Abstract
Background and Purpose
Responses to oral appliances (OAs) in obstructive sleep apnea (OSA) vary, and have not been fully evaluated in Korean patients. In this study we aimed to determine the efficacy of OAs for the first-line treatment of Korean patients with moderate or severe OSA.
Methods
This multicenter prospective observational study included 45 patients with moderate or severe OSA that had been newly diagnosed between March 2017 and May 2018 and who underwent OA treatment for 1 month. Questionnaires were completed and polysomnography (PSG) was performed before and after OA treatment. The primary outcome measures were improvement in the absolute apnea-hypopnea index (AHI) and the percentage reduction in the AHI. The secondary outcomes were improvements in the questionnaire scores related to sleep-associated symptoms and PSG parameters.
Results
The patients were aged 47.4±12.1 years (mean±SD), only two of them were female, and their AHI at baseline was 29.7±10.9/h. After OA treatment the AHI had reduced by 63.9±25.8%, with the reduction was similar between the patients with moderate OSA and those with severe OSA. Overall 31.1% of the patients achieved a normal AHI (<5/h), and 64.4% had an AHI of ≤10/h after the treatment. The body mass index (BMI) was the most reliable factor for predicting the percentage reduction in the AHI. The OAs also improved the sleep architecture and subjective sleep-related symptoms.
Conclusions
The OAs were effective in patients with moderate or severe OSA. The OAs reduced the mean AHI to 63.9% of the baseline value, and this reduction was influenced by the BMI.
Keywords: oral appliance, obstructive sleep apnea, treatment, apnea, hypopnea
INTRODUCTION
Patients with moderate or severe obstructive sleep apnea (OSA) are prone to cardiovascular and metabolic comorbidities, and so they should receive treatment.1 Although continuous positive airway pressure (CPAP) is a treatment mainstay for moderate and severe OSA, oral appliance (OA) therapy can be a useful alternative option. An OA is a device that advances the mandible in order to reduce the collapsibility of the upper airway, which will improve OSA in most patients.2 OAs are generally recommended for patients with mild or moderate OSA and those who are intolerant of or refuse CPAP therapy.3 Some studies have found that OAs may even be useful for treating severe-OSA patients.4,5
There is only weak evidence for the use of OA treatment as a first-line therapy in Korean patients with moderate or severe OSA. Asian patients with OSA generally have a lower body mass index (BMI), smaller mandibles, and more-collapsible airways.6 Nonanatomical pathophysiological factors such as the arousal threshold, muscle responsiveness, and ventilatory control instability have also been suggested to be less important in Asians.7 Therefore, the influence of race on OA treatment outcomes needs to be clarified. A few retrospective Korean studies found that the rates of successful OA treatment in patients with moderate OSA and those with severe OSA were 71–82% and 70–75%, respectively.8,9 One prospective study performed in Thailand included a subset of these patients, and found success rates of 55% and 75% in individuals with moderate OSA and those with severe OSA, respectively.10
Since the responses to OA treatment vary, careful patient selection is crucial for achieving treatment success in clinical practice. Previous studies have applied various cutoff criteria to indicate successful treatment, such as a follow-up apnea- hypopnea index (AHI) of <5/h or <10/h. Researchers have reported that being younger and having a lower BMI, smaller neck circumference, lower baseline AHI, and positional OSA are predictors of a good outcome, but these assertions have not been consistent across studies.11,12 These variable outcomes might be due to OSA being a heterogeneous disorder. Many factors contribute to OSA, including upper airway anatomical abnormalities, airway dilator muscle dysfunction, an increased loop gain, and a decreased arousal threshold.13 OAs improve the anatomy of the upper airway by decreasing its collapsibility, but they appear to have no effect on muscle function, loop gain, or the arousal threshold.14 The responses to OAs may be greatly affected by nonanatomical characteristics of OSA. We hypothesized that OAs can ameliorate the portion of the AHI that is affected by anatomical factors. Therefore, we evaluated factors that are associated with the percentage reduction in the AHI rather than the cutoff criteria of the AHI.
In this multicenter prospective observational study we evaluated the efficacy of OA treatment in Korean patients with moderate or severe OSA. We evaluated the objective and subjective efficacies at 1 month after treatment initiation, and determined the clinical predictors of the percentage reductions in the AHI.
