Abstract
Background and Purpose
We aimed to determine the patterns and mechanisms of persistent nystagmus (PN) lasting >1 year in lateral medullary infarction (LMI).
Methods
We recruited 13 patients with PN due to LMI and another 13 with transient nystagmus (TN) (<1 year) as control. All patients underwent oculography, rotatory chair test, caloric test, bedside head impulse test, dizziness handicap inventory (DHI), and brain MRI.
Results
All patients had spontaneous, contralesional, horizontal-torsional nystagmus during the acute phase. Although two patients exhibited consistent contralesional torsional nystagmus, most patients (11/13, 85%) with PN evolved from the initial contralesional to ipsilesional nystagmus. During horizontal gaze, the patterns of ipsilesional PN were diverse; torsional (n=5), torsional-downbeat (n=2), horizontal (n=2), and horizontal nystagmus while looking at the lesion side, and torsional nystagmus while looking at the opposite side (n=2). During rotatory chair test, the gains of the vestibulo-ocular reflex in the PN group were lower than those in the TN group to the lesion side at 0.02 and 0.64 Hz. The caudal and ventrolateral parts of the vestibular nuclei were mostly involved in patients with PN. The DHI score did not differ between the groups.
Conclusions
PN patterns frequently change in LMI. Resultant vestibular asymmetry after vestibular afferents or cerebellar inhibitory pathway damage and/or inappropriate vestibular compensation may be responsible for PN in LMI. Impairment of the horizontal or vertical neural integrators may be another cause. The presence of PN does not necessarily indicate more severe dizziness in LMI.
Keywords: nystagmus, lateral medullary syndrome, vestibulo-ocular reflex, dizziness
INTRODUCTION
Most cases of spontaneous nystagmus in patients with acute lateral medullary infarction (LMI) are similar to unilateral vestibular neuritis (uVN) the nystagmus is mixed horizontaltorsional and usually beats to the intact side (pseudo-vestibular neuritis), with or without gaze-evoked nystagmus (GEN).1,2,3 A lesion in the ipsilesional vestibular nuclei and/or vicinity, mainly their cerebellar connections, may be the major cause of nystagmus.2 Rarely, patients with acute LMI may present with ipsilesional nystagmus, which might be explained by the destruction of the rostral part of the vestibular nuclei or damage to the inhibitory pathway of these nuclei.4,5,6 In most cases, the nystagmus in LMI disappears through central vestibular compensation, similar to that in vestibular neuritis. However, spontaneous nystagmus may be observed in some patients with chronic LMI.7,8 Persistent nystagmus (PN) in the chronic phase of LMI has not been studied yet. This study aimed to analyze the dynamic patterns of PN in acute and chronic LMI, and to elucidate the characteristics of patients with PN in vestibular function tests and anatomical lesion location by comparison with patients with transient nystagmus (TN) in whom the initial nystagmus resolved within 1 year.
METHODS
Patients and control subjects
In our institution, patients with acute stroke were admitted for acute stroke management and regularly visited our outpatient clinic for secondary stroke prevention. The diagnosis of LMI was confirmed by diffusion-weighted MRI performed within 1 week of symptom onset. We regularly examined patients with LMI for spontaneous and GEN every 2 or 3 months in the outpatient clinic from January 2012 to December 2012. We classified those with persistent spontaneous and/or GEN over 1 year in the PN group and those with TN lasting only during the acute phase of LMI in the TN group. We excluded patients who visited our hospital after more than 1 week from the attack, those in whom brain MRI was not performed, and those without a description of nystagmus in the medical records at admission. Patients with central or peripheral vestibular diseases before or after the diagnosis of acute LMI were also excluded. As a result, 15 among a total of 109 patients with LMI were excluded. Finally, 13 among 94 patients were enrolled in the PN group. To compare the vestibular function tests and subjective dizziness, we also enrolled 13 consecutive patients with TN in the order of visits among patients with TN and/or GEN. This study's protocol was approved by the Institutional Review Board of the Bucheon Sooncheonhyang University Hospital and received written informed patient consent (IRB No. 2011-21).
