christoph Daniel Treiber, Scott Waddell.
Wellcome Trust Wellcome Principal Research fellowship (200846/Z/16/Z) to Scott Waddell.
Wellcome Trust Wellcome Collaborative Award (209235/Z/17/Z) to Scott Waddell, Stephen F Goodwin.
MRC Graduate Student Fellowship to Sebastian Birtles.
Additional information
Competing interests
No competing interests declared.
Author contributions
Conceptualization, Data curation, Formal analysis, Validation, Investigation, Visualization, Methodology, Writing - original draft, Writing - review and editing.
Conceptualization, Formal analysis, Validation, Investigation, Visualization, Methodology, Writing - original draft, Writing - review and editing.
Formal analysis, Validation, Investigation, Visualization, Methodology, Writing - original draft, Writing - review and editing.
Methodology, Writing - review and editing.
Methodology.
Conceptualization, Supervision, Funding acquisition, Writing - review and editing.
Conceptualization, Supervision, Funding acquisition, Writing - original draft, Project administration, Writing - review and editing.
Additional files
Data availability
Sequencing data have been deposited in GEO under accession code GSE141807.
The following dataset was generated:
Allen AM, Neville MC, Birtles S, Croset V, Treiber C, Waddell S, Goodwin SF. 2019. A single-cell transcriptomic atlas of the adult Drosophila ventral nerve cord. NCBI Gene Expression Omnibus. GSE141807
The following previously published datasets were used:
Leader DP, Krause SA, Pandit A, Davies SA, Dow JAT. 2018. FlyAtlas 2: a new version of the Drosophila melanogaster expression atlas with RNA-Seq, miRNA-Seq and sex-specific data. Omnics DI. PRJEB22205
References
- Agrawal T, Sadaf S, Hasan G. A genetic RNAi screen for IP₃/Ca²⁺ coupled GPCRs in Drosophila identifies the PdfR as a regulator of insect flight. PLOS Genet. 2013;9:e1003849. doi: 10.1371/journal.pgen.1003849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen AM. GitHub; 2020. https://github.com/aaron-allen/VNC_scRNAseq [Google Scholar]
- Anderson MG, Perkins GL, Chittick P, Shrigley RJ, Johnson WA. Drifter, a Drosophila POU-domain transcription factor, is required for correct differentiation and migration of tracheal cells and midline Glia. Genes & Development. 1995;9:123–137. doi: 10.1101/gad.9.1.123. [DOI] [PubMed] [Google Scholar]
- Angelini DR, Kaufman TC. Insect appendages and comparative ontogenetics. Developmental Biology. 2005;286:57–77. doi: 10.1016/j.ydbio.2005.07.006. [DOI] [PubMed] [Google Scholar]
- Baek M, Enriquez J, Mann RS. Dual role for hox genes and hox co-factors in conferring leg motoneuron survival and identity in Drosophila. Development. 2013;140:2027–2038. doi: 10.1242/dev.090902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baek M, Mann RS. Lineage and birth date specify motor neuron targeting and dendritic architecture in adult Drosophila. Journal of Neuroscience. 2009;29:6904–6916. doi: 10.1523/JNEUROSCI.1585-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barolo S, Carver LA, Posakony JW. GFP and beta-galactosidase transformation vectors for promoter/enhancer analysis in Drosophila. BioTechniques. 2000;29728:726–732. doi: 10.2144/00294bm10. [DOI] [PubMed] [Google Scholar]
- Bates AS, Janssens J, Jefferis GS, Aerts S. Neuronal cell types in the fly: single-cell anatomy meets single-cell genomics. Current Opinion in Neurobiology. 2019;56:125–134. doi: 10.1016/j.conb.2018.12.012. [DOI] [PubMed] [Google Scholar]
- Bidaye SS, Machacek C, Wu Y, Dickson BJ. Neuronal control of Drosophila walking direction. Science. 2014;344:97–101. doi: 10.1126/science.1249964. [DOI] [PubMed] [Google Scholar]
- Billeter JC, Villella A, Allendorfer JB, Dornan AJ, Richardson M, Gailey DA, Goodwin SF. Isoform-specific control of male neuronal differentiation and behavior in Drosophila by the fruitless gene. Current Biology. 2006;16:1063–1076. doi: 10.1016/j.cub.2006.04.039. [DOI] [PubMed] [Google Scholar]
- Birkholz O, Rickert C, Nowak J, Coban IC, Technau GM. Bridging the gap between postembryonic cell lineages and identified embryonic neuroblasts in the ventral nerve cord of Drosophila melanogaster. Biology Open. 2015;4:420–434. doi: 10.1242/bio.201411072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossing T, Udolph G, Doe CQ, Technau GM. The embryonic central nervous system lineages of Drosophila melanogaster I Neuroblast lineages derived from the ventral half of the neuroectoderm. Developmental Biology. 1996;179:41–64. doi: 10.1006/dbio.1996.0240. [DOI] [PubMed] [Google Scholar]
- Buchner E, Buchner S, Burg MG, Hofbauer A, Pak WL, Pollack I. Histamine is a major mechanosensory neurotransmitter candidate in Drosophila melanogaster. Cell and Tissue Research. 1993;273:119–125. doi: 10.1007/BF00304618. [DOI] [PubMed] [Google Scholar]
