Sir,
We read with great interest the article by Perez et al. (2018) on the emerging condition of intellectual disability caused by biallelic pathogenic variants in RSRC1 (arginine and serine rich coiled-coil 1). Previous genome wide association studies (GWAS) by Potkin et al. (2009, 2010) had suggested a possible involvement of RSRC1 in non-syndromic intellectual disability and gene regulatory networks in schizophrenia. However, the results of these studies remained elusive and were not confirmed by a subsequent GWAS study by Schizophrenia Working Group of the Psychiatric Genomics Consortium (,2014). Meanwhile, Berndt et al. (2013) identified RSRC1 as a new locus influencing height through a genome-wide meta-analysis. The first family in which a homozygous RSRC1 variant clearly segregated with non-syndromic intellectual disability was only recently reported by Maddirevula et al. (2018). A possible role of RSRC1 in major depressive disorder has been further suggested by a meta-analysis on three large GWAS performed by Li et al. (2018).
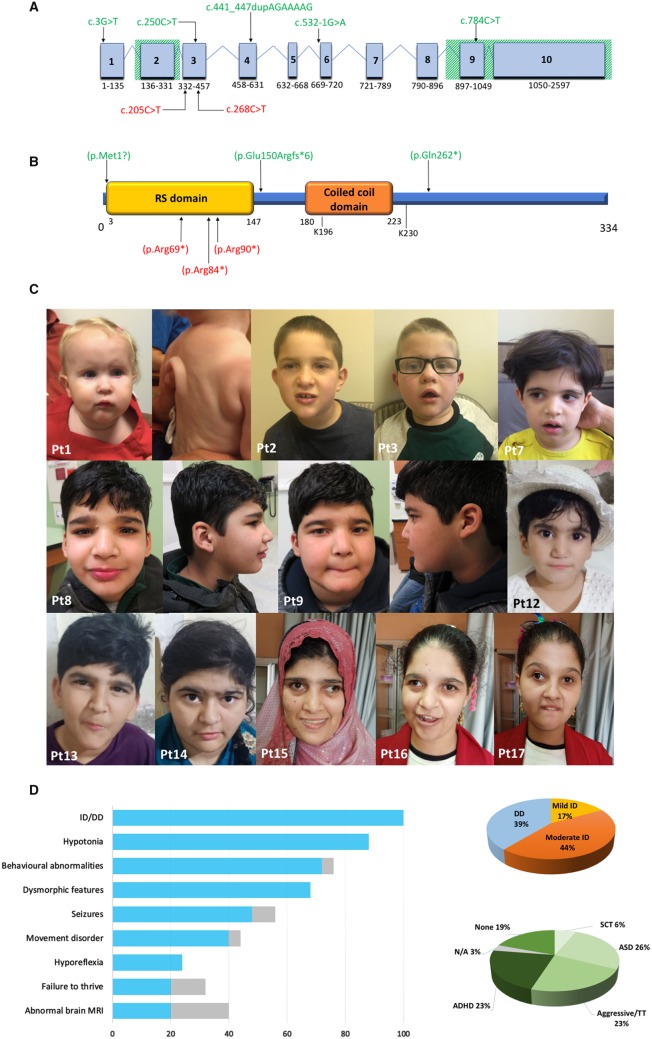
Serine and arginine-rich (SR) proteins are evolutionary conserved co-regulators of constitutive and alternative pre-mRNA splicing. The longest RSRC1 transcript (NM_001271838.1) includes 10 exons (Fig. 1A) and encodes a 334-amino-acid SR-related protein of 53 kDa (SRrp53) (Fig. 1B), localized to the nuclear speckled domain. Interacting with other splicing regulators, RSRC1 plays a relevant role in the second step of pre-mRNA splicing. In addition, it could be involved in post-splicing mRNA processing, shuttling between the nucleus and cytoplasm (Cazalla et al., 2005). RSRC1 further promotes PIAS1-mediated SUMOylation of the pleiotropic transcription factor oestrogen receptor b (ERb), acting as transcriptional regulator (Chen et al., 2015).
