Abstract
Bone health impairment is a frequent detrimental consequence of the high bone tropism of prostate cancer (PCa) cells. It is further worsened by administration of androgen-deprivation therapy (ADT), the current standard of care in the management of advanced PCa, through a rapid and dramatic increase in bone turnover and body mass changes. As a result, patients may experience substantial pain and poor quality of life (QoL) and have an increased risk of death. Notwithstanding the importance of this issue, however, bone health preservation is not yet a widespread clinical goal in daily practice.
To address this urgent unmet need, following a thorough discussion of available data and sharing of their clinical practice experience, a panel of Italian experts in the field of bone health and metabolism formulated a number of practical advices for optimising the monitoring and treatment of bone health in men undergoing ADT during all phases of the disease. The rationale behind the venture was to raise awareness on the importance of bone preservation in this complex setting, while providing an instrument to support physicians and facilitate the management of bone health.
Current evidence regarding the effects on bone health of ADT, of novel hormone therapies (which improve progression delay, pain control and QoL while consistently carrying the risk of non-pathological fractures in both non-metastatic and metastatic PCa) and of bone turnover inhibitors (whose use is frequently suboptimal) is reviewed. Finally, the expert opinion to optimise bone health preservation is given.
Keywords: androgen-deprivation therapy, advanced prostate cancer, bone health, novel hormone therapy, management
Introduction
Prostate cancer (PCa) remains the most frequent male cancer in Italy (1 in 9 men; 19% of all cancer diagnoses), with 37 000 new cases estimated in 2019, and represents the third leading cause of death in the population, the mortality rate being 2.4% among those diagnosed with PCa.1 However, registry data indicate that incidence is decreasing and survival improving, with a 5-year survival rate of 92% and a 10-year survival rate of 90%.1
Advanced PCa exhibits a high bone tropism, which is responsible for the skeletal involvement observed in up to 90% of the cases.2 For this reason, bone must be a target of clinical management throughout the course of the disease.
Androgen-deprivation therapy (ADT) represents a standard of care in the management of advanced PCa.3 Despite the potential benefits associated to its use, however, ADT causes a number of side effects, including a detrimental effect on bone health4–7; this is even more concerning considering the longer life expectancy achieved in these patients, and the possible changes on the bone fostered also by ageing and comorbidities. Due to the sequelae of bone health impairment on the individual’s quality of life (QoL) and health status, together with the considerable burden imposed on healthcare resources, preserving bone health in PCa men on ADT must be a clinical goal across the disease continuum. Notwithstanding the importance of this issue, however, several aspects of bone health are not yet supported by strong evidence. Consequently, they are not completely accounted for in many important international guidelines.8
Here, the available evidence on bone health during ADT and the effects of novel hormone therapies (NHTs) and bone turnover inhibitors (BTTs) on the bone are reviewed; in addition, the advices of a panel of Italian experts are provided to optimise bone health monitoring and treatment in advanced PCa.
Bone health during ADT
Effects of ADT on bone loss and fragility
In patients with PCa, bone health is frequently suboptimal already before commencing ADT: indeed, the prevalence of osteoporosis/osteopenia among ADT-naive patients ranges between 35% and 58%, with similar rates between localised and disseminated disease; still, this condition remains undiagnosed in the majority of cases,9 and approximately 30% of patients displaying ≥1 grade-2 fracture before starting ADT have normal bone mineral density (BMD).10 Moreover, PCa itself is associated to a high risk of fractures (OR (95% CI) for all fractures: 1.8 (1.6 to 2.1) in PCa vs age‐matched control men), which is further increased by the use of ADT (OR 1.7 (1.2 to 2.5), p<0.01).11
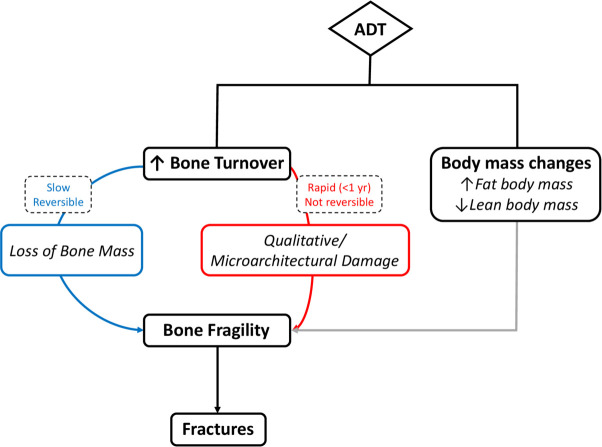
In men, bone remodelling and microstructure are directly affected by testosterone (T) levels, whereas the development and maintenance of the skeleton are predominantly regulated by estradiol (E2), acting as the main inhibitor of bone resorption. By reducing serum T levels to a castration range of values (<5% of the normal range) and serum E2 levels to <20% of the normal range,12 ADT causes a rapid and dramatic increase of bone turnover that results in bone loss (generally slow and reversible) and in qualitative/microarchitectural damage (often rapid and not reversible)13 (figure 1, left). Accordingly, the rate of bone loss recorded immediately after the start of ADT is 4%–4.6% per year, higher than the normal rate of approximately 0.5%–2% per year.14–16 Of interest, among the ADT regimens tested, addition of bicalutamide to gonadotropin-releasing hormone (GnRH) agonists did not worsen BMD loss compared with GnRH agonists alone.17
Figure 1.
ADT-induced bone impairment. ADT augments bone fragility, and thus the risk of fracture, through two mechanisms: (1) bone turnover increase, which leads to bone mass loss via a slow, reversible process, and to qualitative/microarchitectural alterations via a rapid, non-reversible mechanism; (2) body mass changes, namely increased fat body mass and decreased lean body mass. ADT, androgen-deprivation therapy.
Both mechanisms (ie, bone loss and qualitative/microarchitectural damage) increase bone fragility that may ultimately cause fractures.7 13 18–21 A large population-based study demonstrated that, of men surviving at least 5 years after diagnosis, a significantly higher proportion of those treated with ADT versus without experienced a fracture (19.4% vs 12.6%, p<0.001), and the fracture risk increased with the number of ADT doses administered during the first year after diagnosis.20 In this regard, however, it is worth noting that the use of intermittent versus continuous ADT in older men did not yield a significant reduction in bone events (26% vs 31%, respectively, p=0.15).22 23
In men affected by PCa on ADT, similarly to women with breast cancer treated with aromatase inhibitors (AIs), fractures (especially vertebral21) typically occur during the first year of therapy19 20 24 25 as a consequence of the rapid qualitative damage determined by elevated bone turnover. Other risk factors for fractures are older age, a history of fracture, osteoporosis and the rate of bone loss during treatment.26 27 Yet, it must be pointed out that the risk of fracture is often independent of BMD13 and it is frequently misclassified when based only on dual-energy X-ray absorptiometry (DEXA) measurements (see section 5)18 28; this observation reinforces the fact that skeletal fragility is prominently dependent on the poor quality of bone microarchitecture rather than on the low bone mass.
In patients with PCa with bone disease (both hormone-sensitive PCa (HSPC) and castration-resistant PCa (CRPC)), the rate of pathological fractures ranges between 5% and 48%.29–32 They are associated with increased risk of QoL impairment and death in men with malignant bone disease.33 34 Regardless of the setting (HS or CR), however, men with metastatic (M1) disease may also experience fractures in non-metastatic sites as a consequence of long-term ADT: yet, since these fractures can be asymptomatic,30 they are often overlooked and underdiagnosed. Furthermore, bone fragility may predispose patients with bone metastases to skeletal-related events (SREs). Therefore, preventing fragility fractures is an important goal also in patients with bone metastases considered at risk for skeletal complications. In this regard, it is likely that bone health is more preserved in men with M1 CRPC and bone metastases compared with those with non-metastatic (M0) PCa, due to the frequent concomitant administration of BTTs, which may protect also from fragility fractures (see section 4).
Besides the quantitative and qualitative alterations of bone that increase the fracture risk, ADT is associated with consistent changes in body composition, namely increased fat body mass and decreased lean body mass,35 36 that may impair bone health (figure 1, right). Indeed, obesity may negatively affect bone quality via several mechanisms, including alteration of bone-regulating hormones, increased oxidative stress and inflammation. In healthy subjects, the negative effect of adiposity on bone health is blunted by the higher oestrogen levels (due to enhanced aromatase activity) that increase BMD (the so-called ‘obesity paradox’). Obese men on ADT may be at higher risk of bone fractures because of the loss of the protection associated with oestrogens and to the detrimental changes in bone quality associated with adiposity. In a large single institution cross-sectional study recently published,37 fat body mass assessed by DEXA scan had a protective effect on morphometric vertebral fractures in patients with breast cancer not undergoing AIs, whereas it was associated with an increased risk of fragility fractures in women on AIs. Evidence supports the obesity paradox even in advanced PCa,38 where early increase in fat body mass has recently been shown to predict a higher risk of SRE (HR 3.024, 95% CI 1.004 to 10.353, p<0.02), a higher risk of death (HR 2.373, 95% CI 1.012 to 5.567, p=0.04) and a non-significant higher risk of disease recurrence (HR 2.219, 95% CI 0.956 to 5.150, p=0.13).39 As for ADT-associated sarcopenia,40 it further increases the risk of fractures through falls and directs effects on the skeleton geometry and microstructure. When decreased muscle mass, strength and function occur concomitantly to BMD reduction, osteosarcopenia is diagnosed.40
In clinical practice, since bone fragility may be present already before the start of ADT and throughout the disease continuum, close attention should be paid to bone health. The early onset of fractures should be taken into account when managing the fracture risk and treatment timing. Moreover, it is important to plan strategies to prevent, assess and treat both osteoporosis and sarcopenia, to reduce the associated risk of falls, fractures and consequent disability.40
Skeletal effects of ADT
SREs (ie, pathological fractures, radiotherapy to bone, bone surgery and spinal cord compression) are associated with worse outcomes, including increased pain, poorer QoL, morbidity and shorter survival, and may occur throughout the entire course of the disease.
