Abstract
Background and Purpose
Hyperekplexia (HPX), a rare neurogenetic disorder, is classically characterized by neonatal hypertonia, exaggerated startle response provoked by the sudden external stimuli and followed by a shortly general stiffness. Glycine receptor alpha 1 (GLRA1) is the major pathogenic gene of the disease. We described the clinical manifestations of genetically confirmed HPX patients and made a literature review of GLRA1-related HPX to improve the early recognition and prompt the management of the disorder.
Methods
Extensive clinical evaluations were analyzed in 4 Chinese HPX patients from two unrelated families. Next generation sequencing was conducted in the probands. Sanger sequence and segregation analysis were applied to confirm the findings.
Results
All four patients including 3 males and 1 female presented with excessive startle reflex, a cautious gait and recurrent falls. Moreover, startle episodes were dramatically improved with the treatment of clonazepam in all cases. Exome sequencing revealed 2 homozygous GLRA1 mutations in the patients. The mutation c.1286T>A p.I429N has been previously reported, while c.754delC p.L252* is novel.
Conclusions
HPX is a treatable disease, and clonazepam is the drug of choice. By studying and reviewing the disorder, we summarized the phenotype, expanded the genotype spectrum, and discussed the possible pathogenic mechanisms to enhance the understanding and recognition of the disease. Early awareness of the disease is crucial to the prompt and proper administration, as well as the genetic counseling.
Keywords: hyperekplexia, startle reaction, glycine receptor alpha 1, mutation, clonazepam
INTRODUCTION
Hyperekplexia (HPX), also referred to as Startle disease, is a rare inherited neurological disorder which is clinically characterized by neonatal hypertonia, generalized muscle stiffness and exaggerated startle reflexes provoked by sudden, unexpected auditory, tactile, and visual stimuli.1,2 In 1958 Kirstein and Silfverskiold firstly described a family of which affected members suffered sudden falls precipitated by ‘emotional’ stimuli.3 In 1966 Suhren investigated a large Dutch pedigree with the similar symptoms and firstly named the disorder “HPX.”4 Clinically, a generalized stiffness is always noted early after birth, which may be associated with apnea attacks and sudden infant death syndrome,5 or some may be gradually improved in the first few years of life. While excessive startling may last throughout life which can occasionally cause serious traumatic injuries and impaired social interactions in older children.1 Periodic limb movements in sleep and a characteristic head retraction reflex (nose-tapping test) can be observed in most patients with HPX.6 Genetically, the disorder shows genetic heterogeneity.7 Among the causative genes, GLRA1 is the major one, which accounts for about 80% of all cases.8 To date, the pathogenic mechanism of the disease is still not fully understood. However, HPX has relatively a good prognosis with clonazepam to effectively respond to the startle episodes.9
Although potentially treatable, HPX is not necessarily a benign condition. In practice, it can be easily misdiagnosed and missed the prompt and appropriate treatment due to limited attention understanding of the disease.8 Herein, clinical and genetic investigation of 4 Chinese patients from two unrelated families with novel GLRA1 mutations were described, and a further detailed literature review of the GLRA1-related HPX was made to summarize the clinical manifestations, expand the genetic spectrum, and discuss the possible pathogenic mechanisms to enhance the early recognition, shed light on the pathogenetic studies and improve the systematic management for the disorder.
METHODS
Patients
A total of 4 patients from 2 unrelated families were enrolled in this study. Clinical diagnosis of HPX was based on the following performances: exaggerated startle reflex, muscle stiffness, a positive nose-tapping test, and a good response to clonazepam. All patients came from the neurology department of RuiJin Hospital and were evaluated by two senior neurologists at least. The ethics committee of Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China approved the study (2017-38). All participants or their guardians provided written informed consents.
Exome sequencing and data analysis
Genomic DNA was extracted using the standardized phenol/chloroform extraction protocol. Exome sequencing was performed on the proband of each family. The variants were analyzed as follows: firstly, the 1,000 Genomes Project (http://www.internationalgenome.org), dbSNP database (http://www.ncbi.nlm.nih.gov/projects/SNP), and the Exome Aggregation Consortium (ExAC, http://exac.broadinstitute.org/) were as references to exclude all variants present in the population at greater than 5% frequency. Then, the pathogenicity of the nucleotide and amino acid conservation was predicted by Mutationtaster (http://www.mutationtaster.org), PolyPhen-2 (http://genetics.bwh.harvard.edu/pph2), and SIFT (http://sift.jcvi.org). Finally, the pathogenic of the variant was interpreted and classified following the American College of Medical Genetics and Genomics Standards and Guidelines.10 Putative pathogenic variants were further confirmed by Sanger sequencing, as well as co-segregation analysis among family members.
