Abstract
Context
The effects of dietary intake of different fatty acids and pharmacological use of fatty acids, specifically long-chain n-3 polyunsaturated fatty acids (LC n-3 PUFAs), on cardiovascular health and atherosclerotic cardiovascular disease (ASCVD) prevention have been examined in a large number of observational studies and clinical trials. This review summarizes recent data and discusses potential mechanisms.
Evidence acquisition
The review is based on the authors’ knowledge of the field supplemented by a PubMed search using the terms seafood, fish oil, saturated fatty acids, omega-3 fatty acids, eicosapentaenoic acid, docosahexaenoic acid, polyunsaturated fatty acids, monounsaturated fatty acids, and ASCVD.
Evidence synthesis
We mainly discuss the recent clinical trials that examine the effects of different types of dietary fatty acids and pharmacological use of n-3 PUFA products on ASCVD prevention and the potential mechanisms.
Conclusions
While replacement of dietary saturated fat with unsaturated fat, polyunsaturated fat in particular, or intake of LC n-3 PUFA–rich seafood has generally shown benefit for ASCVD prevention and is recommended for cardiovascular benefits, data on effects of n-3 PUFA products on ASCVD health are inconsistent. However, recent clinical trials support benefits of prescription EPA in ASCVD prevention. n-3 PUFAs may contribute to ASCVD prevention through multiple mechanisms, including lowering plasma triglyceride levels, anti-inflammatory effects, antithrombotic effects, and effects on endothelial function.
Keywords: cardiovascular disease, fish oil, n-3 fatty acids, omega-3 fatty acids, prevention
Atherosclerotic cardiovascular disease (ASCVD) remains the leading cause of death and disability worldwide (1). Both diet and medication have been used for prevention and treatment of ASCVD. In this review, we discuss recent updates on dietary fat, including saturated fatty acids (SFAs), polyunsaturated fatty acids (PUFAs), and monounsaturated fatty acids (MUFAs) (Fig. 1), and pharmacological fatty acids, specifically n-3 PUFAs, in ASCVD prevention and treatment.
Figure 1.
Chemical Structure of Key Fatty Acids. From: (3). Abbreviations: DHA, docosahexaenoic acid; DPA, docosapentaenoic acid; EPA, eicosapentaenoic acid.
Dietary Fat and ASCVD Prevention
SFAs
In the United States, SFAs account for approximately 11% of daily energy intake on average (2). Studies show that replacing dietary carbohydrates with SFAs increases both low-density lipoprotein cholesterol (LDL-C) and high-density lipoprotein cholesterol (HDL-C) without significantly changing total cholesterol/HDL-C ratio (4). In contrast, replacing SFAs with PUFAs and MUFAs decreases total cholesterol and LDL-C but only slightly decreases HDL-C, resulting in decreased total cholesterol/HDL-C ratio (4). In prospective observational studies, replacing SFAs with carbohydrates, especially carbohydrates from refined grains and added sugars, did not significantly reduce coronary heart disease (CHD) risk (5–7), whereas replacing SFAs with whole grains or unsaturated FAs, PUFAs in particular, did reduce CHD risk (7). Replacing SFAs with PUFAs (mainly linoleic acid [LA]) or MUFAs also reduced CVD and all-cause mortality (8). However, SFA consumption was not associated with total stroke (9) or ischemic stroke (10) in meta-analyses of cohort studies.
PUFAs
n-3 PUFAs.
n-3 PUFAs include alpha-linolenic acid (18:3n-3), which is derived from plants, and those derived from animals, particularly seafood (fish oil), mainly eicosapentaenoic acid (EPA; 20:5n-3), docosahexaenoic acid (DHA; 22:6n-3), and docosapentaenoic acid (22:5n-3) (11–17). Although alpha-linolenic acid appeared to have cardiovascular benefits in observational studies, overall evidence is inconclusive (17–21). In contrast, seafood intake has been negatively correlated with CHD risk in primary prevention (22, 23). In a primary-prevention meta-analysis, risk for acute coronary syndrome was 22% lower in participants who ate seafood ≥4 times weekly than in participants who ate seafood <1 time monthly, with higher consumption associated with greater benefit (23).
In addition, the association of seafood n-3 PUFAs with recurrent events in individuals with CHD has been assessed in observational studies. In the Cardiovascular Health Study (24), plasma phospholipid n-3 PUFA level was inversely associated with mortality, and red blood cell content of EPA plus DHA was inversely correlated with mortality in the Framingham Heart Study (25), Women’s Health Initiative Memory Study (26, 27), and Heart and Soul Study (28), with similar results in several meta-analyses (29–31).
In the secondary-prevention Diet and Reinfarction Trial (12), 2033 men with recent myocardial infarction (MI) were randomized to receive or not to receive dietary advice on fish (2 servings of fatty fish per week; ~20% took EPA + DHA 855 mg/day), fat (total fat intake <30% of calories, PUFA/SFA ratio 1.0), and fiber (cereal fiber intake 18 g/day). At 2-year follow-up, no significant effects were seen with reduced total fat or increased cereal fiber, but patients randomized to increased fish had significantly lower total mortality (relative risk [RR] 0.71; 95% confidence interval [CI], 0.54–0.93) and CHD mortality (RR 0.84; 95% CI, 0.66–1.07) than those without fish advice.
A meta-analysis showed a negative association of high fish intake with total stroke risk (32). The inverse association was stronger for ischemic than for hemorrhagic stroke.
n-6 PUFAs.
The major n-6 PUFAs are LA and arachidonic acid. LA, found mainly in vegetable oils, nuts, and seeds, is the predominant dietary n-6 PUFA, whereas arachidonic acid, found mainly in red meat, eggs, algae, and fish oil, has low consumption (~100 mg/day compared with 15 g/day of LA). Controlled feeding trials, prospective cohort studies, and randomized clinical trials demonstrate that high intake of n-6 PUFAs, predominantly LA in substitution for SFAs, was associated with reduced CHD risk (33–38). Consistently, a recent meta-analysis of 30 prospective observational studies, including 68659 participants, indicated that higher circulating and tissue levels of n-6 PUFAs, LA in particular, were associated with reduced total CVD, cardiovascular mortality, and ischemic stroke (39). Another pooled analysis, which included 39740 participants, showed that higher percentages of LA biomarkers in total fatty acids in individual lipid compartments (phospholipids, plasma, cholesteryl esters, and adipose tissue) were correlated with reduced risk for type 2 diabetes; in contrast, arachidonic acid biomarkers were not significantly associated with type 2 diabetes risk (40).
MUFAs
The most common MUFA in human diets is oleic acid, accounting for approximately 90% of all dietary MUFAs (41). MUFAs comprise approximately 16% to 29% of total energy intake in a typical Mediterranean diet, mostly from olive oil, and epidemiological studies found low CHD prevalence in Mediterranean populations despite high total fat intake (42). MUFA intake improves a number of CVD risk factors including lipid profile, insulin sensitivity, inflammatory markers, and thrombogenic markers (4, 43–46). Whereas early prospective cohort studies on the association of MUFA intake with CHD risk were inconsistent (6–8, 31, 47–49), in part because of confounding variables such as concomitant SFA intake in common foods, a more recent study showed that substituting MUFAs for carbohydrates reduced risk for CVD and total mortality, and during the 32-year follow-up, the major food source of FAs in this study changed from red meat to olive oil and nuts (8). Olive oil intake was inversely associated with CVD risk in several studies. Increasing olive oil intake by 25 g/day reduced CVD risk by 18% in a meta-analysis of 9 studies including 1 clinical trial (50). MUFA intake was associated with reduced risk for hemorrhagic stroke but not total or ischemic stroke in a meta-analysis of 10 prospective cohort studies including more than 300000 individuals (51).
In the Prevención con Dieta Mediterránea study, Mediterranean-type diets supplemented with extra virgin olive oil or mixed nuts significantly lowered CVD events by 31% or 28%, respectively, compared with a low-fat diet, as primary prevention in individuals at high CVD risk at 4.8-year median follow-up (52). This study provides the strongest evidence to date for the benefit of dietary MUFAs for primary prevention of ASCVD in high-risk individuals.
Taken together, these studies indicate that reducing SFA intake and replacing SFAs with PUFAs and MUFAs are associated with reduced rates of CVD and total mortality. In contrast, replacement of SFAs with refined carbohydrates and sugars showed no benefit on lowering CVD risk.
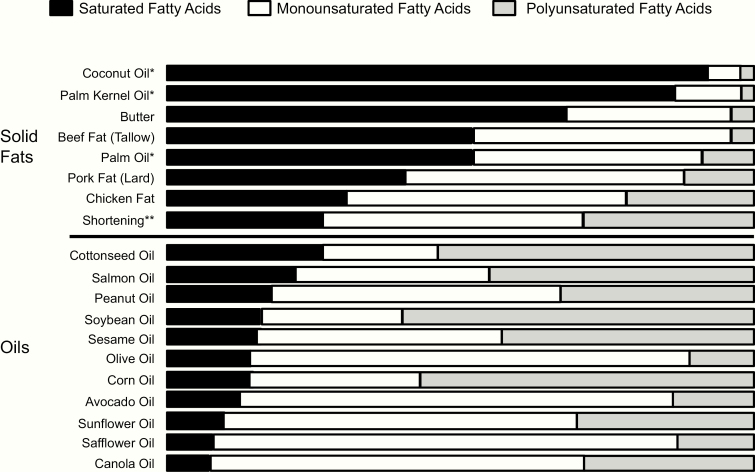
Accordingly, the American Heart Association recommends lowering intake of SFAs and replacing SFAs with unsaturated FAs, especially PUFAs, to reduce risk of CVD and also to include 1 to 2 seafood meals per week to reduce risk of CHD, ischemic stroke, and sudden cardiac death (53). Consistently, the 2015–2020 Dietary Guidelines for Americans (2) and the Scientific Report of the 2015 US Dietary Guidelines Advisory Committee (54) recommend using nonhydrogenated vegetable oils high in unsaturated fats and relatively low in SFAs (soybean, corn, olive, canola) instead of animal fats or tropical oils (palm, palm kernel, coconut) (Fig. 2) (2). Instead of reducing total fat, the type of fat should be optimized. At least 2 servings (3.5 oz per serving) of seafood should be consumed weekly to provide approximately 250 mg/day EPA plus DHA (55) and replace other animal sources of protein (Table 1). While salmon, swordfish, and mussel contain high amounts of EPA and DHA, some other commonly consumed fish, such as tilapia, have very little EPA and DHA.
Figure 2.
Fatty Acid Profiles of Common Fats and Oils. * Coconut, palm kernel, and palm oil are plant derived but are solid or semisolid at room temperature because of high saturated-fat content and are considered solid fats for nutrition. ** Shortening may be made from partially hydrogenated vegetable oil, which contains trans fatty acids. From: (2).
Table 1.