Fragility fractures are associated to increased mortality both in the general population41 and in patients with PCa on ADT.6 42–44 Van Hemelrijck et al demonstrated that men with a hip fracture were 2.4 times more likely to die than the control cohort of all PCa men (95% CI 2.29 to 2.60), and the risk was higher especially in the first month after the fracture (HR 5.64 (95% CI 4.16 to 7.48)).45 In another study, men who developed a fracture within 48 months of cancer diagnosis had a significantly lower survival than men who did not (log-rank test: p<0.001), and the mortality risk increased by 40% after experiencing a fracture.42 Moreover, ADT has been associated with a significantly increased risk of any fracture and hip fracture requiring hospitalisation: the excess risk was partly driven by pathological fractures and spinal cord compression, which are associated with decreased survival in ADT users.6
In clinical practice, accounting for patient risk before prescribing ADT for long-term use together with the close monitoring of bone health during ADT may reduce the risk of fracture and improve QoL and survival.6 42
Bone health during NHT
As already mentioned, the propensity of PCa cells to metastasise to the bone increases the risk of SREs, which, in turn, increase mortality and substantial pain, and reduce patient QoL.
In the last decade, NHTs have been approved for the treatment of advanced PCa based on the survival benefit demonstrated in pivotal phase III randomised controlled trials (RCTs). Table 1 summarises the agents currently available in Europe. Moreover, the European Medicines Agency has recently received a marketing authorisation application for the selective AR antagonist darolutamide.46 47
Table 1.
New hormone therapies currently approved by EMA in the different settings of PCA
| Drug | Setting | Phase III trial | Year of EMA approval |
| M0 disease | |||
| Enza | CRPC at high risk of metastases* | PROSPER48 | 2018 |
| Apa | CRPC at high risk of metastases* | SPARTAN49 | 2019 |
| M1 disease | |||
| AAP | Post-CT CRPC | COU-AA-30199 | 2011 |
| AAP | CT-naive CRPC | COU-AA-30267 | 2012 |
| AAP | Newly diagnosed high-risk HSPC | LATITUDE57 58 | 2017 |
| Enza | Post-CT CRPC | AFFIRM59 | 2012 |
| Enza | CT-naive CRPC | PREVAIL60 | 2014 |
*Baseline PSA level of 2 ng per millilitre or greater, and a PSA doubling time of 10 months or less.
AAP, abiraterone acetate plus prednisone; Apa, apalutamide; CRPC, castration-resistant prostate cancer; CT, chemotherapy; EMA, European Medicines Agency; Enza, enzalutamide; HSPC, hormone-sensitive prostate cancer; M0, non-metastatic; M1, metastatic; PSA, prostate-specific antigen.
Registration trials included bone-related efficacy endpoints, namely radiographic progression-free survival (rPFS) or metastasis-free survival (MFS), time to first skeletal-related event (tSRE), pain control and QoL deterioration, and the rate of non-pathological fractures for safety. In particular, MFS has been used as primary endpoint in alternative to overall survival (OS) in recent trials conducted in the setting of CRPC without overt metastatic disease detected by instrumental staging.48–50 Indeed, in some diseases and treatment settings in which patients have a long life expectancy, post-progression survival (PPS) increases and, consequently, the likelihood that an advantage in terms of progression-free survival (PFS) translates into a significant prolongation of OS (defined as the sum of PFS and PPS) over an acceptable time frame decreases substantially.51 Therefore, in practice, the use of surrogate endpoints may overcome the need for a much larger sample size and longer follow-up (thus expediting trial completion), as well as the ‘dilution’ effect determined by subsequent post-progression treatments that may confound the measurement of OS. Notably, a high correlation between MFS and OS has been demonstrated both at trial and patient level in a meta-analysis based on individual patient data from 12 712 men included in 19 studies.52 However, as acknowledged also by the Food and Drug Administration (FDA), the benefit yielded by alternative endpoints must go beyond statistical significance and be clinically meaningful.53 In the case of MFS, for example, the magnitude of the benefit provided by denosumab (DNB) was not deemed as valid for FDA approval of a new indication,50 54 while it was in the case of apalutamide and enzalutamide: in these cases, in fact, median MFS was dramatically higher than the few-month difference yielded by DNB in the same clinical setting (table 2).48 49 However, some caution must be taken when interpreting MFS results, as occurrence of a bone metastasis (the main contributor to MFS in PCa) per se is not always a clinically meaningful event.55 Due to the psychological implications of being diagnosed with metastatic disease, it is important to use patient-reported outcomes to match the instrumental data of MFS with the actual benefit in terms of both the delay of time to symptom worsening and global QoL. It is worth noting that in the most recent trials on PCa, the advantage in MFS is well supported by evidence of clinical benefit in terms of improvement in PFS on next-line therapy (PFS2),49 56 symptom delay and pain progression46 49 and QoL.48 49 57–63
Table 2.
Bone-related efficacy endpoints
| Drug | Trial | Endpoint | NHT vs placebo |
| M0 CRPC at high risk for metastases | |||
| Enzalutamide | PROSPER48 | MFS PROs |
36.3 vs 14.7 months (HR for metastasis or death 0.29, 95% CI 0.24 to 0.35, p<0.001) Similar clinically meaningful deterioration of HRQoL |
| Apalutamide | SPARTAN49 56 | MFS PFS2 Median time to symptomatic progression PROs |
40.5 vs 16.2 months (HR for metastasis or death 0.28, 95% CI 0.23 to 0.35, p<0.001) 55.6 vs 43.8 months (HR 0.55, 95% CI 0.45 to 0.68, p<0.0001) NR vs NR (HR 0.45, 95% CI 0.32 to 0.63, p<0.001) Stable overall HRQoL over time, similar between groups |
| Darolutamide | ARAMIS46 | Median MFS | 40.4 vs 18.4 months (HR for metastasis or death 0.41, 95% CI 0.34 to 0.50, p<0.001) |
| Median time to pain progression | 40.3 vs 25.4 months (HR 0.65, 95% CI 0.53 to 0.79, p<0.001) | ||
| Median time to first symptomatic SRE | NR in either group (16 vs 18 events, HR 0.43, 95% CI 0.22 to 0.84, p=0.01) | ||
| M1 HSPC | |||
| AAP | LATITUDE57 58 61 62 100 | Median rPFS Median time until pain progression Median time to next symptomatic skeletal events PROs |
33.0 vs 14.8 months (HR 0.47, 95% CI 0.39 to 0.55, p<0.001) 47.4 vs 16.6 months (HR 0.72, 95% CI 0.61 to 0.86, p=0.0002) NR vs NR (HR 0.75, 95% CI 0.60 to 0.95, p=0.0181) Clinical benefit in pain progression, PCa symptoms, fatigue, functional decline and overall HRQoL |
| Apalutamide | TITAN101 | Median rPFS 2-year OS rate |
NR vs 22.1 months (HR 0.48, 95% CI 0.39 to 0.60, p<0.0001) 82% vs 73% |
| M1 CRPC | |||
| AAP | COU-AA-30199 102 | Median rPFS Median time to the first SRE Pain |
5.6 vs 3.6 months (HR 0.67, 95% CI 0.58 to 0.78, p<0.001) 25.0 vs 20.3 months (HR 0.62, 95% CI 0.48 to 0.80, p=0.0001) Significant improvement in pain relief and delay of pain progression |
| AAP | COU-AA-30267 103 104 | Median rPFS Median time to opiate use for cancer-related pain PROs |
16.5 vs 8.2 months (HR 0.52, 95% CI 0.45 to 0.61, p<0.0001) NR vs 23.7 months (HR 0.71, 95% CI 0.59 to 0.85, p=0.0002) Consistent pattern of delays in pain progression and significant delayed degradation in FACT-P total scores (p=0.005) |
| Enzalutamide | AFFIRM59 | rPFS Time to the first SRE QoL response rate |
8.3 vs 2.9 months (HR 0.40, 95% CI 0.35 to 0.47, p<0.001) 16.7 vs 13.3 months (HR 0.69, 95% CI 0.57 to 0.84, p<0.001) 43% vs 18%, p<0.001 |
| Enzalutamide | PREVAIL60 | Median rPFS First SRE occurrence Median time to QoL deterioration |
NR vs 3.9 months (HR 0.19, 95% CI 0.15 to 0.23, p<0.001) 32% vs 37% at 31 months, (HR 0.72, p<0.001) 11.3 vs 5.6 months (HR 0.63, 95% CI 0.54 to 0.73, p<0.001) |
AAP, abiraterone acetate plus prednisone; CRPC, castration-resistant prostate cancer; FACT-P, Functional Assessment of Cancer Therapy–Prostate; HRQoL, health-related quality of life; HSPC, hormone-sensitive prostate cancer; M0, non-metastatic; M1, metastatic; MFS, metastasis-free survival; NHT, novel hormone therapy; NR, not reached; OS, overall survival; PCa, prostate cancer; PFS2, progression-free survival on next-line therapy; PRO, patient-reported outcome; QoL, quality of life; rPFS, radiographic progression-free survival; SRE, skeletal-related event.