METHODS
Patients
This multicenter prospective observational study was performed in three sleep centers in South Korea (Kyung Hee University Hospital at Gangdong, Keimyung University Dongsan Medical Center, and Soonchunhyang University Hospital Cheonan). Patients who underwent overnight polysomnography (PSG) and were newly diagnosed with moderate or severe OSA between March 2017 and May 2018 were considered for inclusion. Moderate OSA was defined as an AHI of 15–29.9/h, and severe OSA was defined as an AHI ≥30/h. The exclusion criteria were 1) OA contraindications (periodontal disease, insufficient teeth, or temporomandibular joint dysfunction), 2) predominantly central sleep apnea, and 3) significant pulmonary or cardiac disease. This study was approved by the Institutional Review Board of each center (IRB No. Kyung Hee University Hospital at Gangdong: 2017-02-013-010, Keimyung University Dongsan Medical Center: 2017-02-046, Soonchunhyang University Hospital Cheonan: 2017-03-027), and written informed consent to participate was obtained from all of the enrolled patients.
Procedures
At baseline we obtained medical histories, performed physical examinations, collected self-reported questionnaires, and gathered overnight PSG data. PSG was performed using a digital polygraph system (Grass-Telefactor twin version 2.6, West Warwick, RI, USA) according to standard protocols. Airflow was measured using both an oronasal thermal sensor and a nasal pressure sensor. The data were manually scored according to version 2.2 of the Manual for the Scoring of Sleep and Associated Events published by American Academy of Sleep Medicine.15 The patients who met the inclusion criteria received a customized two-piece OA (SomnoDent, SomnoMed, Sydney, Australia). The treatment protocol for the OA was identical in all centers: the OA was incrementally titrated to the maximum comfortable limit over an acclimatization period lasting 4–6 weeks, and the results were confirmed by a qualified dentist. The degree of mandibular advancement was set by the dentist according to the maximum comfortable (or tolerable) limit for each patient. The patients were followed up 1 month after the completion of OA titration, and the questionnaires and PSG were repeated. Compliance during OA treatment was determined objectively after 1 month using a temperature-sensitive microsensor (Dentitrac, Braebon, Ontario, Canada) embedded in the upper right side of the OA device.
Questionnaires
Questionnaires were completed by the participants prior to each PSG session. Sleep-related symptoms were evaluated using the Korean version of the Pittsburgh Sleep Quality Index (PSQI), the Epworth Sleepiness Scale (ESS), and the Insomnia Severity Index (ISI). The PSQI measures the quality and patterns of sleep over a 4-week period, the ESS is an eight-item self-reported questionnaire that evaluates the level of daytime sleepiness, and the ISI is a seven-item questionnaire that measures insomnia as perceived by the patient. Depression was evaluated using Beck Depression Inventory-II (BDI-II), which includes 21 multiple-choice questions, each of which is scored from 0 to 3 points.
Outcomes
The primary outcomes were the improvement in the AHI after the treatment, as quantified by the absolute change and the percentage reduction. The improvement was classified into the following three groups according to the 1-month follow-up AHI: 1) <5/h, 2) <10/h, and 3) >50% reduction. The secondary outcomes were the improvement in sleep architecture and the improvements in questionnaire scores evaluating sleep, depression, and OA compliance.
Statistical analysis
All analyses were performed on the intention-to-treat principle, with dropouts and missing values excluded from the analysis. Continuous data were tested for conformity to a normal distribution using the Kolmogorov-Smirnov test, and are presented as mean±SD values. Continuous data were compared using the t-test or Mann-Whitney U-test as appropriate, and the chi-square test was used to analyze categorical data. We used the paired t-test or Wilcoxon signed-rank test to evaluate changes from baseline to 1 month after treatment titration. An initial linear regression was applied to identify factors associated with the percentage change in AHI, and the results are expressed as Pearson correlation coefficients. A multivariate regression model was then developed using stepwise selection, with age and the p-value criterion for inclusion in the model set at <0.10. The criterion for statistical significance was set at p<0.05. All statistical comparisons were performed with SPSS (version 22.0, Armonk, NY, USA).
RESULTS
Clinical features and demographics
Fifty patients (25 with moderate OSA and 25 with severe OSA) were considered for enrollment, of which three patients were excluded for the following reasons: 2 had dental problems and 1 withdrew consent. Two further patients (1 with moderate OSA and 1 with severe OSA) were lost during the 1-month follow-up, and so the remaining 45 patients (AHI=15.0–49.0/h; 21 with moderate OSA and 24 with severe OSA) were included in the analysis (Supplementary Fig. 1 in the online-only Data Supplement). They were aged 47.4±12.1 years, and only two of them were female. Their BMI was 26.8±3.3 kg/m2, and 15.6% of the patients were obese (BMI ≥30 kg/m2), with one patient being morbidly obese (BMI ≥35 kg/m2). Their neck circumference was 39.1±2.5 cm, which was similar between the patients with moderate OSA and those with severe OSA. The mean OA compliance rate (the percentage of days on which the OA was worn for more than 4 h) was 63.4±28.4%, which was higher in the severe-OSA patients than in the moderate-OSA patients (72.8±24.6% vs. 52.7±29.2%, p=0.018); however, the average daily usage time was similar between the two groups (Table 1).