- Bürglin TR, Affolter M. Homeodomain proteins: an update. Chromosoma. 2016;125:497–521. doi: 10.1007/s00412-015-0543-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burrows M. Local circuits for the control of leg movements in an insect. Trends in Neurosciences. 1992;15:226–232. doi: 10.1016/0166-2236(92)90040-F. [DOI] [PubMed] [Google Scholar]
- Card G, Dickinson MH. Visually mediated motor planning in the escape response of Drosophila. Current Biology. 2008;18:1300–1307. doi: 10.1016/j.cub.2008.07.094. [DOI] [PubMed] [Google Scholar]
- Carrillo RA, Özkan E, Menon KP, Nagarkar-Jaiswal S, Lee PT, Jeon M, Birnbaum ME, Bellen HJ, Garcia KC, Zinn K. Control of synaptic connectivity by a network of Drosophila IgSF Cell Surface Proteins. Cell. 2015;163:1770–1782. doi: 10.1016/j.cell.2015.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavodeassi F, Modolell J, Gómez-Skarmeta JL. The iroquois family of genes: from body building to neural patterning. Development. 2001;128:2847–2855. doi: 10.1242/dev.128.15.2847. [DOI] [PubMed] [Google Scholar]
- Chang YH, Keegan RM, Prazak L, Dubnau J. Cellular labeling of endogenous retrovirus replication (CLEVR) reveals de novo insertions of the gypsy retrotransposable element in cell culture and in both neurons and glial cells of aging fruit flies. PLOS Biology. 2019;17:e3000278. doi: 10.1371/journal.pbio.3000278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Choi MS, Mizoguchi A, Veenstra JA, Kang K, Kim YJ, Kwon JY. Isoform-specific expression of the neuropeptide orcokinin in Drosophila melanogaster. Peptides. 2015;68:50–57. doi: 10.1016/j.peptides.2015.01.002. [DOI] [PubMed] [Google Scholar]
- Chen CL, Hermans L, Viswanathan MC, Fortun D, Aymanns F, Unser M, Cammarato A, Dickinson MH, Ramdya P. Imaging neural activity in the ventral nerve cord of behaving adult Drosophila. Nature Communications. 2018;9:4390. doi: 10.1038/s41467-018-06857-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chintapalli VR, Wang J, Herzyk P, Davies SA, Dow JA. Data-mining the FlyAtlas online resource to identify core functional motifs across transporting epithelia. BMC Genomics. 2013;14:518. doi: 10.1186/1471-2164-14-518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clyne PJ, Certel SJ, de Bruyne M, Zaslavsky L, Johnson WA, Carlson JR. The odor specificities of a subset of olfactory receptor neurons are governed by Acj6, a POU-domain transcription factor. Neuron. 1999;22:339–347. doi: 10.1016/S0896-6273(00)81094-6. [DOI] [PubMed] [Google Scholar]
- Clyne JD, Miesenböck G. Sex-specific control and tuning of the pattern generator for courtship song in Drosophila. Cell. 2008;133:354–363. doi: 10.1016/j.cell.2008.01.050. [DOI] [PubMed] [Google Scholar]
- Cognigni P, Felsenberg J, Waddell S. Do the right thing: neural network mechanisms of memory formation, expression and update in Drosophila. Current Opinion in Neurobiology. 2018;49:51–58. doi: 10.1016/j.conb.2017.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole SH, Carney GE, McClung CA, Willard SS, Taylor BJ, Hirsh J. Two functional but noncomplementing Drosophila tyrosine decarboxylase genes: distinct roles for neural tyramine and octopamine in female fertility. The Journal of Biological Chemistry. 2005;280:14948–14955. doi: 10.1074/jbc.M414197200. [DOI] [PubMed] [Google Scholar]
- Court RC, Armstrong J, Börner J, Card G, Costa M, Dickinson M, Duch C, Korff W, Mann R, Merritt D. A systematic nomenclature for the Drosophila ventral nervous system. bioRxiv. 2017 doi: 10.1101/122952. [DOI] [PMC free article] [PubMed]
- Crickmore MA, Vosshall LB. Opposing dopaminergic and GABAergic neurons control the duration and persistence of copulation in Drosophila. Cell. 2013;155:881–893. doi: 10.1016/j.cell.2013.09.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croset V, Treiber CD, Waddell S. Cellular diversity in the Drosophila midbrain revealed by single-cell transcriptomics. eLife. 2018;7:e34550. doi: 10.7554/eLife.34550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crow M, Gillis J. Single cell RNA-sequencing: replicability of cell types. Current Opinion in Neurobiology. 2019;56:69–77. doi: 10.1016/j.conb.2018.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crozatier M, Valle D, Dubois L, Ibnsouda S, Vincent A. Collier, a novel regulator of Drosophila head development, is expressed in a single mitotic domain. Current Biology. 1996;6:707–718. doi: 10.1016/S0960-9822(09)00452-7. [DOI] [PubMed] [Google Scholar]
- Cully DF, Paress PS, Liu KK, Schaeffer JM, Arena JP. Identification of a Drosophila melanogaster glutamate-gated chloride channel sensitive to the antiparasitic agent avermectin. Journal of Biological Chemistry. 1996;271:20187–20191. doi: 10.1074/jbc.271.33.20187. [DOI] [PubMed] [Google Scholar]