Hereinafter, the main clinical trial results are summarised, together with the real-world evidence available.
Bone-related efficacy endpoints during NHT
In all, the available data demonstrate that patients with CRPC or with M1 HSPC and overt bone disease may benefit from the use of NHT as for progression delay, pain control and QoL improvement (table 2). With regard to the combined use of radiopharmaceuticals and NHT, caution must be taken, as demonstrated by the recent ERA 223 study in which men with chemotherapy (CT)-naive asymptomatic or mildly symptomatic M1 CRPC treated with abiraterone acetate plus prednisone/prednisolone had a median symptomatic skeletal event-free survival of 26.0 months (95% CI 21.8 to 28.3).30 However, adding the bone-seeking calcium mimetic radium 223 (Rad-223) increased fractures (29% vs 11%) and deaths (39% vs 36%), while it did not improve skeletal event-free survival (22.3 months (95% CI 20.4 to 24.8)), so that the combination is not recommended.30
In the setting of M1 CRPC, no direct comparison exists between abiraterone and enzalutamide; yet, a recent meta-analysis of registration trials demonstrated no significant difference in terms of rPFS and of tSRE.64 In the real-world setting, a retrospective study on 1516 M1 CRPC men reported that those who had initiated on abiraterone acetate first had better SRE outcomes than those who had initiated on enzalutamide, who had a higher incidence rate (1.86 with enzalutamide vs 1.47 with abiraterone acetate; incidence rate ratio 1.27, p=0.044) and a higher hazard of SREs (HR 1.34 (95% CI 1.06 to 1.69); p=0.015).65 The effectiveness of abiraterone acetate plus prednisone was investigated in the large Italian multicentre, prospective observational study ABITUDE66: in patients with CT-naive M1 CRPC, the 1-year probability of no radiographic progression was 73.9%, and a reduction in pain intensity and worst pain perception together with improvement in daily activity interference was observed, in line with the findings from COU-AA-302.67
As for the effects of NHT on the levels of bone biomarkers, few data are available and are mostly limited to abiraterone acetate. Treatment with this agent plus prednisone significantly reduced the levels of serum C-terminal cross-linked telopeptide of type I collagen (CTX) and bone-specific alkaline phosphatase (BALP) after 6 and 12 months from the start of therapy, likely because of the decrease of bone turnover activity and of bone tumour burden, respectively (unpublished data from ABITUDE). It is worth noting that preclinical data have suggested a direct effect of abiraterone acetate on bone microenvironment: indeed, in an in vitro model of human primary osteoclasts (OCLs)/osteoblasts (OBLs), non-cytotoxic doses of abiraterone acetate inhibited OCL differentiation and activity and stimulated OBL differentiation and bone matrix deposition.68
Bone safety of NHT: rate of non-pathological fractures
Despite the clinical benefit provided by NHT, evaluation of bone health in terms of non-pathological fracture rate in both M0 and M1 settings has unveiled that these consistently represent a common adverse event during treatment with all drugs tested. The rates recorded in the phase III trials are presented in table 3.
Table 3.
Rate of non-pathological fractures in phase III trials of NHT by setting, grade (all and 3–4) and treatment arm (NHT vs placebo)
| Trial | Non-pathological fractures | |||
| All grade (%) | Grades 3–4 (%) | |||
| NHT | Placebo | NHT | Placebo | |
| M0 CRPC | ||||
| SPARTAN (Apa, n=806; placebo, n=401)49 | 11.7 | 6.5 | 2.7 | 0.8 |
| PROSPER (Enza, n=933; placebo, n=468)105 | 11.0 | 4.1 | 1.3 | 0.6 |
| M1 CRPC | ||||
| Post-CT | ||||
| COU-AA-301 (AAP, n=791; placebo, n=394)106 | 5.9 | 2.3 | 1.4 | 0.0 |
| AFFIRM (Enza, n=800; placebo, n=399)105 | 4.0 | 0.8 | 1.4 | 0.3 |
| Pre-CT | ||||
| PREVAIL (Enza, n=871; placebo, n=844)105 | 8.8 | 3.0 | 2.1 | 1.1 |
| EORTC 1333/PEACE III (Enza+Rad-223, n=38; Enza, n=38)82 | *12.4 | |||
| ERA-223 (AAP+Rad-223, n=401; vs AAP+placebo, n=405)30† | 11 | |||
Only currently approved agents are reported.
*IThe rate reported refers to the 1-year cumulative incidence of non-pathological fractures in the Enza arm.
†The rate reported refers to the rate of non-pathological fracures in the AAP+placebo arm.
AAP, abiraterone acetate plus prednisone; Apa, apalutamide; M0 CRPC, non-metastatic castration-resistant prostate cancer; M1 CRPC, metastatic castration-resistant prostate cancer; CT, chemotherapy; Enza, enzalutamide; NHT, novel hormone therapy.
Overall, results from SPARTAN49 and PROSPER suggest that treatment with new-generation HT further increases the fracture risk in men with M0 CRPC receiving long-term ADT. The rate of non-pathological fractures was higher on NHT, compared with placebo, also in men with M1 CRPC in both pre-CT and post-CT settings. Although the mechanisms causing non-pathological fractures remain unclear, it is possible that a more potent inhibition of testosterone activity may enhance bone turnover, ultimately causing fragility. It is also possible that patients receiving NHT were exposed to prolonged treatment, and therefore observed for a longer period compared with those given placebo.
Effects of different bone turnover inhibitors
Cancer treatment–induced bone loss (CTIBL) is generally more rapid and severe than bone loss associated with ageing in men and women or menopause.15 Among the agents tested for their ability to attenuate CTIBL in patients with PCa, there are oral (alendronate and risedronate) or intravenous (pamidronate and zoledronic acid (ZA)) bisphosphonates (BPs) and DNB.
M0 HS disease
In the setting of M0 HS disease on ADT, all BTTs at all schedules and doses used were able to prevent bone loss and/or improve BMD compared with placebo.69–74 However, whether this translates into reduced fractures remains unclear, as no RCTs were designed with fracture risk reduction as primary endpoint. Furthermore, the majority of RCTs include a relatively small number of patients and short follow-up periods, underpowered to detect evidence of any fracture reduction.75–77 The only agent that demonstrated effective in reducing the incidence of new vertebral fractures is DNB (1.5% vs 3.9% with placebo at 36 months; RR 0.38, 95% CI 0.19 to 0.78; p=0.006), in a large RCT where this was a secondary endpoint.25 Therefore, more trials are needed in this population to evaluate the effects of BTTs on fracture outcomes as well as on other outcomes relevant to patients, such as QoL, pain and disability.69 75
M1 HSPC disease
In men with M1 HSPC and bone metastases, early treatment with ZA in the CALGB 90202 study yielded no benefit, compared with placebo, in terms of time to first SRE and OS.78 In the same setting, no benefit with regard to the time of treatment failure, tSRE and OS was provided by ZA in the ZAPCA trial, except for a significant delay of treatment failure in patients with baseline prostate-specific antigen <200 ng/mL.79 Accordingly, current guidelines do not recommend the use of ZA or DNB in patients with M1 HSPC.3 80 As ZA 4 mg monthly is no more suggested in SRE prevention in M1 HSPC and DNB 120 mg monthly has not been studied, it is likely that these patients, who are exposed to the risk of CTIBL at a similar or higher extent than those with M0 disease, do not receive any protection from fragility fracture risk.
M1 CRPC disease
As for patients with M1 CRPC and bone metastases, a post hoc analysis of the COU-AA-302 trial demonstrated that, in CT-naive men, the concomitant use of BTTs, compared with no BTT use, further increased the clinical advantage observed on abiraterone acetate plus prednisone compared with prednisone alone in terms of OS, time to ECOG deterioration and time to opiate use for cancer-related pain.81 Moreover, the recent ERA-223 trial demonstrated that, in patients with CT-naive asymptomatic or paucisymptomatic M1 CRPC on abiraterone acetate plus prednisone/prednisolone and randomised to receive Rad-223 or placebo, the use of BPs or DNB halved the number of patients with osteoporotic fractures in both arms (from 37% in the Rad-223 arm and 15% in the placebo arm without BTTs, to 15% and 7%, respectively, with BTTs).30 Similarly, early data from the EORTC 1333/PEACE III trial comparing enzalutamide and Rad-223 versus enzalutamide alone show that the risk of fractures is very well controlled in both arms, the cumulative 1-year risk of fracture being 37.4% and 12.4%, respectively, without BTTs, and 0% in both arms with BTTs.82
Moreover, in patients with M1 CRPC with bone metastases, ZA proved inferior to DNB in delaying occurrence of the first SRE,83 and it ameliorated PFS, skeletal pain and SRE only in men with a Gleason score ≥8.84 Yet, in the setting of M1 disease, no other data on prevention of fragility fractures are available, and those regarding prevention of BMD loss and the effects on pathological fractures versus fragility fractures among SREs are completely lacking.