Table 1. Baseline characteristics of moderate and severe OSA patients.
| Total (n=45) | Moderate OSA (n=21) | Severe OSA (n=24) | p | |
|---|---|---|---|---|
| Age, years | 47.4±12.1 | 44.6±13.4 | 49.8±10.4 | 0.159 |
| Sex, male | 43 (95.6) | 20 (95.2) | 23 (95.8) | 0.923 |
| Height, cm | 170.6±7.8 | 172.0±7.5 | 169.3±7.9 | 0.253 |
| Weight, kg | 77.9±11.2 | 79.6±11.5 | 76.5±10.9 | 0.357 |
| BMI, kg/m2 | 26.8±3.3 | 26.8±2.9 | 26.7±3.8 | 0.871 |
| BMI ≥30 kg/m2 | 7 (15.6) | 3 (14.3) | 4 (16.7) | 0.826 |
| Neck circumference, cm | 39.1±2.5 | 39.5±2.3 | 38.7±2.7 | 0.298 |
| SBP, mm Hg | 129.9±16.0 | 129.0±16.8 | 130.8±15.5 | 0.720 |
| DBP, mm Hg | 81.6±14.9 | 80.4±14.3 | 82.7±15.6 | 0.612 |
| HTN | 11 (24.4) | 5 (23.8) | 6 (25.0) | 0.926 |
| DM | 4 (8.9) | 1 (4.8) | 3 (12.5) | 0.363 |
| HL | 5 (11.1) | 1 (4.8) | 4 (16.7) | 0.205 |
| CVD | 4 (8.9) | 2 (9.5) | 2 (8.3) | 0.889 |
| OA compliance*, % | 63.4±28.4 | 52.7±29.2 | 72.8±24.6 | 0.018 |
| OA use, h/day | 6.3±1.2 | 6.1±1.3 | 6.5±1.0 | 0.253 |
Data are mean±SD or n (%) values.
*Percentage of days that OA worn for >4 h.
BMI: body mass index, CVD: cardiovascular disease, DBP: diastolic blood pressure, DM: diabetes mellitus, HL: hyperlipidemia, HTN: hypertension, OA: oral appliance, OSA: obstructive sleep apnea, SBP: systolic blood pressure.
Primary outcomes (changes in respiratory indices)
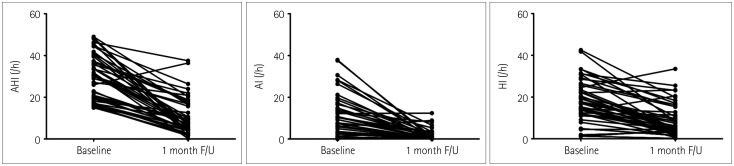
The overall mean percentage reduction in the AHI was 63.9±25.8% (range=40.0–99.4%), which was similar between the patients with moderate OSA and those with severe OSA. The AHI decreased from 29.8±11.0/h to 11.6±10.7/h, the apnea index decreased from 10.5±10.4/h to 1.6±2.7/h, and the hypopnea index decreased from 18.6±9.9/h to 9.0±7.7/h 1 month after OA therapy compared with the same parameters at baseline (all p<0.001) (Fig. 1, Table 2).
Fig. 1. Changes in the AHI, AI, and HI. AHI: apnea-hypopnea index, AI: apnea index, F/U: follow-up, HI: hypopnea index.
Table 2. Respiratory outcome after 1 month of OA therapy.
| Total (n=45) | Moderate OSA (n=21) | Severe OSA (n=24) | p | |
|---|---|---|---|---|
| 1-month F/U AHI <5/h | 14 (31.1) | 9 (42.9) | 5 (20.8) | 0.111 |
| 1-month F/U AHI <10/h | 29 (64.4) | 17 (81.0) | 12 (50.0) | 0.030 |
| Reduction in AHI, /h | 19.0±11.1 | 11.1±6.0 | 25.9±10.0 | <0.001 |
| Reduction in AI, /h | 8.9±10.3 | 3.1±4.6 | 13.9±11.4 | <0.001 |
| Reduction in HI, /h | 9.6±8.5 | 8.0±6.8 | 11.0±9.7 | 0.245 |
| Reduction, % | 63.9±25.8 | 59.8±29.5 | 67.4±22.0 | 0.340 |
| >50% reduction in AHI | 34 (75.6) | 16 (76.2) | 18 (75.0) | 0.685 |
Data are mean±SD or n (%) values.
