Abstract
Components of the cellular and the humoral arm of the immune system are essential elements of the tumor microenvironment (TME). The TME includes tumor-associated macrophages (TAM) which have served as a paradigm for the cancer-promoting inflammation. Cytokines, IL-1 in particular, and Complement have emerged as important players in tumor promotion. On the other hand, myeloid cells, innate lymphoid cells and Complement have the potential, if unleashed, to mediate anticancer resistance. Targeting checkpoints restraining innate immunity, macrophages and NK cells in particular, holds promise as a therapeutic strategy.
Keywords: Tumor microenvironment, Innate immunity, Inflammation, Macrophages, Interleukin-1
1. Introduction
The tumor microenvironment (TME) represents an ecological niche in which carcinogenesis and cancer progression occur [1–5]. At the turn of the millennium a cancer cell-centric view dominated oncology [6]. The TME is now recognized as an important component of cancer [1–5, 7].
Immunity-related components of the TME include tumor promoting inflammation, reflecting innate immunity and taming of effective adaptive immunity. In general, inflammatory cells in the TME contribute to an immunosuppressive microenvironment.
Components of cancer-related inflammation include myeloid cells (macrophages and neutrophils), basophils, eosinophils [2]. Moreover, elements of the humoral arm of innate immunity are also present in situ and play a role in the TME [8, 9]. These include fluid phase pattern recognition molecules and regulators of the Complement cascade such as PTX3 [8] and Complement components [9].
Macrophages have served as a paradigm for the diversity, complexity and protumor role of inflammation in the TME [4]. Tumor-associated macrophages (TAM) are present in all tumors, contribute to progression and inhibit effective innate and adaptive responses.
Here major selected aspects of innate immunity and inflammation in cancer will be summarized. Recent results on therapeutic targeting will be emphasized. Moreover, the emerging relevance of innate lymphoid cell and myeloid cell checkpoints will be discussed.
2. Local and Systemic Inflammation in Tumor Promotion
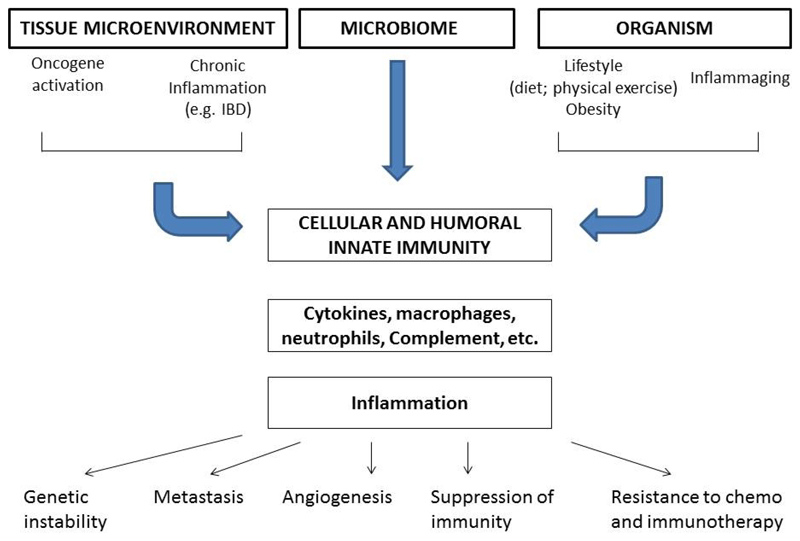
Inflammation and cancer are closely linked. The identification of this connection was in a way an intuition that can be traced to Virchow in the 19th century [5]. The links at a tissue level and at a systemic organism level connecting inflammation and cancer are schematically depicted in Fig 1. Selected chronic non-resolving inflammatory conditions are a risk factor for developing tumors [2]. For instance, inflammatory bowel disease predisposes to colorectal carcinoma and chronic obstructive pulmonary disease to lung cancer.
Figure 1.
Links between inflammation and cancer at tissue and organism level.
In the so called intrinsic pathway, dominant and recessive oncogenes orchestrate an inflammatory microenvironment in cancers with no epidemiological connection to inflammatory conditions (e.g. gliomas). Indeed, cancer-driving genetic events determine the build up of an inflammatory microenvironment [2]. Tumors unrelated in their natural history to clinical inflammatory conditions diseases are characterized by a TME with inflammatory cells and mediators. For instance, in the TME of breast cancer macrophages are present and enhance growth and metastasis [10]. In particular, in triple negative breast cancer tumor-associated macrophages (TAM) are abundant and their levels are associated with poor prognosis [11]. The tyrosine kinase MER causes epithelial to mesenchymal transition and is also an important driver of TAM accumulation in breast cancer [12].
At a systemic level, obesity causes a subclinical inflammation state and is associated to an increased risk to develop cancer, in this same extrinsic pathway perspective (Fig. 1). In addition, inflammation is a crucial component of senescence at a cellular and organism (inflammaging) level and cancer is a disease of aging [13]. Irrespective of the pathways involved, smoldering, non-resolving inflammation acts as a propeller of malignant progression [14].
Therefore, the TME is now considered an essential element of cancer and the field moved from a prevailing cancer cell-centric view of the essence of cancer [2, 6, 15] to one that encompasses the TME. The intrinsic and extrinsic pathway intersect at the level of transcription factors (e.g. NFkB; STAT3), cytokines (e.g. IL-1; TNF) and chemokines. Thus, inflammatory cells and mediators are components of the TME and are now considered an essential property of cancer [2, 7].
The TME has specific features in cancers in different organs contexts and in tumors of different types in the same organ or tissue. With the same gross histologic type, tumors can be distinguished based on widely different TME. For instance, in colorectal cancer four TME phenotypes have been identified based on profiling [1].
