Abstract
Background and Purpose
Sleep disturbance is common in patients with primary headache disorders. We were interest in whether poor sleep quality affects patients directly or via increases in the frequency and severity of headaches. To that end, we investigated the direct and indirect effects of sleep quality on the headache-related impact among patients with primary headache disorders.
Methods
We analyzed migraine and tension-type headache (TTH) in patients included in the headache registry of our headache clinic from October 2015 to May 2018. We collected information on the headache frequency, severity, and psychological status. Sleep quality and headache-related impact were measured using the Pittsburgh Sleep Quality Index and Headache Impact Test-6, respectively. We performed path analyses with headache frequency and severity as covariates to determine the direct effect of sleep quality on the headache-related impact, and the indirect effects mediated by increases in the headache frequency and severity.
Results
This study included 915 patients: 784 with migraine and 131 with TTH. Worse sleep quality was independently associated with greater headache-related impact in both patients with migraine and those with TTH. Path analysis revealed a direct effect (β=0.207, p<0.001) of sleep quality and an indirect effect mediated by headache frequency and severity (β=0.067, p=0.004) on the headache-related impact in migraine. In TTH, only direct effects of sleep quality on the headache-related impact were significant (β=0.224, p=0.004).
Conclusions
We suggest that poor sleep quality can directly increase the headache-related impact in both patients with migraine and TTH as well as indirectly by increasing the headache frequency and severity in patients with migraine.
Keywords: migraine, tension-type headache, sleep quality, Pittsburgh Sleep Quality Index, Headache Impact Test-6
INTRODUCTION
Migraine and tension-type headache (TTH) are the most-common primary headache disorders, with migraine affecting 14.4% of people worldwide and the prevalence of TTH ranging from 26.1% to 86.0% depending on the study population.1,2,3 The burden of these common headache disorders is still problematic in the global population, with migraine ranking 2nd and TTH ranking 28th among the neurologic disorders that cause disability.1,2 Reducing the burden of headache disorders requires a better understanding of the factors affecting the headache-related impact.4
The prevalence of poor sleep quality is higher in patients with migraine and TTH,5 and this is associated with a higher headache frequency and severity in both types of patient.6,7,8,9,10,11 Consequently, a greater headache-related impact is reported among patients with headache disorders and comorbid poor sleep quality. However, whether a poor sleep quality affects the headache-related impact directly or indirectly via increases in the headache frequency and severity has not been reported previously.
In this study we hypothesized that worse sleep quality is associated with greater headache-related impact, and we aimed to determine whether sleep affects headache-related impact directly or indirectly by increasing the headache frequency and severity in patients with primary headache disorders. We investigated the direct and indirect effects of sleep quality on the headache-related impact by analyzing data from a large number of patients visiting our headache clinic. We also established path models that included the sleep quality, headache frequency, and headache severity to investigate the direct and indirect effects of sleep quality on the headache-related impact.
METHODS
Patients
This retrospective, cross-sectional analysis of a headache clinic registry included Korean patients with headache who visited Samsung Medical Center, Seoul, South Korea from October 2015 to May 2018. The included patients with migraine and TTH 1) completed questionnaires and medical interviews and 2) agreed to their enrollment in the registry and data collection. The diagnoses of migraine and TTH were based on the third edition (beta version) of the International Classification of Headache Disorders (ICHD-3 beta).12 The study was approved by Samsung Medical Center Institutional Review Board, and all participants signed an informed consent at the inclusion visit.
Clinical evaluation
All patients who visited our headache clinic were first asked to complete a self-reported, structured headache questionnaire about headache characteristics and patient demographics. They were then interviewed by headache specialists (M.J.L. and C.S.C.) to evaluate the diagnosis of the headache disorder. To maintain diagnostic consistency, all of the patients were seen by a single investigator (M.J.L.) again to classify their headache disorders based on the ICHD-3-beta. After the interview, the study coordinator met the patients and asked if they agreed to being enrolled in the registry. If patients gave informed consent, collected data were deposited in our headache registry. After enrollment in the registry, the patients completed a structured questionnaire specifically designed for each headache disorder depending on their specific diagnosis.
This study extracted data from our headache registry on patients with episodic migraine with or without aura, chronic migraine, and probable migraine (ICHD-3 beta categories 1.1, 1.2, 1.3, and 1.5, respectively) and those with infrequent TTH, frequent TTH, and chronic TTH (ICHD-3 beta categories 2.1, 2.2, and 2.3, respectively). Patients with migraine fulfilling the criterion for chronic daily headache (more than 15 headache days per month over a 3-month period) but not chronic migraine were diagnosed with episodic migraine.