- Dason JS, Romero-Pozuelo J, Marin L, Iyengar BG, Klose MK, Ferrús A, Atwood HL. Frequenin/NCS-1 and the Ca2+-channel alpha1-subunit co-regulate synaptic transmission and nerve-terminal growth. Journal of Cell Science. 2009;122:4109–4121. doi: 10.1242/jcs.055095. [DOI] [PubMed] [Google Scholar]
- Davie K, Janssens J, Koldere D, De Waegeneer M, Pech U, Kreft Ł, Aibar S, Makhzami S, Christiaens V, Bravo González-Blas C, Poovathingal S, Hulselmans G, Spanier KI, Moerman T, Vanspauwen B, Geurs S, Voet T, Lammertyn J, Thienpont B, Liu S, Konstantinides N, Fiers M, Verstreken P, Aerts S. A Single-Cell transcriptome atlas of the aging Drosophila brain. Cell. 2018;174:982–998. doi: 10.1016/j.cell.2018.05.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis FP, Nern A, Picard S, Reiser MB, Rubin GM, Eddy SR, Henry GL. A genetic, genomic, and computational resource for exploring neural circuit function. eLife. 2020;9:e50901. doi: 10.7554/eLife.50901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deneris ES, Hobert O. Maintenance of postmitotic neuronal cell identity. Nature Neuroscience. 2014;17:899–907. doi: 10.1038/nn.3731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeSalvo MK, Hindle SJ, Rusan ZM, Orng S, Eddison M, Halliwill K, Bainton RJ. The Drosophila surface Glia transcriptome: evolutionary conserved blood-brain barrier processes. Frontiers in Neuroscience. 2014;8:346. doi: 10.3389/fnins.2014.00346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiAntonio A, Burgess RW, Chin AC, Deitcher DL, Scheller RH, Schwarz TL. Identification and characterization of Drosophila genes for synaptic vesicle proteins. The Journal of Neuroscience. 1993;13:4924–4935. doi: 10.1523/JNEUROSCI.13-11-04924.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doherty J, Logan MA, Taşdemir OE, Freeman MR. Ensheathing Glia function as phagocytes in the adult Drosophila brain. Journal of Neuroscience. 2009;29:4768–4781. doi: 10.1523/JNEUROSCI.5951-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enriquez J, Rio LQ, Blazeski R, Bellemin S, Godement P, Mason C, Mann RS. Differing strategies despite shared lineages of motor neurons and Glia to achieve robust development of an adult neuropil in Drosophila. Neuron. 2018;97:538–554. doi: 10.1016/j.neuron.2018.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estacio-Gómez A, Hassan A, Walmsley E, Lee L, Southall TD. Dynamic neurotransmitter specific transcription factor expression profiles during Drosophila development. bioRxiv. 2019 doi: 10.1101/830315. [DOI] [PMC free article] [PubMed]
- Etheredge J. Transcriptional Profiling of Drosophila Larval Ventral Nervous System Hemilineages and Neuroscience. University of Cambridge Development Physiology; 2017. [DOI] [Google Scholar]
- Gowda SBM, Paranjpe PD, Reddy OV, Thiagarajan D, Palliyil S, Reichert H, VijayRaghavan K. GABAergic inhibition of leg motoneurons is required for normal walking behavior in freely moving Drosophila. PNAS. 2018;115:E2115–E2124. doi: 10.1073/pnas.1713869115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greer CL, Grygoruk A, Patton DE, Ley B, Romero-Calderon R, Chang HY, Houshyar R, Bainton RJ, Diantonio A, Krantz DE. A splice variant of the Drosophila vesicular monoamine transporter contains a conserved trafficking domain and functions in the storage of dopamine, serotonin, and octopamine. Journal of Neurobiology. 2005;64:239–258. doi: 10.1002/neu.20146. [DOI] [PubMed] [Google Scholar]
- Grillner S. Biological pattern generation: the cellular and computational logic of networks in motion. Neuron. 2006;52:751–766. doi: 10.1016/j.neuron.2006.11.008. [DOI] [PubMed] [Google Scholar]
- Gu Z, Gu L, Eils R, Schlesner M, Brors B. Circlize implements and enhances circular visualization in R. Bioinformatics. 2014;30:2811–2812. doi: 10.1093/bioinformatics/btu393. [DOI] [PubMed] [Google Scholar]
- Hamanaka Y, Park D, Yin P, Annangudi SP, Edwards TN, Sweedler J, Meinertzhagen IA, Taghert PH. Transcriptional orchestration of the regulated secretory pathway in neurons by the bHLH protein DIMM. Current Biology. 2010;20:9–18. doi: 10.1016/j.cub.2009.11.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanlon CD, Andrew DJ. Outside-in signaling--a brief review of GPCR signaling with a focus on the Drosophila GPCR family. Journal of Cell Science. 2015;128:3533–3542. doi: 10.1242/jcs.175158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardie RC. Is histamine a neurotransmitter in insect photoreceptors? Journal of Comparative Physiology A. 1987;161:201–213. doi: 10.1007/BF00615241. [DOI] [PubMed] [Google Scholar]
- Harris RM, Pfeiffer BD, Rubin GM, Truman JW. Neuron hemilineages provide the functional ground plan for the Drosophila ventral nervous system. eLife. 2015;4:e09443. doi: 10.7554/eLife.04493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartenstein V, Cruz L, Lovick JK, Guo M. Developmental analysis of the dopamine-containing neurons of the Drosophila brain. The Journal of Comparative Neurology. 2017;525:363–379. doi: 10.1002/cne.24069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartenstein V. Development of the Nervous System of Invertebrates. In: Byrne J. H, editor. The Oxford Handbook of Invertebrate Neurobiology. Oxford University Press; 2018. [DOI] [Google Scholar]