Figure 1.
Genetic findings and clinical pictures of RSRC1 patients. (A) Schematic diagram of the longer RSRC1 transcript (NM_001271838.1) consisting of 2597 nucleotides in 10 exons. The deletions encompassing exons 2 and 9–10 are represented by diagonal green lines. Single nucleotide variants are shown in red (previously reported patients) or in green (this study). (B) The RSRC1 protein (NP_001258767.1) consists of 334 amino acids encompassing an RS domain rich in arginine (R) and serine (S) that mediates the interactions with other RS-rich proteins involved in splicing regulation (SF2/ASF and U2AF35) and a coiled coil domain required for RSRC1/ERβ interaction as well as the enhancement of ERβ SUMOylation. The residues K196 and K 230 are necessary for RSRC1 SUMOylation by SUMO1 and the E3 ligases PIAS1 and PIAS3. Pathogenic amino acid changes reported in previous papers and identified in this study are shown in red and green, respectively. (C) Sequential pictures from selected patients. Patients from the Amish family (Patients 1–3) show prominent forehead, deep set eyes, depressed nasal bridge, protruding ears, and overbite with drooling. Redundant skin is evident in Patient 1. The Persian patient (Patient 7) shows straight eyebrows with mild synophrys, deep set eyes, and protruding ears. In the first Pakistani family (Patients 8 and 9) dysmorphic features include straight eyebrows with mild medial flaring, deep set eyes, wide nasal base, short philtrum, uplifted earlobes, and prominent chin. Subjects from the second Pakistani family (Patients 12–14) also show straight eyebrows with mild synophrys and deep set eyes, in addition to protruding ears with uplifted lobes (Patient 14). Patients from the Egyptian family (Patients 15–17) show thick eyebrows with medial sparing, deep set eyes, and prominent columella. (D) Graphic illustrations of the most common clinical findings in RSRC1 patients in our cohort: the bar graph shows the percent distribution of the cardinal features of RSRC1-related intellectual disability, with the grey lines representing not available data; the pie charts show the percentage distribution of developmental delay/intellectual disability and the different behavioural abnormalities observed in RSRC1 patients. Gene transcript and protein details available at: https://www.ensembl.org (RSRC1-215, transcript ID ENST00000611884.5), https://www.nextprot.org (NX_Q96IZ7), https://www.uniprot.org (Q96IZ7), https://www.proteomicsdb.org (Q96IZ7). ADHD = attention deficit hyperactivity disorder; ASD = autism spectrum disorder; DD = developmental delay; ID = intellectual disability; N/A = not available; Pt = patient; SCT = sluggish cognitive tempo; TT = temper tantrums.
The involvement of RSRC1 in cancer was first suggested by the identification of the recurrent PTPLB-RSRC1 in-frame gene fusion in nasopharyngeal carcinoma by Valouev et al. (2014). Teplyuk et al. (2016) showed that RSRC1 is a target gene of the microRNA‐10b (miR‐10b), an oncogenic microRNA that is highly expressed in glioblastoma and represents a candidate for the development of targeted therapies. More recently, several studies have implicated RSRC1 in cancer predisposition and progression. The RSRC1 intronic polymorphism rs6441201 G > A has been associated with neuroblastoma susceptibility (McDaniel et al., 2017; Tang et al., 2018). A possible role of RSRC1 in non-small-cell lung cancer tumorigenesis and progression has been hypothesized based on the data from RNA sequencing data of tumour-educated platelets (Sheng et al., 2018). RSRC1 has been also shown to suppress gastric cancer cell proliferation and migration through the regulation of PTEN expression, acting as tumour suppressor (Yu et al., 2019).
