Dear Editor,
Of the 17 subunits of RNA polymerase III (POLR3), POLR3A and POLR3B are the largest and second largest, respectively, which together form the catalytic core of the polymerase. Most cases of POLR3-related leukodystrophy are caused by mutations in POLR3A or POLR3B, with POLR1C mutations causing about 5% of cases.1 Distinctively, POLR1C is a shared subunit of both RNA polymerase I (POLR1) and POLR3, and molecular defects selectively modify the availabilities of these enzymes, leading to two distinct clinical conditions: Treacher Collins syndrome with autosomal recessive inheritance, and POLR3-related leukodystrophy.1 Only 20 POLR1C mutations presenting with the spectrum of POLR3-related leukodystrophy have been reported worldwide. Here we report two Korean siblings with ataxia and leukodystrophy caused by novel POLR1C mutations.
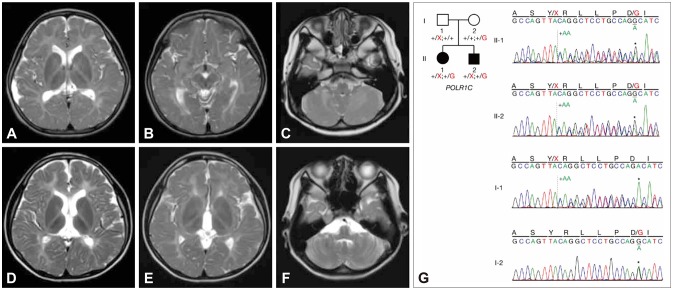
A 5-year-old girl visited our clinic with an ataxic gait. She showed tremor and clumsiness affecting her hands, and mild dysarthria. An ocular examination revealed myopia with mild optic nerve atrophy. Neurocognitive profiles revealed mild mental retardation with an intelligence quotient of 65. Brain MRI showed diffuse hypomyelination, except for T2-weighted hypointensities in the ventrolateral thalamus and optic radiation, without cerebellar atrophy (Fig. 1A, B, and C). She underwent menarche at 13 years of age, without evidence of hypogonadotropic hypogonadism. No dental or skeletal abnormalities were found. Her ataxia, tremor, and dysarthria were worsening at her current age of 15 years. She scored 28/40 on the Scale for the Assessment and Rating of Ataxia (SARA).
Fig. 1. Neuroimaging (A–F) and Sanger sequencing (G) findings. Besides diffuse hypomyelination, relative T2-weighted MRI hypointensities in the ventrolateral thalamus and optic radiation are evident in the proband (A and B) and her affected brother (D and E). Hypomyelination of the cerebellar white matter is visible, without cerebellar atrophy (C and F). (G) Compound heterozygous variants in POLR1C are shown in the pedigree: a frameshift mutation (c.698_699insAA: p.Tyr233fs) and a missense mutation (c.713A>G: p.Asp238Gly). Solid symbols indicate clinically affected and genetically confirmed patients: the proband (II-1) and the affected brother (II-2). One variant was inherited from their mother, the other from their father.
The younger brother of the proband also showed slow progressive ataxia, tremor, and dysarthria from 5 years of age with similar MRI findings (Fig. 1D, E, and F). He had difficulty walking, with ataxia and myoclonus (Supplementary Video 1 in the online-only Data Supplement). Myopia with mild optic nerve atrophy was also noted. No dental or endocrine abnormalities have been found at his current age of 12 years. He had a SARA score of 13/40. Neither sibling exhibited spasticity, dystonia, or seizures.