- Hewes RS, Park D, Gauthier SA, Schaefer AM, Taghert PH. The bHLH protein dimmed controls neuroendocrine cell differentiation in Drosophila. Development. 2003;130:1771–1781. doi: 10.1242/dev.00404. [DOI] [PubMed] [Google Scholar]
- Hobert O, Kratsios P. Neuronal identity control by terminal selectors in worms, flies, and chordates. Current Opinion in Neurobiology. 2019;56:97–105. doi: 10.1016/j.conb.2018.12.006. [DOI] [PubMed] [Google Scholar]
- Honegger B, Galic M, Köhler K, Wittwer F, Brogiolo W, Hafen E, Stocker H. Imp-L2, a putative homolog of vertebrate IGF-binding protein 7, counteracts insulin signaling in Drosophila and is essential for starvation resistance. Journal of Biology. 2008;7:10. doi: 10.1186/jbiol72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard CE, Chen CL, Tabachnik T, Hormigo R, Ramdya P, Mann RS. Serotonergic modulation of walking in Drosophila. Current Biology. 2019;29:4218–4230. doi: 10.1016/j.cub.2019.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang daW, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nature Protocols. 2009;4:44–57. doi: 10.1038/nprot.2008.211. [DOI] [PubMed] [Google Scholar]
- Huang Y, Ng FS, Jackson FR. Comparison of larval and adult drosophila astrocytes reveals stage-specific gene expression profiles. G3: Genes, Genomes, Genetics. 2015;5:551–558. doi: 10.1534/g3.114.016162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito K, Shinomiya K, Ito M, Armstrong JD, Boyan G, Hartenstein V, Harzsch S, Heisenberg M, Homberg U, Jenett A, Keshishian H, Restifo LL, Rössler W, Simpson JH, Strausfeld NJ, Strauss R, Vosshall LB, Insect Brain Name Working Group A systematic nomenclature for the insect brain. Neuron. 2014;81:755–765. doi: 10.1016/j.neuron.2013.12.017. [DOI] [PubMed] [Google Scholar]
- Jois S, Chan YB, Fernandez MP, Leung AK. Characterization of the sexually dimorphic fruitless Neurons That Regulate Copulation Duration. Frontiers in Physiology. 2018;9:780. doi: 10.3389/fphys.2018.00780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonsson T, Kravitz EA, Heinrich R. Sound production during agonistic behavior of male Drosophila Melanogaster. Fly. 2011;5:29–38. doi: 10.4161/fly.5.1.13713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph RM, Carlson JR. Drosophila chemoreceptors: a molecular interface between the chemical world and the brain. Trends in Genetics. 2015;31:683–695. doi: 10.1016/j.tig.2015.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karaiskos N, Wahle P, Alles J, Boltengagen A, Ayoub S, Kipar C, Kocks C, Rajewsky N, Zinzen RP. The Drosophila embryo at single-cell transcriptome resolution. Science. 2017;358:194–199. doi: 10.1126/science.aan3235. [DOI] [PubMed] [Google Scholar]
- Kerner P, Ikmi A, Coen D, Vervoort M. Evolutionary history of the iroquois/Irx genes in metazoans. BMC Evolutionary Biology. 2009;9:74. doi: 10.1186/1471-2148-9-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikuta S, Hagiwara-Komoda Y, Noda H, Kikawada T. A novel member of the trehalose transporter family functions as an h(+)-dependent trehalose transporter in the reabsorption of trehalose in malpighian tubules. Frontiers in Physiology. 2012;3:290. doi: 10.3389/fphys.2012.00290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim B, Shortridge RD, Seong C, Oh Y, Baek K, Yoon J. Molecular characterization of a novel Drosophila gene which is expressed in the central nervous system. Molecules and Cells. 1998;8:750–757. [PubMed] [Google Scholar]
- Kim H, Kirkhart C, Scott K. Long-range projection neurons in the taste circuit of Drosophila. eLife. 2017;6:e23386. doi: 10.7554/eLife.23386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura K, Sato C, Koganezawa M, Yamamoto D. Drosophila ovipositor extension in mating behavior and egg deposition involves distinct sets of brain interneurons. PLOS ONE. 2015;10:e0126445. doi: 10.1371/journal.pone.0126445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knauf F, Rogina B, Jiang Z, Aronson PS, Helfand SL. Functional characterization and immunolocalization of the transporter encoded by the life-extending gene indy. PNAS. 2002;99:14315–14319. doi: 10.1073/pnas.222531899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konstantinides N, Kapuralin K, Fadil C, Barboza L, Satija R, Desplan C. Phenotypic convergence: distinct transcription factors regulate common terminal features. Cell. 2018;174:622–635. doi: 10.1016/j.cell.2018.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacin H, Chen HM, Long X, Singer RH, Lee T, Truman JW. Neurotransmitter identity is acquired in a lineage-restricted manner in the Drosophila CNS. eLife. 2019;8:e43701. doi: 10.7554/eLife.43701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacin H, Truman JW. Lineage mapping identifies molecular and architectural similarities between the larval and adult Drosophila central nervous system. eLife. 2016;5:e13399. doi: 10.7554/eLife.13399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laughlin SB, de Ruyter van Steveninck RR, Anderson JC. The metabolic cost of neural information. Nature Neuroscience. 1998;1:36–41. doi: 10.1038/236. [DOI] [PubMed] [Google Scholar]