Major Fish and Other Seafood Sources of EPA and DHA
| Fish/Seafood | Measure | EPA, g | DHA, g | EPA + DHA, g |
|---|---|---|---|---|
| Cod, Atlantic, canned, solids, and liquid | 3 oz | 0.003 | 0.13 | 0.13 |
| Crab, queen, cooked, moist heat | 3 oz | 0.282 | 0.123 | 0.41 |
| Flatfish (flounder and sole species), cooked, dry heat | 1 fillet | 0.213 | 0.168 | 0.38 |
| Flatfish (flounder and sole species), raw, boneless | 1 oz | 0.039 | 0.031 | 0.07 |
| Grouper, mixed species, cooked, dry heat | 3 oz | 0.03 | 0.181 | 0.21 |
| Grouper, mixed species, raw | 3 oz | 0.023 | 0.187 | 0.21 |
| Haddock, raw | 3 oz | 0.036 | 0.076 | 0.11 |
| Herring, Atlantic, raw, boneless | 1 oz | 0.201 | 0.244 | 0.45 |
| Herring, Pacific, raw | 3 oz | 0.824 | 0.586 | 1.41 |
| Mackerel, jack, canned, drained solids, boneless | 1 oz | 0.123 | 0.226 | 0.35 |
| Mackerel, Spanish, raw | 3 oz | 0.28 | 0.86 | 1.14 |
| Mahi-mahi, cooked, dry heat | 3 oz | 0.022 | 0.096 | 0.12 |
| Mussel, blue, raw | 1 cup | 0.282 | 0.38 | 0.66 |
| Ocean perch, Atlantic, cooked, dry heat | 1 fillet | 0.037 | 0.093 | 0.13 |
| Oyster, Eastern, cooked, breaded and fried | 3 oz | 0.172 | 0.185 | 0.36 |
| Oyster, Eastern, farmed, raw | 3 oz | 0.16 | 0.173 | 0.33 |
| Oyster, Eastern, wild, cooked, moist heat | 3 oz | 0.3 | 0.23 | 0.53 |
| Oyster, Pacific, raw | 1 medium | 0.219 | 0.125 | 0.34 |
| Pike, northern, cooked, dry heat | 3 oz | 0.036 | 0.081 | 0.12 |
| Pollock, Alaska, cooked | 3 oz | 0.088 | 0.193 | 0.28 |
| Roe, mixed species, cooked, dry heat | 1 oz | 0.357 | 0.495 | 0.85 |
| Salmon, chum, cooked, dry heat | 3 oz | 0.254 | 0.429 | 0.68 |
| Salmon, coho, wild, cooked, moist heat | 3 oz | 0.462 | 0.706 | 1.17 |
| Salmon, pink, canned, drained solids | 3 oz | 0.284 | 0.632 | 0.92 |
| Salmon, pink, canned, with bone and liquid | 3 oz | 0.718 | 0.685 | 1.4 |
| Spiny lobster, mixed species, cooked, moist heat | 3 oz | 0.29 | 0.118 | 0.41 |
| Swordfish, cooked, dry heat | 3 oz | 0.108 | 0.656 | 0.76 |
| Tilapia, raw | 1 fillet | 0.006 | 0.1 | 0.11 |
| Trout, mixed species, cooked, dry heat | 1 fillet | 0.161 | 0.42 | 0.58 |
| Trout, rainbow, farmed, cooked, dry heat | 1 fillet | 0.184 | 0.437 | 0.62 |
| Tuna, skipjack, fresh, cooked, dry heat | 3 oz | 0.077 | 0.201 | 0.28 |
| Tuna, white, canned in oil, drained solids | 3 oz | 0.056 | 0.151 | 0.21 |
From: (55).
Effects and Mechanisms of n-3 PUFA on ASCVD Risks
Unsaturated FAs, LC n-3 PUFAs in particular, have been studied for their effects on CVD risk and tested in clinical trials for CVD prevention. Tissue culture, animal, and human studies indicate that n-3 PUFAs have multiple effects that may benefit CVD prevention (56).
Reductions of plasma triglycerides
n-3 PUFAs, particularly high-dose prescription formulations, lower plasma triglyceride (TG) levels (57–60). This effect may result from reduced hepatic very-low-density lipoprotein synthesis, which involves multiple mechanisms (56) of which decreased de novo lipogenesis appears to be predominant (61–64); other mechanisms include increased fatty acid β-oxidation, decreased hepatic nonesterified fatty acid delivery, reduced hepatic lipogenic enzyme activity, and increased hepatic synthesis of phospholipids in place of TGs (61, 65–68). The TG-lowering effect of n-3 PUFAs is generally linear and dose dependent but varies between individuals and is usually greater in individuals with higher TG levels at baseline (69, 70).
Anti-inflammatory effect
Inflammation contributes to ASCVD. The recent Canakinumab Anti-inflammatory Thrombosis Outcomes Study showed benefits of a therapy targeting interleukin-1β in secondary prevention of ASCVD (71). n-3 PUFAs lower several inflammatory markers associated with CVD risk. In clinical studies in patients with hypertriglyceridemia, prescription EPA as compared with placebo reduced plasma high-sensitivity C-reactive protein (hs-CRP), lipoprotein-associated phospholipase A2, and oxidized LDL levels and arachidonic acid/EPA ratio (72–77). TG reductions may contribute to decreasing inflammatory markers and other effects with n-3 PUFAs. However, n-PUFAs may also exert these effects independent of TG reductions. In our and others’ studies, short-term (2-week) treatment with EPA alone, with mild effects on plasma TG levels, improved monocyte phenotypes in individuals with hypertriglyceridemia (57, 78). Compared with DHA, EPA reduces the expression of genes linked to CVD, including those of the interferon pathway (79), 3',5'-cyclic adenosine monophosphate–responsive element protein 1, and hypoxia inducible factor 1 (79) and decreases production of inflammatory markers such as tumor necrosis factor–α and interleukin-1β in macrophages with lipopolysaccharide stimulation (80). All of these effects are involved in TG-independent benefits of EPA treatment on ASCVD events as revealed by the recent Reduction of Cardiovascular Events with EPA–Intervention Trial (REDUCE-IT) described below.
Antithrombotic effect
Hyper-reactivity of platelets leading to thrombus formation is the major cause for MI. Therefore, antiplatelet and antithrombotic therapies are effective in reducing ASCVD mortality. n-3 PUFAs reduce platelet aggregation and may therefore have antithrombotic effects, likely because of their incorporation into platelet membrane (see the following sections) and their role in reducing production and signaling of thromboxane A2, which plays a crucial role in platelet activation (81–83). Indeed, animal studies indicated that n-3 PUFA supplementation inhibited platelet activation and attenuated thrombus formation (84, 85). However, early human trials showed inconsistent effects of n-3 PUFA consumption on platelet aggregation and coagulation factors (86, 87). More recent studies indicated that adding n-3 PUFAs potentiated platelet response to clopidogrel in patients treated with combined aspirin and clopidogrel antiplatelet therapy after percutaneous coronary intervention and decreased lipoprotein-associated phospholipase A2, also known as platelet-activating factor acetylhydrolase, in patients with stable angina undergoing percutaneous coronary intervention (88–90). Overall, at the pharmacological dose of 4 g/day, antithrombotic effects do not appear to be the major mechanism by which n-PUFAs lower ASCVD risk, although subtle effects cannot be excluded.
Effect on endothelial function
Endothelial cell dysfunctions play a crucial role in development of ASCVD. Earlier tissue culture and animal studies indicate that n-3 PUFA improves endothelial function and reduces expression of adhesion molecules, thereby decreasing endothelial-leukocyte interactions and subsequent leukocyte infiltration (91), a key step in atherosclerosis. Several clinical studies showed that n-3 PUFAs improve flow-mediated vasodilation (92–95), a common noninvasive measure of endothelial function, and lower circulating levels of endothelial dysfunction markers such as E-selectin, vascular cell adhesion molecule–1, and intercellular adhesion molecule–1 (96–98). These endothelial function–improving effects may also contribute to n-3 PUFA–mediated protection against ASCVD.
Molecular Mechanisms
The molecular mechanisms whereby n-PUFAs exert the above effects are multifactorial and not fully understood. First, n-3 PUFAs are incorporated into the cell and organelle membrane and alter membrane lipid environments and fluidity, which are involved in various cellular functions such as signal transduction and protein trafficking (99–102) and may contribute to the observed beneficial effects of n-3 PUFAs on inflammation, platelet function, and monocyte phenotypes in humans with hypertriglyceridemia (78). Second, formation of specialized proresolving mediators derived from n-3 PUFAs such as EPA-derived E-series resolvins and DHA-derived D-series resolvins and protectins may play an important role in the resolution of inflammation (103–107). Third, n-3 PUFAs reduce the generation of intracellular secondary messengers such as diacylglycerol and ceramide, resulting in decreased inflammatory response (108). Further, n-3 PUFAs bind to and activate several nuclear receptors and transcription factors (109, 110), which are involved in regulating expression of multiple genes that participate in various functions related to ASCVD such as inflammation, glucose–insulin homeostasis, lipid metabolism, and production of adipocytokines (111–116). Activation of peroxisome proliferator-activated receptor–gamma by n-3 PUFAs reduces nuclear factor–κB translocation to the nucleus, thereby decreasing generation of multiple inflammatory cytokines (117).
Clinical Trials of Pharmacological LC n-3 PUFAs for ASCVD Prevention
Because of their effects as discussed above, n-3 PUFAs have been used in numerous clinical trials (12, 118–123) for ASCVD prevention (Table 2). However, considerable debate remains about the potential cardiovascular benefits of n-3 PUFAs.
Table 2.
Reviewed Outcomes Trials of n-3 PUFAs
| Trial | Population | N | Follow-up, y | Interventions | Baseline Statin Use, % | Baseline LDL-C, mg/dL | Baseline TG, mg/dL | Primary Endpoint | Risk Ratio (95% CI) | Adverse Eventsa |
|---|---|---|---|---|---|---|---|---|---|---|
| Diet and/or supplement | ||||||||||
| DART (12) | Recent MI (men) | 2033 | 2 | Fatty fish 2 servings/week or supplement EPA + DHA 855 mg/d; total fat 30% of calories, PUFA/ SFA ratio 1.0; cereal fiber 18 g/d; no diet advice | NR | TC 250 | NR | Total mortality | RR 0.71 (0.54–0.93) | NR |
| CHD mortality | RR 0.84 (0.66–1.07) | |||||||||
| EPA + DHA (low dose) | ||||||||||
| GISSI-P (118) | Recent MI | 11,324 | 3.5 | EPA + DHA 850 mg/d; vitamin E 300 mg/d; n-3 + vitamin E; no supplement | Cholesterol-lowering agents: 5 (end of study: 46) | 137 | 162 | Death or nonfatal MI or nonfatal stroke | RR 0.90 (0.82–0.99) [2-way analysis]; RR 0·85 (0.74–0.98) [4-way analysis] | GI disturbance, nausea |
| GISSI-HF (119) | Chronic HF | 6975 | 3.9 | EPA + DHA 850 mg; placebo (unspecified) | 23 | TC 188 | 126 | Death | HR 0.91 (0.833–0.998) | GI disturbance |
| Death or CV hosp | HR 0.92 (0.849–0.999) | |||||||||
| OMEGA (124) | Recent MI | 3851 | 1 | DHA + EPA 840 mg; placebo | 94–95 | NR | NR | SCD | OR 0.95 (0.56–1.60) | Neoplasms (19 vs 8), cardiac device therapeutic procedures (16 vs 2) |
| Alpha-omega-3 (18) | Prior MI | 4837 | 3.3 | 400 mg EPA + DHA ± 2 g ALA; 2 g ALA; placebo | Baseline lipid- lowering agents: 85–87 | 99–102 | 144–150 | Fatal/nonfatal CV events or revasc | HR: 1.01 (0.87–1.17) | GI symptoms, prostate cancer incidence, death |
| SU.FOL.OM3 (125) | Recent acute coronary or cerebral ischemic event | 2501 | 4.7 | EPA + DHA 600 mg; placebo + vitamin B | Baseline lipid- lowering agents: 83–87 | 101–104 | 108 (no coronary event); 125 (coronary event) | MACE | HR 1.08 (0.79–1.47) | GI disturbance, nausea, cutaneous reactions |
| ORIGIN (126) | Dysglycemia or high-risk CVD | 12,536 | 6.2 | EPA + DHA 840 mg; placebo (olive oil) | 53–54 | 112 | 140–142 | CV death | HR 0.98 (0.87–1.10) | NR |
| ORIGINALE (127) | ORIGIN participants | 4771 | 8.9 | EPA + DHA 840 mg; placebo (olive oil) | 57–59 | 108–109 | 142 | CV death | HR 0.98 (0.88–1.09) | NR |
| Risk & Prevention (128) | High-risk CVD | 12,513 | 5 | EPA + DHA 850 mg; placebo | 41 | 132 | 150 | CV death or CV hosp | HR 0.97 (0.88–1.08) | GI symptoms |
| ASCEND (120) | Diabetes without CVD | 15,480 | 7.4 | DHA + EPA 840 mg; placebo (olive oil) | 75 | TC 161 | NR | Nonfatal MI, nonfatal stroke, TIA, or vascular death | RR 0.97 (0.87–1.08) | Similar between groups |
| VITAL (121) | Healthy, no history of CVD | 25,871 | 5.3 | DHA + EPA 840 mg; placebo | Cholesterol-lowering agents: 37 | NR | NR | MI, stroke, or CV death | HR 0.97 (0.85–1.12) | GI symptoms |
| EPA (high dose) | ||||||||||
| JELIS (122) | TC >250 mg/dL | 18,645 | 4.6 | EPA 1800 mg + statin; statin only | 97 | 182 | 151 | Sudden cardiac death, fatal or nonfatal MI, unstable angina, or revasc | HR 0.81 (0.69–0.95) | GI, skin, hemorrhage (cerebral, fundal, epistaxis, subcutaneous) (0.6% vs 1.1%, P = 0.0006) |
| REDUCE-IT (123) | CVD or diabetes + additional CVD risk | 8179 | 4.9 | EPA 4000 mg; placebo (mineral oil) | 100 | 75 | 216 | CV death, nonfatal MI, nonfatal stroke, revasc, or unstable angina | HR 0.75 (0.68–0.83) | Hosp for atrial fibrillation and atrial flutter (3.1% vs 2.1%, P = 0.004), serious bleeding events (2.7% vs 2.1%, P = 0.06) |
aNot significantly different between treatment groups except as noted.
