Dear Editor,
Spontaneous intracranial hypotension (SIH) has recently been reported to be an additional risk factor for cerebral venous thrombosis (CVT).1,2 The existence of SIH also increases the risk of developing subdural hematoma (SDH) due to brain sagging and subdural fluid collection with fragile and dilated new dural vessels.1,3 However, the occurrence of SIH in patients with CVT followed by SDH is rare, and it challenges treatment decision-making by clinicians. Here we report a CVT patient with SIH who developed SDH, which are both known complications of SIH.
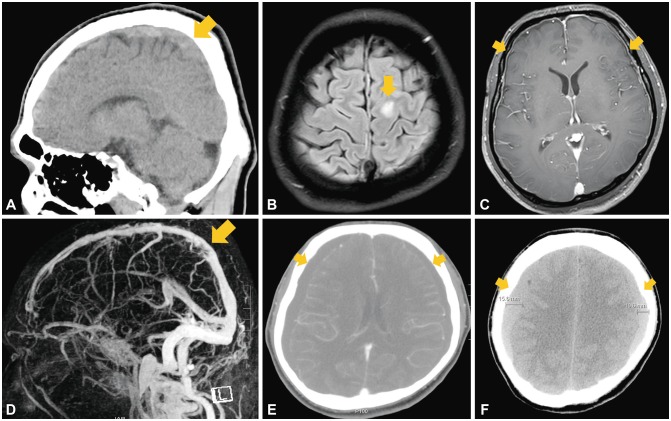
A 35-year-old man presented with a new-onset generalized tonic-clonic seizure. During the previous 7 days he had complained of a progressive headache, mild fever, and neck stiffness. He had suffered from chronic headache following mild head trauma with a brief loss of consciousness after a bicycle accident 10 years previously. A neurological examination revealed that he was drowsy and disoriented without apparent focal neurological deficit. Routine blood tests revealed leukocytosis of 18,930 cells/mm3 with D-dimer elevated at 2.74 µg/mL. The other biochemistry findings and the coagulation profile were unremarkable. The cerebrospinal fluid (CSF) was yellowish with a reduced opening pressure (62 mmH2O), and pleocytosis was absent with red blood cells correction.4 Noncontrast brain CT (NCCT) revealed a positive cord sign with hyperdensity in the superior sagittal sinus (SSS) (Fig. 1A). Fluid-attenuated inversion recovery imaging showed a hyperintense lesion in the left frontal cortex (Fig. 1B). Postgadolinium T1-weighted MRI demonstrated diffuse pachymeningeal enhancement (Fig. 1C). Cerebral magnetic resonance venography demonstrated a filling defect in the SSS consistent with CVT (Fig. 1D).
Fig. 1. Radiologic findings of the patient. A: Initial sagittal NCCT revealing a cord sign with hyperdensity of the SSS (arrow). B: Fluid-attenuated inversion recovery imaging shows a hyperintense lesion in the left frontal cortex (arrow), suggestive of a cerebral venous infarction. C: Postgadolinium T1-weighted MRI demonstrates diffuse pachymeningeal enhancement (arrows). D: Cerebral magnetic resonance venography reveals filling defects due to a thrombus within the SSS (arrow). E: Contrast-enhanced CT performed 5 days after the onset of orthostatic headache shows bilateral subdural hygromas (arrows). F: Follow-up NCCT at 5 days after an epidural blood patch demonstrates bilateral subdural hematomas (arrows). NCCT: noncontrast CT, SSS: superior sagittal sinus.
After treatment with subcutaneous low-molecular-weight heparin (Fraxiparine®, Sanofi-Aventis, Paris, France; at 5,700 IU anti-Xa bid) for 14 days and switching to warfarin (3 or 4.5 mg qd) for 5 days, in addition to levetiracetam (Keppra®, UCB, Brussels, Belgium; at 750 mg bid) for 20 days, he was discharged without neurological deficits. He was admitted again 4 days later with progressive positional headache that worsened when standing and improved soon after lying down. He was found to have bilateral subdural hygromas (Fig. 1E). His anticoagulation treatment was stopped and an epidural blood patch (EBP) was administered in the L3–L4 interspace. However, his headache worsened even when lying down, and 5 days later his level of consciousness deteriorated to a score of 13 on the Glasgow Coma Scale. Follow-up NCCT at 5 days after the first EBP revealed bilateral subdural hematomas with a clot thickness of 15.6 mm, which required neurosurgical treatment (Fig. 1F). He was able to walk with complete resolution of headache at 20 days after burr-hole surgery.