RESULTS
Clinical findings
Clinical profiles of the 4 patients are summarized in Table 1. Total 4 patients from 2 unrelated families, including 3 males and 1 female were enrolled. All patients had no remarkable neonatal problems. Muscle stiffness and/or excessive startle response were noticed during the neonatal period. The abnormal startle response was usually triggered by unexpected noises and constituted sudden stiffness, recurrent rigid falls to the ground with arms by two sides, but without loss of consciousness. All cases showed a cautious gait with a wide stride in adult life. Interestingly, all patients dared not to walk on hard ground, but could walk freely without fear on the greensward, or with something relied on, such as wall, wheelchair, umbrella or stick (the video of the cautious gait and the improved therapeutic effects in siblings of the family 111). The non-specific abnormalities in electroencephalogram (focal spike or slow waves) were found in two patients (family 1). All patients were previously judged as epilepsy or dystonia, and endured long time fear and frequent traumatic falls before correctly diagnosed. There was no developmental delay or intellectual disturbance in all patients. The other examinations such as, routine laboratory test, brain imaging showed no notable abnormalities in all cases. Moreover, clonazepam was administrated to all patients soon after the consideration of HPX, stiffness and startle reflex were dramatically or partially improved.
Table 1. Clinical, medications, and genetic aspects of the patients.
| Patient no. | Sex | Age when noticed (year) | Age at diagnosis (year) | Inheritance | Phenomenology | Complications | EEG | Previous diagnosis | Responsive to CZP | GLRA1 mutations/ACMG classification |
|---|---|---|---|---|---|---|---|---|---|---|
| Family 1 | ||||||||||
| IV:1 | M | Neonate | 48 | Autosomal recessive | Neonatal hypertonia, exaggerated startle reflexes, stiff in 4 limbs, expressionless face, timid | Cerebral hemorrhage and skull fractures caused by recurrent falls | Focal spike and slow waves | Epilepsy, dystonia | Significantly improved (1 mg/day) | c.754delC p.L252* (Hm) “pathogenic” |
| IV:2 | F | Neonate | 41 | Autosomal recessive | Neonatal hypertonia, exaggerated startle reflexes, stiff in 4 limbs, expressionless face, timid | Head trauma caused by recurrent falls | Focal slow waves | Epilepsy, dystonia | Significantly relieved (1 mg/day) | c.754delC p.L252* (Hm) “pathogenic” |
| Family 2 | ||||||||||
| II:1 | M | Neonate | 51 | Autosomal recessive | Exaggerated startle reflexes, timid | Recurrent falls and dizziness | Not done | PNKD | Partially relieved (0.5 mg/day) | c.1286T>A p.I429N (Hm) “pathogenic” |
| II:4 | M | Neonate | 46 | Autosomal recessive | Exaggerated startle reflexes, timid | Recurrent falls | Not done | - | Partially relieved (0.5 mg/day) | c.1286T>A p.I429N (Hm) “pathogenic” |
Responsive to CZP: significantly improved (can walk freely with nothing rely on, startle reflexes almost vanished); partially relieved (maintain a slight sense of alertness, mild cautious gait); nonresponsive/insensitive (still with strong startle response when the dosage was increased to 2–5 mg daily).
ACMG: American College of Medical Genetics and Genomics Standards and Guidelines, CZP: clonazepam, EEG: electroencephalogram, F: female, Hm: homozygous, M: male, PNKD: paroxysmal non-kinesigenic dyskinesia.
