Abstract
Background and Purpose
This study was designed to determine the prevalence, pattern, lesion location, and etiology of dissociation in the results of the bithermal caloric test and the horizontal video head impulse test (vHIT) in dizzy patients with various etiologies and disease durations.
Methods
We analyzed the results of bithermal caloric tests and vHITs performed over 26 months in 893 consecutive patients who underwent both tests within a 10-day period.
Results
Dissociation in the results of the two tests was found in 162 (18.1%) patients. Among them, 123 (75.9%) had abnormal caloric tests (unilateral paresis in 118 and bilateral paresis in 5) but normal vHITs. Peripheral lesions were identified in 105 (85.4%) of these patients, with the main underlying diseases being Meniere's disease (62/105, 59%) and vestibular neuritis/labyrinthitis (29/105, 27.6%). In contrast, central pathologies of diverse etiologies were found only in 18 (14.6%) patients. Abnormal vHIT (bilaterally positive in 18, unilaterally positive in 19, and hyperactive in 2) and normal caloric responses were found in 39 patients, with an equal prevalence of central (n=19) and peripheral (n=20) lesions. The peripheral lesions included vestibular neuritis/labyrinthitis in seven patients and Meniere's disease in another seven. The central lesions had diverse etiologies.
Conclusions
Dissociation in the results between caloric tests and horizontal vHITs is not uncommon. The present patients with abnormal caloric tests and normal vHITs mostly had peripheral lesions, while central lesions were likely to underlie those with abnormal vHITs and normal caloric tests.
Keywords: vertigo, vestibulo-ocular reflex, caloric test, head impulse test, vestibular diseases
INTRODUCTION
The bithermal caloric test is the traditional tool used to evaluate the function of horizontal semicircular canals.1 The more-recent video head impulse test (vHIT) allows moreconvenient evaluations of the horizontal angular vestibulo-ocular reflex (AVOR) during high-velocity and high-acceleration stimuli.2,3 Both tests usually produce concordant results for the horizontal AVOR functions, but may show dissociated results in certain pathological conditions.4 For example, vestibular neuritis may produce an abnormal horizontal vHIT but a normal caloric test during the acute phase.5 Central lesions may also produce an abnormal horizontal vHIT with preserved caloric responses.6,7,8 Conversely, abnormal caloric responses but a normal horizontal vHIT may be observed in Meniere's disease9,10,11 and chronic vestibular neuritis.12 These dissociated results between the caloric test and the horizontal vHIT have also been reported in other vestibular disorders.13 This means that the dissociated results between the tests might be a surrogate marker for specific vestibular disorders or lesion location, but previous suggestions for their utilization in this way have mostly been based on small numbers of patients with specific disorders. This study explored the actual prevalence, pattern, lesion location, and etiology of dissociation in the results of caloric tests and vHITs in a large number of consecutive patients with dizziness.
