Abstract
Background
Clinical trials have shown the ability of therapeutic vaccines to generate immune responses to tumor-associated antigens (TAAs). What is relatively less known is if this translates into immune-cell (IC) infiltration into the tumor microenvironment. This study examined whether neoadjuvant prostate-specific antigen (PSA)-targeted vaccination with PROSTVAC could induce T-cell immunity, particularly at the tumor site.
Methods
An open-label, phase II study of neoadjuvant PROSTVAC vaccine enrolled 27 patients with localized prostate cancer awaiting radical prostatectomy (RP). We evaluated increases in CD4 and CD8 T-cell infiltrates (RP tissue vs baseline biopsies) using a six-color multiplex immunofluorescence Opal method. Antigen-specific responses were assessed by intracellular cytokine staining after in vitro stimulation of peripheral blood mononuclear cells with overlapping 15-mer peptide pools encoding the TAAs PSA, brachyury and MUC-1.
Results
Of 27 vaccinated patients, 26 had matched prevaccination (biopsy) and postvaccination (RP) prostate samples available for non-compartmentalized analysis (NCA) and compartmentalized analysis (CA). Tumor CD4 T-cell infiltrates were significantly increased in postvaccination RP specimens compared with baseline biopsies by NCA (median 176/mm² vs 152/mm²; IQR 136–317/mm² vs 69–284/mm²; p=0.0249; median ratio 1.20; IQR 0.64–2.25). By CA, an increase in both CD4 T-cell infiltrates at the tumor infiltrative margin (median 198/mm² vs 151/mm²; IQR 123–500/mm² vs 85–256/mm²; p=0.042; median ratio 1.44; IQR 0.59–4.17) and in CD8 T-cell infiltrates at the tumor core (median 140/mm² vs 105/mm²; IQR 91–175/mm² vs 83–163/mm²; p=0.036; median ratio 1.25; IQR 0.88–2.09) were noted in postvaccination RP specimens compared with baseline biopsies. A total of 13/25 patients (52%) developed peripheral T-cell responses to any of the three tested TAAs (non-neoantigens); five of these had responses to more than one antigen of the three evaluated.
Conclusion
Neoadjuvant PROSTVAC can induce both tumor immune response and peripheral immune response.
Trial registration number
Keywords: vaccination; immunotherapy, active; tumor microenvironment; clinical trials as topic; urologic neoplasms
Background
Therapeutic vaccines have been investigated in patients with cancer. Clinical trials have shown their ability to generate immune responses to tumor-associated antigens (TAAs),1 2 but relatively little is known about whether this translates into more tumor-targeted immune cells getting into the tumor and, if it does, what the other effects are of this new balance of cells in the tumor microenvironment. One prior study in patients with localized prostate cancer (PC) suggested an increase in immune infiltrates at the interface of tumor and normal tissue following intravenous administration of the therapeutic vaccine sipuleucel-T.3
PROSTVAC is a therapeutic cancer vaccine designed to activate T cells specific against prostate-specific antigen (PSA).4 Previous trials have shown PROSTVAC vaccine to be safe and associated with peripheral immune responses.1 5–7 The focus of this study was to determine whether neoadjuvant vaccination with PROSTVAC could induce T-cell immunity, particularly at the tumor site, as well as any correlations with peripheral and clinical outcomes. This study was designed before the results of the recently reported phase III study of PROSTVAC in the metastatic setting with no survival benefit were known.8 CD4 and CD8 T-cell infiltrates were chosen as primary outcome measures.
Methods
Eligibility
Eligible patients had histopathologically verified localized PC and were surgical candidates for RP based on a standard workup of PSA measurement, biopsy results and imaging as needed. Patients must have chosen RP as their definitive treatment and needed to have evaluable biopsy tissue available for analysis or be willing to undergo a targeted prevaccination prostate biopsy. Patients were ≥18 years of age, had an ECOG performance status of 0–1 and had adequate organ function as defined by liver, kidney and hematological laboratory tests. Patients could have no evidence of immunocompromise or significant autoimmune disease that was active or potentially life-threatening if activated.
Study design
This phase II, single-arm study evaluated the impact of PROSTVAC vaccination in the neoadjuvant PC setting. Patients received recombinant vaccinia (rV)-PSA(L155)-TRICOM (2×108 IU subcutaneously) as a priming vaccination, followed by monthly boosts on weeks 5, 9 and 13 with recombinant fowlpox (rF)-PSA(L155)-TRICOM (1×109 IU subcutaneously). After completing the vaccination series, patients underwent RP. The boosting schedule was subsequently amended to weeks 3, 5 and 9 to allow for earlier surgeries. A final follow-up safety evaluation was performed 12–15 months postoperatively. AEs were monitored using Common Terminology Criteria for Adverse Events V.4.0. The primary objective was to examine changes in CD4 and CD8 T-cell infiltrates in tumor tissue postvaccination. Secondary objectives included investigating changes in peripheral PSA-specific T-cell responses, intraprostatic Treg infiltrates, PSA and MRI parameters postvaccination.
