Abstract
Background and Purpose
The prevalence of dementia is increasing in South Korea. Multidomain interventions may be useful for preventing dementia. Such programs need to be disseminated to elderly Koreans throughout the country. We have developed programs of the SoUth Korean study to PrEvent cognitive impaiRment and protect BRAIN health through lifestyle intervention in at-risk elderly people (SUPERBRAIN), which consists of a facility-based multidomain intervention (FMI) program and a home-based multidomain intervention (HMI) program suitable for elderly Koreans. We aim to determine the feasibility of the SUPERBRAIN programs before a large-scale randomized controlled trial.
Methods
We will recruit 150 participants among those without dementia aged 60–79 years with at least 1 modifiable dementia risk factor. They will be randomly assigned in a 1:1:1 ratio to the FMI, HMI, and the waiting-list control arm. The 6-month multidomain intervention consists of management of metabolic and vascular risk factors, cognitive training and social activity, physical exercise, nutritional guidance, and motivational enhancement programs. The primary outcomes are adherence and retention rates and changes in the total scale index score of the Repeatable Battery for the Assessment of Neuropsychological Status from baseline to the study end. The main secondary outcomes are disability, depressive symptoms, quality of life, vascular risk factors, physical performance, nutritional assessment, and motivation questionnaire. There will be an exploratory evaluation of neurotrophic, neurodegeneration, and neuroinflammation factors, microbiome, telomere length, electroencephalography, and neuroimaging measures.
Conclusions
The results obtained will provide information on the applicability of these multidomain intervention programs to at-risk elderly people.
Keywords: cognitive impairment, dementia, lifestyle, prevention, randomized controlled trial
INTRODUCTION
The number of people with dementia worldwide was estimated to be about 47 million in 2015, and the prevalence is expected to triple by 2050.1 Alzheimer's disease (AD) is the most common cause of dementia and is characterized by an insidious onset and progressive deteriorations in cognition, functional ability, and behavior.2 The currently available pharmacological interventions for AD include cholinesterase inhibitors and the N-methyl-D-aspartate receptor antagonist memantine, whose primary goals are merely symptomatic improvement.3 Studies have investigated drugs that target AD pathophysiology in the early stages such as mild cognitive impairment (MCI) and cognitively unimpaired individuals, with the goal of slowing or preventing the progression of AD.4,5 However, effective therapies for modifying the progression of AD have not yet been developed.
While no disease-modifying treatment for any common type of dementia is available, there are interventions that might delay or prevent one-third of dementia cases by targeting the modifiable risk factors of dementia, including increasing education and exercise, improving the diet, maintaining social engagements, reducing smoking, and managing hypertension, obesity, hearing loss, dyslipidemia, depression, and diabetes mellitus (DM).1,6
There is currently an extensive body of literature reporting on both observational and single-domain intervention studies related to the modifiable risk factors for dementia.1,7,8,9 Those studies have led to multidomain intervention studies of dementia prevention in at-risk populations being performed over the past decade.10,11,12 These interventions target multiple risk factors simultaneously and are expected to produce additive or synergistic preventive effects compared to interventions targeting one risk factor alone. In particular, the Finnish Geriatric Intervention Study to Prevent Cognitive Impairment and Disability (FINGER) trial found that an intensive multidomain intervention targeting a healthy diet based on the Finnish Nutrition Recommendations, exercise, cognitive training, and vascular risk monitoring significantly improved cognition compared to a control group.10
There are some challenges in applying a multidomain intervention program that has been demonstrated to be effective in Western populations to elderly Koreans. In addition to cultural and language differences, many elderly Koreans rarely eat dairy products, olive oil, cereals, and wines that are common in the Western diet, which makes such a diet difficult to apply to daily Korean life.8 Conversely, the proportions of carbohydrate, protein, and fat in the Mediterranean-Dietary Approaches to Stop Hypertension diet Intervention for Neurodegenerative Delay (MIND) diet are closer to those in the typical Korean diet, and removing specific recommendations regarding dairy products makes it easier for elderly Koreans to follow the MIND diet.9 Moreover, rather than using fitness centers, many elderly Koreans go to public health centers or senior citizens' welfare centers for leisure activities, or perform walking exercises around their houses alone. These characteristics mean that multidomain intervention programs need to include exercise programs that can be implemented at public facilities or in the home.
We developed a facility-based multidomain intervention (FMI) program and a home-based multidomain intervention (HMI) program suitable for elderly Koreans and modified based on the FINGER. The SoUth Korean study to PrEvent cognitive impaiRment and protect BRAIN health through lifestyle intervention in at-risk elderly people (SUPERBRAIN) consists of a feasibility randomized controlled trial (RCT) and a subsequent large-scale RCT for these multidomain intervention programs. Ensuring the feasibility of the SUPER-BRAIN programs is very important because the ultimate aim is to spread the programs for preventing dementia throughout the country. Correcting problems with the programs through a feasibility study will also help the success of a subsequent main study.
The present study will aim to determine the feasibility of these intervention programs before a large-scale RCT is performed to investigate the efficacy of the programs. The tested hypothesis is that adherence and retention rates will be at least 75% in the FMI and HMI arms, respectively, and that there will be at least no differences in the changes in cognitive function from baseline to post-intervention between each intervention arm and the control arm.
METHODS
Study design
This study is a multicenter, outcome assessor-blinded, randomized controlled trial with a three parallel-arm design. The FMI and HMI arms are the two experimental arms, and the control arm is the comparator. The multidomain intervention period is 24 weeks. Participants will be enrolled in three hospitals and five public health centers across South Korea. Participants will be selected from people who visit outpatient clinics or public health centers for memory problems, and those recruited through advertising. The trial has been registered with www.ClinicalTrials.gov (NCT03980392). This protocol relies on version 2.5 and was written in line with the SPIRIT (Standard Protocol Items: Recommendations for Interventional Trials) guidelines13 (Supplementary Table 1 in the online-only Data Supplement) and included all items from the World Health Organization Trial Registration Data Set. The first participant in this ongoing study was registered on May 29, 2019.
Participants
The inclusion criteria are summarized in Table 1.14,15,16 Modifiable dementia risk factors will be diagnosed following standard criteria and guidelines, including hypertension,17 DM,18 dyslipidemia,19 obesity,20 abdominal obesity,21 and metabolic syndrome.22 A current smoker is defined as a participant who has smoked more than 100 cigarettes throughout their lifetime and smokes more than 1 cigarette each month.23 Physical inactivity is defined as moderate intensity physical activity lasting <150 minutes per week.24 Social inactivity is defined as fewer than two social activities per week.25 The exclusion criteria are presented in Table 1.