METHODS
Patients and evaluation
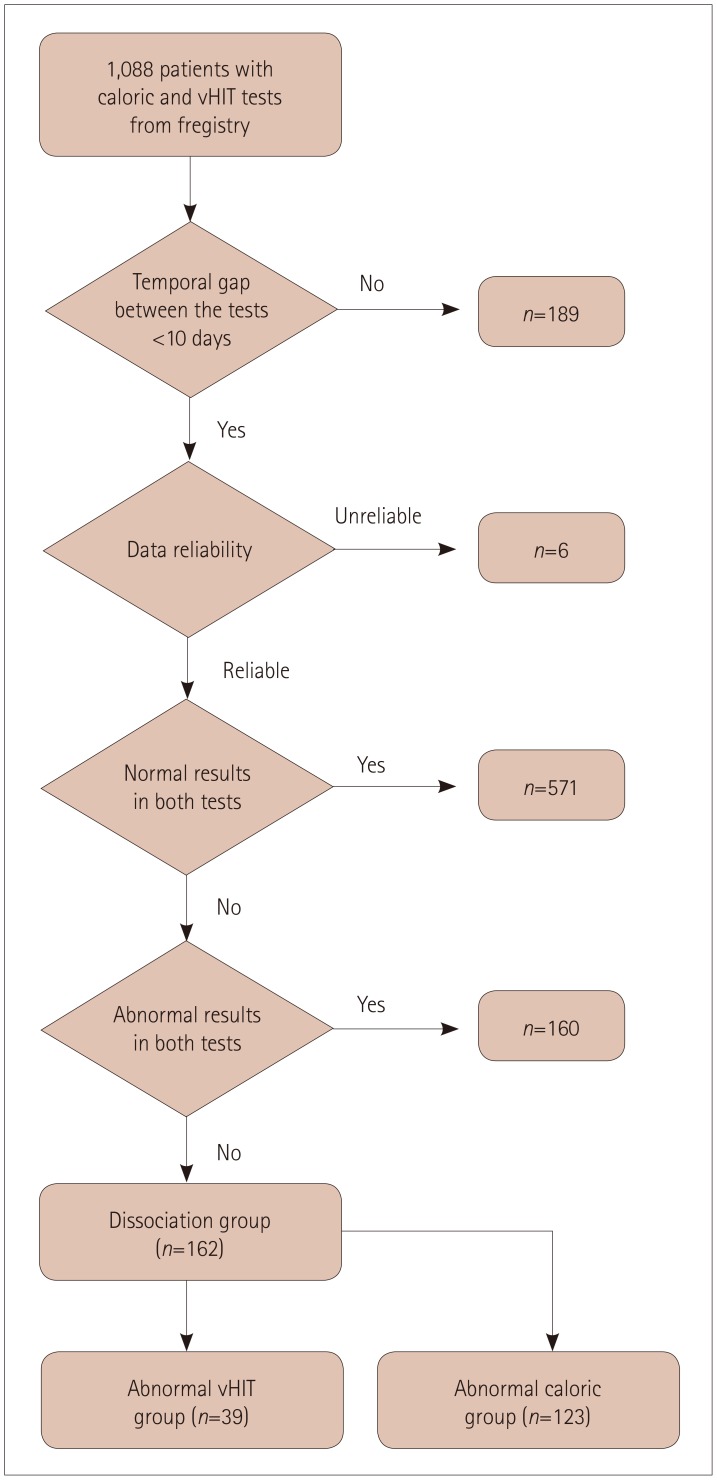
The study protocol, which included no requirement to obtain patient consent, was approved by the Institutional Review Board of Seoul National University Bundang Hospital (B-1810-499-109). For a period of 26 months from March 2016 to May 2018, we recruited consecutive patients who underwent both a caloric test and a vHIT from the registry of the Dizziness Center in Seoul National University Bundang Hospital. Initially, 1,088 patients with dizziness were screened. When the number of patients were plotted as a function of the interval between the studies, the screened patients could clearly be divided into 2 groups: 1) most of them (899 patients) had a peak for an interval of 0 days with a tail at 10 days, while 2) the others were dispersed widely, with a mean interval of 68.8 days. Considering that tests performed with an interval longer than 10 days would not reflect the same functional status of the semicircular canals, we excluded the 189 patients with test interval of 10 days or more. A further 6 with an unreliable caloric test or vHIT were also excluded, and so 893 patients were finally enrolled in the study. The interval between the tests was 0.8±1.5 days (mean plus-minus standard deviation; median=0 days, interquartile range=0–1 day). Patients who had concordant results in the two tests (i.e., normal or abnormal in both tests) were further excluded, and finally the patients with dissociated results between caloric tests and horizontal vHITs were included in the analyses. These patients were further classified into the abnormal caloric or the abnormal vHIT group (Fig. 1).
Fig. 1. Flow chart of patient inclusion.
The protocols of the caloric tests and vHITs of our dizziness center are described elsewhere.14 We defined the cutoff value for a caloric abnormality as more than 25% of caloric paresis according to Jongkee's formula, or less than 20°/s of the sum of four absolute slow-eye velocities obtained from both ears with bithermal irrigation.14,15 An abnormal horizontal vHIT was defined as a gain less than 0.8 or more than 1.2 with corrective catch-up or back-up saccades. The vHIT gain was calculated as the ratio of the eye velocity relative to the head velocity during a 40-ms window centered at the peak head acceleration.14 In addition to the absolute vHIT gain, we also calculated the asymmetry index (AI) for the vHIT gain following a previously described method.8 Other evaluations for audiovestibular functions included pure-tone audiometry, cervical and ocular vestibular-evoked myogenic potentials, subjective visual vertical, and the rotational chair test were performed on a selective basis. Brain or internal auditory canal MRI was additionally performed in selected patients.
Pattern of dissociation, etiology, and lesion location
The patients with abnormal caloric tests were subdivided into unilateral and bilateral paresis groups. The patients with abnormal horizontal vHITs were divided into bilaterally positive, unilaterally positive, and hyperactive vHIT groups.