Multiplex immunofluorescence staining and multispectral imaging
We stained 5 µm-thick formalin-fixed paraffin-embedded sections from prostate biopsies and RP specimens using Opal multiplex 6-plex kits, according to the manufacturer’s protocol (PerkinElmer), for a panel of DAPI, CD4, CD8, FOXP3, Ki67, PanCK and PD-L1 (online supplementary methods).
H&E and multiplex immunofluorescence scans were captured by a PerkinElmer Vectra Polaris. Both biopsies and RP tissue slide scans were virtually segmented by a research pathologist into three distinct compartments: CT, tumor IM and NL. Five randomly selected regions of interest (ROI) (mm2, original magnification 40×, 0.25 µm/pixel resolution) from each compartment were then selected from each slide for multispectral imaging (MSI). Vectra Polaris captures spectral information from an MSI image using a multispectral camera, and the intensity of each fluorescent target is extracted from the multispectral data using linear unmixing. Unmixed MSI images were analyzed using inForm OS 2.3.1 software (PerkinElmer). A common algorithm was built based on five representative images, with adjustment from one batch to another due to batch variation. The algorithm consisted of cell segmentation, phenotyping and thresholding. All immune-cell infiltrates were measured as cell counts/mm² of tissue (density).
Antigen-specific T-cell responses
Antigen-specific responses were assessed by intracellular cytokine staining following a period of in vitro stimulation (IVS) of PBMCs with overlapping 15-mer peptide pools encoding the TAA PSA. The PSA peptide pool contained a previously identified agonist epitope9; pools encoding for human leukocyte antigen (HLA) and CEFT (a mixture of cytomegalovirus, Epstein-Barr virus, influenza and tetanus toxin) served as negative and positive controls, respectively. PBMCs from patients before and 60 days after therapy were stimulated by IVS and stained with antibodies to identify the absolute number of CD4+ or CD8+ lymphocytes producing cytokine (IFN-γ, TNF-α or IL-2) or positive for CD107a, as previously described.10 The background signal (obtained with the HLA peptide pool) and values obtained prevaccination were subtracted from those obtained postvaccination. Values >250 were scored as positive for TAA-specific immune response postvaccination if they were also at least twofold greater than that obtained with HLA. To explore evidence of cross-priming and development of an immune response to TAAs not found in the vaccine (antigen cascade or antigen spreading), responses to the cascade antigens MUC-1 and brachyury were tested using the same method. Peptide pools of MUC-1 and brachyury contained previously identified agonist epitopes.11 12
MRI responses
When feasible, an MRI was performed at baseline and after the last vaccination to assess for changes in the prostate and PC tumors prevaccination and postvaccination, including prostate volume, number of lesions, largest lesion length, PI-RADS score and ADC mapping.13 ADC values have been found to be decreased in various malignancies due to hypercellularity, and lower pretreatment ADC values in cervical cancer have been associated with a higher risk of recurrence.14
Statistical analysis
The primary objective was to determine whether there were changes in CD4 or CD8 cells overall, as well as within subsets based on compartments or peaks within ROIs. Ratios were formed to compare results post/pre. They were tested for whether the ratio=1 by the Wilcoxon signed rank test. Changes in averaged values over five randomly selected ROIs for the CD4 and CD8 T-cell infiltrates were considered the primary end point (p<0.025 would be significant following Bonferroni correction for these two tests, per protocol). Other measures were considered exploratory and were interpreted in the context of the number of tests performed, but without formal adjustment for multiple comparisons. All p values are reported as obtained, without adjustment for multiple comparisons. With 24 evaluable patients with paired data, the trial had 80% power to detect a change from baseline equal to 0.67 SD of the change using a 0.025 significance level paired t-test for each of the two primary measures. In practice, a Wilcoxon signed rank test was to be used if the ratios were not normally distributed. To allow for a small number of non-evaluable patients, the accrual ceiling for the trial was 27 patients.
