Abstract
Background/objectives:
Patients who receive Roux-en-Y gastric bypass (RYGB) lose more weight than those who receive vertical sleeve gastrectomy (VSG). RYGB and VSG alter hedonic responses to sweet flavor, but whether baseline differences in hedonic responses modulate weight loss after RYGB or VSG remains untested.
Participants/methods:
Male and female candidates (n=66) for RYGB or VSG were recruited and tested for their subjective liking and wanting ratings of sucrose solutions and flavored beverages sweetened with aspartame. Participants were classified by unsupervised hierarchical clustering for their liking and wanting ratings of sucrose and aspartame. Participant liking ratings were also used in a supervised classification using pre-established categories of liking ratings (liker, disliker, and inverted u-shape). Effects of categories obtained from unsupervised or supervised classification on body weight loss and their interaction with surgery type were analyzed separately at 3 and 12 months after surgery using linear models corrected for sex and age.
Results:
RYGB participants lost more body weight compared to VSG participants at 3 and 12 months after surgery (P < 0.001 for both time points). Unsupervised clustering analysis identified clusters corresponding to high and low wanting or liking ratings for sucrose or aspartame. RYGB participants in high-wanting clusters based on sucrose, but not aspartame, lost more weight than VSG at both 3 (P = 0.01) and 12 months (P = 0.03), yielding a significant cluster by surgery interaction. Categories based on supervised classification using liking ratings for sucrose or aspartame showed no significant effects on body weight loss between RYGB and VSG participants.
Conclusions:
Classification of patients into high/low wanting ratings for sucrose before surgery can predict differential body weight loss after RYGB or VSG in adults and could be used to advise on surgery type.
Keywords: sweet taste, wanting, liking, bariatric surgery, body weight, cluster analysis
Introduction
Roux-en-Y gastric bypass (RYGB) and vertical sleeve gastrectomy (VSG) are the most effective treatments for severe obesity1. Both procedures can result in up to a 30% loss in body weight2, 3. Compared to VSG, RYGB leads to greater body weight loss4–7. Proposed mechanisms for the effectiveness of bariatric surgeries include: changes in levels of gut hormones (e.g. GLP-1)8 and the resultant changes in perceived hunger and fullness; increased energy expenditure; and changes in vagal nerve signaling due to increased gastric distention9, 10. Despite the effectiveness of bariatric surgery on weight loss, not all participants are equally successful at long-term weight loss, and there is often significant weight regain starting 2 years after either RYGB or VSG surgery2, 11. Thus, understanding which pre-operative factors can influence the weight loss outcome of bariatric surgery is of great potential benefit.
Potential pre-operative predictors of weight loss in RYGB and VSG include anthropomorphic variables (body mass index (BMI), age, sex, race)12–16 and behavioral variables, including self-restraint17, 18, the latter potentially predicting increased dietary adherence after surgery19, 20. RYGB and VSG reduce sweet taste response and drive for intake mediated by pleasure21–24. However, it is not clear whether these predictors reflect changes in eating behavior25, 26. Some investigators suggest that pre-operative responses to sweet taste could predict body weight loss in RYGB and VSG24.
Classifications of participants by intake of sweet tasting fluids are inconsistent predictors of body weight loss after RYGB or VSG21, 27–30. An alternative approach to the classification of participants, based on estimates of caloric intake of sweet, is to focus on the different aspects of the hedonic response to sweet flavors. In animals, intake of sweets (and more broadly experience of any reward) triggers two behavioral and neurological processes: liking and wanting31. Liking is the hedonic or pleasurable aspect of a reinforcer, and is often measured with subjective ratings of enjoyment, or evaluations of facial responses. Wanting is the motivational aspect of a response to some stimuli, and is usually measured by effort expended to obtain a fixed amount of a reinforcer32, but has also been measured by intake33. Whether pre-operative liking or wanting for sweet tastes can predict and account for body weight loss after RYGB of VSG remains unknown.
In humans, both two34 and three35 sweet tasting categories for sucrose are recognized: 1) sweet likers (increased hedonic ratings over increasing sweetener concentrations), 2) sweet dislikers (decreased hedonic ratings over increasing sweetener concentrations) and 3) an inverted u-shape (increased hedonic rating at lower sweetener concentrations and decreased hedonic ratings at higher sweetener concentrations)35. The two group classification relied on either a drop or rise in pleasantness above 0.4 M sucrose34. An advantage of the two-cluster classification is that it has a potential genetic basis as shown in studies of sweet taste classifications in conjunction with alcoholism36, 37. Beyond the identification of these three basic categories and the widespread use of sucrose as sweet taste, a wide variety in the concentrations of sucrose and classification methods have been employed38. Further, to properly analyze sweet taste response, it is also necessary to isolate sweet taste per se from the post-ingestive consequences of foods or beverages that contain nutritive sweeteners such as sucrose39.
The choice of sucrose and aspartame solutions was based on prior studies whose purposes were to test the hypotheses that individuals with eating disorders would increase their effortful responses (sham drinking) as sweetness, without added energy, increased40, 41, and that they would work harder to obtain a sweetened vs. a non-sweetened beverage42. The two concentrations of sucrose were used in order to characterize the participants by their sweet response profiles with both low and high concentrations. We chose concentrations of aspartame that were in the range of equivalence to the sucrose tested and in common use in beverages. There were limits on high aspartame concentrations as at higher concentrations there is a bitter component to aspartame [35]. The current study was conducted in conjunction with an effort-requiring task that utilized, a flavor-containing non-caloric beverage42.
Among the different methodological approaches for classification of participants, unsupervised hierarchical clustering analysis (HCA) has gained recent attention as a tool due to its potential for unbiased cluster discovery38, 43, 44. The application of HCA to “liking” responses to sucrose concentrations ranging up to 1M has generated likers, dislikers, and inverted U groups38. However, there is no current standard in the literature for the method or experimental approach to analysis of sweet liking; we chose the HCA method as most likely to elucidate differential responses. The aim of these studies was to determine the extent to which pre-surgery sweet taste reactions predicted weight loss after RYGB or VSG.