- Leader DP, Krause SA, Pandit A, Davies SA, Dow JAT. FlyAtlas 2: a new version of the Drosophila melanogaster expression atlas with RNA-Seq, miRNA-Seq and sex-specific data. Nucleic Acids Research. 2018;46:D809–D815. doi: 10.1093/nar/gkx976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee G, Villella A, Taylor BJ, Hall JC. New reproductive anomalies in fruitless-mutant Drosophila males: extreme lengthening of mating durations and infertility correlated with defective serotonergic innervation of reproductive organs. Journal of Neurobiology. 2001;47:121–149. doi: 10.1002/neu.1021. [DOI] [PubMed] [Google Scholar]
- Lee KM, Daubnerová I, Isaac RE, Zhang C, Choi S, Chung J, Kim YJ. A neuronal pathway that controls sperm ejection and storage in female Drosophila. Current Biology. 2015;25:790–797. doi: 10.1016/j.cub.2015.01.050. [DOI] [PubMed] [Google Scholar]
- Lee PT, Zirin J, Kanca O, Lin WW, Schulze KL, Li-Kroeger D, Tao R, Devereaux C, Hu Y, Chung V, Fang Y, He Y, Pan H, Ge M, Zuo Z, Housden BE, Mohr SE, Yamamoto S, Levis RW, Spradling AC, Perrimon N, Bellen HJ. A gene-specific T2A-GAL4 library for Drosophila. eLife. 2018;7:e35574. doi: 10.7554/eLife.35574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee G, Hall JC. Abnormalities of male-specific FRU protein and serotonin expression in the CNS of fruitless mutants in Drosophila. The Journal of Neuroscience. 2001;21:513–526. doi: 10.1523/JNEUROSCI.21-02-00513.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Horns F, Wu B, Xie Q, Li J, Li T, Luginbuhl DJ, Quake SR, Luo L. Classifying Drosophila olfactory projection neuron subtypes by Single-Cell RNA sequencing. Cell. 2017;171:1206–1220. doi: 10.1016/j.cell.2017.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limmer S, Weiler A, Volkenhoff A, Babatz F, Klämbt C. The Drosophila blood-brain barrier: development and function of a glial endothelium. Frontiers in Neuroscience. 2014;8:365. doi: 10.3389/fnins.2014.00365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Littleton JT, Ganetzky B. Ion channels and synaptic organization: analysis of the Drosophila genome. Neuron. 2000;26:35–43. doi: 10.1016/s0896-6273(00)81135-6. [DOI] [PubMed] [Google Scholar]
- Macosko EZ, Basu A, Satija R, Nemesh J, Shekhar K, Goldman M, Tirosh I, Bialas AR, Kamitaki N, Martersteck EM, Trombetta JJ, Weitz DA, Sanes JR, Shalek AK, Regev A, McCarroll SA. Highly parallel Genome-wide expression profiling of individual cells using nanoliter droplets. Cell. 2015;161:1202–1214. doi: 10.1016/j.cell.2015.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamiya A, Gurung P, Tuthill JC. Neural coding of leg proprioception in Drosophila. Neuron. 2018;100:636–650. doi: 10.1016/j.neuron.2018.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin CA, Krantz DE. Drosophila melanogaster as a genetic model system to study neurotransmitter transporters. Neurochemistry International. 2014;73:71–88. doi: 10.1016/j.neuint.2014.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurange C, Cheng L, Gould AP. Temporal transcription factors and their targets schedule the end of neural proliferation in Drosophila. Cell. 2008;133:891–902. doi: 10.1016/j.cell.2008.03.034. [DOI] [PubMed] [Google Scholar]
- McGinnis W, Krumlauf R. Homeobox genes and axial patterning. Cell. 1992;68:283–302. doi: 10.1016/0092-8674(92)90471-N. [DOI] [PubMed] [Google Scholar]
- McIlroy G, Foldi I, Aurikko J, Wentzell JS, Lim MA, Fenton JC, Gay NJ, Hidalgo A. Toll-6 and Toll-7 function as neurotrophin receptors in the Drosophila Melanogaster CNS. Nature Neuroscience. 2013;16:1248–1256. doi: 10.1038/nn.3474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendes CS, Bartos I, Akay T, Márka S, Mann RS. Quantification of gait parameters in freely walking wild type and sensory deprived Drosophila Melanogaster. eLife. 2013;2:e00231. doi: 10.7554/eLife.00231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendes CS, Rajendren SV, Bartos I, Márka S, Mann RS. Kinematic responses to changes in walking orientation and gravitational load in Drosophila Melanogaster. PLOS ONE. 2014;9:e0109204. doi: 10.1371/journal.pone.0109204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monastirioti M, Linn CE, White K. Characterization of Drosophila tyramine beta-hydroxylase gene and isolation of mutant flies lacking octopamine. The Neurosci. 1996;16:3900–3911. doi: 10.1523/JNEUROSCI.16-12-03900.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Namiki S, Dickinson MH, Wong AM, Korff W, Card GM. The functional organization of descending sensory-motor pathways in Drosophila. eLife. 2018;7:e34272. doi: 10.7554/eLife.34272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nässel DR, Holmqvist MH, Hardie RC, Håkanson R, Sundler F. Histamine-like immunoreactivity in photoreceptors of the compound eyes and ocelli of the flies Calliphora erythrocephala and Musca Domestica. Cell and Tissue Research. 1988;253:639–646. doi: 10.1007/BF00219755. [DOI] [PubMed] [Google Scholar]