For the etiological evaluation, we initially collected the diagnoses of patients from the registry. Then, to increase the diagnostic accuracy, two authors (J.Y. Lee and J.Y. Choi) reviewed the medical records, history, and imaging and laboratory findings of the patients. All etiologies were categorized into either central or peripheral vestibulopathy according to the extent of the lesions. Patients who had both peripheral and central lesions were classified into central vestibulopathy. For example, a vestibular schwannoma or herpes zoster oticus were classified as central vestibulopathy when MRI or ocular motor findings were suggestive of cerebellar or brainstem involvement. The central signs in ocular motor findings were gaze-evoked nystagmus or saccadic pulse-step mismatch, as suggested previously.16 The etiological diagnosis for each condition was as follows. The diagnosis of vestibular neuritis was made only when the patients had first-ever spontaneous vertigo lasting for longer than 24 hours, spontaneous horizontal and torsional nystagmus beating in the direction opposite to the positive vHIT (gain <0.8), and/or caloric weakness (>25%), without hearing loss or central signs.17 The stage of vestibular neuritis was divided into acute, subacute, and chronic phases. Acute and subacute phases were defined in this study as within 4 days and 5–14 days after onset, respectively. Chronic vestibular neuritis was defined when the patients still had not recovered fully from dizziness at >2 weeks after the disease onset. Meniere's disease and bilateral vestibulopathy were diagnosed in accordance with previously proposed criteria.18
Statistical analysis
The degree of caloric paresis, the vHIT gain, and the AI of the horizontal vHIT gain were estimated and compared in each subgroup according to the lesion locations, except when the subgroup contained fewer than five patients. To compare categorical or continuous values between different subgroups, we applied the chi-square or Mann-Whitney test, with the criterion for statistical significance being a p value of <0.05. All statistical analyses were performed using IBM SPSS for Windows, version 20.0 (IBM Corp., Armonk, NY, USA). Data are presented in the tables as mean±standard deviation or median and interquartile range values. However, data are only presented as mean±standard deviation values in the text for the sake of readability.
RESULTS
Prevalence of dissociation in the results between caloric tests and horizontal vHITs
Most of the patients showed consistent results in the caloric test and vHIT, being normal on both tests in 571 (63.9%) and abnormal on both tests in 160 (17.9%). Thus, dissociated results between the two tests were found in 162 (18.1%) patients: an abnormal caloric test but a normal vHIT in 123 (75.9%), and a normal caloric test but an abnormal vHIT in 39 (24.1%).
Abnormal caloric test group: pattern, etiology, and lesion location
The 123 patients in the abnormal caloric test group comprised 118 with unilateral caloric paresis and 5 with bilateral caloric paresis. Peripheral vestibulopathy predominated over central vestibulopathy (85.4% vs. 14.6%, p<0.001) in this group. The etiologies and the results of the caloric tests and vHITs in this group are presented in Tables 1 and 2.
Table 1. Etiology and lesions for the dissociation between caloric tests and vHITs.
| Peripheral lesions (n=105) | Central lesions (n=18) | |
|---|---|---|
| Abnormal caloric test group (n=123) | ||
| Unilateral caloric paresis (n=118) | n=101 | n=17 |
| Meniere's disease (n=58) | Stroke (n=7) | |
| Definite (n=51) | Brainstem infarction (n=6) | |
| Possible (n=7) | Cerebellar infarction (n=1) | |
| Inflammation (n=29) | Inflammation (n=4) | |
| Acute VN/labyrinthitis (n=7) | Meningoencephalitis (n=4) | |
| Subacute VN/labyrinthitis (n=2) | Degeneration or tumor (n=4) | |
| Chronic VN/labyrinthitis (n=20) | Cerebellar ataxia (n=2) | |
| Others (n=5) | Toxic cerebellar degeneration (n=1) | |
| Labyrinthine concussion (n=3) | Vestibular schwannoma (n=1) | |
| Vestibular schwannoma (n=1) | Undetermined cause (n=2) | |
| Toxic vestibulopathy (n=1) | ||
| Undetermined cause (n=9) | ||
| Bilateral caloric paresis (n=5) | n=4 | n=1 |
| Meniere's disease (n=4) fulfilling the criteria for BVP | Degeneration (n=1) | |
| Definite (n=2) | Cerebellar ataxia (n=1) | |
| Possible (n=2) |
BVP which only refers to conditions with caloric unresponsiveness (<20°/s for the sum of four absolute slow-eye velocities) or decreased bilateral horizontal vHIT gain (<0.6) in the absence of central pathology.
BVP: bilateral vestibulopathy, vHIT: video head impulse test, VN: vestibular neuritis.