Table 1. Inclusion and exclusion criteria applied in the study.
| Inclusion criteria |
| 1. Aged 60 to 79 years. |
| 2. Having at least one modifiable dementia risk factor from among hypertension, diabetes mellitus, dyslipidemia, obesity, abdominal obesity, meta- bolic syndrome, smoking, educational level ≤9 years, physical inactivity, and social inactivity. |
| 3. MMSE14 Z score* ≥-1.5. |
| 4. Able to independently perform the activities of daily living, as defined by K-IADL15 score <0.4. |
| 5. Can read and write Korean, as defined by a literacy test.16 |
| 6. Having a reliable informant who can provide investigators with requested information. |
| 7. Providing written informed consent. |
| Exclusion criteria |
| 1. Major psychiatric illness such as major depressive disorder. |
| 2. Dementia. |
| 3. Substantial cognitive decline. |
| 4. Other neurodegenerative disease (e.g., Parkinson's disease). |
| 5. Malignancy within the previous 5 years. |
| 6. Cardiac stent or revascularization within the previous 1 year. |
| 7. Serious or unstable symptomatic cardiovascular disease. |
| 8. Other serious or unstable medical disease such as acute or severe asthma, active gastric ulcer, severe liver disease, or severe renal disease. |
| 9. Severe loss of vision or hearing, or communication disability. |
| 10. Any conditions preventing cooperation as judged by the study physician. |
| 11. Significant laboratory abnormality that may result in cognitive impairment. |
| 12. Unable to participate in the exercise program safely. |
| 13. Simultaneous participation in any other intervention trial. |
*Based on the means and standard deviations in the age- and education-matched normal elderly Korean population.14
K-IADL: Korean Instrumental Activities of Daily Living, MMSE: Mini-Mental State Examination.
Randomization
Participants will be randomly assigned at baseline in a 1:1:1 ratio to the FMI, HMI, and control arms using the permuted block randomization method with SAS macro programming (SAS Institute, Cary, NC, USA), stratified by the participating centers. Each center will therefore include participants receiving FMI, participants receiving HMI, and controls. The allocation sequence is known only to an independent statistics specialist. To randomize participants, a file bearing the participant's research identification number is emailed to the statistics specialist by the principal investigator or coordinator of the participating center, and a file containing the participant's assignment information is received via email from the statistics specialist. Cognitive outcome assessors will remain blind to the treatment allocation.
Intervention
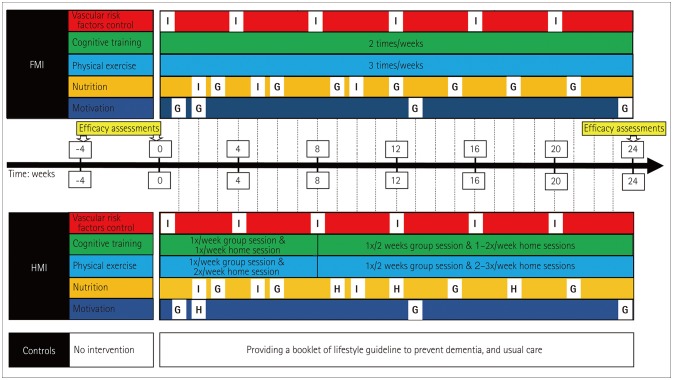
The participants in the FMI and HMI arms will receive all five components of the intervention: 1) monitoring and management of metabolic and vascular risk factors, 2) cognitive training and social activity, 3) physical exercise, 4) nutritional guidance, and 5) motivational training (Fig. 1).
Fig. 1. The South Korean study to PrEvent cognitive impaiRment and protect BRAIN health through lifestyle intervention in at-risk elderly people (SUPERBRAIN) protocol. Participants are classified into the FMI arm, the HMI arm, and the control arm. Cognitive training will be performed twice weekly and physical exercise will be performed three times weekly in the FMI and HMI arms, while control of vascular risk factors, nutritional education, and motivational enhancement programs will be offered several times according to the schedules shown. FMI: facility-based multidomain intervention, G: group intervention, H: home-based session using a tablet or workbook, HMI: home-based multidomain intervention, I: individual intervention.
Facility-based multidomain intervention
A group will consist of 5 or 10 persons depending on the size of the study center. The metabolic and vascular risk factors will be assessed by blood tests and anthropometric measurements (weight, blood pressure, and waist circumference) before the intervention. Hypertension, DM, dyslipidemia, obesity, abdominal obesity, smoking, and high alcohol consumption will be monitored and managed. Each participant will meet a study doctor at baseline and week 12. The study doctors will inform participants of their risk factors, and will prescribe medications if necessary. Participants will be educated at baseline by a study nurse and given education booklets describing their risk factors and a booklet of lifestyle guidelines to prevent dementia. They will also meet the study nurse every 4 weeks for anthropometric measurements and the monitoring of the smoking status and alcohol consumption. Measurements will be recorded at each visit in the participants' notebooks for SUPERBRAIN, so that they can be motivated by the changes. If the risk factors for a participant do not improve, the study nurse will again educate the participant at week 12 with the aid of the booklets.
Cognitive training targets the cognitive domains of episodic memory, executive function, attention, working memory, calculation, and visuospatial function. Cognitive training will be conducted in group sessions by trained health professionals (psychologists, occupational therapists, and study nurses) using a tablet-based application. The composition and content of the cognitive training application are presented in Table 2. If a participant has difficulty in using a tablet, they will be provided with workbooks to be completed under the supervision of trained health professionals. Each workbook for one session contains six pages and is based on homework materials that were found to be effective in MCI patients in a previous study.7 Three or four pages of each workbook consist of memory tasks, and the other two or three pages consist of tasks in other domains, such as executive function, attention, visuospatial function, calculation, or language (Table 3). The content of the workbook is similar to the cognitive training application. The memory strategies applied to the cognitive training application are also practiced in the workbook. The workbooks comprise two levels of difficulty, and the health professionals will determine the level to be used according to the ability of each participant at baseline. Cognitive training will be provided twice weekly for 50 minutes per session.