AHI: apnea-hypopnea index, AI: apnea index, F/U: follow-up, HI: hypopnea index, OSA: obstructive sleep apnea.
The patients with moderate or severe OSA included 31.1% with a normal AHI and 64.4% with an AHI of <10/h at the 1-month follow-up. The proportion of patients who had an AHI of <10/h at the follow-up was higher among those with moderate OSA (81.0% vs. 50.0%, p=0.03). However, the proportion of patients with a normal AHI at the follow-up was similar between the two groups. Three-quarters of the patients exhibited a >50% reduction in the AHI (Table 2).
Secondary outcomes (questionnaire scores and PSG results)
Some of the PSQI, ESS, ISI, and BDI-II scores improved significantly after 1 month of OA treatment. The ESS and BDI-II scores improved only in the patients with moderate OSA. The treatment increased the proportions of time spent in sleep stages N1 and N3, and also the minimum oxygen saturation, and decreased the incidence of wake after sleep onset (WASO) and the arousal index. The changes in WASO and the minimum oxygen saturation were significant only in the severe-OSA patients. All of the respiratory indices decreased, but the proportion of hypopnea in the AHI increased after the treatment (Table 3 and Supplementary Table 1 in the online-only Data Supplement).
Table 3. Questionnaire scores and PSG results at baseline and after 1 month of OA therapy.
| Baseline | 1-month F/U | p | |
|---|---|---|---|
| Questionnaires | |||
| PSQI score | 8.1±3.3 | 6.1±2.9 | <0.001 |
| ESS score | 8.0±4.0 | 7.1±4.7 | 0.029 |
| ISI score | 11.1±5.8 | 7.4±4.7 | <0.001 |
| BDI-II score | 9.5±7.2 | 6.9±6.0 | 0.001 |
| PSG | |||
| TST, minutes | 303.4±72.2 | 308.3±65.4 | 0.404 |
| N1 sleep, % | 23.2±12.8 | 15.6±8.6 | <0.001 |
| N2 sleep, % | 46.7±11.8 | 48.6±12.1 | 0.096 |
| N3 sleep, % | 14.5±13.4 | 19.1±15.7 | 0.001 |
| REM sleep, % | 15.6±6.4 | 16.5±7.3 | 0.41 |
| WASO, minutes | 59.9±52.3 | 38.8±31.8 | 0.009 |
| Sleep efficacy, % | 82.7±12.2 | 86.7±10.1 | 0.040 |
| Arousal index, /h | 39.7±14.5 | 24.2±10.9 | <0.001 |
| Sleep latency, minutes | 5.5±5.6 | 8.1±13.8 | 0.909 |
| REM sleep latency, minutes | 115.2±65.1 | 98.2±58.4 | 0.053 |
| AHI, /h | 29.7±10.9 | 10.7±8.8 | <0.001 |
| AI, /h | 10.5±10.4 | 1.6±2.7 | <0.001 |
| HI, /h | 18.6±9.9 | 9.0±7.7 | <0.001 |
| Hypopnea, % | 65.6±29.1 | 85.3±20.1 | <0.001 |
| Min sat, % | 81.0±8.2 | 84.5±6.3 | 0.001 |
Data are mean±SD or n (%) values.
AHI: apnea-hypopnea index, AI: apnea index, BDI-II: Beck Depression Inventory-II, ESS: Epworth Sleepiness Scale, F/U: follow-up, HI: hypopnea index, ISI: Insomnia Severity Index, Min sat: minimum oxygen saturation, OA: oral appliance, PSG: polysomnography, PSQI: Pittsburgh Sleep Quality Index, REM: rapid eye movement, TST: total sleep time, WASO: wake after sleep onset.
Clinical factors associated with the AHI percentage reduction
In the initial linear regression analysis, the percentage reduction in the AHI was negatively correlated with the BMI (r=−0.368, p=0.013) and positively correlated with the apnea index (r=0.336, p=0.024). The percentage reduction in the AHI tended to be negatively correlated with the proportion of time spent in REM sleep (r=−0.265, p=0.079) and the hypopnea index (r=−0.259, p=0.085), and tended to be positively correlated with the arousal index (r=0.273, p=0.070). Stepwise multivariate linear regression analysis revealed an independent negative correlation between the BMI and the percentage reduction in the AHI (β=−0.368, p=0.013) (Table 4, Fig. 2).