Methods
Participants
Participants were individuals scheduled to undergo bariatric surgery (Mount Sinai St. Luke’s Hospital, New York, NY). Inclusion criteria were: Undergoing either RYGB or VSG procedures, BMI ≥ 35 kg/m2, age 18–65 years, and blood pressure below 160/100 mmHg. Exclusion criteria were: Fasting triglyceride >600 mg/dL; type 2 diabetes; taking any psychotropic medications; or current smoking or pregnancy. Individuals of all racial/ethnic backgrounds and both sexes were recruited. Control participants (BMI between 18.5–24.9 kg/m2) were age- and sex-matched to patients. Table 1 shows demographic information for all participants. Body weight was recorded two weeks before, and at 3 and 12 months after surgery. All experimental procedures were approved by the Institutional Review Boards of Mount Sinai St. Luke’s Hospital and Columbia University Medical Center, and all participants provided informed written consent.
TABLE 1.
Baseline characteristics and body weight loss
| All | ||||
|---|---|---|---|---|
| control | RYGB | VSG | ||
| Age (years)1 | 34.0 ± 1.95a | 33.0 ± 1.96a | 36.0 ± 1.52a | P=0.46 |
| Baseline Body Weight (kg)1 | 59.5 ±1.22 (n=31)a | 124.39 ± 4.45 (n=23)b | 120.1 ± 2.88 (n=43)b | P<0.001 |
| 3 month BW change (kg)1 | 0.37 ± 0.4a | −23.1 ± 1.08b | −19.7 ± 0.74c | P<0.0022 |
| 12 month BW change (kg) | ---- | −43.0 ± 2.22 (n=15) | −35.7 ± 2.33 (n=28) | P=0.0062 |
Different letters indicates p<0.005 for pairwise comparisons.
P-value for differences between groups on body weight loss adjusted for baseline BW, age and sex.
Taste test procedures
Patients were studied at baseline (1–2 weeks before surgery) and controls were studied on their first visit. All participants were told to consume only water after 8 PM the night prior to each scheduled visit. Participants arrived between 09:00 and 11:00 for testing under fasting conditions. Laboratory testing consisted of tasting and rating perceived liking and wanting for three sucrose concentrations in distilled water (0%, 6.1% and 34% weight/volume) and three aspartame concentrations (0%, 10% and 20% sucrose equivalent – 0%, 5.6% 7.5% weight/volume aspartame; Ajinomoto, NA Inc.) in cherry-flavored Kool Aid mixed with distilled water (0.19% weight/volume; Kraft Foods, Inc; Northfield, USA). 6.1% sucrose was selected as it is close to the threshold for detection, and 34% sucrose has been used to discriminate likers from dislikers34, 35. Aspartame concentrations were flavored to simulate a beverage (since pure aspartame solutions are not typically consumed). The low concentration approximates concentrations used in commercial beverages, while the higher concentration is twice that concentration and mimics the sweet taste of the higher sucrose concentration.
After tasting each beverage, participants reported their liking and wanting ratings using visual analog scales (Supplementary Figure 1). Detailed description of the taste test and use of visual analog scales can be found in the Supplementary Methods. Liking and wanting were tested in separate sequences, but in the same order for each participant.
Statistical Analysis
Statistical analysis was performed with R version 3.5.2 and data are presented as mean ± SEM, except for baseline anthropometric data at baseline, where mean ± SD was used. Statistical significance was set at P < 0.05. All pairwise comparisons were corrected for multiple comparisons using Tukey’s HSD. The R scripts used to analyze the data are available upon request.
Analysis of hedonic ratings.
The wanting ratings data were transformed with the following: to reduce the influence of extreme values on the data45. Adjusted liking and wanting ratings were calculated for each participant by subtracting the baseline (0% sucrose or 0% aspartame) ratings from each of the ratings of the above zero concentrations. Differences in hedonic ratings over sucrose or aspartame concentrations (Figures 1, 2a,d and 3a,d) were analyzed with linear mixed models.
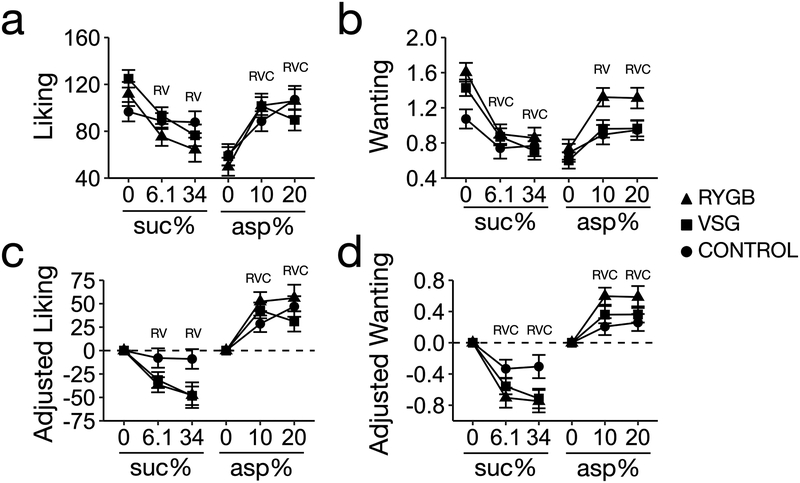
Figure 1. Liking and wanting ratings for sucrose and flavored aspartame.
Letters for each group, R (RGYB patients, n = 23; 3 males and 20 females), V (VSG patients, n = 43; 3 males and 20 females) and C (controls, n = 31; 6 males and 25 females), indicate a p-value < 0.05 for each beverage compared to baseline (0% sucrose or 0% aspartame) within its respective group. All pairwise comparisons corrected by Tukeýs HSD.