Mononuclear phagocytes have provided a paradigm of cancer-related inflammation. Other inflammatory cells in the TME are neutrophils, eosinophils and basophils [16]. Inflammatory cells, TAM in particular, promote tissue invasion, intravasation and metastasis, serving as a component of the niche for tumor cells disseminating at distant sites [17, 18]. Immunocompetent cells in tumors produce produce cytokines and chemokines that stimulate epithelial-to-mesenchymal (EMT) transition, which can be the first step of tumor invasion. Besides promoting EMT, TAM secrete mediators which stimulate cell migration and dissemination, such as proteolytic enzymes (serine proteases and cathepsins), cytokines (IL-1), growth factors (epidermal growth factor; EGF) [4, 19–22].
Neutrophils also contribute to the metastatic process, enhancing extravasation, tissue invasion, survival and growth of tumor cell at secondary sites [18, 23]. Other cells in the TME favour dissemination, including immature myeloid cells or T regulatory (Treg) cells. These cells are potent inhibitors of adaptive immune responses to growing tumors. Cancer-associated fibroblasts serve more than a scaffold for the tumor tissue because they regulate immunity and interact with cancer cells. Finally, platelets affect thrombosis, inflammation and tumor cell survival [24, 25]. In summary the TME contains a variety of immunocompetent cells which enhance dissemination and metastasis.
Cells of the monocyte-macrophage lineage are a major driver of immunosuppression in the microenvironment [4]. Mononuclear phagocytes are endowed with a complex armamentarium of immunosuppressive mediators such as cytokines (IL-10 and TGFβ), amino acid metabolites, prostaglandins, NO and triggers of checkpoint blockade such as PD-L1 [4].
Analysis of cellular elements present in the TME can have prognostic value. T cell infiltration (“Immunoscore”) is an independent positive parameter associated with more favourable prognosis in colorectal cancer and other tumors [26]. In contrast, TAM infiltration usually has bad prognostic significance [27]. colorectal cancer is an exception because evidence suggests that TAM infiltration predicts response to 5-fluorouracil containing therapeutic regimens [28].
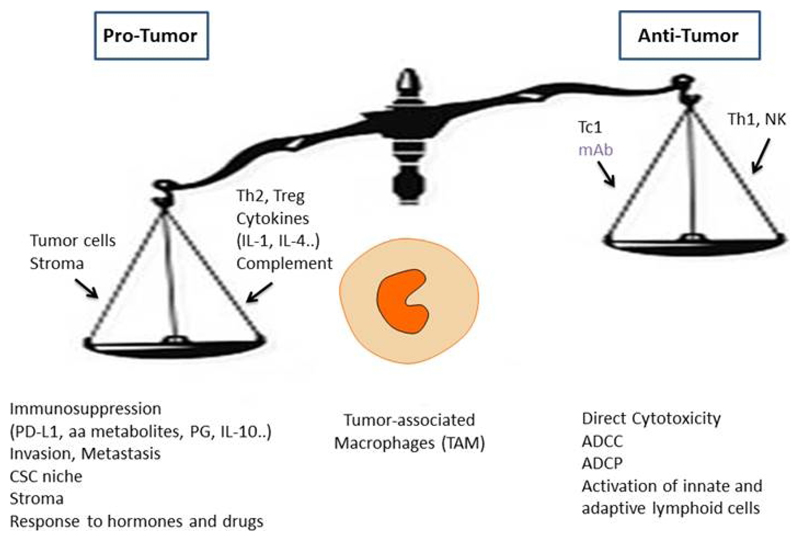
TAM are considered targets for therapy [4]. Targeting TAM plays an important role in the therapeutic activity of Trabectedin, a chemotherapeutic drug approved by the European Medicines Agency (EMA) and by the Food and Drug Administration (FDA) [29, 30]. This finding provided proof of principle for the potential value of TAM directed strategies. Following work in mouse models, antibodies or simple molecules targeting the colony-stimulating factor-1 (CSF1) pathway have entered clinical assessment together with checkpoint blockade inhibitors [31]. Macrophages can also mediate antitumor activity, when “re-educated”, in the presence of antitumor antibodies, or when checkpoints are removed [4] (Fig. 2). As true in general for inflammation and innate immunity, macrophages can act as a double edged sword (Fig. 2). Emerging innate immunity checkpoints will be discussed below in Section 5.
Figure 2.
The macrophage balance. Macrophages can exert a dual function in the tumor microenvironment. In established clinical tumors the pro- tumor function of TAM prevail. Macrophages serve as a paradigm for tumor promoting inflammation
3. Humoral Innate Immunity, Complement, PTX3 and Cancer
Molecules belonging to the humoral arm of innate immunity are components of the TME and have recently been shown to contribute to tumor progression [8]. Complement can kill cancer cells. However preclinical and clinical results now indicate that Complement can contribute to tumor promoting inflammation [9]. Consistently with the concept of Complement as an enhancer of tumor promotion, the humoral pattern recognition molecule PTX3 has been identified to act as an extrinsic oncosuppressor gene [8]. In 3-Methylcholanthrene (3-MCA)-, and 7,12-dimethylbenz [α] anthracene/terephthalic acid (DMBA/TPA)-induced skin carcinogenesis, myeloid cells and endothelial cells produce PTX3. In PTX3-deficient hosts tumors displayed increased macrophage infiltration, cytokine production, angiogenesis, complement C3 deposition and C5a levels. This picture indicated enhanced cancer-related inflammation and complement activation. C3-genetic inactivation and CCL2-inhibition inhibited the enhanced susceptibility to 3-MCA carcinogenesis and the M2-like characteristics and recruitment of TAM in PTX3-deficient hosts. PTX3 acted as a regulator of C3-deposition on cancer cells by interacting with the Complement inhibitor Factor H [8]. Interestingly, PTX3-deficiency resulted in increased DNA damage, as shown by more mutations of Trp53, one of the genes targeted by 3-MCA, oxidative DNA damage and expression of DNA damage markers [8]. Importantly, in selected human mesenchymal and epithelial tumors, the PTX3 promoter and regulatory regions were highly methylated and this epigenetic modification resulted in transcriptional inactivation and silencing of PTX3 expression. In colorectal cancer PTX3 gene methylation and silencing was detected as an early event, already identified in adenomas and stage 1 neoplastic lesions, an observation consistent with an important role in pathogenesis [8].