Analyses of nystagmus, other vestibular functions, and imaging
We retrospectively reviewed the patients' clinical records and brain MRI scans (Signa HDxt 1.5T, General Electric Company, Boston, MA, USA) obtained at admission, in the acute phase. PN in the chronic phase was evaluated by video-oculography (SMI, Teltow, Germany). Head-shaking nystagmus was induced by passive horizontal head shaking at a rate of 2–3 Hz with an amplitude of about ±10° for 15 s.9 Bedside head impulse test, rotatory chair test (Micromedical System 2000, Micromedical Technologies, Chatham, IL, USA), and caloric response were evaluated. Gains of the vestibulo-ocular reflex (VOR) were calculated by dividing the slow-phase velocity of the eye by the velocity of the head (measured by chair velocity). Asymmetry of VOR was a ratio between the slow-phase velocity of the eye to the right and that to the left. The total scores of the dizziness handicap inventory (DHI) of the two groups were compared to assess the effect of PN on the activities of daily living and dizziness. The DHI consists of 25 questions representing the impact of dizziness on the physical, functional, and emotional domains of daily life.10 We analyzed the diffusion-weighted and T1-weighted MRI scans of all patients using MRIcron (www.mccauslandcenter.sc.edu/mricro/mricron). The right-sided lesions were flipped to the left and were overlaid on a spatial template of the cerebellum and brainstem.
Statistical analyses
Patients' demographic data and the results of the vestibular function tests were compared between the two groups using Student's t-test or Mann-Whitney's U test for continuous variables and Pearson's χ2 test for categorical variables. Statistical analyses were performed with a commercially available software package (IBM SPSS Statistics version 24, IBM Corporation, Armonk, NY, USA). A p-value <0.05 was considered significant.
RESULTS
Clinical characteristics
The clinical characteristics and nystagmus patterns of the PN group are presented in Table 1. The clinical characteristics of all enrolled patients and comparisons of the two groups are presented in Table 2. There were 26 patients with chronic LMI included, with 13 in the PN group (9 men and 4 women) and 13 in the TN group (10 men and 3 women). Their ages ranged from 34 to 61 years (mean, 52.77±11.51). The mean follow-up period for all patients was 4.2 years (1–10 years). There was no significant difference in sex, age, time from onset, and laterality of the lesions between the two groups. All patients presented severe vertigo with typical symptoms of LMI during the acute stage. According to the admission records, all patients exhibited spontaneous nystagmus beating to the contralesional side and/or GEN in the acute phase, with various components (horizontal, torsional, mixed, and vertical). One patient with PN had a small ipsilesional cerebellar border zone involvement. Since video-oculography was not performed for most patients in the acute phase, we could not determine if the patients also had GEN. There were more patients without symptoms of dizziness (DHI 0) in the TN group (five patients) than in the PN group (one patient). However, the total DHI scores did not show a significant difference between the two groups (16.15±12.37 in the PN and 10.15±14.78 in the TN group).
Table 1. Clinical characteristics and nystagmus patterns of PN in lateral medullary infarction.
| Age/sex | Lesion side | TO (years) | Persistent nystagmus | HSN | DHI | |||
|---|---|---|---|---|---|---|---|---|
| Ipsilesional | Center | Contralesional | ||||||
| PN 1 | 50/M | Left | 2 | cT | cT | cT | i | 8 |
| PN 2* | 57/M | Left† | 8 | cT | cT | cT, D | i | 4 |
| PN 3 | 60/F | Right | 8 | iT | iT | iT | i | 16 |
| PN 4 | 60/F | Left | 7 | iT | - | iT | - | 34 |
| PN 5 | 44/M | Right | 5 | iT | - | iT | i | 22 |
| PN 6 | 36/F | Right | 2 | iT | - | iT | D | 30 |
| PN 7 | 49/F | Left | 2 | iT | - | - | i | 30 |
| PN 8 | 48/M | Right | 6 | iT, D | iT | iT, D | i | 0 |
| PN 9 | 41/M | Left | 1 | iT, D | - | - | i | 10 |
| PN 10* | 44/M | Right | 8 | iH | iH | - | i | 6 |
| PN 11* | 56/M | Right | 1 | iH | - | - | i | 12 |
| PN 12* | 36/M | Left | 4 | iH | - | iT | i | 4 |
| PN 13 | 38/M | Right | 1 | iH | - | iTD | - | 34 |
*Supplementary video files are attached for these four patients, †This patient had a concomitant ipsilateral cerebellar infarction in the border zone area of the posterior inferior cerebellar artery.
c: contralesional, D: down, DHI: dizziness handicap inventory, H: horizontal transient nystagmus, HSN: post-headshaking nystagmus, i: ipsilesional, PN: persistent nystagmus, T: torsional, TO: time from onset.