The extracted variables and the collection process were defined as follows: We collected information on headache characteristics, disease duration (years since diagnosis), headache frequency (headache days per month), headache severity (subjective headache pain rating on a numeric rating scale ranging from 0 to 10), and psychological status. According to ICHD-3 beta, the number of headache days corresponded to those that were affected by headache for all or part of the day. The psychological status was assessed using the Hospital Anxiety and Depression Scale (HADS) to measure levels of anxiety and depression.13 The HADS is a 14-item scale with 7 items related to anxiety (HADS-A) and 7 items related to depression (HADS-D). Each item is rated on a 4-point scale (from 0 to 3), giving a maximum subscale score of 21 for each of anxiety and depression. Allodynia was evaluated with the Allodynia Symptom Checklist-12 (ASC-12) among patients with migraine.14 Allodynia was defined as a ASC-12 score of 3 or more.14
Assessment of sleep quality
The Pittsburgh Sleep Quality Index (PSQI) was used to assess the sleep quality.15 This index uses 19 questions to estimate the sleep quality during the previous month. These questions are combined into the following seven subitems: subjective sleep quality, sleep latency, sleep duration, habitual sleep insufficiency, sleep disturbance, use of sleeping medication, and daytime dysfunction. The sum of the seven subitem scores produces a global score of subjective sleep quality ranging from 0 to 21, where higher scores indicate worse subjective sleep quality. A total PSQI >5 was defined as poor sleep quality in this study.15
Assessment of headache-related impact
The impact of headache on quality of life was assessed using the Headache Impact Test-6 (HIT-6).16 This tool assesses the subjective impact of headache during the previous month. It consists of a self-reported six-item list that assesses pain, social functioning, role functioning, cognitive functioning, psychological distress, and vitality.
Statistical analysis
Data are presented as mean (SD) and number (percentage) values except where indicated otherwise. The Kolmogorov-Smirnov test revealed that all of the obtained data conformed to a normal distribution. For comparisons between migraine and TTH, the chi-square (χ2) test was used for categorical variables while the independent t-test was used for continuous variables.
Univariable and multivariable linear regression analyses were used to assess the relationships between sleep quality and headache-related impact in migraine and TTH. In addition to the PSQI, we also included age, sex, disease duration, headache frequency, chronic subtype of headache, headache severity, HADS-D score, and HADS-A score as covariates. Missing data were not imputed and so were excluded from the associated analysis.
We conducted path analysis to identify the potential direct and indirect effects of sleep quality (PSQI) on the headache-related impact (HIT-6 score). Headache frequency and headache severity were included as intermediary variables, and the same model was applied to migraine and TTH. The AMOS program (version 21.0, IBM Corp., Armonk, NY, USA)17 was used to fit models with the maximum-likelihood method. Confidence intervals and p values were calculated using 500 bootstrap replications. The effect size was estimated by calculating the standardized regression coefficient (β) and standard error. We measured the direct effect of the PSQI on the HIT-6 score, as well as the indirect effects mediated by headache frequency and headache severity. The indirect effect mediated by headache frequency or headache severity was calculated by multiplying the coefficients on each path. The total indirect effects were quantified by summing up the indirect effects mediated by headache frequency and headache severity.
The model fit was assessed using the χ2 value, goodnessof-fit index (GFI), adjusted goodness-of-fit index (AGFI), normed fit index (NFI), comparative fit index (CFI), and root-mean-square error of the approximation (RMSEA). Statistical analyses were performed using IBM SPSS (version 22.0, IBM Corp.) and AMOS software. A two-tailed probability value of p<0.05 was considered significant.
RESULTS
Patients
The analysis was applied to 915 patients with migraine or TTH. The 784 patients with migraine comprised 245 (31.3%) with chronic migraine, 114 (14.5%) with migraine with aura, and 36 (4.5%) with probable migraine, while chronic TTH was present in 67 (51.1%) of the 131 patients with TTH. The demographics and characteristics of participants are presented in Table 1. Data were missing for disease duration in 13 patients and for the HADS score in 35 patients. Patients with migraine were younger and more likely to be female compared to those with TTH. The mean headache frequency was lower for migraine patients than for TTH patients. When stratified into episodic and chronic subtypes, the mean headache frequency did not differ significantly between migraine and TTH (episodic migraine vs. episodic TTH: 10.0 vs. 11.3 days, p=0.281; chronic migraine vs. chronic TTH, 24.9 vs. 25.9 days, p=0.239). Poor sleep quality (PSQI >5) was found in 624 migraine patients (79.6%) and 84 TTH patients (64.1%). The mean HIT-6 score, PSQI, and HADS-D score were significantly higher in patients with migraine than in those with TTH (64.6 vs. 54.7, p<0.001; 8.8 vs. 7.4, p<0.001; and 7.7 vs. 6.8, p=0.019; respectively). The sleep duration and HADS-A score did not differ between the groups.
Table 1. Demographics and characteristics of patients.