The first RSRC1 pathogenic variant segregating with intellectual disability was reported by Maddirevula et al. (2018) in three affected siblings from a consanguineous Malaysian family (Supplementary Table 3). The homozygous truncating variant c.268C>T (p.Arg90*) (NM_001271838.1) resulted in a full loss of function, causing the natural knockout of the gene. All the reported patients showed developmental delay and variable degree of intellectual disability. One subject also suffered from febrile seizures. Brain MRI was normal in the 10-year-old male (Patient 1), whereas temporal lobe atrophy was found in his 4-year-old brother (Patient 2). No distinctive neurological features or facial dysmorphism were observed. More recently, Perez et al. (2018) reported five further individuals from consanguineous Bedouin kindred with early developmental delay, intellectual disability, hypotonia, behavioural abnormalities, and mild facial dysmorphic features. Brain MRI was normal (Supplementary Table 3). Exome sequencing revealed the homozygous c.205C>T (p.Arg69*) in RSRC1 (NM_001271838.1) in all patients, a nonsense variant leading to nonsense-mediated mRNA decay. RSRC1 knock-down SH-SY5Y cells showed impaired alternative splicing. Specific differential expression of genes associated with intellectual disability, hypotonia, schizophrenia, and dementia was also observed, supporting the pivotal role of RSRC1 in transcriptional regulation (Perez et al., 2018).
We report 17 additional subjects from seven consanguineous families with intellectual disability, behavioural abnormalities, and facial dysmorphism, harbouring homozygous RSRC1 loss-of-function variants (Table 1 and Supplementary Table 1). The families were of different ancestries (European/Middle Eastern, Saudi, Egyptian, Old Order Amish, Pakistani, and Persian) (Supplementary Fig. 1). The collaboration among the involved study centres was managed through GeneMatcher (Sobreira et al., 2015). After informed consent was obtained from the parents, photographic material was collected and genetic testing through exome sequencing was performed. Genetic methods are provided in detail in the Supplementary material. Trio-exome sequencing was conducted in Patient 10, the family quartet was sequenced for Patients 8 and 9, and proband-exome sequencing was performed in all the remaining individuals, followed by variants validation through Sanger sequencing. No relevant single nucleotide variant was identified in the siblings of Amish ancestry (Patients 1–4), who were further studied through comparative genomic hybridization (CGH) array. Five RSRC1 sequence variants (including an intragenic duplication) and two partial deletions were identified.
Table I.
Summary of genetic findings and clinical features of RSRC1 patients
| Family | Family I (Amish) | Family II (Persian) | Family III (Pakistani) | Family IV (Saudi) | Family V (EUR/ ME) | Family VI (Pakistani) | Family VII (Egyptian) | Maddirevula et al.2018 (Malaysian) | Perez et al.2018 (Bedouin) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient | 1(II-2) | 2(II-3) | 3(II-4) | 4(II-5) | 5(II-3) | 6(II-1) | 7(II-2) | 8(II-2) | 9(II-1) | 10(II-2) | 11(II-1) | 12(II-6) | 13(II-4) | 14(II-3) | 15(II-5) | 16 (II-8) | 17(II-9) | 3 patients | 5 patients |
| Age/sex | 3 y/F | 6 y/M | 5 y/M | 11 mo/M | 6 y/M | 14 y/F | 3 y/F | 9 y/M | 11 y/M | 4 y/M | 16 y/F | 6 y/F | 15 y/M | 16 y/F | 22 y/F | 16 y/F | 12 y/F | 4–10 y/2M, 1F | 0.5–8 y/3F, 2M |
| Homozygous RSRC1 variants [NM_001271838.1] | c.158,256,914_158,338,237dela | c.784 C>T (p.Gln262*) | c.157,839,811_ 157,840, 314delb | c.250 C>T (p.Arg84*) | c.441_447dupAGAAAAG (p.Glu150Argfs*6) | c.532-1 G>A | c.3G>T(p.Met1?) | c.268C>T (p.Arg90*) | c.205C>T (p.Arg69*) | ||||||||||
