Abstract
Triterpenoids are a powerful group of phytochemicals derived from plant foods and herbs. Many reports have shown that they possess chemopreventive and chemotherapeutic effects not only in cell lines and animal models but also in clinical trials. Because epigenetic changes could potentially occur in the early stages of carcinogenesis preceding genetic mutations, epigenetics are considered promising targets in early interventions against cancer using epigenetic bioactive substances. The biological properties of triterpenoids in cancer prevention and in health have multiple mechanisms, including antioxidant and anti-inflammatory activities, cell cycle regulation, as well as epigenetic/epigenomic regulation. In this review, we will discuss and summarize the latest advances in the study of the pharmacological effects of triterpenoids in cancer chemoprevention and in health, including the epigenetic machinery.
Keywords: phytochemicals, triterpenoids, chemoprevention, cancer, epigenetics
Graphical Abstract
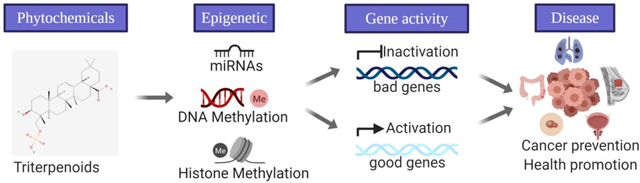
1. Introduction
Cancer has become one of the major global health problems and rank as leading killers around the world in the 21st century [1, 2]. In 2018, there were an estimated 18.1 million new cases and 9.6 million cancer deaths worldwide [2]. Carcinogenesis is a complex, multistep process starting from tumor initiation, promotion to progression that includes a continuous accumulation of transformative events driven by genetic mutations and epigenetic changes that affect major cellular processes and pathways, such as cell growth, and survival, differentiation, metastasis, migration, and invasion [3]. Inflammation is one of the mechanisms and drivers that can cause cancer development, through processes that involve genotoxicity, aberrant tissue repair, proliferative responses, invasion and metastasis [4]. Fortunately, it is estimated that from one-third to two-fifths of new cancer cases can be prevented through lifestyle changes by eliminating or reducing exposure to known environmental risk factors [5–7]. The risk of specific cancers has been reported to be negatively correlated with higher vegetables and fruit intake [8].
Phytochemicals are often used to describe plant compounds that are not scientifically defined as essential nutrients [8]. Many phytochemicals derived from plants have been reported to play a role in the prevention and treatment of cancer [3, 8, 9]. The latest advances in phytochemical research also implicate the health benefits not only in the biology of tumor cells but also in the nervous system [10, 11], diabetes [12, 13], cardiovascular diseases [14, 15], anti-aging [16], and obesity [17–19]. Triterpenoids are terpenoid derivatives of triterpene molecules including oleanolic acid (OA) derived from garlic, java apple, ursolic acid (UA) found in apples, bilberries, cranberries, lavender, moronic acid derived from mistletoe, and betulinic acid [20]. More than 20,000 triterpenoids have been found in nature [21]. Triterpenoids are potent phytochemicals that are beneficial to many human diseases, including different types of cancer [20–22].
Epigenetics/epigenomics has been hypothesized as one of the major mechanisms linking phytochemicals and cancer development [23–25]. Epigenetic modifications ranging from DNA methylation, histone modification to microRNA-mediated modification contribute to changes in gene regulation and expression [26, 27]. The dysregulation of epigenetic processes is often found to be a driving factor in cancer [28]. Many bioactive dietary ingredients including triterpenoids have been gaining attention because their activities on epigenetic modifications and epigenome profiles may play a role in preventing cancers [29]. However, the studies on interactions between phytochemical and epigenetic/epigenomic machinery in cancer prevention and in health are still at its early stage of research. In this review, we will summarize recent advances in the potential mechanisms exerted by triterpenoids in chemoprevention and in health by mainly focusing on the epigenetic/epigenomic regulation of triterpenoids in lung, breast, colon, prostate, skin cancers and other diseases.
2. Epigenetic Modifications as A Bridge Between Phytochemicals and Diseases
Cancer development is based on the crosstalk between the genome and the epigenome which is influenced by lifestyle factors, such as environmental pollutants and diet. Dietary factors affecting epigenetic modification to turn on/off gene expression and signaling pathways is a new strategy to prevent cancer, cardiovascular disease, and other diseases. It has been reported that plant-based compounds target epigenetic modifications and show great potential in the prevention and treatment of cancers and various chronic diseases [3, 30, 31]. For instance, curcumin, a bioactive polyphenol from turmeric, has been shown to remodel chromatin through histone modifications, modulate transcription via alteration in DNA methylation and undergo post-transcriptional regulation by microRNAs (miRNAs) modifications [32, 33].
Other phytochemicals including polyphenols [quercetin, apigenin, epigallocatechin-3-gallate (EGCG), genistein, resveratrol, and curcumin], organosulfur compounds [sulforaphane (SFN), phenethyl isothiocyanate (PEITC), diallyl disulfide (DADS)], and indoles [diindolylmethane (DIM)] may play a significant role in chemopreventive effects through targeting multiple anticancer pathways as well as epigenetic mechanisms [3, 30, 34]. Compared to conventional single-targeted chemotherapeutic drugs with high toxicity, regular intake of multifunctional and relatively non-toxic phytochemicals would be logical in early prevention and long-term treatment of various chronic diseases. Furthermore, the combination of bioactive phytochemicals with chemotherapies has shown a synergistic effect [35].
Triterpenoid biosynthesized from squalene is one of the most potent types of phytochemicals targeting anti-oxidative, anti-inflammatory and anti-tumor pathways in which nuclear factor erythroid-2-related factor 2 (Nrf2), nuclear factor kappa B (NF-κB), signal transducer and activator of transcription 3 (STAT3) are some of the major regulators [36]. The cytotoxic effect of triterpenoids in cancer cells, as well as antitumor efficacy in animal models and human trials, have been investigated widely [21, 22]. The cancer prevention mechanisms by which triterpenoids inhibit cell proliferation and migration, induce apoptosis, regulate cell cycle progression, and inhibit angiogenesis vary with different cancer models (Table 1).
Table 1.
Chemoprevention Effect of Triterpenoids Targeting Signaling Pathway and Epigenetic Modifications
| in vitro/in vivo | Cell line/animal model | Phytochemical | Concentration/dose | Molecular targets and epigenetic modification(s) | Effect(s) | Reference | |
|---|---|---|---|---|---|---|---|
| Lung cancer | in vitro | A549 and patient-derived primary lung cancer cell lines | OA | 30 μg/ml | Activated miR-122/Cyclin G1/CCNG1/MEF2D pathway | Inhibited cell proliferation via cell cycle arrest pathway | [50] |
| in vivo | Lung carcinoma xenografts bearing primary lung cancer cells | OA | 120mg/kg | Activated miR-122/Cyclin G1/CCNG1/MEF2D pathway | Suppressed tumor volume | [50] | |
| in vitro | H1299 and A549 | UA | 30 μM | Mediated SAPK/JNK-induced suppression of SP1 and downregulation of DNMT1 and EZH2 | Inhibited cell proliferation; induced apoptosis via caspase 3/7 activation | [44] | |
| in vitro | A549 | Ginsenoside Rh2 | 40 μg/ml | Restored miR-148a and miR-196b and inhibited miR-100, miR-23b and miR-21 | Inhibited cell proliferation | [38] | |
| Breast cancer | in vitro | MCF-7 | Ginsenoside Rg3 | 20, 50 μM | Induced global hypomethylation, methylation of NOX4 and demethylation of KDM5A | Inhibited cell proliferation, anchorage-independent growth, and induced apoptosis | [42] |
| in vitro | MDA-MB-231 and MCF-7 | Cucurbitacin B | 5μM | Enhanced DNMT1 expression to methylate CpG in the promoter regions of c-Myc, cyclin D1, and survivin | Induced cancer cell shrinkage and inhibited anchorage-independent growth | [39] | |
| in vitro | paclitaxel-resistant MDA-MB-231 | UA | 20 μM | Upregulated miR-149–5p to inhibit MyD88 dependent Akt signaling pathway | Reversed paclitaxel-chemoresistance | [44] | |
| Colorectal cancer | in vivo | AOM/DSS model of CAC (C57BL/6 mice) | Triterpenoid-rich extract from Ilex rotunda Thunb | 25 mg/kg | Inhibited miR-31–5p/LATS2/YAP pathway and downregulated gene expression of inflammatory mediators including TNF-α, IL-6, iNOS, and COX-2 | Rescued dysplasia, shortened colon length and the thickened muscle layer, and improved survival rate in mice | [106] |
| in vitro | Caco2 cells | Triterpenoid-rich extract from Ilex rotunda Thunb | 1–10 μg/ml | Decreased miR-31–5p via inhibition of IL-6 and TNF-α through NF-κB signaling pathway | Exerted anti-inflammatory effects | [106] | |
| in vitro | HCT116andHCT-8 | UA | 10–40 μM | Downregulated TGF-β1 signaling and its target gene Zeb, thus increasing miR-200a/b/c expression | Induced morphology change; inhibited cell proliferation, migration, and invasion; prevented EMT-induced TGF-β1 signaling | [45] | |
| in vitro | HT29 | Ginsenoside compound K | 20 μg/ml | Activated Bim-induced apoptosis by suppressing ERK-DNMT signaling and thus restoring RUNX3-Smad expression by demethylating RUNX3 promoter | Inhibited cell proliferation; induced apoptosis pathway | [40] | |
| Skin cancer | in vitro | JB6 P+ cell induced by TPA | UA | 2.5 μM | Demethylated 15 CpG sites at Nrf2 promoter via downregulating DNMT1, DNMT3a, HDAC2 and HDAC8 | Inhibited cell proliferation and TPA-induced anchorage-independent growth; activated Nrf2 defense pathway | [147] |
| in vivo | UVA (60 mJ/cm2)-induced NMSC model (SKH-1 mice) | UA | 2 μmol in 200 μl of acetone | Modulated oxidation and inflammation-related signaling such as Nrf2, NF-κB, and IL-8 by inverting methylation status and activating/inhibiting associated gene expression at the early stage (2 weeks) of NMSC carcinogenesis | Suppresses UVB-mediated NMSC by tumor volume and number | [47] | |
| Prostate cancer | in vitro | TRAMP-C1 | CRA | 2–8 μM | Demethylated Nrf2 promoter and decrease regulation of H3K27me3, and increase regulation of H3K27ac via regulating DNMTs and HDACs | Inhibited cell proliferation and anchorage-independent growth; activated Nrf2 defense pathway | [48] |
| Glioma | in vitro | U251 | Ginsenoside Rh2 | 12 μg/ml | Induced miR-128/E2F3a pathway | Inhibited cell proliferation; induced apoptosis via caspase 3 activation | [41] |
| Acute myeloid leukemia | in vitro | HL60 | UA | 5–20 ug/ml | Increased the acetylation of histone H3 via decreasing HDAC activity, and enhance cleavage of Bax and PARP for apoptosis | Inhibited cell proliferation; induced apoptosis pathway | [49] |
| Pancreatic cancer | in vitro | MiaPaCa-2 and Panc-1 | CDDO-Me | 0.125 to 0.5 μM | Inhibited hTERT expression by suppressing DNMT1, DNMT3a and active chromatin markers interacting with hTERT promoter (H3K9ac, H4ac, H3K4me2, and H3K9me3) | Inhibited cell proliferation; induced apoptosis pathway; repressed telomerase by decreasing hTERT expression | [148] |
| in vitro | Panc1, Panc28 and L3.6pL | CDDO-Me | 0.5–1.25 μM | Activated ROS and thus regulating the miRNA-27a-ZBTB10-Sp pathway | Inhibited cell proliferation; induced apoptosis and antiangiogenic response pathway; decreased expression of Sp and Sp-regulated genes overexpressed in tumor | [51] | |
| in vivo | Orthotopic pancreatic cancer model bearing L3.6pL cells (athymic nude mice) | CDDO-Me | 7.5 mg/kg | Activated ROS and thus regulating the miRNA-27a-ZBTB10-Sp pathway | Suppressed tumor volume and weight; decreased expression of Sp and Sp-regulated genes overexpressed in tumor | [51] |
Recent evidence suggests that triterpenoids modulate epigenetic mechanisms in exerting cancer preventive effects as summarized in Figure 1 and Table 1. Triterpenoids can be classified into two main categories including tetracyclic and pentacyclic triterpenoids, with ursane, oleanane, lupane are the three major families in pentacyclic triterpenoids [37]. Cucurbitacin, ginsenoside Rg3, ginsenoside Rh2, ginsenoside compound K, which are tetracyclic triterpenoids, have shown to induced global hypomethylation, enhance promoter hypermethylation/hypomethylation of oncogenes and tumor suppressor genes, and modify miRNA by targeting DNA methyltransferase 1 (DNMT1) [38–42]. The epigenetic modifications can contribute to growth inhibition and apoptosis induction in lung cancer, breast cancer, colorectal cancer, and glioma. Ursane family of pentacyclic triterpenoids, including UA and corosolic acid (CRA), can modulate histone modification, miRNA, and CpG methylation in the promoter of transcription factors including (specific protein 1) Sp1, Nrf2 through regulating DNMTs, histone deacetylases (HDACs) and histone methyltransferases (HMTs) [43–49]. These epigenetic mechanisms lead to the inhibition of malignant transformation at an early stage as well as suppression of tumor survival, growth, migration, and invasion at the promotion and progression stages in non-small cell lung cancer (NSCLC), breast cancer, colorectal cancer, skin, prostate cancer, and acute myeloid leukemia. Oleanane type of pentacyclic triterpenoids including OA and bardoxolone methyl (CDDO-Me) activate reactive oxygen species (ROS), induce cell cycle arrest, suppress tumor growth through histone modifications, promoter demethylation of human telomerase reverse transcriptase (hTERT) and miRNA regulation via inhibiting DNMT1 and DNMT3a in lung and pancreatic cancers [50–52]. In the following sections, we will address the triterpenoids-regulated epigenetic mechanisms and signaling pathways in various diseases in more detail.