Here we describe a patient who experienced an orthostatic headache association of SIH with CVT. CVT can develop after a head trauma, lumbar puncture, or spinal anesthesia associated with SIH.5 Reductions in CSF volume and pressure lead to a compensatory increase in the dural venous blood volume and slow the blood flow, which is due to venous stasis.2 CVT with a low CSF pressure and diffuse pachymeningeal enhancement may be related to the underlying SIH. Although anticoagulation is the initial treatment applied in most CVT cases,5 it could be more important to perform screening of a leakage site and an EBP in CVT with SIH.
A few days after the lumbar puncture he developed orthostatic headache with subdural hygroma despite partial recanalization of the CVT. The brain sagging due to a low CSF pressure produces headache while sitting or standing due to traction to pain-sensitive structures, and tears in bridging dural veins leading to SDH.3 Although the incidence of subdural collections associated with SIH is ~20%, large SDHs (clot thickness >10 mm) requiring urgent neurosurgical drainage are very rare.6 Subdural hygroma with SIH probably predisposes patients to SDH during anticoagulation treatment. Thus, the underlying SIH should first be sufficiently managed with the targeted EBP before starting anticoagulation treatment for CVT or surgical evacuation for SDH in patients with SIH. In particular, burr-hole trephination for SDH should be performed together with EBP since SDH can recur if SIH is left untreated.7
The SDH in our patient was associated with a change in the headache pattern, becoming persistent both when upright and lying down. Increased intracranial pressure usually leads to persistent headache, which is often worse when lying down. These changes in the headache pattern in SIH patients could be an important sign associated with complications such as SDH and CVT.
SIH can share common risk factors with CVT and SDH. This case highlights the complex pathophysiological complications of SIH, as well as its risk stratification and treatment.
Acknowledgements
None.
Footnotes
- Conceptualization: Young-Soo Kim, Soo-Kyoung Kim.
- Data curation: Min Ok Kim, Chang Hun Kim, Jongsoo Kang.
- Supervision: Oh-Young Kwon, Nack-Cheon Choi.
- Validation: Juhyeon Kim.
- Visualization: Min Ok Kim.
- Writing—original draft: Min Ok Kim, Soo-Kyoung Kim.
- Writing—review & editing: Heeyoung Kang, Soo-Kyoung Kim.
Conflicts of Interest: The authors have no potential conflicts of interest to disclose.
References
- 1.Zhang D, Wang J, Zhang Q, He F, Hu X. Cerebral venous thrombosis in spontaneous intracranial hypotension: a report on 4 cases and a review of the literature. Headache. 2018;58:1244–1255. doi: 10.1111/head.13413. [DOI] [PubMed] [Google Scholar]
- 2.Sinnaeve L, Vanopdenbosch L, Paemeleire K. Association of cerebral venous thrombosis and intracranial hypotension: review of 3 cases. J Stroke Cerebrovasc Dis. 2017;26:e165–e169. doi: 10.1016/j.jstrokecerebrovasdis.2017.05.015. [DOI] [PubMed] [Google Scholar]
- 3.Lai TH, Fuh JL, Lirng JF, Tsai PH, Wang SJ. Subdural haematoma in patients with spontaneous intracranial hypotension. Cephalalgia. 2007;27:133–138. doi: 10.1111/j.1468-2982.2006.01249.x. [DOI] [PubMed] [Google Scholar]
- 4.Mayefsky JH, Roghmann KJ. Determination of leukocytosis in traumatic spinal tap specimens. Am J Med. 1987;82:1175–1181. doi: 10.1016/0002-9343(87)90221-x. [DOI] [PubMed] [Google Scholar]
- 5.Saposnik G, Barinagarrementeria F, Brown RD, Jr, Bushnell CD, Cucchiara B, Cushman M, et al. Diagnosis and management of cerebral venous thrombosis: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2011;42:1158–1192. doi: 10.1161/STR.0b013e31820a8364. [DOI] [PubMed] [Google Scholar]
- 6.Chen YC, Wang YF, Li JY, Chen SP, Lirng JF, Hseu SS, et al. Treatment and prognosis of subdural hematoma in patients with spontaneous intracranial hypotension. Cephalalgia. 2016;36:225–231. doi: 10.1177/0333102415585095. [DOI] [PubMed] [Google Scholar]
- 7.Schievink WI. Spontaneous spinal cerebrospinal fluid leaks and intracranial hypotension. JAMA. 2006;295:2286–2296. doi: 10.1001/jama.295.19.2286. [DOI] [PubMed] [Google Scholar]