| Consanguinity | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + (3) | + (5) |
| Dysmorphic features | + | + | + | + | + | + | + | – | – | + | + | + | + | + | – | – | – | – | + (5) |
| Global DD/ID | + | + | + | + | ++ | ++ | + | ++ | ++ | + | + | ++ | ++ | ++ | ++ | ++ | ++ | + (5) | +/++ (5) |
| Speech delay | N/A | + | + | + | N/A | N/A | N/A | N/A | N/A | + | + | + | + | – | – | + | – | + (1) | + (4) |
| Behavioural abnormalities | – | ASD | ADHD, ASD | – | ASD | ASD | ASD | ADHD | ADHD | – | SCT | Agg | Agg | SCT | ASD | Agg | ASD | – | TT (4), ASD (1), ADHD (4) |
| Hypotonia | +++ | ++ | + | ++ | ++ | ++ | ++ | + | + | + | ++ | + | + | + | + | + | + | – | + (5) |
| Movement disorders | – | Gait ataxia | Gait ataxia | – | Gait ataxia | Gait ataxia | – | – | – | Truncal ataxia | Brady-kinesia, ataxic gait | – | – | – | – | – | – | – | Fine motor impairment (4) |
| Seizures | – | – | – | – | N/A | – | FS, GTCS | FS | – | GTCS | – | FS, GTCS | FS, GTCS | N/A | – | – | FS | FS (1) | FS (5), epilepsy (1) |
| Musculo-skeletal abnormalities | – | PP, CV | PP, CV | – | – | – | – | PP | PP | – | PP, CV, short toes | PP, short fifth toes | CV, genu valgum | PP | PP | PP | – | Foot deformities (2) | – |
| Brain MRI | N | PSS | PSS | N/A | MCA | N | N | N | N | Left temporo- parietal atrophy | N | N/A | N/A | N/A | N | N | N | Temporal lobes atrophy (1) | N (5) |
ADHD = attention deficit hyperactivity disorder; Agg = aggressive; ASD = autism spectrum disorder; CV = cubitus valgus; DD = developmental delay; EUR = European; FS = febrile seizures; GTCS = generalized tonic-clonic seizures; ID = intellectual disability (+, mild; ++, moderate); MCA = mild cerebral atrophy; ME = Middle Eastern; mo = months; N = normal; N/A = not available; PP = pes planus; PSS = prominent subarachnoid spaces; SCT = sluggish cognitive tempo; y = years.
hg19; 81-kb deletion encompassing exons 9–10 of RSRC1 and whole MLF1.
hg19; 500-bp deletion encompassing exon 2 of RSRC1.
In all the enrolled subjects, global psychomotor developmental delay and mild-to-moderate intellectual disability were observed. Twelve patients were diagnosed with variable behavioural disorders (Table 1 and Supplementary Table 1). Neurological examination revealed generalized hypotonia in all patients and five of them had a history of hypotonia at birth. Decreased deep tendon reflexes were found in six patients (35%). Five patients showed gait ataxia, which was associated with bradykinesia in Patient 11. Truncal ataxia was observed in Patient 10. Seizures occurred in six individuals (35%), including four cases of febrile seizures. Afebrile generalized tonic-clonic seizures in addition to febrile seizures were observed in Patients 7, 12, and 13. Patient 10 experienced three episodes of generalized tonic-clonic seizures before becoming seizure-free. His EEG showed occasional epileptiform discharges in the centrofrontal and in the left parietocentral regions during sleep. No recurrent epileptic phenotype or peculiar EEG features were recognizable in our cohort. Psychomotor regression was not observed in any case. The most common dysmorphic features were deep-set eyes, broad nasal base, and ogival palate (Fig. 1C). Associated congenital anomalies were extremely variable, ranging from simple pes planus to redundant skin. Other isolated clinical features included mitral valve prolapse, recurrent respiratory infections in the first year of life, and tracheomalacia. When available, brain MRI did not reveal any distinctive finding. Non-specific mild cerebral atrophy was observed in Patients 5 and 6, whereas enlargement of subarachnoid spaces was found in Patients 2 and 3. Delayed myelination, dysmorphic lateral ventricles, and unilateral focal polymicrogyria were observed in Patient 10.