Genetic findings
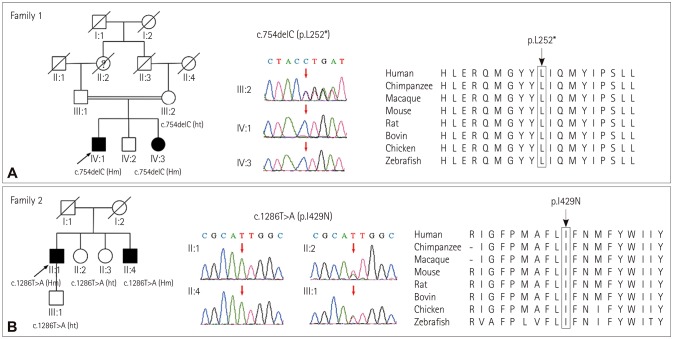
Exome sequencing revealed 2 homozygous mutations in GLAR1 (NM_000171) (Fig. 1). In consanguineous family 1, the two siblings had a novel homozygous deletion mutation c.754delC (p.L252*), while their asymptomatic mother is a heterozygous carrier. In family 2, the proband and his younger brother carried a documented homozygous missense mutation c.1286T>A (p.I429N), his asymptomatic sister and son are heterozygous carriers. All the patients showed an autosomal recessive inheritance mode. The mutations above were not found in 1,000 Genomes Project, dbSNP, and ExAC database, and Mutationtaster predicted the mutations to be disease-causing.
Fig. 1. The family pedigrees with a diagnosis of hyperekplexia. The family trees, the chromatograms and the mutations located in the highly-conserved region of proteins are shown from left to right respectively. A: Homogenous GLRA1 c.754delC (p.L252*) identified in the two siblings (IV:1 and IV:3), their asymptomatic mother (III:2) is a heterozygous carrier. B: Homogenous GLRA1 c.1286T>A (p.I429N) identified in the proband and his younger brother (II:1 and II:4), his asymptomatic sister and son are heterozygous mutation carriers (II:2 and III:1).
DISCUSSION
In this study, we investigated the clinical features of 4 patients from 2 unrelated families with genetically confirmed diagnosis of HPX. All cases were onset shortly after birth and showed an excessive startle response to unexpected stimuli, along with a wide-based and stiff gait in adulthood. While delayed diagnosis of all patients due to lack of awareness. Two GLRA1 homozygous mutations in the patients have been detected, including 1 novel nonsense mutation (c.754delC p.L252*), 1 documented missense mutation (c.1286T>A p.I429N).12 All mutations were highly conservative in the related species. HPX is a rare neuromotor disorder classically characterized by neonatal hypertonia and an exaggerated startle reflex, which is caused by defects in mammalian glycinergic neurotransmission. Only a few cases have been reported in China, including 6 patients from 5 unrelated families carry GLRA1 mutations,12,13,14,15,16 one patient carry GLRB mutation.17 There are two clinical forms of the disorder: a major and a minor form. The major form is typically characterized by generalized stiffness after birth, excessive startling to a sudden stimulus and generalized stiffness after a startle reflex, while the minor form only has excessive startle response.1,9 HPX can also be complicated by umbilical/inguinal hernia, hip dislocation, epilepsy, myoclonus, periodic limb movement during sleep, delayed motor development or social dysfunction.6,9,18 Hypertonicity is noted during neonatal or early infancy time, which may cause prolonged apnea and even sudden death, or gradually diminished spontaneously during the first few years of life. Excessive startle reflexes may persist throughout life, although the severity differs among the patients. The disorder always has a neonatal onset, which requires more attention in paediatrician's clinical practice. In our study, the electroencephalogram (EEG) abnormalities may be a non-specific pathological change, which might due to the severe craniocerebral trauma caused by the recurrent falls. As reported in previous study, fast spikes followed by slow background activity and flattening can be observed on EEG without epileptic discharges of the patient.19 In addition, phenotype disparities existed between the affected members in the same pedigree, which is consisted with the previous reports,1,8,20 suggesting an underling mechanism of variable expressivity. Currently, clonazepam is the drug of choice that dramatically improves the exaggerated startling, which through enhancing GABA-gated chloride channel function and presumably compensating for the defective glycine-gated chloride channel function.9
