Abstract
Background
The Surveillance of Rare Cancers in Europe (RARECARE) project proposed a definition and a list of rare cancers. The Joint Action on Rare Cancers (JARC), launched by the European Union and involving 18 member states and 34 partners, promoted a wide consensus effort to review the list.
Patients and methods
A group of experts was set up, including scientific societies, member state representatives of JARC, representatives of the European Reference Networks dedicated to rare cancers and rare cancer patient advocates. The definition and the list of rare clinical entities, based on the incidence data provided by two European projects (RARECARE and RARECAREnet), were rediscussed through a consensus meeting of the expert panel.
Results
By consensus, it was reiterated that the best criterion for a definition of rare cancers is incidence, rather than prevalence. By consensus, the experts slightly modified the composition of the tiers of rare cancers, according to the definition based on an incidence threshold <6/100 000/year, and grouped all rare cancers within 12 families of rare cancers. Even when defined conservatively this way, rare cancers are not rare collectively, since they correspond to 10%–20% of all cancer cases.
Conclusions
The list of rare cancers reviewed by JARC should be viewed as a tool in the fight against rare cancers and rare diseases. It may help to appreciate that rare cancers are cancers and rare diseases at the same time, combining issues and difficulties of both. We hope that refinements to the list and a wider understanding of its implications may contribute to increase awareness of problems posed by rare cancers and to improve quality of care in a large group of patients with cancer, who may be discriminated against just because of the low frequency of their diseases.
Keywords: rare cancers, incidence, prevalence, cancer registries
Key questions.
What is already known about this subject?
In Europe, rare diseases are often defined as those with a prevalence of <50/100 000. In the USA, the Orphan Drug Act defined rare diseases as those affecting <200 000 persons. The project Surveillance of Rare Cancers in Europe (RARECARE) proposed a list of rare cancers and developed a new incidence-based definition of rare cancers setting the rarity threshold at <6/100 000. In 2016, the European Union (EU) launched the Joint Action on Rare Cancers (JARC). Within the JARC, a consensus effort to re-examine the list of rare cancers as developed within the RARECARE project took place, with a view also to the rare cancer families.
What does this study add?
This paper conveys the notion of rare cancer ‘families’ (12, overall) that we believe may now explain effectively which cancers are actually ‘rare’. As a matter of fact, this notion has not been published yet, but it was already used as a standard reference in the EU for the appointment of European Reference Networks. We also provide a wider insight into the rationale of the list.
The JARC is an EU project aimed to advance the quality of care and research for rare cancers in the EU, with 18 member states and 34 partners involved. The EU launched the JARC to parallel a Joint Action on Rare Diseases, underlying the recognition that rare diseases and rare cancers have commonalities but also belong to different clinical domains. There is a wide movement ongoing on rare cancers, at the moment, in Europe. In particular, the European rare cancer community has been very active on these items through the European Action Against Rare Cancers (www.rarecancers.org), launched in 2008 and coordinated by the European Society for Medical Oncology.
This paper could become a reference paper on the partitioning of the rare cancer ‘families’. The original article on the RARECARE list was published by the European Journal of Cancer in 2011, while the paper on the epidemiological update that was the output of the subsequent project RARECARENET was published by The Lancet Oncology in 2017.
Key questions.
How might this impact on clinical practice?
It is crucial to have a widely shared comprehensive list of rare cancers, since they constitute a prominent issue for cancer care organisation and research. The list of rare cancers reviewed by JARC should be viewed as a tool in the fight against rare cancers. The refinements to the list and a wider understanding of its implications may contribute to increase awareness of problems posed by rare cancers and to improve quality of care in a large group of patients with cancer, who may be discriminated against just because of the low frequency of their diseases.
Introduction
Rare cancers are the rare diseases of oncology. In fact, they are both ‘rare diseases’ and ‘cancers’. However, they are peculiar in comparison to both. They are different from rare diseases because they share all the hallmarks of cancer, so that they are relatively homogeneous, while rare diseases are a highly variegated group of mainly chronic diseases, with different natural histories as well as causes and pathogenetic mechanisms. In addition to this, rare cancers are handled by the same community of physicians, that is, surgical oncologists, radiation oncologists, medical oncologists, haemato-oncologists, paediatric oncologists at cancer centres. On the other hand, they are different from common cancers, because their number is low, while common cancers are among the two main causes of morbidity and mortality in affluent societies. Thus, it is important to focus on rare cancers as a health problem per se. To this end, it is crucial to have a comprehensive list of these entities, based on a sound definition of rarity in cancer.