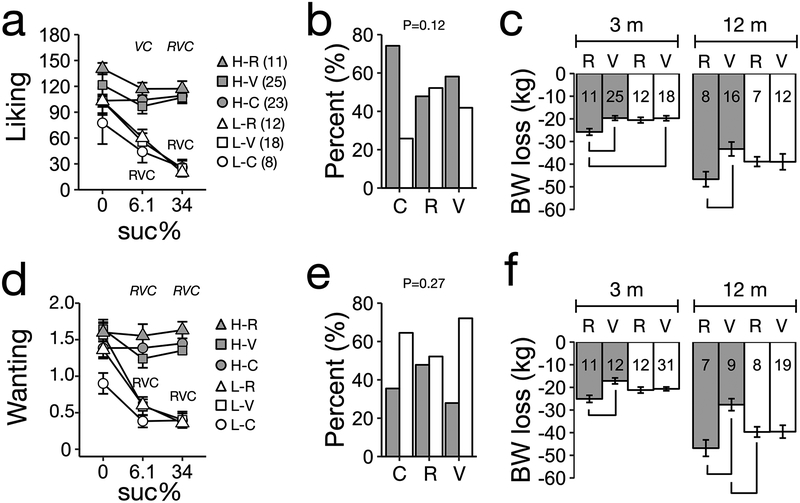
Figure 2. Unsupervised clusters based on unadjusted liking and wanting ratings for sucrose predict weight loss between RYGB and VSG patients.
A HCA identified two clusters (H: HIGH, L: LOW) using as input baseline unadjusted (a) liking or (d) wanting ratings for 6.1% and 34% sucrose solutions. The ratings for 0% ratings were not included in the input data for the HCA but are included in the figure. Numbers next to groups labels indicate sample size. (b,e) Percent distribution of participants within categories (HIGH cluster, dark; LOW cluster, white bars). (c,f) Weight loss at 3 and 12 m based on clusters for (a) liking or (d) wanting ratings for sucrose. Numbers in bars indicate sample size per surgery by cluster combination at each time point. Panels (a,d) letters (R, RGYB patients; V, VSG patients; C, controls) indicate a P-value < 0.05 for each beverage compared to baseline (0%) within participant category. Italic letters above plot indicate a P-value < 0.05 between HIGH and LOW clusters for each group. Panels (b,d) indicate P-value for X2 test. Panels (c,d), bracket indicates p-value<0.05 for pairwise comparison. All pairwise comparisons corrected by Tukey’ HSD.
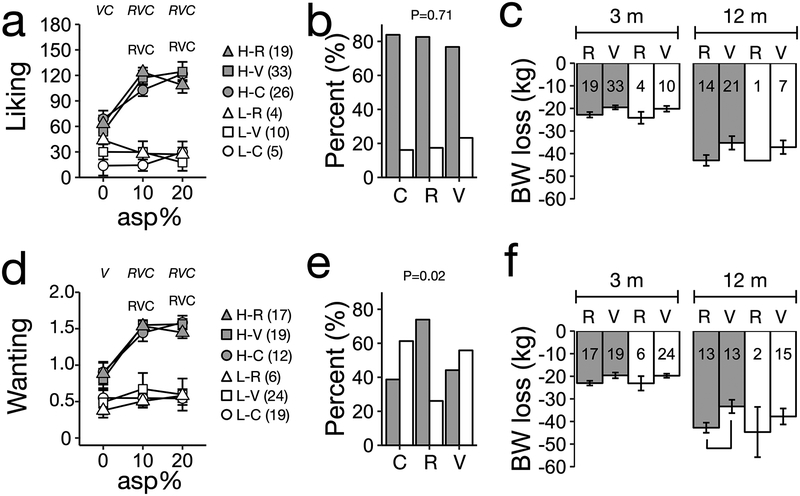
Figure 3. Unsupervised clusters based on unadjusted liking and wanting ratings for aspartame do not predict weight loss between RYGB and VSG patients.
A HCA identified two clusters (H: HIGH, L: LOW) using as input unadjusted (a) liking or (d) wanting ratings for 10% and 20% aspartame. The ratings for 0% aspartame were not included in the input data for the HCA but are included in the figure. (b,e) Percent distribution of participants within categories (HIGH cluster, dark; LOW cluster, white bars). (c,f) Weight loss at 3 and 12 m based on clusters for (a) liking or (d) wanting ratings for aspartame. Numbers in bars indicate sample size per surgery by cluster combination at each time point. Panels (a,d) letters (R, RGYB patients; V, VSG patients; C, controls) indicate a p-value < 0.05 for each beverage compared to baseline (0% aspartame) within participant category. Italic letters P-value < 0.05 between HIGH and LOW clusters for each group. Panels (b,d) indicate P-value for X2 test. Panels (c,d), bracket indicates p-value<0.05 for pairwise comparison. All pairwise comparisons corrected by Tukeýs HSD.
Body weight analysis in patients
The dependent variable for analysis of body weight was body weight loss (kg), calculated as the difference in kilograms between initial pre-surgery body weight and at 3 or 12 months after surgery. Body weight loss was analyzed separately at 3 and 12 months after surgery using linear models (ANOVA Type III SS) including, as main effects, type of surgery (RYGB, VSG), baseline body weight (kg), age (years) and sex (male, female), and followed by planned comparisons. As age did not have a significant effect on body weight loss (Table 1), all subsequent linear models of body weight loss (kg) were corrected only for sex and baseline body weight.