Figure 1.
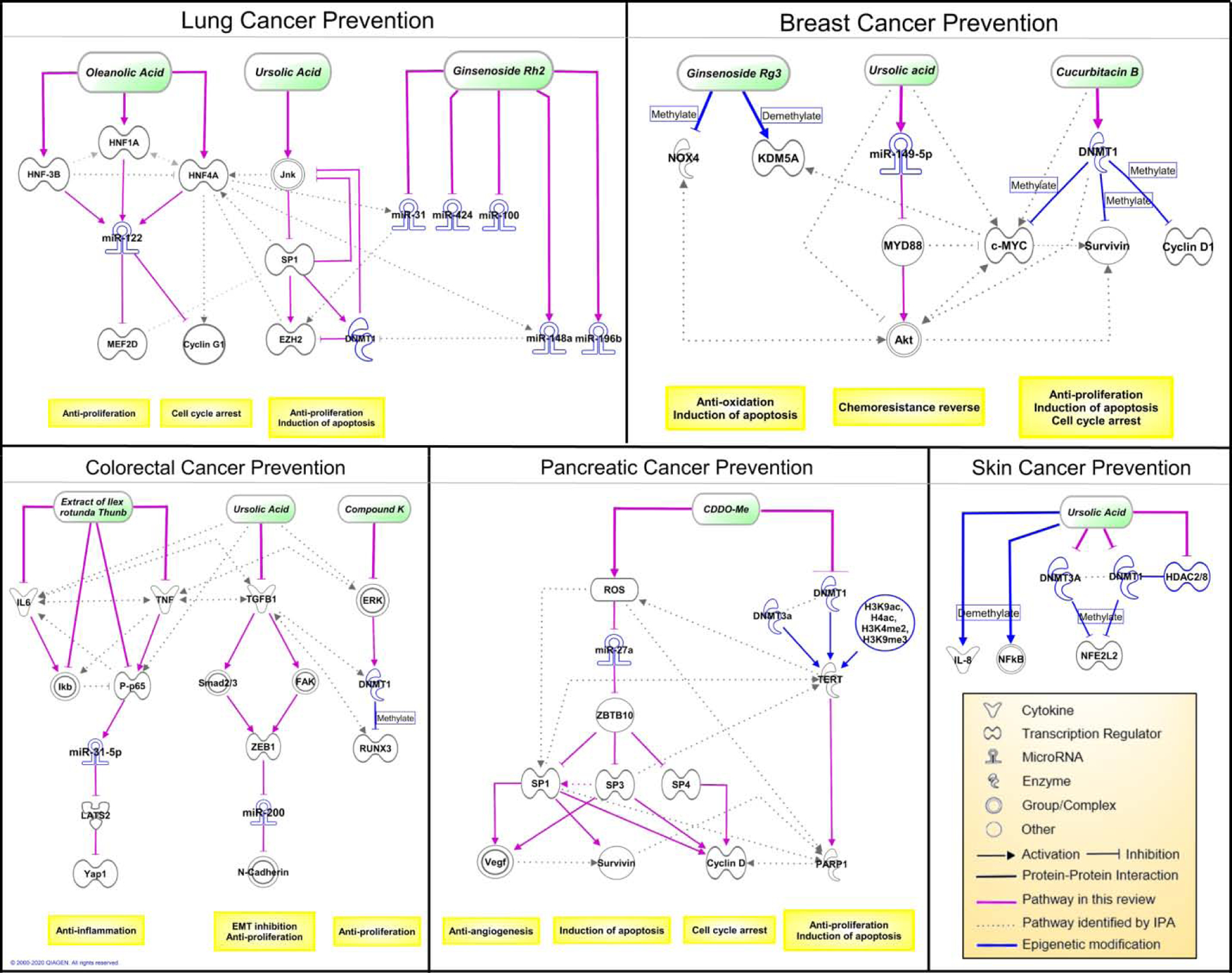
The major cancer prevention pathways of triterpenoids involved in cell proliferation and migration, oxidative stress balance, inflammation, apoptosis, cell cycle progression and angiogenesis are represented in these signaling pathways. In each cancer model, the mechanism reported in the literatures are depicted by solid lines. The pathways and interactions determined using Ingenuity Pathway Analysis (IPA) are depicted by dash lines as potential pathways to be investigated in future research. The epigenetic regulators and mechanisms are highlighted in blue.
3. Triterpenoids Structure Consideration
Triterpenoids represent a large and diverse group of organic compounds characterized by the basic backbone of a 30-carbon isoprenoid molecule and consists of six isoprene units [53]. Triterpenoids share a similar carbon skeleton with triperpene, the pentacyclic structure with different bioactive subgroups [54]. Most of the triterpenoids are secondary metabolites naturally synthesized in the plant and distributed widely in fruits and vegetables such as apple, cranberry, and blueberry [55–58]. The bioavailability of triterpenoids is low due to high first-pass metabolism [59–63]. Several studies have shown that triterpenoids significantly suppress various cellular processes including chronic inflammation by modulating proinflammatory mediators, cell cycle arrest, apoptosis, phase II detoxifying enzymes. [64]. Studies on biological functions of triterpenoids extracted from plants are conducted but remain not fully understood [65, 66]. Among the numerous triterpenoid derivatives, several compounds such as UA (3-beta-3-hydroxy-urs-12-ene-28-oic-acid), OA (3/3-hydroxy-olea-12-en-28-oic acid) and CDDO (2-cyano-3,12-dioxooleana-1,9(11)-dien-28-oic acid), and maslinic acid, lupeol, botulin and betulinic acid show strong pharmacological and medical properties in the prevention and treatment against chronic diseases, particularly on cancers [20, 67, 68]. UA exhibited strong antioxidant, anti-inflammatory and anticancer activities through multiple molecular mechanisms including free radical scavenging, cell cycle regulation, cell apoptosis induction, and some signaling pathways such as NF-κB pathway [59]. OA is the isomer of UA shows a protective effect in leukemia and colon carcinogenesis [69, 70]. The synthetic oleanane triterpenoid CDDO and CDDO derivatives such as methyl ester and ethyl amide also show cancer-preventive effects against various cancers such as lung and prostate cancer [71–73]. Based on these findings, an important structure and activity relationship is revealed. For example, CDDO’s structure of 2-cyano-1-en-3-one subgroup and 9(11)-en-12-one subgroup presented on the specific ring location is highly associated with its strong bioactivity [20]. Besides, the keto group at C-16, l-rhamnose group at R5 and acetyl group at OH-6 of the glucose shows significantly increasing cytotoxicity on human adenocarcinomic alveolar basal epithelial A549 cells and human ileocecal colorectal adenocarcinoma HCT-8 cells, and decreasing cytotoxicity on human papillomavirus-related endocervical adenocarcinoma Bel-7402 cells [74]. The angeloyl group at C-21 or C-22 is also associated with triterpenoid cytotoxic activity and potentially bounds to enzymes such as human DNA topoisomerase I [75]. Multiple functional groups at one carbon location on the same triterpenoid skeleton also reveal the various bioactivity of triterpenoids. For instance, the acetyl group at C-3 and amine group at C-28 shows a combined structure-activity relationship in regulating cell cycle [76]. Other groups such as carboxylic acid, ester of hydroxymethyl propanediol at these carbon locations would also affect the bioactivity of triterpenoids. To sum up, the chemical structure-activity relationship reveals important evidence and provides details for elucidating the molecular mechanism of anticancer activities in human cancers.