Abbreviations: ALA, alpha-linolenic acid; ASCEND, A Study of Cardiovascular Events in Diabetes; CHD, coronary heart disease; CI, confidence interval; CV, cardiovascular; CVD, cardiovascular disease; DART, Diet and Reinfarction Trial; DHA, docosahexaenoic acid; EPA, eicosapentaenoic acid; GI, gastrointestinal; GISSI-P, Gruppo Italiano per lo Studio della Sopravvivenza nell’Infarto Miocardico Prevenzione; GISSI-HF, GISSI–Heart Failure; HF, heart failure; hosp, hospitalization; HR, hazard ratio; JELIS, Japan EPA Lipid Intervention Study; LDL-C, low-density lipoprotein cholesterol; MACE, major adverse cardiac events; MI, myocardial infarction; NR, not reported; OR, odds ratio; ORIGIN, Outcome Reduction with an Initial Glargine Intervention; ORIGINALE, Outcome Reduction with an Initial Glargine Intervention Legacy Effects; PUFA, polyunsaturated fatty acid; REDUCE-IT, Reduction of Cardiovascular Events with Icosapent Ethyl–Intervention Trial; revasc, revascularization; RR, relative risk; SCD, sudden coronary death; SFA, saturated fatty acid; SU.FOL.OM3, Supplementation with Folate, Vitamin B6 and B12 and/or Omega-3 Fatty Acids trial; TC: total cholesterol; TG, triglyceride; TIA, transient ischemic attack; VITAL, Vitamin D and Omega-3 Trial; y, years.
Outcomes trials of mixtures of EPA and DHA at low doses
The Gruppo Italiano per lo Studio della Sopravvivenza nell’Infarto Miocardico Prevenzione (GISSI-P) study assessed effects of combined EPA and DHA ethyl esters 850 mg/day on recurrent ASCVD events in 11324 patients with recent MI (118). After a median follow-up of 3.5 years, the primary endpoint (death, nonfatal MI, and nonfatal stroke) was reduced by 15% (95% CI, 2%–6%; P = 0.02) with n-3 PUFAs compared with placebo. Of note, a low proportion (5%) of patients used concomitant statins at baseline, although 46% of patients used statins at the end of the study.
Similarly, a mixture of EPA and DHA ethyl esters 850 g/day was evaluated in 6975 patients with chronic heart failure in the GISSI–Heart Failure (GISSI-HF) study (119), in which approximately 22% of patients used statin therapy at baseline. Median TG level was 126 mg/dL at baseline and decreased only slightly during treatment. After a median follow-up of 3.9 years, n-3 PUFAs significantly reduced the coprimary endpoints of all-cause mortality by 9% (P = 0.041) and all-cause mortality or hospital admission for cardiovascular reasons by 8% (P = 0.009) compared with placebo.
However, several later studies showed no significant efficacy of n-3 PUFAs in lowering ASCVD risk in patients on statin treatment. A meta-analysis of 10 trials, which included 77917 individuals, showed no significant differences between n-3 PUFA and control groups for CHD, stroke, revascularization, and major vascular events in individuals with previous CHD (60).
In the Outcome Reduction with an Initial Glargine Intervention (ORIGIN) trial (126), 12536 patients at high risk for CVD and with impaired fasting glucose, impaired glucose tolerance, or diabetes were randomized to receive 1 g/day of n-3 PUFAs (containing 465 mg of EPA and 375 mg of DHA) or 1 g/day of placebo (olive oil). At a median follow-up of 6.2 years, TG levels were reduced by 14.5 mg/dL more with n-3 PUFAs than placebo (P < 0.001). However, the n-3 PUFA group did not show significant decreases in the incidence of the primary outcome of cardiovascular death (P = 0.72), major vascular events (P = 0.81), death from any cause (P = 0.63), or death from arrhythmia (P = 0.26) when compared with the placebo group.
The ORIGIN and Legacy Effects (ORIGINALE) study (127) extended follow-up for an additional 2.7 years to examine potential posttreatment impact of n-3 PUFAs. Of the 4771 individuals with extended follow-up, 2368 had been randomized to n-3 PUFAs and 2403 to placebo. At the end of ORIGIN, 89% of the initial n-3 PUFA group and 1% of the placebo group (P < 0.001) were taking n-3 PUFAs, whereas only 11% and 9% of the respective groups (P = 0.04) were taking n-3 PUFAs at the end of ORIGINALE. As in the main trial, at extended follow-up no difference was seen between the initial randomized treatment groups for the primary outcome of cardiovascular death (P = 0.68) or the other cardiovascular outcomes assessed.
More recently, 2 large clinical trials of n-3 PUFAs for primary prevention of ASCVD, A Study of Cardiovascular Events in Diabetes (ASCEND) (120) and Vitamin D and Omega-3 Trial (VITAL) (121), also failed to show benefit on the primary endpoint. In ASCEND, 15480 patients with diabetes were randomized to receive combined EPA and DHA 840 mg/day or olive oil placebo (120). At baseline, approximately 75% of patients were on statin therapy, and non-HDL-C level was 113 mg/dL. After a mean follow-up of 7.4 years, no significant difference was observed in the rates of primary outcome events (nonfatal MI, nonfatal stroke, transient ischemic attack, and vascular death; RR 0.97; 95% CI, 0.87–1.08; P = 0.55) and all-cause mortality between the n-3 PUFA and placebo groups. However, vascular death was modestly reduced with n-3 PUFAs compared with placebo (respective event rates 2.4% and 2.9%; RR 0.81; 95% CI, 0.67–0.99). VITAL used combined EPA and DHA 840 mg/day with or without vitamin D3 supplementation in 25871 healthy elderly individuals (≥50-year-old men and ≥55-year-old women) (121). After a median follow-up of 5.3 years, n-3 PUFAs did not reduce the primary endpoint of major CVD events (MI, stroke, and CVD death) compared with placebo. However, subgroup analyses demonstrated a significant interaction based on median weekly fish consumption, supporting benefits of pharmacological n-3 PUFAs versus placebo among individuals consuming <1.5 servings of fish per week at baseline. Secondary analyses from this trial also showed significant reductions of total MI, fatal MI, number of percutaneous coronary interventions, and total CHD (a composite of MI, coronary revascularization, and CHD death) in the n-3 PUFA group. Subgroup analysis of the total MI secondary endpoint suggested that n-3 PUFA supplementation may have greater coronary benefits in African Americans (~20% of the study population) than in other racial groups in the study. However, because the primary endpoint was not significantly achieved, the results generated from the secondary analyses should be interpreted cautiously.
Outcomes trials of EPA alone
EPA and DHA have biochemical differences including their membrane locations, lipid interactions, and effects on platelet function, membrane bilayer structure, and formation of cholesterol crystalline domains (129–132). Outcomes trials have examined effects of EPA alone on ASCVD prevention, but no trials of DHA alone with ASCVD endpoints have been reported or (according to ClinicalTrials.gov) are ongoing.
The open-label Japan EPA Lipid Intervention Study (JELIS) compared purified EPA at 1.8 g/day and low-intensity statin with low-intensity statin alone in 18645 Japanese patients with hypercholesterolemia (mean baseline total cholesterol ~275 mg/dL; median baseline TG levels ~154 mg/dL) (122). At a mean follow-up of 4.6 years, the primary endpoint of major coronary events (sudden cardiac death, fatal and nonfatal MI, unstable angina pectoris, angioplasty, stenting, and coronary artery bypass grafting) was significantly reduced by 19% in the EPA group compared with controls, and TG level was significantly decreased by 9% with EPA compared with 4% in controls; LDL-C levels were not significantly different between treatment groups (25% reduction from baseline in both groups). Of the component outcomes, unstable angina, but not sudden cardiac death or fatal MI, was significantly decreased with EPA. In subgroup analyses, reduction in risk for the primary endpoint was similar in primary and secondary prevention but was statistically significant only in secondary prevention (~20% of the study population). JELIS patients with high baseline TG level, low baseline HDL-C level, and impaired glucose metabolism had higher risk for CHD and greater benefit on CHD reduction with EPA (133, 134). Event reduction was associated with higher blood levels of EPA and higher ratios of EPA to arachidonic acid (135).
REDUCE-IT evaluated whether treatment with a highly purified EPA ethyl ester, icosapent ethyl, at 2 g twice daily (4 g/day), compared with placebo (mineral oil), reduces ASCVD events in statin-treated patients with relatively well-controlled LDL-C levels (>40 mg/dL and ≤100 mg/dL), high baseline TG levels (≥135 mg/dL and <500 mg/dL), and elevated risk for cardiovascular events (123). Of the 8179 participants randomized, 71% had ASCVD and 29% had diabetes with additional ASCVD risk factors but did not have established ASCVD; at baseline, 31% of patients took high-intensity statin, 62% took moderate-intensity statin, and 59% had diabetes. Baseline median (interquartile range) TG levels were 216 (176–272) mg/dL in the icosapent ethyl group and 216 (176–274) mg/dL in the placebo group; respective LDL-C levels were 74 mg/dL (62–88) mg/dL and 76 (63–89) mg/dL.