Because of the extreme genetic heterogeneity of leukodystrophy and ataxia, we performed whole-exome sequencing and analyzed the candidate genes causing hypomyelination and ataxia, including POLR3A, POLR3B, PLP1, GJC2, and TUBB4A, which did not reveal any pathogenic variants in them. We identified compound heterozygous POLR1C mutations: a frameshift mutation (c.698_699insAA: p.Tyr233fs) and a missense mutation (c.713A>G: p.Asp238Gly) validated with Sanger sequencing (Fig. 1G). The identified variants were classified as pathogenic based on the 2015 guidelines of the American College of Medical Genetics and Genomics, with evidence levels of PVS1, PM2, and PM3 for p.Tyr233fs, and PS3, PM1, PM2, PM3, and PP4 for p.Asp238Gly.2
POLR3 synthetizes small noncoding RNAs that play essential roles in cells, including transcription, and RNA processing and translation.3 Therefore, members of the POLR3 family are considered housekeeping genes that require strict regulation. POLR1C mutations, which impair the assembly and nuclear import of POLR3 and result in decreased binding to its target genes, are thought to reduce the transcription of tRNAs or other small noncoding RNAs that are central to the synthesis of proteins essential for central nervous system myelin development; however, the underlying pathophysiology is not yet fully understood. Although previously described as five distinct entities, these are now recognized as various clinical spectra of POLR3-related leukodystrophy.4 Other than diffuse hypomyelination, additional relatively characteristic findings are known to occur with or without cerebellar atrophy, such as T2-weighted MRI hypointensities in the ventrolateral thalamus, dentate nuclei, the posterior limb of the internal capsule, and the optic radiation. These findings suggest the presence of myelinating structures early in development and better preserved myelination of the pyramidal tract.5,6
Here we have expanded the clinical and genetic spectrum of POLR3-related leukodystrophy caused by POLR1C mutations.1,7 Integrating clinical features such as myopia, characteristic MRI patterns, and next-generation sequencing will facilitate the making of definitive diagnoses in unresolved atypical cases with hypomyelination and ataxia.
Our patients' parents gave written informed consent for the video publication.
Acknowledgements
This study was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF), funded by the Korea government (MSIT) (Grant No. 2017R1C1B5017312) and a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (Grant No. HI16C1986).
Footnotes
- Conceptualization: Jin Sook Lee, Jong-Hee Chae.
- Data curation: Ji Yeon Han, Jin Sook Lee.
- Formal analysis: Soo Yeon Kim, Jung-Eun Cheon, Murim Choi.
- Funding acquisition: Jin Sook Lee, Jong-Hee Chae.
- Supervision: Jin Sook Lee, Jong-Hee Chae.
- Visualization: Ji Yeon Han.
- Writing—original draft: Ji Yeon Han.
- Writing—review & editing: Jin Sook Lee, Jong-Hee Chae.
Conflicts of Interest: The authors have no potential conflicts of interest to disclose.
Supplementary Material
The online-only Data Supplement is available with this article at https://doi.org/10.3988/jcn.2020.16.2.338.
This video of the affected brother at 11 years of age shows his difficulty in walking with ataxia and myoclonus.
References
- 1.Thiffault I, Wolf NI, Forget D, Guerrero K, Tran LT, Choquet K, et al. Recessive mutations in POLR1C cause a leukodystrophy by impairing biogenesis of RNA polymerase III. Nat Commun. 2015;6:7623. doi: 10.1038/ncomms8623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015;17:405–424. doi: 10.1038/gim.2015.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Park JL, Lee YS, Kunkeaw N, Kim SY, Kim IH, Lee YS. Epigenetic regulation of noncoding RNA transcription by mammalian RNA polymerase III. Epigenomics. 2017;9:171–187. doi: 10.2217/epi-2016-0108. [DOI] [PubMed] [Google Scholar]
- 4.Wolf NI, Vanderver A, van Spaendonk RM, Schiffmann R, Brais B, Bugiani M, et al. Clinical spectrum of 4H leukodystrophy caused by POLR3A and POLR3B mutations. Neurology. 2014;83:1898–1905. doi: 10.1212/WNL.0000000000001002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Steenweg ME, Vanderver A, Blaser S, Bizzi A, de Koning TJ, Mancini GM, et al. Magnetic resonance imaging pattern recognition in hypomyelinating disorders. Brain. 2010;133:2971–2982. doi: 10.1093/brain/awq257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cayami FK, Bugiani M, Pouwels PJW, Bernard G, van der Knaap MS, Wolf NI. 4H leukodystrophy: lessons from 3T imaging. Neuropediatrics. 2018;49:112–117. doi: 10.1055/s-0037-1608780. [DOI] [PubMed] [Google Scholar]
- 7.Kraoua I, Karkar A, Drissi C, Benrhouma H, Klaa H, Samaan S, et al. Novel POLR1C mutation in RNA polymerase III-related leukodystrophy with severe myoclonus and dystonia. Mol Genet Genomic Med. 2019;7:e914. doi: 10.1002/mgg3.914. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
This video of the affected brother at 11 years of age shows his difficulty in walking with ataxia and myoclonus.