Pathogenic variants in 5 genes relating to the glycinergic neurotransmission system have been identified in HPX. GLRA1 and GLRB encode the α1 and β subunit of the postsynaptic inhibitory glycine receptor respectively. SLC6A5 encodes the cognate presynaptic glycine transporter 2. It was reported that patients with GLRB and SLC6A5 mutations are more likely to have apnea attacks, or mild to severe delay in development or speech acquisition.7,21 While the mutations in GPHN and ARHGEF9, of which the encoding proteins gephyrin and collybistin are involved in GlyR synaptic clustering, have also been reported to result in more complex or atypical phenotype.22,23 All these proteins are implicated in the normal functioning of inhibitory glycinergic synapses, which are located predominantly in the spinal cord and brainstem.24 In human, four α subunits (α1–α4) and a single β subunit formed the GlyRs, which belong to the Cys-loop family of pentameric ligand-gated ion channel receptors. Binding with its ligand glycine, which is an essential inhibitory neurotransmitter in the adult nervous system, the glycinergic signaling plays a vital role in neural development, including spinal cord, brain, retinal.24 The abnormalities in electrophysiological studies also indicated that abnormal reciprocal inhibition or increased excitability in pontomedullary reticular neurons may contribute to generalized stiffness in hypereplexia.25,26
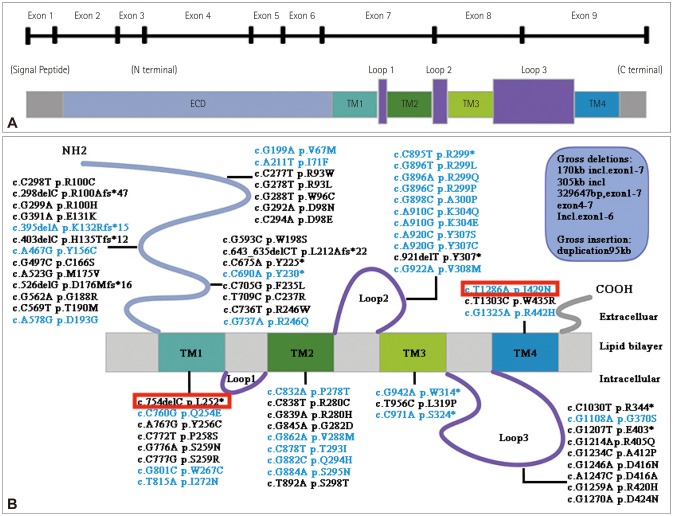
The causative gene GLRA1 is the most common one, which has 9 exons (Fig. 2A). The encoding protein α1 subunit of GlyR contains an extracellular domain (ECD) that harboring the neurotransmitter binding site and a transmembrane domain (TMD) that comprising 4 α-helices, termed TM1–TM427 (Fig. 2A). It demonstrated both dominant and recessive inheritance in GLRA1. So far, about 77 various mutations have been reported, including 64 missense/nonsense mutations, 7 small deletions (including this study), 5 gross deletions and 1 gross insertion. The mutations are distributed in all domains, clustering in ECD, TM1, TM2 domains and Loop2 (Fig. 2B). Among all the mutations, we found that 40.0% (30/77) are dominant, 50.6% (39/77) are recessive (missense and nonsense), 7.8% (6/77) are recessive frameshift variants. Dominant mutations are mainly located in and around TM2 domain and Loop2, while the recessive or compound variants are scattering in all the domains. Localized at the extracellular end of TM2 domain, R299 is the most frequent mutant locus. Numerous studies suggested that dominant mutations could compromise glycine ligand binding, decrease the glycine sensitivity by disrupting the hydrogen bond, or cause chloride conductance defects.28 Besides, a few dominant mutations which located in ECD, TM1, TM2 domain could cause fast desensitization to limit chloride flux.29,30 For many recessive mutations, reduced expression or a deficiency of cell surface targeting of GlyRs might be implicated, in which the related glycine-activated currents could be observed when co-expressed with α1 and/or β wild-type subunits.28,29 Additionally, as zinc is known to have potentiating effect on glycinergic currents upon neuronal stimulation.31 Studies showed that the mutant residues which is to be the binding sites for Zn2+ may involve in the loss of zinc potentiation.27,32 Except for the loss-of-function mechanism above, some gain-of-function mutations can also cause HPX. To date, four dominant mutations (Y156C, Q254E, V308M, R442H) have been demonstrated to induce spontaneous GlyR activation, which result in enhanced sodium and calcium influx rates, and directly contribute to the reduction in the glycine-induced current amplitude.28,29 Nevertheless, the exact mechanism of GLRA1 mutations still beyond fully understand and requires more investigation.
Fig. 2. Schematic drawing of the GLRA1 gene and protein domains, and distribution of all GLRA1 mutations. A: Schematic drawing of the GLRA1 gene and protein with domains. B: Diagram of all GLRA1 mutations (NM_000171). Mutations in black: recessive or compound heterozygous mutations; mutations in blue: dominant mutations. The mutations detected in this paper are marked in red. The five gross deletion mutations, including 170 kb inclusive exon 1–7 (−93.3 kb upstream of ATG), 305 kb incl, 329647 bp exon 1–7, exon 4–7 and inclusive exon 1–6. One insertion: duplication 95 kb. ECD: extracellular domain, TM: transmembrane domain.
