Abstract
Background:
Surveys suggest that most research participants desire access to secondary (incidental) genomic findings. However, few studies clarify whether preferences vary by the nature of the finding.
Methods:
We surveyed members of the Jackson Heart Study (JHS, n=960), the Framingham Heart Study (FHS, n=955), and African-American members of the FHS Omni cohort (n=160) who had consented to genomic studies. Each factorial survey included 3 vignettes, randomly selected from a set of 64, that described a secondary genomic result. Vignettes varied systematically by 5 factors identified by expert panels as salient: phenotype severity, actionability (preventability), reproductive significance, and relative and absolute risk of the phenotype. Respondents indicated whether they would want to receive the result. Data were analyzed separately by cohort using generalized linear mixed models.
Results:
Response rates ranged from 67-73%. Across vignettes, 88–92% of respondents would definitely or probably want to learn the result. In multivariate analyses among JHS respondents, desire for results was associated with positive attitudes towards genetic testing, lower education, higher subjective numeracy, and younger age, but not with any of the 5 factors. Among FHS respondents, desire for results was associated with higher absolute risk, preventability, reproductive risk, and positive attitudes towards genetic testing. Among FHS Omni respondents, desire for results was associated with positive attitudes towards genetic testing and younger age.
Conclusions:
Most genetic research participants desire return of secondary genetic results. Several factors identified by expert panels as salient are associated with preferences among FHS, but not JHS or FHS Omni, participants.
Keywords: genetics, ethics, cohort study, return of research results, secondary findings
Journal Subject Terms: Ethics and Policy, Genetics
Introduction
The increasing use of genomic technologies for discovery and translational research confronts investigators, institutional review boards (IRBs), policymakers and the public with a dilemma: should investigators offer to return clinically informative secondary (or incidental) findings—defined by Wolf et al as “a finding concerning an individual research participant that has potential health or reproductive importance and is discovered in the course of conducting research but is beyond the aims of the study”1–to study participants? The ascendant view, including from panels convened by the National Heart, Lung, and Blood Institute (NHLBI), is that investigators are ethically obligated to offer participants access to a subset of “actionable” genomic findings1-14 or to recontact participants if initial interpretations subsequently change.15 Controversy persists, however, as some take a more cautious view on ethical, legal, logistical, or cost grounds.16-18 Several authors warn against transposing ethical duties appropriate to the clinical context to the research setting, arguing for more limited obligations in the latter case.19, 20
Empirical data on study participants’ and the public’s views regarding return of secondary genomic findings, derived from both quantitative and qualitative studies, indicate strong support for access to results.21-25 Data further suggest that the offer of return of individual results is associated with increased willingness among members of the public to participate in genetic research.26 More research is needed, however, to understand the basis for the desire for return of results and to inform relevant policies.27 Few studies have sought to clarify whether participants’ and the public’s preferences for access to results vary depending on the nature of the particular finding.22, 25, 28-31
To address these questions, we conducted a mailed survey of participants in two long-standing cardiovascular disease cohort studies, the Framingham Heart Study (FHS) and the Jackson Heart Study (JHS), who had agreed to the use of their samples for genetic research. Through the survey, we sought to elucidate participants’ views on return of secondary genomic findings. In particular, we sought to understand whether factors identified by consensus panels as relevant to decisions about return of genetic results, such as phenotype severity and actionability (preventability), influence participants’ desires to receive results.4, 7 We focused on these factors because guidance from professional societies and funding agencies identifies them as central to decisions and policies regarding return, and yet whether they map onto participants’ preferences remains unclear. We also examined whether sociodemographic and attitudinal variables are associated with the desire for results. Qualitatively, we anticipated finding that: (1) the association of two factors, phenotype severity and preventability, with participants’ desire for results are stronger than those of absolute risk, relative risk or reproductive significance; (2) the association of severity with desire for results is greater when the condition is preventable than when it is not; and (3) attitudinal characteristics of participants, such as favorable attitudes toward and greater knowledge about genetic testing, correlate positively with desire for results.
Methods
In accordance with the Journal’s Transparency and Openness Promotion (TOP) Guidelines, the JHS and FHS Coordinating Centers will post their respective data from this study with the NHLBI Biologic Specimen and Data Repository Information Coordinating Center (https://biolincc.nhlbi.nih.gov/home/).
The study was approved by the IRBs at the Dana-Farber Cancer Institute, Education Development Center, Jackson State University, and Boston University. Consistent with the requirements of the Common Rule, return of a completed questionnaire was considered evidence of consent.
See the supplemental material for a detailed description of study methods and for a representative survey booklet.
Results
Description of Study Samples
Response rates were 67%, 73%, and 71%, among JHS, FHS, and FHS Omni participants, respectively. There were no statistically significant age or sex differences between JHS respondents and non-respondents. FHS respondents were slightly older than non-respondents (mean age 58 vs. 56 years), but did not differ by sex. Omni respondents were older than non-respondents (mean age 64 vs. 60 years), but did not differ by sex.
