Berron et al. show that functional connectivity between the medial temporal lobe and the anterior-temporal system is reduced in the earliest stages of Alzheimer’s disease, before connectivity with the posterior-medial system is affected. The reduced functional connectivity is associated with impaired memory performance.
Keywords: functional connectivity, PMAT systems, medial temporal lobe, Alzheimer’s disease, fluid biomarkers
Abstract
Human episodic memory critically depends on subregions of the medial temporal lobe, which are part of functional brain systems such as the anterior-temporal and the posterior-medial system. Here we analysed how Alzheimer’s pathology affects functional connectivity within these systems. Data from 256 amyloid-β-negative cognitively unimpaired, 103 amyloid-β-positive cognitively unimpaired, and 83 amyloid-β-positive individuals with mild cognitive impairment were analysed. Amyloid-β and tau pathology were measured using the CSF amyloid-β42/40 ratio and phosphorylated tau, respectively. We found that amyloid-β-positive cognitively unimpaired individuals were mainly characterized by decreased functional connectivity between the medial temporal lobe and regions in the anterior-temporal system, most prominently between left perirhinal/entorhinal cortices and medial prefrontal cortex. Furthermore, correlation analysis in this group revealed decreasing functional connectivity between bilateral perirhinal/entorhinal cortices, anterior hippocampus and posterior-medial regions with increasing levels of phosphorylated tau. The amyloid-β-positive individuals with mild cognitive impairment mostly exhibited reduced connectivity between the medial temporal lobe and posterior-medial regions, predominantly between the anterior hippocampus and posterior cingulate cortex. In addition, they showed hyperconnectivity within the medial temporal lobe and its immediate proximity. Lower medial temporal-cortical functional connectivity networks resulting from the group comparisons of cognitively unimpaired individuals were associated with reduced memory performance and more rapid longitudinal memory decline as shown by linear mixed-effects regression analysis. Finally, we found that reduced medial temporal-cortical connectivity in mildly cognitively impaired individuals was related to reduced entorhinal thickness and white matter integrity of the parahippocampal cingulum and the fornix. No such relationships were found in cognitively unimpaired individuals. In conclusion, our findings show that the earliest changes in preclinical Alzheimer’s disease might involve decreased connectivity within the anterior-temporal system, and early changes in connectivity might be related to memory impairment, but not to structural changes. With disease progression and increased tau pathology, medial temporal functional connectivity with posterior-medial regions seems to be increasingly impaired. In individuals with mild cognitive impairment, reduced functional connectivity is associated with structural brain changes as well as the emergence of locally increased connectivity patterns. Thus, functional connectivity between the medial temporal lobe and the anterior-temporal and posterior-medial system could serve as stage-specific functional markers in early Alzheimer’s disease.
Introduction
The human medial temporal lobe (MTL) is critically involved in episodic memory (Eichenbaum et al., 2007). At the same time, the MTL is vulnerable to accumulation of Alzheimer’s disease pathology (Braak and Braak, 1997; Schöll et al., 2016). However, the MTL is an inhomogeneous brain structure which consists of several subregions including the hippocampus and substructures along the parahippocampal gyrus. While the anterior parahippocampal gyrus comprises the perirhinal and entorhinal cortices, the posterior section comprises the parahippocampal cortex (Insausti et al., 1998; Ding and Van Hoesen, 2010). Similarly, the hippocampus can be separated in anterior and posterior sections (Poppenk et al., 2013; Strange et al., 2014). MTL subregions are densely connected with each other and with other cortical and subcortical brain regions (Burwell, 2000; van Strien et al., 2009). Neuroimaging studies of spontaneous fluctuations in blood oxygen level-dependent (BOLD) signal measured by resting-state functional MRI have consistently found that the MTL forms a crucial subsystem in the default mode network (DMN), linking it with regions such as the precuneus, posterior cingulate cortex, lateral inferior parietal cortex and medial prefrontal cortex (Raichle, 2013; Ward et al., 2014; Buckner and Di Nicola, 2019). Importantly, the anterior and posterior MTL segments are differentially involved in functional brain systems (Ranganath and Ritchey, 2012). While the perirhinal cortex is part of the anterior-temporal (AT) system including the temporal pole, orbitofrontal cortex, medial prefrontal cortex and the amygdala, the parahippocampal cortex is part of the posterior-medial system (PM) including the retrosplenial cortex, posterior cingulate cortex and precuneus (Kahn et al., 2008; Libby et al., 2012). Both systems are also differentially engaged in cognitive processes. While the AT system is critically involved in object memory, the PM system plays a key role in scene and spatial memory (Ranganath and Ritchey, 2012; Berron et al., 2018). Similarly, anterior and posterior sections of the hippocampus seem to be differentially connected to PM and AT regions and involved in different cognitive functions (Poppenk et al., 2013; Strange et al., 2014; Collin et al., 2015; Brunec et al., 2018). However, to achieve successful memory encoding and retrieval, these cortical systems need to interact with each other and the hippocampus (Collins and Dickerson, 2019; Cooper and Ritchey, 2019).
To understand alterations in subregional MTL-cortical connectivity in the early stages of Alzheimer’s disease, more anatomically detailed approaches are necessary. Recently, studies have focused on changes in anterior and posterior MTL subregional networks (Das et al., 2015; Salami et al., 2016) and showed that both MTL-cortical networks undergo changes with higher age and in subjects with mild cognitive impairment (MCI) and are related to concurrent cognition as well as future cognitive decline. However, they did not focus on early (preclinical) Alzheimer’s disease and did not leverage biomarker information. Thus, it remains unclear which effects in cognitively unimpaired individuals can be attributed to Alzheimer’s disease pathology instead of other ageing related processes.
To address this caveat in the literature, we characterized detailed alterations in MTL-cortical connectivity networks in 442 non-demented participants from the Swedish BioFINDER study. We compared cognitively unimpaired individuals without amyloid-β pathology to amyloid-β+ cognitively unimpaired cases and amyloid-β+ cases with MCI. In addition, we investigated how increasing levels of tau pathology in CSF affect MTL-cortical connectivity networks. To assess the clinical relevance of the observed functional alterations, we related them to measures of baseline cognition and cognitive changes over 8 years. Changes in interregional functional connectivity can result from structural alterations in white matter tracts or grey matter structures but could in principle also precede and possibly be independent of axonal damage (Mollink et al., 2019). Consequently, we related our functional connectivity findings to structural connectivity measures of the main fibre tracts connecting the MTL with the respective cortical systems, i.e. the parahippocampal cingulum, the fornix and the uncinate fasciculus as well as thickness of the entorhinal cortex. Our findings further our understanding of the mechanisms underlying cognitive decline in early stages of Alzheimer’s disease by highlighting differential effects of amyloid-β pathology on critical memory systems and suggest stage-specific functional connectivity as marker for disease progression.
Materials and methods
Participants
We analysed data from 442 individuals from the Swedish BioFINDER study, which was approved by the ethical review board in Lund, Sweden. Participants were enrolled between 2010 and 2015, and gave their written informed consent to participate in the study. Only non-demented individuals with CSF and resting-state functional MRI data were selected for this study. For further study details, see http://biofinder.se and Janelidze et al. (2016, 2017b). Briefly, healthy elderly volunteers were recruited using the following inclusion criteria: (i) absence of memory complaints or any other cognitive symptoms; (ii) preservation of general cognitive functioning; and (iii) no active neurological or psychiatric disease. Participants with subjective or mild cognitive complaints were enrolled consecutively at three memory outpatient clinics in Sweden. The patients were referred for assessment of their cognitive complaints, and thoroughly assessed by physicians with a special interest in dementia disorders. The inclusion criteria were: (i) cognitive symptoms; (ii) not fulfilling the criteria for dementia; (iii) a Mini-Mental State Examination score of 24–30 points; (iv) age 60–80 years; and (v) fluent in Swedish. The exclusion criteria were: (i) cognitive impairment that without doubt could be explained by another condition (other than prodromal dementias); (ii) severe somatic disease; and (iii) refusing lumbar puncture or neuropsychological investigation. A senior neuropsychologist then stratified all patients into those with subjective cognitive decline (i.e. no measurable cognitive deficits) or MCI according to the consensus criteria suggested by (Petersen, 2004). This resulted in a cohort consisting of cognitively unimpaired elderly subjects [n = 359, of whom 138 experienced subjective cognitive decline in line with the recent NIA-AA research framework (Jack et al., 2018)] and patients with MCI (n = 83).
CSF biomarkers
Amyloid-β status was determined using the Elecsys CSF amyloid-β42/amyloid-β40 ratio, which has been validated against amyloid PET status with high agreement (>90%) (Janelidze et al., 2016, 2017a). An unbiased cut-off of <0.059 was used to define amyloid-β positivity based on mixture modelling statistics, which has proven to provide robust and accurate thresholds (Bertens et al., 2017; Palmqvist et al., 2017). We also used Elecsys CSF p-tau levels to estimate individual tau pathology without applying any explicit threshold.
Group classifications
Participants were categorized into different groups according to their CSF amyloid-β42/40 status and their clinical presentation, which resulted in the following groups: 256 amyloid-β− cognitively unimpaired [mean age = 72.2 (standard deviation, SD = 5.6), 163 female], 103 amyloid-β+ cognitively unimpaired [mean age = 72.4 (SD = 5.4), 54 female] and 83 amyloid-β+ patients with MCI [mean age = 72.2 (SD = 4.8), 43 female]. See Table 1 for demographics of the individual subgroups.
Table 1.