As a biomarker in the clinic PTX3 was found to act as a local or systemic indicator of cancer-related inflammation. In particular, PTX3 was present at high levels in soft tissue sarcomas [32], lung cancer [33, 34], myeloproliferative neoplasms [35], pancreatic carcinoma [36], gliomas [37], and hepatocellular carcinoma [38], in some settings correlating with cancer progression. In the case of lung cancer, different studies reported increased systemic and local PTX3 levels and a correlation with disease aggressiveness and progression [33, 34]. Interestingly, in contrast with other epithelial cells that are poor producers of PTX3, lung epithelial cells express PTX3 in inflammatory conditions via JNK [39]. These results indicate that cancer-related inflammation may impact on PTX3 production in lung cancer cells. Along the same line, in myeloproliferative neoplasms, PTX3 levels correlated with mutant JAK2 (JAK2V617F) allele burden [35, 40], which is well esteblished to sustain leukocyte activation.
In conclusion, in preclinical models and in some human tumors (e.g. colorectal cancer) PTX3 functions as an extrinsic oncosuppressor gene, taming complement-driven macrophage-mediated tumor promotion. In other cancers, elevated PTX3 levels reflect systemic inflammation or genetic events that drive carcinogenesis as is the case for JAK2 in myeloproliferative neoplasms.
4. IL-1 in Tumor Promotion and its Clinical Translation
IL-1 is a major mediator connecting inflammation and tumor promotion. IL-1α and IL-1β in cancer are a major mechanism of tumor promotion although early in carcinogenesis, IL-1α may trigger an anti-tumor role as an anti-tumor response [41]. It was originally shown that IL-1β increased metastasis in mouse models [42–45]. IL-1α and IL-1β were found to be induced by RAS and RET-PTC oncogenes [46, 47]. IL-1β was also found in the process of carcinogenesis driven by chronic inflammation in the gastrointestinal tract [48]. In skin carcinogenesis IL-1α was downstream of RAS, affecting transformed cells and the TME [46].
In different murine and human tumor types, including sarcomas, melanoma, pancreatic carcinoma [49–52], myelomas [53], and breast carcinomas [54], a major mechanism of IL-1-mediated promotion has been shown to be the expansion and immunosuppressive function of myeloid cells [55, 56]. In mouse and human melanoma, IL-1 caused upregulation of TET2 in myeloid cells. TET2 is a DNA methylcytosine dioxygenase which induced immunosuppression in M2-like TAM [56]. Endothelial cells are regulated by IL-1 by promoting angiogenesis. IL-1 induced endothelial cell adhesion molecules E-selectin and vascular cell adhesion molecule-1 (VCAM-1) resulting in augmentation of metastasis.
An IL-1β signature was identified in the peripheral blood mononuclear cells from 145 patients with metastatic, hormone-negative breast cancer [54]. When treated with daily IL-1 receptor antagonist (Il-1Ra, Anakinra) for two weeks, the IL-1β signature decreased [54]. Thymic stromal cell lymphopoietin (TSLP) is associated with poor prognosis not only in breast cancer but also in other epithelial cancers [57] and correlated with IL-1β [54, 57]. Human genetics is consistent with a role of IL-1 and related molecules in carcinogenesis [58–63]. Therefore, mouse evidence and human genetics suggests that IL-1 is a driver of tumor promotion.
These results provided a rational for therapeutic translation of IL-1 blocking strategies using Anakinra or anti-IL-1α or IL-1β mAb. Anakinra with dexamethasone in 47 patients with smoldering myeloma resulted in significantly increase in survival [53, 64]. Anakinra was also added to the standard of therapy with flurouracil in advanced metastatic colorectal cancer [65], hormone negative breast cancer [54] and in advanced pancreatic cancer [66, 67]. IL-1α had long been known to mediate muscle loss and cachexia [48]. Three trials have administered anti-IL-1α to patients with advanced cancers of various origins [68, 69] as well as patients with colorectal cancer [70]. Blocking IL-1 resulted in an increase in lean body mass, improved parameters of quality of life, decreased pain and decreased constitutional symptoms. [71]. Reducing IL-1α may also reduce inflammation-mediated immunosuppression, both impacting on increased survival and immune-mediated tumor regression [2].
Preclinical and clinical data since 1990 provided a background for assessing the impact of blocking IL-1β in human cancer development. In the seminal CANTOS study with 10,061 patients with atherosclerosis and high CRP levels, anti-IL-1β (Canakinumab) resulted in a major (>50%) reduction in the incidence and mortality from lung cancer [72]. Blocking IL-1-driven recruitment and immunosuppressive function of macrophages is likely to play a major role in these impressive results.
