Abstract
Introduction
Device-based hypertension treatment is a complementary and possibly alternative approach to conventional lifestyle changes combined with antihypertensive medications to achieve and maintain optimal blood pressure (BP) control. A series of clinical trials assessing novel technologies are currently ongoing for device-based hypertension treatment, including endovascular catheter-based renal denervation (RDN), baroreceptor activation therapy, endovascular baroreflex amplification, and cardiac pacemaker-mediated hypertension treatment.
Only a few years ago, RDN was written off as ineffective after results of the sham-controlled SYMPLICITY HTN-3 trial1 failed to confirm early trials’ reports of significant BP reductions in patients resistant to guideline-based combination drug therapy. However, the trial’s design and execution has been questioned by many. Later-on, following recommendations provided by European and US expert groups,2–4 defining optimal trial design and methodology and the population of patients to be included, three carefully designed, randomized sham-controlled RDN trials (SPYRAL HTN-OFF MED, SPYRAL HTN-ON MED, and RADIANCE-HTN SOLO)5–8 reported consistent, plausible, and clinically meaningful ambulatory and office BP reductions in the short- (2 to 3 months) and mid-term (6 months) with radiofrequency (RF)- or highly focused ultrasound (US)-based RDN while reporting no serious adverse events. These exploratory phase II studies included selected patients with uncontrolled hypertension who were both on and off medications to demonstrate both biological proof of principle (off medications) but also context in routine clinical practice (on medications). With rigorous trial oversight and methods, these studies provided consistent and robust proof for BP lowering efficacy of RDN.
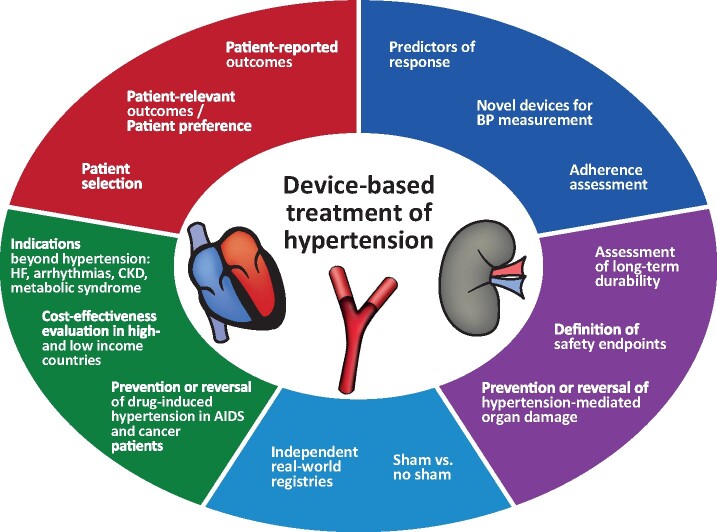
Against this background, the 3rd European Clinical Consensus Conference for device-based therapies for hypertension was held in July 2019 in Mainz, Germany. The aims were to identify key issues and provide consensus recommendations for the design and conduct of new, clinically more relevant, pragmatic trials to move forward with the investigation of device-based hypertension therapies, and to establish what evidence is needed for potential clinical adoption of these technologies (Figure 1). Considerations on how to interact with regulatory and reimbursement authorities were also discussed. The current document summarizes the discussion and consensus views. It focuses on research and development issues and is not a guide to clinical practice (Take home figure).
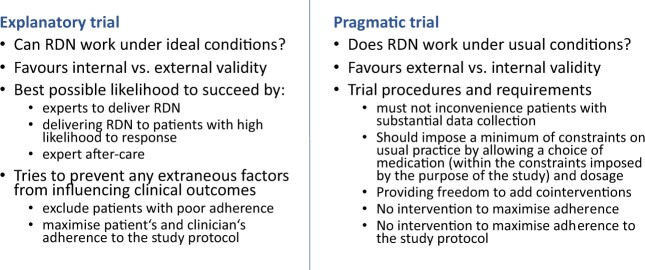
Figure 1.
Explanatory vs. pragmatic trial on the example of RDN. Adapted from Thorpe et al.9
Take home Figure.
Summary of research and development challenges in device-based hypertension treatment.
Current and emerging treatments for renal denervation
Multipolar RF- and US-based RDN were shown to be safe and effective in randomized, controlled trials with the use of second-generation devices (Table 1).5–8 The choice of these newer technologies and ablation techniques has the potential to provide more consistent and effective renal nerve ablation than the first-generation of catheters. The failure of efficacy with RDN in the SYMPLICITY HTN-3 trial was extensively discussed by experts in the field and was attributed to various possible factors, including patient selection, changes in medication prescription and adherence, insufficient and varying operator experience, and suboptimal procedural performance with the first-generation monopolar Symplicity flex catheter.4 Using a modified multielectrode RF-RDN catheter, SPYRAL HTN-OFF MED5 and SPYRAL HTN-ON MED8 included blood and urine sampling to ensure adherence with ‘on drug’ or ‘off drug’ designs. SPYRAL HTN-OFF MED (n = 80) was a randomized, sham-controlled feasibility trial with a 3- to 4-week drug washout period and a 3-month follow-up period in the absence of antihypertensive medications. SPYRAL HTN ON-MED (n = 80) was a randomized, sham-controlled feasibility study with a 6-month follow-up period in which patients were treated with one to three commonly prescribed antihypertensive drugs. A pivotal trial with the multielectrode Spyral RF-catheter in the off medications indication is ongoing (NCT02439749), in addition to a large, randomized trial in the on medications setting. RADIANCE-HTN SOLO6 was a powered, sham-controlled trial with a US-based RDN device which randomized 146 patients with combined systolic and diastolic hypertension after a 4-week washout period and who remained off antihypertensive medication during the first 2-month follow-up. The study met its primary efficacy and safety endpoints at 2 months. At 6-month, results confirmed the maintenance and the durability of BP lowering efficacy with US-based RDN. Fewer protocol-defined antihypertensive medications were administered from to 2 to 6 months in the RDN than in the sham group, and after accounting for these medication differences, RDN had greater ambulatory BP lowering efficacy than the sham procedure.10 Three other adequately powered trials with US-RDN are ongoing, including the RADIANCE TRIO (NCT02649426) trial in patients with resistant hypertension despite receiving three antihypertensive medications in a single combination,11 the REQUIRE trial evaluates patients with resistant hypertension on standard of care medication in Japan and Korea (NCT02918305), and the pivotal RADIANCE II trial in patients with uncontrolled hypertension while on one or two medications (NCT03614260). These two methods of renal artery ablation were compared in patients with resistant hypertension in the open label randomized RADIOSOUND trial.12 This trial showed that US-based RDN of the main renal arteries had (i) a similar BP lowering efficacy as RF-based RDN of the main renal artery plus its branches as well as accessory renal arteries and (ii) a greater BP lowering efficacy than RF-based RDN of the main renal arteries only.
Table 1.
Blood pressure reductions with RDN in recently completed sham-controlled trials
| SPYRAL HTN-OFF MED | SPYRAL HTN ON-MED | RADIANCE-HTN SOLO | REDUCE HTN: REINFORCE | |
|---|---|---|---|---|
| Follow-up data published | 3 months | 6 months | 6 months | 6 months |
| Sham-control | Yes | Yes | Yes | Yes |
| Adherence measurements | Yes | Yes | No | No |
| Feasibility/powered | Feasibility | Feasibility | Powered, primary endpoint (2 months) met | Powered, primary endpoint (2 months) not met |
| Patients randomized | 80 | 80 | 146 | 51 |
| Difference in BP RDN vs. sham (mmHg) | ||||
| 24-h SBP | −5.0 | −7.0 | −4.1 | −7.2 |
| P-value | 0.041 | 0.006 | 0.006 | 0.083 |
| 24-hr DBP | −4.4 | −4.3 | −1.8 | −3.6 |
| P-value | 0.002 | 0.017 | 0.07 | 0.172 |
| Daytime SBP | −5.4 | −6.1 | −6.3 | −9.7 |
| P-value | 0.057 | 0.018 | <0.001 | 0.021 |
| Daytime DBP | −3.9 | −4.1 | −2.6 | |
| P-value | 0.039 | 0.03 | 0.01 | |
| Office SBP | −7.7 | −6.6 | −6.5 | −11.4 |
| P-value | 0.016 | 0.025 | 0.007 | 0.006 |
| Office DBP | −4.9 | −4.2 | −4.1 | −5.4 |
| P-value | 0.008 | 0.02 | 0.005 | 0.037 |
Despite demonstrated efficacy of both approaches, there was large between-patient variation of responses to RDN even in these optimally designed trials. This might be due still to the variable degree of renal nerve ablation achieved with these technologies in the absence of a peri-procedural marker of success or due to a lesser contribution of sympathetic renal nerve activity to the underlying pathophysiology of hypertension in individual patients.13
Further information about the efficacy of RDN came from the REDUCE HTN:REINFORCE trial with the balloon-based bipolar RF-based Vessix system (NCT02392351). Recruitment to this study with ‘off drug’ design was stopped for futility of achieving a significant difference between the RDN- and the sham-group at the predefined 8-week primary endpoint. At 6 and 12 months, however, a significant difference in office BP reduction in favour of RDN was reported.14 A number of ablative and non-ablative technologies are in development and may complement today’s devices in the future (Table 2).
Table 2.
Available and developmental device-based hypertension technologies
| Technology | Demonstrated efficacy and safety |
|---|---|
| Radiofrequency RDN | Yes |
| Ultrasound RDN | Yes |
| Alcohol-mediated RDN | Under investigation |
| Arteriovenous anastomosis (ROX Coupler) | Withdrawn because of high rates of venous stenosis and volume overload (heart failure) |
| Baroreceptor reflex stimulation (CVRx) | Under investigation |
| Baroreceptor reflex amplification (MobiusHD) | Under investigation |
| Pace-maker-mediated hypertension treatment | Under investigation |
Endovascular catheter-based alcohol-mediated RDN is currently under investigation in patients with and without concomitant antihypertensive medication (TARGET BP-OFF, NCT03503773, TARGET BP I, NCT02910414). The results from the open-label, feasibility study in 45 patients with uncontrolled hypertension on multiple antihypertensive medication indicate significant reductions in office and ambulatory BP 6 months following bilateral injection of 0.6 mL alcohol in the perivascular space.15
In comparison to RDN, alternative methods of device-based therapies for treatment of hypertension are presently being studied. Asymmetric ventricular pacing using a dedicated dual-chamber pacemaker aims to reduce BP through varying atrioventricular coupling intervals. The effect can be modulated by modifiable pacing parameters. The open-label, single-arm Moderato study showed promising positive results.16 In an uncontrolled, proof-of-concept study, endovascular baroreceptor amplification with the Mobius HD device (Vascular Dynamics, Mountain View, CA, USA) implanted in patients with resistant hypertension taking at least three antihypertensive agents showed a significant reduction in BP at 6 months.17 A randomized, sham-controlled study in patients with resistant hypertension in currently ongoing (NCT03179800).