- Nässel DR, Pirvola U, Panula P. Histaminelike immunoreactive neurons innervating putative neurohaemal Areas and central neuropil in the thoraco-abdominal ganglia of the flies Drosophila and Calliphora. Journal of Comparative Neurology. 1990;297:525–536. doi: 10.1002/cne.902970406. [DOI] [PubMed] [Google Scholar]
- Nässel DR. Substrates for neuronal cotransmission with neuropeptides and small molecule neurotransmitters in Drosophila. Frontiers in Cellular Neuroscience. 2018;12:83. doi: 10.3389/fncel.2018.00083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nässel DR, Zandawala M. Recent advances in Neuropeptide signaling in Drosophila, from genes to physiology and behavior. Progress in Neurobiology. 2019;179:101607. doi: 10.1016/j.pneurobio.2019.02.003. [DOI] [PubMed] [Google Scholar]
- Niven JE, Graham CM, Burrows M. Diversity and evolution of the insect ventral nerve cord. Annual Review of Entomology. 2008;53:253–271. doi: 10.1146/annurev.ento.52.110405.091322. [DOI] [PubMed] [Google Scholar]
- Noordermeer JN, Kopczynski CC, Fetter RD, Bland KS, Chen WY, Goodman CS. Wrapper, a novel member of the ig superfamily, is expressed by midline Glia and is required for them to ensheath commissural axons in Drosophila. Neuron. 1998;21:991–1001. doi: 10.1016/S0896-6273(00)80618-2. [DOI] [PubMed] [Google Scholar]
- Nusbaum MP, Blitz DM, Marder E. Functional consequences of neuropeptide and small-molecule co-transmission. Nature Reviews Neuroscience. 2017;18:389–403. doi: 10.1038/nrn.2017.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overend G, Luo Y, Henderson L, Douglas AE, Davies SA, Dow JA. Molecular mechanism and functional significance of acid generation in the Drosophila midgut. Scientific Reports. 2016;6:27242. doi: 10.1038/srep27242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Özkan E, Carrillo RA, Eastman CL, Weiszmann R, Waghray D, Johnson KG, Zinn K, Celniker SE, Garcia KC. An extracellular interactome of immunoglobulin and LRR proteins reveals receptor-ligand networks. Cell. 2013;154:228–239. doi: 10.1016/j.cell.2013.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan Y, Robinett CC, Baker BS. Turning males on: activation of male courtship behavior in Drosophila Melanogaster. PLOS ONE. 2011;6:e21144. doi: 10.1371/journal.pone.0021144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park D, Veenstra JA, Park JH, Taghert PH. Mapping peptidergic cells in Drosophila: where DIMM fits in. PLOS ONE. 2008;3:e1896. doi: 10.1371/journal.pone.0001896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauls D, Chen J, Reiher W, Vanselow JT, Schlosser A, Kahnt J, Wegener C. Peptidomics and processing of regulatory peptides in the fruit fly Drosophila. EuPA Open Proteomics. 2014;3:114–127. doi: 10.1016/j.euprot.2014.02.007. [DOI] [Google Scholar]
- Pauls D, Blechschmidt C, Frantzmann F, El Jundi B, Selcho M. A comprehensive anatomical map of the peripheral octopaminergic/tyraminergic system of Drosophila Melanogaster. Scientific Reports. 2018;8:15314. doi: 10.1038/s41598-018-33686-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlou HJ, Lin AC, Neville MC, Nojima T, Diao F, Chen BE, White BH, Goodwin SF. Neural circuitry coordinating male copulation. eLife. 2016;5:e20713. doi: 10.7554/eLife.20713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philippidou P, Dasen JS. Hox genes: choreographers in neural development, architects of circuit organization. Neuron. 2013;80:12–34. doi: 10.1016/j.neuron.2013.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pipes GC, Lin Q, Riley SE, Goodman CS. The beat generation: a multigene family encoding IgSF proteins related to the beat axon guidance molecule in Drosophila. Development. 2001;128:4545–4552. doi: 10.1242/dev.128.22.4545. [DOI] [PubMed] [Google Scholar]
- Prokop A, Technau GM. The origin of postembryonic neuroblasts in the ventral nerve cord of Drosophila Melanogaster. Development. 1991;111:79–88. doi: 10.1242/dev.111.1.79. [DOI] [PubMed] [Google Scholar]