| Migraine (n=784) | TTH (n=131) | p | |
|---|---|---|---|
| Age, years | 42.2 (12.92) | 56.4 (13.19) | <0.001 |
| Female sex, n (%) | 605 (77.2) | 71 (54.2) | <0.001 |
| HTN, n (%) | 85 (10.8) | 37 (28.2) | <0.001 |
| DM, n (%) | 15 (1.5) | 11 (8.2) | <0.001 |
| Disease duration, years | 11.9 (10.39) | 4.9 (6.61) | <0.001 |
| Headache frequency, days/month | 14.6 (10.40) | 18.8 (11.38) | <0.001 |
| Chronic migraine/chronic TTH, n (%) | 245 (31.3) | 67 (51.1) | <0.001 |
| Headache severity, NRS score (range 0–10) | 7.7 (1.53) | 5.4 (1.88) | <0.001 |
| HIT-6 score (range 36–78) | 64.6 (11.57) | 54.7 (10.18) | <0.001 |
| Sleep duration, hours | 6.3 (1.38) | 6.2 (1.27) | 0.472 |
| PSQI (range 0–21) | 8.8 (3.62) | 7.4 (3.47) | <0.001 |
| C1 (subjective sleep quality) | 1.6 (0.82) | 1.3 (0.94) | <0.001 |
| C2 (sleep latency) | 2.4 (0.69) | 2.2 (0.74) | <0.001 |
| C3 (sleep duration) | 1.0 (1.03) | 1.0 (0.97) | 0.874 |
| C4 (habitual sleep insufficiency) | 0.6 (0.91) | 0.5 (0.93) | 0.952 |
| C5 (sleep disturbance) | 1.2 (0.58) | 1.1 (0.48) | 0.018 |
| C6 (use of sleeping medication) | 0.5 (1.01) | 0.4 (0.95) | 0.101 |
| C7 (daytime dysfunction) | 1.5 (1.02) | 1.0 (1.03) | <0.001 |
| HADS-D score (range 0–21) | 7.7 (4.03) | 6.8 (3.94) | 0.019 |
| HADS-A score (range 0–21) | 7.4 (4.09) | 6.9 (4.41) | 0.208 |
| Migraine with aura, n (%) | 114 (14.5) | - | - |
| Allodynia (ASC-12 score ≥3), n (%) | 159 (20.3) | - | - |
Data are mean (SD) or number (percentage) values.
ASC-12: Allodynia Symptom Checklist-12, DM: diabetes mellitus, HADS-A: Hospital Anxiety and Depression Scale—Anxiety, HADS-D: Hospital Anxiety and Depression Scale—Depression, HIT-6: Headache Impact Test-6, HTN: hypertension, NRS: numeric rating scale, PSQI: Pittsburgh Sleep Quality Index, TTH: tension-type headache.
Factors associated with HIT-6 score among patients with migraine
The results of linear regression analysis are summarized in Table 2. The factors associated with the HIT-6 score among patients with migraine in the univariable linear regression analysis were age (β=-0.074, p=0.038), PSQI (β=0.274, p<0.001), headache frequency (β=0.188, p<0.001), chronic migraine (β=0.157, p<0.001), headache severity (β=0.238, p<0.001), HADS-D score (β=0.215, p<0.001), and HADS-A score (β=0.209, p<0.001). The factors that were independently associated with a higher HIT-6 score in the multivariable linear analysis were the PSQI (β=0.146, p=0.001), headache frequency (β=0.116, p=0.003), and headache severity (β=0.174, p<0.001).
Table 2. Univariable and multivariable linear regression analyses of the predictors of headache-related impact in migraine.
| Univariable analysis | Multivariable analysis | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| B | SE | β | 95% CI | p | B | SE | β | 95% CI | p | |
| Age | -0.067 | 0.032 | -0.074 | -0.129 to -0.004 | 0.038 | -0.047 | 0.036 | -0.053 | -0.118 to 0.024 | 0.194 |
| Female sex | -0.126 | 0.985 | -0.005 | -2.058 to 1.807 | 0.899 | 0.815 | 0.985 | 0.030 | -1.119 to 2.749 | 0.408 |
| Disease duration | -0.028 | 0.042 | -0.026 | -0.110 to 0.053 | 0.493 | -0.007 | 0.043 | -0.006 | -0.092 to 0.078 | 0.869 |
| Headache frequency | 0.209 | 0.039 | 0.188 | 0.132 to 0.285 | <0.001 | 0.130 | 0.043 | 0.116 | 0.045 to 0.216 | 0.003 |
| Chronic migraine* | 3.713 | 0.837 | 0.157 | 2.070 to 5.356 | <0.001 | - | - | - | - | - |
| Headache severity | 1.798 | 0.263 | 0.238 | 1.282 to 2.313 | <0.001 | 1.335 | 0.282 | 0.174 | 0.783 to 1.888 | <0.001 |
| PSQI | 0.875 | 0.110 | 0.274 | 0.659 to 1.090 | <0.001 | 0.466 | 0.133 | 0.146 | 0.205 to 0.728 | 0.001 |
| HADS-D score | 0.622 | 0.103 | 0.215 | 0.420 to 0.825 | <0.001 | 0.261 | 0.134 | 0.092 | -0.002 to 0.523 | 0.052 |
| HADS-A score | 0.594 | 0.102 | 0.209 | 0.394 to 0.793 | <0.001 | 0.172 | 0.136 | 0.061 | -0.095 to 0.440 | 0.206 |
| Allodynia | 3.566 | 1.011 | 0.126 | 1.581 to 5.551 | <0.001 | 1.205 | 1.063 | 0.042 | -0.882 to 3.293 | 0.257 |
*Chronic migraine was not included in the multivariable model because the disease duration, headache frequency, and chronic migraine are closely linked.
B: unstandardized regression coefficient, CI: confidence interval, HADS-A: Hospital Anxiety and Depression Scale–Anxiety, HADS-D: Hospital Anxiety and Depression Scale–Depression, PSQI: Pittsburgh Sleep Quality Index, SE: standard error of B, β: standardized regression coefficient.