During a median follow-up of 4.9 years, primary endpoint events (first occurrence of cardiovascular death, nonfatal MI [including silent MI], nonfatal stroke, coronary revascularization, or unstable angina) occurred in 17% of icosapent ethyl patients and 22% of placebo patients (hazard ratio [HR] 0.75, 95% CI, 0.68–0.83; P < 0.001; absolute risk reduction [ARR] 4.8%) (123). Moreover, compared with placebo, the treatment group had significant reductions in secondary endpoint events including cardiovascular death (HR 0.80; 95% CI, 0.66–0.98; P = 0.03; ARR 0.9%), fatal or nonfatal MI (HR 0.69; 95% CI, 0.58–0.81; P < 0.001; ARR 2.6%), fatal or nonfatal stroke (HR 0.72; 95% CI, 0.55–0.93; P = 0.01; ARR 0.9%), unstable angina (HR 0.68; 95% CI, 0.53–0.87; P = 0.002; ARR 1.2%), and urgent or emergent revascularization (HR 0.65; 95% CI, 0.55–0.78; P < 0.001; ARR 2.5%), but all-cause mortality was not significantly different between groups (HR 0.87; 95% CI, 0.74–1.02).
In REDUCE-IT, icosapent ethyl treatment also improved lipids and other biomarkers. TG levels decreased by 18% (−39.0 mg/dL) at 1 year and by 22% (−45.0 mg/dL) at the final visit in the icosapent ethyl group, compared with a 2% (4.5 mg/dL) increase and 6% (−13.0 mg/dL) decrease in the placebo group at the respective timepoints. LDL-C decreased by 1% (−1.0 mg/dL) at both 1 year and the final visit with icosapent ethyl, compared with 11% (9.3 mg/dL) and 6% (5.7 mg/dL) increases with placebo at these timepoints. At the final visit, hs-CRP was reduced by 13% (−0.2 mg/L) with icosapent ethyl but increased by 30% (0.4 mg/L) with placebo.
In a subsequent analysis of REDUCE-IT, icosapent ethyl lowered the RR for total (first and subsequent) ischemic events included in the primary composite endpoint by 30%, with a 25% RR reduction for first events, 32% for second events, 31% for third events, and 48% for fourth and subsequent events (136). Total key secondary endpoint events (cardiovascular death, nonfatal MI, nonfatal stroke) were also reduced with icosapent ethyl compared with placebo (RR 0.72; 95% CI, 0.63–0.82; P < 0.0001).
Discussion of n-3 PUFA Outcomes Trials
Multiple reasons may have contributed to the discrepant results among the trials. The earlier GISSI trials were designed for secondary prevention and included low proportions of patients taking concomitant statins. ASCEND and VITAL were designed for primary prevention of ASCVD and included high proportions of patients on statins as did several other later trials that did not show significant efficacy of n-3 PUFAs (18,124,125,128,137,138).
In contrast, treatment with prescription EPA at high doses has shown great potential in ASCVD prevention, including in statin-treated patients with acceptable LDL-C levels. Despite different EPA doses in REDUCE-IT (4 g/day) and JELIS (1.8 g/day), circulating EPA levels were similar in these 2 trials (144 mg/L at 1 year in REDUCE-IT and 169 mg/L at 5 years in JELIS), which likely reflect greater fish consumption in the Japanese study. Treatment with EPA 4 g/day in the evaluation of the effect of two doses of AMR101 (Ethyl Icosapentate) on fasting serum triglyceride levels in patients with persistent high triglyceride levels (≥200 mg/dL and <500 mg/dL) despite statin therapy (ANCHOR) study resulted in a >6-fold increase in EPA and doubling of docosapentaenoic acid with no change in DHA levels (139). Prior studies involving EPA and DHA mixtures, including ASCEND and VITAL, used formulations containing much less EPA (460 mg/day in ASCEND and VITAL). The discontinued outcomes study to assess statin residual risk reduction with epanova in high CV risk patients with hypertriglyceridemia (STRENGTH) trial examining the efficacy and safety of high-dose (4 g/day) n-3 carboxylic acids containing EPA and DHA is expected to provide further insight into how n-3 PUFA formulation and dosage affect cardiovascular outcomes (140).
In addition, whereas ASCEND and VITAL focused on primary prevention, REDUCE-IT included a large proportion of secondary-prevention patients and higher TG levels at baseline, both higher-risk populations, which increases the power to detect risk reductions. Indeed, in REDUCE-IT, although no significant difference was observed between secondary and primary prevention, a trend toward greater risk reduction in secondary prevention suggests that higher-risk patients may benefit more from n-3 PUFA treatment.
TG levels at baseline were higher in REDUCE-IT (median 216 mg/dL) than in JELIS (median 153 mg/dL), and EPA led to greater TG reductions in REDUCE-IT than in JELIS. However, subgroup analysis from REDUCE-IT found consistent risk reduction with EPA in patients with baseline TG levels ≥150 mg/dL or <150 mg/dL as well as in patients with 1-year TG levels ≥150 mg/dL or <150 mg/dL, suggesting that EPA may have TG-independent benefits for ASCVD prevention. Nevertheless, REDUCE-IT showed significantly greater benefit in patients with baseline TG ≥200 mg/dL in conjunction with baseline HDL-C <35 mg/dL (HR 0.62; 95% CI, 0.51–0.77; compared with HR 0.79; 95% CI, 0.71–0.88 in patients without both lipid values; P = 0.04 for interaction). Similarly, in JELIS, patients with TG ≥150 mg/dL combined with HDL-C <40 mg/dL at baseline had a significantly greater risk for coronary events (HR 1.71; 95% CI, 1.11–2.64; P = 0.014) and a significantly greater risk reduction with EPA treatment (HR 0.47; 95% CI, 0.23–0.98; P = 0.043) compared with patients with neither of these dyslipidemias (134), consistently suggesting greater benefit of EPA treatment in higher-risk patients.
In addition to lowering TG, n-3 PUFAs have other effects that may benefit ASCVD prevention. The reduction of hs-CRP with EPA in REDUCE-IT substantiates previous reports on anti-inflammatory effects of EPA (141, 142). EPA also improves circulating monocyte phenotypes in individuals with hypertriglyceridemia (78). In addition, EPA may reduce atherothrombotic events by inhibiting platelet aggregation (81, 132), which may also explain the tendency for more bleeding events with EPA observed in REDUCE-IT. Additional analyses from this trial, including more information on the association of clinical event reduction with changes in EPA levels, lipids and lipoproteins, and inflammatory biomarkers, may provide more insight into the mechanism(s) of benefit with EPA treatment.
One limitation of REDUCE-IT was the use of mineral oil as a placebo (123). If mineral oil affected statin absorption in some placebo patients, this might have contributed to differences between treatment groups, but the small differences in LDL-C would not explain the observed 25% risk reduction, and a post hoc analysis suggested similar benefits regardless of whether there was an increase in LDL-C among placebo patients.
Safety of n-3 PUFAs
In clinical studies, gastrointestinal events were the main adverse effects with DHA-containing products (131, 143), whereas arthralgia was the main adverse event reported with EPA (130, 144). Some clinical studies showed that treatment with DHA-containing n-3 PUFA products was associated with increased LDL-C in individuals with severe hypertriglyceridemia (145, 146) while other studies involving subjects with moderate hypertriglyceridemia did not show this effect (147–155).
In REDUCE-IT, EPA treatment was associated with increased rates of atrial fibrillation (5.3% vs 3.9% with placebo) and peripheral edema (6.5% vs 5.0% with placebo). The mechanisms for these observations are unknown, but in vitro and animal studies demonstrated that n-PUFAs may affect ion channels of cardiac tissue (156–158). Previous observational studies and randomized trials that examined potential antiarrhythmic effects of n-3 PUFAs had inconsistent results (159). Some studies found trends toward increased atrial fibrillation with n-3 PUFAs (160, 161). In REDUCE-IT, the higher incidence of atrial fibrillation in the EPA-treated group was not associated with increased heart failure or stroke. Additional studies are needed to examine other potential consequences related to atrial fibrillation with EPA treatment.
Both JELIS and REDUCE-IT reported modest increases in bleeding events with EPA treatment compared with controls. Bleeding rates were lower in JELIS (1.1% with EPA; 0.6% in controls) than REDUCE-IT (2.7% with EPA; 2.1% with placebo); at baseline, antiplatelet therapy was used by 13% and 14% of the respective treatment groups in JELIS, compared with 79.7% and 79.1% of the respective groups in REDUCE-IT (136). Although no severe clinical bleeding events were observed in REDUCE-IT or other clinical trials of n-3 PUFAs, including in participants who used aspirin or warfarin and those who had surgery or percutaneous interventions (162–164), the potential interaction between EPA and antithrombotic medications warrants further investigation.
Conclusion
Accumulated evidence supports the recommendation of lowering intake of SFAs, replacing them with unsaturated FAs, especially PUFAs, and consuming seafood containing significant amounts of EPA and DHA at least 2 times per week (ideally ≥4 times per week (23)) to reduce the risk of ASCVD. Accordingly, a healthy diet for ASCVD prevention should include nonhydrogenated vegetable oils that are high in unsaturated FAs with a relatively low proportion of SFAs as well as seafood containing high amounts of LC n-3 PUFAs. With the increased popularity of low-carbohydrate, high-fat diets such as the ketogenic diet, it is important that health care providers educate patients on differential effects of FAs on lipids as MUFAs and PUFAs from plants (165) may avoid the frequent and highly variable LDL-C increases that may occur with the ketogenic diet (166). While statins alone or combined with ezetimibe or proprotein convertase subtilisin/kexin type 9 inhibitors reduce ASCVD risk by lowering LDL-C, other factors such as hypertriglyceridemia and inflammation still contribute residual ASCVD risk. Over the last few years, multiple trials of n-3 PUFAs have examined effects of various dosages and formulations in different patient populations, providing increasing evidence on the pharmacological and clinical effects of n-3 PUFAs in ASCVD prevention. In JELIS and REDUCE-IT, prescription EPA used in conjunction with statin therapy provided further benefit on reducing ASCVD events in high-risk patients compared with statin alone. On the other hand, n-3 PUFAs at low doses have not shown consistent efficacy in reducing ASCVD risk, particularly for primary prevention. Additional trials of n-3 PUFAs are ongoing and will provide further insights on the effectiveness of this class of medication in ASCVD prevention.
Acknowledgments
We thank Kerrie Jara for editorial assistance.
Financial Support: This work was supported by a National Institutes of Health grant (R01 HL098839, to H.W.), an American Heart Association award (AHA16GRNT30410012, to H.W.), and an American Diabetes Association award (1-17-IBS-082, to H.W.).