Table 2. Parameters for caloric tests and vHITs according to the subgroup and lesion location.
| Peripheral lesions | Central lesions | p | |
|---|---|---|---|
| Abnormal caloric test group (n=123) | |||
| Prevalence | 105 (85.4) | 18 (14.6) | <0.001 |
| Unilateral caloric paresis subgroup | |||
| Prevalence | 101 (85.6) | 17 (14.4) | <0.001 |
| Caloric paresis, % | 49.7±21.0 | 48.8±21.9 | 0.788 |
| 43 (33–61) | 42 (29–63) | ||
| vHIT gain for the ear with caloric paresis | 1.00±0.08 | 0.94±0.08 | 0.004 |
| 1.03 (0.95–1.07) | 0.91 (0.89–1.02) | ||
| vHIT gain for the healthy ear in the caloric test | 1.03±0.06 | 1.03±0.06 | 0.687 |
| 1.05 (1.00–1.07) | 1.04 (1.01–1.07) | ||
| AI of vHIT gain, % | 3.0±3.3 | 5.3±3.6 | 0.007 |
| 2 (1–4.5) | 6 (2–7.5) | ||
| Abnormal horizontal vHIT group (n=39) | |||
| Prevalence | 20 (54.1) | 19 (45.9) | 0.001 |
| Bilaterally positive vHIT group | |||
| Prevalence | 9 (50.0) | 9 (50.0) | 0.001 |
| Caloric paresis, % | 9.9±6.0 | 7.7±4.5 | 0.489 |
| 9 (4–15.5) | 8 (3–11.5) | ||
| vHIT gain for both ears | 0.69±0.08 | 0.63±0.10 | 0.101 |
| 0.72 (0.63–0.75) | 0.66 (0.62–0.70) | ||
| AI of vHIT gain, % | 4.4±2.30 | 6.1±6.30 | 0.489 |
| 4.0 (3–5) | 3 (2–13) | ||
| Unilaterally positive vHIT group | |||
| Prevalence | 11 (57.9) | 8 (42.1) | 0.491 |
| Caloric paresis, % | 9.6±8.2 | 11.4±7.7 | 0.840 |
| 8 (2–18) | (5–19) | ||
| vHIT gain for weak ear | 0.62±0.18 | 0.69±0.09 | 0.492 |
| 0.69 (0.56–0.73) | 0.71 (0.60–0.78) | ||
| vHIT gain for healthy ear | 0.97±0.11 | 0.92±0.10 | 0.206 |
| 0.93 (0.89–1.07) | 0.87 (0.84–1.03) | ||
| AI of vHIT gain, % | 23.3±20.2 | 14.0±7.2 | 0.395 |
| 14 (9–28.0) | 15 (8–19.5) |
Data are mean±standard deviation and median (interquartile range) or n (%) values. Statistical analysis was not performed for subgroups with bilateral caloric paresis and hyperactive vHIT due to the small number of patients (<5).
AI: asymmetry index, vHIT: video head impulse test.
Unilateral caloric paresis group (n=118)
The caloric paresis was 50.0±20.9% in this group. In contrast, the horizontal vHIT gain was 1.00±0.08 to the lesion side and 1.00±0.06 to the intact side. Peripheral and central vestibulopathies were diagnosed in 101 (85.6%) and 17 (14.4%) of the patients, respectively (p<0.001), but the degree of caloric paresis did not differ between them (49.7±21.0% vs. 48.8±21.9%, p=0.788). However, the AI of the horizontal vHIT gain was smaller in patients with peripheral vestibulopathy than in those with central vestibulopathy (3.0±3.3% vs. 5.3±3.6%, p=0.007), and the difference resulted from a higher horizontal vHIT gain to the lesion side in peripheral vestibulopathy than in central vestibulopathy (1.00±0.08 vs. 0.94±0.08, p=0.004). In contrast, the horizontal vHIT gain to the intact side did not differ significantly between the peripheral and central vestibulopathies (1.03±0.06 vs. 1.03±0.06, p=0.687). The peripheral vestibulopathies mainly comprised 58 cases of Meniere's disease (51 with definite and 7 with possible) with a duration of 1,174±1,519.3 days and 29 vestibular neuritis/labyrinthitis mostly in the chronic phases. The central vestibulopathies included seven patients with stroke, four with meningoencephalitis, and three with degenerative disorders.
Bilateral caloric paresis (n=5)
The horizontal vHIT gain was 1.02±0.11 in the right ear and 0.99±0.07 in the left ear in the five patients with bilateral caloric paresis. The AI of the horizontal vHIT gain was 3.0±2.0%. The underlying etiology was Meniere's disease in four patients and cerebellar ataxia with vestibular areflexia in the fifth.