Cohort demographics
| Amyloid-β− CU | Amyloid-β+ CU | Amyloid-β+ MCI | |
|---|---|---|---|
| n | 256 | 103 | 83 |
| Age, years | 72.2 ± 5.6 | 72.4 ± 5.4 | 72.2 ± 4.8 |
| Sex, % female | 64% | 52% | 52% |
| MMSE | 28.9 ± 1.1 | 28.7 ± 1.4 | 26.7 ± 1.7 |
| ADAS-cog immediate recall | 2.6 ± 1.3 | 3.2 ± 1.4 | 5.4 ± 1.3 |
| ADAS-cog delayed recall | 2.3 ± 2 | 3.1 ± 2.2 | 7.6 ± 1.7 |
| Education in years | 12.5 ± 3.2 | 12.2 ± 3.8 | 11.5 ± 3.3 |
| CSF p-tau | 17.5 ± 5.1 | 28.52 ± 11.9 | 32.7 ± 13.5 |
| CSF amyloid-β42/40 | 91.4 ± 16.1 | 42.1 ± 9.1 | 39.6 ± 9.5 |
| DTI data, availability in % | 76.2 | 66 | 72.3 |
Data are presented as mean values ± SD unless otherwise stated. ADAS-cog immediate and delayed recall are presented in number of errors while MMSE is presented in points. ADAS-cog = Alzheimer’s disease assessment scale – Cognitive subscale; CU = cognitively unimpaired; DTI = diffusion tensor imaging; MMSE = Mini-Mental State Examination; n = number of participants; p-tau = phosphorylated tau.
Imaging data acquisition
MRI was performed on a 3 T Siemens Tim Trio scanner (Siemens Medical Solutions) (Hahn et al., 2019). The 3D T1-weighted volume used for segmentation and normalization was acquired using an MPRAGE sequence (in-plane resolution = 1 × 1 mm2, slice thickness = 1.2 mm, repetition time = 1950 ms, echo time = 3.37 ms, flip-angle = 9°). Spontaneous BOLD oscillations were acquired with a gradient-echo planar sequence (eyes closed, in-plane resolution = 3 × 3 mm2, slice thickness = 3 mm, repetition time = 2000 ms, echo time = 30 ms, flip-angle = 90°, 180 dynamic scans, 6 min).
Diffusion weighted images (DWI) were acquired using a single-shot echo planar imaging (EPI) sequence (repetition time = 8200 ms, echo time = 86 ms) with 64 diffusion encoding directions using b-values of 0 and 1000 s/mm2. In total, 60 contiguous axial slices with a spatial resolution of 2 × 2 × 2 mm3 were acquired.
To derive cortical regions of interest from an independent dataset, MRI data acquired on a Siemens Prisma scanner were used. To that end, the 3D T1-weighted volume used for segmentation and normalization was acquired using an MPRAGE sequence (in-plane resolution = 1 × 1 mm2, slice thickness = 1 mm, repetition time = 1900 ms, echo time = 2.54 ms, flip-angle = 9°) and spontaneous BOLD oscillations were acquired with a gradient-echo planar sequence (eyes closed, in-plane resolution = 3 × 3 mm2, slice thickness = 3.6 mm, repetition time = 1020 ms, echo time = 30 ms, flip-angle = 63°, 462 dynamic scans, 7.85 min).
Medial temporal lobe region of interest definition
Parahippocampal and hippocampal regions of interest in the MTL were first defined based on the Harvard-Oxford subcortical atlas in both hemispheres in MNI space (Desikan et al., 2006). Afterwards, hippocampal and parahippocampal masks were manually adjusted and regions of interest were separated in anterior and posterior sections. The hippocampus was separated at the uncal apex (Poppenk et al., 2013). The parahippocampal gyrus was separated so that the anterior section contained perirhinal/entorhinal cortices while the posterior section contained parahippocampal cortex (Berron et al., 2017).
Resting state functional MRI preprocessing
Resting state functional MRI data preprocessing was performed with a pipeline composed of FSL (Jenkinson et al., 2012), AFNI (Cox, 1996) and ANTS (Avants et al., 2014). Anatomical processing involved skull stripping, segmentation of CSF, white and grey matter, and normalization to MNI152 space (Grabner et al., 2006). After bulk motion and slice timing correction, nuisance regression compensated white matter/CSF signal, physiological noise (Behzadi et al., 2007), motion parameters (Johnstone et al., 2006), and scanner drift. Finally, the functional data were band-pass filtered (0.01–0.1 Hz) and transformed to MNI space. Frames causing outliers in total frame-to-frame signal variation (75 percentile + 1.5 × interquartile range) were censored (Power et al., 2012). Subjects with a mean/maximum frame-wise displacement exceeding 0.7/3.0 mm were excluded.
The processed functional MRI data were resampled to 6 × 6 × 6 mm3 and masked with grey matter derived from a cortical resting-state network atlas (Yeo et al., 2011), Harvard-Oxford subcortical atlas (Desikan et al., 2006) for the basal ganglia and our manually defined MTL regions. The variance stabilized Fisher z-transformed Pearson correlation between the resulting grey matter BOLD voxel time series yielded our functional connectivity measure.
Characterization of medial temporal lobe-neocortical connectivity in amyloid-β− cognitively unimpaired controls
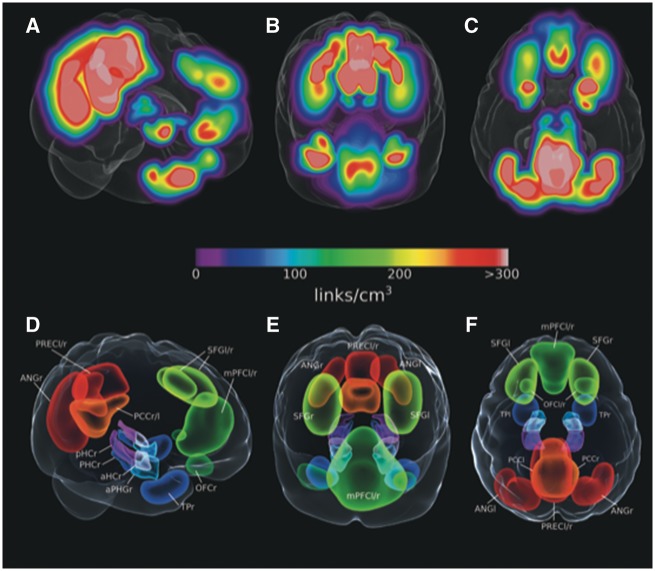
To identify cortical brain regions with a strong functional relationship to the MTL regions of interest, we used an independent sample of 155 cognitively unimpaired amyloid-β− individuals [mean age = 66 (SD = 10.3), 85 female]. First, we applied a threshold to all links converging on the MTL corresponding to correlations with P < 0.01. From this connectivity graph we calculated the localized link densities in 1 × 1 × 1 mm3 space by convoluting each 6 × 6 × 6 mm3 voxels’ strength (sum of weighted links, i.e. BOLD signal correlations, converging on it) with a 3D Gaussian kernel of full-width at half-maximum (FWHM) equal to the average voxel neighbour distance. The link density volume (Fig. 1A–C) was then thresholded. Using these link density measures we identified clusters with high density that were manually delineated with the aim to derive cortical regions of interest of similar size that represent the anterior-temporal and posterior-medial system, which have been characterized using functional connectivity approaches in earlier studies (Libby et al., 2012; Schröder et al., 2015). To that end, a big cluster spanning across posterior cingulate cortex/precuneus was cut in two smaller clusters while parts of the angular gyrus cluster that were extending towards the posterior temporal lobes were cut (Fig. 1). The resulting regions of interest were subsequently incorporated in 6 × 6 × 6 mm3 space for use in all analyses (Fig. 1D–F). As the primary focus of this work is MTL connectivity, we restricted our visualizations to links within and converging on the MTL, even though connectivity between all regions were used when calculating network components (see Supplementary Figs 5 and 6 for the full connectograms).
Figure 1.
MTL-whole brain link densities in amyloid-β− cognitively unimpaired participants and derived regions of interest from MTL-to-whole brain link densities in cognitively unimpaired participants from an additional independent dataset. (A–C) MTL-to-whole brain density maps were calculated for a separate sample of cognitively unimpaired amyloid-β− participants. (D–F) Cortical regions of interest were selected and delineated around coordinates with highest connectivity with the MTL. aHC = anterior hippocampus; ANG = angular gyrus; aPHG = anterior parahippocampal gyrus including entorhinal and perirhinal cortices; l = left; mPFC = medial prefrontal cortex; OFC = orbitofrontal cortex; PCC = posterior cingulate cortex; PHC = parahippocampal cortex; pHC = posterior hippocampus; PREC = precuneus; r = right; SFG = superior frontal gyrus; TP = temporal pole.
Functional connectivity statistical analysis
Note that the regions of interest are only used to assign labels to the corresponding group of voxels. The network component analysis was performed using voxel-wise connectivity maps, which were entered into a group analysis gauging differences in amyloid-β+ MCI and amyloid-β+ cognitively unimpaired relative to amyloid-β− cognitively unimpaired using a code similar to the NBS algorithm (Zalesky et al., 2010) (Supplementary material). Notably, these cortical regions of interest were derived from a fully independent and separate dataset. Briefly, given control and pathological groups, we calculated the largest network component C (defined as a connected set of links), for which tij > t0, where tij is the t-statistic for the link wij (BOLD signal correlation) between voxel i and j, and t0 controls the component size and significance level of constituent links (set to match P < 0.001 given group sizes). The calculation of all the links’ t-values was done in a General Linear model framework modelling connectivity as a function of groups and regressing out age and sex. The component size (number of links) was compared to a permutation-generated null distribution of sizes, thus controlling for the family-wise error rate in the weak sense at α = 0.05. Applying a two-sided suprathreshold (|tij| > t0) simultaneously capturing both hyper- and hypoconnectivity, is crucial in isolating the full progression patterns across groups.