In our cohort, we identified homozygous RSRC1 variants or deletions leading to loss of function (Table 1 and Supplementary Table 1). Variants’ nomenclatures are given with reference to the RSRC1 transcript NM_001271838.1. The four Amish siblings (Patients 1–4) carried an 81-kb deletion encompassing exons 9–10 of RSRC1 and the entire MLF1 gene. Although MLF1 is involved in haematopoiesis and leukemogenesis, a possible pathogenic role for this gene in some of the dysmorphic features and congenital anomalies in the Amish patients cannot be excluded (Yoneda-Kato et al., 1996; Nakamae et al., 2017). A second 500-bp deletion involving exon 2 of RSRC1 was identified in the siblings from the Pakistani family (Patients 8 and 9). Both these rearrangements are novel and are not reported in ClinVar and DECIPHER databases (Supplementary Table 1). The three patients from the second Pakistani family (Patients 12–14) carried the splicing variant c.532-1G>A. This amino acid change is predicted to cause aberrant splicing through the alteration of the splice acceptor site at exon 6 of RSRC1 (Supplementary Table 3). In the remaining families, homozygous stop-gain or start-loss variants were identified. The 16-year-old female of European ancestry (Patient 11) harboured the intragenic duplication c.441_447dupAGAAAAG (p.Glu150Argfs*6). The stop-gain variants c.784C>T (p.Gln262*) and c.250C>T (p.Arg84*) were identified in the siblings from the Persian (Patients 5–7) and Middle East (Patient 10) families, respectively. In the Egyptian family (Patients 15–17), the variant c.3G>T (p.Met1?) causing a start loss and leading to full loss of function (null variant) was found.
All the variants fully segregated with the phenotype and were not found in the most common genome databases, including the Genome Aggregation Database (gnomAD), Iranome, Greater Middle East Variome Project (GME Variome), and our database of 10 000 in-house control exomes. The only exception was the splicing variant c.532-1G>A, which was observed in heterozygous state in 3 of 166 052 in gnomAD (allele frequency 0.00001806). Furthermore, the start loss variant affecting the same nucleotide of the c.3G>T (p.Met1?) variant observed in our Egyptian family is reported in heterozygous state in gnomAD (genomes allele frequency 0.00003186). However, neither variant has been reported in homozygous state in healthy individuals. The analysis of sequence conservation through Genomic Evolutionary Rate Profiling (GERP) score revealed good conservation of the affected residues and in silico prediction analysis through CADD score calculation revealed high-deleterious scores (Supplementary Table 3). All variants were predicted to be damaging or likely damaging by several bioinformatic tools (e.g. SIFT, MutationTaster, and Human Splice Finder) and were classified as pathogenic (class 5) or likely pathogenic (class 4) according to the American College of Medical Genetics and Genomics (ACMG) guidelines (Richards et al., 2015).
This study supports the idea that RSRC1 pathogenic variants cause a non-syndromic disorder characterized by mild-to-moderate intellectual disability, generalized hypotonia, and variable neurological and behavioural features (Fig. 1D). Despite a few minor dysmorphic features being observed (especially deep-set eyes and broad nasal base), a consistent recurrent facial gestalt could not be recognized. Furthermore, the extremely variable associated non-neurological features were not suggestive of a syndromic condition. Even though seizures are common in RSRC1 patients, most of them only suffer from febrile seizures, lacking a distinctive epileptic phenotype. Definite epileptic seizures were only diagnosed in Patient 12 and in a case reported by Perez et al. (2018). This patient suffered from focal seizures with impaired awareness occasionally progressing to tonic-clonic and was treated with valproic acid. Behavioural abnormalities are frequent, although extremely variable and ranging from attention deficit hyperactivity disorder (ADHD) to autism spectrum disorder (ASD) (Fig. 1D). Besides the non-specific neuroradiological abnormalities observed in our patients, bilateral symmetric temporal lobe atrophy has been described in a single case by Maddirevula et al. (2018). Even though further neuroimaging studies will be necessary, the limited data available support the lack of a peculiar neuroradiological phenotype. According to these observations, biallelic RSRC1 variants should be considered the cause of a non-syndromic intellectual disability mainly associated with generalized hypotonia and behavioural disturbances. Facial dysmorphism and other minor clinical features have limited diagnostic relevance, likewise neuroimaging is of limited value.