Table 2. Composition and content of the tablet-based cognitive training application.
| Cognitive domain | Structure | Contents |
|---|---|---|
| Memory | First, information on a subject is provided. Second, a learning process is carried out by using memory strategies and processbased training. Third, quizzes are provided on the subject. The number of memory tasks is 48. | The memory tasks cover the following subjects: Korean folk tales and myths, history and features of Jeju Island and Gangwon Province, health knowledge (dental hygiene, stroke, dementia prevention strategies, Alzheimer's disease, sleep, and air pollution), local festivals, foreign greetings, food (brain foods, seasonal foods, history of kimchi, and Korean traditional liquors according to regions), and national flags. |
| Frontal executive function and attention | Each task comprises a tutorial and a main task. Process-based training is performed. There are 10 levels ranging from level 1 (the easiest) to level 10 (the most difficult). The number of frontal executive function and attention tasks is 12. | Placing footprints forward or backward, placing numbers in ascending or descending order, placing all pictures with the features presented, tapping blocks in the order presented, catching fish with suggested characteristics among various fish, picking up fruit as fast as possible, n-back, Sudoku, performing the directed tasks by remembering where the tomato sprouts disappeared from, and finding matching cards from several face-down cards. |
| Visuospatial function | Each task comprises a tutorial and a main task. Process-based training is performed. There are 10 levels ranging from level 1 (the easiest) to level 10 (the most difficult). The number of visuospatial tasks is 4. | Following the arrows and finding a treasure or acorn, visualizing a picture as two pictures combined, and finding the same figures oriented in different directions. |
| Calculation | A tutorial and a main task are provided. Process-based training is performed. There are 10 levels ranging from level 1 (the easiest) to level 10 (the most difficult). The number of calculation tasks is 4. | Calculation of arrival time, breaking honeycomb through calculations, summation after rotating numbers, and calculating the total cost of shopping items. |
Table 3. Structure and implementation of the tablet-based application and workbooks for cognitive training.
| Cognitive training application | Workbook for cognitive training | |
|---|---|---|
| Training cognitive domain | Memory, frontal function, attention, visuospatial function, and calculation. | Memory, frontal function, attention, visuospatial function, calculation, and language. |
| Training memory strategies | Spaced retrieval, visual imagery, story making, association, chunking, categorization, sequencing, acronyms, keyword method, rehearsal, and rhyming. | Spaced retrieval, visual imagery, story making, association, chunking, categorization, sequencing, acronyms, keyword method, rehearsal, and rhyming. |
| Structure | The tablet-based cognitive training application has 48 memory tasks, 12 frontal/attention tasks, 4 visuospatial tasks, and 4 calculation tasks. | There are 48 workbooks, each of which consists of 3 or 4 pages for 1 memory task and 2 or 3 pages for tasks in different cognitive domains. |
| Level of difficulty | Ten levels of difficulty in all except memory tasks, which have a single level of difficulty. | Two levels of difficulty |
| Session configuration | 1. Welcome, and checking homework (10 minutes). | 1. Welcome, and checking homework (10 minutes). |
| 2. Four tasks using the tablet application: one memory task (20 minutes), two frontal function/attention tasks (10 minutes), and one visuospatial or calculation task (5 minutes). | 2. Cognitive training using one workbook (30–35 minutes). | |
| 3. Wrap-up (5 minutes). | 3. Wrap-up (5 minutes). | |
| Therapist | Psychologist, occupational therapist, or study nurse. | Psychologist, occupational therapist, or study nurse. |
| Adherence | Adherence is assessed using the administrative website that stores information such as whether a participant uses cognitive content. | Adherence is assessed by checking the workbook. |
Writing a diary using a structured form twice weekly will be assigned as homework to the FMI and HMI arms. The diary consists of seven sections: today's date and weather, how you are feeling on the day, meals consumed during the day, today's people and places (places visited, and the traveling method, purpose, and persons met), the amount and details of expenditure, my thoughts on today's news, and tomorrow's plan. Writing a diary is beneficial in providing training related to orientation, mindfulness, episodic memory, healthy diet, calculation, and prospective memory. Social activities will be stimulated through the numerous group meetings of all intervention components and additional social activities at outside institutions (e.g., theaters and meeting friends) at least once a month. Detailed information about the additional social activities available will be provided, and participants will document their social activities in their SUPERBRAIN notebooks.
The physical exercise program will consist of aerobic exercise, exercise to enhance balance and flexibility, muscle-strengthening activities involving major muscle groups, and finger-and-toe movements. Exercise training will be provided three times weekly for 60 minutes per session and will cover different kinds of exercise (Table 4, Supplementary Table 2 in the online-only Data Supplement). The physical performance of the participants will be evaluated based on the Korean National Physical Performance Evaluation Program for testing strength, flexibility, coordination, and balance, as well as the cardiopulmonary endurance before the intervention. Based on the results for their physical performance as well as their physical condition, participants will perform one among a strength-intensive program designed specifically for those with good physical fitness, a strength-intensive program designed specifically for those with poor physical fitness, an aerobic exercise-intensive program designed specifically for those with good physical fitness, and an aerobic exercise-intensive program designed specifically for those with poor physical fitness. Poor physical fitness will be defined as the recorded physical performance in any test being below the 30th percentile of the norms for the age- and sex-matched normal population. The exercise programs will be guided by trained exercise professionals at a gym. The physical exercise programs will use portable tools such as elastic bands, nine floor plates with numbers, and chairs. Every 2 months, the exercise intensity will be increased and the exercise content will be changed in the FMI and HMI arms. To confirm the effectiveness of the exercise and to adjust the exercise plan appropriately, participants in the intervention groups will receive an additional assessment of their physical performance at week 12 using the Korean National Physical Performance Evaluation Program.
Table 4. Progression of the exercise program.
| Weeks 0–8 | Weeks 9–16 | Weeks 17–24 | |
|---|---|---|---|
| Structured exercise program | Level 1 | Level 2 | Level 3 |
| Exercise frequency, per week | 3 | 3 | 3 |
| Percentage of maximum heart rate | 40–50% | 45–55% | 50–60% |
| Duration of exercise (minutes/session) | 60 | 60 | 60 |
| Resistance exercise (minutes/session) | 20–25 | 20–25 | 20–25 |
| Number of muscle groups | 10 | 12 | 15 |
| Number of sets 1 or 2 | 1–3 | 2–4 | |
| Aerobic exercise (minutes/session) | 20–25 | 20–25 | 20–25 |
| Balance exercise (minutes/session) | 5 | 5 | 5 |
| Finger-and-toe movements (minutes/session) | 5 | 5 | 5 |
| Stretching exercise (minutes/session) | 5 | 5 | 5 |
The nutritional intervention includes three individual counseling sessions (each lasting 30 minutes) and seven group sessions (each lasting 50 minutes) led by study nutritionists. Individual sessions include tailoring the participant's daily diet and providing education on customized diets to manage individual vascular risk factors. Group sessions provide discussions, practical exercises for facilitating changes in diet, and advice on how to make meals with recommended ingredients via a cooking lesson. According to the recommendation of the MIND diet, participants will be advised to consume a diet that includes at least three servings of whole grains each day; at least one dark-green salad, another vegetable, and one ounce of nuts each day; beans or legumes at least every other day; berries and poultry at least twice a week; fish at least once a week; olive oil instead of butter or margarine; a glass of wine each day; cheese, fried food, and fast food no more than once a week; and pastries and sweets fewer than five times a week.9 The participants will be motivated by writing a MIND diet checklist by themselves every 2 weeks.