Table 2. Comparison of the demographic characteristics and DHI score between the PN and TN groups in lateral medullary infarction.
| Total (n=26) | PN (n=13) | TN (n=13) | p | |
|---|---|---|---|---|
| Sex* | 1.000 | |||
| Male | 19 (73.1) | 9 (69.2) | 10 (76.9) | |
| Lesion† | 0.234 | |||
| Right | 11 (42.3) | 4 (30.8) | 7 (53.8) | |
| Left | 15 (57.7) | 9 (69.2) | 6 (46.2) | |
| Age (years)‡ | 52.77±11.51 | 55.23±11.86 | 50.31±11.05 | 0.287 |
| Duration (years)‡ | 4.19±2.79 | 4.15±2.79 | 4.23±2.89 | 0.960 |
| DHI score‡ | 13.15±13.70 | 16.15±12.37 | 10.15±14.78 | 0.091 |
Data are n (%) or mean±SD values.
*Fisher's exact test, †Chi-square test, ‡Mann-Whitney U test.
DHI: dizziness handicap inventory, PN: persistent nystagmus, TN: transient nystagmus.
Various patterns of persistent nystagmus
Fig. 1. Various patterns and mechanisms of persistent nystagmus development in lateral medullary infarction. The various patterns of persistent nystagmus can be described by vestibular tone imbalance from the SCCs. iH nystagmus is caused by overactivation of the iHC. This figure also explains the possible mechanisms of iT, D nystagmus caused by iPC, iT nystagmus caused by iVC, and ipsiversive iH and contraversive iT caused by iHC and iVC activation. One patient showed cT nystagmus caused by hypoactivation of the iVCs. AC: anterior canal, c: contralesional, D: down, H: horizontal, HC: horizontal canal, i: ipsilesional, n: number, PC: posterior canal, SCC: semicircular canal, T: torsional, VC: vertical canals.

All patients with PN showed nystagmus on horizontal gazes. Five patients also showed spontaneous nystagmus (center position). Two patients (2/13, 8%) showed direction-fixed, consistent, contralesional torsional nystagmus from the acute phase (first pattern, PN 1, 2). Regarding the contralesional nystagmus (third pattern) in PN 2, the torsional component was observed in all positions, but the downward component was noticeable on horizontal gaze only (Supplementary Video 1 in the online-only Data Supplement). Except these two, all other patients (11/13, 85%) with PN showed evolution of the initial contralesional nystagmus within the first year of infarction. The direction of the PN underwent reversal from 1 week to several months after LMI onset. The patterns of the ipsilesional PN were torsional in five patients (PN 3, 4, 5, 6, and 7), torsional downbeat (TD) in two (PN 8 and 9), horizontal in two (PN 10 and 11), and horizontal while looking at the lesion side and torsional while looking at the opposite side in two (PN 12 and 13). Nystagmus was augmented when the patients were not fixating. The ipsilesional torsional nystagmus (second pattern) in PN 3 aggravated on lateral gaze; thus, it did not follow Alexander's law. The ipsilesional horizontal nystagmus (fourth pattern) in PN 10 was observed in the central position (1 deg/sec, linear slow phase) and on ipsilesional gaze (Supplementary Video 2 in the online-only Data Supplement). However, the ipsilesional horizontal nystagmus (decreasing slow phase) in PN 11 showed when looking at the lesion side (Supplementary Video 3 in the online-only Data Supplement). In PN 12, the ipsilesional horizontal nystagmus (7 deg/sec, linear slow phase) when looking at the lesion side changed to a torsional component (2.5 deg/sec, linear slow phase) during contralesional gazing (fifth pattern, Supplementary Video 4 in the online-only Data Supplement, Fig. 2).