4. Triterpenoids in Cancer Prevention: Regulation of signaling pathways and epigenetic modifications
4.1. Lung Cancer
The use of natural and synthetic triterpenoids in lung cancer prevention studies is increasing annually. From the pharmacokinetic aspect, triterpenoids including UA has shown rapid absorption and elimination as well as better distribution to lung compared with other tissues [61]. In NSCLC lines and a human lung adenocarcinoma cell line, UA has been shown to induce the release of apoptosis-inducing factor and Endo G through mitochondrial dependence pathway, thus causing apoptosis [77–80]. UA can also exert lung cancer prevention activities by weakening the DNA damage protection induced by vaccinia related kinase 1 [81], inhibiting epithelial-mesenchymal transition by downregulation of astrocyte-elevated gene-1 [82] and inducing stress-activated kinase/c-Jun N-terminal kinase (SAPK/JNK) pathway [43]. SAPK/JNK-mediated suppression of Sp1 by UA leads to the downregulation of epigenetic modifiers DNMT1 and EZH2 which contributes to inhibition of NSCLC growth in H1299 and A549 cells [43]. The synthetic triterpenoids CDDO-Me and CDDO-Ea (ethyl amide) can inhibit the expression of malignant phenotypes during the development of lung cancer induced by vinyl carbamate in A/J mouse [72, 83]. CDDO-Ea with HDAC inhibitor vorinostat (SAHA) demonstrated a synergistic effect in the inhibition of expression of cyclin D1 and lung carcinogenesis in VC-1 lung cancer cells and A/J mice [84]. It can also enhance the acetylation of histone H3 when combined with SAHA in VC-1 lung cancer cells. The synthetic triterpenoid CDDO-Im (imidazolide) inhibits phosphorylation of transcription factor STAT, suppresses proliferation and induces apoptosis in lung cancer cells [85]. CDDO-Me, CDDO-Im, and various amide derivatives of CDDO are strong inducers of Nrf2/ARE signaling in various organs, including the lung [86, 87]. CDDO-Me has been shown to induce rapid apoptosis involving cytochrome c-triggered caspase activation pathways in an array of human NSCLC cells [88]. Betulinic acid is another plant-derived pentacyclic triterpenoid with a strong anticancer ability and targets the mitochondrial apoptosis pathway [89]. In in vitro and in vivo lung cancer models, betulinic acid induces cell cycle arrest through increasing the sumoylation of Sp1, thereby reducing the level of Sp1 and inhibiting cyclin A2/Rb signaling [90]. Another study suggests that the protective effect of betulinic acid on the lungs is related to inflammatory cytokine responses [91]. Recent reports have suggested that underexpression of miR-126, miR-200c, and overexpression of miR-21 contribute to the malignancy of NSCLC [92–95]. Ginsenoside Rh2 has been shown to modulate an array of miRNA to reduce lung cancer cell A549 growth [38]. Restoration of miR-148a and miR-196b and inhibition of miR-100, miR-23b and miR-21 appear to be the most significant modifications (more than 2-fold changes) observed during ginsenoside Rh2 treatment. OA also exhibits anti-tumor effects through miRNA regulation. OA induces miR-122-regulating transcriptional factors hepatocyte nuclear factors (HNF)1α, 3β, 4α, and 6 to activate the miR-122/Cyclin G1/Cyclin G1 (CCNG1)/Myocyte Enhancer Factor 2D (MEF2D) axis [96]. These mechanisms contribute to inhibition of proliferation induced by cell cycle arrest pathway in vitro as well as suppression of tumor volume in lung carcinoma xenografts. Santos et al. showed that OA improves lung morphology and function by regulating the release of inflammatory mediators and oxidative stress in experimental acute lung injury [97]. In summary, triterpenoids are promising lung cancer prevention compounds through regulating DNA and histone methylation via DNMT and HMT as well as modifying miRNAs.
4.2. Breast Cancer
It is widely recognized that the accumulation of genetic mutations contributes to the development of breast cancer, the most common cancer in women worldwide [98]. A genome-wide study has revealed 189 genes that are frequently mutated in breast and colorectal carcinomas, and DNA methylation and chromatin remodeling have been implicated in the gene dysregulations [99]. Increasingly many studies have established that gene dysregulations induced by global and loci-specific epigenetic modifications play a crucial role in human breast carcinogenesis [100]. The application of multifunctional phytochemicals such as triterpenoids could be an effective and less cytotoxic approach for the prevention and treatment of breast cancers. Ginsenoside Rg3, an anticancer triterpenoid saponin, has induced global hypomethylation in MCF-7 breast cancer cells in a dose-dependent manner (0–50 μM) as examined by bisulfite PCR of long interspersed nucleotide elements (LINE)-1 [42, 101]. Gene-specific methylation profile has suggested the methylation of NOX4 and demethylation of KDM5A as a key epigenetic mechanism regulated by Rg3 to suppress MCF-7 cell proliferation. The expression of NOX4 and KDM5A altered by Rg3 were validated by PCR and western blot. The inhibitory effect of Rg3 on cell proliferation was enhanced when NOX4 is downregulated by siRNA and was decreased via silencing of KDM5A by siRNA. Dittharot et al. showed that triterpenoid cucurbitacin B suppressed the survival and the anchorage-independent growth of MDA-MB-231 and MCF-7 cells through inversion of CpG methylation status in the promoter regions into hypermethylation in oncogene c-Myc, cyclin D1, and survivin [39]. The enhanced methylation of oncogenes was elucidated by increased RNA and protein expression of DNMT1 by cucurbitacin B as an epigenetic regulatory mechanism. UA can sensitize paclitaxel-resistant MDA-MB-231 breast cancer cells through the upregulation of miR-149–5p while silencing of the miR-149–5p gene by shRNA abrogated the effects of UA on paclitaxel resistance. Upregulated by UA, miR-149–5p can further suppress the expression of the MyD88 gene through direct binding to its 3’UTR and then inhibit MyD88-dependent Akt signaling pathway in paclitaxel-resistant MDA-MB-231 cells [44]. Inflammatory cytokines can attract tumor-associated macrophages (TAMs) to the tumor microenvironment to promote the production of various inflammatory and angiogenesis mediators, such as inducible nitric oxide synthase (iNOS), matrix metalloproteinases (MMP) and vascular endothelial growth factor (VEGF) [102]. Tran et al. demonstrated that CDDO-Me and CDDO-Ea downregulate the expression of M-CSF and MMP-9 to prevent the infiltration of TAMs and thereby suppress ER-negative mammary tumor development in PyMT mice [84, 103]. The effect of infiltration of TAMs on tumor growth is associated with various epigenetic mediators including DNMTs, HMTs, histone demethylases (HDMs) and HDACs [104]. The combination of CDDO-Me or CDDO-Ea with HDAC inhibitor SAHA presented the synergistic effect in mammary tumor prevention [84]. Eades et al. have reported that miR-200a is significantly inhibited in breast cancer cell lines MDA-MB-231 and Hs578T in comparison with the non-tumorigenic MCF-10A cell line by profiling expression of 88 miRNAs [105]. SAHA as the epigenetic regulator can restore miR-200a expression and thus destabilizing Keap1 to activate Nrf2-ARE signaling, contributing to the suppression of anchorage-independent growth of breast cancer cells. In conclusion, triterpenoids are potential epigenetic modulators for breast cancer prevention which can regulate global and gene-specific DNA methylation via DNMT as well as modify miRNAs.
4.3. Colorectal Cancer
Accumulating evidence indicates that triterpenoids can prevent colon cancer through epigenetic modifications. It’s well recognized that miRNAs play important roles in the pathogenesis of colitis-associated cancer (CAC). Chen et al. showed that triterpenoid-rich fraction extracted from Ilex rotunda Thunb attenuates upregulation of miR-31–5p and its target LSTS2/YAP genes, and reduce iNOS, interleukin (IL)-11, and IL-17A in azoxymethane/dextran sodium sulfate model of CAC [106]. The mechanism results in rescued of dysplasia, shortened colon length and the thickened muscle layer, and improved survival rate in vivo. The parallel in vitro study suggests that the extract inhibits miR-31–5p expression via down-regulating tumor necrosis factor (TNF)-α and IL-6 in both thp1 and Caco2 cells. Another triterpenoid in nature, UA, regulates several pathways to prevent colon cancer. At the early stage of colorectal cancer, the epithelial-mesenchymal transition (EMT) plays an important role in promoting proliferation and metastasis. In HCT116 and SW620 cells, UA significantly restores E-cadherin expression for maintaining epithelial morphology and decreases the expression of EMT-promoting genes, such as integrin, Vimentin, Twist, Zeb1 [107]. A study by Zhang et al. further showed that UA inhibits migration and invasion through the downregulation of TGF-β1/Smad and TGF-β1/FAK signaling pathways and its target gene Zeb1 in human colon cancer cells HCT116 and HCT-8. The mechanism is associated with increased miR-200a/c expression which is negatively controlled by CpG methylation of the miR-200 promoter [45, 108]. Choi et al. showed that oral administration of the synthetic triterpenoid CDDO-Me notably decreases the expression of proinflammatory cytokines (iNOS, IFN-γ, TNF-α, IL-6, and IL-1β), STAT1/3 as well as NAD-dependent 15-hydroxyprostaglandin dehydrogenase (15-PGDH) in mice with SMAD4-deficient T cells. 15-PGDH induction effect by CDDO-Me was not presented in Smad3 KO mice and reversed by TGF-β signaling inhibitors. Thus, the authors conclude CDDO-Me can prevent colitis-associated cancers through the 15-PGDH/(SMAD2/3)/TGF-β pathway [109]. Another review by Bai et al. further investigate the relationship between TGF-β signaling and epigenome. Activation of the transcription factor of TGF-β, SMAD2/3, can recruit a variety of epigenetic regulators [110]. Recruitment of SMAD-BRG1 SWItch/Sucrose Non-Fermentable (SWI/SNF) complex or SMAD-KDM6B complex to the promoter as well as acetylation of SMAD by P300/CREB binding protein (CBP) can increase the expression of TGF-β signaling target genes, whereas recruitment of HDACs has the opposite effect. Kang et al. reported that a tetracyclic triterpenoid, ginsenoside compound K, can activating Bim-induced apoptosis in colorectal cancer cell HT29 by the restoration of RUNX3-Smad expression [40]. The mechanism is mediated by CpG demethylation in the RUNX3 promoter induced by downregulation of DNMT1. The suppression of DNMT1 by compound K is via inhibition of the ERK pathway. To sum up, triterpenoids are multifunctional agents against colorectal cancers in vitro and in vivo which targets various inflammatory response pathways especially Smad2/3-mediated TGF-β and RUNX signaling. The epigenetic regulation by triterpenoids including gene-specific methylation via DNMT and miRNA modifications are highly involved in the prevention of colorectal cancers.
4.4. Skin Cancer
Skin cancer, like many other cancers, has been linked to aberrant epigenetic modifications [111]. Studies using melanoma cancer cell lines reveal that CpG island promoter regions of potential human tumor suppressor genes, Ras association domain family 1 isoform A and human mutL homolog 1, are significantly hypermethylated and cancer-associated genes such as Melanoma-associated antigen A1 and mammary serine protease inhibitor (maspin) are found to be hypomethylated [112, 113]. UA has shown potential results in activating Nrf2 and blocking cellular transformation by 12-O-tetradecanoylphorbol-13-acetate (TPA) in mouse epidermal JB6 P+ cells [46]. At a concentration of 2.5 μM, UA is shown to downregulate DNMT1, DNMT3a, HDAC2, and HDAC8. Under this condition, 15 CpG sites of the Nrf2 promoter region are demethylated, inducing expression of Nrf2 mediated detoxifying enzymes, including heme oxygenase-1 (HO-1), NAD(P)H: quinone oxidoreductase 1 (NQO1), and UDP-glucuronosyltransferase 1A1. Cho et al. have reported UA alone or in combination with resveratrol can prevent skin tumor development and epidermal hyperproliferation in the ICR mouse model of two-stage skin carcinogenesis [114]. The multi-functional mechanism is showed by inhibition of inflammatory and growth factor pathways, inclusive of STAT3, Fas, Src, Akt, p38 mitogen-activated protein kinases (MAPK), cyclooxygenase-2, NF-κB, JNK1/2, and epidermal growth factor receptor (EGFR) pathways, through modulating binding and nuclear translocation activity of NF-κB, early growth response protein 1, and activator protein 1 transcription factors. A systematic epigenomic study by Yang et al. reveals that UA suppresses UVB-mediated nonmelanoma skin cancers (NMSC) with a decrease in tumor number and volume coupled with alterations in DNA CpG methylome with RNA expression changes in transcriptome [47]. The CpG methylome profile shows that oxidation- and inflammation-related signaling pathways such as Nrf2, NF-κB, and IL-8 are highly modulated by UA with associated gene expression changes at the early stage (2 weeks) of NMSC carcinogenesis [47]. OA, an isomer of UA, has been shown to downregulate EGFR activity and induces mitochondria-dependent caspase 3-mediated apoptosis which is considered to play an important role in the development of skin cancer [115]. RTA 408, a synthetic oleanane compound is found to be an inducer of the Nrf2-antioxidant response element signaling pathway and induces cytoprotective genes [116, 117]. Overall, triterpenoids prevent skin cancers through epigenetically demethylating promoter of genes involved in oxidative stress and inflammation regulatory pathways such as Nrf2/ARE pathway via regulation of epigenetic modifiers DNMTs, HDACs and others [46].