Glossary
Abbreviations
- ARR
absolute risk reduction
- ASCEND
A Study of Cardiovascular Events in Diabetes
- ASCVD
atherosclerotic cardiovascular disease
- CHD
coronary heart disease
- CI
confidence interval
- DHA
docosahexaenoic acid
- EPA
eicosapentaenoic acid
- FA
fatty acid
- GISSI
Gruppo Italiano per lo Studio della Sopravvivenza nell’Infarto Miocardico Prevenzione
- HDL-C
high-density lipoprotein cholesterol
- HR
hazard ratio
- hs-CRP
high-sensitivity C-reactive protein
- JELIS
Japan EPA Lipid Intervention Study
- LA
linoleic acid
- LC
long-chain
- LDL
low-density lipoprotein cholesterol
- MI
myocardial infarction
- MUFA
monounsaturated fatty acid
- ORIGIN
Outcome Reduction with an Initial Glargine Intervention
- ORIGINALE
Outcome Reduction with an Initial Glargine Intervention Legacy Effects
- PUFA
polyunsaturated fatty acid
- REDUCE-IT
Reduction of Cardiovascular Events with EPA–Intervention Trial
- RR
relative risk
- SFA
saturated fatty acid
- TG
triglyceride
- VITAL
Vitamin D and Omega-3 Trial
Additional Information
Disclosure Summary: L.X. and H.W. have nothing to declare. C.M.B received grant/research support from Akcea, Amgen, Esperion, Novartis, Regeneron, and Sanofi-Synthelabo (all paid to Baylor College of Medicine) and consults for Akcea, Amarin, Amgen, Arrowhead, Astra Zeneca, Boehringer Ingelheim, Denka Seiken, Esperion, Intercept, Janssen, Matinas BioPharma Inc, Merck, Novartis, Novo Nordisk, Regeneron, and Sanofi-Synthelabo. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Data Availability: Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
References
- 1. Roth GA, Forouzanfar MH, Moran AE, et al. Demographic and epidemiologic drivers of global cardiovascular mortality. N Engl J Med. 2015;372(14):1333–1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. U.S. Department of Health and Human Services, U.S. Department of Agriculture. 2015–2020 Dietary Guidelines for Americans,8th ed. https://health.gov/dietaryguidelines/2015/guidelines/. Accessed April 8, 2019. [Google Scholar]
- 3. US National Library of Medicine, National Center for Biotechnology Information. PubChem https://pubchem.ncbi.nlm.nih.gov. Accessed May 20, 2019. [Google Scholar]
- 4. Mensink RP, Zock PL, Kester AD, Katan MB. Effects of dietary fatty acids and carbohydrates on the ratio of serum total to HDL cholesterol and on serum lipids and apolipoproteins: a meta-analysis of 60 controlled trials. Am J Clin Nutr. 2003;77(5):1146–1155. [DOI] [PubMed] [Google Scholar]
- 5. Farvid MS, Ding M, Pan A, et al. Dietary linoleic acid and risk of coronary heart disease: a systematic review and meta-analysis of prospective cohort studies. Circulation. 2014;130(18):1568–1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Jakobsen MU, O’Reilly EJ, Heitmann BL, et al. Major types of dietary fat and risk of coronary heart disease: a pooled analysis of 11 cohort studies. Am J Clin Nutr. 2009;89(5):1425–1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Li Y, Hruby A, Bernstein AM, et al. Saturated fats compared with unsaturated fats and sources of carbohydrates in relation to risk of coronary heart disease: a prospective Cohort study. J Am Coll Cardiol. 2015;66(14):1538–1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wang DD, Li Y, Chiuve SE, et al. Association of specific dietary fats with total and cause-specific mortality. JAMA Intern Med. 2016;176(8):1134–1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Siri-Tarino PW, Sun Q, Hu FB, Krauss RM. Meta-analysis of prospective cohort studies evaluating the association of saturated fat with cardiovascular disease. Am J Clin Nutr. 2010;91(3):535–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. de Souza RJ, Mente A, Maroleanu A, et al. Intake of saturated and trans unsaturated fatty acids and risk of all cause mortality, cardiovascular disease, and type 2 diabetes: systematic review and meta-analysis of observational studies. BMJ. 2015;351:h3978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kromhout D, Bosschieter EB, de Lezenne Coulander C. The inverse relation between fish consumption and 20-year mortality from coronary heart disease. N Engl J Med. 1985;312(19):1205–1209. [DOI] [PubMed] [Google Scholar]
- 12. Burr ML, Fehily AM, Gilbert JF, et al. Effects of changes in fat, fish, and fibre intakes on death and myocardial reinfarction: diet and reinfarction trial (DART). Lancet. 1989;2(8666):757–761. [DOI] [PubMed] [Google Scholar]
- 13. Yuan JM, Ross RK, Gao YT, Yu MC. Fish and shellfish consumption in relation to death from myocardial infarction among men in Shanghai, China. Am J Epidemiol. 2001;154(9):809–816. [DOI] [PubMed] [Google Scholar]
- 14. Daviglus ML, Stamler J, Orencia AJ, et al. Fish consumption and the 30-year risk of fatal myocardial infarction. N Engl J Med. 1997;336(15):1046–1053. [DOI] [PubMed] [Google Scholar]
- 15. Albert CM, Hennekens CH, O’Donnell CJ, et al. Fish consumption and risk of sudden cardiac death. JAMA. 1998;279(1):23–28. [DOI] [PubMed] [Google Scholar]
- 16. Hu FB, Bronner L, Willett WC, et al. Fish and omega-3 fatty acid intake and risk of coronary heart disease in women. JAMA. 2002;287(14):1815–1821. [DOI] [PubMed] [Google Scholar]
- 17. Brouwer IA, Katan MB, Zock PL. Dietary alpha-linolenic acid is associated with reduced risk of fatal coronary heart disease, but increased prostate cancer risk: a meta-analysis. J Nutr. 2004;134(4):919–922. [DOI] [PubMed] [Google Scholar]
- 18. Kromhout D, Giltay EJ, Geleijnse JM; Alpha Omega Trial Group n-3 fatty acids and cardiovascular events after myocardial infarction. N Engl J Med. 2010;363(21):2015–2026. [DOI] [PubMed] [Google Scholar]
- 19. Mente A, de Koning L, Shannon HS, Anand SS. A systematic review of the evidence supporting a causal link between dietary factors and coronary heart disease. Arch Intern Med. 2009;169(7):659–669. [DOI] [PubMed] [Google Scholar]
- 20. Mozaffarian D. Does alpha-linolenic acid intake reduce the risk of coronary heart disease? A review of the evidence. Altern Ther Health Med. 2005;11(3):24–30; quiz 31, 79. [PubMed] [Google Scholar]
- 21. Wang C, Harris WS, Chung M, et al. n-3 Fatty acids from fish or fish-oil supplements, but not alpha-linolenic acid, benefit cardiovascular disease outcomes in primary- and secondary-prevention studies: a systematic review. Am J Clin Nutr. 2006;84(1):5–17. [DOI] [PubMed] [Google Scholar]
- 22. Balk E, Adams G, Langberg V, et al. Omega-3 fatty acids and cardiovascular disease: an updated systematic review. Evid Rep Technol Assess (Full Rep). 2016:1–1252. [DOI] [PubMed] [Google Scholar]
- 23. Leung Yinko SS, Stark KD, Thanassoulis G, Pilote L. Fish consumption and acute coronary syndrome: a meta-analysis. Am J Med. 2014;127(9):848–57.e2. [DOI] [PubMed] [Google Scholar]
- 24. Mozaffarian D, Lemaitre RN, King IB, et al. Plasma phospholipid long-chain ω-3 fatty acids and total and cause-specific mortality in older adults: a cohort study. Ann Intern Med. 2013;158(7):515–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Harris WS, Tintle NL, Etherton MR, Vasan RS. Erythrocyte long-chain omega-3 fatty acid levels are inversely associated with mortality and with incident cardiovascular disease: the Framingham Heart Study. J Clin Lipidol. 2018;12(3):718–727.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Harris WS, Luo J, Pottala JV, et al. Red blood cell polyunsaturated fatty acids and mortality in the Women’s Health Initiative Memory Study. J Clin Lipidol. 2017;11(1):250–259.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Harris WS, Zotor FB. n-3 fatty acids and risk for fatal coronary disease. Proc Nutr Soc. 2019;1–6. [DOI] [PubMed] [Google Scholar]
- 28. Pottala JV, Garg S, Cohen BE, Whooley MA, Harris WS. Blood eicosapentaenoic and docosahexaenoic acids predict all-cause mortality in patients with stable coronary heart disease: the Heart and Soul study. Circ Cardiovasc Qual Outcomes. 2010;3(4):406–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Harris WS, Del Gobbo L, Tintle NL. The omega-3 index and relative risk for coronary heart disease mortality: estimation from 10 cohort studies. Atherosclerosis. 2017;262:51–54. [DOI] [PubMed] [Google Scholar]
- 30. Del Gobbo LC, Imamura F, Aslibekyan S, et al. ; Cohorts for Heart and Aging Research in Genomic Epidemiology (CHARGE) Fatty Acids and Outcomes Research Consortium (FORCe) ω-3 polyunsaturated fatty acid biomarkers and coronary heart disease: pooling project of 19 cohort studies. JAMA Intern Med. 2016;176(8):1155–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Chowdhury R, Warnakula S, Kunutsor S, et al. Association of dietary, circulating, and supplement fatty acids with coronary risk: a systematic review and meta-analysis. Ann Intern Med. 2014;160(6):398–406. [DOI] [PubMed] [Google Scholar]
- 32. Zhao W, Tang H, Yang X, et al. Fish consumption and stroke risk: a meta-analysis of prospective cohort studies. J Stroke Cerebrovasc Dis. 2019;28(3):604–611. [DOI] [PubMed] [Google Scholar]
- 33. Dayton S, Pearce ML, Hashimoto S, Dixon WJ, Tomiyasu U. A controlled clinical trial of a diet high in unsaturated fat in preventing complications of atherosclerosis. Circulation. 1969;40:II-1–II-63. [Google Scholar]
- 34. Leren P. The Oslo diet-heart study. Eleven-year report. Circulation. 1970;42(5):935–942. [DOI] [PubMed] [Google Scholar]
- 35. Controlled trial of soya-bean oil in myocardial infarction. Lancet. 1968;2:693–699. [PubMed] [Google Scholar]
- 36. Turpeinen O, Pekkarinen M, Miettinen M, Elosuo R, Paavilainen E. Dietary prevention of coronary heart disease: the Finnish mental hospital study. Int J Epidemiol. 1979;8:99–118. [DOI] [PubMed] [Google Scholar]
- 37. Miettinen M, Turpeinen O, Karvonen MJ, Elosuo R, Paavilainen E. Effect of cholesterol-lowering diet on mortality from coronary heart-disease and other causes. A twelve-year clinical trial in men and women. Lancet. 1972;2(7782):835–838. [DOI] [PubMed] [Google Scholar]
- 38. Miettinen M, Turpeinen O, Karvonen MJ, Pekkarinen M, Paavilainen E, Elosuo R. Dietary prevention of coronary heart disease in women: the Finnish mental hospital study. Int J Epidemiol. 1983;12(1):17–25. [DOI] [PubMed] [Google Scholar]