Table 1 reports the demographic and attitudinal characteristics of respondents. Fewer than 15% of respondents in each cohort reported prior personal experience with genetic testing, but most respondents reported positive attitudes towards testing (medians 4.3-5.0/5 for all cohorts). Knowledge of genetic testing was moderate, with mean scores of 3.5/7 (JHS), 4.1/7 (FHS) and 3.9/7 (Omni).
Table 1:
Characteristics of participants by site
| Characteristic | JHS (n=960) |
FHS (n=955) |
FHS Omni (n=160) |
|---|---|---|---|
| Age | |||
| N (% of total) with valid response* | 956 (99%) | 955 (100%) | 160 (100%) |
| Mean Age (standard deviation) | 63 (12) | 58 (14) | 64 (13) |
| Median Age (minimum-maximum) | 63 (29-95) | 58 (27-93) | 65 (27-89) |
| Gender | |||
| N (% of total) with valid response | 956 (99%) | 955 (100%) | 160 (100%) |
| Female | 66.2% | 55.2% | 61.9% |
| Race† | |||
| N (% of total) with valid response | 960 (100%) | 931 (97%) | 155 (97%) |
| African-American | 100% | 0% | 88.4% |
| White | 0 | 98.7% | 0.6% |
| Other‡ | 0 | 1.3% | 11.0% |
| Ethnicity | |||
| N (% of total) with valid response | 879 (92%) | 928 (97%) | 149 (93%) |
| Hispanic | 2.5% | 1.9% | 0.7% |
| Education | |||
| N (% of total) with valid response | 921 (96%) | 947 (99%) | 154 |
| High school graduate or less | 31.4% | 18.2% | 9.1% |
| Some college or technical school | 22.7% | 21.1% | 15.6% |
| College graduate or higher | 45.9% | 60.7% | 75.3% |
| Employment Status | |||
| N (% of total) with valid response | 927 (97%) | 939 (98%) | 156 (98%) |
| Employed | 41.9% | 59.0% | 43.6% |
| Retired | 38.8% | 30.1% | 48.7% |
| Other§ | 19.3% | 10.9% | 7.7% |
| Marital Status | |||
| N (% of total) with valid response | 913 (95%) | 940 (98%) | 151 (94%) |
| Single, never married | 11.5% | 7.9% | 10.6% |
| Married or living with partner | 48.5% | 75.0% | 55.6% |
| Divorced or separated | 20.4% | 10.3% | 19.9% |
| Widowed | 19.6% | 6.8% | 13.9% |
| Children | |||
| N (% of total) with valid response | 930 (97%) | 943 (99%) | 157 (98%) |
| Yes | 89.6% | 80.5% | 86.0% |
| Attendance at religious activities | |||
| N (% of total) with valid response | 901 (94%) | 888 (93%) | 151 (94%) |
| Nearly every day | 19.6% | 2.6% | 9.3% |
| At least once a week | 59.6% | 20.4% | 43.7% |
| Few times a month | 14.1% | 12.2% | 9.9% |
| Few times a year | 4.8% | 27.1% | 13.9% |
| Less than once a year or not at all | 1.9% | 37.7% | 23.2% |
| Religious preference | |||
| N (% of total) with valid response∥ | 960 (100%) | 955 (100%) | 160 (100%) |
| Protestant, Baptist | 59.4% | 4.7% | 29.4% |
| Protestant, Mainline | 13.2% | 10.1% | 22.5% |
| Protestant, other | 5.1% | 6.2% | 11.2% |
| Catholic | 2.5% | 51.4% | 8.1% |
| Other | 11.4% | 8.1% | 10.0% |
| None | 1.4% | 12.7% | 12.5% |
| Private | 4.4% | 5.8% | 3.8% |
| No response | 2.7% | 1.1% | 2.5% |
| Personal knowledge of someone with | |||
| N (% of total) with valid response | 941 (98%) | 953 (100%) | 158 (99%) |
| Blood Clot | 53.9% | 57.1% | 55.7% |
| N (% of total) with valid response | 937 (98%) | 946 (99%) | 159 (99%) |
| Alzheimer Disease | 67.0% | 77.4% | 79.3% |
| Personal experience with genetic testing | |||
| N (% of total) with valid response | 941 (98%) | 939 (98%) | 157 (98%) |
| Yes | 13.4% | 8.2% | 5.7% |
| Attitude toward genetic testing (continuous, range 1-5) | |||
| N (% of total) with valid response | 935 (97%) | 935 (98%) | 157 (98%) |
| Mean (standard deviation) | 4.4 (0.9) | 4.4 (0.8) | 4.1 (0.9) |
| Median (25th, 75th percentile) | 5.0 (3.7, 5.0) | 4.7 (4.0, 5.0) | 4.3 (3.3, 5.0) |
| Knowledge of genetic testing (continuous, range 0-7) | |||
| N (% of total) with valid response | 936 (97%) | 937 (98%) | 157 (98%) |
| Mean (standard deviation) | 3.5 (1.7) | 4.1 (1.5) | 3.9 (1.7) |
| Subjective numeracy (continuous, range 1-6) | |||
| N (% of total) with valid response | 944 (98%) | 945 (99%) | 159 (99%) |
| Mean (standard deviation) | 3.5 (1.3) | 4.3 (1.2) | 4.1 (1.3) |
| Median (25th, 75th percentile) | 3.7 (2.5, 4.5) | 4.5 (3.5, 5.3) | 4.3 (3.0, 5.3) |
Represents the number of nonmissing responses used in the denominator of each cell
The JHS survey did not ask about race because all members of the JHS are African-American
Includes mixed, Native American, and Asian-American
Includes disabled, unemployed, in school, and homemaker
To accurately represent response to the Religious preference item and to avoid more substantial amounts of missing data, responses of “prefer not to answer” were coded as a separate category (Private) and respondents who did not select any option were coded as a “No response” category.