Abnormal horizontal vHIT group: pattern, etiology, and lesion location
The 39 patients in the abnormal horizontal vHIT group comprised 18 with bilaterally positive vHIT, 19 with unilaterally positive vHIT, and 2 with hyperactive horizontal vHIT. Diagnoses of peripheral and central vestibulopathies were made in 20 and 19 patients, respectively. Except for a hyperactive vHIT that solely belonged to central vestibulopathy, the proportions of peripheral and central vestibulopathies were comparable in the patients with bilaterally and unilaterally positive vHITs. The underlying etiologies and results of the caloric tests and vHITs are presented in Tables 1 and 2.
Bilaterally positive vHIT (n=18)
The horizontal vHIT gain was 0.69±0.10 to the right and 0.63±0.10 to the left. The AI of the horizontal vHIT gain was 5.3±4.7% in this subgroup. Nine of these patients had peripheral vestibulopathy and the other nine had central vestibulopathy. Meniere's disease was the main cause of peripheral vestibulopathy (n=6, 66%), and its duration was 918.0±820.9 days. Two of the nine patients with peripheral lesions satisfied the criteria for bilateral vestibulopathy (one with Meniere's disease and the other had idiopathic vestibulopathy). Four of the patients with central vestibulopathy had inflammatory disorders such as meningoencephalitis and neuromyelitis optica. The horizontal vHIT gain did not differ between the peripheral and central vestibulopathies (0.69±0.08 vs. 0.63±0.10, p=0.101).
Unilaterally positive vHIT (n=19)
The vHIT gain was 0.95±0.10 to the normal side and 0.65±0.15 to the abnormal side. The AI of the horizontal vHIT gain was 19.8±16.4%. This subgroup comprised 11 patients (57.9%) with peripheral vestibulopathy and 8 (42.1%) with central vestibulopathy (p=0.491). Vestibular neuritis/labyrinthitis was the most-common peripheral etiology (n=7, 63.6%). There was only one case of Meniere's disease, and its duration was <240 days. In contrast, the central etiologies included similar numbers of strokes, tumors, and degenerative disorders. The horizontal vHIT gains to the normal side were 0.97±0.11 and 0.92±0.10 in patients with peripheral and central vestibulopathies, respectively (p=0.206), while the gains to the abnormal side were 0.62±0.18 and 0.69±0.09 (p=0.492). The AI of the vHIT gain did not differ between the peripheral and central vestibulopathies (23.3±20.2% vs. 14.0±7.2%, p=0.395), and there was also no difference in caloric paresis (9.6±8.2% vs. 11.4±7.7%, p=0.840).
Hyperactive vHIT (n=2)
These two patients had bilaterally increased gains with corrective back-up saccades. The gain on both sides and the AI of the vHIT ranged from 1.21 to 1.40 and from 0 to 0.02, respectively. One patient had a degenerative disorder and the other had meningoencephalitis.
DISCUSSION
This study defined the prevalence, patterns, lesion locations, and etiologies in a large number of patients with dizziness and dissociated results between caloric tests and vHITs. Dissociated results were observed in about one out of every six patients with dizziness. The prevalence was three times higher than that (5.9%) found in a recent study.19 This discrepancy may have resulted from differences in the study populations, given that the present study included a higher proportion of patients with central lesions from various etiologies. We found that patients with abnormal caloric tests but normal horizontal vHITs usually have peripheral lesions, while those with abnormal horizontal vHITs but normal caloric tests are at an increased risk of central lesions.
Unilateral caloric paresis was mostly attributable to peripheral lesions in the abnormal caloric group. However, there were also a few central lesions with a degree of caloric paresis similar to those of peripheral lesions. This suggests that the interpretation of this type of dissociation should be based on other associated neurological findings. The most-common etiology in peripheral lesions was Meniere's disease, followed by chronic vestibular neuritis/labyrinthitis. The dissociation in Meniere's disease may result from local convection flow of the endolymph during caloric stimulation due to hydrostatic expansion of the endolymph space,11 or from the vulnerability of type II hair cells in Meniere's disease.20 Indeed, the neural pathway that is mainly involved in low-frequency or low-acceleration and velocity stimuli during caloric stimulation are the connections from type II hair cells to regular vestibular afferents.21,22
In chronic vestibulopathy, the predominant vestibular compensation for high-frequency stimuli associated with daily activities may enhance the recovery of head impulse AVOR behavior but not the recovery of caloric responses.12 Likewise, the mechanism underlying this dissociation in central lesions might be an ongoing compensation for high-frequency AVOR after an insult.12 The lesions in stroke patients mostly occur in the lower brainstem and involve the vestibular nerve root or nucleus. Therefore, both the caloric test and horizontal vHIT would initially be abnormal in these patients,23 but high-frequency AVOR might improve earlier during the recovery. This would also account for the dissociation observed in other central lesions with the clinical course of progression, stabilization, and adaptation. However, the heterogeneity of the etiology, lesion locations, and stage at evaluation make it very difficult to draw definitive conclusions. One particularly interesting observation was that the patients with peripheral lesions had a smaller AI of the horizontal vHIT gain, possibly due to the horizontal vHIT gain being higher on the side of smaller caloric responses than that in patients with central lesions. This small difference might not help in distinguishing between peripheral and central lesions, but the lower vHIT gain to the side of caloric paresis in central lesions would reflect the importance of central mechanisms in normal head impulse behavior.24,25 Bilateral caloric paresis was relatively rare in the abnormal caloric test group, and the main cause was bilateral vestibulopathy associated with Meniere's disease, as expected from the findings for unilateral caloric paresis.