In clinical practice, all patients with M1 CRPC and bone metastases should be given supportive treatment to preserve bone health. Yet, data regarding the real-world patterns of use of BTTs in subjects with bone metastases have unveiled that there is a considerable proportion of patients who do not receive adequate treatment to prevent SREs or manage pain. For example, in the Italian observational study ABITUDE, only approximately 14% of patients were given ZA.66 Moreover, a recent multinational European study reported that 26% of patients with bone metastases did not receive a bone-targeting agent (BTA), and only 53% received treatment within 3 months of bone metastasis (BM) diagnosis.85 Interestingly, oncologists more than urologists prescribed BTAs (78% vs 60%) and initiated treatment within 3 months of BM diagnosis (56% vs 43%). Bone pain was common and undertreated, as demonstrated by the fact that although most patients with BMs (97%) were on analgesics, with 30% receiving strong opioids, 70% were experiencing bone pain, which was moderate to severe in 28%.85 In another recent retrospective study of 2559 men with M1 CRPC, overall, 34% of patients did not use bone health agents at any time. Notably, DNB was used more frequently than ZA (48% vs 24%, respectively86); 58.2% vs 41.8% in another study including 3816 men with PCa and bone metastases.87
Altogether, these data suggest that awareness must be raised on the importance of using BTTs in the management of patients with M1 CRPC to improve care.85 Importantly, to avoid underuse or misuse, BTTs should be employed in selected cases taking into consideration that the effects on bone fragility (ie, prevention) and SRE prevention in metastatic disease depend on the dose given.
Optimising bone health management: an expert opinion
Bone health preservation throughout the continuum of PCa disease represents a prerequisite for acceptable QoL and optimal disease outcome. However, in clinical practice, this is not yet a widespread clinical goal.8
With this in mind, in April 2019, a panel of Italian experts (all authors of the present document) in the field of bone health and metabolism at the national and international level gathered in an advisory board meeting to address this urgent unmet need. The rationale behind the venture was to raise awareness on the importance of bone preservation in this complex setting while providing an instrument to support physicians and facilitate the management of bone heath. Following a thorough discussion of available data and sharing of their clinical practice experience, the experts formulated a number of advices for optimising the monitoring and treatment of patients with PCa on ADT to preserve bone health. Importantly, bone health preservation was addressed in all the phases of the disease, that is, M0 HSPC, M0 CRPC, M1 HSCP and M1 CRPC. The opinions for which the experts reached a 100% agreement are reported hereinafter and the advices are summarised in tables 4 and 5. As they pointed out, the implementation of the experts’ suggestions depends on the reimbursement policy adopted by each country.
Table 4.
Experts’ advices on monitoring modalities by setting
| Non-metastatic disease | Metastatic disease |
| Early management of bone health is mandatory from the start of hormonal therapy and at least throughout its course, regardless of the blockade scheme | Monitor metastases by scintigraphy, NMR or any other evaluation at physician’s discretion and pay closer attention to bone health |
| Assess the risk of fracture | Assess the risk of fracture |
|
Same as for non-metastatic disease |
|
|
| When feasible, perform the following evaluations at baseline and every 12–18 months afterwards | When feasible, perform the following evaluations at baseline and every 12–18 months afterwards |
|
Same as for non-metastatic disease However, when assessing vitamin D, serum calcium and PTH, pay closer attention to the serum levels of these prognostic markers since ongoing administration of BPs or DNB therapies (at the dose for SRE prevention) may cause hypocalcemia |
| Do not overlook pain | Do not overlook pain |
|
Same as for non-metastatic disease |
| In the adjuvant setting of M0 HSPC, reassess the fracture risk at the end of hormonal therapy: if the patient experienced no fracture during treatment, no particular monitoring will be necessary; otherwise, monitoring should be continued; if the patient presents any additional risk factor (eg, new fracture), monitoring and therapy must be carried on | |
| In case of M0 CRPC, it is strongly advised to continue with the same monitoring scheme adopted in case of M0 HS disease, but with closer attention to bone health |
Unless specified, advices are valid for both settings. For detailed explanation, see the text.
HSPC hormone-sensitive prostate cancer; ALP, alkaline phosphatase; BMD, bone mineral density; BMI, body mass index; BP, bisphosphonate; CRPC, castration-resistant prostate cancer; CTX, C-terminal cross-linked telopeptide of type I collagen; DEXA, dual-energy X-ray absorptiometry; DNB, denosumab; M0, non-metastatic; MXA, morphometric X-ray absorptiometry; NMR, nuclear magnetic resonance; P1NP, procollagen type 1 N-terminal propeptide; PTH, parathyroid hormone; SRE, skeletal-related event.
Table 5.
Experts’ advices on treatment modalities by setting
| Non-metastatic disease | Metastatic disease |
| Therapeutic thresholds and modalities are the same for M0 HSPC and M0 CRPC | In the setting of M1 HSPC, the therapeutic schedule of BTTs is that used for osteoporosis (the same of M0 CRPC), not for metastases |
| Before starting any therapy specifically targeting the bone, evaluate and normalise the levels of vitamin D (≥30 ng/mL) during hormonal therapy, regardless of the bone-modifying agent | Intervention for metastatic disease in M1 CRPC is indicated at the time of diagnosis of the first metastasis as per all guidelines, and it is aimed at reducing SREs; the regimen employed both for ZA and DNB will widely cover also the possibility to reduce the risk of fragility fractures (benign fractures) |
| Vitamin D supplementation during bone-modifying agents is mandatory | In case of M1 CRPC, consider the opportunity to continue therapy with bone-modifying agents adjusting the dosages for bone health in case of discontinuation of SRE-specific treatment. In particular, caution must be paid when using DNB |
| Do not consider vitamin D and calcium supplementation as sufficient to maintain bone health or prevent fragility fractures | |
| Physical activity and an adequate calcium intake are advised to avoid weight gain, reduce the risk of fall and for the likely positive impact on bone health | |
| The posology used for DNB is the same used in case of osteoporosis in both men and women; for a BP, a wide spectrum of doses has been proposed, sometimes even higher than those used for osteoporosis | |
| Start treatment with bone-modifying agents as soon as possible regardless of BMD even in M0 HSPC (no strict recommendations exist on PCa) |
Unless specified, advices are valid for both settings. For detailed explanation, see the text.
BMD, bone mineral density; BP, bisphosphonate; BTT, bone turnover inhibitor; CRPC, castration-resistant prostate cancer; DNB, denosumab; HSPC, hormone-sensitive prostate cancer; M0, non-metastatic; M1, metastatic; PCa, prostate cancer; SRE, skeletal-related event; ZA, zoledronic acid.
In general, the experts advise, whenever possible and regardless of the setting, to evaluate bone health in a multidisciplinary context including other ‘bone specialists’ (rheumatologists, endocrinologists, geriatrics, orthopaedics) besides oncologists, radiotherapists and urologists. Importantly, the collaboration with a ‘bone specialist’ does not spare oncologists and urologists from monitoring and treating bone health.
Monitoring of bone health
Androgen and oestrogen deprivation increase bone loss, which is currently measured by BMD through DEXA scan. Indeed, BMD is considered a valid surrogate parameter of fracture risk in osteoporotic but otherwise healthy women and men, and current guidelines recommend the use of BMD as a parameter in the assessment of fracture risk among men on ADT and early breast cancer women on aromatase inhibitor therapy. In particular, international guidelines recommend that patients with PCa eligible for ADT should undergo basal and follow-up evaluation of BMD, as well as assessment of the 10-year fracture risk through the FRAX score. The latter takes into consideration the following risk factors, besides BMD: age, sex, weight, height, previous fracture, parent fractured hip, current smoking, glucocorticoids, rheumatoid arthritis, secondary osteoporosis and alcohol (≥3 units/day). Moreover, many guidelines have adopted a DEXA T-score threshold <−2.5 for treatment.15 76 88–90 It should be noted, however, that in patients with PCa undergoing ADT, the increased risk of fracture is often independent of BMD13 and, as fractures occur even with BMD T-score ranging between normal to osteopenic values, calculating the fracture risk based only on DEXA measurements can be misleading.18 28 Therefore, it is not surprising that the FRAX algorithm91 for fracture risk prediction underestimates the risk in patients with PCa on ADT when BMD is used, and it performs better when used without imputing BMD. Recently, a dedicated algorithm for the assessment of bone microarchitecture at the lumbar spine (LS), the trabecular bone score (TBS), has been introduced. TBS is a textural index based on the evaluation of the pixel grey-level variations in the LS DEXA image, and, thus, represents an indirect index of bone architecture that can assess bone quality and provide information about the fracture risk independently of BMD.92 Therefore, TBS seems to be a better measure of bone fragility in individuals who are obese/overweight, and useful in assessing the osteoporotic fracture risk, with lower TBS values associated to a higher risk. Also, it could be suitable to improve the fracture risk definition in patients with CTIBL and could be usefully combined with FRAX and BMD to optimise the identification of patients with breast cancer and elevated risk.93 However, it has not been validated in PCa, and, therefore, no recommendation for its routine use can be made. Finally, the experts underlined that, in case of metastatic HS disease, no study has demonstrated the efficacy of DNB and BTT in pathological SRE reduction. For this reason, in the setting of M1 HS disease, the goal of bone health preservation (bone fragility protection) can be achieved using the same strategy as in M0 HS disease.