Fig. 2. MRI and persistent nystagmus in patient 4 with left lateral medullary infarction. A: Initial T2-weighted axial MRI demonstrates acute left lateral medullary infarction. B: Oculography of patient 4 shows counter-clockwise torsional nystagmus (2.5°/sec) while looking to the contralesional side (right) and left beating nystagmus (slow-phase velocity 7°/sec) while looking to the ipsilesional side (left). H: horizontal, i: ipsilesional, T: torsional, V: vertical.

Comparison of horizontal VOR tests between the groups
The ipsilesional VOR gains of the PN group were significantly lower than those of the TN group at 0.02 Hz (p=0.039) and 0.64 Hz (p=0.001) during the rotatory chair test (Table 3). Asymmetry of horizontal VOR was significantly greater in the PN group than in the TN group at 0.64 Hz (p=0.016) and 0.02 Hz (p=0.034). The mean and contralesional VOR gain at 0.02 Hz and contralesional 0.64 Hz were not significantly different between the two groups. After the head-shaking maneuver, 10 patients in the PN group showed ipsilesional horizontal nystagmus and one patient had downbeat nystagmus. In the TN group, post-head shaking ipsilesional nystagmus was observed in seven and downbeat nystagmus was observed in three patients. The results of the bedside head impulse test and caloric test were not significantly different between the two groups.
Table 3. Results of the VOR test for patients with PN and TN.
| PN (n=13) | TN (n=13) | p* | ||||
|---|---|---|---|---|---|---|
| Mean±SD | Mean±SD | |||||
| VOR | 0.64 Hz | Gain | Mean | 0.65±0.18 | 0.77±0.11 | 0.029 |
| i | 0.55±0.19 | 0.78±0.12 | 0.001 | |||
| c | 0.74±0.22 | 0.76±0.13 | 0.713 | |||
| Asymmetry | 17.05±15.98 | 5.90±5.93 | 0.016 | |||
| 0.02 Hz | Gain | Mean | 0.39±0.13 | 0.45±0.12 | 0.362 | |
| i | 0.36±0.14 | 0.47±0.12 | 0.039 | |||
| c | 0.42±0.16 | 0.44±0.14 | 0.74 | |||
| Asymmetry | 21.46±22.89 | 7.48±6.75 | 0.034 | |||
| Canal paresis (i) | 3 | 2 | 0.619† | |||
| Abnormal HIT (i, bedside) | 1 | 1 | 1.000† | |||
Canal paresis [(WR+CR)−(WL+CL)/WR+WL+CR+CL] ≥25%.
*t-test, †Chi-square.
c: contralesional, CL: cool stimulus in left ear, CR: cool stimulus in right ear, HIT: head impulse test, i: ipsilesional, PN: persistent nystagmus, TN: transient nystagmus, VOR: vestibulo-ocular reflex, WL: warm stimulus in left ear, WR: warm stimulus in right ear.
Analysis of brain MRI of acute LMI
Ischemic lesions in the lateral medulla were confirmed by diffusion-weighted MRI performed in the acute phase (Fig. 3). All patients had caudal lesions in the dorsolateral medulla including the medial vestibular nucleus. In the PN group, one patient had a concomitant ipsilateral cerebellar lesion corresponding with the border-zone area of the posterior inferior cerebellar artery. In the PN group, the lesion analysis on MRI along with the clinical findings suggested damage to the ventrolateral part of the vestibular nuclei, such as the afferent pathway from the semicircular canals (SCCs) or the cerebellovestibular inhibitory pathway through the inferior cerebellar peduncle (Fig. 3). In addition, subtraction imaging revealed that patients in the PN group had relatively preserved rostral part of the vestibular nuclei and more ventrolateral damage than those in the TN group.