In addition to the group difference analysis, we sought connected network components correlating with CSF levels. Instead of thresholding link t-values for two groups, we applied a suprathreshold rij > r0 to Spearman correlations of all wij with metadata variables (Supplementary material). The result of the algorithm is a component on which connectivity correlates significantly higher than for randomized sets of subject-metadata pairs (at α = 0.05). We selected the initial link threshold corresponding to P < 0.001 and controlled for age and sex via partial correlation.
We also gauged the BOLD series’ information content using an entropy measure for discrete time series called sample entropy (Richman and Moorman, 2000) (setting embedding dimension m = 2 and the range in SD from all time series). This information theoretical entropy-based measure should be higher for time series with lower predictability and random disorder, and conversely reduced for more ordered and predictable time series. After calculating the whole brain entropy for each individual using the processed 6 × 6 × 6 mm3 resting-state functional MRI voxels, we looked for significant voxel-wise group differences in a permutation-based threshold free clustering calculation using FSLs randomize (Winkler et al., 2014). Results are displayed using 3D visualizations of significant network components of altered connectivity and summarizing connectograms. While the 3D visualizations show binary sets of links of the component converging on the MTL, the connectograms reveal the total number of links between voxels constituting the respective region of interest. For the analyses of the relationship between functional connectivity and cognition, white matter tract integrity as well as entorhinal thickness, we have extracted the sum of the functional connectivity (Fisher z-transformed person correlation between BOLD time series) on the parts of the significant network components involving regions of the PM/AT system respectively. Supplementary Fig. 1 summarizes our analysis approach.
Tractography
DTI data were available in ∼70% of subjects in each group (Table 1). Tractography using deterministic tracking based on constrained spherical deconvolution was performed to generate the left and right parahippocampal cingulum, the fornix as well as the left and right uncinate fasciculus (Tournier et al., 2008; Jones et al., 2013). See the Supplementary material for further method description, details on seed regions and example visualizations of the respective tracts (Supplementary Fig. 7).
Entorhinal thickness
Entorhinal thickness data were derived from individual T1 scans (Mattsson et al., 2019) using Freesurfer (version 6.0; http://surfer.nmr.mgh.harvard.edu).
Cognitive measures and longitudinal analyses
We used measures representing episodic memory [immediate/delayed word list recall tests from the Alzheimer’s Disease Assessment Scale–Cognitive subscale (ADAS-cog), measured on scales from 0 to 10, with 0 being least impaired]. Longitudinal cognitive measures were modelled using linear mixed-effects regression with a random intercept and slope using the lme4 package in R v3.3.2 (www.r-project.org). We had cognitive follow-up data for all participants for up to 8 years, and in order to investigate the relationship of baseline functional connectivity estimates and future cognitive decline, the models included the interaction between baseline functional connectivity and time. Multiple regression analyses were carried out between baseline measures of structural tract integrity (functional anisotropy) and baseline functional connectivity estimates to investigate whether alterations in structural tract integrity were associated with baseline functional connectivity. All models were adjusted for sex and age, models including cognitive measures were also adjusted for years of education. Results were corrected for multiple comparisons using Bonferroni-Holm correction where appropriate.
Data availability
Anonymized data will be shared by request from a qualified academic investigator for the sole purpose of replicating procedures and results presented in the article and as long as data transfer is in agreement with EU legislation on the general data protection regulation and decisions by the Ethical Review Board of Sweden and Region Skåne.
Results
Anterior and posterior medial temporal lobe connectivity networks in ageing
Using the above described method of thresholding the average cognitively unimpaired amyloid-β− connectivity converging on the MTL, we identified cortical regions with the highest functional connectivity with MTL subregions (see Fig. 1A–C for the resulting link density). Note that this has been done in an independent dataset that was not used for the following group comparisons. The resulting regions of interest included bilateral masks in orbitofrontal cortex, superior frontal gyrus, medial prefrontal cortex, temporal polar cortex, posterior cingulate cortex, precuneus, angular gyrus, lateral occipital cortex and the thalamus (Fig. 1D–F), which represent important nodes of the PM and AT system. While the parahippocampal, posterior cingulate and lateral occipital cortices as well as the precuneus and thalamus represent critical nodes within the PM system, perirhinal/entorhinal cortices, temporal polar, orbitofrontal and medial prefrontal cortices have been associated with the AT system (Libby et al., 2012; Schröder et al., 2015).
Medial temporal lobe-anterior temporal connectivity is reduced in amyloid-β+ cognitively unimpaired individuals
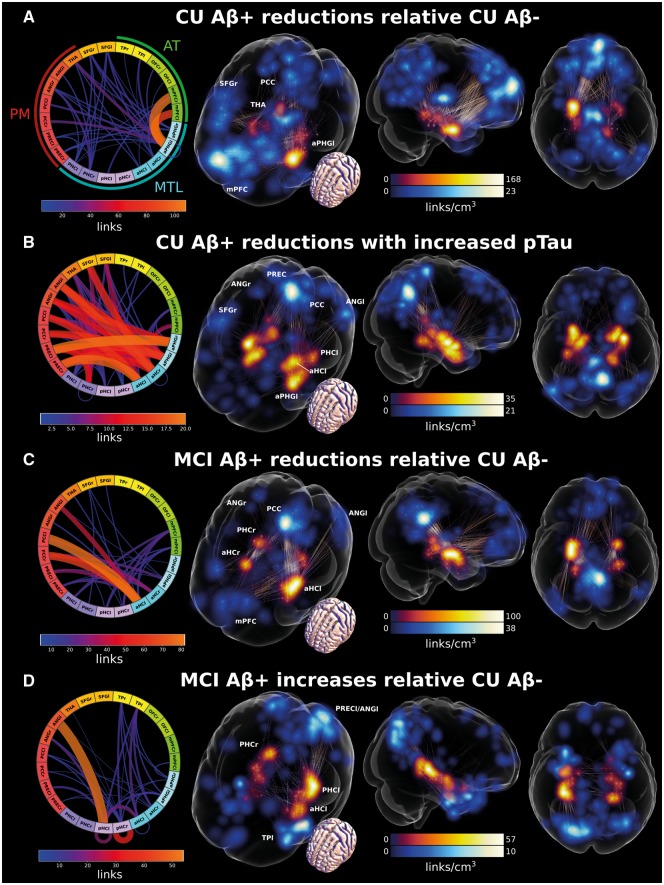
We first investigated group differences between the amyloid-β− and amyloid-β+ cognitively unimpaired connectivity of the MTL regions of interest and its functionally connected cortical regions. The amyloid-β+ cognitively unimpaired group had significantly reduced connectivity mostly between subregions of the anterior MTL and regions of interest in the AT system (Fig. 2A, see Supplementary material for 3D visualizations). In addition, there was some reduced connectivity between the anterior MTL and the posterior cingulate cortex as well as the thalamus. Interestingly, the strongest difference between the groups was characterized by reduced connectivity between the left perirhinal/entorhinal cortex and the bilateral medial prefrontal cortex (see Fig. 2A connectogram).
Figure 2.
Changes in MTL-cortical functional connectivity. (A) Reduced MTL-cortical connectivity in amyloid-β+ compared to amyloid-β− cognitively unimpaired (CU) participants. (B) Decreased MTL-cortical connectivity with increasing levels of CSF p-tau in amyloid-β+ cognitively unimpaired individuals. (C) Reduced MTL-cortical connectivity in amyloid-β+ MCI patients compared to amyloid-β− cognitively unimpaired participants. (D) Increased MTL-cortical connectivity in amyloid-β+ MCI compared to amyloid-β− cognitively unimpaired participants. Significant effects are visualized as set of binary links. The end-point transparency and maximal intensity projection colour fields are proportional to the number of links converging on the voxel location. End-points in MTL regions of interest are displayed in red while end-points in cortical regions of interest are displayed in blue. The connectograms give an overview of the 3D plots, where line width and colour are proportional to the number of links between voxels constituting the respective regions of interest. Please see Supplementary Videos 1–4 for 3D visualizations. aHC = anterior hippocampus; ANG = angular gyrus; aPHG = anterior parahippocampal gyrus including entorhinal and perirhinal cortices; l = left; mPFC = medial prefrontal cortex; OFC = orbitofrontal cortex; PCC = posterior cingulate cortex; PHC = parahippocampal cortex; pHC = posterior hippocampus; PREC = precuneus; r = right; SFG = superior frontal gyrus; TP = temporal pole.
Relationship of CSF p-tau levels and altered MTL-neocortical connectivity in amyloid-β+ cognitively unimpaired individuals
With disease progression, there is increasing accumulation of neurofibrillary tangle (NFT) pathology in the MTL (Braak and Braak, 1991). In early disease stages, tau accumulation is most pronounced in anterior sections of the parahippocampal gyrus containing the perirhinal and entorhinal cortices. In later stages, increased accumulation can also be observed in the hippocampus (Braak and Braak, 1991; Braak et al., 2006). Thus, we were interested which MTL-neocortical connections would be altered with increasing levels of CSF p-tau (Mattsson et al., 2017). We found that increasing levels of p-tau correlated with reduced connectivity on a network component dominated by decreasing connectivity between the bilateral MTL and PM regions (Fig. 2B). In line with the results from the group comparisons, we found that the strongest effects were found in perirhinal/entorhinal cortices and the anterior hippocampus. In addition, the anterior hippocampus showed decreased connectivity with superior frontal gyrus (see Fig. 2C connectogram). No significant network component was found for increased connectivity with increased p-tau.