For several of these technologies, the lack of a control group in the initial trials places them at a similar promising stage as RF-based RDN in the early 2010s and with the same need for additional rigorous confirmatory trials. Finally, not only efficacy but above all safety needs to be demonstrated when these devices are used especially in patients with the milder forms of hypertension who are at lower risk for cardiovascular (CV), cerebrovascular, and renal events in the short- and mid-term.18 Even a very small risk of adverse events (e.g. >1/1000 patients) would be unacceptable for patients with mild hypertension. For example, with the ROX coupler, which adds a low-resistance, high-compliance venous segment to the central arterial tree to exploit the natural mechanical effects, significant BP lowering efficacy was shown at 6 months and sustained at 12 months.19 , 20 However, up to 30% of patients developed venous stenosis at the insertion site and a heart failure (HF) signal was observed during long-term follow-up in the active group vs. control patients, which led to termination of the pivotal ROX HTN 2 trial and an uncertain future for this approach.
Both the 2017 North-American and the 2018 European Society of Cardiology (ESC)/European Society of Hypertension (ESH) Guidelines21 for the management of arterial hypertension state that the use of device-based therapies is not recommended for the routine treatment of hypertension outside of clinical studies as the evidence available at the time of writing of the guidelines was considered not informative enough but called for further sham-controlled trials of device-based therapies.
Who should be investigated in device-based hypertension trials?
The primary guiding rule when selecting the appropriate patient population for each specific device/technology is that the procedural risk must not exceed the underlying risk of the untreated condition for each individual patient. This is particularly relevant since a class effect likely does not exist with device therapies for hypertension, and each much be considered on their individual risks. As priority candidates for further clinical trials, this group considered targeting high-risk hypertensive patients with coronary artery disease (CAD), chronic kidney disease (CKD), diabetes, and other comorbidities, followed by studies in patients with masked hypertension or white-coat hypertension. For everyday clinical care, patients with difficult-to-control hypertension remain at the greatest need for device-based hypertension therapies, particularly in the setting of widespread non-adherence with drug therapy; whereas a second priority in daily care may be to provide access for high-risk patients, as well as those with mild-to-moderate hypertension in presence of hypertension-mediated organ damage (HMOD) or severe comorbidities.
Given the mid-term safety and efficacy of RDN in patients with mild–moderate-risk documented recently,5–8 it may be reasonable to start investigating device-based therapies in other populations. A non-exhaustive list of potential candidates for future research activities is shown in Table 3.
Table 3.
Current and potential candidate hypertensive populations for RDN therapy
| Patient group | Pro | Con |
|---|---|---|
| Current candidates | ||
| Difficult-to-control hypertensive patients (with office SBP between 140 and 170 mmHg or DBP between 90 and 109 mmHg) |
|
Narrow group |
| Potential candidates | ||
| CAD patients |
|
Prolonged procedure (PCI + RDN) |
| Cancer survivors |
|
Need for RDN not proven |
| HIV patients |
|
|
|
White-coat hypertension on medications Masked hypertension off and on medications |
|
|
| White-coat hypertension off medications |
|
|
CAD, coronary artery disease; HIV, human immunodeficiency virus; HTN, hypertension; PCI, percutaneous coronary intervention.
In addition, a number of other potential candidates have been discussed:
Patients with CAD undergoing percutaneous coronary revascularization represent a high-risk group with an unmet need for efficient therapies to achieve recommended rates of BP control.21 , 22 Coronary artery disease patients have increased CV risk, which makes this a particularly interesting population for future studies, also to investigate the impact of device-based hypertension therapy on the residual CV risk and CV events within a short period of follow-up. Specifically, there is evidence of antiarrhythmic effects of RDN which would be particularly suitable in this patient population.
An increasing number of patients express a strong preference to be treated with a non-pill based therapy instead of receiving pharmacotherapy. Patient preference for an alternative to pharmaceutical therapy because of drug intolerance/side effects, social, economic, or other reasons suggests these patients may be appropriate candidates for device-based hypertension treatments.
Due to improved treatments, cancer survivors are more prevalent, live longer with better quality of life, however, are exposed to long-term consequences of CV toxicity including hypertension and HF as a number of targeted anticancer treatments, old as well as new, can induce hypertension.24
With the widespread use of antiretroviral therapy and thereby improved prognosis, patients with human immunodeficiency virus (HIV) experience CV illnesses, with high rates of uncontrolled and complicated hypertension.25 , 26 As HIV therapy already imposes a substantial pill burden with a risk of various drug–drug interactions, device-based therapies may be a favourable option in these individuals.
Both ‘white-coat’ hypertension (elevated clinic and normal 24-h ambulatory BP) found in 30–40% of patients (and >50% in the very old) and untreated masked hypertension (normal clinic and elevated 24-h ambulatory BP) found in approximately 15% of patients with a normal office BP, have been shown to be associated with elevated mortality risk,21 , 23 with masked hypertension conferring even a greater risk than sustained hypertension.27 , 28 It should be discussed whether intervention in white-coat hypertension might provide an early prevention strategy to avoid entering the stage of sustained or masked hypertension.
Of note, a number of additional factors need to be considered to treat these potential new populations with device-based therapies of hypertension. Many of these individuals are rarely seen by physicians specialized in hypertension treatment and the unmet need is often not felt as such; this also includes assessment of patients’ own preferences. Similarly, regulatory and reimbursement authorities may have a greater understanding of the indication of device-based therapies in patients with severe, complicated or resistant forms of hypertension, and may not feel comfortable with discussing expanded indications at the present time and cost of the therapy. Targeted studies will have to be carefully designed in order to demonstrate relevant efficacy, safety and value (cost effectiveness) in populations beyond those included in the successful clinical trials to date. For example, for higher-risk individuals, the option of baroreflex amplification is being investigated while awaiting the results of the ongoing RDN trials listed above, bearing in mind that the safety profiles of these devices have not yet been fully established.
Consensus statement 1: Selection of hypertensive patients for device-based therapies
When selecting the appropriate device-based hypertension therapy for each individual patient, the procedural risk must not exceed the risk from the underlying condition itself.
In everyday clinical care, patients with difficult-to-control hypertension may benefit most from device-based hypertension therapies. Patients need to be provided with balanced information concerning the variability of the BP response and the unknown BP lowering effect and safety in the long-term.
In the short-term perspective (2–3 years), the most appropriate population for further clinical trials in device-based hypertension treatment should be high-risk hypertensive patients with comorbidities including CAD, diabetes, and CKD.
A changed focus from severe and resistant hypertension towards embracing other groups with mild to moderately elevated BP should be considered in the future.
Design of clinical trials in renal denervation
Endpoint selection beyond blood pressure reduction
Appropriate pivotal trials to demonstrate BP lowering efficacy is a sine qua non requirement for any emerging treatment. To demonstrate value beyond BP reduction, trials should target the common endpoints for CV therapies, e.g. CV morbidity/mortality, improvement in HMOD, as shown for the different classes of antihypertensive medications.29 These endpoints further need to allow for a treatment effect to be ascertained within reasonable follow-up. However, with increasing attention being paid to patients’ perspectives these outcomes alone are unlikely to be sufficient in future. Indeed, endpoints which matter most to patients are not necessarily the same as those prioritized by physicians. For device-based hypertension therapies, this becomes especially important, as devices compete with non-invasive treatments which have lower acceptance threshold, particularly in lower-risk patients. Among BP-related events, patients care about stroke, dementia and cognitive deficits, myocardial infarction and HF, and end-stage renal disease which makes these core endpoints very relevant for inclusion in future trials of device-based hypertension therapies.30 For device-based hypertension therapies to become widely acceptable to patients and clinicians, it will need to be demonstrated that the CV benefits conferred by device therapies do not come at the cost of patient-reported side effects or other adverse outcomes (including side-effects to medications), and adapting treatment to their preferences and context.31 There is a growing realization among medical professionals that patient-reported outcomes (PROs) reflect a key dimension of overall disease burden. Patient-reported outcome measurements are increasingly included in clinical trials.32 Patient-reported outcomes need to be handled and analysed as stringently as any other data and should be reported in accordance with the CONSORT PRO Extension.33 They must be reported by the patient and not by the physician. The use of PRO measures is discussed further below. We are lacking validated PRO questionnaire for HTN, accounting for importance and different relevance in various cultures. Patients should be involved in the trial design and endpoint selection of studies using PRO.
Consensus statement 2: Endpoints and outcomes
Evidence is needed on patient-relevant outcome benefits from BP reduction with device-based hypertension therapies. The risk profile of the study population and the relevant outcome measures of such trials need to be defined.
An unstudied opportunity is to survey PRO measures in hypertension. Device-based hypertension therapy needs to deliver outcomes that matter to patients. The attention to patient preferences in future clinical trial design should be encouraged.
Patient preference information and quality of life assessments are mandatory to further determine the potential benefits and risks for devices. In addition, there remains a need to develop measures of patient-reported health status specific to device-based therapies for hypertension.