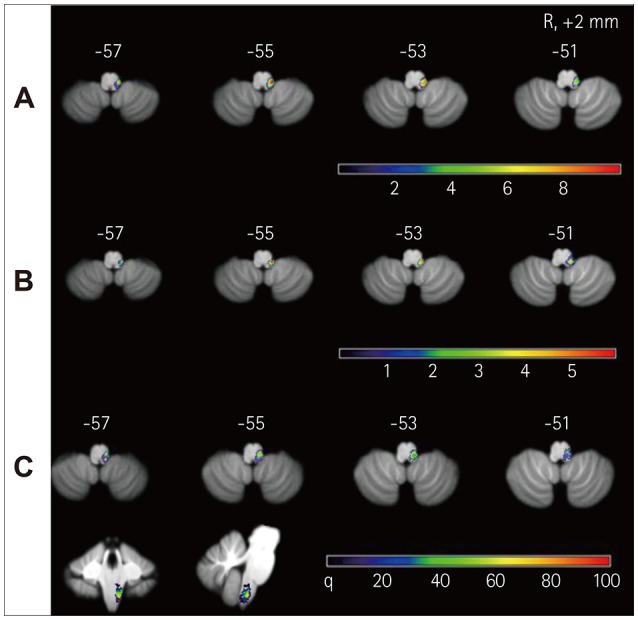
Fig. 3. Lesion analyses of the patients with LMI. Overlay images of 11 patients with PN (A), overlay images of 13 patients with TN (B), and subtraction images (C). A: The overlapping areas of the 11 LMIs with PN were located in the dorsolateral medulla, mainly in the vestibular nuclei and vicinity. The numbers of overlapping lesions are illustrated by different colors, from violet (n=1) to red (n=10). B: The lesions of 13 patients with TN were also located in the dorsolateral medulla. The numbers of overlapping lesions are illustrated by different colors, from violet (n=0) to red (n=6). C: Subtractions of B from A shows that the PN group had relatively preserved rostral part of the vestibular nuclei compared to the TN group and more ventrolateral damage. These findings are probably related with damage of the afferent pathway from the vestibular nerve or the inferior cerebellar peduncle; color-coded presentation from violet (0%) to red (100%). LMI: lateral medullary infarction, PN: persistent nystagmus, TN: transient nystagmus.
DISCUSSION
LMI causes symptoms, including vertigo, tilt illusion, skew deviation, nausea/vomiting, diplopia, and severe gait ataxia due to damage to the vestibular nuclei or vestibular cerebellar connections. Most cases of spontaneous nystagmus in acute LMI might result from central vestibular imbalance by stimulation of the contralesional posterior and horizontal SCCs or by damage to the central SCC pathways from the ipsilesional horizontal and anterior SCCs.2 Therefore, most cases of spontaneous nystagmus in acute LMI are contralesional mixed horizontal type, similar to that in unilateral uVN. However, the direction and components of spontaneous nystagmus in lesions of the vestibular nuclei are less predictable than those in peripheral vestibulopathy and depend on the location and extent of the lesion.11 GEN is also observed in most patients with LMI and is mostly horizontal (bilateral, ipsilesional, or contralesional).3 LMI can damage the caudal aspects of the vestibular nuclear complex, the projections between the vestibular nuclei and the cerebellum, the olivocerebellar fibers in the inferior cerebellar peduncles, and the vestibulocerebellum.12 Ipsilesional nystagmus is observed in some patients with acute LMI, which is thought to be caused by direct damage to the rostral part of the superior or medial vestibular nuclei.3,4,5
Most cases of spontaneous nystagmus in LMI resolve through central vestibular compensation. Some patients in the chronic phase of LMI have PN and most of them showed ipsilesional nystagmus through the evolution of the initial nystagmus within the first year of infarction. A few earlier reports have described ipsilesional, contralesional, or bidirectional PN in the chronic stage of LMI.7,8,13
Compared with these reports, our study demonstrated greater variation in the patterns of PN in the chronic phase of LMI. Contralesional torsional nystagmus was observed for over 1 year in two patients, which may be related to incomplete vestibular compensation, followed by hypoactivation of the vertical SCCs pathway. Similarly, two cases reported by Amari et al.8 in 2016 exhibited contralesional nystagmus in chronic LMI. However, they described only the horizontal direction of the nystagmus without fixation; thus, the vertical and torsional components are unknown.
Most cases of PN in our study showed reversal of the nystagmus direction to the ipsilesional side within 1 year. Most cases of spontaneous nystagmus without fixation disappear over days or weeks through central compensation in the chronic phase of uVN. Rarely, there is minute spontaneous nystagmus without fixation to the same direction 1 year after uVN onset.14,15 However, there was no report of evolution of the spontaneous nystagmus in uVN.