- Rezával C, Nojima T, Neville MC, Lin AC, Goodwin SF. Sexually dimorphic octopaminergic neurons modulate female postmating behaviors in Drosophila. Current Biology. 2014;24:725–730. doi: 10.1016/j.cub.2013.12.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rideout EJ, Dornan AJ, Neville MC, Eadie S, Goodwin SF. Control of sexual differentiation and behavior by the doublesex gene in Drosophila Melanogaster. Nature Neuroscience. 2010;13:458–466. doi: 10.1038/nn.2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ro J, Pak G, Malec PA, Lyu Y, Allison DB, Kennedy RT, Pletcher SD. Serotonin signaling mediates protein valuation and aging. eLife. 2016;5:e16843. doi: 10.7554/eLife.16843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinow S, Campos AR, Yao KM, White K. The elav gene product of Drosophila, required in neurons, has three RNP consensus motifs. Science. 1988;242:1570–1572. doi: 10.1126/science.3144044. [DOI] [PubMed] [Google Scholar]
- Satija R, Farrell JA, Gennert D, Schier AF, Regev A. Spatial reconstruction of single-cell gene expression data. Nature Biotechnology. 2015;33:495–502. doi: 10.1038/nbt.3192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid A, Chiba A, Doe CQ. Clonal analysis of Drosophila embryonic neuroblasts: neural cell types, axon projections and muscle targets. Development. 1999;126:4653–4689. doi: 10.1242/dev.126.21.4653. [DOI] [PubMed] [Google Scholar]
- Schor IE, Bussotti G, Maleš M, Forneris M, Viales RR, Enright AJ, Furlong EEM. Non-coding RNA expression, function, and variation during Drosophila Embryogenesis. Current Biology. 2018;28:3547–3561. doi: 10.1016/j.cub.2018.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schützler N, Girwert C, Hügli I, Mohana G, Roignant JY, Ryglewski S, Duch C. Tyramine action on motoneuron excitability and adaptable tyramine/octopamine ratios adjust Drosophila locomotion to nutritional state. PNAS. 2019;116:3805–3810. doi: 10.1073/pnas.1813554116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scruggs BS, Gilchrist DA, Nechaev S, Muse GW, Burkholder A, Fargo DC, Adelman K. Bidirectional transcription arises from two distinct hubs of transcription factor binding and active chromatin. Molecular Cell. 2015;58:1101–1112. doi: 10.1016/j.molcel.2015.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeds AM, Ravbar P, Chung P, Hampel S, Midgley FM, Mensh BD, Simpson JH. A suppression hierarchy among competing motor programs drives sequential grooming in Drosophila. eLife. 2014;3:e02951. doi: 10.7554/eLife.02951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sellami A, Agricola HJ, Veenstra JA. Neuroendocrine cells in Drosophila Melanogaster producing GPA2/GPB5, a hormone with homology to LH, FSH and TSH. General and Comparative Endocrinology. 2011;170:582–588. doi: 10.1016/j.ygcen.2010.11.015. [DOI] [PubMed] [Google Scholar]
- Shepherd D, Harris R, Williams DW, Truman JW. Postembryonic lineages of the Drosophila ventral nervous system: neuroglian expression reveals the adult hemilineage associated fiber tracts in the adult thoracic neuromeres. Journal of Comparative Neurology. 2016;524:2677–2695. doi: 10.1002/cne.23988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd D, Sahota V, Court R, Williams DW, Truman JW. Developmental organization of central neurons in the adult Drosophila ventral nervous system. Journal of Comparative Neurology. 2019;527:2573–2598. doi: 10.1002/cne.24690. [DOI] [PubMed] [Google Scholar]
- Shih M-FM, Davis FP, Henry GL, Dubnau J. Nuclear Transcriptomes of the Seven Neuronal Cell Types That Constitute the Drosophila Mushroom Bodies. G3: Genes, Genomes, Genetics. 2019;9:81–94. doi: 10.1534/g3.118.200726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirangi TR, Stern DL, Truman JW. Motor control of Drosophila courtship song. Cell Reports. 2013;5:678–686. doi: 10.1016/j.celrep.2013.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sievers F, Higgins DG. Clustal omega for making accurate alignments of many protein sequences. Protein Science. 2018;27:135–145. doi: 10.1002/pro.3290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smarandache-Wellmann CR. Arthropod neurons and nervous system. Current Biology. 2016;26:R960–R965. doi: 10.1016/j.cub.2016.07.063. [DOI] [PubMed] [Google Scholar]
- Smith SJ, Sümbül U, Graybuck LT, Collman F, Seshamani S, Gala R, Gliko O, Elabbady L, Miller JA, Bakken TE, Rossier J, Yao Z, Lein E, Zeng H, Tasic B, Hawrylycz M. Single-cell transcriptomic evidence for dense intracortical neuropeptide networks. eLife. 2019;8:e47889. doi: 10.7554/eLife.47889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahl BA, Peco E, Davla S, Murakami K, Caicedo Moreno NA, van Meyel DJ, Keene AC. The taurine transporter Eaat2 functions in ensheathing Glia to modulate sleep and metabolic rate. Current Biology. 2018;28:3700–3708. doi: 10.1016/j.cub.2018.10.039. [DOI] [PubMed] [Google Scholar]