Medial temporal lobe-posterior medial connectivity is predominantly reduced in amyloid-β+ patients with mild cognitive impairment
Next, we examined group differences in MTL-neocortical functional connectivity between amyloid-β+ MCI patients and amyloid-β− cognitively unimpaired subjects. In contrast to the amyloid-β+ cognitively unimpaired results, amyloid-β+ MCI patients showed strong reductions in functional connectivity between regions in the anterior MTL and the PM system (Fig. 2C). The strongest difference could be observed between the anterior hippocampus and the bilateral posterior cingulate as well as the left lateral occipital cortices (see Fig. 2B, connectogram). In addition, there were significantly reduced connections between the MTL and the medial prefrontal cortex. Similar to the amyloid-β+ cognitively unimpaired group, the results in this group contrast appear to be left lateralized.
Amyloid-β+ MCI subjects show increased connectivity within hippocampus and between the hippocampus and angular gyrus
Earlier reports have not only shown decreased but also increased functional connectivity in ageing and neurodegenerative diseases (Das et al., 2013; Salami et al., 2014, 2016; Pasquini et al., 2015; Harrison et al., 2019). While we found almost no increased connectivity in the amyloid-β+ cognitively unimpaired group, the network component of significantly altered connectivity in the amyloid-β+ MCI group was clearly characterized by increased functional connectivity within the hippocampus, especially within the bilateral posterior hippocampi, and some increase in connectivity between left and right hippocampus. We also found increased functional connectivity between the left posterior hippocampus and the angular gyrus, and additionally between hippocampus, temporo-polar and perirhinal/entorhinal cortices (Fig. 2D).
To elucidate the nature of the increased connectivity in MCI patients, we investigated entropy as a measure of BOLD time series predictability (Yao et al., 2013). To that end, we computed the entropy-based information theoretical measure (SampEn) in amyloid-β− cognitively unimpaired and amyloid-β+ MCI patients. As not all MCI patients were characterized by strongly increased connectivity, we used a median split to identify the half of the MCI sample with the most pronounced increase. To evaluate the spatial correspondence between resulting functional connectivity and entropy differences (i.e. the reduced predictability of the BOLD time series gauged by the entropy-based measure of information), we calculated the functional connectivity contrast between amyloid-β− cognitively unimpaired and the amyloid-β+ MCI subset with the strongest connectivity increase. The results clearly show spatial correspondence between the two measures indicating that increased functional connectivity appears to be characterized by less regular and unpredictable BOLD time series (i.e. increased entropy) (see overlap of significant P < 0.001 entropy isosurface and network component link density in Fig. 3 and compare with Fig. 2D for the full MCI amyloid-β+ group).
Figure 3.
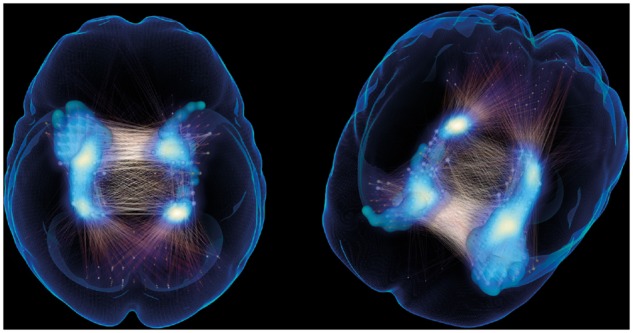
Reduced functional entropy overlaps with increased functional connectivity in a subset of amyloid-β+ MCI patients relative to amyloid-β+ cognitively unimpaired subjects. The isosurface corresponds to significantly increased entropy (calculated in 6 × 6 × 6 mm3 voxel space and convoluted with Gaussian of 6-mm FWHM to generate the visualized surface). The blue maximal intensity projection is proportional to the link density of the MCI amyloid-β+ versus cognitively unimpaired amyloid-β− functional connectivity contrast visualized.
Analyses including a summary statistic for the anatomical alignment between the individual and group masks show that our results are not affected by anatomical alignment quality (Supplementary Fig. 3). Likewise, analyses including global temporal signal-to-noise and mean fractional displacement as covariates suggest that these factors are not driving the results (Supplementary Fig. 4).
Relationship between functional connectivity group contrasts and cognitive performance
To assess the relevance of alterations in MTL-cortical functional connectivity to cognition, we tested the relationship between individual levels of baseline connectivity and baseline memory as well as memory decline within the following 8 years.
Relationship between baseline functional connectivity and baseline cognition
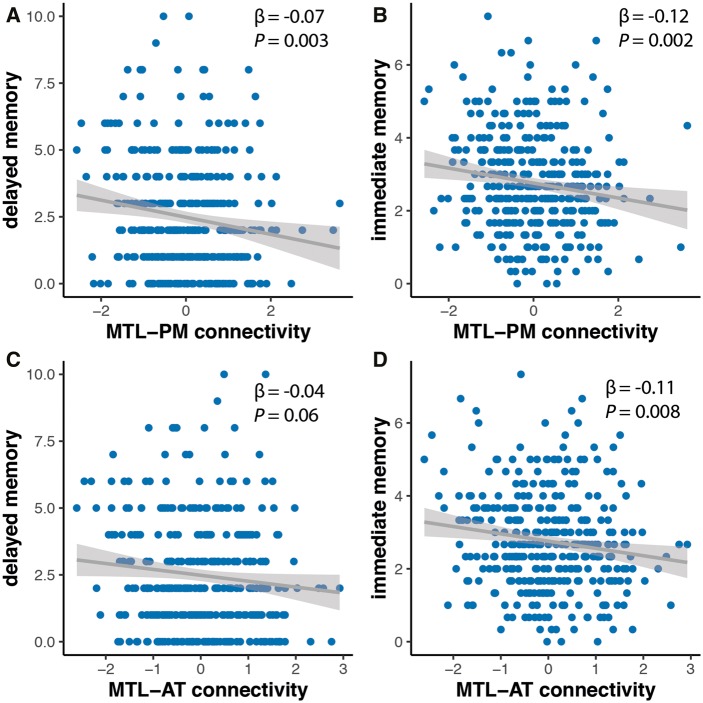
In the cognitively unimpaired groups (amyloid-β+ and amyloid-β−), we found that performance in the delayed memory task was associated with MTL-PM connectivity [MTL-PM: β = −0.07, standard error (SE) = 0.03, P = 0.003; MTL-AT: β = −0.04, SE = 0.03, P = 0.06] (Fig. 4A and C). This indicates that participants with lower levels of MTL-PM connectivity did perform worse in the delayed memory test. On the other hand, performance in the immediate memory task was related to both MTL-PM and MTL-AT connectivity (MTL-AT: β = −0.11, SE = 0.04, P = 0.008; MTL-PM: β = −0.12, SE = 0.04, P = 0.002) (Fig. 4B and D). All models remain significant when we include individual CSF amyloid-β42/40 levels.
Figure 4.
Relationship of baseline memory scores and MTL-cortical connectivity estimates. Delayed and immediate recall were associated with MTL-PM functional connectivity (A and B) while only immediate recall was related to MTL-AT functional connectivity (C and D).
Relationship between baseline functional connectivity and future memory decline
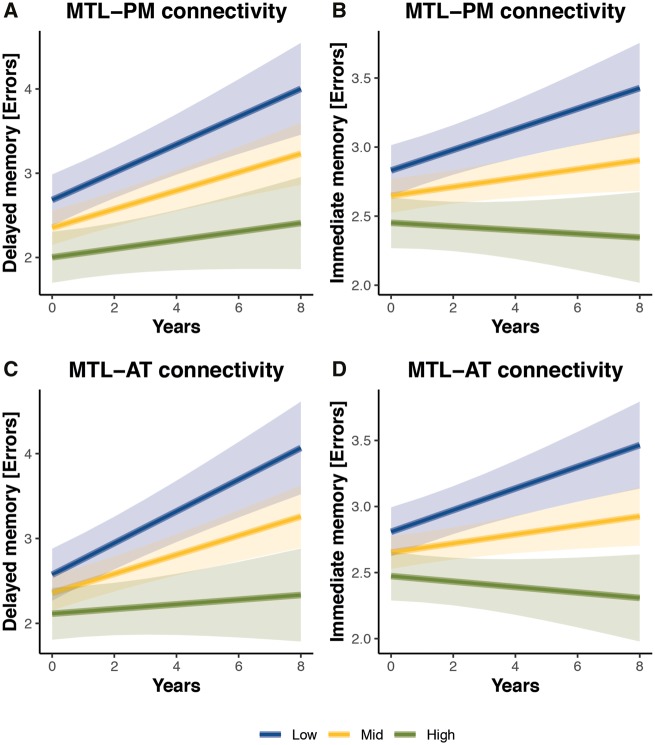
Changes in functional connectivity are likely to arise very early in the course of Alzheimer’s disease and might precede symptoms such as cognitive decline. Thus, we set out to test whether changes in MTL-neocortical connectivity were associated with future memory decline in the cognitively unimpaired group by testing for a significant baseline functional connectivity × future cognitive decline interaction. We found that both MTL-AT and MTL-PM connectivity at baseline were associated with future cognitive decline in immediate (MTL-AT: β = −0.05, SE = 0.014, P = 0.001; MTL-PM: β = −0.04, SE = 0.014, P = 0.004) and delayed memory (MTL-AT: β = −0.07, SE = 0.022, P = 0.001; MTL-PM: β = −0.05, SE = 0.022, P = 0.019) in cognitively unimpaired (Fig. 5). All models remain significant when we include individual CSF amyloid-β42/40 levels.