Unsupervised classification using hierarchical clustering analysis (HCA)
Patients and controls were included together in the HCA without regard to surgical condition. The HCA was based on adjusted or non-adjusted ratings for different concentrations of sucrose or aspartame and was done by means of the Ward algorithm, which used as input a matrix of pairwise Euclidean distance46 of the hedonic ratings of all participants (i.e. patients and controls) combined in a single clustering procedure. Unadjusted or adjusted liking and wanting ratings for 6.1% and 34% sucrose were used together (not averaged) or separately for each sucrose concentration. Unadjusted or adjusted liking and wanting ratings for 10% and 20% sucrose-intensity-equivalent aspartame were used together (not averaged). The liking and wanting ratings for 0% sucrose and 0% aspartame were not used as input data for the HCA. The number of clusters selected for analysis was based on visual inspection of the dendrogram, scree plots, and completeness of body weight data. For all datasets, we selected the solution with two clusters for all analyses, as this was a compromise between balanced datasets and taste phenotypes of interest. The statistical significance of the two cluster solutions was analyzed using a randomization test47 by comparing the original data set against two randomized data sets generated using two different permutation strategies: In one model, hedonic ratings were randomized across all concentrations and participants; in the second model, the hedonic ratings were only randomized across concentrations but within participants.
Supervised classification of participants using pre-established sweet liking categories
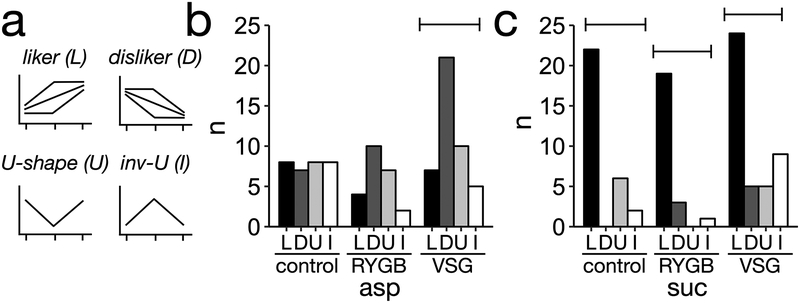
Patients and controls were used in this analysis without regard to surgery. A set of rules based on change in ratings over increasing sucrose or aspartame concentrations was used to classify participants into five different categories (Figure 4a and Supplementary Table 1): Liker (increased ratings), disliker (decreased ratings), neutral (no change), and inverted u-shape (increased liking ratings over low concentrations and decreased hedonic ratings high sucrose or aspartame concentrations). Participants that did not conform to either rule were classified as u-shape (Figure 4a). Since only one participant was classified as neutral, this category was not considered for further analysis. Differences between RYGB, VSG, and controls in liking ratings were statistically analyzed between taste categories containing at least three participants, leaving out the inverted U-shape sweet taste category for aspartame and sucrose.
Figure 4. Classification of patients and controls into sweet tasting categories.
(a) Description of sweet tasting categories (b) Distribution of controls within sweet taste categories (c) Distribution of patients within sweet taste categories. Brackets indicate p-value < 0.05 for chi-square within group.
Results
Baseline characteristics and body weight loss per surgery.
Participants’ characteristics at baseline and body weight loss after surgery are reported in Table 1. RYGB patients (n=23; 3 males and 20 females) lost significantly more weight (adjusted for age, sex, and baseline body weight) compared to VSG patients (n=43; 4 males and 39 females) at 3 (difference of 2.59 ± 0.95 kg) and 12 months (difference of 7.31 ± 2.53 kg) after surgery.
Liking and wanting ratings for sucrose and aspartame.
In RYGB and VSG patients, unadjusted or adjusted liking ratings for sucrose, measured before surgery, decreased significantly across increasing sucrose concentrations (P < 0.001 for all analyses, Figure 1a,c). Conversely, in controls, liking ratings did not change across sucrose concentrations (P = 0.58, Figure 1a,c). All participants’ (patients and controls) wanting ratings for sucrose decreased over increasing sucrose concentrations (P < 0.001 for all analysis, Figure 1b,d). In all participants, adjusted and unadjusted liking and wanting ratings for aspartame increased over increasing aspartame concentrations (P < 0.001 for all analysis, Figure 1a–d). In all baseline ratings noted above, there were no significant differences between operation groups (RYGB and VSG) (P > 0.05 for all analyses).
Ratings and prediction of weight loss from clusters for sucrose
Visual inspection of the scree-plot for the clusters obtained from the HCA liking and wanting ratings of controls and patients before surgery (Supplementary Figures 2 and 3), led us to select the two main clusters as the most parsimonious model for further analysis. The distribution of participants within these clusters was not random (Supplementary Table 2). The two clusters corresponded to high and low liking (Figure 2a) and wanting ratings (Figure 2d). There were no differences in ratings, across sucrose concentrations, between RYGB, VSG, and controls, in the high-liking or high-wanting clusters (P > 0.05 for all analysis, Figure 2a,d). Conversely, liking and wanting ratings were significantly lower for 6.1% and 34% sucrose than for 0% sucrose in the low-liking and low-wanting clusters (P < 0.05 for all analysis, Figure 2a,d). There were no significant differences in all groups in the frequencies of participants within clusters for liking (Figure 2b) or wanting ratings data (Figure 2e).
Regardless of cluster membership, RYGB patients lost more weight than VSG patients (P <0.01 at 3 and 12 months). For clusters based on liking ratings, there was neither a significant effect of cluster on body weight loss (3 months: P = 0.08, 12 months: P = 0.79), nor a significant interaction with surgery type (3 months: P = 0.1, 12 months: P = 0.29, Figure 2c). For clusters based on wanting ratings, there was no significant effect of cluster on body weight loss (3 months: P = 0.92, 12 months: P = 0.64), but there was a significant interaction with surgery at 3 (P = 0.01) and 12 months (P = 0.03, Figure 2f). In the high-wanting cluster only, RYGB patients lost more weight compared to VSG patients at 3 months (P = 0.003) and 12 months (difference of 14.97 ± 3.92 kg, P = 0.002) after surgery. At 12 months, RYGB patients in the low-wanting cluster lost more weight than VSG patients in the high-wanting cluster (difference of 10.17 ± 3.71 kg, P = 0.04). When 6.1% or 34% sucrose were analyzed by HCA separately there was no significant interaction between cluster and surgery type on weight loss (Supplementary Figure 4). Unsupervised classification using adjusted liking or wanting ratings for sucrose did not predict differential body weight loss between RYGB and VSG patients (Supplementary Figure 5, See Supplementary Material for detailed results).