Capturing PROs in clinical trials should be encouraged and measures should be reported in accordance with the CONSORT PRO Extension.33
Screening for potential candidates
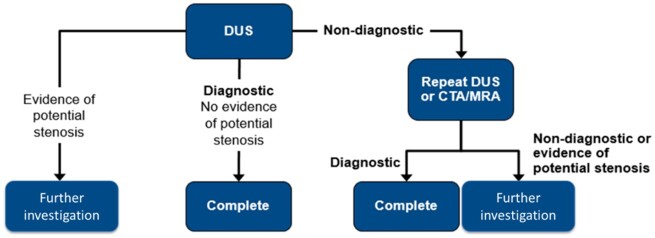
The main anatomical exclusion criterion for RDN is renal artery stenosis of any origin (atherosclerosis, fibromuscular dysplasia, and others). Although the prevalence of renal artery stenosis is very low in mild to moderate hypertension, in referral groups with higher uncontrolled BP despite antihypertensive treatment, the prevalence may be up to 30%.34 , 35 Patients considered for RDN undergo renal artery anatomical screening to confirm anatomical eligibility criteria. Even though the screening method preferred by most physicians is duplex ultrasound (DUS), which has the advantages of lack of exposure to radiation or contrast dye, it has several limitations including operator-dependency, and often lack of visualization of the main renal artery over its entire length.36 For clinical diagnosis and follow-up, DUS may not represent the best option, as computed tomography angiography (CTA) or magnetic resonance angiography may be needed for confirmation of anatomical eligibility as well as detection of adrenal tumour or hyperplasia, completing thus the necessary diagnostic workup for secondary hypertension especially in the context of resistant hypertension.21 However, pulmonary computed tomography (CT) was reported to carry a non-negligible cancer risk of 1/1250 to ∼1/500037 which would be unacceptable for screening purposes. The newer third-generation dual-source CT systems for CV imaging purposes are associated with much lower radiation exposure and contrast injection than conventional CTA. Magnetic resonance angiography with gadolinium-based contrast agents may be an alternative to CTA but the technology is hindered by many false positives for renal artery stenosis, and exposure to the unknown long-term consequences of gadolinium accumulation in the brain.38 For the future, fusion of post-processed images in conjunction with real-time fluoroscopy is an emerging modality which promises short procedural time, low contrast volume, and catheter manipulation. Whenever possible, core laboratories, blinded to patient’s characteristics, should be used for imaging data analyses. A reasonable screening pathway with current technologies is depicted in Figure 2.
Figure 2.
Suggested algorithm for identification of RDN candidates for clinical trials. CTA, computed tomography angiography; DUS, duplex ultrasound; MRA, magnetic resonance angiography.
Predictors of response
A difficulty is the lack of standardized definitions of response for use in patient identification and selection for device-based treatment of hypertension. Ambulatory BP and office BP are both used as primary efficacy parameters in device-based trials of hypertension. The group earlier recommended using 10 mmHg reduction in office systolic BP (SBP) or 6–7 mmHg in daytime or 24-h ambulatory SBP for the purpose of power calculations in hypertension device trials. These BP reductions are considered clinically meaningful4 since a 10 mmHg reduction in office SBP is associated with reduced risk of major CV disease events by up to 20%, coronary heart disease by 17%, stroke by 27%, and HF by 28%.29 The time to the response analysis may also affect how a responder is characterized and should ideally be comparable between trials. Most often the timepoint for primary endpoint evaluation spanned over from 2–3 months to 6 months. The futility analysis in REDUCE HTN: REINFORCE was performed on an 8-week endpoint, whereas a similar analysis at 6 months would have permitted the trial to continue and in all probability report a positive result.39 , 40
The variability in BP response in the clinical trials was rather large, with 20–30% of patients experiencing above-average BP reductions within 3 months after the procedure.5 , 6 It has been pointed out that this holds true even in patients not treated with concomitant medication.41 A number of possible confounders may influence the short- and long-term treatment effect, e.g. the variable contribution of the sympathetic nervous system (SNS) to BP elevation in the individual patient, genetic background, comorbidities, or accompanying treatments. Currently available study data do not provide much information to help predict response. A large number of potential predictors are available (Table 4) but of those, the association between baseline BP and increased BP reduction has been the only consistent finding in RDN trials, which is a well-known phenomenon observed with any type of antihypertensive treatment.42 , 43 It remains elusive how much of this effect can be attributed to regression to the mean.44 In RADIANCE-HTN SOLO,6 there was correlation between a greater number of previous antihypertensive medications before washout and greater reduction in ambulatory SBP, but the number of drugs may be a proxy for severity of BP.
Table 4.
Potential predictors of response to RDN therapy
Baseline characteristics
|
Procedural variables
|
Biomarkers
|
Invasive/provocative testing
|
Imaging
|
In SPYRAL HTN-OFF MED, ambulatory heart rate (HR) above the median (>73.5 b.p.m.) was predictive of reduction in average daytime SBP, daytime diastolic BP, and office SBP.45 Heart rate may represent a sign of SNS activation and is predictive of incident HF in hypertensive patients.46 HF patients with elevated HR are at increased risk for adverse outcomes.47 There are thus several reasons to study HR as predictor of response in clinical trials. The signal may be confounded by treatment with beta blockers.
We now have three published contemporary sham-controlled studies with the RF Spyral catheter and the US Paradise catheter, and a study-level meta-analysis of these data in addition to other sham-controlled studies has demonstrated a clear reduction in BP with RDN.5–8 These studies, along with the Radiosound trial,12 which allowed a direct, between-group comparison of these technologies, provide an opportunity for a more detailed meta-analysis of individual participant data by a third-party independent academic institution. Indeed, the use of individual participant data instead of aggregate data in meta-analyses has many potential advantages, both statistically and clinically. The aim of such a meta-analysis would be to search for predictors of the BP response to RDN in a larger population of patients using newer machine learning and unsupervised methods.
Consensus statement 3: Considerations for trial design
A practical, predictable, non-invasive, and pre-procedural measure to identify optimal RDN candidates remains an unmet need (similar markers that predict response to other device-based therapies are also desired).
Current recommendations to demonstrate a minimum of 10 mmHg reduction in office SBP or 6–7 mmHg in 24-h or daytime ambulatory SBP in hypertension device-based trials remain unchanged. There remains no consensus regarding what magnitude of BP reduction and at what time point defines a responder.
Greater consistency among RDN trials of definitions and timing of response is desirable. The most suitable time point for analysis of the BP endpoint appears to be between 2 and 6 months.
Heart rate as predictor of response should be further studied in clinical trials.
A meta-analysis of individual participant data of contemporary trials of RDN should be conducted, e.g. to identify potential predictors of BP response to RDN.
Blood pressure and adherence measurements
The need for consistent and validated BP measurement methods in device-based hypertension trial is of major importance. Ambulatory BP, home BP, and office BP measurements provide complementary information. An ideal trial should include all three methods to optimize the precision of the analysis with validated devices according to guidelines.48 Most wearable digital tools were developed as consumer products for fitness purposes and are not suitable for medical use. Whichever measurement is used, it is important not to allow BP measurements to influence patient behaviour in the trial setting.
Patient adherence to the medication is an important confounding factor in hypertension trials and should be assessed in any device-based hypertension therapy trial. Taking adherence into account can also save costs by reducing the need to enroll additional patients to compensate for low adherence. Only three good alternatives for adherence monitoring exist at the time of writing: drug assay, electronic monitoring, and digital monitoring. Interview questionnaires, pill count, and refill data are all unreliable and selective. Directly observed treatment is useful but logistically burdensome, and requires the patient to attend the caregiver that day.49 Mobile health applications are appealing and may have value to patients,50 but there is evidence from other clinical fields of ‘technology fatigue’ over time.51 Drug assays have been used successfully in device-based hypertension therapy trials.52 Absence of a medication in a urine sample is a strong sign of non-adherence. Drug levels will depend on the pharmacological profile of the drug as well as on the metabolic profile of a patient. It is important to sample frequently, to avoid measurement bias from patients’ modifying their behaviour before a prespecified visit, a phenomenon known as ‘white-coat adherence’.49 Electronic monitoring is one of the most reliable techniques to diagnose poor adherence and seems to be similarly accurate as well as cheaper than drug assays.53 The most recent method, digital monitoring relies on tiny ingestible sensors incorporated in the pill which are activated in the stomach and generating a unique message on medication and dose which is recorded together with date and time by a wearable patch worn by the patient.54 While highly accurate, such systems remain prohibitively expensive to date.
Consensus statement 4: Tools for measuring blood pressure
Ambulatory BP, home BP, and office BP measurements provide complementary information; at least one office and one out-of-office BP measurement technique should be utilized.
Adherence assessment by direct methods should be included in device-based hypertension therapy trial protocols.
Sham or no sham in 2020?
The most recent trials in RDN were all sham-controlled.5 , 6 , 8 A sham-control group as well as allocation concealment are necessary to avoid selection and allocation biases, performance and evaluation bias, and cointerventions (provision of unintended care to either comparison group) especially for proof of concept studies designed to establish BP lowering efficacy in small groups of highly selected patients. A sham design trial is necessary when assessing the efficacy of even established device-based techniques on parameters other than BP in hypertensive patients or extending the indication of a device-based approach to other diseases [CKD, HF, atrial fibrillation (AF), etc.]. However, a sham group would not be appropriate for future trials using technologies which have already proven their BP lowering efficacy in pivotal sham-controlled studies. In pharmacotherapy trials, a placebo is not ethically defensible once a treatment has been shown to be beneficial.55 Ethical considerations are highly relevant with device-based therapies because of the associated procedural risk. Although no safety signals were observed in the studies published up to date, a sham procedure is not as risk-free as a placebo pill.
Consensus statement 5: To sham or not to sham
Assuming the ongoing pivotal trials of the SPYRAL (NCT02439775; NCT02439749) and RADIANCE TRIO, REQUIRE, and RADIANCE II (NCT03614260) programmes confirm the efficacy and safety of RDN in 2020/2021, the group considers sham controls to be no longer necessary in trials using established RDN technologies examining BP lowering efficacy.
On the same assumptions, the Group considers sham control trials to be no longer or very rarely necessary in established device-based hypertension technologies evaluating procedural safety.
Sham control should remain a requirement for newer indications (CKD, HF, AF etc.) or for outcomes not previously assessed in prior trials using a given technology.
Sham control should remain a requirement for newer, unproven technologies.