Motivational enhancement includes four group counseling sessions lasting 50 minutes each and led by a psychologist (at 1, 2, 13, and 24 weeks). The purpose of the motivational enhancement program is to induce, maintain, and strengthen motivation, thereby acting as a psychological resource to help maintain the performance of dementia prevention activities. Group meetings provide information and support for facilitating dementia prevention program activities, and include discussions on the importance of change, ambivalence, and self-efficacy, as well as family education using video clips. We developed three video clips for participants, families, and facility workers based on learning and nudge theories.26,27 In addition, through the family-coach program, a family member can participate in reinforcing the motivation of a participant. Encouraging pop-up video messages made by participants' families and self-rated achievement pop-up messages will be provided every week before the tablet-based cognitive intervention. Participants in workbook-type cognitive interventions will be sent the encouraging pop-up video messages on their cell phones and will perform self-rated achievement assessments on paper.
Home-based multidomain intervention
The management program for metabolic and vascular risk factors, the social activity programs, and the motivational enhancement programs in the HMI arm are identical to those in the FMI arm. The difference is that the motivational enhancement program at week 2 is performed alone at home using a tablet or workbook.
The contents of the cognitive training and physical exercise programs in the HMI arm are identical to those in the FMI arm. Those in the HMI arm will participate in one group cognitive training session (each lasting 50 minutes) and one homebased cognitive training session (each lasting 30–40 minutes) per week, and one group exercise session (each lasting 60 minutes) and two home-based exercise sessions (each lasting 60 minutes) per week during the first 2 months of the trial. For the remainder of the 6-month study, participants in the HMI arm will attend one group cognitive training session and one group exercise session every 2 weeks. For the weeks that include group sessions, participants will perform one cognitive training session and two exercise sessions alone at home each week. For weeks that do not include group sessions, participants will perform two cognitive training sessions and three exercise sessions alone at home each week.
Whenever participants attend the group sessions, exercise professionals will provide tips on how to incorporate the exercises at home. Participants will perform a tablet-based cognitive training program and will exercise at home watching videos on the tablet PC. If a participant has difficulty using the tablet, they will complete a workbook for cognitive training in a group session and at home, and will exercise at home while following instructions on a poster or in a booklet. Participants will be given appropriate equipment to enable them to perform the exercises.
The exercise behaviors of the participants will be monitored by wearing a Fitbit smartwatch (Fitbit, Inc., San Francisco, CA, USA) on their wrists while they perform their daily activities. Because the Fitbit smartwatch does not capture all types of physical activity and is unable to discriminate between the activities of our exercise program and other daily activities, participants will write down and report the day and time when they perform the exercise program. We will subsequently cross-check their written self-report with the recorded Fitbit activity. During the nonvisiting week, a study coordinator will motivate participants in the HMI arm to complete their homework well and perform a safety assessment via telephone conversations.
The contents of the nutritional programs are identical in the HMI and FMI arms. The nutritional intervention program consists of three individual counseling sessions and four group education sessions by the study nutritionist, and three home-based sessions using a tablet or workbook (Fig. 1).
Control condition
At baseline, participants in the control group will meet a study doctor, be prescribed medication if necessary, and receive a booklet on lifestyle guidelines to prevent dementia. The participants will also be informed that they can participate in the multidomain intervention program after this study ends and will receive their usual care during the study period.
Outcome measures
The primary outcomes are adherence and retention rates, and the change in the total scale index score of the Repeatable Battery for the Assessment of Neuropsychological Status (RBANS) from baseline to the end of the study. The RBANS consists of tests A, B, C, and D that have an identical degree of difficulty. Each test includes 12 subtests and evaluates the following 5 cognitive domains: attention, language, visuospatial/constructional abilities, immediate memory, and delayed memory.28 Participants will perform tests A and D ofthe RBANS at baseline and postintervention, respectively. The intervention program will be determined feasible if the following success criteria are met: 1) a minimum retention rate of 75% at week 24 (based on a previous study),7 2) a minimum adherence to the intervention program of 75%, and 3) at least no difference from the control arm in the RBANS analysis.29 Feasibility will be determined separately in the FMI and HMI arms.
Adherence with all of the group and individual interventions carried out at the institution will be assessed during the time that they are participating in the intervention. The tablet-based cognitive application is configured to allow administrators to view all of the data of the participants in user access. Information such as whether or not a participant uses the cognitive content and their correct answer rate and attendance rate is stored. The study coordinators will assess adherence with the cognitive program home sessions on the administration homepage, or by checking the last workbook homework in the HMI arm. The study coordinators will check adherence with the exercise program home sessions by comparing the written self-reports with the recorded Fitbit activity. The study coordinator will assess adherence by checking the homework of the nutritional and motivational home sessions. The study coordinator will assess adherence of the additional social activity by checking the written self-reports in participants' SUPERBRAIN notebooks. The retention rate will be calculated as the percentage of participants in each group who have not dropped out from the study at its end.
The secondary outcome measures are summarized in Table 5,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48 which include the measures to evaluate the effectiveness of each of the five components of the intervention.
Table 5. Primary and secondary outcome measures.