Due to the overlapping clinical signs, HPX can be initially misdiagnosed as epilepsy, cerebral palsy in infancy period, or adult-onset anxiety neurosis. The disorder has a neonatal onset, when newborns showed diffuse muscular rigidity, episodic tonic spasm, apnea, aspiration pneumonia, it should be considered the possibility of HPX. Nose-tapping test can make a preliminary judgement. Once acute hypertonia and apnea episodes occur, a simple intervention called the Vigevano action (flexing of the head and limbs toward the trunk) can relieve the event.33 Fortunately, HPX is a potentially treatable disease. Clonazepam, which can specifically upgrade the GABARA1 chloride channels, is the main and most effective administration for HPX patients.9 The treatment is recommended to start with a dose of 0.5 mg daily, adjust dosage based on effects, up to 6 mg daily if necessary.34 Some other antiepileptic drugs, like carbamazepine or phenobarbital, can also be used while their therapeutic effect is still in debate. For the psychological problems like social anxiety, timidity and self-basement, psychotherapy and family support are crucial for personality development and alleviating mental problems.
In conclusions, HPX is a treatable neurogenetic disorder, and clonazepam is the drug of choice. Mutations in GLRA1 account for the most. By studying and reviewing the disorder, we summarized the phenotype, expanded the genotype spectrum, discussed the possible pathogenic mechanisms, to enhance learning, awareness-raising of the HPX and shed light on the pathogenetic studies. Early recognition of the disease is helpful for prompt and appropriate treatment, to avoid the serious adverse events and improve the quality of life. Moreover, prompt genetic analysis may be useful for early definite diagnosis, genetic counseling and safer care for affected neonates.
Acknowledgements
This study was supported by the grants from the National Natural Science Foundation of China (No.81571086 and 81870889), National Key R&D Program of China (2017YFC1310200), Shanghai Municipal Education Commission-Gaofeng Clinical Medicine Grant (20161401) and Interdisciplinary Project of Shanghai Jiao Tong University (YG2016MS64).
We would like to thank the patients for their cooperation.
Footnotes
- Conceptualization: Li Cao.
- Data curation: Feixia Zhan, Chao Zhang, Shige Wang, Zeyu Zhu, Guang Chen, Mingliang Zhao, Li Cao.
- Formal analysis: Feixia Zhan, Chao Zhang, Li Cao.
- Funding acquisition: Li Cao.
- Investigation: Feixia Zhan, Chao Zhang, Shige Wang, Zeyu Zhu, Guang Chen, Mingliang Zhao, Li Cao.
- Methodology: Feixia Zhan, Chao Zhang, Li Cao.
- Project administration: Li Cao.
- Resources: Li Cao.
- Software: Feixia Zhan, Chao Zhang.
- Supervision: Li Cao.
- Validation: Li Cao.
- Writing—original draft: Feixia Zhan.
- Writing—review & editing: Li Cao.
Conflicts of Interest: The authors have no potential conflicts of interest to disclose.