Responses to Factorial Vignettes
Across the three cohorts, very few respondents skipped or choose “prefer not to answer” for any of the three factorial vignettes (5% of JHS, 3% of FHS, and 2% of FHS Omni respondents). Consequently, analyses of the factorial vignettes were based on a total of 5980 responses (98% of all possible responses) to factorial vignettes provided by 2042 respondents.
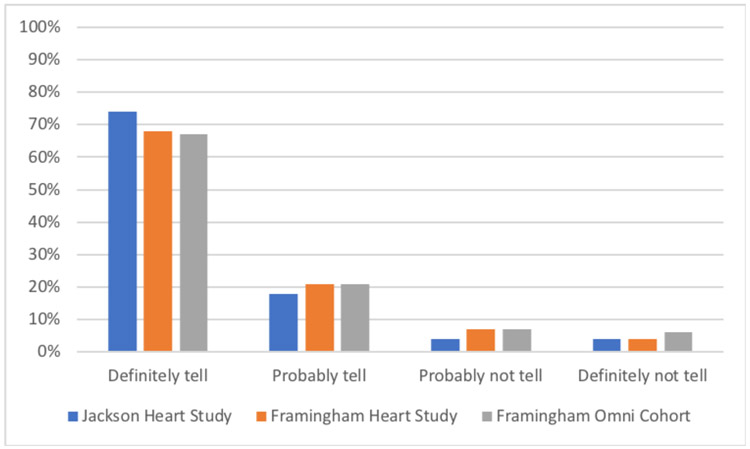
Across all 64 vignette combinations, most respondents said that they would definitely (JHS 74%; FHS 68%; FHS Omni 67%) or probably (JHS 18%; FHS 21%; FHS Omni 21%) want the researchers to tell them about their increased genetic risk (Figure 1).
Figure 1.
Responses to factorial vignettes. Bars represent the proportions of respondents who said they would definitely, probably, probably not, or definitely not want to be told about their genetic risk across all 64 factorial vignette combinations. The data represent 2736 responses from 942 unique JHS respondents, 2773 responses from 941 unique FHS respondents, and 471 responses from 159 unique FHS Omni Cohort respondents.
Among JHS respondents, none of the expert-identified factors embedded in the factorial vignettes (severity, preventability, relative risk, absolute risk, reproductive implications) was associated with preference for return of genetic results. JHS respondents with more favorable attitudes to genetic testing were significantly more likely than other respondents to report a desire for return of results. The odds ratio (OR) associated with each 1-point increase in favorable attitude was 1.61 (95% confidence interval [CI] 1.29–2.00, p<0.0001), implying that the odds of desiring results among respondents with the most favorable attitudes was approximately 7 times that of otherwise identical respondents with the least favorable attitudes. Respondents with higher subjective numeracy were marginally more likely to report a desire for return of results, whereas those who were older and who had graduated from college were less likely than other respondents to desire return of results (Table 2).