Withdrawal of medication in responders
Another topic of discussion was the withdrawal of antihypertensive medication in patients on multidrug regimens who are controlled after the procedure. A recent meta-analysis of patients on pharmacotherapy suggests a substantial proportion of successfully treated patients, mostly elderly, might be withdrawn from antihypertensive therapies without rebound effects and with only a small risk for minor side effects.56 Successful drug withdrawal in patients after device-based hypertension treatment would reduce risks with the long-term use of antihypertensive drugs, with additional benefit of lower medication burden. Although most antihypertensive drugs are generic and of low cost to payers, the cost of medications and their side effects as well as the treatment burden might impact reimbursement decisions. In the majority of patients, antihypertensive medication will be re-introduced or intensified after primary endpoint collection to achieve BP control which may complexify the assessment of efficacy. There was consensus among the delegates on the desirability to include a limited drug-withdrawal period (e.g. after 1 or 2 years) in clinical trials with device-based hypertension therapies to address the issue of long-term efficacy. A drug-withdrawal assessment must be formalized (typically 4–6 weeks) and closely monitored, and it would need patient’s consent. The Group felt this would be the only way to assess long-term efficacy with minimal confounding.
Consensus statement 6: Assessment of durability
Assessment of long-term efficacy is challenging. Clinical trials with device-based hypertension therapies should include a limited drug-withdrawal period at longer-term follow-up (1–2 years) to address the issue of long-term efficacy in the absence of antihypertensive medication, when considered safe.
Safety
For any device-based approach, injury of the treated vessel (acute or long-term), vascular access site and access-related complications, as well as organ injury (e.g. kidney and brain) and renal artery injury need to be ascertained. Given the relative novelty of device-based hypertension treatment there is no current consensus on standardized definitions of safety criteria for trials or registries, although needed. Standardized definitions of safety criteria for trials and registries in device-based hypertension therapies need to be developed. Bleeding complications after cardiac procedures are typically classified using Valve Academic Research Consortium (VARC-2) criteria57 for TAVI or Bleeding Academic Research Consortium (BARC) criteria58 for patients receiving antithrombotic therapy and undergoing coronary revascularization. Valve Academic Research Consortium-2 has included the BARC classification in the system. It would seem reasonable to include VARC-2-like criteria in a classification system for device-based hypertension trials and expand with standardized criteria for acute kidney and vascular injuries. The time frame for safety evaluation should also be standardized. For acute events, it may be appropriate to use a similar definition as for other procedures: an event is any encounter with the healthcare system within 30 days.57 , 58 For vascular safety a time period of 6–12 months seems reasonable. Long-term safety data should be collected at 1 and 3 years.
Consensus statement 7: Definition of safety endpoints
Standardized definitions of safety criteria for trials and registries need to be developed. Based on VARC-2 criteria and expanded with acute kidney and vascular injuries these standardized definitions would enable safety assessments to be comparable within and between device-based hypertension therapies.
For acute events, a similar definition as for surgical procedures would be appropriate, defining an event as any encounter with the healthcare system within 30 days.
For vascular safety, a time period of 6–12 months appears reasonable. The time point for long-term safety analysis is longer and should be between 1 and 3 years.
Long-term data and registries
The group underlined the value of well-defined registries monitored regularly for data accuracy and completeness. These registries are particularly relevant for detection of rare events and for collecting information on novel devices use in real-world patients treated across multiple geographies. Registries are an irreplaceable source of clinical research data to support long-term safety and effectiveness claims. The global SYMPLICITY Registry has provided data on renal RF ablation for up to 3 years.59 Such information is becoming critical: the European Union’s medical device regulation came into force in 2017 with a transition time of 3 years. The new rules confer notified bodies increased post-market surveillance authority. Annual safety and performance reporting are increasingly required by device manufacturers. For registries from clinical trials to be valuable, many matters need to be decided. Data collecting needs to be comparable between trials. Therefore, a consensus on what data should be collected, sample size, follow-up duration, timing of data collection, and selection of outcomes has to be provided. To design and implement such standards, a dedicated effort from the community of clinical trialists is needed. Registries have well-known disadvantages compared with controlled trials: the information collected is typically less detailed, there is greater loss of follow-up and adherence to protocols is usually handled less strictly. A way to increase data collection is to link reimbursement to registry participation, as has been trialled in some contexts, e.g. the German Aortic Valve Registry (GARY).60
Consensus statement 8: Real-world registries
Registries should be set up whenever possible with standardized protocols for what data to collect, follow-up duration, timing of data collection, outcomes, and more.
To drive this standardization a dedicated effort from the community of clinical trialists will be needed.
Ideally, and when feasible, these registries should be connected to national administrative health databases to automatically retrieve information about vital status, causes of hospitalization and causes of death.
Cross country comparisons are valuable, they require special attention to the requirements of the General Data Protection Regulation.
Patient-related outcomes
As noted above, regulatory bodies are paying increasing attention to patients’ experience of illness and treatment when considering marketing approvals and claims. Early interaction with regulatory agencies is highly recommended when planning to include PROs in support for labelling claims or for use in reimbursement discussions. Healthcare professionals owe it to patients to inform appropriately on matters such as what to expect of a treatment; differences between average results and individual results, and how to judge risk. Patients’ expectations and attitudes will influence PROM. Terms need to be presented in ways the general public understands, including much that is self-evident to physicians. There may also be discrepancies between effects that the physician may consider a positive outcome and what patients may experience—positively or negatively—as PROs. The community needs to build the tools necessary to capture PROs and ensure they are validated and standardized. This should be done starting from existing tools for consistency. A number of publications have concerned themselves with the process for constructing quality PRO instruments, from conceptual model development through instrument validation.61 As highlighted in guidance from the US Food and Drug Administration (FDA)62 substantial patient input should be included in PRO development. Of note, the hierarchy between PRO and traditional endpoints is not clear.
Patient preferences are different from PROM, although both may be considered as part of patient experience. Patient-reported outcome instruments measure a patient’s perception of health status before, during, and after therapy. In contrast, patient-preference studies examine how patients rank treatment decisions and how treatment attributes may influence treatment choices. The FDA guidance on this topic63 states that reviewers may consider ‘patient tolerance for risk and perspective on benefit’ as an additional factor during reviews of approval applications when this information is available and qualifies as scientific evidence. Patient-preference data can measure the likely size of the population for whom the benefits of a treatment outweigh the risks.
Consensus statement 9: Patient-related outcome metrics
PROs reflect a key dimension of overall disease burden and should be a primary aim of disease management to improve patient well-being. An opportunity for future study relates to the development of PRO models specific to device-based therapies for hypertension.
PRO and patient-preference should be part of future clinical studies.
Physicians should be trained on eliciting and respecting patients’ preference in shared decision processes and on the application and interpretation of PROs.
Considerations for regulatory and reimbursement authorities
Any discussion of the future role of device-based hypertension treatment needs to take into account the changing health care landscape, where value-based systems are becoming ever more important. Value in health care has been defined as outcomes relative to cost.64 The possible relationships between cost and effectiveness are outlined in Figure 3. The ideal scenario of lower cost for greater effectiveness compared with existing treatments is unlikely to be observed in hypertension treatment. Reimbursement authorities will have to set an effectiveness-cost threshold, which is decided at national levels in Europe.
Figure 3.
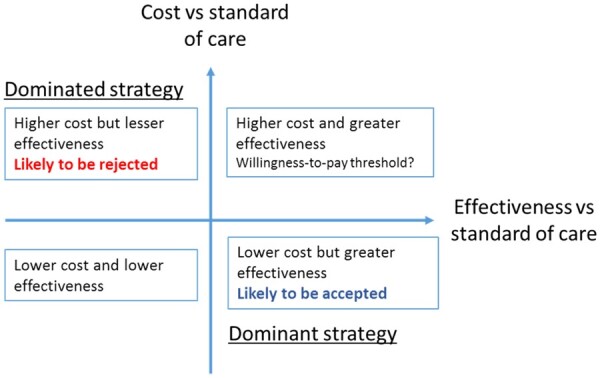
Cost-effectiveness plane. Adapted from Bulsei et al.65
After the SYMPLICITY HTN trials, a number of health-economic studies were conducted modelling the cost-effectiveness of RDN in various countries and healthcare systems.66–70 With the advent of new, proven technologies, an explosion of such activity can be expected. A number of challenges to such analyses have recently been summarized.65 For trialists, the challenge is to demonstrate value by maximizing outcomes. This means selecting those patients most likely to respond and in whom the treatment affects high-impact outcomes, typically high-risk groups. A value-focused selection criterion may be the potential for the greatest efficiency of procedure, to reduce the cost factor in economic modelling. Reimbursement bodies may appreciate endpoints which predict the development of HF, AF or end-stage renal disease, but the time horizon needs to be considered. Some long-term endpoints may be more suitable for inclusion in a registry, although evidence of key benefits beyond BP reduction may need demonstration in a controlled trial, at least initially.
Two important assumptions are usually made when modelling hypertension data: that treatment remains effective long-term and that treatment-induced reductions in BP reduces the risk of events in an expected manner per unit reduction of BP. For device-based hypertension therapies both assumptions need to be proven. The effectiveness of a drug investigated in a trial tends to be higher than in real life, however,71 , 72 which indicates the necessity for registry data to complement the evidence-base.
Consensus statement 10: Cost-effectiveness evaluation
Additional clinical evidence is needed for the assumptions that device-based hypertension treatment remains effective long-term and that device treatment-induced reductions in BP reduces the risk of events similar to drug treatment reductions in BP as well as to estimate the cost effectiveness in the various candidate populations.
The time point for economic evaluations and to identify changes in resource utilization should be between 1 and 3 years.
Potential future indications for neuromodulation therapies
Most clinical trials in device-based hypertension therapies to date have focused on demonstrating BP reductions in patients with uncontrolled hypertension. After the recent crop of consistently successful trials, the question arises whether the time has come to explore device-based autonomic modulation in other CV diseases such as HF, arrhythmias or in hypertensive patients with CKD. Such trials would signal a shift in focus from reducing BP towards neuromodulation, which is a factor in the aetiology of both HF and AF but also metabolic syndrome.73
Renal denervation has been studied in smaller trials in HF but the evidence to date remains inconclusive.74–76 Hypertension is the most relevant risk factor for onset and recurrence of AF.77 , 78 In patients with AF and HTN, RDN was investigated as adjunct to pulmonary vein isolation (PVI) with favourable results on AF recurrence and BP.77 It remains elusive whether the neurohormonal effect of RDN or its BP lowering effect is the main factor driving these results. New evidence may be provided by the multicentre SYMPLICITY-AF (NCT02064764) and ASAF (NCT02115100) trials, which evaluate the safety and effectiveness of performing both RDN and PVI simultaneously in patients with paroxysmal and persistent AF and uncontrolled hypertension (SBP >140 mm Hg, despite ≥1 antihypertensive drug).