In light of these data, the experts formulated the following advices, valid regardless of the setting and hormonal therapy:
The use of the WHO risk assessment tool FRAX91 as the most frequently used tool in clinical practice to evaluate the 10-year probability of osteoporotic fractures is discouraged, as it was not specifically designed for men receiving ADT and, indeed, it does not account for important clinical factors unique for this vulnerable population (eg, hormonal therapy); besides, it does not allow an adequate risk stratification.90 The FRAX score should integrate the following.
-
Evaluate the following independent factors of fracture risk:
BMD.
Familiarity for fragility fractures.
Corticosteroid therapy (>5 mg/prednisone equivalent in the past for more than 3 months consecutively or ongoing).
Metabolic bone diseases or fragilising disease/treatment.
Disability or high risk of fall.
Age.
Anamnesis for low-energy trauma fractures.
Non-metastatic disease
Early management of bone health is mandatory from the start of hormonal therapy and at least throughout its course, regardless of the blockade scheme.
-
When feasible, it is advised to perform the following evaluations at baseline and every 12–18 months afterwards:
Bone turnover markers (bone ALP).
Vitamin D, serum calcium and parathyroid hormone (PTH) (the latter in combination with calcium and vitamin D to differentiate between primary or secondary hyperparathyroidism).
DEXA scan (for BMD and if available vertebral morphometry (MXA)).
Height, weight and body mass index (BMI).
If feasible, evaluate body composition (by DEXA, bioelectrical impedance or plicometry) besides BMI.
Do not overlook back pain.
-
In case of back pain or height loss (as a reduction by ≥1 cm/year predicts a 98% probability of vertebral fracture),94 perform a spine radiography for early identification of prevalent vertebral fractures.
In the adjuvant setting of M0 HSPC, it is suggested to reassess the fracture risk at the end of hormonal therapy: if the patient experienced no fracture during treatment, no particular monitoring will be necessary; otherwise, monitoring should be continued if the patient present any additional risk factor (eg, new fracture) and therapy must be carried on.
In case of M0 CRPC, it is strongly advised to continue with the same monitoring scheme adopted in case of M0 HS disease, but with closer attention to bone health, as the longer duration of hormonal therapy exposes patients to a higher risk of bone impairment.
Metastatic disease
It is advised to pay close attention to bone health also in the metastatic setting.
Besides monitoring metastases (by scintigraphy, NMR or any other evaluation at physician’s discretion), the monitoring strategy for bone health is the same as for M0 PCa, but with closer attention when evaluating the serum levels of vitamin D, serum calcium and PTH as prognostic markers, since ongoing administration of BPs or DNB therapies (at the dose for SRE prevention) may cause hypocalcemia.
In case of back pain or height loss, perform a spine radiography with the aim to early identify morphometric fractures.
Options of treatment for bone health
Available therapies in the different settings have been described above. As for BTTs, in Italy they are used, in the M0 setting, also in primary prevention and reimbursement is higher than abroad. The use of such agents is mandatory in case of T-score <2.5, but it can be suggested even with normal T-score in patients on ADT, based on the relevance of hormonotherapy as a risk factor for fractures, independently from basal T-score levels. As summarised in section 4, the use of BTTs in M1 CRPC disease may help preserve bone health in terms of fragility fracture prevention.
Non-metastatic disease
Therapeutic thresholds and modalities are the same for M0 HS and M0 CR disease.
Before starting any therapy specifically targeting the bone, evaluate and normalise the levels of vitamin D (≥30 ng/mL) during hormonal therapy, regardless of the bone-modifying agent.
Vitamin D supplementation during bone-modifying agents is mandatory. Regardless of the threshold to be reached, it is difficult to suggest simple rules to follow that can be adapted to all individuals. The Italian Society of Osteoporosis and Mineral and Skeletal Metabolism, in line with the Endocrine Society, suggests the administration of a daily dose of 1500–2000 IU, to reach and maintain the value of 30 ng/mL (75 nmol/L).95 96 A rapid correction of hypovitaminosis D is indicated in candidates for potent anti-resorptive therapy (ie, with a rapid effect), such as BPPs and DNB. The initial loading dose could be calculated on the basis of the half-life of the drug multiplied by the maintenance dose.97
Do not consider vitamin D and calcium supplementation alone as sufficient to maintain bone health or prevent fragility fractures.
Due to the initial evidence of detrimental effect of sarcopenic obesity on bone health, physical activity and an adequate calcium intake are advised to avoid weight gain, reduce the risk of fall and for the likely positive impact on bone health.
BPs and DNB prevent bone loss and increase bone mass, but only DNB has been shown to decrease the fracture risk independently of bone mass effects. The posology used for DNB is the same used in case of osteoporosis in both men and women; for a BP, a wide spectrum of doses has been proposed, sometimes even higher than those used for osteoporosis.76
It is advised to start treatment with bone-modifying agents, as primary prevention, as soon as possible regardless of BMD even in this setting (no strict recommendations exist on PCa).
Metastatic disease
Due to the current paucity of evidence in the setting of M1 HSPC, the therapeutic schedule of BTTs is not the same used for metastases, but it is that for osteoporosis (the same of M0 CR). In fact, in M1 HS disease, the schedule used for BTT in M1 CRPC setting was not effective in reducing SREs and for DNB there are no data in support.
Intervention for metastatic disease in M1 CRPC is indicated at the time of diagnosis of the first metastasis as per all guidelines, and it is aimed at reducing SREs; the regimen employed both for ZA and DNB will widely cover also the possibility to reduce the risk of fragility fractures (benign fractures).
In case of M1 CRPC, consider the opportunity to continue therapy with bone-modifying agents adjusting the dosages for bone health in case of discontinuation of SRE-specific treatment. In particular, caution must be paid when using DNB, as, unlike for BPS, rapid bone loss occurs following treatment interruption, along with a potential rebound in the risk of vertebral fractures (pathological and osteoporotic fractures).98
Conclusion
Bone health preservation in PCa men undergoing ADT must be a clinical goal across the whole disease continuum because of the sequelae of bone health impairment on the individual’s QoL and health status, as well as the considerable burden imposed on healthcare resources. Yet, it remains an urgent unmet need not yet given adequate attention from the scientific community. For this reason, it is crucial to raise awareness on the importance of bone preservation in this complex setting and optimise the management of bone health possibly through a multidisciplinary approach. This document is intended to be a tool to support physicians when managing bone health in their daily practice; still, the applicability of the advices formulated depends on the reimbursement policy of each individual country and region.
Acknowledgments
Medical writing support and editorial assistance was provided by Clara Ricci, PhD (Edra S.p.A., Milan, Italy), and unconditionally funded by Janssen-Cilag S.p.A.
Footnotes
Twitter: @MassimoDiMaio75
Correction notice: Affiliation of Toni Ibrahim has been revised to ‘Osteoncology and Rare Tumors Center, Istituto Scientifico Romagnolo per lo Studio e la Cura dei Tumori (IRST) IRCCS, Meldola, Italy.’
Contributors: All authors made substantial contributions to the conception of the work; they all drafted the manuscript, revised it critically for important intellectual content and gave final approval of the version published. All authors agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Funding: This editorial project was supported by Janssen-Cilag S.p.A.
Competing interests: DS reports personal fees from Janssen during the conduct of the study; grants and personal fees from Amgen, Novartis and Astellas; personal fees from Boehringer, Roche and Pfizer outside the submitted work. AB reports personal fees from Janssen-Cilag during the conduct of the study. MDM reports personal fees from Janssen during the conduct of the study; personal fees from Janssen, Bristol Myers Squibb, Merck Sharp & Dohme, AstraZeneca, Takeda, Eisai and Pfizer outside the submitted work. GP reports fees as consultant/for advisory board from Bayer, Janssen, MSD and Novartis. SB reports fees for advisory board from Astellas, Janssen and Bayer. TI reports personal fees from Eisai; other from Novartis, Ipsen and PharmaMar; and grants from Novartis outside the submitted work. FB reports personal fees from Janssen during the conduct of the study, grants and personal fees from Amgen and Abiogen, and grants from Chiesi outside the submitted work.
Patient consent for publication: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Intermedia Editore I Numeridel Cancroin Italia 2019. Available: https://www.aiom.it/wp-content/uploads/2019/09/2019_Numeri_Cancro-operatori-web.pdf [Accessed 5 Jan 2020].
- 2.Bubendorf L, Schöpfer A, Wagner U, et al. . Metastatic patterns of prostate cancer: an autopsy study of 1,589 patients. Hum Pathol 2000;31:578–83. 10.1053/hp.2000.6698 [DOI] [PubMed] [Google Scholar]
- 3.Parker C, Gillessen S, Heidenreich A, et al. . Cancer of the prostate: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2015;26:v69–77. 10.1093/annonc/mdv222 [DOI] [PubMed] [Google Scholar]
- 4.Rhee H, Gunter JH, Heathcote P, et al. . Adverse effects of androgen-deprivation therapy in prostate cancer and their management. BJU Int 2015;115:3–13. 10.1111/bju.12964 [DOI] [PubMed] [Google Scholar]
- 5.Nguyen PL, Alibhai SMH, Basaria S, et al. . Adverse effects of androgen deprivation therapy and strategies to mitigate them. Eur Urol 2015;67:825–36. 10.1016/j.eururo.2014.07.010 [DOI] [PubMed] [Google Scholar]
- 6.Wang A, Obertová Z, Brown C, et al. . Risk of fracture in men with prostate cancer on androgen deprivation therapy: a population-based cohort study in New Zealand. BMC Cancer 2015;15:837 10.1186/s12885-015-1843-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Taylor LG, Canfield SE, Du XL. Review of major adverse effects of androgen-deprivation therapy in men with prostate cancer. Cancer 2009;115:2388–99. 10.1002/cncr.24283 [DOI] [PubMed] [Google Scholar]
- 8.ESMO Cancer of the prostate: ESMO clinical practice guidelines. Available: https://www.esmo.org/Guidelines/Genitourinary-Cancers/Cancer-of-the-Prostate [Accessed 11 Jun 2019].