The vestibular nuclei are hubs for vestibular compensation and multisensory interaction.15 Therefore, damage to the vestibular nuclei and their connections may alter the direction and components of nystagmus due to inappropriate compensation, unlike uVN. Additionally, severe damage to the horizontal, rather than the vertical SCC pathway, can cause ipsilesional torsional nystagmus with or without a downbeat component via disproportionate cerebellar or commissural fiber inhibition. In 2017, Jeong et al.7 reported a case with LMI, similar to ours (PN 8 and 9), demonstrating the evolution and persistence of TD nystagmus over several years. The torsional nystagmus in five patients was probably caused by relative hyperactivation of the ipsilateral vertical SCCs, and the TD nystagmus in two patients may be explained by relative hyperactivation of the ipsilateral anterior canal pathway. The low gain of the ipsilesional horizontal VOR and the spared rostral part of the vestibular nuclei in the PN group indicate that PN is caused by damage to the afferent vestibular pathway from the horizontal SCCs, followed by overcompensation of the vertical SCC pathways.
Ipsilesional horizontal nystagmus may be caused by overcompensation of the horizontal VOR due to damage to the inhibitory cerebellovestibular pathway (PN 10, linear waveform) or severe damage to the vertical canals, followed by overcompensation of the horizontal SCC pathways. In addition, ipsilesional horizontal nystagmus during ipsilesional gaze can be caused by abnormal gaze-holding mechanisms (PN 11, decreasing waveform). Ipsilesional horizontal (on ipsilesional gaze) and torsional (on contralesional gaze) nystagmus found in two patients is a unique phenomenon in LMI and cannot be explained simply by asymmetry of VOR. However, it is known that the vestibular tone imbalance from SCC or otolith organs may change depending on the eye position. Although it is triggered by horizontal gaze, it differs from gaze-holding nystagmus owing to dysfunction of the neural integrators.16 This is similar to the aggravation or appearance of downbeat nystagmus on horizontal gaze.17 According to Listing's law, the eye position has a zero torsional component when expressed as a single rotation.18 Therefore, if vertical and torsional neural integrational deficits do not follow Listing's law, vertical or torsional nystagmus can be observed in the horizontal gaze.19 Large amplitude blips were also found pathologically in patients with a dorsolateral medullary lesion.20
Considering all above, we can assume the pathophysiology of PN in LMI. First, damage of the vestibular afferents pathway and incomplete vestibular compensation results in contralesional nystagmus. Second, selective damage to the vestibular nuclei or vestibular afferents and inappropriate compensation of sparing SCC pathways can result in ipsilesional PN in LMI. Third, damage to the cerebellar inhibitory fibers or increased velocity storage can cause high gain of VOR and ipsilesional PN. Fourth, impaired vertical or horizontal neural integrators can cause horizontal, vertical, or torsional nystagmus in the horizontal gaze.
The presence of spontaneous nystagmus was not related to subjective dizziness in our study. In 2016, Amari et al.8 reported spontaneous head-shaking and positional evaluation for post-LMI dizziness. They argued that chronic dizziness in LMI is associated with greater ipsilateral VOR gains caused by the increased velocity-storage mechanism in the dizziness group. We were unable to discover the correlation between patterns of spontaneous or post-head shaking nystagmus and subjective dizziness. We believe that various factors, including damage to the VOR (emotional component, vestibulospinal reflexes etc.), may be responsible for subjective dizziness in chronic LMI. We also did not analyze the quantitative data of nystagmus in the acute phase of LMI, vertical canal functions through the video head impulse, and otolith function through the vestibular evoked myogenic potential tests. Additionally, the inability to include all 81 patients with TN in the control group is considered a limitation of this study. We expect to determine the pathomechanism of PN in LMI through documentation of the nystagmus change from the acute phase and analysis of the vertical SCCs or otolith functions in the future.