5. Novel Checkpoints in Innate Immunity
Adaptive T cell orchestrated immunity and its subversion are central in the control of carcinogenesis and progression. Recent results have shed new light on the long overlooked role of innate lymphoid cells (ILC) [73–75] NK cells are a population of ILCs which has not been credited to play a major role in resistance against solid tumor carcinogenesis. NK cells are a component of resistance against leukemia and lymphomas and restrain hematogenous metastasis. The differentiation and activity of NK cells is also controlled by negative regulators. The member of the IL-1 receptor family IL-1R8 has recently been shown to serve as a checkpoint for IL-18-induced differentiation and activation of NK cells and ILC1 cells [76]. In an independent study it was found that IL-37, produced by Treg cells, suppressed NK function via IL-1R8 [77]. Inhibition of IL-1R8 unleashed NK cell-mediated resistance against liver and lung carcinogenesis and metastasis, two NK cell rich anatomical sites [76]. The checkpoint activity of IL-1R8 was also detected in human NK cells. These results suggest that IL-1R8 can serve as a double edged sword in carcinogenesis. On the one hand it inhibits tumor promoting inflammation; on the other hand, it acts as a checkpoint for NK cells which, if unleashed, can mediate anticancer immunity at distant organs rich in NK cells. In agreement with these results, in breast cancer IL-1R8 expression was found to be associated with an NK cell inflamed molecular signature [78]. These results suggest that targeting the IL-1R8 checkpoint may of value in particular in the context of liver metastasis. IL-1R8 adds to the diverse number of negative regulators which keep NK cells under control [74, 79].
As discussed for NK cells and generally true for immunocompetent cells, the function of myelomonocytic cells is held in check by molecular brakes (checkpoints). CD47 is a “don’t eat me” signal recognized by SIRPα on macrophages. It regulates the phagocytosis of effete normal cells, erytrocytes in particular [80]. Interestingly, the c-myc oncogene induces expression of CD47 and PD-L1 [81]. Preclinical evidence showed that blocking the CD47-SIRPα checkpoint unleashed antibody-dependent cellular phagocytosis (ADCP). The antitumor activity of ADCP can synergize with anti-CD20 in lymphoma killing [82]. Indeed, it has been known since the late ‘70 that macrophages are potent effectors of antibody-dependent tumor cell killing [4] and preclinical and clinical evidence is consistent with the in vivo relevance of this function [4, 83]. Interestingly, signals which orient TAM in a protumor direction (M2-like) do not inhibit their ADCC and ADCP effector function, or actually increase it [83]. Thus, seemingly paradoxically, in the presence of anti-tumor mAb TAM may well be suited to mediate ADCC-ADCP after checkpoint blockade.
In a Phase 1b [84] a monoclonal antibody (mAb, 5F9) blocking the CD47 checkpoint of macrophage function in concert with anti-CD20 (rituximab) had significant antitumor activity in refractory diffuse large B-cell lymphoma and follicular lymphoma. These results [84] are noteworthy not only because of their own clinical merit (mild side effects; frequency, rapidity and durability of responses) but also because they have more in general implications for cancer immunotherapy [85].
6. Concluding Remarks
Immunity and inflammation are essential elements of the tumor TME which provides a nurturing niche for cancer [1, 5, 7] Inflammatory cells, TAM in particular, promote invasion dissemination and metastasis. Progression of cancer to invasion and metastasis and evasion from immunity are associated with immunosuppressive pathways on innate and adaptive anti-tumor immune responses. Immunosuppression is mediated by suppressive cells of the myeloid lineage, triggering of checkpoint blockade, induction and recruitment of Treg cells.
Quantitative analysis of the immune and inflammatory components of the TME has resulted in the identification of novel prognostic factors associated with clinical cancer progression as illustrated by the immunoscore for T cells and by TAM analysis. Genomic approaches have added vistas to the analysis of the TME and to candidate new classifications of cancers. Finally, better understanding of the mechanism of action of conventional chemotherapeutic strategies, the therapeutic outcome of checkpoint blockade inhibitors, the introduction of therapeutic antibodies and, very recently, therapies based on adoptive transfer of immunocompetent cells for leukemias and lymphomas [86–88] have unequivocally shown that the immune responses can be manipulated to cure metastatic tumors.
The emergence of novel checkpoints acting on myeloid and innate lymphoid cells [76, 84, 85] may provide tools for innovative approaches. In the same vein, the impressive results discussed above blocking IL-1 in patients [89] offer promise for new approaches blocking tumor- promoting inflammation.
Acknowledgements
Alberto Mantovani is supported by European Commission (ERC project PHII-669415), Ministero dell’Istruzione, dell’Università e della Ricerca (MIUR) (project PRIN 2015YYKPNN), Associazione Italiana Ricerca sul Cancro (AIRC IG 19014; AIRC 5x1000 9962 and 21147), and Italian Ministry of Health. Sebastien Jaillon is supported by a grant from the Associazione Italiana per la Ricerca sul Cancro (AIRC ID18475); Antonio Inforzato is supported by Italian Ministry of Health (GR-2011-02349539) and Fondazione Beppe e Nuccy Angiolini; Andrea Ponzetta is recipient of a fellowship from the Fondazione Umberto Veronesi (FUV).