- 9.Poulsen MH, Frost M, Abrahamsen B, et al. . Osteoporosis and prostate cancer: a cross-sectional study of Danish men with prostate cancer before androgen deprivation therapy. Scand J Urol 2014;48:350–5. 10.3109/21681805.2014.884160 [DOI] [PubMed] [Google Scholar]
- 10.Mistry R, Hughes D, Wadhwa V, et al. . Lateral spine radiographs before androgen deprivation treatment detect a high incidence of undiagnosed vertebral fragility fractures in men with advanced prostate cancer. J Urol 2011;186:474–81. 10.1016/j.juro.2011.03.149 [DOI] [PubMed] [Google Scholar]
- 11.Abrahamsen B, Nielsen MF, Eskildsen P, et al. . Fracture risk in Danish men with prostate cancer: a nationwide register study. BJU Int 2007;100:749–54. 10.1111/j.1464-410X.2007.07163.x [DOI] [PubMed] [Google Scholar]
- 12.Leuprolide Study Group Leuprolide versus diethylstilbestrol for metastatic prostate cancer. N Engl J Med 1984;311:1281–6. 10.1056/NEJM198411153112004 [DOI] [PubMed] [Google Scholar]
- 13.Boivin G, Meunier PJ. Changes in bone remodeling rate influence the degree of mineralization of bone. Connect Tissue Res 2002;43:535–7. 10.1080/03008200290000934 [DOI] [PubMed] [Google Scholar]
- 14.Alibhai SMH, Mohamedali HZ, Gulamhusein H, et al. . Changes in bone mineral density in men starting androgen deprivation therapy and the protective role of vitamin D. Osteoporos Int 2013;24:2571–9. 10.1007/s00198-013-2343-4 [DOI] [PubMed] [Google Scholar]
- 15.Body JJ, Bergmann P, Boonen S, et al. . Management of cancer treatment-induced bone loss in early breast and prostate cancer—a consensus paper of the Belgian Bone Club. Osteoporos Int 2007;18:1439–50. 10.1007/s00198-007-0439-4 [DOI] [PubMed] [Google Scholar]
- 16.Morote J, Orsola A, Abascal JM, et al. . Bone mineral density changes in patients with prostate cancer during the first 2 years of androgen suppression. J Urol 2006;175:1679–83. Discussion 1683 10.1016/S0022-5347(05)00999-7 [DOI] [PubMed] [Google Scholar]
- 17.Kim SH, Joung JY, Kim S, et al. . Comparison of bone mineral loss by combined androgen block agonist versus GnRH in patients with prostate cancer: a 12 month-prospective observational study. Sci Rep 2017;7:39562 10.1038/srep39562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Greenspan SL, Wagner J, Nelson JB, et al. . Vertebral fractures and trabecular microstructure in men with prostate cancer on androgen deprivation therapy. J Bone Miner Res 2013;28:325–32. 10.1002/jbmr.1771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wallander M, Axelsson KF, Lundh D, et al. . Patients with prostate cancer and androgen deprivation therapy have increased risk of fractures—a study from the fractures and fall injuries in the elderly cohort (FRAILCO). Osteoporos Int 2019;30:115–25. 10.1007/s00198-018-4722-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shahinian VB, Kuo Y-F, Freeman JL, et al. . Risk of fracture after androgen deprivation for prostate cancer. N Engl J Med 2005;352:154–64. 10.1056/NEJMoa041943 [DOI] [PubMed] [Google Scholar]
- 21.Smith MR, Lee WC, Brandman J, et al. . Gonadotropin-releasing hormone agonists and fracture risk: a claims-based cohort study of men with nonmetastatic prostate cancer. J Clin Oncol 2005;23:7897–903. 10.1200/JCO.2004.00.6908 [DOI] [PubMed] [Google Scholar]
- 22.Hussain M, Tangen CM, Berry DL, et al. . Intermittent versus continuous androgen deprivation in prostate cancer. N Engl J Med 2013;368:1314–25. 10.1056/NEJMoa1212299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hershman DL, Unger JM, Wright JD, et al. . Adverse health events following intermittent and continuous androgen deprivation in patients with metastatic prostate cancer. JAMA Oncol 2016;2:453–61. 10.1001/jamaoncol.2015.4655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.López AM, Pena MA, Hernández R, et al. . Fracture risk in patients with prostate cancer on androgen deprivation therapy. Osteoporos Int 2005;16:707–11. 10.1007/s00198-004-1799-7 [DOI] [PubMed] [Google Scholar]
- 25.Smith MR, Egerdie B, Hernández Toriz N, et al. . Denosumab in men receiving androgen-deprivation therapy for prostate cancer. N Engl J Med 2009;361:745–55. 10.1056/NEJMoa0809003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alibhai SMH, Duong-Hua M, Cheung AM, et al. . Fracture types and risk factors in men with prostate cancer on androgen deprivation therapy: a matched cohort study of 19,079 men. J Urol 2010;184:918–24. 10.1016/j.juro.2010.04.068 [DOI] [PubMed] [Google Scholar]
- 27.Saylor PJ, Morton RA, Hancock ML, et al. . Factors associated with vertebral fractures in men treated with androgen deprivation therapy for prostate cancer. J Urol 2011;186:482–6. 10.1016/j.juro.2011.03.111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sullivan S, Wagner J, Resnick NM, et al. . Vertebral fractures and the misclassification of osteoporosis in men with prostate cancer. J Clin Densitom 2011;14:348–53. 10.1016/j.jocd.2011.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Onukwugha E, Yong C, Mullins CD, et al. . Skeletal-related events and mortality among older men with advanced prostate cancer. J Geriatr Oncol 2014;5:281–9. 10.1016/j.jgo.2014.03.002 [DOI] [PubMed] [Google Scholar]
- 30.Smith M, Parker C, Saad F, et al. . Addition of radium-223 to abiraterone acetate and prednisone or prednisolone in patients with castration-resistant prostate cancer and bone metastases (ERA 223): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 2019;20:408–19. 10.1016/S1470-2045(18)30860-X [DOI] [PubMed] [Google Scholar]
- 31.Nørgaard M, Jensen Annette Østergaard, Jacobsen JB, et al. . Skeletal related events, bone metastasis and survival of prostate cancer: a population based cohort study in Denmark (1999 to 2007). J Urol 2010;184:162–7. 10.1016/j.juro.2010.03.034 [DOI] [PubMed] [Google Scholar]
- 32.Sathiakumar N, Delzell E, Morrisey MA, et al. . Mortality following bone metastasis and skeletal-related events among men with prostate cancer: a population-based analysis of US Medicare beneficiaries, 1999–2006. Prostate Cancer Prostatic Dis 2011;14:177–83. 10.1038/pcan.2011.7 [DOI] [PubMed] [Google Scholar]
- 33.Fizazi K, Massard C, Smith M, et al. . Bone-related parameters are the main prognostic factors for overall survival in men with bone metastases from castration-resistant prostate cancer. Eur Urol 2015;68:42–50. 10.1016/j.eururo.2014.10.001 [DOI] [PubMed] [Google Scholar]
- 34.Saad F, Eastham J. Zoledronic acid improves clinical outcomes when administered before onset of bone pain in patients with prostate cancer. Urology 2010;76:1175–81. 10.1016/j.urology.2010.05.026 [DOI] [PubMed] [Google Scholar]
- 35.Berruti A, Dogliotti L, Terrone C, et al. . Changes in bone mineral density, lean body mass and fat content as measured by dual energy X-ray absorptiometry in patients with prostate cancer without apparent bone metastases given androgen deprivation therapy. J Urol 2002;167:2361–7. Discussion 2367 10.1016/S0022-5347(05)64985-3 [DOI] [PubMed] [Google Scholar]
- 36.Smith MR. Changes in fat and lean body mass during androgen-deprivation therapy for prostate cancer. Urology 2004;63:742–5. 10.1016/j.urology.2003.10.063 [DOI] [PubMed] [Google Scholar]
- 37.Pedersini R, Amoroso V, Maffezzoni F, et al. . Association of fat body mass with vertebral fractures in postmenopausal women with early breast cancer undergoing adjuvant aromatase inhibitor therapy. JAMA Netw Open 2019;2:e1911080 10.1001/jamanetworkopen.2019.11080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Antoun S, Bayar A, Ileana E, et al. . High subcutaneous adipose tissue predicts the prognosis in metastatic castration-resistant prostate cancer patients in post chemotherapy setting. Eur J Cancer 2015;51:2570–7. 10.1016/j.ejca.2015.07.042 [DOI] [PubMed] [Google Scholar]
- 39.Buttigliero C, Vana F, Bertaglia V, et al. . The fat body mass increase after adjuvant androgen deprivation therapy is predictive of prostate cancer outcome. Endocrine 2015;50:223–30. 10.1007/s12020-015-0525-x [DOI] [PubMed] [Google Scholar]
- 40.Hirschfeld HP, Kinsella R, Duque G. Osteosarcopenia: where bone, muscle, and fat collide. Osteoporos Int 2017;28:2781–90. 10.1007/s00198-017-4151-8 [DOI] [PubMed] [Google Scholar]
- 41.Bliuc D, Nguyen ND, Nguyen TV, et al. . Compound risk of high mortality following osteoporotic fracture and refracture in elderly women and men. J Bone Miner Res 2013;28:2317–24. 10.1002/jbmr.1968 [DOI] [PubMed] [Google Scholar]
- 42.Shao Y-H, Moore DF, Shih W, et al. . Fracture after androgen deprivation therapy among men with a high baseline risk of skeletal complications. BJU Int 2013;111:745–52. 10.1111/j.1464-410X.2012.11758.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Svedbom A, Borgstöm F, Hernlund E, et al. . Quality of life for up to 18 months after low-energy hip, vertebral, and distal forearm fractures-results from the ICUROS. Osteoporos Int 2018;29:557–66. 10.1007/s00198-017-4317-4 [DOI] [PubMed] [Google Scholar]
- 44.Oefelein MG, Ricchiuti V, Conrad W, et al. . Skeletal fractures negatively correlate with overall survival in men with prostate cancer. J Urol 2002;168:1005–7. 10.1016/S0022-5347(05)64561-2 [DOI] [PubMed] [Google Scholar]
- 45.Van Hemelrijck M, Garmo H, Michaëlsson K, et al. . Mortality following hip fracture in men with prostate cancer. PLoS One 2013;8:e74492 10.1371/journal.pone.0074492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fizazi K, Shore N, Tammela TL, et al. . Darolutamide in nonmetastatic, castration-resistant prostate cancer. N Engl J Med 2019;380:1235–46. 10.1056/NEJMoa1815671 [DOI] [PubMed] [Google Scholar]
- 47.European Medicines Agency Applications for new human medicines under evaluation by the Committee for Medicinal Products for Human Use. Available: https://www.ema.europa.eu/en/documents/report/applications-new-human-medicines-under-evaluation-chmp-april-2019_en.pdf [Accessed 20 Jun 2019].