4.5. Prostate Cancer
Prostate cancer is one of the most common cancers in the world with the incidence rate increasing annually. Some phytochemicals are potent in inhibiting proliferation and inducing apoptosis epigenetically in the prevention of prostate cancer. Triterpenoids have been demonstrated to exert various pharmacological activities through activation of the Nrf2 pathway and restoration of abnormal epigenetic alterations to prevent neoplasms including prostate cancer [46, 48, 118]. Previous work from our laboratory provided evidence that the suppression of Nrf2 during prostate tumor development in TRAMP mice is regulated by methylation in Nrf2 promoter related to binding of methyl-CpG-binding protein 2 and trimethyl-histone H3K9 [119]. UA can exhibit anti-oxidative, anti-inflammatory and DNA damage reduction effects in various diseases as discussed above [120, 121]. In human prostate cancer cells, UA has shown to facilitate apoptosis through mechanisms elaborated as follows. Mu et al. reported that UA activates caspase-3/9 dependent apoptosis in prostate cancer cells by targeting ROCK1/Phosphatase and tensin homolog to mediate translocation of cofilin-1 and release of cytochrome c from mitochondria [122]. Shin et al. showed that UA enhances apoptosis mediated by tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) via C/EBP homologous protein (CHOP)-dependent DR5 upregulation in several prostate cancer cell lines [123]. Another compound, CRA which has a similar structure as UA can inhibit anchorage-independent growth of TRAMP-C1 prostate cells via activation of the Nrf2 pathway mediated by demethylation of Nrf2 promoter [48]. The mechanism is associated with acetylation of histone H3 lysine 27 (H3K27ac) and demethylation of H3 lysine 27 (H3K27me3) modulated by DNMTs and HDACs. Besides, some other triterpenoids such as CDDO and its synthetic derivatives also play an important role in the prevention of prostate cancers. CDDO-Im suppresses prostate cancer growth in vitro by induction of apoptosis through targeting mitochondrial glutathione (mGSH) [124]. CDDO sensitizes prostate cancer cells to TRAIL-induced apoptosis by enhancing ubiquitination and degradation of FLICE-inhibitory protein (FLIP) protein [125]. In conclusion, the above studies have shown that triterpenoids can exhibit prostate cancer-preventive effect by epigenetically regulation of Nrf2/ARE signaling and activation of a variety of apoptosis pathways.
4.6. Other cancers
Wu et al. showed that ginsenoside Rh2 inhibits proliferation of glioma by targeting miR-128/E2F3a pathway, contributing to increased cytotoxicity, apoptosis, and caspase 3 activation [41]. Chen et al. suggested that UA increases the acetylation of histone H3 and decreased HDAC activity, resulting in cell death in HL-60 human acute myeloid leukemia cells [49]. Deeb et al. reported CDDO-Me can prevent proliferation and induce apoptosis in pancreatic cancer cells via inhibiting expression of hTERT and telomerase activity [52]. The inhibition of hTERT is illustrated by CpGs demethylation in the hTERT promoter, resulting from the inhibition of DNMT1 and DNMT3a expression. The mechanism is further associated with a reduction in acetylated histone H3K9, acetylated histone H4, dimethyl-H3K4, and trimethyl-H3K9 at the hTERT promoter. Moreover, suppression of transcription factors Sp1, c-Myc, NF-κB, CCCTC-binding factor, E2F Transcription Factor 1 and mitotic arrest deficient 1 by CDDO-Me can also regulate the transcriptional response of hTERT. Moreover, Jutooru et al. also revealed the antitumor effect of CDDO-Me in pancreatic cancer in vitro and in vivo [51]. CDDO-Me decreases MMP and induces ROS, thereby inhibiting miR-27a and upregulating zinc finger and BTB domain containing 10, leading to repression of Sp1/3/4 transcription factors. Sp can regulate the transcription of Sp-dependent genes involving in proliferation, apoptosis, and angiogenesis of pancreatic cancers.
5. Triterpenoids Pharmacological Effects in Non-cancer Diseases
Growing evidence connecting the health benefits of eating vegetables and fruits in preventing or treating various non-cancer illnesses, including cardiovascular, metabolic, neurodegenerative and other chronic diseases. Clinical trials and epidemiological studies have shown that these health benefits are closely related to the bioactive phytochemicals. Many studies have shown that triterpenoids have beneficial effects in this regard.
Dysregulated homeostasis in the cardiovascular system often leads to many cardiovascular diseases, such as hypertension, which is characterized by a chronic increase in system arterial pressure above a certain threshold value [126]. Oxidative stress is one of the main mechanisms for the occurrence and development of cardiovascular diseases. Triterpenoids can eliminate damage caused by ROS in various cardiomyopathy models by activating the Nrf2 pathway [127, 128]. By inducing HO-1 expression, CDDO-Im increases the availability of nitric oxide (NO) and reduces the levels of ROS and endothelial nitric oxide synthase (eNOS) in naïve or stressed endothelial cells, thereby mediating the coupling of eNOS and vascular homeostasis. The anti-hypertensive effects of triterpenoid OA and its derivatives have also been reported. For example, the preventive effect of 60 mg/kg OA on glucocorticoid-induced hypertension in rats was evaluated by Bachhav et al. [129]. The use of OA significantly prevented the increase of the systolic blood pressure and cardiac lipid peroxidation level. However, glucocorticoid therapy had no significant effect on changes in body weight or thymus weight. This study suggests that the nitric oxide release of OA may be involved in its anti-hypertensive effect. Nitric oxide is a molecule known to play an important role in cardiovascular regulation [130, 131]. To further understand the mechanism of the antihypertensive action of OA and the involvement of NO-releasing action, Nω-nitro-L-arginine methyl ester (L-NAME)-induced hypertensive rats were used [132]. This study suggested that the effect of OA in L-NAME induced hypertension might be due to the diuresis and nephron-protection. Madlala et al. provided additional insights into the antihypertensive effects of OA and its methyl ester and brominated derivatives; Me-OA and Br-OA, respectively [133]. These compounds displayed vasodilatory activity, which is facilitated by both COX and vascular muscle K+ channels.
Chronic diseases such as diabetes and obesity are closely related to metabolic disorders. Both genetic and pharmacological activation can induce a lot of genes involved in lipid metabolism [134]. CDDO-Im induces aryl hydrocarbon receptor (Ahr) transcription and blocks lipid accumulation in Nrf2(+/+) mouse embryonic fibroblasts (MEFs) in vitro by activating Nrf2, but not in Nrf2(−/−) MEFs [135]. In mice on a high-fat diet or in mice with leptin receptor (Leprdb/db) deficiency, CDDO-Me not only reduces proinflammatory cytokine expression, total body fat, free fatty acid levels, and plasma triglyceride but also improves glucose tolerance and insulin sensitivity. CDDO-Me has an effective antidiabetic effect in diabetic mouse models, mediated at least in part by AMP-activated protein kinase activation [136]. OA can reduce insulin resistance, reduce TNF-α and IL-6, NF-κB, and up-regulate the expression of insulin receptor substrate 1 and glucose transporter 4 in insulin-resistant HepG2 cells [137]. OA supplementation at 25 mg/kg/day for 10 weeks also improves fructose-induced insulin resistance through the IRS-1/phosphatidylinositol 3-kinase/Akt pathway [138]. OA can also inhibit gluconeogenesis and reduce insulin resistance in the liver. Hepatic insulin resistance is considered a major link between type 2 diabetes and nonalcoholic fatty liver disease [139, 140]. The administration of OA in mice (20 mg/kg/day, i.p.) for 14 days can reduce fat weight, protect liver morphology and function, reduce fasting glucose, enhance insulin signaling, and inhibit gluconeogenesis [141].
Neurological disorders include anxiety, depression, stroke, and Alzheimer’s disease, among others [142]. There is evidence of the protective effect of UA on ischemic stroke [143]. A recent report shows that redox-sensitive Nrf2 activation plays a decisive role in improving endogenous defense mechanisms through which the brain protects itself from ischemic damage and recovers from stroke [144]. Sahni et al. suggested that UA derivatives of C-6 (C-17 propyl amide) and C-2 (C-3 methyl ester) showed significant neuroprotective role in vivo models of D-galactose-induced neurotoxicity in rats. Therefore, c-2 and c-6 may have advantages in the treatment of cognitive impairment, for example, Alzheimer’s disease and dementia [145]. Li et al. reported a study on the neuroprotective effects of UA in vivo. This study shows that the anti-inflammatory and antioxidant effects of UA are important and necessary in the mouse brain after middle cerebral occlusion [144]. Parkinson’s disease (PD) is another chronic progressive neurodegenerative disease. Rai et al. studied the neuroprotective effect of UA on PD mice induced by 1-methyl-4-phenyl1,2,3, 6-tetrahydropyridine. These authors found that UA can reduce oxidative stress, protect against neurodegeneration, improve behavior disorders, and is a potential agent for Parkinson’s disease [146].
6. Conclusion
In conclusion, recent studies have shown that many triterpenoid compounds are effective and have desirable pharmacological activities against cancer and other diseases. The pharmacological properties of triterpenoids in cancer prevention and health are attributed to multiple mechanisms, including antioxidant, anti-inflammatory, and cell cycle regulatory properties, as well as epigenetic/epigenomic regulation. Triterpenoids have shown anticancer activities in certain concentration ranges in vitro and in vivo. Cucurbitacin B and Ginsenosides exhibit in vitro anti-cancer effect at concentrations of 5 μM and 12 to 40 μg/ml respectively [38, 39, 41]. UA and CRA have been shown to exert in vitro cancer preventive effect at concentrations of 2.5 to 40 μM and 2 to 8 μM respectively [45, 48, 147]. OA shows in vitro and in vivo efficacy against lung cancer at 30 μg/ml and 120 mg/kg separately [50]. CDDO-me demonstrates anti-tumor effect at much lower concentration range of 0.125 to 1.25 μM in vitro and at 7.5 mg/kg in vivo [51, 148]. The effective concentrations of these pharmacological actions in large part could be attributable to various factors including different cell line models, animal models, molecular targets, and sources of the compounds. In addition, there is evidence that triterpenoids can be used as adjuvant therapy under certain conditions. Further research is needed to translation these results into clinical applications. Future research can focus on studying the in vivo mechanisms, identifying epigenetic regulatory switches, finding new analogs, increasing the bioavailability of triterpenoids, to help identify more effective compounds to prevent chronic diseases such as cancer and cardiovascular diseases. Overall, the studies summarized in this review enhance our understanding of the epigenetic/epigenomic regulation by triterpenoids in preventing cardiovascular diseases, diabetes, neurological disorders as well as a wide range of cancers.