| Instrument or method (with score range, assessor) | |
|---|---|
| Primary outcomes | |
| Feasibility | Retention rate |
| Adherence rate | |
| Total scale index score of the RBANS | |
| Secondary outcomes | |
| Cognition | MMSE (range 0–30)14 |
| Clinical Dementia Rating–Sum of Boxes (range 0–18)30 | |
| Prospective Memory Test (range 0–12)7,31 | |
| Prospective Retrospective Memory Questionnaire (range 16–80, participant & informant)32 | |
| Cognitive Complaint Interview (range 0–10, participant)33 | |
| Mood | Geriatric Depression Scale-15 items (range 0–15, participant)34 |
| Disability | Bayer Activities of Daily Living (range 1–10, informant)35 |
| Quality of life | Quality of Life in Alzheimer's Disease (range 0–52, participant & informant)36 |
| Physical function | Global Physical Activity Questionnaire (participant)37 |
| Short Physical Performance Battery (range 0–12)38 | |
| Korean National Physical Performance Evaluation Program* | |
| Nutrition | Nutrition Quotient for Elderly (range 0–100, participant)39 |
| Mini Nutritional Assessment (range 0–14)40 | |
| Food Frequency Questionnaire | |
| Folate, vitamin B12, homocysteine, and 25-OH vitamin D levels | |
| Vascular risk factors | Blood pressure, body mass index, waist circumference, smoking, alcohol consumption, lipid profile, hemoglobin A1c, glucose, other inflammation biomarkers, and lipid and glucose metabolism |
| Motivation | Motivational Enhancement Program Questionnaire (participant)41,42,43,44 |
| Sleep | Pittsburgh Sleep Quality Index (participant)45 |
| Progression | Conversion to mild cognitive impairment or dementia46,47,48 |
| Exploratory outcomes | Electroencephalography |
| Brain MRI: 3D T1-weighted imaging, DTI, and resting-state functional imaging | |
| Actigraphy | |
| Telomere length | |
| Neurotrophic factors: brain-derived neurotropic factor, VEGF, insulin-like growth factor 1 | |
| Neurodegeneration factors: neurogranin, TREM-2, YKL-40, neurofilament light chain Neuroinflammation factors: interleukin-18, transforming growth factor-β, MCP-1 | |
| Plasma amyloid-β42 | |
| Microbiome |
*Consisting of grip power, sit-to-stand movements for 30 seconds, walking in one place for 2 minutes, bending the upper body forward, standing on one foot while lifting the other knee up, fast walking along a figure-of-eight trajectory, and bioelectrical impedance analysis.
DTI: diffusion-tensor imaging, MCP-1: monocyte chemoattractant protein 1, MMSE: Mini-Mental State Examination, RBANS: Repeatable Battery for the Assessment of Neuropsychological Status, TREM-2: triggering receptor expressed on myeloid cells-2, VEGF: vascular endothelial growth factor, YKL-40: chitinase-3-like protein 1.
Exploratory outcomes
We have planned exploratory studies to investigate the mechanism via which the multidomain intervention program works in the brain (Table 5). Changes in cortical thickness and functional networks will be examined using brain MRI in approximately 60 participants from 3 centers. The brain MRI data will be acquired using a 3.0-Tesla MRI scanner. Electroencephalography will be performed to evaluate changes in functional connectivity after the multidomain intervention.49 In addition, changes in blood biomarkers including plasma amyloid-β42, neurotrophic factors, neurodegeneration factors and neuroinflammation factors, telomere length, stool microbiome, and actigraphy after the multidomain intervention will be investigated.
Study procedure
Before initiating the study, all assessors and other individuals implementing the intervention will participate in a workshop to receive education on how to perform outcome measurements or how to apply the programs. The RBANS and Prospective Memory Test7 will be assessed by the same psychologist at baseline and within 4 weeks after completion of the intervention. Other secondary and exploratory outcomes will be evaluated within 4 weeks before the intervention and within 4 weeks after completion of the intervention. Adherence to the intervention program and the occurrence of adverse events will be evaluated by the study coordinator in the FMI and HMI arms every 4 weeks. The safety committee will meet regularly to assess the occurrence of any adverse events. Participants who withdraw prematurely will be asked to complete all end-point assessments at the time of such early terminations. Antidepressants, antianxiolytics, and acetylcholinesterase inhibitors taken in stable doses for more than 8 weeks prior to the baseline will be continued without changing their doses until the end of the study.
Criteria for early discontinuation
The criteria for early discontinuation are as follows: 1) the participant withdrawing their consent; 2) unavoidable circumstances, such as moving residence or being lost to follow-up; or 3) the investigator deciding to terminate in order to ensure the welfare or health of the participant. Nonadherence will not be a reason for terminating participation, and any progression to dementia during the study will not preclude participation.
Data management
Data entry will be completed by study coordinators at each center. The quality of data will be improved by automatically examining the ranges of data values. The data collected in this study will be monitored by an independent monitor in accordance with Good Clinical Practice (GCP) guidelines and clinical research protocols. Only authorized users will be able to access the data system.
Estimating the sample size
Since the objectives of this feasibility trial are to estimate retention and adherence rates, the sample size was calculated using the width of the 95% confidence interval for the rate. Based on recommendations on sample-size calculations for feasibility studies from the National Institute for Health Research,50 we can estimate retention and adherence rates of 75% when the sample size is 50 within a 95% confidence interval of ±12%. Therefore, the required sample size was calculated to be 150, with 50 participants in each arm.
Statistical analysis
The end points of the study will be assessed using a modified intention-to-treat (ITT) population, defined as all randomized participants who undergo a baseline evaluation and at least one postbaseline assessment, and so participate at least once in the intervention program if they are in the FMI or HMI arm. Additional analyses on per-protocol populations will also be performed. The conclusion of the trial will use the modified ITT analysis. Analysis of covariance with a baseline score as a covariate will be used to compare changes in the RBANS and secondary outcomes from baseline to the end of the study between each intervention arm and the control arm. The safety analysis population will comprise participants who undergo at least one safety evaluation after baseline, and participate at least once in the intervention program if they are in the FMI or HMI arm. The chi-squared test will be used to compare the prevalence of adverse events between each intervention arm and the control arm. The retention rate and adherence to the intervention program will be assessed in each arm at the end of the study.
Ethics and dissemination
The study will be conducted in accordance with the International Conference on Harmonization GCP Guideline. This study has been approved by Inha University Hospital Institutional Review Board (IRB)(INHAUH-2018-11-022), Ewha Womans University Mokdong Hospital IRB (EUMC-2019-04-013), Ajou University Hospital IRB (AJIRB-BMR-SUR-19-070, AJIRB-BMR-SUR-19-077), Dong-A University Hospital IRB (DAUHIRB-19-078), and Chonnam National University Hospital IRB (CNUH-2019-139). Protocol modifications will be reported to and approved by the IRB of each center. Written informed consent and additional consent for the collection and use of biological specimens will be obtained from all potential participants by a study doctor before they are enrolled in the study. All names of the participants will be kept confidential and their privacy will be assured, with participants identified by research identification numbers assigned during the study. The study results will be presented at national and international conferences and published in peer-reviewed journals. The principal investigator will consider which researchers are eligible for inclusion as authors. No professional writers will be used. Access to raw data will be available upon reasonable request.
Patient and public involvement
Patients and other members of the public will not be involved in the design, conduct, reporting, or dissemination of our research.
DISCUSSION
South Korea became an aged society in 2017, when more than 14% of the population was aged 65 years or older, and it is projected to become a postaged society by 2025, when 20% of the population will be older than 65 years.51 The number of dementia patients is increasing rapidly along with this unprecedented rate of population aging in South Korea. We aim to address this situation by developing a multidomain intervention program that will be easy to disseminate to large numbers of older people nationwide. The physical exercise in the SUPERBRAIN programs can be performed at public facilities, hospitals, and in the home using portable tools. Those who experience difficulty using the Internet-based tablet application can also perform workbook-type cognitive training in the SUPERBRAIN programs. Since there are restrictions on the number of people who can receive intensive multidomain interventions at any given facility, we have developed the HMI program so that more elderly people can benefit from such interventions. The HMI program will also be useful to those who have difficulty in regularly attending sessions at a facility and to those that are reluctant to take part in group-based classes.