In 2008, the European Commission funded a project, ‘Surveillance of Rare Cancers in Europe’ (RARECARE). RARECARE delivered a list of rare cancers, based on a definition which was felt to be relevant for oncology (setting rare cancers as those with a crude incidence rate <6/100 000/year in Europe).1
In 2016, the European Union (EU) launched the Joint Action on Rare Cancers (JARC), paralleling a Joint Action on Rare Diseases. This underlined the recognition that rare diseases and rare cancers have commonalities but also belong to different clinical domains. This consensus effort to re-examine the list of rare cancers as developed within the RARECARE project took place within JARC, with a view also to the rare cancer families.
Rationale, definition and open issues
Tiers and families
In order to get to a list of rare cancers, one must have a list of cancers. The most obvious list of cancers is based on their topography and histological classification (morphology), that is, the International Classification of Diseases for Oncology (ICD-O). However, the morphologicl entities enlisted therein need to be grouped into clinically distinct entities, which in turn may be gathered into families of neoplastic diseases. An effort to better define these groupings was made within the RARECARE project through its consensus process. A panel of experts, including clinicians, pathologists and epidemiologists, was set up in 2007 to work out a tentative proposal of both a definition of rare cancers and a list.
The panel of experts agreed to build the list of clinically relevant entities on the basis of all the combination of topography and morphologies coded in the ICD-O3. Biologically and clinically, epithelial tumours of one site differ from epithelial tumours of another site much more than, say, a sarcoma of different sites may do. Thus, one will find that epithelial tumours are apparently broken down by site, while sarcomas, neuroendocrine tumours and haematological neoplasms are not, or only secondarily they are. Actually, it is all about distinguishing neoplasms with a different natural history, clinical approach and, as a matter of fact, a lung adenocarcinoma differs from a colorectal adenocarcinoma because they are two different diseases. In the end, according to the ICD-O, each tumour will be identified by a combination of morphology and topography. Depending on the tumour type, grouping will be based on one of them, or the other, or both.
Tier 2: The experts were asked to group the ICD-O3 morphological entities, which thus constitutes the bottom tier of the list (‘tier 3’), to give rise to a second tier of clinically distinct entities (‘tier 2’), by using morphologies and topographies (eg, ‘squamous cell carcinoma of nasal cavity and sinuses’, ‘soft tissue sarcoma of limb’, etc). These entities had to be viewed as clinically relevant by clinicians. In general, these diagnoses had to correspond to consistent diagnostic and therapeutic approaches (eg, these entities could be used as eligibility criteria in a clinical trial).1
Tier 1: The ‘tier 2’ entities were assembled into a smaller number of ‘tier 1’ entities. Tier 1 entities were intended to be major cancer entities in a clinical sense (eg, ‘epithelial tumours of nasal cavity and sinuses’, ‘soft tissue sarcoma’) and to have an organisational importance, for example, they could underlie patient referral policies.1
Families of rare cancers. Focusing on referral of patients, ‘tier 1’ entities were grouped, by the JARC panel of experts (see online supplementary appendix 1), into gross partitions, which give rise to what were called ‘families’ of rare cancers, identifying major groups of rare cancer diseases (eg, ‘rare cancers of head and neck’, ‘sarcomas’, etc). These are dealt with by the same disease-based communities of physicians and clinical researchers.
esmoopen-2019-000666supp001.pdf (32.5KB, pdf)
To define the major families of rare cancers, the JARC consensus panel combined the ‘tier 1’ entities with an incidence rate <6/100 000/year. Box 1 enlists ‘tier 1’ entities with an incidence below 6/100 000/year, and the corresponding major families of rare cancers. To put all this into context, box 2 enlists ‘tier 1’ entities having an incidence above 6/100 000/year.
Box 1. Rare cancers: RARECARE ‘families’ and ‘tier 1’ entities with an incidence <6/100 000.
Head and neck
Epithelial tumours of the larynx.
Epithelial tumours of the hypopharynx.
Epithelial tumours of the nasal cavity and sinuses.
Epithelial tumours of the nasopharynx.
Epithelial tumours of major salivary glands and salivary gland type tumours.
Epithelial tumours of the oropharynx.
Epithelial tumours of the oral cavity and lip.
Epithelial tumours of the eye and adnexa.
Epithelial tumours of the middle ear.
Digestive
Epithelial tumours of the small intestine.
Epithelial tumours of the anal canal.
Epithelial tumours of the gallbladder and extrahepatic biliary duct.