Factors associated with HIT-6 score among patients with TTH
The factors associated with the HIT-6 score among patients with TTH in the univariable linear regression analysis were headache frequency (β=0.357, p<0.001), chronic TTH (β=0.266, p=0.002), headache severity (β=0.549, p<0.001), PSQI (β=0.289, p=0.001), and HADS-D score (β=0.248, p=0.005) (Table 3). The factors that were independently associated with a higher HIT-6 score in the multivariable analysis were PSQI (β=0.217, p=0.006), headache frequency (β=0.292, p<0.001), and headache severity (β=0.466, p<0.001).
Table 3. Univariable and multivariable linear regression analyses of the predictors of headache-related impact in TTH.
| Univariable analysis | Multivariable analysis | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| B | SE | β | 95% CI | p | B | SE | β | 95% CI | p | |
| Age | -0.042 | 0.068 | -0.055 | -0.177 to 0.092 | 0.532 | -0.075 | 0.056 | -0.097 | -0.187 to 0.037 | 0.189 |
| Female sex | 0.706 | 1.791 | 0.035 | -2.837 to 4.249 | 0.694 | 1.079 | 1.502 | 0.054 | -1.898 to 4.055 | 0.474 |
| Disease duration | 0.122 | 0.141 | 0.078 | -0.157 to 0.401 | 0.388 | 0.017 | 0.112 | 0.011 | -0.205 to 0.239 | 0.879 |
| Headache frequency | 0.319 | 0.074 | 0.357 | 0.174 to 0.465 | <0.001 | 0.254 | 0.064 | 0.292 | 0.128 to 0.381 | <0.001 |
| Chronic TTH* | 5.404 | 1.721 | 0.266 | 1.999 to 8.810 | 0.002 | - | - | - | - | - |
| Headache severity | 2.974 | 0.398 | 0.549 | 2.186 to 3.762 | <0.001 | 2.554 | 0.401 | 0.466 | 1.759 to 3.349 | <0.001 |
| PSQI | 0.849 | 0.247 | 0.289 | 0.360 to 1.339 | 0.001 | 0.619 | 0.222 | 0.217 | 0.179 to 1.059 | 0.006 |
| HADS-D score | 0.624 | 0.220 | 0.248 | 0.188 to 1.060 | 0.005 | 0.291 | 0.241 | 0.115 | -0.188 to 0.769 | 0.231 |
| HADS-A score | 0.265 | 0.202 | 0.118 | -0.135 to 0.664 | 0.192 | -0.114 | 0.211 | -0.051 | -0.533 to 0.305 | 0.592 |
*Chronic TTH was not included in the multivariable model because the disease duration, headache frequency, and chronic migraine are closely linked. B: unstandardized regression coefficient, CI: confidence interval, HADS-A: Hospital Anxiety and Depression Scale–Anxiety, HADS-D: Hospital Anxiety and Depression Scale–Depression, PSQI: Pittsburgh Sleep Quality Index, SE: standard error of B, TTH: tension-type headache, β: standardized regression coefficient.
Path analysis between PSQI and HIT-6 score in migraine
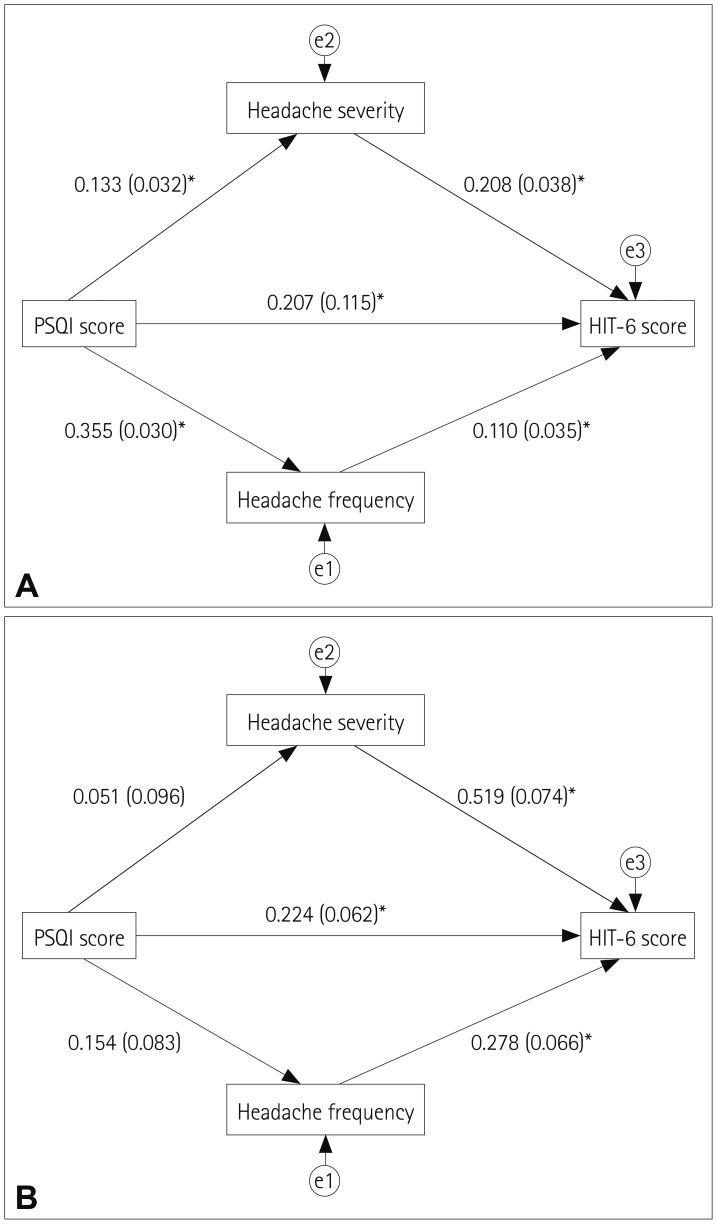
All of the parameter estimates (standardized regression weights) of each path model are depicted in Fig. 1A. The total, direct, and indirect effects are summarized in Table 4. The hypothesized model fit was excellent (χ2=0.656, degrees of freedom=1, p=0.418, GFI=1, AGFI=0.996, NFI=0.997, CFI=1, and RMSEA=0). In patients with migraine, poor sleep quality exerted a significant direct effect on the headache-related impact (β=0.207, p<0.001) as well as significant indirect effects mediated by headache frequency (β=0.039, p=0.004) and headache severity (β=0.028, p=0.004). The direct effect was greater than the sum of the indirect effects (β=0.207 vs. 0.067).
Fig. 1. Path-analysis models from the Pittsburgh Sleep Quality Index (PSQI) to the Headache Impact Test-6 (HIT-6) score mediated by headache frequency and severity. A: Path analysis in migraine shows significant direct and indirect effects. B: Path analysis in tension-type headache shows a significant direct effect without an indirect effect. Numbers along each path indicate standardized regression coefficients (standard errors). A single arrow represents the direction of the presumed influence. An asterisk indicates that the path is significant (p<0.05). Error terms e1, e2, and e3 represent residual variances within variables not accounted for by the hypothesized path-analysis model.