The cognitive training application and the paper-and-pencil-based workbook program are comparable in terms of the training cognitive domains, memory strategy, and session configuration and time; their contents are also similar. The tablet application has more levels of difficulty than the workbooks, while the content is more varied in the workbooks than in the tablet application. Gamification is an important factor for the tablet application and writing is an important factor for the workbook. A previous study found that the effectiveness of homework materials similar to the workbooks was comparable to the conventional cognitive training in MCI patients.7 The tablet application and the workbooks consisted of the most appropriate contents in each setting of the intervention.
The SUPERBRAIN programs are also characterized by the motivational enhancement program. In the FINGER, adherence was highest for cardiovascular monitoring and nutritional counseling, and lowest for unsupervised computer-based cognitive training.52 In the present study, it is likely that participants in the HMI arm will have a low adherence with homebased cognitive training and exercise. We tried to overcome this problem by utilizing motivational programs.
This study will be subject to some limitations. First, it has a cognitive assessor-blinded design rather than a double-blinded design. Participants will be instructed not to discuss their study involvement with the assessor, but there will be a potential to influence the masking of the assessments and introduce rater bias. The study coordinators who will check adherence will not be blinded. Second, the FINGER enrolled 1,260 elderly people, and the multidomain intervention lasted for 2 years.10 The intervention period is relatively short and the sample is small in the present feasibility study, and both of these factors may influence the potential for negative efficacy results. If participants in each intervention arm show significant improvement in the RBANS score compared to the control arm at the end of the study, the intervention program will be considered to have a high tendency to be effective. Third, the Fitbit smartwatch does not capture data from finger-and-toe exercises or from resistance exercises performed in a seated position. This makes it likely that the assessments of adherence with some exercise programs will be incorrect in the HMI arm.
In addition to the efficacy of the FMI and HMI programs, feasibility is also an important factor affecting the ability to implement programs nationwide, which also makes this study meaningful. If our primary outcomes are not reached, we will need to find the cause, fix the SUPERBRAIN programs, and apply the modified SUPERBRAIN programs in a subsequent large-scale randomized controlled trial to investigate the efficacy of the modified programs. The results obtained in this study will represent useful information for applying these multidomain intervention programs aimed at preventing cognitive impairment and protecting brain health to at-risk elderly people.
Acknowledgements
This study was supported by a grant from the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (HI18C0479). The funder is not involved in the study design or operations, such as the selection and management of sites, data management, or the drafting of any manuscripts submitted for publication.
Footnotes
- Conceptualization: Seong Hye Choi, Jee Hyang Jeong, So Young Moon, Yoo Kyoung Park, Chang Hyung Hong, Hae Ri Na.
- Funding acquisition: Seong Hye Choi, Chang Hyung Hong, So Young Moon, Jee Hyang Jeong, Yoo Kyoung Park.
- Investigation: Seong Hye Choi, Jee Hyang Jeong, Hee Kyung Park, Chang Hyung Hong, So Young Moon, Yoo Kyoung Park, Hae Ri Na, Hong-Sun Song, Sun Min Lee, Muncheong Choi, Kyung Won Park, Byeong C. Kim, Soo Hyun Cho, Soo Hyun Cho.
- Methodology: Seong Hye Choi, Jee Hyang Jeong, Hee Kyung Park, So Young Moon, Yoo Kyoung Park, Chang Hyung Hong, Hae Ri Na, Hong-Sun Song, Sun Min Lee, Muncheong Choi, Soo Hyun Cho.
- Writing—original draft: Hee Kyung Park, Jee Hyang Jeong.
- Writing—review & editing: Seong Hye Choi, Chang Hyung Hong, So Young Moon, Yoo Kyoung Park, Hae Ri Na, Hong-Sun Song, Sun Min Lee, Muncheong Choi, Kyung Won Park, Byeong C. Kim, Soo Hyun Cho, Soo Hyun Cho.
Conflicts of Interest: The authors have no potential conflicts of interest to disclose.
Supplementary Materials
The online-only Data Supplement is available with this article at https://doi.org/10.3988/jcn.2020.16.2.292.
SPIRIT (Standard Protocol Items: Recommendations for Interventional Trials) 2013 checklist: recommended items to address in a clinical trial protocol and related documents
Details of the structured exercise programs tailored according to physical performance as well as physical condition
References
- 1.Livingston G, Sommerlad A, Orgeta V, Costafreda SG, Huntley J, Ames D, et al. Dementia prevention, intervention, and care. Lancet. 2017;390:2673–2734. doi: 10.1016/S0140-6736(17)31363-6. [DOI] [PubMed] [Google Scholar]
- 2.Reisberg B, Burns A, Brodaty H, Eastwood R, Rossor M, Sartorius N, et al. Diagnosis of Alzheimer's disease. Report of an International Psychogeriatric Association Special Meeting Work Group under the cosponsorship of Alzheimer's Disease International, the European Federation of Neurological Societies, the World Health Organization, and the World Psychiatric Association. Int Psychogeriatr. 1997;9 Suppl 1:11–38. doi: 10.1017/s1041610297004675. [DOI] [PubMed] [Google Scholar]
- 3.Tan CC, Yu JT, Wang HF, Tan MS, Meng XF, Wang C, et al. Efficacy and safety of donepezil, galantamine, rivastigmine, and memantine for the treatment of Alzheimer's disease: a systematic review and meta-analysis. J Alzheimers Dis. 2014;41:615–631. doi: 10.3233/JAD-132690. [DOI] [PubMed] [Google Scholar]