References
- 1.Fridman WH, Zitvogel L, Sautes-Fridman C, Kroemer G. The immune contexture in cancer prognosis and treatment. Nat Rev Clin Oncol. 2017;14:717–34. doi: 10.1038/nrclinonc.2017.101. [DOI] [PubMed] [Google Scholar]
- 2.Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454:436–44. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 3.Mantovani A, Romero P, Palucka AK, Marincola FM. Tumour immunity: effector response to tumour and role of the microenvironment. Lancet. 2008;371:771–83. doi: 10.1016/S0140-6736(08)60241-X. [DOI] [PubMed] [Google Scholar]
- 4.Mantovani A, Marchesi F, Malesci A, Laghi L, Allavena P. Tumour-associated macrophages as treatment targets in oncology. Nature reviews Clinical oncology. 2017;14:399–416. doi: 10.1038/nrclinonc.2016.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357:539–45. doi: 10.1016/S0140-6736(00)04046-0. [DOI] [PubMed] [Google Scholar]
- 6.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 7.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–74. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 8.Bonavita E, Gentile S, Rubino M, et al. PTX3 is an extrinsic oncosuppressor regulating complement-dependent inflammation in cancer. Cell. 2015;160:700–14. doi: 10.1016/j.cell.2015.01.004. [DOI] [PubMed] [Google Scholar]
- 9.Reis ES, Mastellos DC, Ricklin D, Mantovani A, Lambris JD. Complement in cancer: untangling an intricate relationship. Nature Reviews Immunology. 2018;18:5–18. doi: 10.1038/nri.2017.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Medrek C, Ponten F, Jirstrom K, Leandersson K. The presence of tumor associated macrophages in tumor stroma as a prognostic marker for breast cancer patients. BMC Cancer. 2012;12:306. doi: 10.1186/1471-2407-12-306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang WJ, Wang XH, Gao ST, et al. Tumor-associated macrophages correlate with phenomenon of epithelial-mesenchymal transition and contribute to poor prognosis in triple-negative breast cancer patients. J Surg Res. 2018;222:93–101. doi: 10.1016/j.jss.2017.09.035. [DOI] [PubMed] [Google Scholar]
- 12.Schoumacher M, Burbridge M. Key Roles of AXL and MER Receptor Tyrosine Kinases in Resistance to Multiple Anticancer Therapies. Curr Oncol Rep. 2017;19:19. doi: 10.1007/s11912-017-0579-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bottazzi B, Riboli E, Mantovani A. Aging, inflammation and cancer. Seminars in immunology. 2018;40:74–82. doi: 10.1016/j.smim.2018.10.011. [DOI] [PubMed] [Google Scholar]
- 14.Balkwill F, Charles KA, Mantovani A. Smoldering and polarized inflammation in the initiation and promotion of malignant disease. Cancer cell. 2005;7:211–7. doi: 10.1016/j.ccr.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 15.Mantovani A. Cancer: Inflaming metastasis. Nature. 2009;457:36–7. doi: 10.1038/457036b. [DOI] [PubMed] [Google Scholar]
- 16.Galdiero MR, Varricchi G, Loffredo S, Mantovani A, Marone G. Roles of neutrophils in cancer growth and progression. J Leukoc Biol. 2018;103:457–64. doi: 10.1002/JLB.3MR0717-292R. [DOI] [PubMed] [Google Scholar]
- 17.Noy R, Pollard JW. Tumor-Associated Macrophages: From Mechanisms to Therapy. Immunity. 2014;41:49–61. doi: 10.1016/j.immuni.2014.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.de Mingo Pulido A, Ruffell B. Immune Regulation of the Metastatic Process: Implications for Therapy. Adv Cancer Res. 2016;132:139–63. doi: 10.1016/bs.acr.2016.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Qian BZ, Li J, Zhang H, et al. CCL2 recruits inflammatory monocytes to facilitate breast-tumour metastasis. Nature. 2011;475:222–5. doi: 10.1038/nature10138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ruffell B, Affara NI, Coussens LM. Differential macrophage programming in the tumor microenvironment. Trends in immunology. 2012;33:119–26. doi: 10.1016/j.it.2011.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wyckoff J, Wang W, Lin EY, et al. A paracrine loop between tumor cells and macrophages is required for tumor cell migration in mammary tumors. Cancer research. 2004;64:7022–9. doi: 10.1158/0008-5472.CAN-04-1449. [DOI] [PubMed] [Google Scholar]
- 22.Mason SD, Joyce JA. Proteolytic networks in cancer. Trends in cell biology. 2011;21:228–37. doi: 10.1016/j.tcb.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liang W, Ferrara N. The Complex Role of Neutrophils in Tumor Angiogenesis and Metastasis. Cancer Immunol Res. 2016;4:83–91. doi: 10.1158/2326-6066.CIR-15-0313. [DOI] [PubMed] [Google Scholar]
- 24.Gay LJ, Felding-Habermann B. Contribution of platelets to tumour metastasis. Nat Rev Cancer. 2011;11:123–34. doi: 10.1038/nrc3004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Joyce JA, Pollard JW. Microenvironmental regulation of metastasis. Nature reviews Cancer. 2009;9:239–52. doi: 10.1038/nrc2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pages F, Mlecnik B, Marliot F, et al. International validation of the consensus Immunoscore for the classification of colon cancer: a prognostic and accuracy study. Lancet. 2018;391:2128–39. doi: 10.1016/S0140-6736(18)30789-X. [DOI] [PubMed] [Google Scholar]