Thanks to the remarkable advances in gene discovery achieved through exome sequencing, the large group of known genes causing non-syndromic intellectual disability is rapidly expanding with relevant impact on diagnosis and patient management. Our findings support the pathogenic role of biallelic loss-of-function RSRC1 variants in autosomal recessive intellectual disability, in addition to contributing to the phenotypic delineation of this emerging condition. Global developmental delay, mild-to-moderate intellectual disability, behavioural abnormalities, and generalized hypotonia represent the cardinal features of RSRC1-related intellectual disability. Other neurological features may be less frequently observed, especially hyporeflexia and febrile seizures. Further studies will help clarify the possible role of neuroimaging in the diagnostic process. In conclusion, we suggest that the involvement of RSRC1 should be considered in the differential diagnosis in intellectually disabled children with hypotonia and behavioural disturbances, and that RSRC1 should be included in next generation sequencing (NGS) panels for intellectual disability.
Data availability
Data sharing is not applicable to this article, as no new data were created or analysed in this study.
Web resources
The following URLs were used for data presented herein:
ClinVar; https://www.ncbi.nlm.nih.gov/clinvar
Combined Annotation Dependent Depletion (CADD); http://cadd.gs.washington.edu
DECIPHER; https://decipher.sanger.ac.uk
Ensembl; https://www.ensembl.org/index.html
Gene Cards; http://www.genecards.org
Gene Matcher; http://www.genematcher.org
Genome Aggregation Database (GnomAD); http://gnomad.broadinstitute.org
Greater Middle East (GME) Variome Project; http://igm.ucsd.edu/gme/
Human Splice Finder; http://www.umd.be/HSF
Iranome; http://www.iranome.ir
Mutalyzer; https://mutalyzer.nl
Mutation Taster; http://www.mutationtaster.org
NeXtProt; https://www.nextprot.org
Online Mendelian Inheritance in Man; http://www.ncbi.nlm.nih.gov/Omim
Proteomics DB; https://www.proteomicsdb.org
PubMed; http://www.ncbi.nlm.nih.gov/pubmed
RefSeq; https://www.ncbi.nlm.nih.gov/refseq
SIFT; https://sift.bii.a-star.edu.sg
The 1000 Genomes Browser; http://browser.1000genomes.org/index.html
The Greater Middle East (GME) Variome Project; http://igm.ucsd.edu/gme/index.php
UniProt; https://www.uniprot.org
UCSC Human Genome Database; http://www.genome.ucsc.edu
Varsome; https://varsome.com
Supplementary Material
Acknowledgements
The authors would like to thank the patients’ families for their support and consent to the publication of this study. This research was conducted as part of the Queen Square Genomics group at University College London, supported by the National Institute for Health Research University College London Hospitals Biomedical Research Centre.
Funding
The work at University of Maryland, Baltimore, USA was supported by National Institute of Neurological Disorders and Stroke (NINDS) (R01NS107428) (to S.R.). This study was funded by the Medical Research Council (MRC) (MR/S01165X/1, MR/S005021/1, G0601943), The National Institute for Health Research University College London Hospitals Biomedical Research Centre, Rosetree Trust, Ataxia UK, Multiple System Atrophy Trust, Brain Research UK, Sparks Great Ormond Street Hospital Charity, Muscular Dystrophy UK (MDUK), Muscular Dystrophy Association (MDA USA).
Competing interests
J.C. is consultant to Invitae. R.E.P. is an employee of GeneDx, Inc. The Department of Molecular and Human Genetics at Baylor College of Medicine receives revenue from clinical genetic testing completed at Baylor Genetics.