Acknowledgments
This study was supported in part by Institutional Funds, R01CA200129 from the National Cancer Institute (NCI) and R01AT009152 from the National Center for Complementary & Integrative Health (NCCIH). The authors appreciate all the members of Dr. Kong’s laboratory for their invaluable support and assistance.
Abbreviations:
- CAC
colitis-associated cancer
- CBP
CREB binding protein
- CDDO
2-cyano-3,12-dioxooleana-1,9(11)-dien-28-oic acid
- CDDO-Me
bardoxolone methyl
- CRA
corosolic acid
- DADS
diallyl disulfide
- DNMT1
DNA methyltransferase 1
- DIM
diindolylmethane
- EGFR
epidermal growth factor receptor
- EGCG
epigallocatechin-3-gallate
- EMT
epithelial-mesenchymal transition
- eNOS
endothelial nitric oxide synthase
- HDMs
histone demethylases
- HDACs
histone deacetylases
- HMTs
histone methyltransferases
- hTERT
human telomerase reverse transcriptase
- HO-1
heme oxygenase-1
- iNOS
inducible nitric oxide synthase
- MEFs
mouse embryonic fibroblasts
- MEF2D
Myocyte Enhancer Factor 2D
- MMP
matrix metalloproteinases
- NQO1
NAD(P)H: quinone oxidoreductase 1
- NF-κB
nuclear factor kappa B
- NSCLC
non-small cell lung cancer
- NMSC
nonmelanoma skin cancers
- Nrf2
nuclear factor erythroid-2-related factor 2
- OA
oleanolic acid
- PD
Parkinson’s disease
- PEITC
phenethyl isothiocyanate
- ROS
reactive oxygen species
- STAT3
signal transducer and activator of transcription 3
- SFN
sulforaphane
- TAMs
tumor-associated macrophages
- TPA
12-O-tetradecanoylphorbol-13-acetate
- UA
ursolic acid
- VEGF
vascular endothelial growth factor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest
The authors declare no conflict of interest.
References
- [1].Siegel RL, Miller KD, Jemal A, Cancer statistics, 2019, CA: a cancer journal for clinicians 69(1) (2019) 7–34. [DOI] [PubMed] [Google Scholar]
- [2].Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A, Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries, CA: a cancer journal for clinicians 68(6) (2018) 394–424. [DOI] [PubMed] [Google Scholar]
- [3].Schnekenburger M, Dicato M, Diederich M, Plant-derived epigenetic modulators for cancer treatment and prevention, Biotechnology advances 32(6) (2014) 1123–1132. [DOI] [PubMed] [Google Scholar]
- [4].Elinav E, Nowarski R, Thaiss CA, Hu B, Jin C, Flavell RA, Inflammation-induced cancer: crosstalk between tumours, immune cells and microorganisms, Nature reviews. Cancer 13(11) (2013) 759–71. [DOI] [PubMed] [Google Scholar]
- [5].Wilson LF, Antonsson A, Green AC, Jordan SJ, Kendall BJ, Nagle CM, Neale RE, Olsen CM, Webb PM, Whiteman DC, How many cancer cases and deaths are potentially preventable? Estimates for Australia in 2013, International journal of cancer 142(4) (2018) 691–701. [DOI] [PubMed] [Google Scholar]
- [6].Islami F, Goding Sauer A, Miller KD, Siegel RL, Fedewa SA, Jacobs EJ, McCullough ML, Patel AV, Ma J, Soerjomataram I, Proportion and number of cancer cases and deaths attributable to potentially modifiable risk factors in the United States, CA: a cancer journal for clinicians 68(1) (2018) 31–54. [DOI] [PubMed] [Google Scholar]
- [7].Brown KF, Rumgay H, Dunlop C, Ryan M, Quartly F, Cox A, Deas A, Elliss-Brookes L, Gavin A, Hounsome L, The fraction of cancer attributable to modifiable risk factors in England, Wales, Scotland, Northern Ireland, and the United Kingdom in 2015, British journal of cancer 118(8) (2018) 1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Surh Y-J, Cancer chemoprevention with dietary phytochemicals, Nature Reviews Cancer 3(10) (2003) 768. [DOI] [PubMed] [Google Scholar]
- [9].Lee KW, Bode AM, Dong Z, Molecular targets of phytochemicals for cancer prevention, Nature reviews. Cancer 11(3) (2011) 211–8. [DOI] [PubMed] [Google Scholar]
- [10].Lee J, Jo D-G, Park D, Chung HY, Mattson MP, Adaptive cellular stress pathways as therapeutic targets of dietary phytochemicals: focus on the nervous system, Pharmacological reviews 66(3) (2014) 815–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Kumar GP, Khanum F, Neuroprotective potential of phytochemicals, Pharmacognosy reviews 6(12) (2012) 81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Dembinska-Kiec A, Mykkänen O, Kiec-Wilk B, Mykkänen H, Antioxidant phytochemicals against type 2 diabetes, British Journal of Nutrition 99(E-S1) (2008) ES109–ES117. [DOI] [PubMed] [Google Scholar]
- [13].Tiwari AK, Rao JM, Diabetes mellitus and multiple therapeutic approaches of phytochemicals: Present status and future prospects, Current science (2002) 30–38. [Google Scholar]
- [14].Kruger MJ, Davies N, Myburgh KH, Lecour S, Proanthocyanidins, anthocyanins and cardiovascular diseases, Food Research International 59 (2014) 41–52. [Google Scholar]
- [15].Chan JYY, Yuen ACY, Chan RYK, Chan SW, A review of the cardiovascular benefits and antioxidant properties of allicin, Phytotherapy Research 27(5) (2013) 637–646. [DOI] [PubMed] [Google Scholar]
- [16].Ogle WO, Speisman RB, Ormerod BK, Potential of treating age-related depression and cognitive decline with nutraceutical approaches: a mini-review, Gerontology 59(1) (2013) 23–31. [DOI] [PubMed] [Google Scholar]
- [17].Bradford PG, Curcumin and obesity, Biofactors 39(1) (2013) 78–87. [DOI] [PubMed] [Google Scholar]
- [18].Williams DJ, Edwards D, Hamernig I, Jian L, James AP, Johnson SK, Tapsell LC, Vegetables containing phytochemicals with potential anti-obesity properties: A review, Food Research International 52(1) (2013) 323–333. [Google Scholar]
- [19].Chuang C-C, McIntosh MK, Potential mechanisms by which polyphenol-rich grapes prevent obesity-mediated inflammation and metabolic diseases, Annual review of nutrition 31 (2011) 155–176. [DOI] [PubMed] [Google Scholar]
- [20].Petronelli A, Pannitteri G, Testa U, Triterpenoids as new promising anticancer drugs, Anti-Cancer Drug 20(10) (2009) 880–892. [DOI] [PubMed] [Google Scholar]
- [21].Liby KT, Yore MM, Sporn MB, Triterpenoids and rexinoids as multifunctional agents for the prevention and treatment of cancer, Nature Reviews Cancer 7(5) (2007) 357. [DOI] [PubMed] [Google Scholar]
- [22].Bishayee A, Ahmed S, Brankov N, Perloff M, Triterpenoids as potential agents for the chemoprevention and therapy of breast cancer, Frontiers in bioscience: a journal and virtual library 16 (2011) 980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Montgomery M, Srinivasan A, Epigenetic Gene Regulation by Dietary Compounds in Cancer Prevention, Advances in Nutrition 10(6) (2019) 1012–1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Supic G, Jagodic M, Magic Z, Epigenetics: a new link between nutrition and cancer, Nutr Cancer 65(6) (2013) 781–92. [DOI] [PubMed] [Google Scholar]
- [25].Bishop KS, Ferguson LR, The interaction between epigenetics, nutrition and the development of cancer, Nutrients 7(2) (2015) 922–947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Dupont C, Armant DR, Brenner CA, Epigenetics: definition, mechanisms and clinical perspective, Seminars in reproductive medicine, © Thieme Medical Publishers, 2009, pp. 351–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Bird A, Perceptions of epigenetics, Nature 447(7143) (2007) 396. [DOI] [PubMed] [Google Scholar]
- [28].Bennett RL, Licht JD, Targeting epigenetics in cancer, Annual review of pharmacology and toxicology 58 (2018) 187–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Hardy TM, Tollefsbol TO, Epigenetic diet: impact on the epigenome and cancer, Epigenomics 3(4) (2011) 503–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Thakur VS, Deb G, Babcook MA, Gupta S, Plant phytochemicals as epigenetic modulators: role in cancer chemoprevention, The AAPS journal 16(1) (2014) 151–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Malireddy S, Kotha SR, Secor JD, Gurney TO, Abbott JL, Maulik G, Maddipati KR, Parinandi NL, Phytochemical antioxidants modulate mammalian cellular epigenome: implications in health and disease, Antioxidants & Redox Signaling 17(2) (2012) 327–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Teiten MH, Dicato M, Diederich M, Curcumin as a regulator of epigenetic events, Molecular nutrition & food research 57(9) (2013) 1619–1629. [DOI] [PubMed] [Google Scholar]
- [33].Fu S, Kurzrock R, Development of curcumin as an epigenetic agent, Cancer 116(20) (2010) 4670–4676. [DOI] [PubMed] [Google Scholar]
- [34].Guo Y, Su Z-Y, Kong A-NT, Current perspectives on epigenetic modifications by dietary chemopreventive and herbal phytochemicals, Current pharmacology reports 1(4) (2015) 245–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Shukla S, Meeran SM, Katiyar SK, Epigenetic regulation by selected dietary phytochemicals in cancer chemoprevention, Cancer letters 355(1) (2014) 9–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Yadav VR, Prasad S, Sung B, Kannappan R, Aggarwal BB, Targeting inflammatory pathways by triterpenoids for prevention and treatment of cancer, Toxins 2(10) (2010) 2428–2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Sk P, Tp S, Airao V, So R, Ap G, Neuropharmacological effects of triterpenoids, 2013.