Consensus statement 11: Extended potential indications for neuromodulation therapies, beyond hypertension
The confirmation of the biological proof-of-principle of RDN has provided the rationale for further study of device-based neuromodulation in other indications such as HF, arrhythmias, CKD, and metabolic syndrome.
The next step should be carefully designed, sham-controlled, feasibility studies in these new indications with blinded evaluation of both efficacy and safety endpoints.
Outlook
After several years in the doldrums, device-based therapy for hypertension has returned as one of the promising and novel treatment approaches on the horizon. However, it should be remembered that other effects beyond BP lowering remain to be demonstrated: improvement in outcomes, health-economic value, and benefits on PROs, to name just a few. For RDN to be ready for a wider uptake among patients, physicians and health authorities, reliable predictors of response should be identified. Identifying a reliable and uncomplicated intra-procedural validation method to assess the completeness of the ablation remains a fundamental challenge. Approaches to identify candidate most likely to respond to RDN include BP response to high-frequency renal nerve stimulation, reflex elicitation using a physiological stimulus and other methods,79–81 but no simple front-runner technique has emerged to date. Other devices need to provide biological proof of principle and safety, before pivotal studies can be initiated. If data continue to support device-based hypertension therapies and the critical needs can be fulfilled, the technologies may be applied to a number of vulnerable groups of patients with hypertension. With the help of local health care systems, of international bodies and device companies, a development plan of device-based treatment at low cost should be implemented in low-income countries to treat the most vulnerable patients with hypertension at very high risk of hypertension-related complications.
Acknowledgements
The authors thank Pelle Stolt (Basel, Switzerland) for his valuable help with this manuscript. Organization Committee: Michel Azizi, Sebastian Ewen, Felix Mahfoud, Atul Pathak, Roland E. Schmieder, Costas Tsioufis, and WilliamWijns.
Funding
The European Expert meeting was supported by an unrestricted educational grant from Europa Organization, Toulouse, France.
Conflict of interest: F.M. is supported by Deutsche Hochdruckliga, Deutsche Gesellschaft für Kardiologie, and Deutsche Forschungsgemeinschaft (TRR SFB-219) and has received speaker honoraria from Medtronic, ReCor, Bayer, and Boehringer Ingelheim. M.A. reports grants from French Ministry of Health; grants and non-financial support from Recor, grants from Idorsia, Novartis, Quantum Genomics, and French Federation of Cardiology; speaker honoraria from CVRx and Novartis, outside the submitted work. S.E.: received scientific support or/and speaker honoraria from Medtronic, Recor, Bristol Myers Squibb, Daiichi Sankyo, and Bayer. A.P.: reports grants and non-financial support from Recor, Medtronic, Ablative Solution, CVRx; speaker honoraria from CVRx, Novartis, Medtronic, Ablative Solution, and Recor. C.U.: Medtronic and Recor. M.B.: reports support from Abbott, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Cytokinetics, Servier, Medtronic, Novartis, and Vifor. M.B. is supported by the Deutsche Forschungsgemeinschaft (TRR SFB-219, S-01, M-03, M-05). M.B.: research grants and speakers fees from Servier, Sanofi, Menarini, Novartis, Medtronic, Actelion, Idorsia, and Amgen. I.D.Z.: reports grants from French Ministry of Health; advisory board and speaker honoraria from Boston Scientific, Medtronic, Pfizer, BMS, Sanofi outside the submitted work. D.E.K.: institutional research/grant support from Medtronic CardioVascular and Ablative Solutions; personal consulting honoraria from Medtronic CardioVascular. A.K.: institutional funding to Columbia University and/or Cardiovascular Research Foundation from Medtronic, Boston Scientific, Abbott Vascular, Abiomed, CSI, Philips, and ReCor Medical. S.E.K.: lecture honoraria from Merck (Darmstadt), MSD, Sanofi, and Takeda. M.D.L. is funded by the Barts Charity and is a consultant to: Medtronic, Ablative Solutions, ReCor Medical, Vascular Dynamics, ROX Medical, Tarilian Laser Technologies and has received speaker fees from CVRx. T.F.L.: research grants form Abbott, Medtronic, and Ablative Solutions to the institution and honoraria from Ablativ solutions. G.P.: honoraria for lectures from Omron Healthcare and Sanofi. P.R.: reports personal fees/consulting for Idorsia and G3P, AstraZeneca, Bayer, CVRx, Fresenius, Grunenthal, Novartis, NovoNordisk, Servier, Stealth Peptides, Ablative Solutions, Corvidia, Relypsa, Vifor and Vifor Fresenius Medical Care Renal Pharma. P.R. is the cofounder of CardioRenal. L.R.: Speaker/advisor for Medtronic. M.P.S. is supported by an NHMRC Senior Research Fellowship and has received consulting fees, and/or travel and research support from Medtronic, Abbott, Novartis, Servier, Pfizer, and Boehringer-Ingelheim. A.S.P.S.: reports consultancy and speaker’s fees from Medtronic and Recor Medical. He has received research support from Medtronic. H.S.: study honoraria to institution, travel expenses, consulting fees 1 <25 000 €: 4tech Cardio, Abbott, Ablative Solutions, Ancora Heart, Append Medical, Bavaria Medizin Technologie GmbH, Bioventrix, Boston Scientific, Carag, Cardiac Dimensions, Cardimed, Celonova, Comed B.V., Contego, CVRx, Dinova, Edwards, Endologix, Hemoteq, Hangzhou Nuomao Medtech, Holistick Medical, Lifetech, Maquet Getinge Group, Medtronic, Mokita, Occlutech, Recor, Renal Guard, Terumo, Vascular Dynamics, Vectorious Medtech, Venus, Venock, Vivasure Medical. M.A.W.: received consulting fees from Medtronic, ReCor, Ablative Solutions, Johnson and Johnson, Abbvie and Urovant. R.E.S.: has received speaker and consulting honoraria from Medtronic, ReCor, Ablative Solutions, and Rox Medical. C.T.: has received horonaria for advisory boards and lectures from Medtronic, Servier, Bayer, Menarini, Novartis, Astra-Zeneca, Boehringer In, Pfizer, Pythagoras, Sanofi, Amgen. W.W.: institutional grant and honoraria from MicroPort; medical advisor of Rede Optimus Research; cofounder of Argonauts Partners, an innovation accelerator. P.J.B. reports grants from Recor, grants from Medtronic, grants from Ablative Solutions, grants from European Commission, grants from Fresenius, from BBraun, outside the submitted work. All other authors have declared no conflict of interest.
Contributor Information
Felix Mahfoud, Klinik für Innere Medizin III, Kardiologie, Angiologie und Internistische Intensivmedizin, Universitätsklinikum des Saarlandes, Saarland University, Homburg, Germany; Institute for Medical Engineering and Science, Massachusetts Institute of Technology, Cambridge, MA, USA.
Michel Azizi, Université de Paris, INSERM CIC1418, F-75015 Paris, France; APHP, Hôpital Européen Georges Pompidou, Hypertension Unit, F-75015 Paris, France; F-CRIN INI-CRCT Network, Nancy, France.
Sebastian Ewen, Klinik für Innere Medizin III, Kardiologie, Angiologie und Internistische Intensivmedizin, Universitätsklinikum des Saarlandes, Saarland University, Homburg, Germany.
Atul Pathak, F-CRIN INI-CRCT Network, Nancy, France; Department of Cardivascular Medicine, INSERM 1048, Princess Grace Hospital (CHPG), Avenue Pasteur, 98000 Monaco, Monaco.
Christian Ukena, Klinik für Innere Medizin III, Kardiologie, Angiologie und Internistische Intensivmedizin, Universitätsklinikum des Saarlandes, Saarland University, Homburg, Germany.
Peter J Blankestijn, UMC Utrecht, Utrecht, The Netherlands.
Michael Böhm, Klinik für Innere Medizin III, Kardiologie, Angiologie und Internistische Intensivmedizin, Universitätsklinikum des Saarlandes, Saarland University, Homburg, Germany.
Michel Burnier, University of Lausanne, Lausanne, Switzerland.
Gilles Chatellier, Université de Paris, INSERM CIC1418, F-75015 Paris, France; APHP, Hôpital Européen Georges Pompidou, Clinical Trial Unit, F-75015 Paris, France.
Isabelle Durand Zaleski, Assistance Publique Hopitaux de Paris, URCEco Hotel Dieu, Paris, France.
Guido Grassi, Clinica Medica, University of Milano Bicocca, Milan, Italy.
Michael Joner, Deutsches Herzzentrum München, Munich, Germany; Deutsches Zentrum für Herz- und Kreislauf-Forschung (DZHK) e.V. (German Center for Cardiovascular Research), Partner Site Munich, Munich, Germany.
David E Kandzari, Piedmont Heart Institute, Atlanta, GA, USA.
Ajay Kirtane, Columbia University Irving Medical Center/NewYork-Presbyterian Hospital and the Cardiovascular Research Foundation, New York, NY, USA.
Sverre E Kjeldsen, Ullevaal Hospital Oslo University, Oslo, Norway.
Melvin D Lobo, William Harvey Research Institute, Centre for Clinical Pharmacology, Barts NIHR Cardiovascular Biomedical Research Centre, Queen Mary University of London, London, UK.
Thomas F Lüscher, Center for Molecular Cardiology, Schlieren Campus, Zürich, Switzerland; Royal Brompton and Harefield Hospital Trust, Imperial College London, London, UK.
John William McEvoy, National University of Ireland Galway, Galway, Ireland.
Gianfranco Parati, Department of Medicine and Surgery, University of Milano-Bicocca—Istituto Auxologico Italiano, IRCCS, Milano, Italy.
Patrick Rossignol, F-CRIN INI-CRCT Network, Nancy, France; Université de Lorraine, Inserm, Centre d’Investigations cliniques-plurithématique 1433, Inserm U1116, Nancy, France; CHRU Nancy, Nancy, France.