Ratings and prediction of weight loss from clusters for aspartame
For aspartame, two main clusters were present for wanting ratings while three main clusters were present for liking ratings (Supplementary Figure 2c,d). There were less than five groups for both liking and wanting ratings (Supplementary Figure 2). To maintain consistency of analysis of aspartame with sucrose HCA, we selected the two-cluster solution for aspartame liking and wanting ratings. The distribution of participants within these clusters was not random (Supplementary Table 2). For all groups, ratings by participants in the high rating clusters were significantly higher for 10% and 20% aspartame than for 0% aspartame (P < 0.01, Figure 3a,d). Conversely, for all groups, ratings for those in the low rating clusters were not significantly different across aspartame concentrations (Figure 3a,d). There was a higher proportion of participants in the high wanting ratings cluster only for RYGB patients (Figure 3e). Thus, the HCA separated RYGB, VSG and controls into two clusters, one that increased their ratings over increasing aspartame concentrations, and one whose ratings were consistently low across concentrations. There were no significant effects of cluster type and no interaction of cluster by surgery type for aspartame on body weight loss at 3 or 12 months (P > 0.05 for all comparisons). However, there was a significant difference between body weight losses of RYGB and VSG patients at 3 and 12 months (P < 0.05 for all time points). Thus, unsupervised classification of adjusted liking or wanting ratings for aspartame did not predict differential body weight loss between RYGB and VSG patients (Supplementary Figure 6, See Supplementary Material for detailed results).
Supervised clustering of participants did not predict differential body weight loss between RYGB and VSG.
For sucrose, there was a higher proportion of dislikers among VSG patients (P < 0.001), while the distribution was random for controls (P = 0.99) and RYGB patients (P = 0.09). However, for aspartame there was a significantly larger proportion of likers among controls, RYGB and VSG patients (Figure 4b, P < 0.01 for all groups).
Liking ratings were significantly different across sucrose or aspartame concentrations for all sweet taste categories, yet there were no significant differences before surgery between RYGB, VSG, or controls (Figures 5a,c). The distribution of participants, within taste categories, for aspartame, was not uniform for RYGB and VSG participants at 3 and 12 months, thus separate analyses were done for each surgery group. There were no significant differences in body weight loss between sweet taste categories, based on aspartame liking ratings, for RYGB (Figure 5b, 3 months: P = 0.48, 12 months: P = 0.86) or VSG patients (3 months: P = 0.81, 12 months: P = 0.23). Within likers, RYGB patients lost more weight compared to VSG patients at 3 months (P = 0.017) and 12 months (P = 0.02). For sweet taste categories for sucrose, RYGB and VSG participants were present in each of the three taste categories at 3 and 12 months. At 3 months, there was a significant effect of surgery on body weight loss (RYGB patients lost more weight than VSG, P = 0.02), but no significant effects of sweet taste categories (P = 0.35) or the interaction between surgery and taste categories (P = 0.30). At 12 months after surgery, there was no significant effect of surgery (P = 0.41), sweet taste category (P = 0.93), or their interaction (P = 0.91) on body weight loss.
Figure 5. Sweet taste categories do not predict weight loss between RYGB and VSG patients.
Ratings for sweet taste categories based on liking for (a) aspartame and (b) sucrose. Weight loss at 3 and 6 months based on sweet taste categories based on (a) aspartame and (b) sucrose. Numbers in bars indicate sample size per surgery by cluster combination at each time point. Panels (a,c) letters R (RGYB patients), V (SG patients) and C (controls) indicate a P-value < 0.05 for each beverage compared to baseline (0% sucrose or 0% aspartame) within participant category. Brackets with letter above indicate P<0.05 for pairwise comparisons between groups. All pairwise comparisons corrected by Tukeýs HSD.
Discussion
The key finding of this report is that clusters of high wanting and low wanting for sucrose, found by unsupervised HCA, predicted differential body weight loss between RYGB and VSG patients. Since the HCA was conducted without regard to groups or surgery assignment HCA based on sucrose hedonic profiles, could be utilized clinically as a potential predictor of differential success in body weight loss after RYGB or VSG surgeries. A cutoff of 1.0 of the log transformed volume wanting rating of 6.1% and 34% sucrose solutions42, 45 can be used to classify patients into clusters of high and low wanting. Participants with log-transformed wanting ratings above 1 for 6.1% and 34% would be more successful in weight loss under RYGB, but not VSG. The HCA did not generate the three well established taste phenotypes for sucrose: liker, disliker, and u-shape35. This failure could be attributable to the larger range of sucrose concentrations used previously compared to the ones used here or might represent an inherent characteristic of our sample population.
Prior studies of predictors of body weight loss after RYGB or VSG surgery have focused on classification of participants based on intake of sweet foods and have not considered hedonic responses to sweet beverages. In a comparison of RYGB and VSG effectiveness in participants defined as sweets eaters (based on percent or amount of calories consumed from sweets)27, 28, RYGB was equally effective in sweets eaters or non-sweets eaters, while sweets eaters lost less weight after VSG27, 28. Yet, later studies have challenged that sweet eating (defined as amount of sweets consumed relative to total intake) is a reliable predictor of body weight loss after bariatric surgery29. Cravings for sweets, as defined by the Food Craving Inventory, did not predict changes in BMI after RYGB25. Other studies have suggested that decreased consumption of caloric soda was a predictor of larger weight loss after RYGB30. Our data provide direct evidence that hedonic ratings for sucrose, as opposed to self-report or questionnaires, predict differential body weight loss between RYGB and VSG patients. However, whether these hedonic ratings are related to sweet choice and intake outside laboratory conditions remains to be tested.