- 39. Marklund M, Wu JHY, Imamura F, et al. ; Cohorts for Heart and Aging Research in Genomic Epidemiology (CHARGE) Fatty Acids and Outcomes Research Consortium (FORCE) Biomarkers of dietary omega-6 fatty acids and incident cardiovascular disease and mortality: an individual-level pooled analysis of 30 cohort studies. Circulation. 2019;139(21):2422–2436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wu JHY, Marklund M, Imamura F, et al. ; Cohorts for Heart and Aging Research in Genomic Epidemiology (CHARGE) Fatty Acids and Outcomes Research Consortium (FORCE) Omega-6 fatty acid biomarkers and incident type 2 diabetes: pooled analysis of individual-level data for 39 740 adults from 20 prospective cohort studies. Lancet Diabetes Endocrinol. 2017;5(12):965–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Joris PJ, Mensink RP. Role of cis-monounsaturated fatty acids in the prevention of coronary heart disease. Curr Atheroscler Rep. 2016;18(7):38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Keys A. Coronary heart disease in seven countries. Circulation. 1970;41:186–195. [PubMed] [Google Scholar]
- 43. Kris-Etherton PM. AHA science advisory. Monounsaturated fatty acids and risk of cardiovascular disease. American Heart Association. Nutrition Committee. Circulation. 1999;100(11): 1253–1258. [DOI] [PubMed] [Google Scholar]
- 44. McMurray HF, Parthasarathy S, Steinberg D. Oxidatively modified low density lipoprotein is a chemoattractant for human T lymphocytes. J Clin Invest. 1993;92(2):1004–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Pacheco YM, López S, Bermúdez B, Abia R, Villar J, Muriana FJ. A meal rich in oleic acid beneficially modulates postprandial sICAM-1 and sVCAM-1 in normotensive and hypertensive hypertriglyceridemic subjects. J Nutr Biochem. 2008;19(3): 200–205. [DOI] [PubMed] [Google Scholar]
- 46. Witztum JL, Steinberg D. Role of oxidized low density lipoprotein in atherogenesis. J Clin Invest. 1991;88(6):1785–1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Hu FB, Stampfer MJ, Manson JE, et al. Dietary fat intake and the risk of coronary heart disease in women. N Engl J Med. 1997;337(21):1491–1499. [DOI] [PubMed] [Google Scholar]
- 48. Schwingshackl L, Hoffmann G. Monounsaturated fatty acids, olive oil and health status: a systematic review and meta-analysis of cohort studies. Lipids Health Dis. 2014;13:154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Guasch-Ferré M, Babio N, Martínez-González MA, et al. ; PREDIMED Study Investigators Dietary fat intake and risk of cardiovascular disease and all-cause mortality in a population at high risk of cardiovascular disease. Am J Clin Nutr. 2015;102(6):1563–1573. [DOI] [PubMed] [Google Scholar]
- 50. Martínez-González MA, Dominguez LJ, Delgado-Rodríguez M. Olive oil consumption and risk of CHD and/or stroke: a meta-analysis of case-control, cohort and intervention studies. Br J Nutr. 2014;112(2):248–259. [DOI] [PubMed] [Google Scholar]
- 51. Cheng P, Wang J, Shao W. Monounsaturated fatty acid intake and stroke risk: a meta-analysis of prospective cohort studies. J Stroke Cerebrovasc Dis. 2016;25(6):1326–1334. [DOI] [PubMed] [Google Scholar]
- 52. Estruch R, Ros E, Salas-Salvadó J, et al. ; PREDIMED Study Investigators Primary prevention of cardiovascular disease with a mediterranean diet supplemented with extra-virgin olive oil or nuts. N Engl J Med. 2018;378(25):e34. [DOI] [PubMed] [Google Scholar]
- 53. Rimm EB, Appel LJ, Chiuve SE, et al. ; American Heart Association Nutrition Committee of the Council on Lifestyle and Cardiometabolic Health; Council on Epidemiology and Prevention; Council on Cardiovascular Disease in the Young; Council on Cardiovascular and Stroke Nursing; and Council on Clinical Cardiology Seafood long-chain n-3 polyunsaturated fatty acids and cardiovascular disease: a science advisory from the American Heart Association. Circulation. 2018;138(1):e35–e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Dietary Guidelines Advisory Committee. Scientific Report of the 2015 Dietary Guidelines Advisory Committee: Advisory Report to the Secretary of Health and Human Services and the Secretary of Agriculture. Washington, DC: U.S. Department of Agriculture, Agricultural Research Service; 2015. [Google Scholar]
- 55. US Department of Agriculture, Agricultural Research Service. USDA food composition databases: Nutrient search https://ndb.nal.usda.gov/ndb/nutrients/index. Accessed April 12, 2019.
- 56. Mozaffarian D, Wu JH. Omega-3 fatty acids and cardiovascular disease: effects on risk factors, molecular pathways, and clinical events. J Am Coll Cardiol. 2011;58(20):2047–2067. [DOI] [PubMed] [Google Scholar]
- 57. Maki KC, Bobotas G, Dicklin MR, Huebner M, Keane WF. Effects of MAT9001 containing eicosapentaenoic acid and docosapentaenoic acid, compared to eicosapentaenoic acid ethyl esters, on triglycerides, lipoprotein cholesterol, and related variables. J Clin Lipidol. 2017;11(1):102–109. [DOI] [PubMed] [Google Scholar]
- 58. Shaikh SR. Biophysical and biochemical mechanisms by which dietary N-3 polyunsaturated fatty acids from fish oil disrupt membrane lipid rafts. J Nutr Biochem. 2012;23(2):101–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Williams JA, Batten SE, Harris M, et al. Docosahexaenoic and eicosapentaenoic acids segregate differently between raft and nonraft domains. Biophys J. 2012;103(2):228–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Aung T, Halsey J, Kromhout D, et al. ; Omega-3 Treatment Trialists’ Collaboration Associations of omega-3 fatty acid supplement use with cardiovascular disease risks: meta-analysis of 10 trials involving 77 917 individuals. JAMA Cardiol. 2018;3(3):225–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Jump DB. Fatty acid regulation of hepatic lipid metabolism. Curr Opin Clin Nutr Metab Care. 2011;14(2):115–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Hein GJ, Bernasconi AM, Montanaro MA, et al. Nuclear receptors and hepatic lipidogenic enzyme response to a dyslipidemic sucrose-rich diet and its reversal by fish oil n-3 polyunsaturated fatty acids. Am J Physiol Endocrinol Metab. 2010;298(3):E429–E439. [DOI] [PubMed] [Google Scholar]
- 63. Sato A, Kawano H, Notsu T, et al. Antiobesity effect of eicosapentaenoic acid in high-fat/high-sucrose diet-induced obesity: importance of hepatic lipogenesis. Diabetes. 2010;59(10):2495–2504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Tanaka N, Zhang X, Sugiyama E, et al. Eicosapentaenoic acid improves hepatic steatosis independent of PPARα activation through inhibition of SREBP-1 maturation in mice. Biochem Pharmacol. 2010;80(10):1601–1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Harris WS, Bulchandani D. Why do omega-3 fatty acids lower serum triglycerides? Curr Opin Lipidol. 2006;17(4):387–393. [DOI] [PubMed] [Google Scholar]
- 66. Faeh D, Minehira K, Schwarz JM, et al. Effect of fructose overfeeding and fish oil administration on hepatic de novo lipogenesis and insulin sensitivity in healthy men. Diabetes. 2005;54(7):1907–1913. [DOI] [PubMed] [Google Scholar]
- 67. Clarke SD. Polyunsaturated fatty acid regulation of gene transcription: a molecular mechanism to improve the metabolic syndrome. J Nutr. 2001;131(4):1129–1132. [DOI] [PubMed] [Google Scholar]
- 68. Saraswathi V, Morrow JD, Hasty AH. Dietary fish oil exerts hypolipidemic effects in lean and insulin sensitizing effects in obese LDLR-/- mice. J Nutr. 2009;139(12):2380–2386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Harris WS. n-3 fatty acids and serum lipoproteins: human studies. Am J Clin Nutr. 1997;65(5 Suppl):1645S–1654S. [DOI] [PubMed] [Google Scholar]
- 70. Hamazaki K, Itomura M, Huan M, et al. n-3 long-chain FA decrease serum levels of TG and remnant-like particle-cholesterol in humans. Lipids. 2003;38(4):353–358. [DOI] [PubMed] [Google Scholar]
- 71. Ridker PM, Everett BM, Thuren T, et al. ; CANTOS Trial Group Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med. 2017;377(12):1119–1131. [DOI] [PubMed] [Google Scholar]
- 72. Ballantyne CM, Bays HE, Kastelein JJ, et al. Efficacy and safety of eicosapentaenoic acid ethyl ester (AMR101) therapy in statin-treated patients with persistent high triglycerides (from the ANCHOR study). Am J Cardiol. 2012;110(7):984–992. [DOI] [PubMed] [Google Scholar]
- 73. Bays HE, Ballantyne CM, Braeckman RA, Stirtan WG, Soni PN. Icosapent ethyl, a pure ethyl ester of eicosapentaenoic acid: effects on circulating markers of inflammation from the MARINE and ANCHOR studies. Am J Cardiovasc Drugs. 2013;13(1):37–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Bays HE, Ballantyne CM, Kastelein JJ, Isaacsohn JL, Braeckman RA, Soni PN. Eicosapentaenoic acid ethyl ester (AMR101) therapy in patients with very high triglyceride levels (from the Multi-center, plAcebo-controlled, Randomized, double-blINd, 12-week study with an open-label Extension [MARINE] trial). Am J Cardiol. 2011;108(5):682–690. [DOI] [PubMed] [Google Scholar]
- 75. Satoh-Asahara N, Shimatsu A, Sasaki Y, et al. Highly purified eicosapentaenoic acid increases interleukin-10 levels of peripheral blood monocytes in obese patients with dyslipidemia. Diabetes Care. 2012;35(12):2631–2639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Satoh N, Shimatsu A, Kotani K, et al. Purified eicosapentaenoic acid reduces small dense LDL, remnant lipoprotein particles, and C-reactive protein in metabolic syndrome. Diabetes Care. 2007;30(1):144–146. [DOI] [PubMed] [Google Scholar]
- 77. Brinton EA, Ballantyne CM, Bays HE, Kastelein JJ, Braeckman RA, Soni PN. Effects of icosapent ethyl on lipid and inflammatory parameters in patients with diabetes mellitus-2, residual elevated triglycerides (200-500 mg/dL), and on statin therapy at LDL-C goal: the ANCHOR study. Cardiovasc Diabetol. 2013;12:100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Dai Perrard XY, Lian Z, Bobotas G, Dicklin MR, Maki KC, Wu H. Effects of n-3 fatty acid treatment on monocyte phenotypes in humans with hypertriglyceridemia. J Clin Lipidol. 2017;11(6):1361–1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Tsunoda F, Lamon-Fava S, Asztalos BF, Iyer LK, Richardson K, Schaefer EJ. Effects of oral eicosapentaenoic acid versus docosahexaenoic acid on human peripheral blood mononuclear cell gene expression. Atherosclerosis. 2015;241(2):400–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Mickleborough TD, Tecklenburg SL, Montgomery GS, Lindley MR. Eicosapentaenoic acid is more effective than docosahexaenoic acid in inhibiting proinflammatory mediator production and transcription from LPS-induced human asthmatic alveolar macrophage cells. Clin Nutr. 2009;28(1):71–77. [DOI] [PubMed] [Google Scholar]
- 81. Krämer HJ, Stevens J, Grimminger F, Seeger W. Fish oil fatty acids and human platelets: dose-dependent decrease in dienoic and increase in trienoic thromboxane generation. Biochem Pharmacol. 1996;52(8):1211–1217. [DOI] [PubMed] [Google Scholar]