Thoracic
Epithelial tumours of the trachea.
Thymomas and thymic carcinomas.
Malignant mesothelioma.
Female genital
Non-epithelial tumours of the ovary.
Epithelial tumours of the vulva and vagina.
Trophoblastic tumours of the placenta.
Male genital and urogenital
Tumours of the testis and paratestis.
Epithelial tumours of penis.
Extragonadal germ cell tumours.
Epithelial tumours of renal pelvis, ureter and urethra.
Skin cancers and non-cutaneous melanoma
Mucosal melanoma.
Uveal melanoma.
Adnexal skin carcinomas.
Kaposi sarcoma.
Sarcomas
Soft tissue sarcoma.
Bone sarcoma.
Gastrointestinal stromal tumours.
Neuroendocrine tumour (NET)
NET gastrointestinal pancreatic.
NET lung.
NET other sites.
Endocrine organ
Thyroid cancers.
Parathyroid cancer.
Adrenal cortex cancer.
Pituitary gland cancer.
Central nervous system (CNS)
Glial tumours and others.**
Malignant meningioma.
Embryonal tumours of CNS.
Paediatric*
Hepatoblastoma.
Neuroblastoma and ganglioneuroblastoma.
Nephroblastoma.
Odontogenic malignant tumours.
Olfactory neuroblastoma.
Pancreatoblastoma.
Pleuropulmonary blastoma.
Retinoblastoma.
Haematological
Lymphoid malignancies.**
Myelodysplastic syndromes.
Myeloproliferative neoplasms (including mastocytosis).
Myelodysplastic/myeloproliferative neoplasms.
Myeloid/ lymphoid neoplasms with eosinophilia and abnormalities of PDGFRA (platelet derived growth factor receptor alpha), PDGFRB (platelet derived growth factor receptor beta), or FGFR1 (fibroblast growth factor receptor 1), or with PCM1-JAK2
Acute myeloid leukaemia and related neoplasms.
*Other neoplasms which mainly, or also, occur in childhood are included under other labels (eg, Ewing’s sarcoma and osteosarcoma under bone sarcomas; rhabdomyosarcoma under soft tissue sarcoma; medulloblastoma under embryonal tumour of CNS).
**All subgroups (tier 2 entities) within are rare.
Box 2. Tier 1 cancer entities with incidence rate > 6/100 000.
Digestive—common
Epithelial tumours of the oesophagus.
Epithelial tumours of the stomach.
Epithelial tumours of the colon.
Epithelial tumours of the rectum.
Epithelial tumours of the pancreas.
Epithelial tumours of the liver and intrahepatic and bile tract.
Thoracic—common
Epithelial tumours of the lung.
Breast
Epithelial tumours of the breast.
Female genital—common
Epithelial tumours of the corpus uteri.
Epithelial tumours of the ovary and fallopian tube.
Epithelial tumours of the cervix uteri.
Urogenital—common
Epithelial tumours of the kidney.
Epithelial tumours of the bladder.
Epithelial tumours of the prostate.
Skin cancers—common
Epithelial tumours of the skin.
Skin melanoma.
Some ‘tier 1’ entities, namely epithelial tumours of the oesophagus, of the liver, of the ovary and fallopian tube, of the cervix uteri, include only rare ‘tier 2’ entities. These tumours were not included in the families of rare cancers because their ‘tier 1’ is not rare. We do acknowledge that these ‘tier 2’ entities are rare, and that, say, clinical studies will face the same difficulties as for rare cancers. However, patient referral may be closer to what happens for common cancers.
There is a big difference between a rare ‘family’ of cancers and a rare cancer ‘entity’ belonging to a common family of tumours. For example, metaplastic cancers of the breast are a rare cancer entity, with the same incidence as, say, pleomorphic liposarcoma. However, while it may well be equally problematic to do any clinical research exclusively focusing on both, the expertise needed to approach appropriately a metaplastic breast cancer will be relatively easy to find in the community. This does not apply to pleomorphic liposarcoma, for which referral centres, or networks, will inevitably be more difficult to find in the community.
By consensus, in the end, the experts managed to move from more than 600 tier 3 morphological entities to about 200 rare cancers (tier 2) and to 12 major families of rare cancers.