Table 4. Estimates of the effects of the Pittsburgh Sleep Quality Index on the Headache Impact Test-6 score using path analysis models in migraine and TTH.
| Migraine | TTH | |||||
|---|---|---|---|---|---|---|
| β | SE | p | β | SE | p | |
| Total effects | 0.274 | 0.033 | 0.004 | 0.293 | 0.087 | 0.004 |
| Direct effect | 0.207 | 0.115 | <0.001 | 0.224 | 0.062 | 0.004 |
| Total indirect effects | 0.067 | 0.016 | 0.004 | 0.069 | 0.060 | 0.265 |
| Indirect effect via | 0.039 | 0.013 | 0.004 | 0.043 | 0.026 | 0.059 |
| Indirect effect via headache severity | 0.028 | 0.009 | 0.004 | 0.026 | 0.050 | 0.592 |
SE: standard error, TTH: tension-type headache, β: standardized regression coefficient.
Path analysis between PSQI and HIT-6 score in TTH
The path-analysis model in patients with TTH is presented in Table 4 and Fig. 1B. The hypothesized model fit was excellent (χ2=0.999, degrees of freedom=1, p=0.318, GFI=0.996, AGFI=0.962, NFI=0.998, CFI=1, and RMSEA=0). Sleep quality exerted significant direct effects on the headache-related impact (β=0.224, p=0.004) but not indirect effects mediated by headache frequency (β=0.043, p=0.059) or headache severity (β=0.026, p=0.592).
DISCUSSION
The main finding of our study is that sleep quality was independently associated with the headache-related impact in both migraine and TTH patients. However, how the impact of sleep quality mediated the headache-related impact differed between migraine and TTH. Poor sleep quality affected the headache-related impact directly, and increased both the headache frequency and severity of headache, which in turn influenced the headache-related impact in patients with migraine. In contrast, in patients with TTH, sleep quality directly affected the headache-related impact, but this was not mediated by the number or severity of headaches.
Association between sleep quality and headache-related impact
The headache-related impact is theoretically determined by the combination of pain severity and attack frequency.8,18 However, previous studies have shown that factors other than headache frequency and severity can contribute to the headache-related impact. For example, cognitive decline during an attack is associated with increases in the headache-related impact,19 and anxiety and major depressive disorder are also independently associated with a severe headache-related impact.20,21
In addition to cognitive and affective factors, sleep can also be an important determinant of headache-related impact. Primary headache is associated with higher prevalence of sleep problems such as poor sleep quality, short sleep duration, and insomnia.7,8,11,22 The present study found that sleep quality was independently associated with the headache-related impact in both migraine and TTH. This finding is consistent with previous studies showing associations between poor sleep quality, short sleep duration, and insomnia with headache-related impact.7,8,11,22 Our study found that sleep quality is an independent determinant of the headache-related impact after adjusting for both the headache severity and frequency.