Table 4. Results of univariate and multivariate linear regression analyses.
| Univariate | Multivariate stepwise regression | |||
|---|---|---|---|---|
| r | p | β | p | |
| Age, years | -0.178 | 0.242 | -0.165 | 0.249 |
| BMI, kg/m2 | -0.368 | 0.013 | -0.368 | 0.013 |
| Neck circumference, cm | -0.064 | 0.677 | ||
| OA compliance, % | 0.128 | 0.403 | ||
| OA use, h/day | 0.138 | 0.367 | ||
| Polysomnography | ||||
| TST, minutes | -0.062 | 0.686 | ||
| N1 sleep, % | 0.069 | 0.651 | ||
| N2 sleep, % | -0.112 | 0.464 | ||
| N3 sleep, % | 0.161 | 0.292 | -0.135 | 0.393 |
| REM sleep, % | -0.265 | 0.079 | ||
| WASO | -0.018 | 0.907 | ||
| Sleep efficacy | -0.018 | 0.906 | ||
| Arousal index | 0.273 | 0.070 | 0.166 | 0.275 |
| Sleep latency | 0.131 | 0.389 | ||
| REM sleep latency | 0.050 | 0.745 | ||
| AHI | 0.017 | 0.909 | ||
| AI | 0.336 | 0.024 | 0.220 | 0.160 |
| HI | -0.259 | 0.085 | -0.108 | 0.511 |
| Hypopnea, % | -0.249 | 0.099 | -0.106 | 0.510 |
| Min sat, % | 0.125 | 0.414 | ||
AHI: apnea-hypopnea index, AI: apnea index, BMI: body mass index, HI: hypopnea index, Min sat: minimum oxygen saturation, OA: oral appliance, TST: total sleep time, WASO: wake after sleep onset.
Fig. 2. Correlations of the percentage reduction in the AHI with the baseline AHI (A) and BMI (B). AHI: apnea-hypopnea index, BMI: body mass index.
DISCUSSION
This study found OA treatment to be an effective first-line therapy in Korean patients with moderate or severe OSA. The overall percentage reduction in the AHI was 63.9±25.8%, and was similar between patients with moderate OSA and those with severe OSA. The baseline BMI was the best predictive factor for the reduction. Approximately one-third (31.1%) of the patients had a normal AHI of <5/h at 1 month after the initiation of OA therapy, while around two-thirds (64.4%) had an AHI of <10/h. The treatment not only ameliorated respiratory symptoms during sleep but also improved the sleep quality and depression level.
This study evaluated the effect of OAs as a first-line treatment in Korean patients with moderate or severe OSA. The cohorts in previous retrospective9,16,17 and prospective10 studies only included some patients with moderate OSA (30–60%) or severe OSA (6–57%). One study evaluated the effect of OA treatment in 34 Chinese patients with severe OSA who refused CPAP therapy.18 Most of the patients enrolled in the present study were males, who are known to respond less to OSA treatment,19 and most of them were not obese (BMI <30 kg/m2), which is known to be associated with a better response.4 However, the proportions of patients with AHIs of <5/h or <10/h at the follow-up were similar to those in previous studies.9,10,16,17 The objectively measured percentage of days with good adherence (i.e., the percentage of days on which the OA was worn for >4 h) was 63.4±28.4%, and the duration of OA use was 6.3±1.2 h/day, which is similar to the results of previous studies.9,20,21
The present patients with severe OSA benefited from the OA treatment. Half of the patients had an AHI of <10/h, and 20% had a normal AHI after the treatment. The OA improved not only the respiratory indices of the participants but also their sleep architecture and subjective sleep-related symptoms. OAs are known to improve subjective daytime sleepiness,22 but contradictory results have also been reported.9 The subjective sleep quality and insomnia improved in both the patients with moderate OSA and those with severe OSA, but daytime sleepiness and the depression level improved only in those with moderate OSA. Patients with severe OSA had more frequent arousal and a lower minimum oxygen saturation during sleep, which could have led to this discrepancy.
We hypothesized that OAs can improve the part of the AHI associated with anatomical factors. The overall reduction in the AHI after OA treatment in our study was 63.9±25.8%, which was similar between patients with different severities of OSA. The percentage reduction in the AHI after OA treatment was reported previously in small studies or in specific OSA types. One study found that the reduction after OA therapy in seven patients ranged from 98.1% to 100%.23 A reduction of 62.6±7.5% was found during non-REM sleep, with a median reduction of 13.4% during REM sleep.14 Reductions of 74.69±16.92% and 46.03±36.44% were found in patients with positional and nonpositional OSA, respectively.24 OAs can improve airway collapsibility, but they do not affect muscle function, loop gain, or the arousal threshold.14 The heterogeneity in the results obtained might therefore be attributable to the various pathophysiological characteristics underlying OSA, such as increased loop gain and a decreased arousal threshold.