RYGB and VSG lead to significant changes in sweet taste perception. After RYGB, there are reports of decrease in liking for 40% sucrose solution without changes in response to sour, bitter, or salty tastes48, a reduction in threshold for sweet taste detection49 and in the motivation to work for sweet and fatty foods24. Neural responses to food cues have also been found to differ from pre- to post-RYGB surgery, with the largest post-surgical reductions to food cues being observed in corticolimbic areas within the mesolimbic reward pathway50, 51. A study in rats and humans found reduced sucrose intake relative to water, though sucrose exposure prior to surgery attenuated this effect52. Additionally, in rats, gastric bypass altered both mRNA and tissue protein levels of the sugar binding receptor proteins T1R2 and T1R3, which mediate tastes of both natural and artificial sweeteners52. In humans, RYGB patients detected lower concentrations of sucrose when compared to normal weight controls prior to surgery, and after surgery patients were able to detect lower concentrations compared to their pre-surgery levels and compared to controls52. In our study, only wanting ratings (which describe motivation for sucrose) and not liking ratings (which describe hedonic response to sucrose) influenced body weight loss between RYGB and VSG. The fact that difference in body weight loss was not observed with adjusted wanting ratings for sucrose, indicates that the absolute value of wanting ratings, rather than changes in response across concentrations within participants is relevant for body weight loss after bariatric surgery. Future studies should address these and other possible mechanisms by which sweet taste responses predict weight loss.
This was an exploratory study with some limitations. First, our analytical approach to the prediction of body weight loss is one of many, and our conclusions should be tested as a hypothesis using a controlled, randomized study design. Second, increasing the range of sucrose and aspartame concentrations could lead to different clusters. Third, it is important to determine whether use of flavored aspartame solutions has a major effect on hedonic ratings that could lead to different cluster results. Finally, the lack of sex balance in our sample size could limit the applicability of our results and indicates that future studies should aim to explore the existence and extent of any sex differences. Also, we did not take into account menstrual cycle, as it makes only a minor contribution to taste response, and only after, but not before, glucose loads53.
In conclusion, unsupervised classification of patients by means of pre-operative wanting ratings for sucrose can predict differential body weight loss after RYGB or VSG. Thus, wanting but not liking ratings reflect behaviors whose regulatory pathways are altered differentially by RYGB and VSG. The simplicity of our tests indicate that behavioral tests of sweet hedonics could be used in a clinical setting to aid in assignment of patients to surgical procedures.
Supplementary Material
Acknowledgements
This work was supported by NIH 1R01DK108643 (HR Kissileff, PI), FONDECYT Regular 1150274 (Perez-Leighton). Aspartame was a gift from Ajinomoto Co., Inc., Tokyo, Japan
Footnotes
Competing Interests.
The authors declare no competing financial interests.
Supplementary information is available at International Journals of Obesity’s website
References
- 1.Chang SH, Stoll CR, Song J, Varela JE, Eagon CJ, Colditz GA. The effectiveness and risks of bariatric surgery: an updated systematic review and meta-analysis, 2003–2012. JAMA surgery 2014; 149(3): 275–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.King WC, Hinerman AS, Belle SH, Wahed AS, Courcoulas AP. Comparison of the Performance of Common Measures of Weight Regain After Bariatric Surgery for Association With Clinical Outcomes. Jama 2018; 320(15): 1560–1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Osland E, Yunus RM, Khan S, Memon B, Memon MA. Weight Loss Outcomes in Laparoscopic Vertical Sleeve Gastrectomy (LVSG) Versus Laparoscopic Roux-en-Y Gastric Bypass (LRYGB) Procedures: A Meta-Analysis and Systematic Review of Randomized Controlled Trials. Surgical laparoscopy, endoscopy & percutaneous techniques 2017; 27(1): 8–18. [DOI] [PubMed] [Google Scholar]
- 4.Celio AC, Wu Q, Kasten KR, Manwaring ML, Pories WJ, Spaniolas K. Comparative effectiveness of Roux-en-Y gastric bypass and sleeve gastrectomy in super obese patients. Surgical endoscopy 2017; 31(1): 317–323. [DOI] [PubMed] [Google Scholar]
- 5.Rondelli F, Bugiantella W, Vedovati MC, Mariani E, Balzarotti Canger RC, Federici S et al. Laparoscopic gastric bypass versus laparoscopic sleeve gastrectomy: A retrospective multicenter comparison between early and long-term post-operative outcomes. International journal of surgery (London, England) 2017; 37: 36–41. [DOI] [PubMed] [Google Scholar]
- 6.Panagiotou OA, Markozannes G, Adam GP, Kowalski R, Gazula A, Di M et al. Comparative Effectiveness and Safety of Bariatric Procedures in Medicare-Eligible Patients: A Systematic Review. JAMA surgery 2018; 153(11): e183326. [DOI] [PubMed] [Google Scholar]