- 4.Egan MF, Kost J, Voss T, Mukai Y, Aisen PS, Cummings JL, et al. Randomized trial of verubecestat for prodromal Alzheimer's disease. N Engl J Med. 2019;380:1408–1420. doi: 10.1056/NEJMoa1812840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lopez Lopez C, Tariot PN, Caputo A, Langbaum JB, Liu F, Riviere ME, et al. The Alzheimer's Prevention Initiative Generation Program: study design of two randomized controlled trials for individuals at risk for clinical onset of Alzheimer's disease. Alzheimers Dement (N Y) 2019;5:216–227. doi: 10.1016/j.trci.2019.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kivipelto M, Ngandu T, Laatikainen T, Winblad B, Soininen H, Tuomilehto J. Risk score for the prediction of dementia risk in 20 years among middle aged people: a longitudinal, population-based study. Lancet Neurol. 2006;5:735–741. doi: 10.1016/S1474-4422(06)70537-3. [DOI] [PubMed] [Google Scholar]
- 7.Jeong JH, Na HR, Choi SH, Kim J, Na DL, Seo SW, et al. Group- and home-based cognitive intervention for patients with mild cognitive impairment: a randomized controlled trial. Psychother Psychosom. 2016;85:198–207. doi: 10.1159/000442261. [DOI] [PubMed] [Google Scholar]
- 8.Scarmeas N, Stern Y, Mayeux R, Manly JJ, Schupf N, Luchsinger JA. Mediterranean diet and mild cognitive impairment. Arch Neurol. 2009;66:216–225. doi: 10.1001/archneurol.2008.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hosking DE, Eramudugolla R, Cherbuin N, Anstey KJ. MIND not Mediterranean diet related to 12-year incidence of cognitive impairment in an Australian longitudinal cohort study. Alzheimers Dement. 2019;15:581–589. doi: 10.1016/j.jalz.2018.12.011. [DOI] [PubMed] [Google Scholar]
- 10.Ngandu T, Lehtisalo J, Solomon A, Levälahti E, Ahtiluoto S, Antikainen R, et al. A 2 year multidomain intervention of diet, exercise, cognitive training, and vascular risk monitoring versus control to prevent cognitive decline in at-risk elderly people (FINGER): a randomised controlled trial. Lancet. 2015;385:2255–2263. doi: 10.1016/S0140-6736(15)60461-5. [DOI] [PubMed] [Google Scholar]
- 11.Moll van Charante EP, Richard E, Eurelings LS, van Dalen JW, Ligthart SA, van Bussel EF, et al. Effectiveness of a 6-year multidomain vascular care intervention to prevent dementia (preDIVA): a clusterrandomised controlled trial. Lancet. 2016;388:797–805. doi: 10.1016/S0140-6736(16)30950-3. [DOI] [PubMed] [Google Scholar]
- 12.Andrieu S, Guyonnet S, Coley N, Cantet C, Bonnefoy M, Bordes S, et al. Effect of long-term omega 3 polyunsaturated fatty acid supplementation with or without multidomain intervention on cognitive function in elderly adults with memory complaints (MAPT): a randomised, placebo-controlled trial. Lancet Neurol. 2017;16:377–389. doi: 10.1016/S1474-4422(17)30040-6. [DOI] [PubMed] [Google Scholar]
- 13.Chan AW, Tetzlaff JM, Gøtzsche PC, Altman DG, Mann H, Berlin JA, et al. SPIRIT 2013 explanation and elaboration: guidance for protocols of clinical trials. BMJ. 2013;346:e7586. doi: 10.1136/bmj.e7586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Han C, Jo SA, Jo I, Kim E, Park MH, Kang Y. An adaptation of the Korean Mini-Mental State Examination (K-MMSE) in elderly Koreans: demographic influence and population-based norms (the AGE study) Arch Gerontol Geriatr. 2008;47:302–310. doi: 10.1016/j.archger.2007.08.012. [DOI] [PubMed] [Google Scholar]
- 15.Chin J, Park J, Yang SJ, Yeom J, Ahn Y, Baek MJ, et al. Re-standardization of the Korean-Instrumental Activities of Daily Living (K-IADL): clinical usefulness for various neurodegenerative diseases. Dement Neurocogn Disord. 2018;17:11–22. doi: 10.12779/dnd.2018.17.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Choi SH, Shim YS, Ryu SH, Ryu HJ, Lee DW, Lee JY, et al. Validation of the literacy independent cognitive assessment. Int Psychogeriatr. 2011;23:593–601. doi: 10.1017/S1041610210001626. [DOI] [PubMed] [Google Scholar]
- 17.1999 World Health Organization-International Society of Hypertension guidelines for the management of hypertension. Guidelines subcommittee. J Hypertens. 1999;17:151–183. [PubMed] [Google Scholar]
- 18.Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Report of the expert committee on the diagnosis and classification of diabetes mellitus. Diabetes Care. 1997;20:1183–1197. doi: 10.2337/diacare.20.7.1183. [DOI] [PubMed] [Google Scholar]
- 19.National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) Third report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106:3143–3421. [PubMed] [Google Scholar]
- 20.Pan WH, Yeh WT. How to define obesity? Evidence-based multiple action points for public awareness, screening, and treatment: an extension of Asian-Pacific recommendations. Asia Pac J Clin Nutr. 2008;17:370–374. [PubMed] [Google Scholar]
- 21.Nam GE, Park HS. Perspective on diagnostic criteria for obesity and abdominal obesity in Korean adults. J Obes Metab Syndr. 2018;27:134–142. doi: 10.7570/jomes.2018.27.3.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, et al. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120:1640–1645. doi: 10.1161/CIRCULATIONAHA.109.192644. [DOI] [PubMed] [Google Scholar]
- 23.Centers for Disease Control and Prevention (CDC) Cigarette smoking among adults--United States, 1992, and changes in the definition of current cigarette smoking. MMWR Morb Mortal Wkly Rep. 1994;43:342–346. [PubMed] [Google Scholar]
- 24.Minn YK, Choi SH, Suh YJ, Jeong JH, Kim EJ, Kim JH, et al. Effect of physical activity on the progression of Alzheimer's disease: the Clinical Research Center for Dementia of South Korea study. J Alzheimers Dis. 2018;66:249–261. doi: 10.3233/JAD-180333. [DOI] [PubMed] [Google Scholar]
- 25.Akbaraly TN, Portet F, Fustinoni S, Dartigues JF, Artero S, Rouaud O, et al. Leisure activities and the risk of dementia in the elderly: results from the three-city study. Neurology. 2009;73:854–861. doi: 10.1212/WNL.0b013e3181b7849b. [DOI] [PubMed] [Google Scholar]
- 26.Binns C, Low WY. Nobel prizes, nudge theory, and public health. Asia Pac J Public Health. 2017;29:632–634. doi: 10.1177/1010539517743630. [DOI] [PubMed] [Google Scholar]
- 27.Lewis SK, Thompson P. Application of adult learning theory to physician assistant education. J Physician Assist Educ. 2017;28:196–200. doi: 10.1097/JPA.0000000000000172. [DOI] [PubMed] [Google Scholar]