Direct effect of sleep quality on the headache-related impact
We used path analyses to investigate how sleep quality affects the headache-related impact. In migraine, poor sleep quality exerted both direct and indirect effects on headache severity and headache frequency. In contrast, sleep quality directly affected the headache-related impact in patients with TTH. Based on our findings, we suggest that poor sleep quality can directly affect the headache-related impact in migraine and TTH. Sleep quality is related to the overall quality of life.23 Poor sleep quality may contribute to alterations in the neuroendocrine stress response system and metabolic activity during sleep, resulting in impaired daytime function.24 We hypothesized that this potential role of sleep—which induces an overall poor quality of life—also contributes to the headache-related impact. Poor sleep quality also affects psychological and cognitive functions, which can further increase the headache-related impact.
Indirect effect of sleep quality on the headache-related impact
This study found that sleep quality was associated with increases in the frequency and severity of headache in patients with migraine, which in turn mediated the effect of sleep quality on greater headache-related impact. Several studies have found that the headache frequency (but not the severity) was associated with poor sleep quality in migraine.9,10 Recent studies suggest that sleep deprivation was associated with increased brain hyperexcitability, which increases the susceptibility of the brain to a migraine attack.25,26 Sleep deprivation causes changes in inhibition–facilitation balance of the cerebral cortices, which may contribute to increased cortical excitability.27,28 Additionally, poor sleep quality may be associated with cortical sensitization.29 A previous clinical study found an association between the duration of sleep and allodynia in patients with migraine.30 The mechanism underlying how sleep modulates cortical activities remains to be elucidated. A few preclinical studies have shown that the concentrations of pituitary adenylate cyclase-activating peptide and nitric oxide—which are the key molecules in trigeminovascular activation—change with the sleep cycle.31 In addition to these neurotransmitters, the orexinergic neuronal pathway may play a role in both sleep and migraine and may be a key factor linking the two conditions.31
In contrast, the impact of sleep was not mediated by the number and severity of headache in patients with TTH in the present study. Previous studies have produced conflicting results on the association of sleep quality with headache frequency or severity in TTH.6,7,32,33 The activation of hyperexcitable peripheral afferent neurons from head and neck muscles is known to be the main mechanism underlying TTH.34 There is debate on the role of central mechanisms in TTH.35 A few studies of chronic TTH have suggested that central sensitization such as allodynia and hyperalgesia indicates brain involvement in TTH.35,36 In contrast to our study, a recent study showed a direct effect of sleep quality on headache frequency and an indirect effect of emotional burden on headache frequency mediated by sleep quality in chronic TTH using path analysis.33 In our study, the values of statistical parameters such as the β coefficient were similar between migraine and TTH, although the absence of statistical significance in patients with TTH might have been due to the sample smallness. Together these findings suggest that TTH shares a central mechanism with the sleep pathway, but that its direct impact on increasing the headache frequency is less robust than in migraine.
Strengths and limitations
The main strengths of this study are that it considered variables that might affect the headache-related impact, and thoroughly investigated and controlled them in the analysis despite the largeness of the sample. However, our study also had limitations. First, sleep characteristics and sleep disorders were not assessed objectively using polysomnography or actigraphy. Instead, we assessed these parameters using a validated questionnaire such as PSQI. Second, indirect effects in TTH were not statistically significant in our study. However, considering that the β coefficients were similar to those of migraine, nonsignificant results might be due to either a true lack of an effect or the sample smallness. Future studies with large samples of TTH patients are therefore necessary to confirm our findings. Third, we did not collect information on the previous use of antidepressants, hypnotics, anxiolytics, or preventive medications for headaches in our registry. Because medication might be an important covariable between sleep quality and headache-related impact, future studies should consider assessing this variable. Fourth, the headache frequency and severity were not recorded in a prospective headache diary. Due to the retrospective nature of our questionnaire, recall bias might have been present. Fifth, we showed the direct and indirect effects of sleep quality on the headache-related impact in migraine and TTH. However, this finding might not be generalizable because patients were sampled from a specialized headache clinic in a tertiary hospital. Sixth, we used data from patients who agreed to being enrolled in the registry. Although our data showed that that included patients well represented the clinic-based population of migraine and TTH, unmeasured bias might have been present in the enrollment process. Finally, because our study had a cross-sectional design, the results of our path analysis do not indicate causal relationships. Further studies with longitudinal designs are therefore needed.
Conclusions
Poor sleep quality may directly increase the headache-related impact in patients with migraine and TTH as well as indirectly by increasing the headache frequency and severity in patients with migraine. Physicians should make efforts to improve the sleep quality in patients with primary headache disorders, since this would further improve the headache-related impact as well as the headache frequency and severity in migraine and TTH. Our study suggests that the headache-related impact is not merely equal to the sum of the headache frequency and severity, and so other factors affecting headache-related impacts such as poor sleep quality should be taken into consideration in future studies.
Acknowledgements
This study was supported by the National Research Foundation of Korea (NRF) grants funded by the Korean government (MSIP) (Nos. 2017R1A2B2009086 and 2017R1A2B4007254). The Yuhan company and DongA ST partially supported the data management. The funding bodies had no role in study design, data collection, analysis, or interpretation, or in writing this report.
The authors thank M. Jung for data management.