- 7.Ahmed B, King WC, Gourash W, Belle SH, Hinerman A, Pomp A et al. Long-term weight change and health outcomes for sleeve gastrectomy (SG) and matched Roux-en-Y gastric bypass (RYGB) participants in the Longitudinal Assessment of Bariatric Surgery (LABS) study. Surgery 2018; 164(4): 774–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Larraufie P, Roberts GP, McGavigan AK, Kay RG, Li J, Leiter A et al. Important Role of the GLP-1 Axis for Glucose Homeostasis after Bariatric Surgery. Cell reports 2019; 26(6): 1399–1408.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Behary P, Miras AD. Food preferences and underlying mechanisms after bariatric surgery. The Proceedings of the Nutrition Society 2015; 74(4): 419–25. [DOI] [PubMed] [Google Scholar]
- 10.Miras AD, le Roux CW. Mechanisms underlying weight loss after bariatric surgery. Nature reviews. Gastroenterology & hepatology 2013; 10(10): 575–84. [DOI] [PubMed] [Google Scholar]
- 11.Lauti M, Kularatna M, Hill AG, MacCormick AD. Weight Regain Following Sleeve Gastrectomy-a Systematic Review. Obesity surgery 2016; 26(6): 1326–34. [DOI] [PubMed] [Google Scholar]
- 12.Al-Khyatt W, Ryall R, Leeder P, Ahmed J, Awad S. Predictors of Inadequate Weight Loss After Laparoscopic Gastric Bypass for Morbid Obesity. Obesity surgery 2017; 27(6): 1446–1452. [DOI] [PubMed] [Google Scholar]
- 13.Scozzari G, Passera R, Benvenga R, Toppino M, Morino M. Age as a long-term prognostic factor in bariatric surgery. Annals of surgery 2012; 256(5): 724–8; discussion 728–9. [DOI] [PubMed] [Google Scholar]
- 14.Andersen JR, Aadland E, Nilsen RM, Vage V. Predictors of weight loss are different in men and women after sleeve gastrectomy. Obesity surgery 2014; 24(4): 594–8. [DOI] [PubMed] [Google Scholar]
- 15.Parri A, Benaiges D, Schroder H, Izquierdo-Pulido M, Ramon J, Villatoro M et al. Preoperative predictors of weight loss at 4 years following bariatric surgery. Nutrition in clinical practice: official publication of the American Society for Parenteral and Enteral Nutrition 2015; 30(3): 420–4. [DOI] [PubMed] [Google Scholar]
- 16.Baldridge AS, Pacheco JA, Aufox SA, Kim KY, Silverstein JC, Denham W et al. Factors Associated With Long-Term Weight Loss Following Bariatric Surgery Using 2 Methods for Repeated Measures Analysis. American journal of epidemiology 2015; 182(3): 235–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Generali I, De Panfilis C. Personality Traits and Weight Loss Surgery Outcome. Current obesity reports 2018; 7(3): 227–234. [DOI] [PubMed] [Google Scholar]
- 18.Marek RJ, Tarescavage AM, Ben-Porath YS, Ashton K, Merrell Rish J, Heinberg LJ. Using presurgical psychological testing to predict 1-year appointment adherence and weight loss in bariatric surgery patients: predictive validity and methodological considerations. Surgery for obesity and related diseases: official journal of the American Society for Bariatric Surgery 2015; 11(5): 1171–81. [DOI] [PubMed] [Google Scholar]
- 19.Wakayama L, Nameth K, Adler S, Safer DL. Replication and extension of dietary adherence as a predictor of suboptimal weight-loss outcomes in postbariatric patients. Surgery for obesity and related diseases: official journal of the American Society for Bariatric Surgery 2018. [DOI] [PubMed] [Google Scholar]
- 20.Sarwer DB, Wadden TA, Moore RH, Baker AW, Gibbons LM, Raper SE et al. Preoperative eating behavior, postoperative dietary adherence, and weight loss after gastric bypass surgery. Surgery for obesity and related diseases: official journal of the American Society for Bariatric Surgery 2008; 4(5): 640–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ahmed K, Penney N, Darzi A, Purkayastha S. Taste Changes after Bariatric Surgery: a Systematic Review. Obesity surgery 2018; 28(10): 3321–3332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kittrell H, Graber W, Mariani E, Czaja K, Hajnal A, Di Lorenzo PM. Taste and odor preferences following Roux-en-Y surgery in humans. PloS one 2018; 13(7): e0199508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hansen TT, Jakobsen TA, Nielsen MS, Sjodin A, Le Roux CW, Schmidt JB. Hedonic Changes in Food Choices Following Roux-en-Y Gastric Bypass. Obesity surgery 2016; 26(8): 1946–55. [DOI] [PubMed] [Google Scholar]
- 24.Miras AD, Jackson RN, Jackson SN, Goldstone AP, Olbers T, Hackenberg T et al. Gastric bypass surgery for obesity decreases the reward value of a sweet-fat stimulus as assessed in a progressive ratio task. The American journal of clinical nutrition 2012; 96(3): 467–73. [DOI] [PubMed] [Google Scholar]
- 25.Sudan R, Sudan R, Lyden E, Thompson JS. Food cravings and food consumption after Roux-en-Y gastric bypass versus cholecystectomy. Surgery for obesity and related diseases: official journal of the American Society for Bariatric Surgery 2017; 13(2): 220–226. [DOI] [PubMed] [Google Scholar]
- 26.Nielsen MS, Christensen BJ, Ritz C, Rasmussen S, Hansen TT, Bredie WLP et al. Roux-En-Y Gastric Bypass and Sleeve Gastrectomy Does Not Affect Food Preferences When Assessed by an Ad libitum Buffet Meal. Obesity surgery 2017; 27(10): 2599–2605. [DOI] [PubMed] [Google Scholar]
- 27.Sugerman HJ, Londrey GL, Kellum JM, Wolf L, Liszka T, Engle KM et al. Weight loss with vertical banded gastroplasty and Roux-Y gastric bypass for morbid obesity with selective versus random assignment. American journal of surgery 1989; 157(1): 93–102. [DOI] [PubMed] [Google Scholar]
- 28.Sugerman HJ, Starkey JV, Birkenhauer R. A randomized prospective trial of gastric bypass versus vertical banded gastroplasty for morbid obesity and their effects on sweets versus non-sweets eaters. Annals of surgery 1987; 205(6): 613–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Herpertz S, Kielmann R, Wolf AM, Hebebrand J, Senf W. Do psychosocial variables predict weight loss or mental health after obesity surgery? A systematic review. Obesity research 2004; 12(10): 1554–69. [DOI] [PubMed] [Google Scholar]