In conclusion, Rarely, some patients with LMI have PN and most of them showed ipsilesional nystagmus with various components through the evolution of the initial nystagmus within the first year of infarction. We demonstrated five patterns of PN; contralesional torsional, ipsilesional torsional, ipsilesional TD, ipsilesional horizontal, and ipsilesional horizontal when looking at the lesion side and torsional when looking at the opposite side. Resultant vestibular asymmetry due to damage to the vestibular afferents or cerebellar inhibitory pathway as well as impaired neural integrators may be responsible for PN in LMI. The presence of PN does not necessarily indicate more severe dizziness in LMI.
Acknowledgements
This work was supported by the Soonchunhyang University Research Fund.
Footnotes
- Conceptualization: Tae-Kyeong Lee, Ji-Yun Park, HyunAh Kim, Kwang-Dong Choi, Ji-Soo Kim, Ki-Bum Sung.
- Data curation: Tae-Kyeong Lee, Ji-Yun Park.
- Formal analysis: Tae-Kyeong Lee, Ji-Yun Park, HyunAh Kim, Kwang-Dong Choi.
- Funding acquisition: Tae-Kyeong Lee, Ji-Yun Park.
- Investigation: Tae-Kyeong Lee, Ji-Yun Park.
- Methodology: Tae-Kyeong Lee, Ji-Yun Park, HyunAh Kim, Kwang-Dong Choi.
- Project administration: Tae-Kyeong Lee, Ji-Yun Park.
- Resources: Tae-Kyeong Lee, Ji-Yun Park.
- Supervision: Tae-Kyeong Lee, Ji-Yun Park.
- Visualization: Tae-Kyeong Lee, Ji-Yun Park.
- Writing—original draft: Tae-Kyeong Lee, Ji-Yun Park.
- Writing—review & editing: HyunAh Kim, Kwang-Dong Choi, Ji-Soo Kim, Ki-Bum Sung.
Conflicts of Interest: The authors have no potential conflicts of interest to disclose.
Supplementary Material
The online-only Data Supplement is available with this article at https://doi.org/10.3988/jcn.2020.16.2.285.
Contralesional TD nystagmus in PN 2. Torsional components are observed in all positions, and the downbeat components are noticeable on horizontal gaze.
Ipsilesional horizontal nystagmus in PN 10. The nystagmus looks linear in central position (1°/sec, linear slow phase) and ipsilesional gaze.
Ipsilesional horizontal nystagmus in PN 11 shows when looking at the lesion side with decreasing slow phase velocity.
Ipsilesional horizontal nystagmus of PN 12 (7°/sec, linear slow phase) is observed when looking at the lesion side. It changes into a torsional component (2.5°/sec, linear slow phase) when looking at the opposite side.
References
- 1.Brazis PW. Ocular motor abnormalities in Wallenberg's lateral medullary syndrome. Mayo Clin Proc. 1992;67:365–368. doi: 10.1016/s0025-6196(12)61553-5. [DOI] [PubMed] [Google Scholar]
- 2.Rambold H, Helmchen C. Spontaneous nystagmus in dorsolateral medullary infarction indicates vestibular semicircular canal imbalance. J Neurol Neurosurg Psychiatry. 2005;76:88–94. doi: 10.1136/jnnp.2003.031690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dieterich M, Brandt T. Wallenberg's syndrome: lateropulsion, cyclorotation, and subjective visual vertical in thirty-six patients. Ann Neurol. 1992;31:399–408. doi: 10.1002/ana.410310409. [DOI] [PubMed] [Google Scholar]
- 4.Sung KB, Park JY, Park SA, Lee TK. A case of lateral medullary syndrome with ipsilesional nystagmus due to intramedullary hemorrhage. Res Vestib Sci. 2009;8:52–55. [Google Scholar]
- 5.Uemura T, Cohen B. Effects of vestibular nuclei lesions on vestibuloocular reflexes and posture in monkeys. Acta Otolaryngol Suppl. 1973;315:1–71. doi: 10.3109/00016487409129565. [DOI] [PubMed] [Google Scholar]
- 6.Baloh RW, Yee RD, Honrubia V. Eye movements in patients with Wallenberg's syndrome. Ann N Y Acad Sci. 1981;374:600–613. doi: 10.1111/j.1749-6632.1981.tb30904.x. [DOI] [PubMed] [Google Scholar]