Luis Ruilope, Institute of Research i+12 and CIBER CV, Hospital 12 de Octubre and Faculty of Sport Medicine, European University, Madrid, Spain.
Markus P Schlaich, Dobney Hypertension Centre, The University of Western Australia—Royal Perth Hospital Campus, Perth, Australia; Baker Heart and Diabetes Institute, Melbourne, Australia.
Atif Shahzad, National University of Ireland Galway, Galway, Ireland; Galway University Hospital, Galway, Ireland.
Faisal Sharif, National University of Ireland Galway, Galway, Ireland; Galway University Hospital, Galway, Ireland.
Andrew S P Sharp, University Hospital of Wales, Cardiff, UK; University of Exeter, Exeter, UK.
Horst Sievert, CardioVascular Center Frankfurt CVC, Frankfurt, Germany; Anglia Ruskin University, Chelmsford, UK; University California San Francisco UCSF, San Francisco, USA; Yunnan Hospital Fuwai, Kunming, China.
Massimo Volpe, Sapienza University of Rome—Sant’Andrea Hospital Rome and IRCCS Neuromed, Pozzilli, Italy.
Michael A Weber, SUNY Downstate College of Medicine, New York, NY, USA.
Roland E Schmieder, Department of Nephrology and Hypertension, University Hospital, Erlangen, Germany.
Costas Tsioufis, First Cardiology Clinic, Medical School, National and Kapodistrian University of Athens, Hippokration Hospital, Athens, Greece.
William Wijns, The Lambe Institute for Translational Medicine, National University of Ireland Galway, Galway, Ireland.
This paper was guest edited by F. Crea (Roma, Italy).
The opinions expressed in this article are not necessarily those of the Editors of the European Heart Journal or of the European Society of Cardiology.
References
- 1. Bhatt DL, Kandzari DE, O'Neill WW, D'Agostino R, Flack JM, Katzen BT, Leon MB, Liu M, Mauri L, Negoita M, Cohen SA, Oparil S, Rocha-Singh K, Townsend RR, Bakris GL. A controlled trial of renal denervation for resistant hypertension. N Engl J Med 2014;370:1393–1401. [DOI] [PubMed] [Google Scholar]
- 2. White WB, Galis ZS, Henegar J, Kandzari DE, Victor R, Sica D, Townsend RR, Turner JR, Virmani R, Mauri L. Renal denervation therapy for hypertension: pathways for moving development forward. J Am Soc Hypertens 2015;9:341–350. [DOI] [PubMed] [Google Scholar]
- 3. Mahfoud F, Böhm M, Azizi M, Pathak A, Durand Zaleski I, Ewen S, Tsioufis K, Andersson B, Blankestijn PJ, Burnier M, Chatellier G, Gafoor S, Grassi G, Joner M, Kjeldsen SE, Lüscher TF, Lobo MD, Lotan C, Parati G, Redon J, Ruilope L, Sudano I, Ukena C, Leeuwen E. V, Volpe M, Windecker S, Witkowski A, Wijns W, Zeller T, Schmieder RE. Proceedings from the European clinical consensus conference for renal denervation: considerations on future clinical trial design. Eur Heart J 2015;36:2219–2227. [DOI] [PubMed] [Google Scholar]
- 4. Mahfoud F, Schmieder RE, Azizi M, Pathak A, Sievert H, Tsioufis C, Zeller T, Bertog S, Blankestijn PJ, Böhm M, Burnier M, Chatellier G, Durand Zaleski I, Ewen S, Grassi G, Joner M, Kjeldsen SE, Lobo MD, Lotan C, Lüscher Parati FT, Rossignol G, Ruilope P, Sharif L, Leeuwen F, van E, Volpe M, Windecker S, Witkowski A, Wijns W. Proceedings from the 2nd European Clinical Consensus Conference for device-based therapies for hypertension: state of the art and considerations for the future. Eur Heart J 2017;38:3272–3281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Townsend RR, Mahfoud F, Kandzari DE, Kario K, Pocock S, Weber MA, Ewen S, Tsioufis K, Tousoulis D, Sharp ASP, Watkinson AF, Schmieder RE, Schmid A, Choi JW, East C, Walton A, Hopper I, Cohen DL, Wilensky R, Lee DP, Ma A, Devireddy CM, Lea JP, Lurz PC, Fengler K, Davies J, Chapman N, Cohen SA, DeBruin V, Fahy M, Jones DE, Rothman M, Böhm M; SPYRAL HTN-OFF MED trial investigators. Catheter-based renal denervation in patients with uncontrolled hypertension in the absence of antihypertensive medications (SPYRAL HTN-OFF MED): a randomised, sham-controlled, proof-of-concept trial. Lancet 2017;390:2160–2170. [DOI] [PubMed] [Google Scholar]
- 6. Azizi M, Schmieder RE, Mahfoud F, Weber MA, Daemen J, Davies J, Basile J, Kirtane AJ, Wang Y, Lobo MD, Saxena M, Feyz L, Rader F, Lurz P, Sayer J, Sapoval M, Levy T, Sanghvi K, Abraham J, Sharp ASP, Fisher NDL, Bloch MJ, Reeve-Stoffer H, Coleman L, Mullin C, Mauri L; RADIANCE-HTN Investigators. Endovascular ultrasound renal denervation to treat hypertension (RADIANCE-HTN SOLO): a multicentre, international, single-blind, randomised, sham-controlled trial. Lancet 2018;391:2335–2345. [DOI] [PubMed] [Google Scholar]
- 7. Sardar P, Bhatt DL, Kirtane AJ, Kennedy KF, Chatterjee S, Giri J, Soukas PA, White WB, Parikh SA, Aronow HD. Sham-controlled randomized trials of catheter-based renal denervation in patients with hypertension. J Am Coll Cardiol 2019;73:1633–1642. [DOI] [PubMed] [Google Scholar]
- 8. Kandzari DE, Böhm M, Mahfoud F, Townsend RR, Weber MA, Pocock S, Tsioufis K, Tousoulis D, Choi JW, East C, Brar S, Cohen SA, Fahy M, Pilcher G, Kario K; SPYRAL HTN-ON MED Trial Investigators. Effect of renal denervation on blood pressure in the presence of antihypertensive drugs: 6-month efficacy and safety results from the SPYRAL HTN-ON MED proof-of-concept randomised trial. Lancet 2018;391:2346–2355. [DOI] [PubMed] [Google Scholar]
- 9. Thorpe KE, Zwarenstein M, Oxman AD, Treweek S, Furberg CD, Altman DG, Tunis S, Bergel E, Harvey I, Magid DJ, Chalkidou K. A pragmatic-explanatory continuum indicator summary (PRECIS): a tool to help trial designers. J Clin Epidemiol 2009;62:464–475. [DOI] [PubMed] [Google Scholar]
- 10. Azizi M, Schmieder RE, Mahfoud F, Weber MA, Daemen J, Lobo MD, Sharp ASP, Bloch MJ, Basile J, Wang Y, Saxena M, Lurz P, Rader F, Sayer J, Fisher NDL, Fouassier D, Barman NC, Reeve-Stoffer H, McClure C, Kirtane AJ; RADIANCE-HTN Investigators. Six-month results of treatment-blinded medication titration for hypertension control after randomization to endovascular ultrasound renal denervation or a sham procedure in the RADIANCE-HTN SOLO trial. Circulation 2019;139:2542–2553. [DOI] [PubMed] [Google Scholar]
- 11. Mauri L, Kario K, Basile J, Daemen J, Davies J, Kirtane AJ, Mahfoud F, Schmieder RE, Weber M, Nanto S, Azizi M. A multinational clinical approach to assessing the effectiveness of catheter-based ultrasound renal denervation: the RADIANCE-HTN and REQUIRE clinical study designs. Am Heart J 2018;195:115–129. [DOI] [PubMed] [Google Scholar]
- 12. Fengler K, Rommel K-P, Blazek S, Besler C, Hartung P, von RM, Petzold M, Winkler S, Höllriegel R, Desch S, Thiele H, Lurz P. A three-arm randomized trial of different renal denervation devices and techniques in patients with resistant hypertension (RADIOSOUND-HTN). Circulation 2019;139:590–600. [DOI] [PubMed] [Google Scholar]
- 13. Poulter NR, Prabhakaran D, Caulfield M. Hypertension. Lancet 2015;386:801–812. [DOI] [PubMed] [Google Scholar]
- 14. Weber MA. REDUCE HTN REINFORCE: a Randomized, Sham-controlled Trial of Bipolar Radiofrequency Renal Denervation for the Treatment of Hypertension. JACC Cardiovasc Interv 2020;13:461–470. [DOI] [PubMed] [Google Scholar]
- 15. Mahfoud F, Renkin J, Sievert H, Bertog S, Ewen S, Böhm M, Lengelé J-P, Wojakowski W, Schmieder RE, Giet M. V D, Parise H, Haratani N, Pathak A, Persu A. Alcohol-mediated renal denervation using the peregrine system infusion catheter for treatment of hypertension. JACC Cardiovasc Interv 2020;13:471–484. [DOI] [PubMed] [Google Scholar]
- 16. Neuzil P, Merkely B, Erglis A, Marinskis G, Groot JR, de Schmidinger H, Venegas Voskuil RM, Sturmberger M, Petru T, Jongejan J, Aichinger N, Kamzola J, Aidietis G, Gellér A, Mraz L, Osztheimer T, Mika I, Evans Y, Burkhoff S, Kuck K-H; BackBeat Study Investigators. Pacemaker-mediated programmable hypertension control therapy. J Am Heart Assoc 2017;6:e006974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Spiering W, Williams B, Van der Heyden J, van Kleef M, Lo R, Versmissen J, Moelker A, Kroon A, Reuter H, Ansel G, Stone GW, Bates M, Spiering W, Williams B, Stone GW, Bates M; CALM-FIM_EUR investigators. Endovascular baroreflex amplification for resistant hypertension: a safety and proof-of-principle clinical study. Lancet 2017;390:2655–2661. [DOI] [PubMed] [Google Scholar]
- 18. Azizi M. Catheter-based renal denervation for treatment of hypertension. Lancet 2017;390:2124–2126. [DOI] [PubMed] [Google Scholar]
- 19. Lobo MD, Sobotka PA, Stanton A, Cockcroft JR, Sulke N, Dolan E, Giet M, van der Hoyer J, Furniss SS, Foran JP, Witkowski A, Januszewicz A, Schoors D, Tsioufis K, Rensing BJ, Scott B, Ng GA, Ott C, Schmieder RE; ROX CONTROL HTN Investigators. Central arteriovenous anastomosis for the treatment of patients with uncontrolled hypertension (the ROX CONTROL HTN study): a randomised controlled trial. Lancet 2015;385:1634–1641. [DOI] [PubMed] [Google Scholar]