Between October 2014 and May 2016, 27 patients were enrolled in a phase II study at the Center for Cancer Research, National Cancer Institute (table 1). The first four patients received monthly boosting vaccinations; all subsequent patients received boosts on weeks 3, 5 and 9. All patients completed the full series of vaccinations, 26/27 subsequently underwent radical prostatectomy (RP) and 1 patient elected to come off-study prior to surgery.
Table 1.
Baseline characteristics of patients on study (n=27)
| Age (years) | 64.8 (53.7–75.4) |
| Race/ethnicity | |
| White | 23 (85.2%) |
| African-American | 3 (11.1%) |
| Unknown | 1 (3.7%) |
| ECOG | 0 (–) |
| Gleason | |
| 6 | 1 (3.7%) |
| 7 | 19 (70.4%) |
| 8 | 4 (14.8%) |
| 9 | 3 (11.1%) |
| Disease stage | |
| cIA | 3 (11%) |
| cIC | 18 (66.7%) |
| cIIA | 5 (18.5%) |
| cIIB | 1 (3.7%) |
| Prostate-specific antigen (ng/mL) | 6.57 (1.44–55.82) |
| Absolute lymphocyte count (K/μL) | 1.66 (0.83–3.09) |
Data are n (%) or median (range).
ECOG, Eastern Cooperative Oncology Group.
Clinical outcomes
The treatment course was well-tolerated, with no serious adverse events (AEs) or toxicities >grade 2 attributed to vaccine (online supplementary table 1). The most common AE attributed to vaccine was a self-limited injection-site reaction (28 events in 18 patients). Two grade 3 serious AEs occurred: an infected lymphocele and a thromboembolic event, both attributed to surgery. There were no significant changes in baseline and postvaccination PSA values (online supplementary figure 1); 26/27 patients underwent baseline MRI of the prostate, and 24 had follow-up imaging. There were no significant differences between baseline and postvaccination parameters (online supplementary table 2). Apparent diffusion coefficient (ADC) changes were further examined on a per-lesion basis and found to vary in degree and even direction in different lesions from the same subject (online supplementary figure 2). Patients were seen for the final protocol-required follow-up visit 12–15 months postoperatively, after which they had the option of transferring to the care of their local physician. Therefore, long-term data on biochemical or radiographic time to recurrence are limited. A total of 4/26 evaluable patients had biochemical recurrence within 2 years of prostatectomy.
jitc-2020-000655supp001.pdf (137.2KB, pdf)
jitc-2020-000655supp002.pdf (212.5KB, pdf)
jitc-2020-000655supp003.pdf (808.6KB, pdf)
jitc-2020-000655supp004.pdf (179.6KB, pdf)
jitc-2020-000655supp005.pdf (889.4KB, pdf)
Primary end points
Tumor immune response
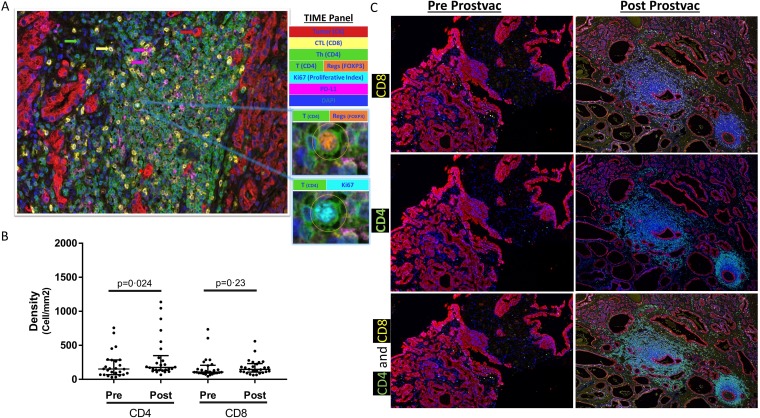
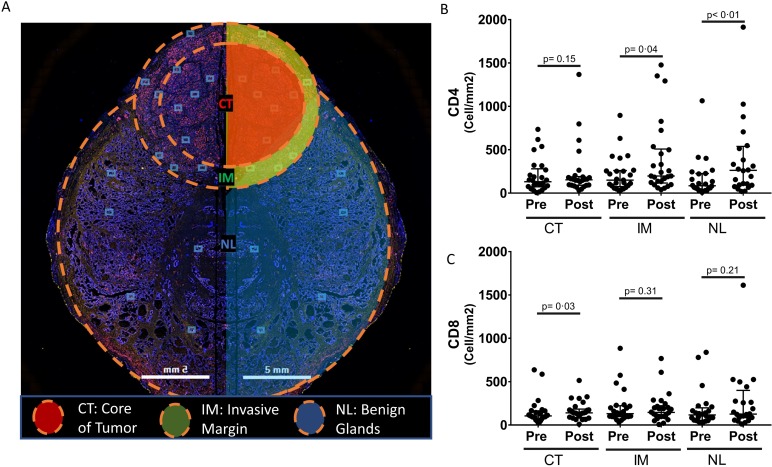
Matched prevaccination (biopsy) and postvaccination (RP) prostate samples from 26 patients were available for evaluation, all of which provided adequate tumor for analysis by two approaches: non-compartmentalized analysis (NCA) and compartmentalized analysis (CA) (see ‘Methods’ section). Two RP cases and two biopsies were missing benign lymph glands (NL), and one biopsy was missing infiltrative margins (IM) due to complete involvement by tumor. Tumor CD4 T-cell infiltrates were significantly increased in postvaccination RP specimens compared with baseline biopsies