Table 2:
Factors associated with preference for return of results in factorial vignettes
| Characteristic of Interest* | JHS† | FHS‡ | FHS Omni§ | |||
|---|---|---|---|---|---|---|
| Odds Ratio (95% CI) |
p-value | Odds Ratio (95% CI) |
p-value | Odds Ratio (95% CI) |
p-value | |
| High relative risk (vs. low) | 0.82 (0.59, 1.15) |
0.252 | 1.29 (.98, 1.71) |
0.090 | 0.89 (0.44, 1.82) |
0.755 |
| High absolute risk (vs. low) | 0.88 (0.63, 1.223) |
0.442 | 1.56 (1.18, 2.07) |
0.001 | 0.88 (0.43,1.78) |
0.716 |
| Death (vs. painless rash) | 0.92 (0.58, 1.48) |
0.739 | 0.72 (0.49, 1.06) |
0.109 | 1.60 (0.60, 4.29) |
0.346 |
| Memory loss (vs. painless rash) | 0.84 (0.53, 1.33) |
0.445 | .81 (0.55, 1.20) |
0.307 | 1.34 (0.51. 3.51) |
0.548 |
| Arthritis (vs. painless rash) | 1.04 (0.64, 1.68) |
0.880 | 0.98 (0.65, 1.47) |
0.948 | 1.51 (0.57, 3.97) |
0.408 |
| Can be prevented (vs. cannot be prevented) | 0.82 (0.59, 1.15) |
0.442 | 3.34 (2.49, 4.49) |
<0.0001 | 1.16 (0.57, 2.34) |
0.688 |
| Reproductive risk of 1 in 2 (vs. unlikely) | 1.22 (0.88, 1.71) |
0.236 | 1.38 (1.05, 1.83) |
0.021 | 1.42 (0.69, 2.92) |
0.333 |
| Attitude towards genetic testing (continuous, range 1-5) | 1.61 (1.29,2.00) |
<0.0001 | 2.32 (1.93, 2.78) |
<0.0001 | 1.79 (1.04, 3.10) |
0.037 |
| College graduate (vs. not) | 0.51 (0.33, 0.81) |
0.005 | 1.08 (0.75, 1.55) |
0.701 | 1.65 (0.52, 5.24) |
0.393 |
| Subjective numeracy (continuous, range 1-6) | 1.19 (1.00, 1.41) |
0.050 | 1.03 (0.89. 1.20) |
0.663 | 1.07 (0.73, 1.59) |
0.725 |
| Age (continuous) | 0.98 (0.96, 1.00) |
0.037 | .99 (0.98, 1.00) |
0.138 | 0.96 (0.92, 0.99) |
0.047 |
Odds ratios represent the adjusted relative odds of desiring return of results comparing subjects who differ only on the covariate of interest. For continuous covariates, odds ratios represent the adjusted relative odds of desiring return of results associated with a 1-point change in the covariate (or one-year increase in age). Odds ratios are derived from generalized linear mixed models that account for multiple responses per participant.
N= 2636 vignette responses from 906 JHS respondents; adjusted R2 0.045.
N=2720 vignette responses from 922 FHS respondents; adjusted R2 0.12.
N=464 vignette responses from 156 FHS Omni respondents; adjusted R2=.049
Among FHS respondents, preventability (OR 3.34, CI 2.49–4.49, p<0.0001) and high absolute risk (OR 1.56, CI 1.18–2.07, p=0.001) were significantly associated with reported desire for results. Respondents were also marginally more likely to report a desire for return of results with reproductive implications (OR 1.38, CI 1.05–1.83, p=0.021). As with JHS, FHS respondents with more favorable attitudes to genetic testing were significantly more likely than other respondents to report a desire for return of results. The OR associated with each 1-point increase in favorable attitude was 2.32 (CI 1.93–2.78, p<0.0001), implying that the odds of desiring results among respondents with the most favorable attitudes was approximately 29 times that of otherwise identical respondents with the least favorable attitudes. No other respondent characteristic was significantly associated with the desire for return of results.
Among FHS Omni respondents, a positive attitude towards genetic testing was associated with an increased likelihood of desiring return of results (OR per 1-point increase 1.79, CI 1.04–3.10, p=0.037), whereas older age was associated with a decreased likelihood of desiring results (OR per year 0.96, CI 0.92-0.99, p=0.047).
Responses to Realistic Vignettes
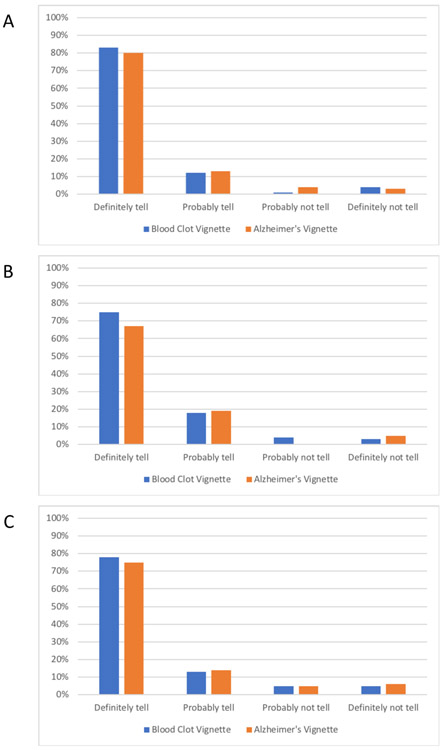
Figure 2 summarizes responses to the two realistic vignettes. As with the factorial vignettes, more than 90% of JHS respondents would definitely or probably want to be told about their risk for a blood clot and for Alzheimer’s disease. Among FHS respondents, 93% would definitely or probably want to be told about their increased risk for a blood clot, and 85% would definitely or probably want to be told about their increased risk for early Alzheimer’s disease. Among FHS Omni respondents, 91% would definitely or probably want to be told about increased their risk for a blood clot, and 89% would definitely or probably want to be told about their increased risk for early Alzheimer’s disease.
Figure 2.
Responses to blood clot risk and early Alzheimer’s disease risk vignettes. Bars represent the proportions of respondents who said they would definitely, probably, probably not, or definitely not want to be told about their genetic risk for the blood clot (black bars) and Alzheimer’s disease (grey bars) vignettes. Figure 2A includes 933 responses to the blood clot vignette and 918 responses to the Alzheimer’s disease vignette from JHS respondents. Figure 2B includes 945 responses to the blood clot vignette and 935 responses to the Alzheimer’s disease vignette from FHS respondents. Figure 2C includes 157 responses to both the blood clot and the Alzheimer’s disease vignette from FHS Omni Cohort respondents.