Another dissociation pattern was an abnormal vHIT and a normal caloric test. This type of dissociation may be further categorized into three subgroups. Patients in the first and second subgroups exhibited a decrease in the vHIT gain bilaterally or unilaterally and comprised a similar proportion of peripheral and central lesions in each subgroup. The gain in the bilaterally positive vHIT subgroup was around 0.6 to 0.7 in either direction regardless of the lesion location. The vHIT gain in the unilaterally positive vHIT subgroup did not differ between peripheral and central lesions. It was therefore reasonable to suspect the presence of central pathologies in these subgroups of patients.
It is noteworthy that there was substantial difference in the etiology between bilaterally and unilaterally positive vHIT subgroups in both peripheral and central lesions. In peripheral lesions, Meniere's disease was the main cause in patients with bilaterally positive vHITs, while vestibular neuritis was mostly responsible for unilaterally positive vHITs. This contrasts with the findings of Meniere's disease in the abnormal caloric test group. Meniere's disease is one of the main causes of bilateral vestibulopathy,26 which means that it may result in ongoing deterioration of the vestibular function. Indeed, the duration of Meniere's disease seems to be longer in a bilaterally positive vHIT than in a unilaterally positive vHIT (1,174 days vs. 240 days), although no statistical comparison was possible because there was only one subject with Meniere's disease in the unilateral vHIT group. Therefore, a bilaterally positive vHIT and relatively spared caloric responses may be observed when the pathological changes are not sufficient to decrease the caloric responses to meet the criterion of bilateral vestibulopathy (i.e., total sum of four absolute peak slow eye velocities <20°/s).
A unilaterally positive vHIT with normal caloric responses is unusual in vestibular neuritis/labyrinthitis. However, the inflammation may gradually worsen and the vestibular function may vary according to the severity of inflammation and the vulnerability of neural structures.5 Given that a normal head impulse AVOR requires weighted neuronal responses over the peripheral and central vestibular systems, which represents a greater coordination of different mechanisms compared to that required for normal caloric responses,24 partial damage of the vestibular nerve during the early phase of inflammation may result in an abnormal vHIT with normal caloric responses.5 However, this dissociation might instead be attributable to different parameter for determining abnormalities of vHITs and caloric tests. Indeed, unilateral caloric paresis is determined by a difference in the responses between the ears. Thus, when the damage to one ear was mild, the caloric paresis would not reach the threshold for abnormality. Indeed, the AI of the vHIT gain calculated by the same method for caloric paresis did not reach 25% in this subgroup.
The etiology also differed between the bilaterally and unilaterally positive vHIT subgroups in central lesions. Diffuse brain lesions resulting from meningoencephalitis, Wernicke's encephalopathy, and metastatic carcinomatosis were common in the bilateral subgroup, while circumscribed lesions including strokes or tumors were common in the unilateral group. Another subgroup of abnormal vHITs showed hyperactive results that have been considered a sign of diffuse cerebellar dysfunction.24 Like in previous observations,27,28 the etiology of hyperactive vHITs in this study also included degenerative or inflammatory disorders.
This study was subject to some limitations. Inherent bias is inevitable in any study with a retrospective design despite the use of consecutively collected registry data. As we found in Meniere's disease and vestibular neuritis patients, the results of vHITs and caloric tests depend on the disease duration. However, temporal evolution in the patterns of dissociation could not be addressed due to the cross-sectional study design. Thus, clinicians should consider the etiology as well as the stage of each disorder when interpreting the results of vHITs and caloric tests, and remember that the mechanisms of dissociation in various subgroups remain uncertain.
In conclusion, dissociation in the results between caloric tests and vHITs is not uncommon in patients with dizziness. Cases of abnormal caloric tests and normal vHITs were mostly attributable to peripheral lesions. In contrast, similar proportions of peripheral and central lesions were present in patients with abnormal horizontal vHITs and normal caloric tests, which underscores the need for careful evaluations of central pathologies.