- [38].An IS, An S, Kwon KJ, Kim YJ, Bae S, Ginsenoside Rh2 mediates changes in the microRNA expression profile of human non-small cell lung cancer A549 cells, Oncol Rep 29(2) (2013) 523–8. [DOI] [PubMed] [Google Scholar]
- [39].Dittharot K, Dakeng S, Suebsakwong P, Suksamrarn A, Patmasiriwat P, Promkan M, Cucurbitacin B Induces Hypermethylation of Oncogenes in Breast Cancer Cells, Planta Med 85(5) (2019) 370–378. [DOI] [PubMed] [Google Scholar]
- [40].Kang KA, Kim HS, Kim DH, Hyun JW, The role of a ginseng saponin metabolite as a DNA methyltransferase inhibitor in colorectal cancer cells, Int J Oncol 43(1) (2013) 228–36. [DOI] [PubMed] [Google Scholar]
- [41].Wu N, Wu G.-c., Hu R, Li M, Feng H, Ginsenoside Rh2 inhibits glioma cell proliferation by targeting microRNA-128, Acta Pharmacol Sin 32(3) (2011) 345–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Ham J, Lee S, Lee H, Jeong D, Park S, Kim SJ, Genome-Wide Methylation Analysis Identifies NOX4 and KDM5A as Key Regulators in Inhibiting Breast Cancer Cell Proliferation by Ginsenoside Rg3, Am J Chin Med 46(6) (2018) 1333–1355. [DOI] [PubMed] [Google Scholar]
- [43].Wu J, Zhao S, Tang Q, Zheng F, Chen Y, Yang L, Yang X, Li L, Wu W, Hann SS, Activation of SAPK/JNK mediated the inhibition and reciprocal interaction of DNA methyltransferase 1 and EZH2 by ursolic acid in human lung cancer cells, Journal of Experimental & Clinical Cancer Research 34(1) (2015) 99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Xiang F, Fan Y, Ni Z, Liu Q, Zhu Z, Chen Z, Hao W, Yue H, Wu R, Kang X, Ursolic Acid Reverses the Chemoresistance of Breast Cancer Cells to Paclitaxel by Targeting MiRNA-149–5p/MyD88, Front Oncol 9 (2019) 501–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Zhang L, Cai QY, Liu J, Peng J, Chen YQ, Sferra TJ, Lin JM, Ursolic acid suppresses the invasive potential of colorectal cancer cells by regulating the TGF-beta1/ZEB1/miR-200c signaling pathway, Oncol Lett 18(3) (2019) 3274–3282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Kim H, Ramirez CN, Su Z-Y, Kong A-NT, Epigenetic modifications of triterpenoid ursolic acid in activating Nrf2 and blocking cellular transformation of mouse epidermal cells, The Journal of nutritional biochemistry 33 (2016) 54–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Yang Y, Yin R, Wu R, Ramirez CN, Sargsyan D, Li S, Wang L, Cheng D, Wang C, Hudlikar R, Kuo H-C, Lu Y, Kong A-N, DNA methylome and transcriptome alterations and cancer prevention by triterpenoid ursolic acid in UVB-induced skin tumor in mice, Molecular carcinogenesis 58(10) (2019) 1738–1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Yang J, Wu R, Li W, Gao L, Yang Y, Li P, Kong A-N, The triterpenoid corosolic acid blocks transformation and epigenetically reactivates Nrf2 in TRAMP-C1 prostate cells, Molecular carcinogenesis 57(4) (2018) 512–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Chen IH, Lu M-C, Du Y-C, Yen M-H, Wu C-C, Chen Y-H, Hung C-S, Chen S-L, Chang F-R, Wu Y-C, Cytotoxic Triterpenoids from the Stems of Microtropis japonica, Journal of Natural Products 72(7) (2009) 1231–1236. [DOI] [PubMed] [Google Scholar]
- [50].Zhao X, Liu M, Li D, Oleanolic acid suppresses the proliferation of lung carcinoma cells by miR-122/Cyclin G1/MEF2D axis, Mol Cell Biochem 400(1–2) (2015) 1–7. [DOI] [PubMed] [Google Scholar]
- [51].Jutooru I, Chadalapaka G, Abdelrahim M, Basha MR, Samudio I, Konopleva M, Andreeff M, Safe S, Methyl 2-cyano-3,12-dioxooleana-1,9-dien-28-oate decreases specificity protein transcription factors and inhibits pancreatic tumor growth: role of microRNA-27a, Mol Pharmacol 78(2) (2010) 226–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Deeb D, Brigolin C, Gao X, Liu Y, Pindolia KR, Gautam SC, Induction of Apoptosis in Pancreatic Cancer Cells by CDDO-Me Involves Repression of Telomerase through Epigenetic Pathways, J Carcinog Mutagen 5 (2014) 177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Connolly JD, Hill RA, Triterpenoids, Nat Prod Rep 22(2) (2005) 230–48. [DOI] [PubMed] [Google Scholar]
- [54].Xu R, Fazio GC, Matsuda SPT, On the origins of triterpenoid skeletal diversity, Phytochemistry 65(3) (2004) 261–291. [DOI] [PubMed] [Google Scholar]
- [55].Cargnin ST, Gnoatto SB, Ursolic acid from apple pomace and traditional plants: A valuable triterpenoid with functional properties, Food Chemistry 220 (2017) 477–489. [DOI] [PubMed] [Google Scholar]
- [56].Liberty AM, Amoroso JW, Neto CC, Hart PE, Cranberry PACs and Triterpenoids: Anti-Cancer Activities in Colon Tumor Cell Lines, Acta Hortic 841 (2009) 61–66. [Google Scholar]
- [57].Escudero J, Lopez JC, Rabanal RM, Valverde S, Secondary Metabolites from Satureja Species - New Triterpenoid from Satureja-Acinos, Journal of Natural Products 48(1) (1985) 128–131. [Google Scholar]
- [58].Saratha V, Subramanian SP, Lupeol, a triterpenoid isolated from Calotropis gigantea latex ameliorates the primary and secondary complications of FCA induced adjuvant disease in experimental rats, Inflammopharmacology 20(1) (2012) 27–37. [DOI] [PubMed] [Google Scholar]
- [59].Yin R, Li T, Tian JX, Xi P, Liu RH, Ursolic acid, a potential anticancer compound for breast cancer therapy, Crit Rev Food Sci Nutr 58(4) (2018) 568–574. [DOI] [PubMed] [Google Scholar]
- [60].Liao Q, Yang W, Jia Y, Chen X, Gao Q, Bi K, LC-MS determination and pharmacokinetic studies of ursolic acid in rat plasma after administration of the traditional chinese medicinal preparation Lu-Ying extract, Yakugaku Zasshi 125(6) (2005) 509–15. [DOI] [PubMed] [Google Scholar]
- [61].Chen Q, Luo S, Zhang Y, Chen Z, Development of a liquid chromatography-mass spectrometry method for the determination of ursolic acid in rat plasma and tissue: application to the pharmacokinetic and tissue distribution study, Anal Bioanal Chem 399(8) (2011) 2877–84. [DOI] [PubMed] [Google Scholar]
- [62].Yin MC, Lin MC, Mong MC, Lin CY, Bioavailability, distribution, and antioxidative effects of selected triterpenes in mice, J Agric Food Chem 60(31) (2012) 7697–701. [DOI] [PubMed] [Google Scholar]
- [63].Zhang C, Wang C, Li W, Wu R, Guo Y, Cheng D, Yang Y, Androulakis IP, Kong A-N, Pharmacokinetics and pharmacodynamics of the triterpenoid ursolic acid in regulating the antioxidant, anti-inflammatory, and epigenetic gene responses in rat leukocytes, Molecular pharmaceutics 14(11) (2017) 3709–3717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Patlolla JM, Rao CV, Triterpenoids for cancer prevention and treatment: current status and future prospects, Curr Pharm Biotechnol 13(1) (2012) 147–55. [DOI] [PubMed] [Google Scholar]
- [65].Di Gioia F, Petropoulos SA, Phytoestrogens, phytosteroids and saponins in vegetables: Biosynthesis, functions, health effects and practical applications, Adv Food Nutr Res 90 (2019) 351–421. [DOI] [PubMed] [Google Scholar]
- [66].Shen SF, Zhu LF, Wu ZJ, Wang GK, Ahmad Z, Chang MW, Production of triterpenoid compounds from Ganoderma lucidum spore powder using ultrasound-assisted extraction, Prep Biochem Biotech (2019). [DOI] [PubMed] [Google Scholar]
- [67].Ramirez CN, Li W, Zhang C, Wu R, Su S, Wang C, Gao L, Yin R, Kong AN, In Vitro-In Vivo Dose Response of Ursolic Acid, Sulforaphane, PEITC, and Curcumin in Cancer Prevention, The AAPS journal 20(1) (2017) 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Furtado RA, Rodrigues EP, Araujo FR, Oliveira WL, Furtado MA, Castro MB, Cunha WR, Tavares DC, Ursolic acid and oleanolic acid suppress preneoplastic lesions induced by 1,2-dimethylhydrazine in rat colon, Toxicol Pathol 36(4) (2008) 576–80. [DOI] [PubMed] [Google Scholar]
- [69].Ovesna Z, Kozics K, Slamenova D, Protective effects of ursolic acid and oleanolic acid in leukemic cells, Mutat Res 600(1–2) (2006) 131–7. [DOI] [PubMed] [Google Scholar]
- [70].Janakiram NB, Indranie C, Malisetty SV, Jagan P, Steele VE, Rao CV, Chemoprevention of colon carcinogenesis by oleanolic acid and its analog in male F344 rats and modulation of COX-2 and apoptosis in human colon HT-29 cancer cells, Pharm Res 25(9) (2008) 2151–7. [DOI] [PubMed] [Google Scholar]
- [71].Deeb D, Gao X, Liu Y, Jiang D, Divine GW, Arbab AS, Dulchavsky SA, Gautam SC, Synthetic triterpenoid CDDO prevents the progression and metastasis of prostate cancer in TRAMP mice by inhibiting survival signaling, Carcinogenesis 32(5) (2011) 757–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Liby K, Risingsong R, Royce DB, Williams CR, Ma T, Yore MM, Sporn MB, Triterpenoids CDDO-methyl ester or CDDO-ethyl amide and rexinoids LG100268 or NRX194204 for prevention and treatment of lung cancer in mice, Cancer Prevention Research 2(12) (2009) 1050–1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Liby KT, Yore MM, Sporn MB, Triterpenoids and rexinoids as multifunctional agents for the prevention and treatment of cancer, Nat Rev Cancer 7(5) (2007) 357–69. [DOI] [PubMed] [Google Scholar]
- [74].Mu LH, Huang CL, Zhou WB, Guo DH, Liu P, Methanolysis of triterpenoid saponin from Ardisia gigantifolia stapf. and structure-activity relationship study against cancer cells, Bioorganic & Medicinal Chemistry Letters 23(22) (2013) 6073–6078. [DOI] [PubMed] [Google Scholar]
- [75].Wang P, Ownby S, Zhang ZZ, Yuan W, Li SY, Cytotoxicity and inhibition of DNA topoisomerase I of polyhydroxylated triterpenoids and triterpenoid glycosides, Bioorganic & Medicinal Chemistry Letters 20(9) (2010) 2790–2796. [DOI] [PubMed] [Google Scholar]
- [76].Kommera H, Kaluderovic GN, Kalbitz J, Drager B, Paschke R, Small structural changes of pentacyclic lupane type triterpenoid derivatives lead to significant differences in their anticancer properties, Eur J Med Chem 45(8) (2010) 3346–3353. [DOI] [PubMed] [Google Scholar]