- 82. Adili R, Hawley M, Holinstat M. Regulation of platelet function and thrombosis by omega-3 and omega-6 polyunsaturated fatty acids. Prostaglandins Other Lipid Mediat. 2018;139:10–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Larson MK, Tormoen GW, Weaver LJ, et al. Exogenous modification of platelet membranes with the omega-3 fatty acids EPA and DHA reduces platelet procoagulant activity and thrombus formation. Am J Physiol Cell Physiol. 2013;304(3):C273–C279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Nieuwenhuys CM, Hornstra G. The effects of purified eicosapentaenoic and docosahexaenoic acids on arterial thrombosis tendency and platelet function in rats. Biochim Biophys Acta. 1998;1390(3):313–322. [DOI] [PubMed] [Google Scholar]
- 85. Adili R, Voigt EM, Bormann JL, et al. In vivo modeling of docosahexaenoic acid and eicosapentaenoic acid-mediated inhibition of both platelet function and accumulation in arterial thrombi. Platelets. 2017:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Wang C, Chung M, Lichtenstein A, et al. Effects of omega-3 fatty acids on cardiovascular disease. Evid Rep Technol Assess (Summ). 2004:1–8. [PMC free article] [PubMed] [Google Scholar]
- 87. Kristensen SD, Iversen AM, Schmidt EB. n-3 polyunsaturated fatty acids and coronary thrombosis. Lipids. 2001;36(Suppl):S79–S82. [DOI] [PubMed] [Google Scholar]
- 88. Gajos G, Rostoff P, Undas A, Piwowarska W. Effects of polyunsaturated omega-3 fatty acids on responsiveness to dual antiplatelet therapy in patients undergoing percutaneous coronary intervention: the OMEGA-PCI (OMEGA-3 fatty acids after pci to modify responsiveness to dual antiplatelet therapy) study. J Am Coll Cardiol. 2010;55(16):1671–1678. [DOI] [PubMed] [Google Scholar]
- 89. Gajos G, Zalewski J, Rostoff P, Nessler J, Piwowarska W, Undas A. Reduced thrombin formation and altered fibrin clot properties induced by polyunsaturated omega-3 fatty acids on top of dual antiplatelet therapy in patients undergoing percutaneous coronary intervention (OMEGA-PCI clot). Arterioscler Thromb Vasc Biol. 2011;31(7):1696–1702. [DOI] [PubMed] [Google Scholar]
- 90. Gajos G, Zalewski J, Mostowik M, Konduracka E, Nessler J, Undas A. Polyunsaturated omega-3 fatty acids reduce lipoprotein-associated phospholipase A(2) in patients with stable angina. Nutr Metab Cardiovasc Dis. 2014;24(4):434–439. [DOI] [PubMed] [Google Scholar]
- 91. De Caterina R, Liao JK, Libby P. Fatty acid modulation of endothelial activation. Am J Clin Nutr. 2000;71(1 Suppl): 213S–223S. [DOI] [PubMed] [Google Scholar]
- 92. Stirban A, Nandrean S, Götting C, et al. Effects of n-3 fatty acids on macro- and microvascular function in subjects with type 2 diabetes mellitus. Am J Clin Nutr. 2010;91(3):808–813. [DOI] [PubMed] [Google Scholar]
- 93. Mori TA, Watts GF, Burke V, Hilme E, Puddey IB, Beilin LJ. Differential effects of eicosapentaenoic acid and docosahexaenoic acid on vascular reactivity of the forearm microcirculation in hyperlipidemic, overweight men. Circulation. 2000;102(11):1264–1269. [DOI] [PubMed] [Google Scholar]
- 94. Haberka M, Mizia-Stec K, Mizia M, et al. N-3 polyunsaturated fatty acids early supplementation improves ultrasound indices of endothelial function, but not through NO inhibitors in patients with acute myocardial infarction: N-3 PUFA supplementation in acute myocardial infarction. Clin Nutr. 2011;30(1):79–85. [DOI] [PubMed] [Google Scholar]
- 95. Schiano V, Laurenzano E, Brevetti G, et al. Omega-3 polyunsaturated fatty acid in peripheral arterial disease: effect on lipid pattern, disease severity, inflammation profile, and endothelial function. Clin Nutr. 2008;27(2):241–247. [DOI] [PubMed] [Google Scholar]
- 96. Robinson JG, Stone NJ. Antiatherosclerotic and antithrombotic effects of omega-3 fatty acids. Am J Cardiol. 2006;98:39i-49i. [DOI] [PubMed] [Google Scholar]
- 97. Kris-Etherton PM, Harris WS, Appel LJ; American Heart Association. Nutrition Committee Fish consumption, fish oil, omega-3 fatty acids, and cardiovascular disease. Circulation. 2002;106(21):2747–2757. [DOI] [PubMed] [Google Scholar]
- 98. Abe Y, El-Masri B, Kimball KT, et al. Soluble cell adhesion molecules in hypertriglyceridemia and potential significance on monocyte adhesion. Arterioscler Thromb Vasc Biol. 1998;18(5):723–731. [DOI] [PubMed] [Google Scholar]
- 99. Kim W, Khan NA, McMurray DN, Prior IA, Wang N, Chapkin RS. Regulatory activity of polyunsaturated fatty acids in T-cell signaling. Prog Lipid Res. 2010;49(3):250–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Dart C. Lipid microdomains and the regulation of ion channel function. J Physiol. 2010;588(Pt 17):3169–3178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Grossfield A, Feller SE, Pitman MC. A role for direct interactions in the modulation of rhodopsin by omega-3 polyunsaturated lipids. Proc Natl Acad Sci U S A. 2006;103(13):4888–4893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Gawrisch K, Soubias O. Structure and dynamics of polyunsaturated hydrocarbon chains in lipid bilayers-significance for GPCR function. Chem Phys Lipids. 2008;153(1):64–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Serhan CN, Clish CB, Brannon J, Colgan SP, Chiang N, Gronert K. Novel functional sets of lipid-derived mediators with antiinflammatory actions generated from omega-3 fatty acids via cyclooxygenase 2-nonsteroidal antiinflammatory drugs and transcellular processing. J Exp Med. 2000;192(8):1197–1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Serhan CN, Petasis NA. Resolvins and protectins in inflammation resolution. Chem Rev. 2011;111(10):5922–5943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Halade GV, Black LM, Verma MK. Paradigm shift - metabolic transformation of docosahexaenoic and eicosapentaenoic acids to bioactives exemplify the promise of fatty acid drug discovery. Biotechnol Adv. 2018;36(4):935–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Bäck M, Yurdagul A Jr, Tabas I, Öörni K, Kovanen PT. Inflammation and its resolution in atherosclerosis: mediators and therapeutic opportunities. Nat Rev Cardiol. 2019;16(7):389–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Wu JHY, Micha R, Mozaffarian D. Dietary fats and cardiometabolic disease: mechanisms and effects on risk factors and outcomes. Nat Rev Cardiol. 2019;16(10):581–601. [DOI] [PubMed] [Google Scholar]
- 108. McMurray DN, Jolly CA, Chapkin RS. Effects of dietary n-3 fatty acids on T cell activation and T cell receptor-mediated signaling in a murine model. J Infect Dis. 2000;182(Suppl 1):S103–S107. [DOI] [PubMed] [Google Scholar]
- 109. Jump DB. N-3 polyunsaturated fatty acid regulation of hepatic gene transcription. Curr Opin Lipidol. 2008;19(3):242–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Adkins Y, Kelley DS. Mechanisms underlying the cardioprotective effects of omega-3 polyunsaturated fatty acids. J Nutr Biochem. 2010;21(9):781–792. [DOI] [PubMed] [Google Scholar]
- 111. Hertz R, Magenheim J, Berman I, Bar-Tana J. Fatty acyl-CoA thioesters are ligands of hepatic nuclear factor-4alpha. Nature. 1998;392(6675):512–516. [DOI] [PubMed] [Google Scholar]
- 112. de Urquiza AM, Liu S, Sjöberg M, et al. Docosahexaenoic acid, a ligand for the retinoid X receptor in mouse brain. Science. 2000;290(5499):2140–2144. [DOI] [PubMed] [Google Scholar]
- 113. Deckelbaum RJ, Worgall TS, Seo T. n-3 fatty acids and gene expression. Am J Clin Nutr. 2006;83(6 Suppl):1520S–1525S. [DOI] [PubMed] [Google Scholar]
- 114. Sheena V, Hertz R, Nousbeck J, Berman I, Magenheim J, Bar-Tana J. Transcriptional regulation of human microsomal triglyceride transfer protein by hepatocyte nuclear factor-4alpha. J Lipid Res. 2005;46(2):328–341. [DOI] [PubMed] [Google Scholar]
- 115. Banga A, Unal R, Tripathi P, et al. Adiponectin translation is increased by the PPARgamma agonists pioglitazone and omega-3 fatty acids. Am J Physiol Endocrinol Metab. 2009;296(3):E480–E489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Itoh M, Suganami T, Satoh N, et al. Increased adiponectin secretion by highly purified eicosapentaenoic acid in rodent models of obesity and human obese subjects. Arterioscler Thromb Vasc Biol. 2007;27(9):1918–1925. [DOI] [PubMed] [Google Scholar]
- 117. Massaro M, Habib A, Lubrano L, et al. The omega-3 fatty acid docosahexaenoate attenuates endothelial cyclooxygenase-2 induction through both NADP(H) oxidase and PKC epsilon inhibition. Proc Natl Acad Sci U S A. 2006;103(41):15184–15189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. GISSI-Prevenzione Investigators. Dietary supplementation with n-3 polyunsaturated fatty acids and vitamin E after myocardial infarction: results of the GISSI-Prevenzione trial. Lancet. 1999;354:447–455. [PubMed] [Google Scholar]
- 119. Tavazzi L, Maggioni AP, Marchioli R, et al. ; Gissi-HF Investigators Effect of n-3 polyunsaturated fatty acids in patients with chronic heart failure (the GISSI-HF trial): a randomised, double-blind, placebo-controlled trial. Lancet. 2008;372(9645):1223–1230. [DOI] [PubMed] [Google Scholar]
- 120. Bowman L, Mafham M, Wallendszus K, et al. Effects of n-3 fatty acid supplements in diabetes mellitus. N Engl J Med. 2018; 379:1540–1550. [DOI] [PubMed] [Google Scholar]
- 121. Manson JE, Cook NR, Lee IM, et al. ; VITAL Research Group Marine n-3 fatty acids and prevention of cardiovascular disease and cancer. N Engl J Med. 2019;380(1):23–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Yokoyama M, Origasa H, Matsuzaki M, et al. ; Japan EPA lipid intervention study (JELIS) Investigators Effects of eicosapentaenoic acid on major coronary events in hypercholesterolaemic patients (JELIS): a randomised open-label, blinded endpoint analysis. Lancet. 2007;369(9567):1090–1098. [DOI] [PubMed] [Google Scholar]
- 123. Bhatt DL, Steg PG, Miller M, et al. ; REDUCE-IT Investigators Cardiovascular risk reduction with icosapent ethyl for hypertriglyceridemia. N Engl J Med. 2019;380(1):11–22. [DOI] [PubMed] [Google Scholar]
- 124. Rauch B, Schiele R, Schneider S, et al. ; OMEGA Study Group OMEGA, a randomized, placebo-controlled trial to test the effect of highly purified omega-3 fatty acids on top of modern guideline-adjusted therapy after myocardial infarction. Circulation. 2010;122(21):2152–2159. [DOI] [PubMed] [Google Scholar]
- 125. Galan P, Kesse-Guyot E, Czernichow S, Briancon S, Blacher J, Hercberg S; SU.FOL.OM3 Collaborative Group Effects of B vitamins and omega 3 fatty acids on cardiovascular diseases: a randomised placebo controlled trial. BMJ. 2010;341:c6273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. ORIGIN Trial Investigators. n-3 fatty acids and cardiovascular outcomes in patients with dysglycemia. N Engl J Med. 2012;367:309–318. [DOI] [PubMed] [Google Scholar]