Acknowledgements
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science and ICT (NRF-2017R1C1B1008582).
Footnotes
- Conceptualization: Jeong-Yoon Choi, Ji-Soo Kim, Hui Jong Oh.
- Data curation: Jeong-Yoon Choi, Eunjin Kwon, Hyo-Jung Kim, Ja-Won Koo.
- Formal analysis: Jeong-Yoon Choi, Ju-Young Lee.
- Funding acquisition: Ji-Soo Kim, Jeong-Yoon Choi.
- Investigation: Jeong-Yoon Choi, Ju-Young Lee, Eunjin Kwon, Hyo-Jung Kim.
- Supervision: Ji-Soo Kim, Hui Jong Oh, Ja-Won Koo.
- Writing—original draft: Jeong-Yoon Choi, Ju-Young Lee.
- Writing—review & editing: Jeong-Yoon Choi, Ji-Soo Kim.
Conflicts of Interest: Dr. JS Kim serves as an associate editor of Frontiers in Neuro-otology and on the editorial boards of the Journal of Korean Society of Clinical Neurophysiology, the Journal of Clinical Neurology, Frontiers in Neuroophthalmology, the Journal of Neuro-ophthalmology, the Journal of Vestibular Research, the Journal of Neurology, and Medicine. Others have no conflicts of interest to disclose.
References
- 1.Baloh RW, Honrubia V. Clinical neurophysiology of the vestibular system. Contemp Neurol Ser. 1979;18:1–21. [PubMed] [Google Scholar]
- 2.Halmagyi GM, Curthoys IS. A clinical sign of canal paresis. Arch Neurol. 1988;45:737–739. doi: 10.1001/archneur.1988.00520310043015. [DOI] [PubMed] [Google Scholar]
- 3.Halmagyi GM, Chen L, MacDougall HG, Weber KP, McGarvie LA, Curthoys IS. The video head impulse test. Front Neurol. 2017;8:258. doi: 10.3389/fneur.2017.00258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van de Berg R, Rosengren S, Kingma H. Laboratory examinations for the vestibular system. Curr Opin Neurol. 2018;31:111–116. doi: 10.1097/WCO.0000000000000526. [DOI] [PubMed] [Google Scholar]
- 5.Lee SU, Park SH, Kim HJ, Koo JW, Kim JS. Normal caloric responses during acute phase of vestibular neuritis. J Clin Neurol. 2016;12:301–307. doi: 10.3988/jcn.2016.12.3.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baek SH, Choi JY, Jung JM, Kwon DY, Park MH, Choi J, et al. Abnormal head impulse test in a unilateral cerebellar lesion. J Clin Neurol. 2015;11:279–282. doi: 10.3988/jcn.2015.11.3.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Park HK, Kim JS, Strupp M, Zee DS. Isolated floccular infarction: impaired vestibular responses to horizontal head impulse. J Neurol. 2013;260:1576–1582. doi: 10.1007/s00415-013-6837-y. [DOI] [PubMed] [Google Scholar]
- 8.Chen L, Todd M, Halmagyi GM, Aw S. Head impulse gain and saccade analysis in pontine-cerebellar stroke and vestibular neuritis. Neurology. 2014;83:1513–1522. doi: 10.1212/WNL.0000000000000906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blödow A, Heinze M, Bloching MB, von Brevern M, Radtke A, Lempert T. Caloric stimulation and video-head impulse testing in Ménière's disease and vestibular migraine. Acta Otolaryngol. 2014;134:1239–1244. doi: 10.3109/00016489.2014.939300. [DOI] [PubMed] [Google Scholar]
- 10.Lee SU, Kim HJ, Koo JW, Kim JS. Comparison of caloric and head-impulse tests during the attacks of Meniere’s disease. Laryngoscope. 2017;127:702–708. doi: 10.1002/lary.26103. [DOI] [PubMed] [Google Scholar]
- 11.McGarvie LA, Curthoys IS, MacDougall HG, Halmagyi GM. What does the dissociation between the results of video head impulse versus caloric testing reveal about the vestibular dysfunction in Ménière’s disease? Acta Otolaryngol. 2015;135:859–865. doi: 10.3109/00016489.2015.1015606. [DOI] [PubMed] [Google Scholar]
- 12.Zellhuber S, Mahringer A, Rambold HA. Relation of video-head-impulse test and caloric irrigation: a study on the recovery in unilateral vestibular neuritis. Eur Arch Otorhinolaryngol. 2014;271:2375–2383. doi: 10.1007/s00405-013-2723-6. [DOI] [PubMed] [Google Scholar]