The BMI and apnea index were associated with the percentage reduction in the AHI in this study. Most previous studies have used different criteria to assess treatment success, including various cutoff values,11 and naturally a lower baseline AHI will be predictive of a good response. The baseline apnea index—but not the AHI—was associated with the AHI percentage reduction in this study. Apnea and hypopnea episodes have different mechanisms, with the former representing the absence of flow due to a static obstruction and the latter representing flow limitations due to a dynamic obstruction.25 Anatomical changes produced by OAs might affect static obstructions more than dynamic obstructions, thus explaining the increased proportion of hypopnea episodes after OA treatment.
The strongest predictive factor for the AHI percentage reduction was the BMI. Most studies have found the BMI to be a predictor of a poor response to OA therapy.20,26 A previous review showed that the BMI has large negative predictive value along with CPAP pressure and cephalometry measures.11 However, some studies performed in Western countries have found that age, the baseline AHI, and cephalometric measures were more significant predictors than the BMI.27 The BMI might be more important for predicting OA treatment responses in Koreans. Higher BMIs are associated with fat deposition, which can narrow pharyngeal wall diameters and increase upper airway collapsibility. Higher BMIs can also increase the loop gain,28 which is an independent predictor of a poor response to OA therapy, and the arousal threshold,29 which is not changed when using an OA. Moreover, a high BMI can cause pharyngeal dilator muscle dysfunction and reduce the respiratory functional residual volume.30 Our results support the idea that a higher BMI can contribute to OSA in more ways than only via affecting the anatomy of the upper airway.
This was the first prospective study of Korean patients with moderate or severe OSA, but the treatment period was only one month, which might have been too short to provide a full evaluation of the compliance and treatment effects. Moreover, the sample was of modest size and predominantly consisted of males, which limits the generalizability of our findings. Moreover, we did not consider cephalometric measures, sleeping position, or REM predominance, which can be important predictors of OA treatment responses.
In conclusion, This study found that OA therapy was effective for patients with either moderate or severe OSA. OA therapy improved not only respiratory indices but also subjective and objective sleep indices. The OAs reduced the mean AHI to 63.9% of the baseline value, and the percentage reduction was lower in patients with higher BMIs. It can therefore be predicted that the follow-up AHI after OA therapy in patients with moderate or severe OSA will be one-third of their baseline AHI, with some variation according to the BMI. Future larger studies are required to confirm the efficacy of OA therapy, treatment predictors for patients with moderate or severe OSA, and the influence of race on outcomes.
Acknowledgements
This study was supported by grants from SomnoMed, but this entity played no role in the study design or in data acquisition, analysis, or interpretation.
Footnotes
- Conceptualization: Jung-Ick Byun, Su-Jin Ahn, Peter A. Cistulli, Won Chul Shin.
- Data curation: Jung-Ick Byun, Dong-Ha Kim, Su-Jin Ahn.
- Formal analysis: Jung-Ick Byun, Dong-Ha Kim.
- Funding acquisition: Won Chul Shin.
- Investigation: Jung-Ick Byun, Dong-Ha Kim, Su-Jin Ahn, Kwang Ik Yang, Yong Won Cho.
- Methodology: Jung-Ick Byun, Su-Jin Ahn, Won Chul Shin.
- Supervision: Won Chul Shin.
- Writing—original draft: Jung-Ick Byun.
- Writing—review & editing: Kwang Ik Yang, Yong Won Cho, Peter A. Cistulli, Won Chul Shin.
Conflicts of Interest: The authors have no potential conflicts of interest to disclose.
Supplementary Materials
The online-only Data Supplement is available with this article at https://doi.org/10.3988/jcn.2020.16.2.215.
Flowchart of the participants. OSA: obstructive sleep apnea.