- 20. Lobo MD, Ott C, Sobotka PA, Saxena M, Stanton A, Cockcroft JR, Sulke N, Dolan E, M van der G, Hoyer J, Furniss SS, Foran JP, Witkowski A, Januszewicz A, Schoors D, Tsioufis K, Rensing BJ, Scott B, Ng GA, Schmieder RE. Central iliac arteriovenous anastomosis for uncontrolled hypertension: one-year results from the ROX CONTROL HTN trial. Hypertension 2017;70:1099–1105. [DOI] [PubMed] [Google Scholar]
- 21. Williams B, Mancia G, Spiering W, Agabiti Rosei E, Azizi M, Burnier M, Clement DL, Coca A, de Simone G, Dominiczak A, Kahan T, Mahfoud F, Redon J, Ruilope L, Zanchetti A, Kerins M, Kjeldsen SE, Kreutz R, Laurent S, Lip GYH, McManus R, Narkiewicz K, Ruschitzka F, Schmieder RE, Shlyakhto E, Tsioufis C, Aboyans V, Desormais I; ESC Scientific Document Group. 2018 ESC/ESH Guidelines for the management of arterial hypertension: the Task Force for the management of arterial hypertension of the European Society of Cardiology and the European Society of Hypertension. Eur Heart J 2018;39:3021–3104. [DOI] [PubMed] [Google Scholar]
- 22. Kotseva K; EUROASPIRE Investigators. The EUROASPIRE surveys: lessons learned in cardiovascular disease prevention. Cardiovasc Diagn Ther 2017;7:633–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cohen JB, Lotito MJ, Trivedi UK, Denker MG, Cohen DL, Townsend RR. Cardiovascular events and mortality in white coat hypertension: a systematic review and meta-analysis. Ann Intern Med 2019;170:853–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Herrmann J, Yang EH, Iliescu CA, Cilingiroglu M, Charitakis K, Hakeem A, Toutouzas K, Leesar MA, Grines CL, Marmagkiolis K. Vascular toxicities of cancer therapies: the old and the new—an evolving avenue. Circulation 2016;133:1272–1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Feinstein MJ, Bahiru E, Achenbach C, Longenecker CT, Hsue P, So-Armah K, Freiberg MS, Lloyd-Jones DM. Patterns of cardiovascular mortality for HIV-infected adults in the United States: 1999 to 2013. Am J Cardiol 2016;117:214–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Dakum P, Kayode GA, Abimiku A, Avong YK, Okuma J, Onyemata E, Ali T, Adekanmbi V, Uthman O. Prevalence of hypertension among patients aged 50 and older living with human immunodeficiency virus. Medicine 2019;98:e15024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Banegas JR, Ruilope LM, Sierra A. D L, Vinyoles E, Gorostidi M, Cruz JJ. D L, Ruiz-Hurtado G, Segura J, Rodríguez-Artalejo F, Williams B. Relationship between clinic and ambulatory blood-pressure measurements and mortality. N Engl J Med 2018;378:1509–1520. [DOI] [PubMed] [Google Scholar]
- 28. Mahfoud F, Ukena C, Schmieder RE, Cremers B, Rump LC, Vonend O, Weil J, Schmidt M, Hoppe UC, Zeller T, Bauer A, Ott C, Blessing E, Sobotka PA, Krum H, Schlaich M, Esler M, Böhm M. Ambulatory blood pressure changes after renal sympathetic denervation in patients with resistant hypertension. Circulation 2013;128:132–140. [DOI] [PubMed] [Google Scholar]
- 29. Ettehad D, Emdin CA, Kiran A, Anderson SG, Callender T, Emberson J, Chalmers J, Rodgers A, Rahimi K. Blood pressure lowering for prevention of cardiovascular disease and death: a systematic review and meta-analysis. Lancet 2016;387:957–967. [DOI] [PubMed] [Google Scholar]
- 30. Hicks KA, Tcheng JE, Bozkurt B, Chaitman BR, Cutlip DE, Farb A, Fonarow GC, Jacobs JP, Jaff MR, Lichtman JH, Limacher MC, Mahaffey KW, Mehran R, Nissen SE, Smith EE, Targum SL; American College of Cardiology, American Heart Association. 2014 ACC/AHA key data elements and definitions for cardiovascular endpoint events in clinical trials: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Data Standards (Writing Committee to Develop Cardiovascular Endpoints Data Standards). Circulation 2015;132:302–361. [DOI] [PubMed] [Google Scholar]
- 31. Tran V-T, Riveros C, Péan C, Czarnobroda A, Ravaud P. Patients’ perspective on how to improve the care of people with chronic conditions in France: a citizen science study within the ComPaRe e-cohort. BMJ Qual Saf 2019;28:875–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Berlowitz DR, Foy CG, Kazis LE, Bolin LP, Conroy MB, Fitzpatrick P, Gure TR, Kimmel PL, Kirchner K, Morisky DE, Newman J, Olney C, Oparil S, Pajewski NM, Powell J, Ramsey T, Simmons DL, Snyder J, Supiano MA, Weiner DE, Whittle J; SPRINT Research Group. Effect of intensive blood-pressure treatment on patient-reported outcomes. N Engl J Med 2017;377:733–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Calvert M, Blazeby J, Altman DG, Revicki DA, Moher D, Brundage MD; CONSORT PRO Group. Reporting of patient-reported outcomes in randomized trials: the CONSORT PRO extension. JAMA 2013;309:814–822. [DOI] [PubMed] [Google Scholar]
- 34. Bloch MJ, Basile J. The diagnosis and management of renovascular disease: a primary care perspective. Part II. Issues in management. J Clin Hypertens (Greenwich) 2003;5:261–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Strandness DE. Doppler and ultrasound methods for diagnosis. Semin Nephrol 2000;20:445–449. [PubMed] [Google Scholar]
- 36. Schäberle W, Leyerer L, Schierling W, Pfister K. Ultrasound diagnostics of renal artery stenosis: stenosis criteria, CEUS and recurrent in-stent stenosis. Gefässchirurgie 2016;21:4–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Rampinelli C, De Marco P, Origgi D, Maisonneuve P, Casiraghi M, Veronesi G, Spaggiari L, Bellomi M. Exposure to low dose computed tomography for lung cancer screening and risk of cancer: secondary analysis of trial data and risk-benefit analysis. BMJ 2017;356:j347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Gulani V, Calamante F, Shellock FG, Kanal E, Reeder SB; International Society for Magnetic Resonance in Medicine. Gadolinium deposition in the brain: summary of evidence and recommendations. Lancet Neurol 2017;16:564–570. [DOI] [PubMed] [Google Scholar]
- 39. Lièvre M, Ménard J, Bruckert E, Cogneau J, Delahaye F, Giral P, Leitersdorf E, Luc G, Masana L, Moulin P, Passa P, Pouchain D, Siest G. Premature discontinuation of clinical trial for reasons not related to efficacy, safety, or feasibility. BMJ 2001;322:603–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Pocock SJ, Clayton TC, Stone GW. Challenging issues in clinical trial design: part 4 of a 4-part series on statistics for clinical trials. J Am Coll Cardiol 2015;66:2886–2898. [DOI] [PubMed] [Google Scholar]
- 41. Kjeldsen SE, Esler MD. Take a blood pressure pill or undergo renal denervation?. Lancet 2018;391:2298–2300. [DOI] [PubMed] [Google Scholar]
- 42. Messerli FH, Rexhaj E. Of headwind and tailwind, regression to the mean and Wilder’s principle. J Hypertens 2019;37:4–5. [DOI] [PubMed] [Google Scholar]
- 43. Messerli FH, Bangalore S, Schmieder RE. Wilder’s principle: pre-treatment value determines post-treatment response. Eur Heart J 2015;36:576–579. [DOI] [PubMed] [Google Scholar]
- 44. Pocock SJ, Bakris G, Bhatt DL, Brar S, Fahy M, Gersh BJ. Regression to the mean in SYMPLICITY HTN-3: implications for design and reporting of future trials. J Am Coll Cardiol 2016;68:2016–2025. [DOI] [PubMed] [Google Scholar]
- 45. Böhm M, Mahfoud F, Townsend RR, Kandzari DE, Pocock S, Ukena C, Weber MA, Hoshide S, Patel M, Tyson CC, Weil J, Agdirlioglu T, Fahy M, Kario K. Ambulatory heart rate reduction after catheter-based renal denervation in hypertensive patients not receiving anti-hypertensive medications: data from SPYRAL HTN-OFF MED, a randomized, sham-controlled, proof-of-concept trial. Eur Heart J 2019;40:743–751. [DOI] [PubMed] [Google Scholar]
- 46. Lonn EM, Rambihar S, Gao P, Custodis FF, Sliwa K, Teo KK, Yusuf S, Böhm M. Heart rate is associated with increased risk of major cardiovascular events, cardiovascular and all-cause death in patients with stable chronic cardiovascular disease: an analysis of ONTARGET/TRANSCEND. Clin Res Cardiol 2014;103:149–159. [DOI] [PubMed] [Google Scholar]
- 47. Böhm M, Swedberg K, Komajda M, Borer JS, Ford I, Dubost-Brama A, Lerebours G, Tavazzi L. Heart rate as a risk factor in chronic heart failure (SHIFT): the association between heart rate and outcomes in a randomised placebo-controlled trial. Lancet 2010;376:886–894. [DOI] [PubMed] [Google Scholar]
- 48. Stergiou GS, Palatini P, Asmar R, Ioannidis JP, Kollias A, Lacy P, McManus RJ, Myers MG, Parati G, Shennan A, Wang J, O’Brien E; European Society of Hypertension Working Group on Blood Pressure Monitoring. Recommendations and Practical Guidance for performing and reporting validation studies according to the Universal Standard for the validation of blood pressure measuring devices by the Association for the Advancement of Medical Instrumentation/European Society of Hypertension/International Organization for Standardization (AAMI/ESH/ISO). J Hypertens 2019;37:459–466. [DOI] [PubMed] [Google Scholar]