- 27.Steidl C, Lee T, Shah SP, et al. Tumor-associated macrophages and survival in classic Hodgkin's lymphoma. The New England journal of medicine. 2010;362:875–85. doi: 10.1056/NEJMoa0905680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Malesci A, Bianchi P, Celesti G, et al. Tumor-associated macrophages and response to 5-fluorouracil adjuvant therapy in stage III colorectal cancer. Oncoimmunology. 2017;6:e1342918. doi: 10.1080/2162402X.2017.1342918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Germano G, Frapolli R, Belgiovine C, et al. Role of macrophage targeting in the antitumor activity of trabectedin. Cancer Cell. 2013;23:249–62. doi: 10.1016/j.ccr.2013.01.008. [DOI] [PubMed] [Google Scholar]
- 30.D'Incalci M, Badri N, Galmarini CM, Allavena P. Trabectedin, a drug acting on both cancer cells and the tumour microenvironment. Br J Cancer. 2014;111:646–50. doi: 10.1038/bjc.2014.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ries CH, Cannarile MA, Hoves S, et al. Targeting tumor-associated macrophages with anti-CSF-1R antibody reveals a strategy for cancer therapy. Cancer Cell. 2014;25:846–59. doi: 10.1016/j.ccr.2014.05.016. [DOI] [PubMed] [Google Scholar]
- 32.Germano G, Frapolli R, Simone M, et al. Antitumor and anti-inflammatory effects of trabectedin on human myxoid liposarcoma cells. Cancer research. 2010;70:2235–44. doi: 10.1158/0008-5472.CAN-09-2335. [DOI] [PubMed] [Google Scholar]
- 33.Planque C, Kulasingam V, Smith CR, Reckamp K, Goodglick L, Diamandis EP. Identification of five candidate lung cancer biomarkers by proteomics analysis of conditioned media of four lung cancer cell lines. Mol Cell Proteomics. 2009;8:2746–58. doi: 10.1074/mcp.M900134-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Infante M, Allavena P, Garlanda C, et al. Prognostic and diagnostic potential of local and circulating levels of pentraxin 3 in lung cancer patients. Int J Cancer. 2016;138:983–91. doi: 10.1002/ijc.29822. [DOI] [PubMed] [Google Scholar]
- 35.Barbui T, Carobbio A, Finazzi G, et al. Inflammation and thrombosis in essential thrombocythemia and polycythemia vera: different role of C-reactive protein and pentraxin 3. Haematologica. 2011;96:315–8. doi: 10.3324/haematol.2010.031070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kondo S, Ueno H, Hosoi H, et al. Clinical impact of pentraxin family expression on prognosis of pancreatic carcinoma. British Journal of Cancer. 2013;109:739–46. doi: 10.1038/bjc.2013.348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Locatelli M, Ferrero S, Martinelli Boneschi F, et al. The long pentraxin PTX3 as a correlate of cancer-related inflammation and prognosis of malignancy in gliomas. Journal of neuroimmunology. 2013;260:99–106. doi: 10.1016/j.jneuroim.2013.04.009. [DOI] [PubMed] [Google Scholar]
- 38.Carmo RF, Aroucha D, Vasconcelos LR, Pereira LM, Moura P, Cavalcanti MS. Genetic variation in PTX3 and plasma levels associated with hepatocellular carcinoma in patients with HCV. J Viral Hepat. 2016;23:116–22. doi: 10.1111/jvh.12472. [DOI] [PubMed] [Google Scholar]
- 39.Han B, Mura M, Andrade CF, et al. TNFalpha-induced long pentraxin PTX3 expression in human lung epithelial cells via JNK. J Immunol. 2005;175:8303–11. doi: 10.4049/jimmunol.175.12.8303. [DOI] [PubMed] [Google Scholar]
- 40.Lussana F, Carobbio A, Salmoiraghi S, et al. Driver mutations (JAK2V617F, MPLW515L/K or CALR), pentraxin-3 and C-reactive protein in essential thrombocythemia and polycythemia vera. J Hematol Oncol. 2017;10:54. doi: 10.1186/s13045-017-0425-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Song X, Voronov E, Dvorkin T, et al. Differential effects of IL-1 alpha and IL-1 beta on tumorigenicity patterns and invasiveness. J Immunol. 2003;171:6448–56. doi: 10.4049/jimmunol.171.12.6448. [DOI] [PubMed] [Google Scholar]
- 42.Giavazzi R, Garofalo A, Bani MR, et al. Interleukin 1-induced augmentation of experimental metastases from a human melanoma in nude mice. Cancer research. 1990;50:4771–5. [PubMed] [Google Scholar]
- 43.Chirivi RG, Garofalo A, Padura IM, Mantovani A, Giavazzi R. Interleukin 1 receptor antagonist inhibits the augmentation of metastasis induced by interleukin 1 or lipopolysaccharide in a human melanoma/nude mouse system. Cancer Res. 1993;53:5051–4. [PubMed] [Google Scholar]
- 44.Vidal-Vanaclocha F, Amezaga C, Asumendi A, Kaplanski G, Dinarello CA. Interleukin-1 receptor blockade reduces the number and size of murine B16 melanoma hepatic metastases. Cancer Res. 1994;54:2667–72. [PubMed] [Google Scholar]
- 45.Voronov E, Shouval DS, Krelin Y, et al. IL-1 is required for tumor invasiveness and angiogenesis. Proc Natl Acad Sci USA. 2003;100:2645–50. doi: 10.1073/pnas.0437939100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cataisson C, Salcedo R, Hakim S, et al. IL-1R-MyD88 signaling in keratinocyte transformation and carcinogenesis. J Exp Med. 2012;209:1689–702. doi: 10.1084/jem.20101355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Borrello MG, Alberti L, Fischer A, et al. Induction of a proinflammatory program in normal human thyrocytes by the RET/PTC1 oncogene. Proc Natl Acad Sci USA. 2005;102:14825–30. doi: 10.1073/pnas.0503039102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Garlanda C, Dinarello CA, Mantovani A. The interleukin-1 family: back to the future. Immunity. 2013;39:1003–18. doi: 10.1016/j.immuni.2013.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ling J, Kang Y, Zhao R, et al. KrasG12D-induced IKK2/beta/NF-kappaB activation by IL-1alpha and p62 feedforward loops is required for development of pancreatic ductal adenocarcinoma. Cancer Cell. 2012;21:105–20. doi: 10.1016/j.ccr.2011.