The indicator and the threshold for cancer rarity
With regard to the best criterion suitable to define rarity, there was a wide consensus on using incidence. The definition for orphan drug designation defines rare diseases as those having a prevalence lower than 50 in 100 000 people in the EU2 and around 70 in 100 000 in the USA.3
Prevalence has shortcomings as a measure of cancer rarity, since some cancers with a low incidence but a good survival will look common as long as their good survival pushes up prevalence. An example is testicular cancer. Similarly, some commonly occurring diseases with a poor survival will look rare because their poor survival lowers prevalence. Examples are adenocarcinoma of stomach or squamous cell carcinoma of lung. In addition, the natural history of any cancer is such that everything tends to happen once: there will be one potentially eradicating surgery, one local radiation therapy, one first chemotherapy, and each of these will take place in discrete time intervals. Thus, incidence, which reflects the yearly number of new cases occurring in a population, might be a better indicator of the burden posed by a cancer. This is not the case with most non-neoplastic chronic rare diseases that, for example, require lifelong treatments. This is the reason why prevalence renders much better the burden posed by non-neoplastic diseases, including rare diseases.
Of course, evolution of therapies may well affect the suitability of incidence as a sound indicator. For example, if in the future anticancer therapies are delivered chronically (lifelong), overcoming the currently limiting factor of tumour resistance, prevalence will become a much more suitable indicator to render the burden of disease on a population scale, at least as far as medical therapy is concerned. At the moment, this is not the case, although an evolution towards prolonged administration of anticancer therapies is in place. Anyway, prevalence is available from population-based cancer registries (www.rarecarenet.eu).
The RARECARE panel of experts was provided with the incidence data for all malignant cancers identified in ‘tiers 1’ and ‘tiers 2’ based the RARECARE dataset.1 Assuming incidence as an indicator, it was felt by clinical experts that cancers with an incidence below 3/100 000/year were definitely rare. However, if the thresholds of <3/100 000/year were adopted, glial tumours, epithelial cancers of the oral cavity and lip, epithelial cancers of gallbladder and extrahepatic biliary tract, soft tissue sarcomas, tumours of testis and paratestis, myeloproliferative neoplasms and acute myeloid leukaemia would all be excluded. Yet experts believed that these forms share all problems of rarity. On the contrary, those tumours with an incidence above 10/100 000/year were definitely felt as common. A working threshold was set at an annual incidence rate of <6/100 000 per year on the basis of the population level in Europe.
The RARECARE list is based on incidences provided by 83 population-based cancer registries thus are robust. However, population-based registries are fed with diagnoses provided by the community. It is well known that the pathological diagnosis of rare cancers may be inappropriate in a significant proportion of cases depending on the cancer type (eg, sarcomas are more exposed to inappropriateness compared with squamous cell head and neck carcinomas). In particular, a number of not otherwise specified (NOS) diagnoses will be found that clearly could be converted if a pathological review was made. In principle, this could lead to underestimate the incidence and prevalence of some entities. In the RARECARE database, no registries showed clearly outlying values of NOS cases. Furthermore, the JARC worked with European registries to develop recommendations on how to ameliorate the quality of registration for rare cancers. The European Reference Network (ERN) on rare solid adult cancers has a dedicated pathological task force that will work to improve the diagnosis of rare adult cancers in Europe. This will improve quality of care and, as a by-product, also quality of registration.
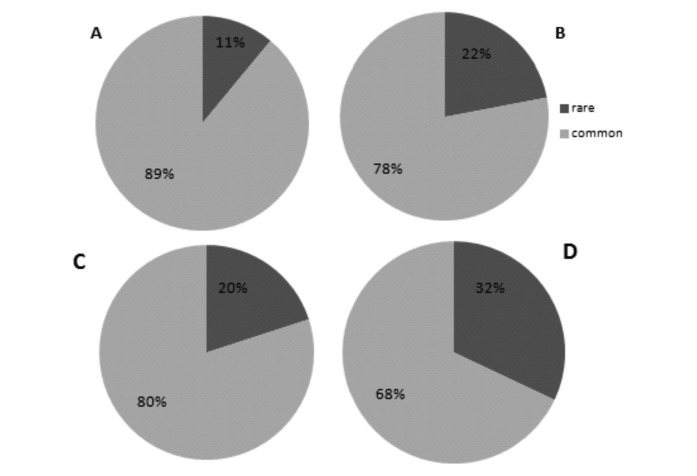
With an incidence of <6/100 000/year applied as a threshold to the ‘tier 1’ entities of the list, 11% of incident cases of malignancies in the RARECARE data base would be selected as rare. If applied on the ‘tier 2’ entities, this threshold would correspond to 22% of new cancer cases (figure 1A, B).
Figure 1.