Figure 5.
Relationship of MTL-cortical connectivity estimates and memory decline. Plots of estimated curves for three groups with different baseline functional connectivity (low, mid, high) and memory outcomes over time. Cognitively unimpaired (CU) individuals are subdivided in tertiles according to their baseline MTL-PM and MTL-AT functional connectivity. The lowest tertile is shown in blue, the middle in yellow and the highest in green. Estimation of curves included all participants regardless of length of follow-up time. Lower baseline MTL-PM connectivity (A and B) and lower baseline MTL-AT functional connectivity (C and D) were associated with steeper decline rates in immediate and delayed memory (increase in number of errors).
Integrity of white matter tracts, entorhinal cortical thickness and baseline functional connectivity
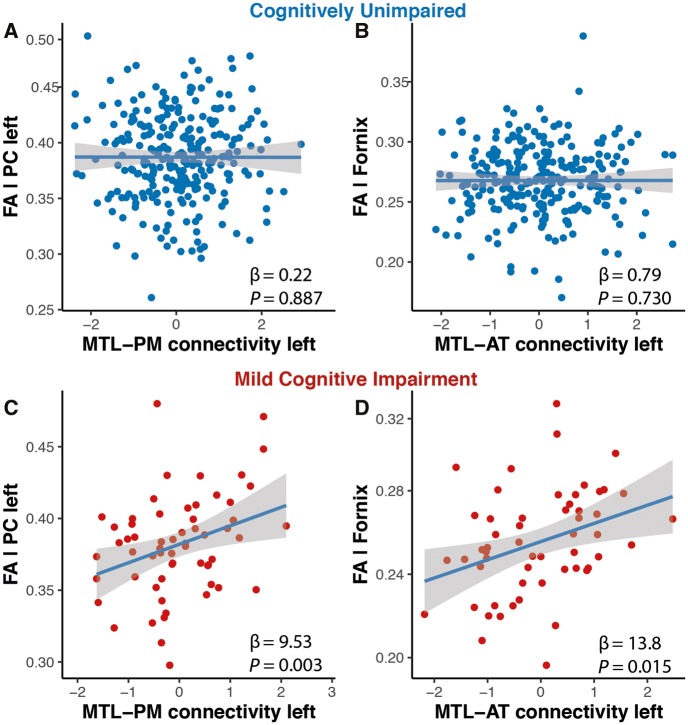
Lower functional connectivity could result from structural alterations in white matter tracts but could in principle also arise earlier and possibly independent from white matter damage (Greicius et al., 2009; Mollink et al., 2019). To test this hypothesis, we calculated fractional anisotropy in the anatomical tracts that connect the MTL with PM (parahippocampal cingulum) and AT regions (uncinate fasciculus and fornix). Given that our results from the functional connectivity group analysis were all strongly left-lateralized, we focused our analyses towards the structural tracts in the left hemisphere and analysed whether estimates of white matter integrity were associated with baseline functional connectivity. In cognitively unimpaired (amyloid-β+ and amyloid-β−, n = 263), we found no relationship between functional connectivity and white matter integrity in the respective brain systems, i.e. we found no correlations between connectivity in the MTL-AT system and fractional anisotropy of the uncincate fasciculus (β = 1.27, SE = 2.1, P = 0.538) or the fornix (β = 0.79, SE = 2.3, P = 0.730) or between connectivity within MTL-PM and fractional anisotropy of the parahippocampal cingulum (β = −0.22, SE = 1.6, P = 0.887) (Fig. 6A and B). In amyloid-β+ MCI (n = 83), however, we found that reduced fractional anisotropy in the left parahippocampal cingulum was associated with decreased left MTL-PM connectivity (β = 9.53, SE = 3.1, P = 0.003) (Fig. 6C). In addition, there was a significant relationship between left MTL-AT connectivity and fornix fractional anisotropy (β = 13.8, SE = 5.5, P = 0.015) (Fig. 6D). We did not find significant relationships between estimates of white matter tract integrity and increased within-MTL connectivity, but a trend for the left hemisphere with fornix fractional anisotropy (β = 56.9, SE = 32.3, P = 0.08).
Figure 6.
Relationship of white matter tract integrity and system-specific functional connectivity estimates. In MCI patients, baseline MTL-PM functional connectivity was associated with fractional anisotropy of the left parahippocampal cingulum (C). Baseline MTL-AT connectivity was associated with fractional anisotropy of the fornix (D). There were no such relationships for cognitively unimpaired individuals (A and B). FA = fractional anisotropy; PC = parahippocampal cingulum.
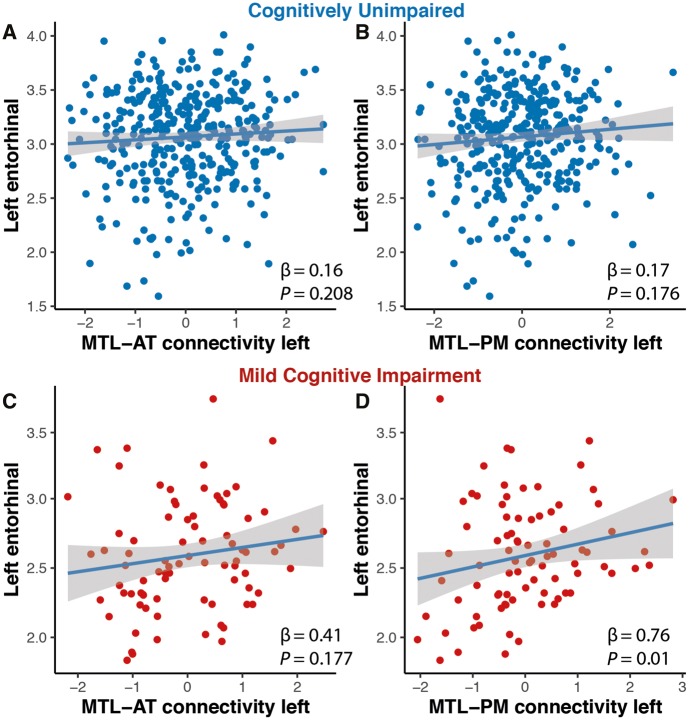
Finally, we tested whether atrophy in the MTL measured by left entorhinal thickness was related to MTL-cortical functional connectivity in amyloid-β+ cognitively unimpaired and MCI subjects. While we did not find any relationship between entorhinal thickness and MTL-cortical functional connectivity in the amyloid-β+ cognitively unimpaired group (AT: β = 0.16, P = 0.208, PM: β = 0.17, P = 0.176) (Fig. 7A and B), entorhinal thickness was significantly related to MTL-PM (β = 0.76, P = 0.01) but not MTL-AT functional connectivity (β = 0.41, P = 0.177) in MCI patients (Fig. 7C and D).
Figure 7.
Relationship of entorhinal thickness and system-specific functional connectivity estimates. In MCI patients, baseline MTL-PM (D) but not MTL-AT functional connectivity was associated with thickness of the left entorhinal cortex (C). There were no such relationships for cognitively unimpaired individuals (A and B).
Discussion
We found that amyloid-β+ compared to amyloid-β− cognitively unimpaired individuals were mainly characterized by decreased functional connectivity between the anterior MTL and regions in the AT system, most prominently between left perirhinal/entorhinal and medial prefrontal cortex. While amyloid-β+ patients with MCI compared to amyloid-β− cognitively unimpaired also exhibited reduced MTL-AT connectivity, we mostly found reductions between the MTL and PM regions, prominently between left anterior hippocampus and posterior cingulate cortex. In addition, MCI patients were characterized by hyperconnectivity within the MTL and with its neighbouring regions. Further examination of MCI with strong hyperconnectivity revealed a significant increase in BOLD signal entropy with a striking spatial overlap with the hyperconnecting regions, suggesting that regions characterized by hyperconnectivity also show less predictable BOLD signal. Linear regression analyses showed that lower MTL-AT/PM functional connectivity in cognitively unimpaired subjects was associated with worse memory at baseline and more rapid future memory decline. Finally, diffusion-weighted imaging showed that MCI subjects’ altered MTL-AT/PM functional connectivity was associated with compromised structural integrity of the parahippocampal cingulum and the fornix, respectively. No relationship between functional connectivity and structural tract integrity was found in cognitively unimpaired individuals. Similarly, entorhinal thickness was related to MTL-PM functional connectivity only in MCI patients.
Medial temporal lobe-anterior temporal connectivity is predominantly affected in preclinical Alzheimer’s disease
There have been several studies showing alterations in functional connectivity networks in cognitively unimpaired amyloid-β+ individuals (Hedden et al., 2009; Sperling et al., 2009; Sheline et al., 2010; Drzezga et al., 2011), mostly focusing on the DMN or precuneus. Here we instead focused on two crucial cortical memory networks, the PM and AT networks, which are densely interconnected with the MTL (Ranganath and Ritchey, 2012). Alzheimer’s disease pathology does not uniformly affect all MTL subregions. In the earliest disease stage, NFT pathology mostly affects the medial bank of the collateral sulcus (area 35, part of perirhinal cortex) and can in later stages be found in entorhinal cortex and hippocampus (especially in cornu ammonis 1) (Braak and Braak, 1997; Braak et al., 2006). Both perirhinal and the anterior-lateral entorhinal cortex are strongly connected to the AT system (Maass et al., 2015; Schröder et al., 2015) and one would therefore expect functional connectivity of the anterior MTL and the AT system to be most strongly affected in the earliest stages of Alzheimer’s disease (Harrison et al., 2019). Here we found that MTL-AT functional connectivity was predominantly affected in amyloid-β+ compared to amyloid-β− cognitively unimpaired subjects. In line with neuropathological studies (Braak and Braak, 1991; Lace et al., 2009), we found the strongest reduction of functional connectivity between the left perirhinal/entorhinal and medial prefrontal cortex. This is also in agreement with ageing studies showing that anterior MTL connectivity is reduced after the age of 60 (Salami et al., 2016) as well as reduced connectivity between perirhinal/entorhinal and hippocampal subfield CA1 (Dalton et al., 2019). Although these studies did not study Alzheimer’s disease pathology biomarkers, neuropathological studies suggest that a significant portion (>60%) of 70-year-old individuals harbour NFT pathology in their MTL (Braak and Braak, 1997). A recent study incorporating Alzheimer’s disease biomarkers reports similar findings where amyloid accumulation in the anterior MTL was related to its anterior functional connectivity in cognitively unimpaired individuals (Song et al., 2015).