- 49. Burnier M, Egan BM. Adherence in hypertension: a review of prevalence, risk factors, impact, and management. Circ Res 2019;124:1124–1140. [DOI] [PubMed] [Google Scholar]
- 50. Parati G, Torlasco C, Omboni S, Pellegrini D. Smartphone applications for hypertension management: a potential game-changer that needs more control. Curr Hypertens Rep 2017;19:48. [DOI] [PubMed] [Google Scholar]
- 51. Subbaraman R, de Mondesert L, Musiimenta A, Pai M, Mayer KH, Thomas BE, Haberer J. Digital adherence technologies for the management of tuberculosis therapy: mapping the landscape and research priorities. BMJ Glob Health 2018;3:e001018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Ewen S, Meyer MR, Cremers B, Laufs U, Helfer AG, Linz D, Kindermann I, Ukena C, Burnier M, Wagenpfeil S, Maurer HH, Böhm M, Mahfoud F. Blood pressure reductions following catheter-based renal denervation are not related to improvements in adherence to antihypertensive drugs measured by urine/plasma toxicological analysis. Clin Res Cardiol 2015;104:1097–1105. [DOI] [PubMed] [Google Scholar]
- 53. Vrijens B, Urquhart J. Methods for measuring, enhancing, and accounting for medication adherence in clinical trials. Clin Pharmacol Ther 2014;95:617–626. [DOI] [PubMed] [Google Scholar]
- 54. Belknap R, Weis S, Brookens A, Au-Yeung KY, Moon G, DiCarlo L, Reves R. Feasibility of an ingestible sensor-based system for monitoring adherence to tuberculosis therapy. PLoS One 2013;8:e53373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Michels KB, Rothman KJ. Update on unethical use of placebos in randomised trials. Bioethics 2003;17:188–204. [DOI] [PubMed] [Google Scholar]
- 56. Wardt V. V D, Harrison JK, Welsh T, Conroy S, Gladman J. Withdrawal of antihypertensive medication: a systematic review. J Hypertens 2017;35:1742–1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Kappetein AP, Head SJ, Généreux P, Piazza N, Mieghem N. V, Blackstone EH, Brott TG, Cohen DJ, Cutlip DE, Es G-A. V, Hahn RT, Kirtane AJ, Krucoff MW, Kodali S, Mack MJ, Mehran R, Rodés-Cabau J, Vranckx P, Webb JG, Windecker S, Serruys PW, Leon MB. Updated standardized endpoint definitions for transcatheter aortic valve implantation: the Valve Academic Research Consortium-2 consensus document. J Am Coll Cardiol 2012;60:1438–1454. [DOI] [PubMed] [Google Scholar]
- 58. Ndrepepa G, Schuster T, Hadamitzky M, Byrne RA, Mehilli J, Neumann F-J, Richardt G, Schulz S, Laugwitz K-L, Massberg S, Schömig A, Kastrati A. Validation of the Bleeding Academic Research Consortium definition of bleeding in patients with coronary artery disease undergoing percutaneous coronary intervention. Circulation 2012;125:1424–1431. [DOI] [PubMed] [Google Scholar]
- 59. Mahfoud F, Böhm M, Schmieder R, Narkiewicz K, Ewen S, Ruilope L, Schlaich M, Williams B, Fahy M, Mancia G. Effects of renal denervation on kidney function and long-term outcomes: 3-year follow-up from the Global SYMPLICITY Registry. Eur Heart J 2019;40:3474–3482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Hamm CW, Möllmann H, Holzhey D, Beckmann A, Veit C, Figulla H-R, Cremer J, Kuck K-H, Lange R, Zahn R, Sack S, Schuler G, Walther T, Beyersdorf F, Böhm M, Heusch G, Funkat A-K, Meinertz T, Neumann T, Papoutsis K, Schneider S, Welz A, Mohr FW; GARY-Executive Board. The German Aortic Valve Registry (GARY): in-hospital outcome. Eur Heart J 2014;35:1588–1598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Rothrock NE, Kaiser KA, Cella D. Developing a valid patient-reported outcome measure. Clin Pharmacol Ther 2011;90:737–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.U.S. Department of Health and Human Services and Food and Drug Administration. Guidance for Industry: Patient-Reported Outcome Measures: Use in Medical Product Development to Support Labeling Claims. 2009. https://www.fda.gov/media/77832/download (26 February 2020).
- 63.Health C for D and R. Factors to consider when making benefit-risk determinations in medical device premarket approval and de novo classifications. US Food Drug Adm. 2019. https://www.fda.gov/media/99769/download (26 February 2020).
- 64. Porter ME. What is value in health care? N Engl J Med 2010;363:2477–2481. [DOI] [PubMed] [Google Scholar]
- 65. Bulsei J, Darlington M, Durand-Zaleski I, Azizi M; DENERHTN Study Group. How to perform a cost-effectiveness analysis with surrogate endpoint: renal denervation in patients with resistant hypertension (DENERHTN) trial as an example. Blood Press 2018;27:66–72. [DOI] [PubMed] [Google Scholar]
- 66. Henry TL, De Brouwer BFE, Van Keep MML, Blankestijn PJ, Bots ML, Koffijberg H. Cost-effectiveness of renal denervation therapy for the treatment of resistant hypertension in The Netherlands. J Med Econ 2015;18:76–87. [DOI] [PubMed] [Google Scholar]
- 67. Gladwell D, Henry T, Cook M, Akehurst R. Cost effectiveness of renal denervation therapy for the treatment of resistant hypertension in the UK. Appl Health Econ Health Policy 2014;12:611–622. [DOI] [PubMed] [Google Scholar]
- 68. Tilden D, McBride M, Whitbourn R, Krum H, Walton T, Gillespie J. Cost effectiveness of catheter-based renal denervation for treatment resistant hypertension—an Australian Payer Perspective. Value Health 2014;17:A762. [DOI] [PubMed] [Google Scholar]
- 69. Kontsevaia AV, Suvorova EI, Khudiakov MB. [Economic efficiency of renal denervation in patients with resistant hypertension: results of Markov modeling]. Kardiologiia 2014;54:41–47. [PubMed] [Google Scholar]
- 70. Dorenkamp M, Bonaventura K, Leber AW, Boldt J, Sohns C, Boldt L-H, Haverkamp W, Frei U, Roser M. Potential lifetime cost-effectiveness of catheter-based renal sympathetic denervation in patients with resistant hypertension. Eur Heart J 2013;34:451–461. [DOI] [PubMed] [Google Scholar]
- 71. Revicki DA, Frank L. Pharmacoeconomic evaluation in the real world. Effectiveness versus efficacy studies. PharmacoEconomics 1999;15:423–434. [DOI] [PubMed] [Google Scholar]
- 72. Pratley RE. The efficacy and effectiveness of drugs for diabetes: how do clinical trials and the real world compare? Diabetologia 2014;57:1273–1275. [DOI] [PubMed] [Google Scholar]
- 73. Böhm M, Linz D, Ukena C, Esler M, Mahfoud F. Renal denervation for the treatment of cardiovascular high risk-hypertension or beyond?’ Circ Res 2014;115:400–409. [DOI] [PubMed] [Google Scholar]
- 74. Böhm M, Ewen S, Mahfoud F. Renal denervation for chronic heart failure: background and pathophysiological rationale. Korean Circ J 2017;47:9–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Nammas W, Koistinen J, Paana T, Karjalainen PP. Renal sympathetic denervation for treatment of patients with heart failure: summary of the available evidence. Ann Med 2017;49:384–395. [DOI] [PubMed] [Google Scholar]
- 76. Fukuta H, Goto T, Wakami K, Ohte N. Effects of catheter-based renal denervation on heart failure with reduced ejection fraction: a systematic review and meta-analysis. Heart Fail Rev 2017;22:657–664. [DOI] [PubMed] [Google Scholar]
- 77.Steinberg JS, Shabanov V, Ponomarev D, Losik D, Ivanickiy E, Kropotkin E, Polyakov K, Ptaszynski P, Keweloh B, Yao CJ, Pokushalov EA, Romanov AB. Effect of Renal Denervation and Catheter Ablation vs Catheter Ablation Alone on Atrial Fibrillation Recurrence Among Patients With Paroxysmal Atrial Fibrillation and Hypertension: The ERADICATE-AF Randomized Clinical Trial. JAMA 2020;323:248–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Pokushalov E, Romanov A, Corbucci G, Artyomenko S, Baranova V, Turov A, Shirokova N, Karaskov A, Mittal S, Steinberg JS. A randomized comparison of pulmonary vein isolation with versus without concomitant renal artery denervation in patients with refractory symptomatic atrial fibrillation and resistant hypertension. J Am Coll Cardiol 2012;60:1163–1170. [DOI] [PubMed] [Google Scholar]
- 79. Jong M. D, Adiyaman A, Gal P, Smit JJJ, Delnoy P, Heeg J-E, Hasselt B. V, Lau EOY, Persu A, Staessen JA, Ramdat Misier AR, Steinberg JS, Elvan A. Renal nerve stimulation-induced blood pressure changes predict ambulatory blood pressure response after renal denervation. Hypertension 2016;68:707–714. [DOI] [PubMed] [Google Scholar]
- 80. Gal P, de Jong MR, Smit JJJ, Adiyaman A, Staessen JA, Elvan A. Blood pressure response to renal nerve stimulation in patients undergoing renal denervation: a feasibility study. J Hum Hypertens 2015;29:292–295. [DOI] [PubMed] [Google Scholar]
- 81. Kiuchi MG, Esler MD, Fink GD, Osborn JW, Banek CT, Böhm M, Denton KM, DiBona GF, Everett TH, Grassi G, Katholi RE, Knuepfer MM, Kopp UC, Lefer DJ, Lohmeier TE, May CN, Mahfoud F, Paton JFR, Schmieder RE, Pellegrino PR, Sharabi Y, Schlaich MP. Renal denervation update from the international sympathetic nervous system summit. J Am Coll Cardiol 2019;73:3006–3017. [DOI] [PMC free article] [PubMed] [Google Scholar]