PSG results at baseline and after 1 month of oral appliance therapy in patients with moderate OSA and those with severe OSA
References
- 1.Flemons WW. Clinical practice. Obstructive sleep apnea. N Engl J Med. 2002;347:498–504. doi: 10.1056/NEJMcp012849. [DOI] [PubMed] [Google Scholar]
- 2.Sutherland K, Vanderveken OM, Tsuda H, Marklund M, Gagnadoux F, Kushida CA, et al. Oral appliance treatment for obstructive sleep apnea: an update. J Clin Sleep Med. 2014;10:215–227. doi: 10.5664/jcsm.3460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ramar K, Dort LC, Katz SG, Lettieri CJ, Harrod CG, Thomas SM, et al. Clinical practice guideline for the treatment of obstructive sleep apnea and snoring with oral appliance therapy: an update for 2015. J Clin Sleep Med. 2015;11:773–827. doi: 10.5664/jcsm.4858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sutherland K, Takaya H, Qian J, Petocz P, Ng AT, Cistulli PA. Oral appliance treatment response and polysomnographic phenotypes of obstructive sleep apnea. J Clin Sleep Med. 2015;11:861–868. doi: 10.5664/jcsm.4934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Doff MHJ, Hoekema A, Wijkstra PJ, van der Hoeven JH, Huddleston Slater JJ, de Bont LGM, et al. Oral appliance versus continuous positive airway pressure in obstructive sleep apnea syndrome: a 2-year follow-up. Sleep. 2013;36:1289–1296. doi: 10.5665/sleep.2948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li KK, Kushida C, Powell NB, Riley RW, Guilleminault C. Obstructive sleep apnea syndrome: a comparison between Far-East Asian and white men. Laryngoscope. 2000;110:1689–1693. doi: 10.1097/00005537-200010000-00022. [DOI] [PubMed] [Google Scholar]
- 7.Lee RWW, Sutherland K, Sands SA, Edwards BA, Chan TO, SS Ng S, et al. Differences in respiratory arousal threshold in Caucasian and Chinese patients with obstructive sleep apnoea. Respirology. 2017;22:1015–1021. doi: 10.1111/resp.13022. [DOI] [PubMed] [Google Scholar]
- 8.Park P, Jeon HW, Han DH, Won TB, Kim DY, Rhee CS, et al. Therapeutic outcomes of mandibular advancement devices as an initial treatment modality for obstructive sleep apnea. Medicine (Baltimore) 2016;95:e5265. doi: 10.1097/MD.0000000000005265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee CH, Mo JH, Choi IJ, Lee HJ, Seo BS, Kim DY, et al. The mandibular advancement device and patient selection in the treatment of obstructive sleep apnea. Arch Otolaryngol Head Neck Surg. 2009;135:439–444. doi: 10.1001/archoto.2009.31. [DOI] [PubMed] [Google Scholar]
- 10.Banhiran W, Kittiphumwong P, Assanasen P, Chongkolwatana C, Metheetrairut C. Adjustable thermoplastic mandibular advancement device for obstructive sleep apnea: outcomes and practicability. Laryngoscope. 2014;124:2427–2432. doi: 10.1002/lary.24607. [DOI] [PubMed] [Google Scholar]
- 11.Okuno K, Pliska BT, Hamoda M, Lowe AA, Almeida FR. Prediction of oral appliance treatment outcomes in obstructive sleep apnea: a systematic review. Sleep Med Rev. 2016;30:25–33. doi: 10.1016/j.smrv.2015.11.007. [DOI] [PubMed] [Google Scholar]
- 12.Ng JH, Yow M. Oral appliances in the management of obstructive sleep apnea. Sleep Med Clin. 2019;14:109–118. doi: 10.1016/j.jsmc.2018.10.012. [DOI] [PubMed] [Google Scholar]
- 13.Shin W, Jen R, Li Y, Malhotra A. Tailored treatment strategies for obstructive sleep apnea. Respir Investig. 2016;54:2–7. doi: 10.1016/j.resinv.2015.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Edwards BA, Andara C, Landry S, Sands SA, Joosten SA, Owens RL, et al. Upper-airway collapsibility and loop gain predict the response to oral appliance therapy in patients with obstructive sleep apnea. Am J Respir Crit Care Med. 2016;194:1413–1422. doi: 10.1164/rccm.201601-0099OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Berry RB, Brooks R, Gamaldo CE, Harding SM, Lloyd RM, Marcus CL, Vaughn BV the American Academy of Sleep Medicine. The AASM manual for the scoring of sleep and associated events: rules, terminology and technical specifications, version 2.2. Darien, IL: American Academy of Sleep Medicine; 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Park P, Jeon HW, Han DH, Won TB, Kim DY, Rhee CS, et al. Therapeutic outcomes of mandibular advancement devices as an initial treatment modality for obstructive sleep apnea. Medicine (Baltimore) 2016;95:e5265. doi: 10.1097/MD.0000000000005265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fukuda T, Tsuiki S, Kobayashi M, Nakayama H, Inoue Y. Selection of response criteria affects the success rate of oral appliance treatment for obstructive sleep apnea. Sleep Med. 2014;15:367–370. doi: 10.1016/j.sleep.2013.12.007. [DOI] [PubMed] [Google Scholar]