- 28.Randolph C, Tierney MC, Mohr E, Chase TN. The Repeatable Battery for the Assessment of Neuropsychological Status (RBANS): preliminary clinical validity. J Clin Exp Neuropsychol. 1998;20:310–319. doi: 10.1076/jcen.20.3.310.823. [DOI] [PubMed] [Google Scholar]
- 29.Walters K, Frost R, Kharicha K, Avgerinou C, Gardner B, Ricciardi F, et al. Home-based health promotion for older people with mild frailty: the HomeHealth intervention development and feasibility RCT. Health Technol Assess. 2017;21:1–128. doi: 10.3310/hta21730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Choi SH, Lee BH, Kim S, Hahm DS, Jeong JH, Yoon SJ, et al. Interchanging scores between clinical dementia rating scale and global deterioration scale. Alzheimer Dis Assoc Disord. 2003;17:98–105. doi: 10.1097/00002093-200304000-00008. [DOI] [PubMed] [Google Scholar]
- 31.Radford KA, Lah S, Say MJ, Miller LA. Validation of a new measure of prospective memory: the Royal Prince Alfred Prospective Memory Test. Clin Neuropsychol. 2011;25:127–140. doi: 10.1080/13854046.2010.529463. [DOI] [PubMed] [Google Scholar]
- 32.Byun E, Kim KK. Prospective and retrospective memory failures in persons with memory complaints: a questionnaire study. Dement Neurocogn Disord. 2009;8:45–52. [Google Scholar]
- 33.Thomas-anterion C, Honore-masson S, Laurent B. The cognitive complaint interview (CCI) Psychogeriatrics. 2006;6(Suppl 1):S18–S22. [Google Scholar]
- 34.Bae JN, Cho MJ. Development of the Korean version of the Geriatric Depression Scale and its short form among elderly psychiatric patients. J Psychosom Res. 2004;57:297–305. doi: 10.1016/j.jpsychores.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 35.Choi SH, Na DL, Lee BH, Kang SJ, Ha CK, Han SH, et al. Validation of the Korean version of the Bayer activities of daily living scale. Hum Psychopharmacol. 2003;18:469–475. doi: 10.1002/hup.505. [DOI] [PubMed] [Google Scholar]
- 36.Shin HY. [A preliminary study on the Korean version of quality of life-Alzheimer's disease (QOL-AD) scale in community-dwelling elderly with dementia] J Prev Med Public Health. 2006;39:243–248. [PubMed] [Google Scholar]
- 37.Lee J, Lee C, Min J, Kang DW, Kim JY, Yang HI, et al. Development of the Korean Global Physical Activity Questionnaire: reliability and validity study. Glob Health Promot. 2019:1757975919854301. doi: 10.1177/1757975919854301. [DOI] [PubMed] [Google Scholar]
- 38.Guralnik JM, Ferrucci L, Simonsick EM, Salive ME, Wallace RB. Lower-extremity function in persons over the age of 70 years as a predictor of subsequent disability. N Engl J Med. 1995;332:556–561. doi: 10.1056/NEJM199503023320902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chung MJ, Kwak TK, Kim HY, Kang MH, Lee JS, Chung HR, et al. Development of NQ-E, Nutrition Quotient for Korean elderly: item selection and validation of factor structure. J Nutr Health. 2018;51:87–102. [Google Scholar]
- 40.Vellas B, Guigoz Y, Garry PJ, Nourhashemi F, Bennahum D, Lauque S, et al. The Mini Nutritional Assessment (MNA) and its use in grading the nutritional state of elderly patients. Nutrition. 1999;15:116–122. doi: 10.1016/s0899-9007(98)00171-3. [DOI] [PubMed] [Google Scholar]
- 41.Kim SY, Kim JM, Yoo JA, Bae KY, Kim SW, Yang SJ, et al. Standardization and validation of Big Five Inventory-Korean version (BFI-K) in elders. Korean J Biol Psychiatry. 2010;17:15–25. [Google Scholar]
- 42.Lee TK, Hwang JY, Kim SY, Jung YC, Kang UG. Reliability and validity of the Korean version of the Readiness to Change Questionnaire Treatment version. J Korean Neuropsychiatr Assoc. 2011;50:139–147. [Google Scholar]
- 43.Hwang HW, Kim BH. The validity of a situational motivation scale based on self-determination theory regarding work. Korean Journal of Counseling. 2013;14:2891–2906. [Google Scholar]
- 44.Song R, Lee H. Effects of a 12-week cardiac rehabilitation exercise program on motivation and health-promoting lifestyle. Heart Lung. 2001;30:200–209. doi: 10.1067/mhl.2001.113282. [DOI] [PubMed] [Google Scholar]
- 45.Sohn SI, Kim DH, Lee MY, Cho YW. The reliability and validity of the Korean version of the Pittsburgh Sleep Quality Index. Sleep Breath. 2012;16:803–812. doi: 10.1007/s11325-011-0579-9. [DOI] [PubMed] [Google Scholar]
- 46.American Psychiatry Association. Diagnostic and Statistical Manual of Mental Disorders. 5th edition. Arlington, VA: American Psychaitry Association; 2013. [Google Scholar]
- 47.Albert MS, DeKosky ST, Dickson D, Dubois B, Feldman HH, Fox NC, et al. The diagnosis of mild cognitive impairment due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7:270–279. doi: 10.1016/j.jalz.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR, Jr, Kawas CH, et al. The diagnosis of dementia due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7:263–269. doi: 10.1016/j.jalz.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lee D, Kang DH, Ha NH, Oh CY, Lee U, Kang SW. Effects of an Online Mind-Body Training Program on the default mode network: an EEG functional connectivity study. Sci Rep. 2018;8:16935. doi: 10.1038/s41598-018-34947-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hooper R. Justifying sample size for a feasibility study [Internet] London: National Institute for Health Research; 2019. [cited 2019 Dec 5]. Available from: https://www.rds-london.nihr.ac.uk/wpcms/wp-content/uploads/2019/02/Justifying-sample-size-for-feasibility-study-updated-22-Feb-2019.pdf. [Google Scholar]
- 51.Han JW, Kim TH, Kwak KP, Kim K, Kim BJ, Kim SG, et al. Overview of the Korean Longitudinal Study on Cognitive Aging and Dementia. Psychiatry Investig. 2018;15:767–774. doi: 10.30773/pi.2018.06.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Coley N, Ngandu T, Lehtisalo J, Soininen H, Vellas B, Richard E, et al. Adherence to multidomain interventions for dementia prevention: data from the FINGER and MAPT trials. Alzheimers Dement. 2019;15:729–741. doi: 10.1016/j.jalz.2019.03.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
SPIRIT (Standard Protocol Items: Recommendations for Interventional Trials) 2013 checklist: recommended items to address in a clinical trial protocol and related documents
Details of the structured exercise programs tailored according to physical performance as well as physical condition