- 30.Fox B, Chen E, Suzo A, Jolles S, Greenberg JA, Campos GM et al. Dietary and psych predictors of weight loss after gastric bypass. The Journal of surgical research 2015; 197(2): 283–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Berridge KC, Ho CY, Richard JM, DiFeliceantonio AG. The tempted brain eats: pleasure and desire circuits in obesity and eating disorders. Brain research 2010; 1350: 43–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Finlayson G, King N, Blundell JE. Liking vs. wanting food: importance for human appetite control and weight regulation. Neuroscience and biobehavioral reviews 2007; 31(7): 987–1002. [DOI] [PubMed] [Google Scholar]
- 33.Berridge KC. Motivation concepts in behavioral neuroscience. Physiology & behavior 2004; 81(2): 179–209. [DOI] [PubMed] [Google Scholar]
- 34.Thompson DA, Moskowitz HR, Campbell RG. Effects of body weight and food intake on pleasantness ratings for a sweet stimulus. Journal of applied physiology 1976; 41(1): 77–83. [DOI] [PubMed] [Google Scholar]
- 35.Iatridi V, Hayes J, Yeomans M. Reconsidering the classification of sweet taste liker phenotypes: A methodological review. Food Quality and Preference 2019; 72: 56–76. [Google Scholar]
- 36.Garbutt JC, Osborne M, Gallop R, Barkenbus J, Grace K, Cody M et al. Sweet liking phenotype, alcohol craving and response to naltrexone treatment in alcohol dependence. Alcohol and alcoholism (Oxford, Oxfordshire) 2009; 44(3): 293–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Garbutt JC, Kampov-Polevoy AB, Kalka-Juhl LS, Gallop RJ. Association of the Sweet-Liking Phenotype and Craving for Alcohol With the Response to Naltrexone Treatment in Alcohol Dependence: A Randomized Clinical Trial. JAMA psychiatry 2016; 73(10): 1056–1063. [DOI] [PubMed] [Google Scholar]
- 38.Iatridi V, Hayes JE, Yeomans MR. Quantifying Sweet Taste Liker Phenotypes: Time for Some Consistency in the Classification Criteria. Nutrients 2019; 11(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sclafani A, Ackroff K. Glucose- and fructose-conditioned flavor preferences in rats: taste versus postingestive conditioning. Physiology & behavior 1994; 56(2): 399–405. [DOI] [PubMed] [Google Scholar]
- 40.Klein DA, Schebendach JS, Devlin MJ, Smith GP, Walsh BT. Intake, sweetness and liking during modified sham feeding of sucrose solutions. Physiology & behavior 2006; 87(3): 602–6. [DOI] [PubMed] [Google Scholar]
- 41.Klein DA, Schebendach JE, Brown AJ, Smith GP, Walsh BT. Modified sham feeding of sweet solutions in women with and without bulimia nervosa. Physiology & behavior 2009; 96(1): 44–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hogenkamp PS, Shechter A, St-Onge MP, Sclafani A, Kissileff HR. A sipometer for measuring motivation to consume and reward value of foods and beverages in humans: Description and proof of principle. Physiology & behavior 2017; 171: 216–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Garneau N, Nuessle T, Mendelsber B, Shepard S, Tucker R. Sweet liker status in children and adults: Consequences for beverage intake in adults. Food Quality and Preference 2018; 65: 175–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kim J, Prescott J, Kim K. Emotional responses to sweet foods according to sweet liker status. Food Quality and Preference 2017; 59: 1–7. [Google Scholar]
- 45.Berry DA. Logarithmic transformations in ANOVA. Biometrics 1987; 43(2): 439–56. [PubMed] [Google Scholar]
- 46.Measures of difference for compositional data and hierarchical clustering methods. Proceedings of IAMG, 1998. [Google Scholar]
- 47.Park P, Manjourides J, Bonetti M, Pagano M. A permutation test for determining significance of clusters with applications to spatial and gene expression data. Computational Statistics & Data Analysis 2009; 53(12): 4290–4300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bray GA, Barry RE, Benfield JR, Castelnuovo-Tedesco P, Rodin J. Intestinal bypass surgery for obesity decreases food intake and taste preferences. The American journal of clinical nutrition 1976; 29(7): 779–83. [DOI] [PubMed] [Google Scholar]
- 49.Mathes CM, Spector AC. Food selection and taste changes in humans after Roux-en-Y gastric bypass surgery: a direct-measures approach. Physiology & behavior 2012; 107(4): 476–83. [DOI] [PubMed] [Google Scholar]
- 50.Ochner CN, Kwok Y, Conceicao E, Pantazatos SP, Puma LM, Carnell S et al. Selective reduction in neural responses to high calorie foods following gastric bypass surgery. Annals of surgery 2011; 253(3): 502–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ochner CN, Stice E, Hutchins E, Afifi L, Geliebter A, Hirsch J et al. Relation between changes in neural responsivity and reductions in desire to eat high-calorie foods following gastric bypass surgery. Neuroscience 2012; 209: 128–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bueter M, Miras AD, Chichger H, Fenske W, Ghatei MA, Bloom SR et al. Alterations of sucrose preference after Roux-en-Y gastric bypass. Physiology & behavior 2011; 104(5): 709–21. [DOI] [PubMed] [Google Scholar]
- 53.Pliner P, Fleming AS. Food intake, body weight, and sweetness preferences over the menstrual cycle in humans. Physiology & behavior 1983; 30(4): 663–6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.