- 48.Hussain M, Fizazi K, Saad F, et al. . Enzalutamide in men with nonmetastatic, castration-resistant prostate cancer. N Engl J Med 2018;378:2465–74. 10.1056/NEJMoa1800536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Smith MR, Saad F, Chowdhury S, et al. . Apalutamide treatment and metastasis-free survival in prostate cancer. N Engl J Med 2018;378:1408–18. 10.1056/NEJMoa1715546 [DOI] [PubMed] [Google Scholar]
- 50.Smith MR, Saad F, Coleman R, et al. . Denosumab and bone-metastasis-free survival in men with castration-resistant prostate cancer: results of a phase 3, randomised, placebo-controlled trial. Lancet 2012;379:39–46. 10.1016/S0140-6736(11)61226-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Broglio KR, Berry DA. Detecting an overall survival benefit that is derived from progression-free survival. J Natl Cancer Inst 2009;101:1642–9. 10.1093/jnci/djp369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Xie W, Regan MM, Buyse M, et al. . Metastasis-free survival is a strong surrogate of overall survival in localized prostate cancer. J Clin Oncol 2017;35:3097–104. 10.1200/JCO.2017.73.9987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tuma R. Progression-free survival remains debatable endpoint in cancer trials. J Natl Cancer Inst 2009;101:1439–41. 10.1093/jnci/djp399 [DOI] [PubMed] [Google Scholar]
- 54.FDA panel rejects denosumab against bone metastasis in prostate cancer. Available: https://www.mdedge.com/endocrinology/article/47400/oncology/fda-panel-rejects-denosumab-against-bone-metastasis-prostate [Accessed 28 Jun 2019].
- 55.Reis LO. Metastasis-free survival—progress or lowering the bar on nonmetastatic prostate cancer? Eur Urol 2018;74:682–3. 10.1016/j.eururo.2018.08.018 [DOI] [PubMed] [Google Scholar]
- 56.Small EJ, Saad F, Chowdhury S, et al. . Apalutamide and overall survival in non-metastatic castration-resistant prostate cancer. Ann Oncol 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fizazi K, Tran N, Fein L, et al. . Abiraterone plus prednisone in metastatic, castration-sensitive prostate cancer. N Engl J Med 2017;377:352–60. 10.1056/NEJMoa1704174 [DOI] [PubMed] [Google Scholar]
- 58.Fizazi K, Tran N, Fein L, et al. . Abiraterone acetate plus prednisone in patients with newly diagnosed high-risk metastatic castration-sensitive prostate cancer (LATITUDE): final overall survival analysis of a randomised, double-blind, phase 3 trial. Lancet Oncol 2019;20:686–700. 10.1016/S1470-2045(19)30082-8 [DOI] [PubMed] [Google Scholar]
- 59.Scher HI, Fizazi K, Saad F, et al. . Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med 2012;367:1187–97. 10.1056/NEJMoa1207506 [DOI] [PubMed] [Google Scholar]
- 60.Beer TM, Armstrong AJ, Rathkopf DE, et al. . Enzalutamide in metastatic prostate cancer before chemotherapy. N Engl J Med 2014;371:424–33. 10.1056/NEJMoa1405095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fizazi K, Chi KN. Abiraterone in metastatic prostate cancer. N Engl J Med 2017;377:1697–8. 10.1056/NEJMc1711029 [DOI] [PubMed] [Google Scholar]
- 62.Chi KN, Protheroe A, Rodríguez-Antolín A, et al. . Patient-reported outcomes following abiraterone acetate plus prednisone added to androgen deprivation therapy in patients with newly diagnosed metastatic castration-naive prostate cancer (LATITUDE): an international, randomised phase 3 trial. Lancet Oncol 2018;19:194–206. 10.1016/S1470-2045(17)30911-7 [DOI] [PubMed] [Google Scholar]
- 63.Cella D, Traina S, Li T, et al. . Relationship between patient-reported outcomes and clinical outcomes in metastatic castration-resistant prostate cancer: post hoc analysis of COU-AA-301 and COU-AA-302. Ann Oncol 2018;29:392–7. 10.1093/annonc/mdx759 [DOI] [PubMed] [Google Scholar]
- 64.Rizzo S, Galvano A, Pantano F, et al. . The effects of enzalutamide and abiraterone on skeletal related events and bone radiological progression free survival in castration resistant prostate cancer patients: an indirect comparison of randomized controlled trials. Crit Rev Oncol Hematol 2017;120:227–33. 10.1016/j.critrevonc.2017.09.008 [DOI] [PubMed] [Google Scholar]
- 65.Engel-Nitz N, Behl AS, Blauer-Peterson C, et al. . Real world skeletal related events (SREs) associated with oral treatments in patients with metastatic castration resistant prostate cancer (mCRPC). JCO 2016;34:e16537 10.1200/JCO.2016.34.15_suppl.e16537 [DOI] [Google Scholar]
- 66.TMJA Abstract, 2018. Available: https://www.aiom.it/wp-content/uploads/2018/05/2018_TMJA_AbstractAIOMXX.pdf [Accessed 25 Jun 2019].