- 13.Shaw B, Raghavan RS. Dissociation between caloric and head impulse testing in patients with congenital abnormalities of the semicircular canals. J Laryngol Otol. 2018;132:932–935. doi: 10.1017/S0022215118001317. [DOI] [PubMed] [Google Scholar]
- 14.Kim HJ, Park SH, Kim JS, Koo JW, Kim CY, Kim YH, et al. Bilaterally abnormal head impulse tests indicate a large cerebellopontine angle tumor. J Clin Neurol. 2016;12:65–74. doi: 10.3988/jcn.2016.12.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Strupp M, Kim JS, Murofushi T, Straumann D, Jen JC, Rosengren SM, et al. Bilateral vestibulopathy: diagnostic criteria consensus document of the Classification Committee of the Bárány Society. J Vestib Res. 2017;27:177–189. doi: 10.3233/VES-170619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Choi JY, Glasauer S, Kim JH, Zee DS, Kim JS. Characteristics and mechanism of apogeotropic central positional nystagmus. Brain. 2018;141:762–775. doi: 10.1093/brain/awx381. [DOI] [PubMed] [Google Scholar]
- 17.Baloh RW. Clinical practice. Vestibular neuritis. N Engl J Med. 2003;348:1027–1032. doi: 10.1056/NEJMcp021154. [DOI] [PubMed] [Google Scholar]
- 18.Lopez-Escamez JA, Carey J, Chung WH, Goebel JA, Magnusson M, Mandalà M, et al. [Diagnostic criteria for Menière’s disease according to the Classification Committee of the Bárány Society] HNO. 2017;65:887–893. doi: 10.1007/s00106-017-0387-z. [DOI] [PubMed] [Google Scholar]
- 19.Hannigan IP, Welgampola MS, Watson SRD. Dissociation of caloric and head impulse tests: a marker of Meniere’s disease. J Neurol. 2019 doi: 10.1007/s00415-019-09431-9. [DOI] [PubMed] [Google Scholar]
- 20.Tsuji K, Velázquez-Villaseñor L, Rauch SD, Glynn RJ, Wall C, 3rd, Merchant SN. Temporal bone studies of the human peripheral vestibular system. Meniere’s disease. Ann Otol Rhinol Laryngol Suppl. 2000;181:26–31. doi: 10.1177/00034894001090s505. [DOI] [PubMed] [Google Scholar]
- 21.Boyle R, Rabbitt RD, Highstein SM. Efferent control of hair cell and afferent responses in the semicircular canals. J Neurophysiol. 2009;102:1513–1525. doi: 10.1152/jn.91367.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pujol R, Pickett SB, Nguyen TB, Stone JS. Large basolateral processes on type II hair cells are novel processing units in mammalian vestibular organs. J Comp Neurol. 2014;522:3141–3159. doi: 10.1002/cne.23625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim HJ, Lee SH, Park JH, Choi JY, Kim JS. Isolated vestibular nuclear infarction: report of two cases and review of the literature. J Neurol. 2014;261:121–129. doi: 10.1007/s00415-013-7139-0. [DOI] [PubMed] [Google Scholar]
- 24.Choi JY, Kim HJ, Kim JS. Recent advances in head impulse test findings in central vestibular disorders. Neurology. 2018;90:602–612. doi: 10.1212/WNL.0000000000005206. [DOI] [PubMed] [Google Scholar]
- 25.Sadeghi SG, Minor LB, Cullen KE. Neural correlates of motor learning in the vestibulo-ocular reflex: dynamic regulation of multimodal integration in the macaque vestibular system. J Neurosci. 2010;30:10158–10168. doi: 10.1523/JNEUROSCI.1368-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Strupp M, Feil K, Dieterich M, Brandt T. Bilateral vestibulopathy. Handb Clin Neurol. 2016;137:235–240. doi: 10.1016/B978-0-444-63437-5.00017-0. [DOI] [PubMed] [Google Scholar]
- 27.Choi JY, Kim JS, Jung JM, Kwon DY, Park MH, Kim C, et al. Reversed corrective saccades during head impulse test in acute cerebellar dysfunction. Cerebellum. 2014;13:243–247. doi: 10.1007/s12311-013-0535-2. [DOI] [PubMed] [Google Scholar]
- 28.Walker MF, Zee DS. Cerebellar disease alters the axis of the high-acceleration vestibuloocular reflex. J Neurophysiol. 2005;94:3417–3429. doi: 10.1152/jn.00375.2005. [DOI] [PubMed] [Google Scholar]