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Melisi D, Niu J, Chang Z, et al. Secreted interleukin-1alpha induces a metastatic phenotype in pancreatic cancer by sustaining a constitutive activation of nuclear factor-kappaB. Mol Cancer Res. 2009;7:624–33. doi: 10.1158/1541-7786.MCR-08-0201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhuang Z, Ju HQ, Aguilar M, et al. IL1 receptor antagonist inhibits pancreatic cancer growth by abrogating NF-kappaB activation. Clin Cancer Res. 2016;22:1432–44. doi: 10.1158/1078-0432.CCR-14-3382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tjomsland V, Bojmar L, Sandstrom P, Bratthall C, Messmer D, Spangeus A, Larsson M. IL-1alpha expression in pancreatic ductal adenocarcinoma affects the tumor cell migration and is regulated by the p38MAPK signaling pathway. PLoS One. 2013;8:e70874. doi: 10.1371/journal.pone.0070874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lust JA, Lacy MQ, Zeldenrust SR, Witzig TE, Moon-Tasson LL, Dinarello CA, Donovan KA. Reduction in C-reactive protein indicates successful targeting of the IL-1/IL-6 axis resulting in improved survival in early stage multiple myeloma. American journal of hematology. 2016;91:571–4. doi: 10.1002/ajh.24352. [DOI] [PubMed] [Google Scholar]
- 54.Wu TC, Xu K, Martinek J, et al. IL1 Receptor Antagonist Controls Transcriptional Signature of Inflammation in Patients with Metastatic Breast Cancer. Cancer research. 2018;78:5243–58. doi: 10.1158/0008-5472.CAN-18-0413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Elkabets M, Ribeiro VS, Dinarello CA, Ostrand-Rosenberg S, Di Santo JP, Apte RN, Vosshenrich CA. IL-1beta regulates a novel myeloid-derived suppressor cell subset that impairs NK cell development and function. Eur J Immunol. 2010;40:3347–57. doi: 10.1002/eji.201041037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pan W, Zhu S, Qu K, et al. The DNA Methylcytosine Dioxygenase Tet2 Sustains Immunosuppressive Function of Tumor-Infiltrating Myeloid Cells to Promote Melanoma Progression. Immunity. 2017;47:284–97 e5. doi: 10.1016/j.immuni.2017.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kuan EL, Ziegler SF. A tumor-myeloid cell axis, mediated via the cytokines IL-1alpha and TSLP, promotes the progression of breast cancer. Nat Immunol. 2018;19:366–74. doi: 10.1038/s41590-018-0066-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.El-Omar EM, Carrington M, Chow WH, et al. Interleukin-1 polymorphisms associated with increased risk of gastric cancer. Nature. 2000;404:398–402. doi: 10.1038/35006081. [DOI] [PubMed] [Google Scholar]
- 59.Lind H, Zienolddiny S, Ryberg D, Skaug V, Phillips DH, Haugen A. Interleukin 1 receptor antagonist gene polymorphism and risk of lung cancer: a possible interaction with polymorphisms in the interleukin 1 beta gene. Lung Cancer. 2005;50:285–90. doi: 10.1016/j.lungcan.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 60.Bhat IA, Naykoo NA, Qasim I, et al. Association of interleukin 1 beta (IL-1beta) polymorphism with mRNA expression and risk of non small cell lung cancer. Meta Gene. 2014;2:123–33. doi: 10.1016/j.mgene.2013.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zienolddiny S, Ryberg D, Maggini V, Skaug V, Canzian F, Haugen A. Polymorphisms of the interleukin-1 beta gene are associated with increased risk of non-small cell lung cancer. Int J Cancer. 2004;109:353–6. doi: 10.1002/ijc.11695. [DOI] [PubMed] [Google Scholar]
- 62.Hu Z, Shao M, Chen Y, et al. Allele 2 of the interleukin-1 receptor antagonist gene (IL1RN*2) is associated with a decreased risk of primary lung cancer. Cancer Lett. 2006;236:269–75. doi: 10.1016/j.canlet.2005.05.015. [DOI] [PubMed] [Google Scholar]
- 63.Khazim K, Azulay EE, Kristal B, Cohen I. Interleukin 1 gene polymorphism and susceptibility to disease. Immunol Rev. 2018;281:40–56. doi: 10.1111/imr.12620. [DOI] [PubMed] [Google Scholar]
- 64.Lust JA, Lacy MQ, Zeldenrust SR, et al. Induction of a chronic disease state in patients with smoldering or indolent multiple myeloma by targeting interleukin 1{beta}-induced interleukin 6 production and the myeloma proliferative component. Mayo Clin Proc. 2009;84:114–22. doi: 10.4065/84.2.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Isambert N, Hervieu A, Hennequin A, et al. Fluorouracil plus bevacizumab plus anakinra for patients with metastatic colorectal cancer refractory to standard therapies (IRAFU): an investigator-initiated, open-label, single-arm, multicenter, phase 2 study. ASCO. 2018 doi: 10.1080/2162402X.2018.1474319. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Becerra C, Paulson AS, Cavaness K, Hoof PD, Celinski S. Gemcitabine, nab-paclitaxel, cisplatin, and anakinra (AGAP) treatment in patients with non-metastatic pancreatic ductal adenocarcinoma (PDAC). NCT02550327. J Clin Oncol. 2018;36(supplement 4) Abstract 449. [Google Scholar]
- 67.Whiteley A, Becerra C, McCollum D, Paulson AS, Goel A. A pilot, non-randomized evaluation of the safety of anakinra plus FOLFIRINOX in metastatic pancreatic ductal adenocarcinoma patients. J Clin Oncol. 2016;34(suppl):e165750. [Google Scholar]