- 67.Ryan CJ, Smith MR, de Bono JS, et al. . Abiraterone in metastatic prostate cancer without previous chemotherapy. N Engl J Med 2013;368:138–48. 10.1056/NEJMoa1209096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Iuliani M, Pantano F, Buttigliero C, et al. . Biological and clinical effects of abiraterone on anti-resorptive and anabolic activity in bone microenvironment. Oncotarget 2015;6:12520–8. 10.18632/oncotarget.3724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Alibhai SMH, Zukotynski K, Walker-Dilks C. Bone health and bone-targeted therapies for nonmetastatic prostate cancer. Ann Intern Med 2018;168:459–60. 10.7326/L17-0702 [DOI] [PubMed] [Google Scholar]
- 70.Kozyrakis D, Paridis D, Perikleous S, et al. . The current role of osteoclast inhibitors in patients with prostate cancer. Adv Urol 2018;2018:1–9. 10.1155/2018/1525832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Choo R, Lukka H, Cheung P, et al. . Randomized, double-blinded, placebo-controlled, trial of risedronate for the prevention of bone mineral density loss in nonmetastatic prostate cancer patients receiving radiation therapy plus androgen deprivation therapy. Int J Radiat Oncol Biol Phys 2013;85:1239–45. 10.1016/j.ijrobp.2012.11.007 [DOI] [PubMed] [Google Scholar]
- 72.Greenspan SL, Nelson JB, Trump DL, et al. . Skeletal health after continuation, withdrawal, or delay of alendronate in men with prostate cancer undergoing androgen-deprivation therapy. J Clin Oncol 2008;26:4426–34. 10.1200/JCO.2007.15.1233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Israeli RS, Rosenberg SJ, Saltzstein DR, et al. . The effect of zoledronic acid on bone mineral density in patients undergoing androgen deprivation therapy. Clin Genitourin Cancer 2007;5:271–7. 10.3816/CGC.2007.n.003 [DOI] [PubMed] [Google Scholar]
- 74.Poon Y, Pechlivanoglou P, Alibhai SMH, et al. . Systematic review and network meta-analysis on the relative efficacy of osteoporotic medications: men with prostate cancer on continuous androgen-deprivation therapy to reduce risk of fragility fractures. BJU Int 2018;121:17–28. 10.1111/bju.14015 [DOI] [PubMed] [Google Scholar]
- 75.Alibhai SMH, Zukotynski K, Walker-Dilks C, et al. . Bone health and bone-targeted therapies for nonmetastatic prostate cancer: a systematic review and meta-analysis. Ann Intern Med 2017;167:341–50. 10.7326/M16-2577 [DOI] [PubMed] [Google Scholar]
- 76.Cianferotti L, Bertoldo F, Carini M, et al. . The prevention of fragility fractures in patients with non-metastatic prostate cancer: a position statement by the International Osteoporosis Foundation. Oncotarget 2017;8:75646–63. 10.18632/oncotarget.17980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ding H, Yang L, Du W, et al. . Bisphosphonates for osteoporosis in nonmetastatic prostate cancer patients receiving androgen-deprivation therapy: a systematic review and meta-analysis. Asian Pac J Cancer Prev 2013;14:3337–43. 10.7314/APJCP.2013.14.5.3337 [DOI] [PubMed] [Google Scholar]
- 78.Smith MR, Halabi S, Ryan CJ, et al. . Randomized controlled trial of early zoledronic acid in men with castration-sensitive prostate cancer and bone metastases: results of CALGB 90202 (Alliance). J Clin Oncol 2014;32:1143–50. 10.1200/JCO.2013.51.6500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kamba T, Kamoto T, Maruo S, et al. . A phase III multicenter, randomized, controlled study of combined androgen blockade with versus without zoledronic acid in prostate cancer patients with metastatic bone disease: results of the ZAPCA trial. Int J Clin Oncol 2017;22:166–73. 10.1007/s10147-016-1037-2 [DOI] [PubMed] [Google Scholar]
- 80.Guida L. Trattamento Delle Metastasi Ossee 2018;106. [Google Scholar]
- 81.Saad F, Shore N, Van Poppel H, et al. . Impact of bone-targeted therapies in chemotherapy-naïve metastatic castration-resistant prostate cancer patients treated with abiraterone acetate: post hoc analysis of study COU-AA-302. Eur Urol 2015;68:570–7. 10.1016/j.eururo.2015.04.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.EORTC Preliminary results confirm that the addition of bone-protecting agents to Radium-233 (Ra-233) treatment can limit fractures in metastatic castration resistant prostate cancer patients. Available: https://www.eortc.org/blog/2019/05/31/preliminary-results-confirm-that-the-addition-of-bone-protecting-agents-to-radium-233-ra-233-treatment-can-limit-fractures-in-metastatic-castration-resistant-prostate-cancer-patients-2/ [Accessed 4 Jul 2019].
- 83.Fizazi K, Carducci M, Smith M, et al. . Denosumab versus zoledronic acid for treatment of bone metastases in men with castration-resistant prostate cancer: a randomised, double-blind study. Lancet 2011;377:813–22. 10.1016/S0140-6736(10)62344-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ueno S, Mizokami A, Fukagai T, et al. . Efficacy of combined androgen blockade with zoledronic acid treatment in prostate cancer with bone metastasis: the ZABTON-PC (zoledronic acid/androgen blockade trial on prostate cancer) study. Anticancer Res 2013;33:3837–44. [PubMed] [Google Scholar]
- 85.Body J-J, von Moos R, Rider A, et al. . A real-world study assessing the use of bone-targeted agents and their impact on bone metastases in patients with prostate cancer treated in clinical practice in Europe. J Bone Oncol 2019;14:100212 10.1016/j.jbo.2018.100212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Higano CS, Sternberg CN, Saad F, et al. . Treatment patterns and outcomes for metastatic castration-resistant prostate cancer (mCRPC) in a real-world setting: a retrospective study of greater than 2500 patients. JCO 2019;37:256 10.1200/JCO.2019.37.7_suppl.256 [DOI] [Google Scholar]
- 87.Qian Y, Bhowmik D, Kachru N, et al. . Longitudinal patterns of bone-targeted agent use among patients with solid tumors and bone metastases in the United States. Support Care Cancer 2017;25:1845–51. 10.1007/s00520-017-3583-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Briot K, Paccou J, Beuzeboc P, et al. . French recommendations for osteoporosis prevention and treatment in patients with prostate cancer treated by androgen deprivation. Joint Bone Spine 2019;86:21–8. 10.1016/j.jbspin.2018.09.017 [DOI] [PubMed] [Google Scholar]
- 89.Graham J, Kirkbride P, Cann K, et al. . Prostate cancer: summary of updated NICE guidance. BMJ 2014;348:f7524 10.1136/bmj.f7524 [DOI] [PubMed] [Google Scholar]
- 90.Saylor PJ, Kaufman DS, Michaelson MD, et al. . Application of a fracture risk algorithm to men treated with androgen deprivation therapy for prostate cancer. J Urol 2010;183:2200–5. 10.1016/j.juro.2010.02.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.WHO Scientific Group Technical Report. Available: https://www.sheffield.ac.uk/FRAX/pdfs/WHO_Technical_Report.pdf [Accessed 16 May 2019].
- 92.Pothuaud L, Carceller P, Hans D. Correlations between grey-level variations in 2D projection images (TBS) and 3D microarchitecture: applications in the study of human trabecular bone microarchitecture. Bone 2008;42:775–87. 10.1016/j.bone.2007.11.018 [DOI] [PubMed] [Google Scholar]
- 93.Mariotti V, Page DB, Davydov O, et al. . Assessing fracture risk in early stage breast cancer patients treated with aromatase-inhibitors: an enhanced screening approach incorporating trabecular bone score. J Bone Oncol 2017;7:32–7. 10.1016/j.jbo.2016.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Moayyeri A, Luben RN, Bingham SA, et al. . Measured height loss predicts fractures in middle-aged and older men and women: the EPIC-Norfolk prospective population study. J Bone Miner Res 2008;23:425–32. 10.1359/jbmr.071106 [DOI] [PubMed] [Google Scholar]
- 95.Rossini M, Adami S, Bertoldo F, et al. . Guidelines for the diagnosis, prevention and management of osteoporosis. Reumatismo 2016;68:1–39. 10.4081/reumatismo.2016.870 [DOI] [PubMed] [Google Scholar]
- 96.Holick MF, Binkley NC, Bischoff-Ferrari HA, et al. . Guidelines for preventing and treating vitamin D deficiency and insufficiency revisited. J Clin Endocrinol Metab 2012;97:1153–8. 10.1210/jc.2011-2601 [DOI] [PubMed] [Google Scholar]
- 97.Vieth R. The pharmacology of vitamin D, including fortification strategies. In: Vitamin D. Elsevier 2005:995–1015. [Google Scholar]
- 98.Tsourdi E, Langdahl B, Cohen-Solal M, et al. . Discontinuation of denosumab therapy for osteoporosis: a systematic review and position statement by ECTS. Bone 2017;105:11–17. 10.1016/j.bone.2017.08.003 [DOI] [PubMed] [Google Scholar]
- 99.de Bono JS, Logothetis CJ, Molina A, et al. . Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med 2011;364:1995–2005. 10.1056/NEJMoa1014618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Today URO. ASCO GU 2019: final analysis of LATITUDE, a phase III in patients with newly diagnosed high-risk metastatic castration-naïve prostate cancer, 2019. Available: https://www.urotoday.com/conference-highlights/asco-gu-2019/asco-gu-2019-prostate-cancer/110261-asco-gu-2019-final-analysis-of-latitude-a-phase-iii-in-patients-with-newly-diagnosed-high-risk-metastatic-castration-naive-prostate-cancer-treated-with-abiraterone-acetate-prednisone-added-to-adt.html [Accessed 27 Sep 2019].
- 101.Meeting Abstracts. Available: https://abstracts.asco.org/239/AbstView_239_252003.html [Accessed 23 Jul 2019].
- 102.Logothetis CJ, Basch E, Molina A, et al. . Effect of abiraterone acetate and prednisone compared with placebo and prednisone on pain control and skeletal-related events in patients with metastatic castration-resistant prostate cancer: exploratory analysis of data from the COU-AA-301 randomised trial. Lancet Oncol 2012;13:1210–7. 10.1016/S1470-2045(12)70473-4 [DOI] [PubMed] [Google Scholar]
- 103.Rathkopf DE, Smith MR, de Bono JS, et al. . Updated interim efficacy analysis and long-term safety of abiraterone acetate in metastatic castration-resistant prostate cancer patients without prior chemotherapy (COU-AA-302). Eur Urol 2014;66:815–25. 10.1016/j.eururo.2014.02.056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ryan CJ, Smith MR, Fizazi K, et al. . Abiraterone acetate plus prednisone versus placebo plus prednisone in chemotherapy-naive men with metastatic castration-resistant prostate cancer (COU-AA-302): final overall survival analysis of a randomised, double-blind, placebo-controlled phase 3 study. Lancet Oncol 2015;16:152–60. 10.1016/S1470-2045(14)71205-7 [DOI] [PubMed] [Google Scholar]
- 105.Xtandi SPC. Available: https://www.astellas.us/docs/12a005-enz-wpi.pdf [Accessed 21 May 2019].
- 106.Highlights of Prescribing Information. Available: https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/202379s024lbl.pdf [Accessed 4 Jul 2019].