Among JHS respondents, those with more favorable attitudes toward genetic testing were significantly more likely than other respondents to desire return of results (Table 3). For the Alzheimer’s disease vignette, college graduates were less likely than other respondents to want to learn of the result.
Table 3:
Factors associated with preference for return of results in response to blood clot and Alzheimer’s disease vignettes
| Characteristic of Interest | Blood Clot Odds Ratio (95% CI)* |
p-value | Alzheimer’s Disease Odds Ratio (95% CI)† |
p-value |
|---|---|---|---|---|
| Jackson Heart Study† | ||||
| Number of valid responses | 861 | 851 | ||
| Attitude towards genetic testing‡ | 1.74 (1.30, 2.34) | 0.0002 | 1.56 (1.20, 2.03) | 0.001 |
| No religious preference (vs. any or private) | 0.43 (0.05, 3.67) | 0.441 | 0.52 (0.06, 4.49) | 0.551 |
| Religious attendance more than once/week | 0.67 (0.29, 1.55) | 0.354 | 0.77 (0.39, 1.52) | 0.452 |
| College graduate or more | 1.02 (0.55, 1.89) | 0.955 | 0.52 (0.31, 0.87) | 0.014 |
| Framingham Heart Study§ | ||||
| Number of valid responses | 861 | 854 | ||
| Attitude towards genetic testing‡ | 1.99 (1.51, 2.62) | <0.0001 | 2.24 (1.80, 2.79) | <0.0001 |
| No religious preference (vs. any or private) | 0.42 (0.22, 0.83) | 0.013 | 0.94 (0.50, 1.78) | 0.854 |
| Religious attendance more than once/week | 0.93 (0.47, 1.83) | 0.833 | 0.59 (0.37, 0.94) | 0.025 |
| College graduate or more | 1.23 (0.71, 2.12) | 0.455 | 1.11 (0.74, 1.68) | 0.612 |
| Framingham Heart Study-Omni∥ | ||||
| Number of valid responses | 145 | 145 | ||
| Attitude towards genetic testing‡ | 1.26 (0.65, 2.43) | 0.500 | 1.39 (0.80, 2.43) | .0248 |
| No religious preference (vs. any or private) | 0.27 (0.04, 2.14) | 0.217 | 0.55 (0.09, 3.41) | 0.524 |
| Religious attendance more than once/week | 0.31 (0.06, 1.54) | 0.153 | 0.55 (0.16, 1.86) | 0.334 |
| College graduate or more | 1.29 (0.36, 4.66) | 0.697 | 1.61 (0.54, 4.83) | 0.395 |
Odds ratios represent the adjusted relative odds of desiring return of results comparing subjects who differ only on the covariate of interest. For continuous covariates, odds ratios represent the adjusted relative odds of desiring return of results associated with a 1-point change in the covariate.
Adjusted R2 for JHS blood clot vignette was 0.0348; adjusted R2 for JHS Alzheimer’s vignette was 0.0185.
Possible range 1 (least favorable) to 5 (most favorable)
Adjusted R2 for FHS blood clot vignette was 0.0579; adjusted R2 for FHS Alzheimer’s vignette was 0.0954.
Adjusted R2 for FHS-Omni blood clot vignette was 0.0041; adjusted R2 for FHS-Omni Alzheimer’s vignette was 0.0057
As with JHS respondents, FHS respondents with more favorable attitudes toward genetic testing were significantly more likely than other respondents to desire return of results. Having no religious preference was marginally associated with a reduced likelihood of desiring return of blood clot results, and participating in religious activities more than once a week was marginally associated with a reduced likelihood of desiring return of Alzheimer’s disease results.
Among FHS Omni respondents, no covariates were associated with the likelihood of desiring genetic results in either the blood clot or Alzheimer’s disease scenario.
Discussion
We surveyed JHS, FHS, and FHS Omni participants to assess their preferences for return of secondary genetic findings and to identify factors that correlate with those preferences. As in previous studies, the vast majority of participants reported a desire for return of results under most circumstances described. Our findings partially confirm our a priori expectations that preventability would have a strong relationship with desire for results, but do not confirm our expectation of an association between severity and desire for results. They also confirm our expectation that favorable attitudes towards genetic testing would correlate with desire for results. Specifically, among FHS respondents, preventability was strongly associated, and high absolute risk and reproductive implications were modestly associated, with the desire for return of results. In contrast, among JHS and FHS Omni respondents, we did not observe significant associations between the factors identified by consensus panels as relevant to the decision to offer results (i.e., severity, preventability, magnitude of increased risk, and reproductive implications) and preferences for return. In all cohorts, participants with more positive attitudes towards genetic testing were much more likely than those with less positive attitudes to desire return of results. Finally, although we observed several associations between sociodemographic factors and the desire for return of results, no consistent patterns emerged across the analyses we performed.