References
- 1.Bakker MJ, van Dijk JG, van den Maagdenberg AMJM, Tijssen MAJ. Startle syndromes. Lancet Neurol. 2006;5:513–524. doi: 10.1016/S1474-4422(06)70470-7. [DOI] [PubMed] [Google Scholar]
- 2.Harvey RJ, Topf M, Harvey K, Rees MI. The genetics of hyperekplexia: more than startle. Trends Genet. 2008;24:439–447. doi: 10.1016/j.tig.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 3.Kirstein L, Silfverskiold BP. A family with emotionally precipitated drop seizures. Acta Psychiatr Neurol Scand. 1958;33:471–476. doi: 10.1111/j.1600-0447.1958.tb03533.x. [DOI] [PubMed] [Google Scholar]
- 4.Suhren O, Bruyn GW, Tuynman JA. Hyperexplexia: a hereditary startle syndrome. J Neurol Sci. 1966;3:577–605. [Google Scholar]
- 5.Giacoia GP, Ryan SG. Hyperekplexia associated with apnea and sudden infant death syndrome. Arch Pediatr Adolesc Med. 1994;148:540–543. doi: 10.1001/archpedi.1994.02170050098025. [DOI] [PubMed] [Google Scholar]
- 6.Mine J, Taketani T, Yoshida K, Yokochi F, Kobayashi J, Maruyama K, et al. Clinical and genetic investigation of 17 Japanese patients with hyperekplexia. Dev Med Child Neurol. 2015;57:372–377. doi: 10.1111/dmcn.12617. [DOI] [PubMed] [Google Scholar]
- 7.Thomas RH, Chung SK, Wood SE, Cushion TD, Drew CJG, Hammond CL, et al. Genotype-phenotype correlations in hyperekplexia: apnoeas, learning difficulties and speech delay. Brain. 2013;136:3085–3095. doi: 10.1093/brain/awt207. [DOI] [PubMed] [Google Scholar]
- 8.Sprovieri T, Ungaro C, Sivo S, Quintiliani M, Contaldo I, Veredice C, et al. Clinical features and genetic analysis of two siblings with startle disease in an Italian family: a case report. BMC Med Genet. 2019;20:40. doi: 10.1186/s12881-019-0779-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhou L, Chillag KL, Nigro MA. Hyperekplexia: a treatable neurogenetic disease. Brain Dev. 2002;24:669–674. doi: 10.1016/s0387-7604(02)00095-5. [DOI] [PubMed] [Google Scholar]
- 10.Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015;17:405–424. doi: 10.1038/gim.2015.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang C, Wang SG, Wang Y, Liu XL, Cao L. Teaching video neuroimages: cautious walking gait in siblings with hereditary hyperekplexia. Neurology. 2019;92:e2068–e2069. doi: 10.1212/WNL.0000000000007375. [DOI] [PubMed] [Google Scholar]
- 12.Huang Z, Lian Y, Xu H, Zhang H. Weird laughing in hyperekplexia: a new phenotype associated with a novel mutation in the GLRA1 gene? Seizure. 2018;58:6–8. doi: 10.1016/j.seizure.2018.03.017. [DOI] [PubMed] [Google Scholar]
- 13.Yang Z, Sun G, Yao F, Tao D, Zhu B. A novel compound mutation in GLRA1 cause hyperekplexia in a Chinese boy- a case report and review of the literature. BMC Med Genet. 2017;18:110. doi: 10.1186/s12881-017-0476-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chan KK, Cherk SWW, Lee HHC, Poon WT, Chan AYW. Hyperekplexia: a Chinese adolescent with 2 novel mutations of the GLRA1 gene. J Child Neurol. 2014;29:111–113. doi: 10.1177/0883073812465338. [DOI] [PubMed] [Google Scholar]
- 15.Poon WT, Au KM, Chan YW, Chan KY, Chow CB, Tong SF, et al. Novel missense mutation (Y279S) in the GLRA1 gene causing hyperekplexia. Clin Chim Acta. 2006;364:361–362. doi: 10.1016/j.cca.2005.09.018. [DOI] [PubMed] [Google Scholar]
- 16.Tsai CH, Chang FC, Su YC, Tsai FJ, Lu MK, Lee CC, et al. Two novel mutations of the glycine receptor gene in a Taiwanese hyperekplexia family. Neurology. 2004;63:893–896. doi: 10.1212/01.wnl.0000138566.65519.67. [DOI] [PubMed] [Google Scholar]
- 17.Li H, Yang ZX, Xue J, Qian P, Liu XY. [Clinical and genetic analysis of hyperekplexia in a Chinese child and literature review] Zhonghua Er Ke Za Zhi. 2017;55:120–124. doi: 10.3760/cma.j.issn.0578-1310.2017.02.013. [DOI] [PubMed] [Google Scholar]