- Suska A, Miguel-Aliaga I, Thor S. Segment-specific generation of Drosophila capability neuropeptide neurons by multi-faceted hox cues. Developmental Biology. 2011;353:72–80. doi: 10.1016/j.ydbio.2011.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tayler TD, Pacheco DA, Hergarden AC, Murthy M, Anderson DJ. A neuropeptide circuit that coordinates sperm transfer and copulation duration in Drosophila. PNAS. 2012;109:20697–20702. doi: 10.1073/pnas.1218246109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thor S, Andersson SG, Tomlinson A, Thomas JB. A LIM-homeodomain combinatorial code for motor-neuron pathway selection. Nature. 1999;397:76–80. doi: 10.1038/16275. [DOI] [PubMed] [Google Scholar]
- Truman JW, Schuppe H, Shepherd D, Williams DW. Developmental architecture of adult-specific lineages in the ventral CNS of Drosophila. Development. 2004;131:5167–5184. doi: 10.1242/dev.01371. [DOI] [PubMed] [Google Scholar]
- Truman JW, Moats W, Altman J, Marin EC, Williams DW. Role of notch signaling in establishing the hemilineages of secondary neurons in Drosophila melanogaster. Development. 2010;137:53–61. doi: 10.1242/dev.041749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truman JW, Bate M. Spatial and temporal patterns of neurogenesis in the central nervous system of Drosophila Melanogaster. Developmental Biology. 1988;125:145–157. doi: 10.1016/0012-1606(88)90067-X. [DOI] [PubMed] [Google Scholar]
- Tsubouchi A, Yano T, Yokoyama TK, Murtin C, Otsuna H, Ito K. Topological and modality-specific representation of somatosensory information in the fly brain. Science. 2017;358:615–623. doi: 10.1126/science.aan4428. [DOI] [PubMed] [Google Scholar]
- Tuthill JC, Wilson RI. Parallel Transformation of Tactile Signals in Central Circuits of Drosophila. Cell. 2016;164:1046–1059. doi: 10.1016/j.cell.2016.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Maaten L. Accelerating t-SNE using Tree-Based algorithms. Journal of Machine Learning Research. 2014;15:3221–3245. [Google Scholar]
- Venkatasubramanian L, Mann RS. The development and assembly of the Drosophila adult ventral nerve cord. Current Opinion in Neurobiology. 2019;56:135–143. doi: 10.1016/j.conb.2019.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkenhoff A, Weiler A, Letzel M, Stehling M, Klämbt C, Schirmeier S. Glial glycolysis is essential for neuronal survival in Drosophila. Cell Metabolism. 2015;22:437–447. doi: 10.1016/j.cmet.2015.07.006. [DOI] [PubMed] [Google Scholar]
- von Philipsborn AC, Liu T, Yu JY, Masser C, Bidaye SS, Dickson BJ. Neuronal control of Drosophila courtship song. Neuron. 2011;69:509–522. doi: 10.1016/j.neuron.2011.01.011. [DOI] [PubMed] [Google Scholar]
- Wilk R, Hu J, Blotsky D, Krause HM. Diverse and pervasive subcellular distributions for both coding and long noncoding RNAs. Genes & Development. 2016;30:594–609. doi: 10.1101/gad.276931.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams MJ, Goergen P, Rajendran J, Klockars A, Kasagiannis A, Fredriksson R, Schiöth HB. Regulation of aggression by obesity-linked genes TfAP-2 and twz through octopamine signaling in Drosophila. Genetics. 2014;196:349–362. doi: 10.1534/genetics.113.158402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witt E, Benjamin S, Svetec N, Zhao L. Testis single-cell RNA-seq reveals the dynamics of de novo gene transcription and germline mutational Bias in Drosophila. eLife. 2019;8:e47138. doi: 10.7554/eLife.47138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wosnitza A, Bockemühl T, Dübbert M, Scholz H, Büschges A. Inter-leg coordination in the control of walking speed in Drosophila. Journal of Experimental Biology. 2013;216:480–491. doi: 10.1242/jeb.078139. [DOI] [PubMed] [Google Scholar]
- Xiong WC, Okano H, Patel NH, Blendy JA, Montell C. Repo encodes a glial-specific homeo domain protein required in the Drosophila nervous system. Genes & Development. 1994;8:981–994. doi: 10.1101/gad.8.8.981. [DOI] [PubMed] [Google Scholar]
- Yang CP, Samuels TJ, Huang Y, Yang L, Ish-Horowicz D, Davis I, Lee T. Imp and syp RNA-binding proteins govern decommissioning of Drosophila neural stem cells. Development. 2017;144:3454–3464. doi: 10.1242/dev.149500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yellman C, Tao H, He B, Hirsh J. Conserved and sexually dimorphic behavioral responses to biogenic amines in decapitated Drosophila. PNAS. 1997;94:4131–4136. doi: 10.1073/pnas.94.8.4131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zappia L, Oshlack A. Clustering trees: a visualization for evaluating clusterings at multiple resolutions. GigaScience. 2018;7:083. doi: 10.1093/gigascience/giy083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou B, Williams DW, Altman J, Riddiford LM, Truman JW. Temporal patterns of broad isoform expression during the development of neuronal lineages in Drosophila. Neural Development. 2009;4:39. doi: 10.1186/1749-8104-4-39. [DOI] [PMC free article] [PubMed] [Google Scholar]