- [77].Chen C-J, Shih Y-L, Yeh M-Y, Liao N-C, Chung H-Y, Liu K-L, Lee M-H, Chou P-Y, Hou H-Y, Chou J-S, Ursolic Acid Induces Apoptotic Cell Death Through AIF and Endo G Release Through a Mitochondria-dependent Pathway in NCI-H292 Human Lung Cancer Cells In Vitro, in vivo 33(2) (2019) 383–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Li Y, Xing D, Chen Q, Chen WR, Enhancement of chemotherapeutic agent-induced apoptosis by inhibition of NF-κB using ursolic acid, International journal of cancer 127(2) (2010) 462–473. [DOI] [PubMed] [Google Scholar]
- [79].Yang K, Chen Y, Zhou J, Ma L, Shan Y, Cheng X, Wang Y, Zhang Z, Ji X, Chen L, Ursolic Acid promotes apoptosis and mediates transcriptional suppression of CT45A2 gene expression in NSCLCs harboring EGFR T790M, British journal of pharmacology (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Hsu Y-L, Kuo P-L, Lin C-C, Proliferative inhibition, cell-cycle dysregulation, and induction of apoptosis by ursolic acid in human non-small cell lung cancer A549 cells, Life sciences 75(19) (2004) 2303–2316. [DOI] [PubMed] [Google Scholar]
- [81].Kim S-H, Ryu HG, Lee J, Shin J, Harikishore A, Jung H-Y, Kim YS, Lyu H-N, Oh E, Baek N-I, Ursolic acid exerts anti-cancer activity by suppressing vaccinia-related kinase 1-mediated damage repair in lung cancer cells, Scientific reports 5 (2015) 14570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Liu K, Guo L, Miao L, Bao W, Yang J, Li X, Xi T, Zhao W, Ursolic acid inhibits epithelial–mesenchymal transition by suppressing the expression of astrocyte-elevated gene-1 in human nonsmall cell lung cancer A549 cells, Anti-cancer drugs 24(5) (2013) 494–503. [DOI] [PubMed] [Google Scholar]
- [83].Liby K, Royce DB, Williams CR, Risingsong R, Yore MM, Honda T, Gribble GW, Dmitrovsky E, Sporn TA, Sporn MB, The synthetic triterpenoids CDDO-methyl ester and CDDO-ethyl amide prevent lung cancer induced by vinyl carbamate in A/J mice, Cancer Research 67(6) (2007) 2414–2419. [DOI] [PubMed] [Google Scholar]
- [84].Tran K, Risingsong R, Royce DB, Williams CR, Sporn MB, Pioli PA, Gediya LK, Njar VC, Liby KT, The combination of the histone deacetylase inhibitor vorinostat and synthetic triterpenoids reduces tumorigenesis in mouse models of cancer, Carcinogenesis 34(1) (2013) 199–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Liby K, Voong N, Williams CR, Risingsong R, Royce DB, Honda T, Gribble GW, Sporn MB, Letterio JJ, The synthetic triterpenoid CDDO-Imidazolide suppresses STAT phosphorylation and induces apoptosis in myeloma and lung cancer cells, Clin Cancer Res 12(14 Pt 1) (2006) 4288–93. [DOI] [PubMed] [Google Scholar]
- [86].Liby K, Hock T, Yore MM, Suh N, Place AE, Risingsong R, Williams CR, Royce DB, Honda T, Honda Y, Gribble GW, Hill-Kapturczak N, Agarwal A, Sporn MB, The synthetic triterpenoids CDDO and CDDO-imidazolide, are potent inducers of heme oxygenase-1 and Nrf2/ARE signaling, Cancer Res 65(11) (2005) 4789–98. [DOI] [PubMed] [Google Scholar]
- [87].To C, Ringelberg CS, Royce DB, Williams CR, Risingsong R, Sporn MB, Liby KT, Dimethyl fumarate and the oleanane triterpenoids, CDDO-imidazolide and CDDO-methyl ester, both activate the Nrf2 pathway but have opposite effects in the A/J model of lung carcinogenesis, Carcinogenesis 36(7) (2015) 769–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Kim KB, Lotan R, Yue P, Sporn MB, Suh N, Gribble GW, Honda T, Wu GS, Hong WK, Sun SY, Identification of a novel synthetic triterpenoid, methyl-2-cyano-3,12-dioxooleana-1,9-dien-28-oate, that potently induces caspase-mediated apoptosis in human lung cancer cells, Mol Cancer Ther 1(3) (2002) 177–84. [PubMed] [Google Scholar]
- [89].Mullauer FB, van Bloois L, Daalhuisen JB, Ten Brink MS, Storm G, Medema JP, Schiffelers RM, Kessler JH, Betulinic acid delivered in liposomes reduces growth of human lung and colon cancers in mice without causing systemic toxicity, Anti-cancer drugs 22(3) (2011) 223–233. [DOI] [PubMed] [Google Scholar]
- [90].Hsu T-I, Wang M-C, Chen S-Y, Huang S-T, Yeh Y-M, Su W-C, Chang W-C, Hung J-J, Betulinic acid decreases specificity protein 1 (Sp1) level via increasing the sumoylation of sp1 to inhibit lung cancer growth, Molecular pharmacology 82(6) (2012) 1115–1128. [DOI] [PubMed] [Google Scholar]
- [91].Lingaraju MC, Pathak NN, Begum J, Balaganur V, Bhat RA, Ramachandra HD, Ayanur A, Ram M, Singh V, Kumar D, Kumar D, Tandan SK, Betulinic acid attenuates lung injury by modulation of inflammatory cytokine response in experimentally-induced polymicrobial sepsis in mice, Cytokine 71(1) (2015) 101–108. [DOI] [PubMed] [Google Scholar]
- [92].Ceppi P, Mudduluru G, Kumarswamy R, Rapa I, Scagliotti GV, Papotti M, Allgayer H, Loss of miR-200c expression induces an aggressive, invasive, and chemoresistant phenotype in non-small cell lung cancer, Molecular cancer research : MCR 8(9) (2010) 1207–16. [DOI] [PubMed] [Google Scholar]
- [93].Zhang JG, Wang JJ, Zhao F, Liu Q, Jiang K, Yang GH, MicroRNA-21 (miR-21) represses tumor suppressor PTEN and promotes growth and invasion in non-small cell lung cancer (NSCLC), Clin Chim Acta 411(11–12) (2010) 846–52. [DOI] [PubMed] [Google Scholar]
- [94].Zhu X, Li H, Long L, Hui L, Chen H, Wang X, Shen H, Xu W, miR-126 enhances the sensitivity of non-small cell lung cancer cells to anticancer agents by targeting vascular endothelial growth factor A, Acta Biochim Biophys Sin (Shanghai) 44(6) (2012) 519–26. [DOI] [PubMed] [Google Scholar]
- [95].Markou A, Tsaroucha EG, Kaklamanis L, Fotinou M, Georgoulias V, Lianidou ES, Prognostic value of mature microRNA-21 and microRNA-205 overexpression in non-small cell lung cancer by quantitative real-time RT-PCR, Clinical chemistry 54(10) (2008) 1696–704. [DOI] [PubMed] [Google Scholar]
- [96].Zhao X, Liu M, Li D, Oleanolic acid suppresses the proliferation of lung carcinoma cells by miR-122/Cyclin G1/MEF2D axis, Molecular and cellular biochemistry 400(1–2) (2015) 1–7. [DOI] [PubMed] [Google Scholar]
- [97].Santos RS, Silva PL, Oliveira GP, Cruz FF, Ornellas DS, Morales MM, Fernandes J, Lanzetti M, Valenca SS, Pelosi P, Effects of oleanolic acid on pulmonary morphofunctional and biochemical variables in experimental acute lung injury, Respiratory physiology & neurobiology 179(2–3) (2011) 129–136. [DOI] [PubMed] [Google Scholar]
- [98].WHO. Breast cancer: prevention and control. Available at: https://www.who.int/cancer/detection/breastcancer/en/index1.html Accessed Jan 2, 2020.
- [99].Sjöblom T, Jones S, Wood LD, Parsons DW, Lin J, Barber TD, Mandelker D, Leary RJ, Ptak J, Silliman N, Szabo S, Buckhaults P, Farrell C, Meeh P, Markowitz SD, Willis J, Dawson D, Willson JKV, Gazdar AF, Hartigan J, Wu L, Liu C, Parmigiani G, Park BH, Bachman KE, Papadopoulos N, Vogelstein B, Kinzler KW, Velculescu VE, The Consensus Coding Sequences of Human Breast and Colorectal Cancers, Science 314(5797) (2006) 268. [DOI] [PubMed] [Google Scholar]
- [100].Pasculli B, Barbano R, Parrella P, Epigenetics of breast cancer: Biology and clinical implication in the era of precision medicine, Seminars in Cancer Biology 51 (2018) 22–35. [DOI] [PubMed] [Google Scholar]
- [101].Yang AS, Estécio MRH, Doshi K, Kondo Y, Tajara EH, Issa J-PJ, A simple method for estimating global DNA methylation using bisulfite PCR of repetitive DNA elements, Nucleic acids research 32(3) (2004) e38–e38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Ono M, Molecular links between tumor angiogenesis and inflammation: inflammatory stimuli of macrophages and cancer cells as targets for therapeutic strategy, Cancer Sci 99(8) (2008) 1501–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Tran K, Risingsong R, Royce D, Williams CR, Sporn MB, Liby K, The synthetic triterpenoid CDDO-methyl ester delays estrogen receptor-negative mammary carcinogenesis in polyoma middle T mice, Cancer Prev Res (Phila) 5(5) (2012) 726–34. [DOI] [PubMed] [Google Scholar]
- [104].de Groot AE, Pienta KJ, Epigenetic control of macrophage polarization: implications for targeting tumor-associated macrophages, Oncotarget 9(29) (2018) 20908–20927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Eades G, Yang M, Yao Y, Zhang Y, Zhou Q, miR-200a Regulates Nrf2 Activation by Targeting Keap1 mRNA in Breast Cancer Cells, 286(47) (2011) 40725–40733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Chen G, Han Y, Feng Y, Wang A, Li X, Deng S, Zhang L, Xiao J, Li Y, Li N, Extract of Ilex rotunda Thunb alleviates experimental colitis-associated cancer via suppressing inflammation-induced miR-31–5p/YAP overexpression, Phytomedicine 62 (2019) 152941. [DOI] [PubMed] [Google Scholar]
- [107].Wang X, Wang T, Yi F, Duan C, Wanwg Q, He N, Zhu L, Li Q, Deng W, Ursolic Acid Inhibits Tumor Growth via Epithelial-to-Mesenchymal Transition in Colorectal Cancer Cells, Biol Pharm Bull 42(5) (2019) 685–691. [DOI] [PubMed] [Google Scholar]
- [108].Gregory PA, Bracken CP, Smith E, Bert AG, Wright JA, Roslan S, Morris M, Wyatt L, Farshid G, Lim YY, Lindeman GJ, Shannon MF, Drew PA, Khew-Goodall Y, Goodall GJ, An autocrine TGF-beta/ZEB/miR-200 signaling network regulates establishment and maintenance of epithelial-mesenchymal transition, Mol Biol Cell 22(10) (2011) 1686–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Choi SH, Kim BG, Robinson J, Fink S, Yan M, Sporn MB, Markowitz SD, Letterio JJ, Synthetic triterpenoid induces 15-PGDH expression and suppresses inflammation-driven colon carcinogenesis, J Clin Invest 124(6) (2014) 2472–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Bai J, Xi Q, Crosstalk between TGF-β signaling and epigenome, Acta Biochimica et Biophysica Sinica 50(1) (2017) 60–67. [DOI] [PubMed] [Google Scholar]