Percentage of rare and common cancers based on: the incidence rate of tier 1 cancer entities (A); incidence rate of tier 2 cancer entities (B); prevalence of tier 1 cancer entities (C); prevalence of tier 2 cancer entities (D).
With a prevalence threshold at <50/100 000, one would identify 20% of cancer cases as rare if applied to ‘tier 1’ entities, and 32% if applied to ‘tier 2’ entities (figure 1C, D).
Overall, the 12 families of rare cancers would comprise 13% of all new cancer cases.
RARECARE provided average incidence estimates for the whole EU, and the list was shaped according to these values. The definition of rare cancers, as the definition of rare diseases, was meant to be EU based. Experts agreed that having a single list of rare cancers is essential to foster collaboration on rare cancers in Europe. However, the incidence rates may differ from country to country. This could be due to several factors, as the prevalence of risk factors in populations (eg, smoking, alcohol, virus), overdiagnosis (eg, thyroid cancers), quality of pathological diagnoses and cancer registration. In any case, RARECARE estimated the incidence rate of all tiers in the countries that contributed to the database. Out of the ‘tiers 2’ with an incidence <6/100 000 at EU level, only up to a maximum of nine ‘tiers 2’ had a different incidence rate, either below or above 6/100 000/year, in single countries. On the other side, estimates in single countries are less robust, especially in rare cancers by definition in small countries.
Open issues
Every definition of rare conditions is subject to limitations. Some of them have already been recalled. Others are mentioned below. In the end, only the flexibility with which the list is used in practice will make such limitations less problematic.
Rare diseases are viewed as problematic because of their low frequency, and this is why their definition must be based on indicators of frequency. In fact, a low frequency may result into discriminations per se. Economies of scale cannot be made, there is not enough market for drugs, benefits in outcomes cannot be demonstrated through conventional studies, medical expertise is hard to build and to find out in the community. However, frequency is not the only problem that a disease may pose under a population perspective: some diseases may well be challenging because, say, they are complex to treat, constitute an unmet clinical need. Public policy measures should take into account these factors, in addition to frequency. For example, regulations on orphan drugs foresee that, in order to be designated as ‘orphan’, a drug addresses a disease that is rare but also life-threatening or chronically debilitating, and the new drug must be of significant benefit in comparison to therapies already available, if any. This said, given our aim, the RARECARE list of rare cancers is only based on frequency.
When focusing on individual patients, the disease entity (ie, its nosographic label) is just one of the attributes that singles out the clinical presentation. In addition to being affected by a given cancer entity, a patient presents with, say, a stage of disease, which, along with sex, age, heritage and several other factors (including concurrent diseases), will eventually determine the treatment choice. Furthermore, in the era of targeted therapies against cancer, the molecular profile will be more and more relevant. It follows that uncountable clinical presentations may constitute rare occurrences even when the tumour entity is common, whatever the definition of rarity. The list devised by RARECARE was based on tumour entities, as coded by the last ICD-O classification, which is the worldwide-recognised classification of cancers. Any list of rare cancers will always be a subset of a standard list of cancers. International agencies preside over such classifications, constantly updating them, and genetic and molecular profile is more and more relevant to cancer partitioning in such classifications. It follows that our list needs to be updated following updates in the ICD-O classification.
A whole group of cancers, paediatric cancers, are rare. With regard to cancers in children and adolescents, the RARECARE list includes some of them under the family of ‘paediatric cancers’ but several have been included under specific families, such as haematological tumours, sarcomas, CNS tumours, head and neck cancers, digestive cancers, thoracic cancers, endocrine tumours. As said, however, all childhood cancers are rare but within this family, there are also very rare paediatric cancers, with additional challenges in terms of access to high-quality expert care and research. Under the JARC framework, a dedicated group; EXPeRT have re-evaluated the definition of paediatric very rare cancers.4 Paediatric cancers are treated in networks of paediatric haemato-oncology centres, and there is a European Reference Network specifically dedicated to paediatric haemato-oncology. In the regulatory context of medicine development, paediatric cancers are also distinct, in that the orphan drug regulation has been demonstrated to be ineffective in this area.5 JARC has been an important opportunity to recognise that there are commonalities between adult rare cancers and paediatric cancers and help cross-communication between the communities.