- 7.Jeong SH, Jo HJ, Lee AY, Kim JM, Kim JS, Sohn MK. Evolution and persistence of torsional downbeat nystagmus in lateral medullary infarction. Can J Neurol Sci. 2017;44:615–617. doi: 10.1017/cjn.2017.34. [DOI] [PubMed] [Google Scholar]
- 8.Amari K, Kudo Y, Watanabe K, Yamamoto M, Takahashi K, Tanaka O, et al. Spontaneous, headshaking, and positional nystagmus in post-lateral medullary infarction dizziness. J Neurol Sci. 2016;368:249–253. doi: 10.1016/j.jns.2016.07.019. [DOI] [PubMed] [Google Scholar]
- 9.Choi KD, Kim JS. Head-shaking nystagmus in central vestibulopathies. Ann N Y Acad Sci. 2009;1164:338–343. doi: 10.1111/j.1749-6632.2008.03737.x. [DOI] [PubMed] [Google Scholar]
- 10.Jacobson GP, Newman CW. The development of the dizziness handicap inventory. Arch Otolaryngol Head Neck Surg. 1990;116:424–427. doi: 10.1001/archotol.1990.01870040046011. [DOI] [PubMed] [Google Scholar]
- 11.Furman JM, Lempert T. Neuro-otology. Amsterdam: Elsevier; 2016. [Google Scholar]
- 12.Fisher CM, Karnes WE, Kubik CS. Lateral medullary infarction-the pattern of vascular occlusion. J Neuropathol Exp Neurol. 1961;20:323–379. doi: 10.1097/00005072-196107000-00001. [DOI] [PubMed] [Google Scholar]
- 13.Morrow MJ, Sharpe JA. Torsional nystagmus in the lateral medullary syndrome. Ann Neurol. 1988;24:390–398. doi: 10.1002/ana.410240307. [DOI] [PubMed] [Google Scholar]
- 14.Choi KD, Oh SY, Kim HJ, Koo JW, Cho BM, Kim JS. Recovery of vestibular imbalances after vestibular neuritis. Laryngoscope. 2007;117:1307–1312. doi: 10.1097/MLG.0b013e31805c08ac. [DOI] [PubMed] [Google Scholar]
- 15.Curthoys IS, Halmagyi GM. Multisensory interaction and vestibular compensation. In: Bronstein A, editor. Oxford textbook of vertigo and imbalance. Oxford: Oxford University Press; 2013. pp. 63–68. [Google Scholar]
- 16.Leigh RJ, Zee DS. Diagnosis of nystagmus and saccadic intrusions. In: Leigh RJ, Zee DS, editors. The neurology of eye movements. 5th ed. Oxford: Oxford University Press; 2015. pp. 657–768. [Google Scholar]
- 17.Yee RD. Downbeat nystagmus: characteristics and localization of lesions. Trans Am Ophthalmol Soc. 1989;87:984–1032. [PMC free article] [PubMed] [Google Scholar]
- 18.Wong AM. Listing's law: clinical significance and implications for neural control. Surv Ophthalmol. 2004;49:563–575. doi: 10.1016/j.survophthal.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 19.Glasauer S, Hoshi M, Kempermann U, Eggert T, Büttner U. Three-dimensional eye position and slow phase velocity in humans with downbeat nystagmus. J Neurophysiol. 2003;89:338–354. doi: 10.1152/jn.00297.2002. [DOI] [PubMed] [Google Scholar]
- 20.Helmchen C, Glasauer S, Büttner U. Pathological torsional eye deviation during voluntary saccades: a violation of Listing's law. J Neurol Neurosurg Psychiatry. 1997;62:253–260. doi: 10.1136/jnnp.62.3.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Contralesional TD nystagmus in PN 2. Torsional components are observed in all positions, and the downbeat components are noticeable on horizontal gaze.
Ipsilesional horizontal nystagmus in PN 10. The nystagmus looks linear in central position (1°/sec, linear slow phase) and ipsilesional gaze.
Ipsilesional horizontal nystagmus in PN 11 shows when looking at the lesion side with decreasing slow phase velocity.
Ipsilesional horizontal nystagmus of PN 12 (7°/sec, linear slow phase) is observed when looking at the lesion side. It changes into a torsional component (2.5°/sec, linear slow phase) when looking at the opposite side.