Footnotes
- Conceptualization: Soohyun Cho, Mi Ji Lee, Hea Ree Park, Eun Yeon Joo, Chin-Sang Chung.
- Formal analysis: Soohyun Cho, Mi Ji Lee, Hea Ree Park, Seonwoo Kim.
- Funding acquisition: Mi Ji Lee, Chin-Sang Chung.
- Investigation: Soohyun Cho, Mi Ji Lee.
- Supervision: Eun Yeon Joo, Chin-Sang Chung.
- Writing—original draft: Soohyun Cho, Mi Ji Lee.
- Writing—review & editing: Soohyun Cho, Mi Ji Lee, Hea Ree Park, Seonwoo Kim, Eun Yeon Joo, Chin-Sang Chung.
Conflicts of Interest: The authors have no potential conflicts of interest to disclose.
References
- 1.GBD 2016 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2017;390:1211–1259. doi: 10.1016/S0140-6736(17)32154-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.GBD 2016 Headache Collaborators. Global, regional, and national burden of migraine and tension-type headache, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2018;17:954–976. doi: 10.1016/S1474-4422(18)30322-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Russell MB, Levi N, Šaltytė-Benth J, Fenger K. Tension-type headache in adolescents and adults: a population based study of 33,764 twins. Eur J Epidemiol. 2006;21:153–160. doi: 10.1007/s10654-005-6031-3. [DOI] [PubMed] [Google Scholar]
- 4.Saunders K, Merikangas K, Low NCP, Von Korff M, Kessler RC. Impact of comorbidity on headache-related disability. Neurology. 2008;70:538–547. doi: 10.1212/01.wnl.0000297192.84581.21. [DOI] [PubMed] [Google Scholar]
- 5.Ødegård SS, Engstrøm M, Sand T, Stovner LJ, Zwart JA, Hagen K. Associations between sleep disturbance and primary headaches: the third Nord-Trøndelag Health Study. J Headache Pain. 2010;11:197–206. doi: 10.1007/s10194-010-0201-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Benito-González E, Palacios-Ceña M, Fernández-Muñoz JJ, Castaldo M, Wang K, Catena A, et al. Variables associated with sleep quality in chronic tension-type headache: a cross-sectional and longitudinal design. PLoS One. 2018;13:e0197381. doi: 10.1371/journal.pone.0197381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim J, Cho SJ, Kim WJ, Yang KI, Yun CH, Chu MK. Insomnia in tension-type headache: a population-based study. J Headache Pain. 2017;18:95. doi: 10.1186/s10194-017-0805-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Seidel S, Hartl T, Weber M, Matterey S, Paul A, Riederer F, et al. Quality of sleep, fatigue and daytime sleepiness in migraine - a controlled study. Cephalalgia. 2009;29:662–669. doi: 10.1111/j.1468-2982.2008.01784.x. [DOI] [PubMed] [Google Scholar]
- 9.Song TJ, Cho SJ, Kim WJ, Yang KI, Yun CH, Chu MK. Poor sleep quality in migraine and probable migraine: a population study. J Headache Pain. 2018;19:58. doi: 10.1186/s10194-018-0887-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Walters AB, Hamer JD, Smitherman TA. Sleep disturbance and affective comorbidity among episodic migraineurs. Headache. 2014;54:116–124. doi: 10.1111/head.12168. [DOI] [PubMed] [Google Scholar]
- 11.Song TJ, Yun CH, Cho SJ, Kim WJ, Yang KI, Chu MK. Short sleep duration and poor sleep quality among migraineurs: a population-based study. Cephalalgia. 2018;38:855–864. doi: 10.1177/0333102417716936. [DOI] [PubMed] [Google Scholar]
- 12.Headache Classification Committee of the International Headache Society (IHS) The International Classification of Headache Disorders, 3rd edition (beta version) Cephalalgia. 2013;33:629–808. doi: 10.1177/0333102413485658. [DOI] [PubMed] [Google Scholar]
- 13.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67:361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 14.Lipton RB, Bigal ME, Ashina S, Burstein R, Silberstein S, Reed ML, et al. Cutaneous allodynia in the migraine population. Ann Neurol. 2008;63:148–158. doi: 10.1002/ana.21211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Buysse DJ, Reynolds CF, 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 16.Kosinski M, Bayliss MS, Bjorner JB, Ware JE, Jr, Garber WH, Batenhorst A, et al. A six-item short-form survey for measuring headache impact: the HIT-6. Qual Life Res. 2003;12:963–974. doi: 10.1023/a:1026119331193. [DOI] [PubMed] [Google Scholar]
- 17.Byrne BM. Structural Equation Modeling with AMOS: Basic Concepts, Applications, and Programming. 3rd ed. New York: Routledge; 2016. [Google Scholar]
- 18.Sauro KM, Rose MS, Becker WJ, Christie SN, Giammarco R, Mackie GF, et al. HIT-6 and MIDAS as measures of headache disability in a headache referral population. Headache. 2010;50:383–395. doi: 10.1111/j.1526-4610.2009.01544.x. [DOI] [PubMed] [Google Scholar]