The inconsistency across cohorts in the associations between characteristics of the result, such as preventability and risk, and desire for return is surprising. Although our data do not provide an explanation for this variation, possibilities include differences in fundamental values and preferences regarding access to personal medical information, in educational materials from or interactions with the respective study investigators and teams, or in understanding of the complex questions posed. In addition, variation in attitudes, beliefs, and cultural factors associated with race and ethnicity may help explain the differences we observed. For example, one might hypothesize that legacies of mistrust in medicine and science among African-Americans might encourage a uniform desire for results, rather than a willingness to defer to investigators to decide which results to return. Further research is needed to clarify the reasons underlying these differences.
Few prior studies have asked whether research participants’ desires for return of secondary results vary according to the nature of the result. In a survey of a random sample of the Swedish public, Hoeyer found that 55% of respondents would want genetic results returned to them only if a therapeutic or prophylactic intervention were available, whereas 29% would want genetic results returned to them under any circumstances.29 Another Swedish study by Viberg Johansson et al of participants ages 50-64 in a single-institution cardiopulmonary cohort at a found that type of disease, penetrance, and effectiveness of preventive measures were associated with a desire to receive genetic research test results, with the latter having the largest effect.30 A mixed-methods study of adult primary-care and cardiology patients by Jamal et al found that most participants would want results of their genetic research tests in most settings, with modest decreases in desire for results if the phenotype was not preventable or treatable, if the result indicated a variant of uncertain significance, or if the variant only slightly increased risk.31 In qualitative interviews, Jamal et al also found that how research participants or the public understand genetic variants does not necessarily match the bins that experts use to categorize them, potentially explaining why participants’ preferences often do not map onto the recommendations of guideline panels. In a cross-sectional sample of the Canadian public, Regier found that respondents attributed positive utility to receiving results with a high penetrance and with associated recommendations for lifestyle change and medical treatment, whereas they attributed negative utility to receiving results with no recommended treatment or with low penetrance.28 In a focus group study, Wright found that some participants in the National Human Genome Research Institute’s ClinSeq cohort did not wish to learn results associated with diseases that were incurable or that had implications for their children’s health.22 Finally, Murphy conducted 15 focus groups with members of the public in five cities across the United States to assess expectations for return of results from large genetic cohort studies.23 She observed that the accuracy or validity of the result and its actionability were the main factors influencing participants’ preferences for return. Taken together, these studies suggest that actionability does increase participants’ and the public’s desires to receive secondary genetic findings, whereas in our study actionability (described as preventability) was associated with the desire for return of results only among FHS participants. Differences from prior work may reflect the wording of questions, the factorial design of the present study, or differences in study populations. Nevertheless, as a large quantitative study using rigorous experimental methods that was conducted among diverse research participants for whom the topic was highly salient, our findings add important information to the existing evidence base.
Several limitations of our study bear mention. First, the issues addressed by the survey are complex, and responses may be influenced by misunderstanding of the questions or by failure to appreciate the issues. However, the fact that we did not observe an association between genetic knowledge and desire for results argues against misunderstanding as an important confounding factor. Participants may also have interpreted some of the factors embedded in the factorial vignettes differently than we intended; for example, with respect to severity, they may have been influenced by the type of disease as distinct from the perceived seriousness of the condition described in the survey. Second, although the response rates in all cohorts were high and we detected minor or no differences between respondents and nonrespondents by sex and age, it is possible that nonrespondents might have reported different preferences from respondents. Third, our findings are applicable only to those participants in the respective cohorts who consented to genetic studies; nevertheless, the question of return of results does not arise for those who decline research genomic testing. In addition, they do not address the important topic of recontact after initial disclosure.15 Fourth, the data were collected approximately 7 years ago, and it is possible that increased public exposure to genetics or concerns about genetic discrimination may have affected attitudes among research participants. Fifth, we did not adjust for multiple testing; weaker statistical associations should be interpreted with this in mind. Also, the Omni cohort was small, limiting power to detect associations. Sixth, the results should not be used to make comparisons between participants from the three cohorts or between the populations from which they were drawn. Finally, it is important to generalize these findings with caution to other settings, as factors specific to the JHS and FHS, such as their population- rather than patient-based recruitment, their focus on cardiovascular health, the high levels of trust between investigators and participants, and the extensive community engagement related to genetics and other aspects of the research may influence preferences for return of results.32-34
In conclusion, among over 2000 participants in three long-standing cardiovascular cohort studies, most respondents reported a definite or probable desire for return of genetic research results. Across the cohorts, a positive attitude towards genetic testing was the most consistent predictor of desire for results. Finally, among participants in FHS but not those in JHS, factors identified as relevant by consensus groups, such as preventability, level of risk and reproductive implications, were associated with desire for results. These findings have important normative implications for policies and practices regarding return of genomic results. If policies are to be driven by participants’ preferences, studies will need to adopt a liberal approach to the offer of return. If, however, investigators and policymakers adopt a more restrictive approach to the offer of return, they will need to justify it based on other considerations such as paternalism, logistical difficulty, or cost.