In later disease stages, when tau pathology progresses towards large portions of the hippocampus and to more widespread lateral and posterior cortical areas, decreases in MTL-PM connectivity are likely to emerge. Indeed, one of the main findings in Alzheimer’s disease is reduced functional connectivity in posterior DMN nodes such as precuneus/posterior cingulate cortex (Badhwar et al., 2017) as well as between the hippocampus and the posterior DMN (Zhou et al., 2008). Here we found that predominantly MTL-PM functional connectivity was decreased in amyloid-β+ MCI, which is in line with previous findings (Sheline et al., 2010). In particular, we found the strongest reduction of functional connectivity between the left anterior hippocampus and the posterior cingulate cortex. The shift from entorhinal/perirhinal cortex to the anterior hippocampus in amyloid-β+ MCI is in agreement with neuropathological studies where NFT pathology in entorhinal/perirhinal cortex (Braak stage I) precedes tau accumulation in the hippocampus (Braak stage II) (Braak and Braak, 1991). In light of this, we also analysed functional connectivity changes associated with increasing CSF p-tau levels in amyloid-β+ cognitively unimpaired. While we found mostly decreased MTL-AT connectivity in the amyloid-β+ versus amyloid-β− cognitively unimpaired group comparison, we mainly found MTL-PM reductions with increasing p-tau levels in amyloid-β+ cognitively unimpaired. This fits well with a recent study showing that structural alterations of the parahippocampal cingulum (connecting the MTL with parietal regions) predict downstream tau accumulation in posterior cingulate cortex (Jacobs et al., 2018). Interestingly, our results appeared to be strongly left lateralized, both for amyloid-β+ cognitively unimpaired and amyloid-β+ MCI compared to cognitively unimpaired amyloid-β−. This fits with earlier studies showing similar left lateralized effects in early Alzheimer’s disease using volumetric grey matter measurements (Shi et al., 2009), FDG-PET (Weise et al., 2018) or amyloid PET (Raji et al., 2008). However, future studies are needed to systematically replicate this effect and investigate potential factors that could be associated with it. Taken together, this suggests MTL-AT connectivity being reduced early on in amyloid-β+ cognitively unimpaired, while MTL-PM functional connectivity is progressively affected with increasing levels of CSF p-tau, a pattern most prominently pronounced in amyloid-β+ MCI.
Amyloid-β+ MCI subjects are characterized by increased medial temporal lobe connectivity
We found that the hallmark of amyloid-β+ subjects with MCI was not only the decreased MTL-PM connectivity, but also hyperconnectivity within MTL subregions, and between MTL and PM/AT regions (cf. Fig. 2C and D). This hyperconnectivity phenomenon has been seen in previous studies (Pasquini et al., 2015; Tahmasian et al., 2015; Harrison et al., 2019) and interpreted in the light of the hippocampus disconnection hypothesis, which states that reduced cortical input may contribute to disinhibition-like changes of hippocampal activity (Salami et al., 2014; Tahmasian et al., 2015). Earlier studies also presented evidence of increased within-MTL functional connectivity associated with worse cognition and clinical decline (Salami et al., 2014; Pasquini et al., 2015). Furthermore, studies in ageing found that increased connectivity could mainly be found in the posterior MTL (Salami et al., 2016). Interestingly, although we did not observe hyperconnectivity in amyloid-β+ cognitively unimpaired subjects, the regions with the highest increase in functional connectivity in amyloid-β+ MCI subjects were within the bilateral posterior hippocampus and between the left posterior hippocampus and angular gyrus. This is further strengthened by a recent ageing study highlighting reduced anterior hippocampal connectivity with increased posterior hippocampal connectivity (Stark et al., 2019).
To elucidate the nature of this increased functional connectivity in MCI, we calculated entropy as a measure of BOLD signal predictability. MCI patients with increased functional connectivity were characterized by increased entropy (i.e. reduced BOLD signal predictability) and showed clear spatial correspondence between regions of significant changes in both measures relative to the amyloid-β− cognitively unimpaired group. This indicates that the BOLD time series in MCI patients with increased functional connectivity is less predictable and more random with increased complexity, which has been shown similarly in ageing (Yao et al., 2013). Although this might indicate suboptimal neural processing, it is difficult to fully understand the implications of this finding at this point and further research will be needed.
Functional connectivity between the medial temporal lobe posterior medial and anterior temporal regions is related to memory performance
Functional connectivity between the MTL and both cortical networks was reduced in amyloid-β+ relative to amyloid-β− subjects. However, it is unclear whether these network-specific alterations in functional connectivity are related to memory functioning. Decreased functional connectivity in the DMN has been shown to be related to memory functioning and cognitive decline in earlier studies, where longitudinal changes in intra-DMN connectivity were related to memory decline (Staffaroni et al., 2018) and decline in the preclinical Alzheimer cognitive composite (Buckley et al., 2017). We found that reductions in functional connectivity were related to baseline memory performance as well as memory decline in cognitively unimpaired where cognitively unimpaired with lower baseline MTL-cortical functional connectivity had worse cognitive scores at baseline and showed steeper decline rates within the following 8 years.
A recent study focused on impaired working memory in ageing and its relation to temporal decoupling of neural codes theorized to constitute a flexible frontotemporal circuit involved in memory functioning (Reinhart and Nguyen, 2019). They reported impaired working memory and concomitant insufficient long-range MTL-prefrontal cortex (i.e. MTL-AT) theta synchronization. This fits well with our findings on reduced MTL-AT connectivity and immediate memory (i.e. working memory) in cognitively unimpaired individuals. Interestingly, coherence as well as memory performance could be restored by a non-invasive intervention procedure targeting these long-range theta interactions, which suggests that memory impairment associated to MTL-AT functional disconnection may indeed be reversible in early stages.
Evidence for dependence on white matter tract integrity in amyloid-β+ MCI but not cognitively unimpaired individuals
Our results suggest that alterations in specific MTL-cortical networks are associated with cognitive performance already in cognitively unimpaired individuals. This opens up the question whether this dysfunction is already related to structural damage or not. While the parahippocampal cingulum connects the MTL with the posterior cingulate, the uncinate fasciculus and the fornix connect the MTL with the prefrontal cortex (Croxson et al., 2005; Villain et al., 2010). We found that amyloid-β+ cognitively unimpaired and amyloid-β+ MCI subjects showed alterations in functional connectivity, predominantly in the left hemisphere. Thus, we set out to test whether changes in left MTL-PM and MTL-AT connectivity were related to white matter tract integrity in both groups. For cognitively unimpaired individuals, we found that white matter tract integrity was not related to reductions in functional connectivity (Fig. 6A and B). However, in amyloid-β+ MCI, we found that fornix integrity was related to MTL-AT connectivity and that parahippocampal cingulum related to MTL-PM (Fig. 6C and D). This is in line with earlier studies showing structural impairment in white matter tracts in early Alzheimer’s disease stages only in neurodegeneration-positive groups and MCI (Villain et al., 2010; Pereira et al., 2016, 2018; Kantarci et al., 2017). Similarly, we found that while reduced MTL-cortical functional connectivity was not related to MTL structural integrity, there was a relationship between MTL-PM connectivity and entorhinal thickness in MCI patients. Although one has to be careful interpreting cross-sectional data, this suggests that reduced connectivity and resulting memory impairment could potentially be reversible in early stages where objective cognitive impairment is not yet evident.
Limitations
Our study comes with some limitations. First, we used cross-sectional MRI data and it is thus important to replicate the changes in MTL-cortical system-specific functional connectivity using longitudinal data to test whether the same individuals will progress between the cross-sectionally observed states (from predominant MTL-AT to predominant MTL-PM reduction in functional connectivity). Second, we used CSF measures to subdivide our sample in amyloid-β+/− as well as to analyse the relationship of functional connectivity with increasing levels of p-tau. However, as CSF measures of Alzheimer’s disease pathology are not regionally specific, we cannot be sure whether our findings are the result of local or global effects of Alzheimer’s disease pathology.
Conclusion
Taken together, our results suggest that amyloid-β related changes in MTL-AT functional connectivity precede those involving MTL and PM regions, and are already associated with memory impairment and future decline in cognitively unimpaired individuals. Our MTL subregional analysis results fit neuropathological studies pointing to early involvement of perirhinal/entorhinal cortices and the anterior hippocampus. With disease progression, MTL-PM connectivity is increasingly impaired and in later MCI stages, this decline prompts increased connectivity within the MTL and to its proximity. Our analyses showing that integrity of key white matter tracts is related to decreased functional connectivity in the MCI stage but not in cognitively unimpaired individuals suggests impairment at this early stage might still be reversible. Thus, MTL-PM/AT functional connectivity could serve as stage-specific functional markers in early Alzheimer’s disease.