- 19.Gil-Gouveia R, Oliveira AG, Martins IP. The impact of cognitive symptoms on migraine attack-related disability. Cephalalgia. 2016;36:422–430. doi: 10.1177/0333102415604471. [DOI] [PubMed] [Google Scholar]
- 20.Tietjen GE, Brandes JL, Digre KB, Baggaley S, Martin V, Recober A, et al. High prevalence of somatic symptoms and depression in women with disabling chronic headache. Neurology. 2007;68:134–140. doi: 10.1212/01.wnl.0000251195.55563.02. [DOI] [PubMed] [Google Scholar]
- 21.Buse D, Manack A, Serrano D, Reed M, Varon S, Turkel C, et al. Headache impact of chronic and episodic migraine: results from the American Migraine Prevalence and Prevention study. Headache. 2012;52:3–17. doi: 10.1111/j.1526-4610.2011.02046.x. [DOI] [PubMed] [Google Scholar]
- 22.Oh JH, Cho SJ, Kim WJ, Yang KI, Yun CH, Chu MK. Insufficient sleep in tension-type headache: a population study. J Clin Neurol. 2018;14:566–573. doi: 10.3988/jcn.2018.14.4.566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zeitlhofer J, Schmeiser-Rieder A, Tribl G, Rosenberger A, Bolitschek J, Kapfhammer G, et al. Sleep and quality of life in the Austrian population. Acta Neurol Scand. 2000;102:249–257. doi: 10.1034/j.1600-0404.2000.102004249.x. [DOI] [PubMed] [Google Scholar]
- 24.Tiemeier H, Pelzer E, Jönck L, Möller HJ, Rao ML. Plasma catecholamines and selective slow wave sleep deprivation. Neuropsychobiology. 2002;45:81–86. doi: 10.1159/000048681. [DOI] [PubMed] [Google Scholar]
- 25.Scalise A, Desiato MT, Gigli GL, Romigi A, Tombini M, Marciani MG, et al. Increasing cortical excitability: a possible explanation for the proconvulsant role of sleep deprivation. Sleep. 2006;29:1595–1598. doi: 10.1093/sleep/29.12.1595. [DOI] [PubMed] [Google Scholar]
- 26.Lang E, Kaltenhäuser M, Neundörfer B, Seidler S. Hyperexcitability of the primary somatosensory cortex in migraine--a magnetoencephalographic study. Brain. 2004;127:2459–2469. doi: 10.1093/brain/awh295. [DOI] [PubMed] [Google Scholar]
- 27.Civardi C, Boccagni C, Vicentini R, Bolamperti L, Tarletti R, Varrasi C, et al. Cortical excitability and sleep deprivation: a transcranial magnetic stimulation study. J Neurol Neurosurg Psychiatry. 2001;71:809–812. doi: 10.1136/jnnp.71.6.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Noseda R, Kainz V, Jakubowski M, Gooley JJ, Saper CB, Digre K, et al. A neural mechanism for exacerbation of headache by light. Nat Neurosci. 2010;13:239–245. doi: 10.1038/nn.2475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smith MT, Edwards RR, McCann UD, Haythornthwaite JA. The effects of sleep deprivation on pain inhibition and spontaneous pain in women. Sleep. 2007;30:494–505. doi: 10.1093/sleep/30.4.494. [DOI] [PubMed] [Google Scholar]
- 30.de Tommaso M, Delussi M, Vecchio E, Sciruicchio V, Invitto S, Livrea P. Sleep features and central sensitization symptoms in primary headache patients. J Headache Pain. 2014;15:64. doi: 10.1186/1129-2377-15-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Holland PR. Headache and sleep: shared pathophysiological mechanisms. Cephalalgia. 2014;34:725–744. doi: 10.1177/0333102414541687. [DOI] [PubMed] [Google Scholar]
- 32.Wang Y, Xie J, Yang F, Wu S, Wang H, Zhang X, et al. Comorbidity of poor sleep and primary headaches among nursing staff in North China. J Headache Pain. 2015;16:88. doi: 10.1186/s10194-015-0571-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Palacios-Ceña M, Fernández-Muñoz JJ, Castaldo M, Wang K, Guerrero-Peral Á, Arendt-Nielsen L, et al. The association of headache frequency with pain interference and the burden of disease is mediated by depression and sleep quality, but not anxiety, in chronic tension type headache. J Headache Pain. 2017;18:19. doi: 10.1186/s10194-017-0730-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schmidt-Hansen PT, Svensson P, Bendtsen L, Graven-Nielsen T, Bach FW. Increased muscle pain sensitivity in patients with tension-type headache. Pain. 2007;129:113–121. doi: 10.1016/j.pain.2006.09.037. [DOI] [PubMed] [Google Scholar]
- 35.Bendtsen L, Jensen R, Olesen J. Decreased pain detection and tolerance thresholds in chronic tension-type headache. Arch Neurol. 1996;53:373–376. doi: 10.1001/archneur.1996.00550040113021. [DOI] [PubMed] [Google Scholar]
- 36.Ashina S, Bendtsen L, Ashina M, Magerl W, Jensen R. Generalized hyperalgesia in patients with chronic tension-type headache. Cephalalgia. 2006;26:940–948. doi: 10.1111/j.1468-2982.2006.01150.x. [DOI] [PubMed] [Google Scholar]