- [111].Saha K, Hornyak TJ, Eckert RL, Epigenetic cancer prevention mechanisms in skin cancer, The AAPS journal 15(4) (2013) 1064–1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Spugnardi M, Tommasi S, Dammann R, Pfeifer GP, Hoon DS, Epigenetic inactivation of RAS association domain family protein 1 (RASSF1A) in malignant cutaneous melanoma, Cancer research 63(7) (2003) 1639–1643. [PubMed] [Google Scholar]
- [113].Tellez CS, Shen L, Estécio MR, Jelinek J, Gershenwald JE, Issa J-PJ, CpG island methylation profiling in human melanoma cell lines, Melanoma research 19(3) (2009) 146–155. [DOI] [PubMed] [Google Scholar]
- [114].Cho J, Rho O, Junco J, Carbajal S, Siegel D, Slaga TJ, DiGiovanni J, Effect of combined treatment with ursolic acid and resveratrol on skin tumor promotion by 12-O-tetradecanoylphorbol-13-acetate, Cancer prevention research 8(9) (2015) 817–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Ghosh S, Bishayee K, Khuda-Bukhsh AR, Oleanolic acid isolated from ethanolic extract of Phytolacca decandra induces apoptosis in A375 skin melanoma cells: drug-DNA interaction and signaling cascade, Journal of integrative medicine 12(2) (2014) 102–114. [DOI] [PubMed] [Google Scholar]
- [116].Reisman SA, Lee C-YI, Meyer CJ, Proksch JW, Ward KW, Topical application of the synthetic triterpenoid RTA 408 activates Nrf2 and induces cytoprotective genes in rat skin, Archives of dermatological research 306(5) (2014) 447–454. [DOI] [PubMed] [Google Scholar]
- [117].Reisman SA, Lee C-YI, Meyer CJ, Proksch JW, Sonis ST, Ward KW, Topical application of the synthetic triterpenoid RTA 408 protects mice from radiation-induced dermatitis, Radiation research 181(5) (2014) 512–520. [DOI] [PubMed] [Google Scholar]
- [118].Li W, Guo Y, Zhang C, Wu R, Yang AY, Gaspar J, Kong AN, Dietary Phytochemicals and Cancer Chemoprevention: A Perspective on Oxidative Stress, Inflammation, and Epigenetics, Chemical research in toxicology 29(12) (2016) 2071–2095. [DOI] [PubMed] [Google Scholar]
- [119].Yu S, Khor TO, Cheung KL, Li W, Wu TY, Huang Y, Foster BA, Kan YW, Kong AN, Nrf2 expression is regulated by epigenetic mechanisms in prostate cancer of TRAMP mice, PLoS One 5(1) (2010) e8579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Gupta MB, Bhalla TN, Gupta GP, Mitra CR, Bhargava KP, Anti-inflammatory activity of natural products. I. Triterpenoids, Eur J Pharmacol 6(1) (1969) 67–70. [DOI] [PubMed] [Google Scholar]
- [121].Ikeda Y, Murakami A, Ohigashi H, Ursolic acid: an anti- and pro-inflammatory triterpenoid, Mol Nutr Food Res 52(1) (2008) 26–42. [DOI] [PubMed] [Google Scholar]
- [122].Mu D, Zhou G, Li J, Su B, Guo H, Ursolic acid activates the apoptosis of prostate cancer via ROCK/PTEN mediated mitochondrial translocation of cofilin-1, Oncology letters 15(3) (2018) 3202–3206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Shin SW, Park J-W, Ursolic acid sensitizes prostate cancer cells to TRAIL-mediated apoptosis, Biochimica et Biophysica Acta (BBA) - Molecular Cell Research 1833(3) (2013) 723–730. [DOI] [PubMed] [Google Scholar]
- [124].Samudio I, Konopleva M, Hail N, Shi Y-X, McQueen T, Hsu T, Evans R, Honda T, Gribble GW, Sporn M, Gilbert HF, Safe S, Andreeff M, 2-Cyano-3,12-dioxooleana-1,9-dien-28-imidazolide (CDDO-Im) Directly Targets Mitochondrial Glutathione to Induce Apoptosis in Pancreatic Cancer, 280(43) (2005) 36273–36282. [DOI] [PubMed] [Google Scholar]
- [125].Kim Y, Suh N, Sporn M, Reed JC, An Inducible Pathway for Degradation of FLIP Protein Sensitizes Tumor Cells to TRAIL-induced Apoptosis, 277(25) (2002) 22320–22329. [DOI] [PubMed] [Google Scholar]
- [126].Giles TD, Materson BJ, Cohn JN, Kostis JB, Definition and classification of hypertension: an update, J Clin Hypertens (Greenwich) 11(11) (2009) 611–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Li J, Ichikawa T, Janicki JS, Cui T, Targeting the Nrf2 pathway against cardiovascular disease, Expert Opin Ther Targets 13(7) (2009) 785–94. [DOI] [PubMed] [Google Scholar]
- [128].Koenitzer JR, Freeman BA, Redox signaling in inflammation: interactions of endogenous electrophiles and mitochondria in cardiovascular disease, Ann N Y Acad Sci 1203 (2010) 45–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Bachhav SS, Patil SD, Bhutada MS, Surana SJ, Oleanolic acid prevents glucocorticoid-induced hypertension in rats, Phytotherapy research : PTR 25(10) (2011) 1435–9. [DOI] [PubMed] [Google Scholar]
- [130].el Karib AO, Sheng J, Betz AL, Malvin RL, The central effects of a nitric oxide synthase inhibitor (N omega-nitro-L-arginine) on blood pressure and plasma renin, Clin Exp Hypertens 15(5) (1993) 819–32. [DOI] [PubMed] [Google Scholar]
- [131].Dominiczak AF, Bohr DF, Nitric oxide and its putative role in hypertension, Hypertension 25(6) (1995) 1202–11. [DOI] [PubMed] [Google Scholar]
- [132].Bachhav SS, Bhutada MS, Patil SP, Sharma KS, Patil SD, Oleanolic Acid Prevents Increase in Blood Pressure and Nephrotoxicity in Nitric Oxide Dependent Type of Hypertension in Rats, Pharmacognosy Res 7(4) (2014) 385–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Madlala HP, Metzinger T, van Heerden FR, Musabayane CT, Mubagwa K, Dessy C, Vascular Endothelium-Dependent and Independent Actions of Oleanolic Acid and Its Synthetic Oleanane Derivatives as Possible Mechanisms for Hypotensive Effects, PLoS One 11(1) (2016) e0147395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Yates MS, Tran QT, Dolan PM, Osburn WO, Shin S, McCulloch CC, Silkworth JB, Taguchi K, Yamamoto M, Williams CR, Liby KT, Sporn MB, Sutter TR, Kensler TW, Genetic versus chemoprotective activation of Nrf2 signaling: overlapping yet distinct gene expression profiles between Keap1 knockout and triterpenoid-treated mice, Carcinogenesis 30(6) (2009) 1024–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Shin S, Wakabayashi N, Misra V, Biswal S, Lee GH, Agoston ES, Yamamoto M, Kensler TW, NRF2 modulates aryl hydrocarbon receptor signaling: influence on adipogenesis, Mol Cell Biol 27(20) (2007) 7188–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Saha PK, Reddy VT, Konopleva M, Andreeff M, Chan L, The triterpenoid 2-cyano-3,12-dioxooleana-1,9-dien-28-oic-acid methyl ester has potent anti-diabetic effects in diet-induced diabetic mice and Lepr(db/db) mice, The Journal of biological chemistry 285(52) (2010) 40581–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Li M, Han Z, Bei W, Rong X, Guo J, Hu X, Oleanolic Acid Attenuates Insulin Resistance via NF-kappaB to Regulate the IRS1-GLUT4 Pathway in HepG2 Cells, Evid Based Complement Alternat Med 2015 (2015) 643102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Li Y, Wang J, Gu T, Yamahara J, Li Y, Oleanolic acid supplement attenuates liquid fructose-induced adipose tissue insulin resistance through the insulin receptor substrate-1/phosphatidylinositol 3-kinase/Akt signaling pathway in rats, Toxicology and applied pharmacology 277(2) (2014) 155–63. [DOI] [PubMed] [Google Scholar]
- [139].Perry RJ, Samuel VT, Petersen KF, Shulman GI, The role of hepatic lipids in hepatic insulin resistance and type 2 diabetes, Nature 510(7503) (2014) 84–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Wanless IR, Lentz JS, Fatty liver hepatitis (steatohepatitis) and obesity: an autopsy study with analysis of risk factors, Hepatology 12(5) (1990) 1106–10. [DOI] [PubMed] [Google Scholar]
- [141].Wang X, Liu R, Zhang W, Zhang X, Liao N, Wang Z, Li W, Qin X, Hai C, Oleanolic acid improves hepatic insulin resistance via antioxidant, hypolipidemic and anti-inflammatory effects, Mol Cell Endocrinol 376(1–2) (2013) 70–80. [DOI] [PubMed] [Google Scholar]
- [142].do Nascimento PG, Lemos TL, Bizerra AM, Arriaga AM, Ferreira DA, Santiago GM, Braz-Filho R, Costa JG, Antibacterial and antioxidant activities of ursolic acid and derivatives, Molecules 19(1) (2014) 1317–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Zhang T, Su J, Wang K, Zhu T, Li X, Ursolic acid reduces oxidative stress to alleviate early brain injury following experimental subarachnoid hemorrhage, Neurosci Lett 579 (2014) 12–7. [DOI] [PubMed] [Google Scholar]
- [144].Li L, Zhang X, Cui L, Wang L, Liu H, Ji H, Du Y, Ursolic acid promotes the neuroprotection by activating Nrf2 pathway after cerebral ischemia in mice, Brain Res 1497 (2013) 32–9. [DOI] [PubMed] [Google Scholar]
- [145].Sahni R, Parcha V, Dobhal Y, Maithani A, Isolation, characterization of ursolic acid & its synthetic modification as new neuro-protective agent for prevention of cognition defects and oxidative damage, 2016 3(1) (2016) 9. [Google Scholar]
- [146].Rai SN, Yadav SK, Singh D, Singh SP, Ursolic acid attenuates oxidative stress in nigrostriatal tissue and improves neurobehavioral activity in MPTP-induced Parkinsonian mouse model, J Chem Neuroanat 71 (2016) 41–9. [DOI] [PubMed] [Google Scholar]
- [147].Kim H, Ramirez CN, Su ZY, Kong AN, Epigenetic modifications of triterpenoid ursolic acid in activating Nrf2 and blocking cellular transformation of mouse epidermal cells, The Journal of nutritional biochemistry 33 (2016) 54–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].Deeb D, Brigolin C, Gao X, Liu Y, Pindolia KR, Gautam SC, Induction of Apoptosis in Pancreatic Cancer Cells by CDDO-Me Involves Repression of Telomerase through Epigenetic Pathways, J Carcinog Mutagen 5 (2014) 177–177. [DOI] [PMC free article] [PubMed] [Google Scholar]