- 127. ORIGIN Trial Investigators. Cardiovascular and other outcomes postintervention with insulin glargine and omega-3 fatty acids (ORIGINALE). Diabetes Care. 2016;39:709–716. [DOI] [PubMed] [Google Scholar]
- 128. Roncaglioni MC, Tombesi M, Avanzini F, et al. n-3 fatty acids in patients with multiple cardiovascular risk factors. N Engl J Med. 2013;368:1800–1808. [DOI] [PubMed] [Google Scholar]
- 129. Sherratt SCR, Mason RP. Eicosapentaenoic acid and docosahexaenoic acid have distinct membrane locations and lipid interactions as determined by x-ray diffraction. Chem Phys Lipids. 2018;212:73–79. [DOI] [PubMed] [Google Scholar]
- 130. Mason RP, Jacob RF, Shrivastava S, Sherratt SCR, Chattopadhyay A. Eicosapentaenoic acid reduces membrane fluidity, inhibits cholesterol domain formation, and normalizes bilayer width in atherosclerotic-like model membranes. Biochim Biophys Acta. 2016;1858(12):3131–3140. [DOI] [PubMed] [Google Scholar]
- 131. Chang CH, Tseng PT, Chen NY, et al. Safety and tolerability of prescription omega-3 fatty acids: a systematic review and meta-analysis of randomized controlled trials. Prostaglandins Leukot Essent Fatty Acids. 2018;129:1–12. [DOI] [PubMed] [Google Scholar]
- 132. Swann PG, Venton DL, Le Breton GC. Eicosapentaenoic acid and docosahexaenoic acid are antagonists at the thromboxane A2/prostaglandin H2 receptor in human platelets. FEBS Lett. 1989;243(2):244–246. [DOI] [PubMed] [Google Scholar]
- 133. Oikawa S, Yokoyama M, Origasa H, et al. ; JELIS Investigators, Japan Suppressive effect of EPA on the incidence of coronary events in hypercholesterolemia with impaired glucose metabolism: sub-analysis of the Japan EPA lipid intervention study (JELIS). Atherosclerosis. 2009;206(2):535–539. [DOI] [PubMed] [Google Scholar]
- 134. Saito Y, Yokoyama M, Origasa H, et al. ; JELIS Investigators, Japan Effects of EPA on coronary artery disease in hypercholesterolemic patients with multiple risk factors: sub-analysis of primary prevention cases from the Japan EPA lipid intervention study (JELIS). Atherosclerosis. 2008;200(1):135–140. [DOI] [PubMed] [Google Scholar]
- 135. Ohnishi H, Saito Y. Eicosapentaenoic acid (EPA) reduces cardiovascular events: relationship with the EPA/arachidonic acid ratio. J Atheroscler Thromb. 2013;20(12):861–877. [DOI] [PubMed] [Google Scholar]
- 136. Bhatt DL, Steg PG, Miller M, et al. ; REDUCE-IT Investigators Effects of icosapent ethyl on total ischemic events: from REDUCE-IT. J Am Coll Cardiol. 2019;73(22):2791–2802. [DOI] [PubMed] [Google Scholar]
- 137. Handelsman Y, Shapiro MD. Triglycerides, atherosclerosis, and cardiovascular outcome studies: focus on omega-3 fatty acids. Endocr Pract. 2017;23(1):100–112. [DOI] [PubMed] [Google Scholar]
- 138. Bosch J, Gerstein HC, Dagenais GR, et al. n-3 fatty acids and cardiovascular outcomes in patients with dysglycemia. N Engl J Med. 2012;367:309–318. [DOI] [PubMed] [Google Scholar]
- 139. Ballantyne CM, Manku MS, Bays HE, et al. Icosapent ethyl effects on fatty acid profiles in statin-treated patients with high triglycerides: the randomized, placebo-controlled ANCHOR study. Cardiol Ther. 2019;8(1):79–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Nicholls SJ, Lincoff AM, Bash D, et al. Assessment of omega-3 carboxylic acids in statin-treated patients with high levels of triglycerides and low levels of high-density lipoprotein cholesterol: rationale and design of the STRENGTH trial. Clin Cardiol. 2018;41(10):1281–1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Mullen A, Loscher CE, Roche HM. Anti-inflammatory effects of EPA and DHA are dependent upon time and dose-response elements associated with LPS stimulation in THP-1-derived macrophages. J Nutr Biochem. 2010;21(5):444–450. [DOI] [PubMed] [Google Scholar]
- 142. Calder PC. Omega-3 fatty acids and inflammatory processes: from molecules to man. Biochem Soc Trans. 2017;45(5):1105–1115. [DOI] [PubMed] [Google Scholar]
- 143. Weintraub HS. Overview of prescription omega-3 fatty acid products for hypertriglyceridemia. Postgrad Med. 2014;126(7):7–18. [DOI] [PubMed] [Google Scholar]
- 144. Blair HA, Dhillon S. Omega-3 carboxylic acids (Epanova): a review of its use in patients with severe hypertriglyceridemia. Am J Cardiovasc Drugs. 2014;14(5):393–400. [DOI] [PubMed] [Google Scholar]
- 145. Harris WS, Ginsberg HN, Arunakul N, et al. Safety and efficacy of Omacor in severe hypertriglyceridemia. J Cardiovasc Risk. 1997;4(5-6):385–391. [PubMed] [Google Scholar]
- 146. Pownall HJ, Brauchi D, Kilinç C, et al. Correlation of serum triglyceride and its reduction by omega-3 fatty acids with lipid transfer activity and the neutral lipid compositions of high-density and low-density lipoproteins. Atherosclerosis. 1999;143(2):285–297. [DOI] [PubMed] [Google Scholar]
- 147. Maki KC, McKenney JM, Reeves MS, Lubin BC, Dicklin MR. Effects of adding prescription omega-3 acid ethyl esters to simvastatin (20 mg/day) on lipids and lipoprotein particles in men and women with mixed dyslipidemia. Am J Cardiol. 2008;102(4):429–433. [DOI] [PubMed] [Google Scholar]
- 148. Mackness MI, Bhatnagar D, Durrington PN, et al. Effects of a new fish oil concentrate on plasma lipids and lipoproteins in patients with hypertriglyceridaemia. Eur J Clin Nutr. 1994;48(12):859–865. [PubMed] [Google Scholar]
- 149. Durrington PN, Bhatnagar D, Mackness MI, et al. An omega-3 polyunsaturated fatty acid concentrate administered for one year decreased triglycerides in simvastatin treated patients with coronary heart disease and persisting hypertriglyceridaemia. Heart. 2001;85(5):544–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Davidson MH, Stein EA, Bays HE, et al. ; COMBination of prescription Omega-3 with Simvastatin (COMBOS) Investigators Efficacy and tolerability of adding prescription omega-3 fatty acids 4 g/d to simvastatin 40 mg/d in hypertriglyceridemic patients: an 8-week, randomized, double-blind, placebo-controlled study. Clin Ther. 2007;29(7):1354–1367. [DOI] [PubMed] [Google Scholar]
- 151. Bays HE, McKenney J, Maki KC, Doyle RT, Carter RN, Stein E. Effects of prescription omega-3-acid ethyl esters on non–high-density lipoprotein cholesterol when coadministered with escalating doses of atorvastatin. Mayo Clin Proc. 2010;85(2):122–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Skulas-Ray AC, Alaupovic P, Kris-Etherton PM, West SG. Dose-response effects of marine omega-3 fatty acids on apolipoproteins, apolipoprotein-defined lipoprotein subclasses, and Lp-PLA2 in individuals with moderate hypertriglyceridemia. J Clin Lipidol. 2015;9(3):360–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Oh PC, Koh KK, Sakuma I, et al. Omega-3 fatty acid therapy dose-dependently and significantly decreased triglycerides and improved flow-mediated dilation, however, did not significantly improve insulin sensitivity in patients with hypertriglyceridemia. Int J Cardiol. 2014;176(3):696–702. [DOI] [PubMed] [Google Scholar]
- 154. Paranandi A, Asztalos BF, Mangili A, et al. Short communication: effects of omega-3 fatty acids on triglycerides and high-density lipoprotein subprofiles in HIV-infected persons with hypertriglyceridemia. AIDS Res Hum Retroviruses. 2014;30(8):800–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Maki KC, Orloff DG, Nicholls SJ, et al. A highly bioavailable omega-3 free fatty acid formulation improves the cardiovascular risk profile in high-risk, statin-treated patients with residual hypertriglyceridemia (the ESPRIT trial). Clin Ther. 2013;35(9):1400–11.e1. [DOI] [PubMed] [Google Scholar]
- 156. Xiao YF, Ke Q, Chen Y, Morgan JP, Leaf A. Inhibitory effect of n-3 fish oil fatty acids on cardiac Na+/Ca2+ exchange currents in HEK293t cells. Biochem Biophys Res Commun. 2004;321(1):116–123. [DOI] [PubMed] [Google Scholar]
- 157. Li GR, Sun HY, Zhang XH, et al. Omega-3 polyunsaturated fatty acids inhibit transient outward and ultra-rapid delayed rectifier K+currents and Na+current in human atrial myocytes. Cardiovasc Res. 2009;81(2):286–293. [DOI] [PubMed] [Google Scholar]
- 158. Leifert WR, McMurchie EJ, Saint DA. Inhibition of cardiac sodium currents in adult rat myocytes by n-3 polyunsaturated fatty acids. J Physiol. 1999;520 Pt 3:671–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. London B, Albert C, Anderson ME, et al. Omega-3 fatty acids and cardiac arrhythmias: prior studies and recommendations for future research: a report from the National Heart, Lung, and Blood Institute and Office Of Dietary Supplements Omega-3 Fatty Acids and their Role in Cardiac Arrhythmogenesis Workshop. Circulation. 2007;116(10):e320–e335. [DOI] [PubMed] [Google Scholar]
- 160. Kowey PR, Reiffel JA, Ellenbogen KA, Naccarelli GV, Pratt CM. Efficacy and safety of prescription omega-3 fatty acids for the prevention of recurrent symptomatic atrial fibrillation: a randomized controlled trial. JAMA. 2010;304(21):2363–2372. [DOI] [PubMed] [Google Scholar]
- 161. Frost L, Vestergaard P. n-3 Fatty acids consumed from fish and risk of atrial fibrillation or flutter: the Danish diet, cancer, and health study. Am J Clin Nutr. 2005;81(1):50–54. [DOI] [PubMed] [Google Scholar]
- 162. Reis GJ, Boucher TM, Sipperly ME, et al. Randomised trial of fish oil for prevention of restenosis after coronary angioplasty. Lancet. 1989;2(8656):177–181. [DOI] [PubMed] [Google Scholar]
- 163. Eritsland J, Arnesen H, Seljeflot I, Kierulf P. Long-term effects of n-3 polyunsaturated fatty acids on haemostatic variables and bleeding episodes in patients with coronary artery disease. Blood Coagul Fibrinolysis. 1995;6(1):17–22. [DOI] [PubMed] [Google Scholar]
- 164. Mozaffarian D, Marchioli R, Gardner T, et al. The ω-3 fatty acids for prevention of post-operative atrial fibrillation trial–rationale and design. Am Heart J. 2011;162(1):56–63.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Jenkins DJ, Wong JM, Kendall CW, et al. Effect of a 6-month vegan low-carbohydrate (‘Eco-Atkins’) diet on cardiovascular risk factors and body weight in hyperlipidaemic adults: a randomised controlled trial. BMJ Open. 2014;4(2):e003505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Retterstøl K, Svendsen M, Narverud I, Holven KB. Effect of low carbohydrate high fat diet on LDL cholesterol and gene expression in normal-weight, young adults: a randomized controlled study. Atherosclerosis. 2018;279:52–61. [DOI] [PubMed] [Google Scholar]