Supplementary Material
Acknowledgments:
The authors wish to thank the participants of the Jackson and Framingham Heart Studies for their ongoing generosity and dedication. They also wish to thank the staff of the JHS Coordinating Center at Jackson State University for their assistance with data collection, Katherine Saylor MS for helpful comments on previous manuscript drafts, and Ree Dawson PhD for statistical guidance.
Sources of Funding: This study was supported by R01HG005083 from the National Human Genome Research Institute (Joffe, PI). The Jackson Heart Study is supported and conducted in collaboration with Jackson State University (HHSN268201300049C and HHSN268201300050C), Tougaloo College (HHSN268201300048C), and the University of Mississippi Medical Center (HHSN268201300046C and HHSN268201300047C) contracts from the National Heart, Lung, and Blood Institute (NHLBI) and the National Institute for Minority Health and Health Disparities (NIMHD). The Framingham Heart Study was supported by contracts to Boston University from the NHLBI (N01-HC-25195 and HHSN268201500001). The views expressed here are those of the authors and do not necessarily represent the views of the National Heart, Lung, and Blood Institute; the National Institutes of Health; or the U.S. Department of Health and Human Services.
Non-standard Abbreviations and Acronyms
- FHS
Framingham Heart Study
- IRB
institutional review board
- JHS
Jackson Heart Study
- SNS
Subjective Numeracy Scale
Footnotes
Disclosures: None
References:
- 1.Wolf SM, Lawrenz FP, Nelson CA, Kahn JP, Cho MK, Clayton EW, Fletcher JG, Georgieff MK, Hammerschmidt D, Hudson K, et al. Managing incidental findings in human subjects research: analysis and recommendations. J Law Med Ethics. 2008;36:219–48, 211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Medical Research Council/Wellcome Trust. Framework on the feedback of health-related findings in research. 2014. Available at https://mrc.ukri.org/documents/pdf/mrc-wellcome-trust-framework-on-the-feedback-of-health-related-findings-in-researchpdf/. Accessibility verified September 10, 2019.
- 3.National Bioethics Advisory Commission. Research Involving Human Biological Materials: Ethical Issues and Policy Guidance. 1999. Available at https://bioethicsarchive.georgetown.edu/nbac/hbm.pdf. Accessibility verified September 10, 2019.
- 4.Bookman EB, Langehorne AA, Eckfeldt JH, Glass KC, Jarvik GP, Klag M, Koski G, Motulsky A, Wilfond B, Manolio TA, et al. Reporting genetic results in research studies: summary and recommendations of an NHLBI working group. Am J Med Genet. 2006;140:1033–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Knoppers BM, Joly Y, Simard J and Durocher F. The emergence of an ethical duty to disclose genetic research results: international perspectives. Eur J Hum Genet. 2006;14:1170–8. [DOI] [PubMed] [Google Scholar]
- 6.Wolf SM, Crock BN, Van Ness B, Lawrenz F, Kahn JP, Beskow LM, Cho MK, Christman MF, Green RC, Hall R, et al. Managing incidental findings and research results in genomic research involving biobanks and archived data sets. Genet Med. 2012;14:361–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fabsitz RR, McGuire A, Sharp RR, Puggal M, Beskow LM, Biesecker LG, Bookman E, Burke W, Burchard EG, Church G, et al. Ethical and Practical Guidelines for Reporting Genetic Research Results to Study Participants: Updated Guidelines From a National Heart, Lung, and Blood Institute Working Group. Circ Cardiovasc Genet. 2010;3:574–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Incidental benefits. Nature. 2012;483:373. [DOI] [PubMed] [Google Scholar]
- 9.Christenhusz GM, Devriendt K, Dierickx K. To tell or not to tell? A systematic review of ethical reflections on incidental findings arising in genetics contexts. Eur J Hum Genet. 2013;21:248–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Presidential Commission for the Study of Bioethical Issues. Anticipate and Communicate: Ethical Management of Incidental and Secondary Findings in the Clinical, Research, and Direct to Consumer Contexts. 2013. Available at https://bioethicsarchive.georgetown.edu/pcsbi/sites/default/files/FINALAnticipateCommunicate_PCSBI_0.pdf. Accessibility verified September 10, 2019. [DOI] [PubMed]
- 11.Jarvik GP, Amendola LM, Berg JS, Brothers K, Clayton EW, Chung W, Evans BJ, Evans JP, Fullerton SM, Gallego CJ, et al. Return of genomic results to research participants: the floor, the ceiling, and the choices in between. Am J Hum Genet. 2014;94:818–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Greely HT. The uneasy ethical and legal underpinnings of large-scale genomic biobanks. Annu Rev Genomics Hum Genet. 2007;8:343–64. [DOI] [PubMed] [Google Scholar]
- 13.Kohane IS, Mandl KD, Taylor PL, Holm IA, Nigrin DJ, Kunkel LM. Reestablishing the researcher-patient compact. Science. 2007;316:836–7. [DOI] [PubMed] [Google Scholar]