Furthermore, some cancers have a hereditary risk component. Some of them are rare cancers as such (eg, sarcomas in Li Fraumeni syndrome), others belong to common entities (eg, colon adenocarcinoma in familial adenomatous polyposis). Currently, there is no specific code for registration of heredofamilial cancers. On the other side, hereditary cancer syndromes may be incorporated into rare diseases. Thus, ICD-O could be used to register the cancers (eg, sarcomas or colon adenocarcinoma), while ORPHA numbers, recommended by the EU to register rare diseases, could be used in parallel to register the hereditary syndromes. A European Reference Network is dealing with such conditions (GENTURIS). Experts stress the importance of a close collaboration between networks dedicated to rare cancers with GENTURIS, as clinical oncologists need to collaborate with medical geneticists at the patient’s bedside.
Conclusions
The rare cancer families identified by JARC should be viewed as a tool in the fight against rare cancers and rare diseases.
Initiatives prompted by the rare cancer community have been ongoing on rare cancers, like the European Action Against Rare Cancers (www.rarecancers.org), launched in 2008 by the European Society for Medical Oncology in partnership with the many stakeholders who must have a say to advance our knowledge and practices in this challenging, ‘orphan’ area of human diseases. The EU recognised the results of RARECARE and supported a second project (RARECAREnet), which updated and enriched the information on rare cancers in Europe. Also following this evolution in the perception of rare cancers as a distinct issue in the world of oncology, the EU prompted JARC, which thus parallels the Joint Action on Rare Diseases. This underlined the recognition that rare diseases and rare cancers have commonalities but belong to different areas. Importantly, the EU created the European Reference Networks in 2017 on several rare diseases, including three European Reference Networks on rare cancers (one on paediatric cancers, one on haematological cancers, one on rare adult solid cancers). The ERNs are virtual networks of healthcare providers across the EU targeting rare or low-prevalence complex diseases or conditions. The main objective of ERNs is improve quality of care, through proper referral and teleconsultations, exploiting centres of expertise throughout the EU. Other objectives of the ERNs include the promotion of medical education and patient information, the development of clinical practice guidelines, the promotion of research as well as epidemiological surveillance advancement. The final aim of ERNs is to bring innovation, knowledge and expertise from centres of excellence to the patient, independent of his/her point of access.
In the face of ongoing efforts on rare cancers, we hope that refinements to the list, a wider understanding of its implications and above all improved awareness of the problems posed by rare cancers may contribute to improving quality of care in a large group of patients with cancer who may be discriminated against just because of the low frequency of their diseases.
Footnotes
Twitter: @casali_pg
Contributors: Both authors provided a substantial contributions to the conception of the work, drafted the work and revised it critically for important intellectual content. They provided the final approval of the version published and agree to be accountable for all aspects of the work.
Funding: This research was supported by the joint action ‘724161/JARC’ which has received funding from the European Union’s Health Programme (2014-2020).
Competing interests: PGC: honoraria for advisory/speaker’s role from Deciphera Pharmaceuticals, Eisai, Eli Lilly, Nektar Ther, Pfizer, PharmaMar; institutional fundings from Amgen Dompé, AROG, Bayer, Blueprint, Eli Lilly, Daiichi Sankyo Pharma, Epizyme, Glaxo SK, Novartis, Pfizer, PharmaMar.
Patient consent for publication: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: All data relevant to the study are included in the article or uploaded as online supplementary information.
References
- 1.Gatta G, van der Zwan JM, Casali PG, et al. Rare cancers are not so rare: the rare cancer burden in Europe. Eur J Cancer 2011;47:2493–511. 10.1016/j.ejca.2011.08.008 [DOI] [PubMed] [Google Scholar]
- 2.Official Journal of the European Communities , 2000. Available: http://eurlex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2000:018:0001:0005:en:PDF
- 3.Food and Drug Administration Available: https://www.ecfr.gov/cgi-bin/textidx?c=ecfr&SID=51cf70689d51f0ea4147c0a8ac649321&rgn=div5&view=text&node=21:5.0.1.1.6&idno=21
- 4.Ferrari A, Brecht IB, Gatta G, et al. Defining and listing very rare cancers of paediatric age: consensus of the Joint Action on Rare Cancers in cooperation with the European Cooperative Study Group for Pediatric Rare Tumors. Eur J Cancer 2019;110:120–6. 10.1016/j.ejca.2018.12.031 [DOI] [PubMed] [Google Scholar]
- 5.Vassal G, Kearns P, Blanc P, et al. Orphan drug regulation: a missed opportunity for children and adolescents with cancer. Eur J Cancer 2017;84:149–58. 10.1016/j.ejca.2017.07.021 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
esmoopen-2019-000666supp001.pdf (32.5KB, pdf)