Supplementary Material
Acknowledgements
First of all, we want to thank all participants of the Swedish BioFINDER study and their families for their participation in the study. Furthermore, we want to thank Joana Pereira and Daniel Svärd for their help regarding the DTI data analysis.
Funding
The work in the present study was supported by the European Research Council (grant no. 311292), the Swedish Research Council (grant no. 2016-00906), the Knut and Alice Wallenberg Foundation (grant no. 2017-0383), the Marianne and Marcus Wallenberg Foundation (grant no. 2015.0125), the Swedish Alzheimer Foundation (grant no. AF-745911), the Swedish Brain Foundation (grant no. FO2019-0326), the Swedish federal government under the ALF agreement (grant no. 2018-Projekt0279) and the Strategic Research Area MultiPark (Multidisciplinary Research in Parkinson’s disease) at Lund University.
Competing interests
O.H. has acquired research support (for the institution) from Roche, GE Healthcare, Biogen, AVID Radiopharmaceuticals and Euroimmun. In the past 2 years, he has received consultancy/speaker fees (paid to the institution) from Biogen and Roche. All other authors report no competing interests.
Glossary
- AT =
anterior-temporal
- BOLD =
blood oxygen level-dependent
- DMN =
default mode network
- MCI =
mild cognitive impairment
- MTL =
medial temporal lobe
- PM =
posterior-medial
References
- Avants BB, Tustison NJ, Stauffer M, Song G, Wu B, Gee JC.. The Insight ToolKit image registration framework. Front Neuroinform 2014; 8: 44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badhwar A, Tam A, Dansereau C, Orban P, Hoffstaedter F, Bellec P.. Resting-state network dysfunction in Alzheimer’s disease: a systematic review and meta-analysis. Alzheimer’s & Dementia: diagnosis. Alzheimers Dement 2017; 8: 73–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behzadi Y, Restom K, Liau J, Liu TT.. A component based noise correction method (CompCor) for BOLD and perfusion based fMRI. Neuroimage 2007; 37: 90–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berron D, Neumann K, Maass A, Schütze H, Fliessbach K, Kiven V, et al. Age-related functional changes in domain-specific medial temporal lobe pathways. Neurobiol Aging 2018; 65: 86–97. [DOI] [PubMed] [Google Scholar]
- Berron D, Vieweg P, Hochkeppler A, Pluta J, Ding S-L, Maass A, et al. A protocol for manual segmentation of medial temporal lobe subregions in 7 Tesla MRI. Neuroimage Clin 2017; 15: 466–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertens D, Tijms BM, Scheltens P, Teunissen CE, Visser P.. Unbiased estimates of cerebrospinal fluid β-amyloid 1–42 cutoffs in a large memory clinic population. Alzheimer’s Res Ther 2017; 9: 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braak H, Alafuzoff I, Arzberger T, Kretzschmar H, Tredici K.. Staging of Alzheimer disease-associated neurofibrillary pathology using paraffin sections and immunocytochemistry. Acta Neuropathol 2006; 112: 389–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braak H, Braak E.. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol 1991; 82: 239–59. [DOI] [PubMed] [Google Scholar]
- Braak H, Braak E.. Frequency of stages of Alzheimer-related lesions in different age categories. Neurobiol Aging 1997; 18: 351–7. [DOI] [PubMed] [Google Scholar]
- Brunec IK, Bellana B, Ozubko JD, Man V, Robin J, Liu Z-X, et al. Multiple scales of representation along the hippocampal anteroposterior axis in humans. Curr Biol 2018; 28: 2129–35.e6. [DOI] [PubMed] [Google Scholar]
- Buckley RF, Schultz AP, Hedden T, Papp KV, Hanseeuw BJ, Marshall G, et al. Functional network integrity presages cognitive decline in preclinical Alzheimer disease. Neurology 2017; 89: 29–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner RL, Di Nicola LM. The brain’s default network: updated anatomy, physiology and evolving insights. Nat Rev Neurosci 2019; 20: 593–608. [DOI] [PubMed] [Google Scholar]
- Burwell R. The parahippocampal region: corticocortical connectivity. Ann N Y Acad Sci 2000; 911: 25–42. [DOI] [PubMed] [Google Scholar]
- Collin SH, Milivojevic B, Doeller CF.. Memory hierarchies map onto the hippocampal long axis in humans. Nat Neurosci 2015; 18: 1562–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins JA, Dickerson BC.. Functional connectivity in category‐selective brain networks after encoding predicts subsequent memory. Hippocampus 2019; 29: 440–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper RA, Ritchey M.. Cortico-hippocampal network connections support the multidimensional quality of episodic memory. Elife 2019; 8: e45591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox R. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res 1996; 29: 162–73. [DOI] [PubMed] [Google Scholar]
- Croxson PL, Johansen-Berg H, Behrens TE, Robson MD, Pinsk MA, Gross CG, et al. Quantitative investigation of connections of the prefrontal cortex in the human and macaque using probabilistic diffusion tractography. J Neurosci 2005; 25: 8854–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalton MA, McCormick C, Maguire EA.. Differences in functional connectivity along the anterior-posterior axis of human hippocampal subfields. Neuroimage 2019; 192: 28–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das SR, Pluta J, Mancuso L, Kliot D, Orozco S, Dickerson BC, et al. Increased functional connectivity within medial temporal lobe in mild cognitive impairment. Hippocampus 2013; 23: 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das SR, Pluta J, Mancuso L, Kliot D, Yushkevich PA, Wolk DA.. Anterior and posterior MTL networks in aging and MCI. Neurobiol Aging 2015; 36: S141–50.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desikan RS, Ségonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage 2006; 31: 968–80. [DOI] [PubMed] [Google Scholar]
- Ding S, Van Hoesen GW.. Borders, extent, and topography of human perirhinal cortex as revealed using multiple modern neuroanatomical and pathological markers. Hum Brain Mapp 2010; 31: 1359–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drzezga A, Becker AJ, Dijk KR, Enivasan A, Talukdar T, Sullivan C, et al. Neuronal dysfunction and disconnection of cortical hubs in non-demented subjects with elevated amyloid burden. Brain 2011; 134: 1635–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichenbaum H, Yonelinas AP, Ranganath C.. The medial temporal lobe and recognition memory. Annu Rev Neurosci 2007; 30: 123–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabner G, Janke AL, Budge MM, Smith D, Pruessner J, Collins D.. Symmetric atlasing and model based segmentation: an application to the hippocampus in older adults. Med Image Comput Comput Assist Interv 2006; 9: 58–66. [DOI] [PubMed] [Google Scholar]
- Greicius MD, Supekar K, Menon V, Dougherty RF.. Resting-state functional connectivity reflects structural connectivity in the default mode network. Cerebral Cortex 2009; 19: 72–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn A, Strandberg T, Stomrud E, Nilsson M, van Westen D, Palmqvist S, et al. Association between earliest amyloid uptake and functional connectivity in cognitively unimpaired elderly. Cereb Cortex 2019; 29: 2173–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison TM, Maass A, Adams JN, Du R, Baker SL, Jagust WJ.. Tau deposition is associated with functional isolation of the hippocampus in aging. Nat Commun 2019; 10: 4900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedden T, Dijk KR, Becker AJ, Mehta A, Sperling RA, Johnson KA, et al. Disruption of functional connectivity in clinically normal older adults harboring amyloid burden. J Neurosci 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insausti R, Juottonen K, Soininen H, Insausti A, Partanen K, Vainio P, et al. MR volumetric analysis of the human entorhinal, perirhinal, and temporopolar cortices. AJNR Am J Neuroradiol 1998; 19: 659–71. [PMC free article] [PubMed] [Google Scholar]
- Jack CR, Bennett D, Blennow K, Carillo MC, Dunn B, Budd Haeberlein S, et al. NIA-AA research framework: toward a biological definition of Alzheimer’s disease. 2018; 14: 535–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs HIL, Hedden T, Schultz AP, Sepulcre J, Perea RD, Amariglio RE, et al. Structural tract alterations predict downstream tau accumulation in amyloid-positive older individuals. 2018; 21: 421–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janelidze S, Hertze J, Nägga K, Nilsson K, Nilsson C, Swedish BioFINDER Study Group, et al. Increased blood-brain barrier permeability is associated with dementia and diabetes but not amyloid pathology or APOE genotype. 2017a; 51: 104–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janelidze S, Pannee J, Mikulskis A, Chiao P, Zetterberg H, Blennow K, et al. Concordance between different amyloid immunoassays and visual amyloid positron emission tomographic assessment. JAMA Neurol 2017b; 12: 1492–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janelidze S, Zetterberg H, Mattsson N, Palmqvist S, Vanderstichele H, Lindberg O, et al. CSF Aβ42/Aβ40 and Aβ42/Aβ38 ratios: better diagnostic markers of Alzheimer disease. Ann Clin Transl Neurol 2016; 3: 154–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson M, Beckmann CF, Behrens T, Woolrich MW, Ith S.. FSL. Neuroimage 2012; 62: 782–90. [DOI] [PubMed] [Google Scholar]