- 18.Lam B, Sam K, Lam JCM, Lai AYK, Lam CL, Ip MSM. The efficacy of oral appliances in the treatment of severe obstructive sleep apnea. Sleep Breath. 2011;15:195–201. doi: 10.1007/s11325-011-0496-y. [DOI] [PubMed] [Google Scholar]
- 19.Ng ATM, Darendeliler MA, Petocz P, Cistulli PA. Cephalometry and prediction of oral appliance treatment outcome. Sleep Breath. 2012;16:47–58. doi: 10.1007/s11325-011-0484-2. [DOI] [PubMed] [Google Scholar]
- 20.Dieltjens M, Verbruggen AE, Braem MJ, Wouters K, Verbraecken JA, De Backer WA, et al. Determinants of objective compliance during oral appliance therapy in patients with sleep-disordered breathing: a prospective clinical trial. JAMA Otolaryngol Head Neck Surg. 2015;141:894–900. doi: 10.1001/jamaoto.2015.1756. [DOI] [PubMed] [Google Scholar]
- 21.Ingman T, Arte S, Bachour A, Bäck L, Mäkitie A. Predicting compliance for mandible advancement splint therapy in 96 obstructive sleep apnea patients. Eur J Orthod. 2013;35:752–757. doi: 10.1093/ejo/cjs092. [DOI] [PubMed] [Google Scholar]
- 22.Ahrens A, McGrath C, Hägg U. Subjective efficacy of oral appliance design features in the management of obstructive sleep apnea: a systematic review. Am J Orthod Dentofacial Orthop. 2010;138:559–576. doi: 10.1016/j.ajodo.2010.01.030. [DOI] [PubMed] [Google Scholar]
- 23.Zhao M, Barber T, Cistulli P, Sutherland K, Rosengarten G. Computational fluid dynamics for the assessment of upper airway response to oral appliance treatment in obstructive sleep apnea. J Biomech. 2013;46:142–150. doi: 10.1016/j.jbiomech.2012.10.033. [DOI] [PubMed] [Google Scholar]
- 24.Chung JW, Enciso R, Levendowski DJ, Morgan TD, Westbrook PR, Clark GT. Treatment outcomes of mandibular advancement devices in positional and nonpositional OSA patients. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2010;109:724–731. doi: 10.1016/j.tripleo.2009.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Farré R, Rigau J, Montserrat JM, Buscemi L, Ballester E, Navajas D. Static and dynamic upper airway obstruction in sleep apnea: role of the breathing gas properties. Am J Respir Crit Care Med. 2003;168:659–663. doi: 10.1164/rccm.200211-1304OC. [DOI] [PubMed] [Google Scholar]
- 26.Zeng B, Ng AT, Qian J, Petocz P, Darendeliler MA, Cistulli PA. Influence of nasal resistance on oral appliance treatment outcome in obstructive sleep apnea. Sleep. 2008;31:543–547. doi: 10.1093/sleep/31.4.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hoekema A, Doff MHJ, de Bont LGM, van der Hoeven JH, Wijkstra PJ, Pasma HR, et al. Predictors of obstructive sleep apnea-hypopnea treatment outcome. J Dent Res. 2007;86:1181–1186. doi: 10.1177/154405910708601208. [DOI] [PubMed] [Google Scholar]
- 28.Sands SA, Eckert DJ, Jordan AS, Edwards BA, Owens RL, Butler JP, et al. Enhanced upper-airway muscle responsiveness is a distinct feature of overweight/obese individuals without sleep apnea. Am J Respir Crit Care Med. 2014;190:930–937. doi: 10.1164/rccm.201404-0783OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Edwards BA, Eckert DJ, McSharry DG, Sands SA, Desai A, Kehlmann G, et al. Clinical predictors of the respiratory arousal threshold in patients with obstructive sleep apnea. Am J Respir Crit Care Med. 2014;190:1293–1300. doi: 10.1164/rccm.201404-0718OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tham KW, Lee PC, Lim CH. Weight management in obstructive sleep apnea: medical and surgical options. Sleep Med Clin. 2019;14:143–153. doi: 10.1016/j.jsmc.2018.10.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Flowchart of the participants. OSA: obstructive sleep apnea.
PSG results at baseline and after 1 month of oral appliance therapy in patients with moderate OSA and those with severe OSA