- 14.Returning Individual Research Results to Participants: Guidance for a New Research Paradigm. Washington (DC): National Academies Press; 2018. [PubMed] [Google Scholar]
- 15.Bombard Y, Brothers KB, Fitzgerald-Butt S, Garrison NA, Jamal L, James CA, Jarvik GP, McCormick JB, Nelson TN, Ormond KE, et al. The responsibility to recontact research participants after reinterpretation of genetic and genomic research results. Am J Hum Genet. 2019;104:578–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Clayton EW, McGuire AL. The legal risks of returning results of genomics research. Genet Med. 2012;14:473–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bledsoe MJ, Clayton EW, McGuire AL, Grizzle WE, O'Rourke PP, Zeps N. Return of research results from genomic biobanks: cost matters. Genet Med. 2013;15:103–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ossorio P Taking aims seriously: repository research and limits on the duty to return individual research findings. Genet Med. 2012;14:461–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Burke W, Evans BJ, Jarvik GP. Return of results: ethical and legal distinctions between research and clinical care. Am J Med Genet C Semin Med Genet. 2014;166C:105–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Beskow LM, Burke W. Offering individual genetic research results: context matters. Sci Transl Med. 2010;2:38cm20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shalowitz DI, Miller FG. Communicating the results of clinical research to participants: attitudes, practices, and future directions. PLoS Med. 2008;5:e91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wright MF, Lewis KL, Fisher TC, Hooker GW, Emanuel TE, Biesecker LG, Biesecker BB. Preferences for results delivery from exome sequencing/genome sequencing. Genet Med. 2014;16:442–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Murphy J, Scott J, Kaufman D, Geller G, LeRoy L, Hudson K. Public expectations for return of results from large-cohort genetic research. Am J Bioeth. 2008;8:36–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mackley MP, Fletcher B, Parker M, Watkins H, Ormondroyd E. Stakeholder views on secondary findings in whole-genome and whole-exome sequencing: a systematic review of quantitative and qualitative studies. Genet Med. 2016; 19:283–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Townsend A, Adam S, Birch PH, Lohn Z, Rousseau F, Friedman JM. “I want to know what’s in Pandora’s Box”: comparing stakeholder perspectives on incidental findings in clinical whole genomic sequencing. Am J Med Genet A. 2012;158A:2519–25. [DOI] [PubMed] [Google Scholar]
- 26.Kaufman D, Murphy J, Scott J, Hudson K. Subjects matter: a survey of public opinions about a large genetic cohort study. Genet Med. 2008;10:831–9. [DOI] [PubMed] [Google Scholar]
- 27.Jackson L, Goldsmith L, O’Connor A, Skirton H. Incidental findings in genetic research and clinical diagnostic tests: a systematic review. Am J Med Genet A. 2012;158A:3159–67. [DOI] [PubMed] [Google Scholar]
- 28.Regier DA, Peacock SJ, Pataky R, van der Hoek K, Jarvik GP, Hoch J, Veenstra D. Societal preferences for the return of incidental findings from clinical genomic sequencing: a discrete-choice experiment. CMAJ. 2015;187:E190–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hoeyer K, Olofsson BO, Mjorndal T, Lynoe N. Informed consent and biobanks: a population-based study of attitudes towards tissue donation for genetic research. Scandinavian Journal of Public Health. 2004;32:224–9. [DOI] [PubMed] [Google Scholar]
- 30.Viberg Johansson J, Langenskiold S, Segerdahl P, Hansson MG, Hosterey UU, Gummesson A, Veldwijk J. Research participants’ preferences for receiving genetic risk information: a discrete choice experiment. Genet Med. 2019;21:2381–9. [DOI] [PubMed] [Google Scholar]
- 31.Jamal L, Robinson JO, Christensen KD, Blumenthal-Barby J, Slashinski MJ, Perry DL, Vassy JL, Wycliff J, Green RC, McGuire AL. When bins blur: Patient perspectives on categories of results from clinical whole genome sequencing. AJOB Empir Bioeth. 2017;8:82–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wilson JG, Rotimi CN, Ekunwe L, Royal CD, Crump ME, Wyatt SB, Steffes MW, Adeyemo A, Zhou J, Taylor HA Jr., et al. Study design for genetic analysis in the Jackson Heart Study. Ethn Dis. 2005;15:S6-30–37. [PubMed] [Google Scholar]
- 33.Walker ER, Nelson CR, Antoine-LaVigne D, Thigpen DT, Puggal MA, Sarpong DE, Smith AM. Research participants’ opinions on genetic research and reasons for participation: a Jackson Heart Study focus group analysis. Ethn Dis. 2014;24:290–7. [PubMed] [Google Scholar]
- 34.Levy D, Splansky GL, Strand NK, Atwood LD, Benjamin EJ, Blease S, Cupples LA, D’Agostino RB Sr., Fox CS, Kelly-Hayes M, et al. Consent for genetic research in the Framingham Heart Study. Am J Med Genet A. 2010;152A:1250–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.