References
- Berndt SI, Gustafsson S, Mägi R, Ganna A, Wheeler E, Feitosa MF, et al. Genome-wide meta-analysis identifies 11 new loci for anthropometric traits and provides insights into genetic architecture. Nat Genet 2013; 45: 501–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cazalla D, Newton K, Cáceres JF.. A novel SR-related protein is required for the second step of Pre-mRNA splicing. Mol Cell Biol 2005; 25: 2969–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Li W, Qiu W, Ren W, Li Q, Han B, et al. RSRC1 SUMOylation enhances SUMOylation and inhibits transcriptional activity of estrogen receptor β. FEBS Lett 2015; 589: 1476–84. [DOI] [PubMed] [Google Scholar]
- Li X, Luo Z, Gu C, Hall LS, McIntosh AM, Zeng Y, et al. Common variants on 6q16.2, 12q24.31 and 16p13.3 are associated with major depressive disorder. Neuropsychopharmacology 2018; 43: 2146–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maddirevula S, Al Zahrani F, Anazi S, Almureikhi M, Ben-Omran T, Abdel-Salam GMH, et al. GWAS signals revisited using human knockouts. Genet Med 2018; 20: 64–8. [DOI] [PubMed] [Google Scholar]
- McDaniel LD, Conkrite KL, Chang X, Capasso M, Vaksman Z, Oldridge DA, et al. Common variants upstream of MLF1 at 3q25 and within CPZ at 4p16 associated with neuroblastoma. PLoS Genet 2017; 13: e1006787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamae I, Kato JY, Yokoyama T, Ito H, Yoneda-Kato N.. Myeloid leukemia factor 1 stabilizes tumor suppressor C/EBPα to prevent Trib1-driven acute myeloid leukemia. Blood Adv 2017; 1: 1682–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez Y, Menascu S, Cohen I, Kadir R, Basha O, Shorer Z, et al. RSRC1 mutation affects intellect and behaviour through aberrant splicing and transcription, downregulating IGFBP3. Brain 2018; 141: 961–70. [DOI] [PubMed] [Google Scholar]
- Potkin SG, Macciardi F, Guffanti G, Fallon JH, Wang Q, Turner JA, et al. Identifying gene regulatory networks in schizophrenia. Neuroimage 2010; 53: 839–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potkin SG, Turner JA, Fallon JA, Lakatos A, Keator DB, Guffanti G, et al. Gene discovery through imaging genetics: identification of two novel genes associated with schizophrenia. Mol Psychiatry 2009; 14: 416–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med 2015; 17: 405–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schizophrenia Working Group of the Psychiatric Genomics Consortium. Biological insights from 108 schizophrenia-associated genetic loci. Nature 2014; 511: 421–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng M, Dong Z, Xie Y.. Identification of tumor-educated platelet biomarkers of non-small-cell lung cancer. Ono Targets Ther 2018; 11: 8143–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobreira N, Schiettecatte F, Valle D, Hamosh A.. GeneMatcher: a matching tool for connecting investigators with an interest in the same gene. Hum Mutat 2015; 36: 928–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang J, Liu W, Zhu J, Zhang J, Wang FH, Liang JH, et al. RSRC1 and CPZ gene polymorphisms with neuroblastoma susceptibility in Chinese children. Gene 2018; 662: 83–7. [DOI] [PubMed] [Google Scholar]
- Teplyuk NM, Uhlmann EJ, Gabriely G, Volfovsky N, Wang Y, Teng J, et al. Therapeutic potential of targeting microRNA-10b in established intracranial glioblastoma: first steps toward the clinic. EMBO Mol Med 2016; 8: 268–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valouev A, Weng Z, Sweeney RT, Varma S, Le QT, Kong C, et al. Discovery of recurrent structural variants in nasopharyngeal carcinoma. Genome Res 2014; 24: 300–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoneda-Kato N, Look AT, Kirstein MN, Valentine MB, Raimondi SC, Cohen KJ, et al. The t(3; 5)(q25.1; q34) of myelodysplastic syndrome and acute myeloid leukemia produces a novel fusion gene, NPM-MLF1. Oncogene 1996; 12: 265–75. [PubMed] [Google Scholar]
- Yu S, Gautam N, Quan M, Gao Y.. RSRC1 suppresses gastric cancer cell proliferation and migration by regulating PTEN expression. Mol Med Rep 2019; 20: 1747–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data sharing is not applicable to this article, as no new data were created or analysed in this study.