- 68.Hong DS, Janku F, Naing A, et al. Xilonix, a novel true human antibody targeting the inflammatory cytokine interleukin-1 alpha, in non-small cell lung cancer. Invest New Drugs. 2015;33:621–31. doi: 10.1007/s10637-015-0226-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hong DS, Hui D, Bruera E, et al. MABp1, a first-in-class true human antibody targeting interleukin-1alpha in refractory cancers: an open-label, phase 1 dose-escalation and expansion study. Lancet Oncol. 2014;15:656–66. doi: 10.1016/S1470-2045(14)70155-X. [DOI] [PubMed] [Google Scholar]
- 70.Hickish T, Andre T, Wyrwicz L, et al. MABp1 as a novel antibody treatment for advanced colorectal cancer: a randomised, double-blind, placebo-controlled, phase 3 study. Lancet Oncol. 2017;18:192–201. doi: 10.1016/S1470-2045(17)30006-2. [DOI] [PubMed] [Google Scholar]
- 71.McDonald JJ, McMillan DC, Laird BJA. Targeting IL-1alpha in cancer cachexia: a narrative review. Curr Opin Support Palliat Care. 2018;12:453–9. doi: 10.1097/SPC.0000000000000398. [DOI] [PubMed] [Google Scholar]
- 72.Ridker PM, MacFadyen JG, Everett BM, Libby P, Thuren T, Glynn RJ. Relationship of C-reactive protein reduction to cardiovascular event reduction following treatment with canakinumab: a secondary analysis from the CANTOS randomised controlled trial. Lancet. 2018;391:319–28. doi: 10.1016/S0140-6736(17)32814-3. [DOI] [PubMed] [Google Scholar]
- 73.Molgora M, Supino D, Mavilio D, Santoni A, Moretta L, Mantovani A, Garlanda C. The yin-yang of the interaction between myelomonocytic cells and NK cells. Scand J Immunol. 2018;88:e12705. doi: 10.1111/sji.12705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Vivier E, Raulet DH, Moretta A, et al. Innate or adaptive immunity? The example of natural killer cells. Science. 2011;331:44–9. doi: 10.1126/science.1198687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Vivier E, Artis D, Colonna M, et al. Innate Lymphoid Cells: 10 Years On. Cell. 2018;174:1054–66. doi: 10.1016/j.cell.2018.07.017. [DOI] [PubMed] [Google Scholar]
- 76.Molgora M, Bonavita E, Ponzetta A, et al. IL-1R8 is a checkpoint in NK cells regulating anti-tumour and anti-viral activity. Nature. 2017;551:110–4. doi: 10.1038/nature24293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sarhan D, Hippen KL, Lemire A, et al. Adaptive NK Cells Resist Regulatory T-cell Suppression Driven by IL37. Cancer Immunol Res. 2018;6:766–75. doi: 10.1158/2326-6066.CIR-17-0498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Campesato LF, Silva APM, Cordeiro L, et al. High IL-1R8 expression in breast tumors promotes tumor growth and contributes to impaired antitumor immunity. Oncotarget. 2017;8:49470–83. doi: 10.18632/oncotarget.17713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bellora F, Castriconi R, Dondero A, et al. The interaction of human natural killer cells with either unpolarized or polarized macrophages results in different functional outcomes. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:21659–64. doi: 10.1073/pnas.1007654108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.McCracken MN, Cha AC, Weissman IL. Molecular Pathways: Activating T Cells after Cancer Cell Phagocytosis from Blockade of CD47 "Don't Eat Me" Signals. Clinical cancer research : an official journal of the American Association for Cancer Research. 2015;21:3597–601. doi: 10.1158/1078-0432.CCR-14-2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Casey SC, Tong L, Li Y, et al. MYC regulates the antitumor immune response through CD47 and PD-L1. Science. 2016;352:227–31. doi: 10.1126/science.aac9935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chao MP, Alizadeh AA, Tang C, et al. Anti-CD47 antibody synergizes with rituximab to promote phagocytosis and eradicate non-Hodgkin lymphoma. Cell. 2010;142:699–713. doi: 10.1016/j.cell.2010.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Leidi M, Gotti E, Bologna L, et al. M2 macrophages phagocytose rituximab-opsonized leukemic targets more efficiently than m1 cells in vitro. J Immunol. 2009;182:4415–22. doi: 10.4049/jimmunol.0713732. [DOI] [PubMed] [Google Scholar]
- 84.Advani R, Flinn I, Popplewell L, et al. CD47 Blockade by Hu5F9-G4 and Rituximab in Non-Hodgkin's Lymphoma. N Engl J Med. 2018;379:1711–21. doi: 10.1056/NEJMoa1807315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mantovani A, Longo DL. Macrophage Checkpoint Blockade in Cancer - Back to the Future. The New England journal of medicine. 2018;379:1777–9. doi: 10.1056/NEJMe1811699. [DOI] [PubMed] [Google Scholar]
- 86.Sadelain M, Riviere I, Riddell S. Therapeutic T cell engineering. Nature. 2017;545:423–31. doi: 10.1038/nature22395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Anderson KG, Stromnes IM, Greenberg PD. Obstacles Posed by the Tumor Microenvironment to T cell Activity: A Case for Synergistic Therapies. Cancer cell. 2017;31:311–25. doi: 10.1016/j.ccell.2017.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gubin MM, Artyomov MN, Mardis ER, Schreiber RD. Tumor neoantigens: building a framework for personalized cancer immunotherapy. The Journal of clinical investigation. 2015;125:3413–21. doi: 10.1172/JCI80008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ridker PM, MacFadyen JG, Thuren T, Everett BM, Libby P, Glynn RJ. Effect of interleukin-1beta inhibition with canakinumab on incident lung cancer in patients with atherosclerosis: exploratory results from a randomised, double-blind, placebo-controlled trial. Lancet. 2017;390:1833–42. doi: 10.1016/S0140-6736(17)32247-X. [DOI] [PubMed] [Google Scholar]