- 18.Lee Y, Kim NY, Hong S, Chung SJ, Jeong SH, Lee PH, et al. Familiar hyperekplexia, a potential cause of cautious gait: a new Korean case and a systematic review of phenotypes. J Mov Disord. 2017;10:53–58. doi: 10.14802/jmd.16044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tohier C, Roze JC, David A, Veccierini MF, Renaud P, Mouzard A. Hyperexplexia or Stiff Baby Syndrome. Arch Dis Child. 1991;66:460–461. doi: 10.1136/adc.66.4.460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zwarts MJ, Willemsen MH, Kamsteeg EJ, Schelhaas HJ. Paroxysmal sensory (spinal) attacks without Hyperexplexia in a patient with a variant in the GLRA1 gene. J Neurol Sci. 2017;378:175–176. doi: 10.1016/j.jns.2017.05.024. [DOI] [PubMed] [Google Scholar]
- 21.Masri A, Chung SK, Rees MI. Hyperekplexia: report on phenotype and genotype of 16 Jordanian patients. Brain Dev. 2017;39:306–311. doi: 10.1016/j.braindev.2016.10.010. [DOI] [PubMed] [Google Scholar]
- 22.Rees MI, Harvey K, Ward H, White JH, Evans L, Duguid IC, et al. Isoform heterogeneity of the human gephyrin gene (GPHN), binding domains to the glycine receptor, and mutation analysis in hyperekplexia. J Biol Chem. 2003;278:24688–24696. doi: 10.1074/jbc.M301070200. [DOI] [PubMed] [Google Scholar]
- 23.Harvey K, Duguid IC, Alldred MJ, Beatty SE, Ward H, Keep NH, et al. The GDP-GTP exchange factor collybistin: an essential determinant of neuronal gephyrin clustering. J Neurosci. 2004;24:5816–5826. doi: 10.1523/JNEUROSCI.1184-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chalphin AV, Saha MS. The specification of glycinergic neurons and the role of glycinergic transmission in development. Front Mol Neurosci. 2010;3:11. doi: 10.3389/fnmol.2010.00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Matsumoto J, Fuhr P, Nigro M, Hallett M. Physiological abnormalities in hereditary hyperekplexia. Ann Neurol. 1992;32:41–50. doi: 10.1002/ana.410320108. [DOI] [PubMed] [Google Scholar]
- 26.Floeter MK, Andermann F, Andermann E, Nigro M, Hallett M. Physiological studies of spinal inhibitory pathways in patients with hereditary hyperekplexia. Neurology. 1996;46:766–772. doi: 10.1212/wnl.46.3.766. [DOI] [PubMed] [Google Scholar]
- 27.Bode A, Lynch JW. The impact of human hyperekplexia mutations on glycine receptor structure and function. Mol Brain. 2014;7:2. doi: 10.1186/1756-6606-7-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chung SK, Vanbellinghen JF, Mullins JGL, Robinson A, Hantke J, Hammond CL, et al. Pathophysiological mechanisms of dominant and recessive GLRA1 mutations in hyperekplexia. J Neurosci. 2010;30:9612–9620. doi: 10.1523/JNEUROSCI.1763-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bode A, Wood SE, Mullins JGL, Keramidas A, Cushion TD, Thomas RH, et al. New hyperekplexia mutations provide insight into glycine receptor assembly, trafficking, and activation mechanisms. J Biol Chem. 2013;288:33745–33759. doi: 10.1074/jbc.M113.509240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Breitinger HG, Villmann C, Becker K, Becker CM. Opposing effects of molecular volume and charge at the hyperekplexia site alpha 1 (P250) govern glycine receptor activation and desensitization. J Biol Chem. 2001;276:29657–29663. doi: 10.1074/jbc.M100446200. [DOI] [PubMed] [Google Scholar]
- 31.Hirzel K, Müller U, Latal AT, Hülsmann S, Grudzinska J, Seeliger MW, et al. Hyperekplexia phenotype of glycine receptor α1 subunit mutant mice identifies Zn2+ as an essential endogenous modulator of glycinergic neurotransmission. Neuron. 2006;52:679–690. doi: 10.1016/j.neuron.2006.09.035. [DOI] [PubMed] [Google Scholar]
- 32.Zhou N, Wang CH, Zhang S, Wu DC. The GLRA1 missense mutation W170S associates lack of Zn2+ potentiation with human hyperekplexia. J Neurosci. 2013;33:17675–17681. doi: 10.1523/JNEUROSCI.3240-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vigevano F, Di Capua M, Dalla Bernardina B. Startle disease: an avoidable cause of sudden infant death. Lancet. 1989;1:216. doi: 10.1016/s0140-6736(89)91226-9. [DOI] [PubMed] [Google Scholar]
- 34.Dreissen YEM, Tijssen MAJ. The startle syndromes: physiology and treatment. Epilepsia. 2012;53 Suppl 7:3–11. doi: 10.1111/j.1528-1167.2012.03709.x. [DOI] [PubMed] [Google Scholar]