- Johnstone T, Walsh KS, Greischar LL, Alexander AL, Fox AS, Davidson RJ, et al. Motion correction and the use of motion covariates in multiple‐subject fMRI analysis. Hum Brain Mapp 2006; 10: 779–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones D, Christiansen K, Chapman R, Aggleton J.. Distinct subdivisions of the cingulum bundle revealed by diffusion MRI fibre tracking: implications for neuropsychological investigations. Neuropsychologia 2013; 51: 67–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn I, Andrews-Hanna JR, Vincent JL, Snyder AZ, Buckner RL.. Distinct cortical anatomy linked to subregions of the medial temporal lobe revealed by intrinsic functional connectivity. J Neurophysiol 2008; 100: 129–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantarci K, Murray ME, Schwarz CG, Reid RI, Przybelski SA, Lesnick T, et al. White-matter integrity on DTI and the pathologic staging of Alzheimer’s disease. Neurobiol Aging 2017; 56: 172–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lace G, Savva G, Forster G, de Silva R, Brayne C, Matthews F, et al. Hippocampal tau pathology is related to neuroanatomical connections: an ageing population-based study. Brain 2009; 5: 1324–34. [DOI] [PubMed] [Google Scholar]
- Libby LA, Ekstrom AD, Ragland DJ, Ranganath C.. Differential connectivity of perirhinal and parahippocampal cortices within human hippocampal subregions revealed by high-resolution functional imaging. J Neurosci 2012; 32: 6550–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maass A, Berron D, Libby L, Ranganath C.. Functional subregions of the human entorhinal cortex. Elife 2015; 4: e06426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattsson N, Insel PS, Donohue M, Jögi J, Ossenkoppele R, Olsson T, et al. Predicting diagnosis and cognition with 18F-AV-1451 tau PET and structural MRI in Alzheimer’s disease. Alzheimer’s & dementia: the journal of the Alzheimer’s. Association 2019; 15: 570–80. [DOI] [PubMed] [Google Scholar]
- Mattsson N, Schöll M, Strandberg O, Smith R, Palmqvist S, Insel PS, et al. 18F‐AV‐1451 and CSF T‐tau and P‐tau as biomarkers in Alzheimer’s disease. EMBO Mol Med 2017; 9: 1212–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mollink J, Ith S, Elliott LT, Kleinnijenhuis M, Hiemstra M, Alfaro-Almagro F, et al. The spatial correspondence and genetic influence of interhemispheric connectivity with white matter microstructure. Nat Neurosci 2019; 22: 809–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmqvist S, Schöll M, Strandberg O, Mattsson N, Stomrud E, Zetterberg H, et al. Earliest accumulation of β-amyloid occurs within the default-mode network and concurrently affects brain connectivity. Nat Commun 2017; 8: 1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasquini L, Scherr M, Tahmasian M, Meng C, Myers NE, Ortner M, et al. Link between hippocampus’ raised local and eased global intrinsic connectivity in AD. Alzheimers Dement 2015; 11: 475–84. [DOI] [PubMed] [Google Scholar]
- Pereira JB, Mijalkov M, Kakaei E, Mecocci P, Vellas B, Tsolaki M, et al. Disrupted network topology in patients with stable and progressive mild cognitive impairment and Alzheimer’s disease. Cereb Cortex 2016; 26: 3476–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira JB, van Westen D, Stomrud E, Strandberg TO, Volpe G, Westman E, et al. Abnormal structural brain connectome in individuals with preclinical Alzheimer’s disease. Cerebral cortex 2018; 28: 3638–49. [DOI] [PubMed]
- Petersen RC. Mild cognitive impairment as adiagnostic entity.J Intern Med2004; 256: 183–94. [DOI] [PubMed] [Google Scholar]
- Poppenk J, Evensmoen HR, Moscovitch M, Nadel L.. Long-axis specialization of the human hippocampus. Trends Cogn Sci 2013; 17: 230–40. [DOI] [PubMed] [Google Scholar]
- Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE.. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage 2012; 59: 2142–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle ME. The brain’s default mode network. Annu Rev Neurosci 2013; 38: 1–15. [DOI] [PubMed] [Google Scholar]
- Raji CA, Becker JT, Tsopelas ND, Price JC, Mathis CA, Saxton JA, et al. Characterizing regional correlation, laterality and symmetry of amyloid deposition in mild cognitive impairment and Alzheimer's disease with Pittsburgh Compound B. J Neurosci Methods 2008; 172: 277–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranganath C, Ritchey M.. Two cortical systems for memory-guided behaviour. Nat Rev Neurosci 2012; 13: 713–26. [DOI] [PubMed] [Google Scholar]
- Reinhart RM, Nguyen JA.. Working memory revived in older adults by synchronizing rhythmic brain circuits. Nat Neurosci 2019; 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richman J, Moorman J.. Physiological time-series analysis using approximate entropy and sample entropy. Am J Physiol Heart Circ Physiol 2000; 278: H2039–49. [DOI] [PubMed] [Google Scholar]
- Stark Q, Frithsen A, Stark CEL. Age-related alterations in functional connectivity along the longitudinal axis of the hippocampus and its subfields. bioRxiv 2019; 577361; doi: 10.1101/577361. [DOI] [PMC free article] [PubMed]
- Staffaroni A, Brown JA, Casaletto KB, Elahi FM, Deng J, Neuhaus J, et al. The longitudinal trajectory of default mode network connectivity in healthy older adults varies as a function of age and is associated with changes in episodic memory and processing speed. J Neurosci 2018; 38: 2809–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salami A, Pudas S, Nyberg L.. Elevated hippocampal resting-state connectivity underlies deficient neurocognitive function in aging. Proc Natl Acad Sci USA 2014; 111: 17654–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salami A, Wåhlin A, Kaboodvand N, Lundquist A, Nyberg L.. Longitudinal evidence for dissociation of anterior and posterior MTL resting-state connectivity in aging: links to perfusion and memory. Cereb Cortex 2016; 26: 3953–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schöll M, Lockhart SN, Schonhaut DR, O’Neil JP, Janabi M, Ossenkoppele R, et al. PET imaging of tau deposition in the aging human brain. Neuron 2016; 89: 971–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schröder T, Haak KV, Jimenez NI, Beckmann CF, Doeller CF.. Functional topography of the human entorhinal cortex. Elife 2015; 4: e06738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheline YI, Raichle ME, Snyder AZ, Morris JC, Head D, Wang S, et al. Amyloid plaques disrupt resting state default mode network connectivity in cognitively normal elderly. Biol Psychiatry 2010; 67: 584–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi F, Liu B, Zhou Y, Yu C, Jiang T. Hippocampal volume and asymmetry in mild cognitive impairment and alzheimer's disease: meta‐analyses of MRI studies. Hippocampus 2009; 19: 1055–64. [DOI] [PubMed] [Google Scholar]
- Song Z, Insel PS, Buckley S, Yohannes S, Mezher A, Simonson A, et al. Brain amyloid-β burden is associated with disruption of intrinsic functional connectivity within the medial temporal lobe in cognitively normal elderly. J Neurosci 2015; 35: 3240–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperling RA, LaViolette PS, O’Keefe K, O’Brien J, Rentz DM, Pihlajamaki M, et al. Amyloid deposition is associated with impaired default network function in older persons without dementia. Neuron 2009; 63: 178–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strange BA, Witter MP, Lein ES, Moser EI.. Functional organization of the hippocampal longitudinal axis. Nat Rev Neurosci 2014; 15: 655–69. [DOI] [PubMed] [Google Scholar]
- van Strien N, Cappaert N, Witter M.. The anatomy of memory: an interactive overview of the parahippocampal–hippocampal network. Nat Rev Neurosci 2009; 10: 272–82. [DOI] [PubMed] [Google Scholar]
- Tahmasian M, Pasquini L, Scherr M, Meng C, Förster S, Bratec S, et al. The lower hippocampus global connectivity, the higher its local metabolism in Alzheimer disease. Neurology 2015; 84: 1956–63. [DOI] [PubMed] [Google Scholar]
- Tournier J-D, Yeh C-H, Calamante F, Cho K-H, Connelly A, Lin C-P.. Resolving crossing fibres using constrained spherical deconvolution: validation using diffusion-weighted imaging phantom data. Neuroimage 2008; 42: 617–25. [DOI] [PubMed] [Google Scholar]
- Villain N, Fouquet M, Baron J-C, Mézenge F, Landeau B, de Sayette V, et al. Sequential relationships between grey matter and white matter atrophy and brain metabolic abnormalities in early Alzheimer’s disease. Brain 2010; 133: 3301–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward AM, Schultz AP, Huijbers W, Dijk K, Hedden T, Sperling RA.. The parahippocampal gyrus links the default‐mode cortical network with the medial temporal lobe memory system. Hum Brain Mapp 2014; 35: 1061–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weise CM, Chen K, Chen Y, Kuang X, Savage CR, Reiman EM et al. Left lateralized cerebral glucose metabolism declines in amyloid-β positive persons with mild cognitive impairment. Neuroimage Clin 2018; 20: 286–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler AM, Ridgway GR, Webster MA, Ith S, Nichols TE.. Permutation inference for the general linear model. Neuroimage 2014; 92: 381–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao Y, Lu W, Xu B, Li C, Lin C, Waxman D, et al. The increase of the functional entropy of the human brain with age. Sci Rep 2013; 3: srep02853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeo B, Krienen FM, Sepulcre J, Sabuncu MR, Lashkari D, Hollinshead M, et al. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J Neurophysiol 2011; 106: 1125–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zalesky A, Fornito A, Bullmore ET.. Network-based statistic: identifying differences in brain networks. Neuroimage 2010; 53: 1197–207. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Dougherty JH, Hubner KF, Bai B, Cannon RL, Hutson KR.. Abnormal connectivity in the posterior cingulate and hippocampus in early Alzheimer’s disease and mild cognitive impairment. Alzheimer Dement 2008; 4: 265–70. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Anonymized data will be shared by request from a qualified academic investigator for the sole purpose of replicating procedures and results presented in the article and as long as data transfer is in agreement with EU legislation on the general data protection regulation and decisions by the Ethical Review Board of Sweden and Region Skåne.