in NCA (median 176/mm² vs 152/mm²; IQR 136–317/mm² vs 69–284/mm²; p=0.0249; median ratio 1.21; IQR 0.64–2.25). Using the same analysis, CD8 T-cell infiltrates did not significantly increase postvaccination compared with baseline (median 147/mm2 vs 107/mm²; IQR 111–226/mm² vs 94–203/mm²; p=0.23 (figure 1); median ratio 1.03; IQR 0.66–1.71 (figure 1, online supplementary figure 3)). The CA showed that both CD4 and CD8 T-cell infiltrates were seen more at the IM compared with the tumor core (CT) (figure 2). In this analysis, increased CD4 T-cell infiltrates at IM (median 198/mm² vs 151/mm²; IQR 123–500/mm² vs 85–256/mm²; p=0.042; median ratio 1.44; IQR 0.59–4.17) and CD8 T-cell infiltrates at CT (median 140/mm² vs 105/mm²; IQR 91–175/mm² vs 83–163/mm²; p=0.036; median ratio 1.25; IQR 0.88–2.09) were noted in postvaccination RP specimens compared with baseline biopsies (figure 2). Similarly, an increase in CD4 T-cell infiltrates was seen postvaccination in RP specimens at NL (median 263/mm² vs 86/mm²; IQR 93–531/mm² vs 41–225/mm²; p=0.0002; median ratio 2.33; IQR 1.05–5.22) compared with baseline biopsies (figure 2, online supplementary figure 3). In fact, 21/25 patients (84%) had a >1.5-fold increase in CD4 T cells and 18/25 patients (72%) had a >1.5-fold increase in CD8 T cells in at least one of three compartments (online supplementary table 3). T-cell infiltrates were increased in the tumor core (CT) in postvaccination RP specimens compared with untreated control RP specimens (n=5) for both CD4 (median 65.17/mm²; IQR 11.79–110.4/mm²; p=0.0132) and CD8 (median 18.3/mm²; IQR 14.29–53.61/mm²; p=0.0007) (online supplementary figure 4).
Figure 1.
Cell densities in prevaccination biopsies (Pre) and in radical prostatectomy sections postvaccination (Post) with PROSTVAC. (A) Representative image of multiplex immunofluorescence panel. Insets show a cell expressing four markers (CD4, FOXP3, Ki67 and DAPI). (B) Immune-cell infiltrates were quantified in both prevaccination and postvaccination sections using inForm software. NCA of both CD4 and CD8 immune-cell density ratios was assessed by the Wilcoxon signed rank test. Median±IQR shown with horizontal lines. (C) Exceptional case with CD4 and CD8 immune-cell infiltrates prevaccination and postvaccination. CTL, cytotoxic T lymphocyte; NCA, non-compartmentalized analysis; PD-L1, programmed death-ligand 1; Th, T helper; TIME: tumor immune microenvironment.
Figure 2.
Compartmental distribution of CD4 and CD8 T cells postvaccination in intraprostatic tissue. (A) Schematic representation of radical prostatectomy (RP) section with three virtually separate compartments: tumor core (CT), invasive margin (IM) and benign glands (NL). (B, C) CD4 and CD8 T-cell infiltrate average densities were quantified as previously described using inForm software in each compartment before and after treatment with PROSTVAC vaccine. CD4 and CD8 immune-cell density ratios were assessed by the Wilcoxon signed rank test. Median±IQR shown with horizontal lines.
jitc-2020-000655supp006.pdf (884.4KB, pdf)
jitc-2020-000655supp007.pdf (211.5KB, pdf)
jitc-2020-000655supp008.pdf (825.9KB, pdf)
Heterogeneity in immune cells, tumor-infiltrating lymphocytes and peak density
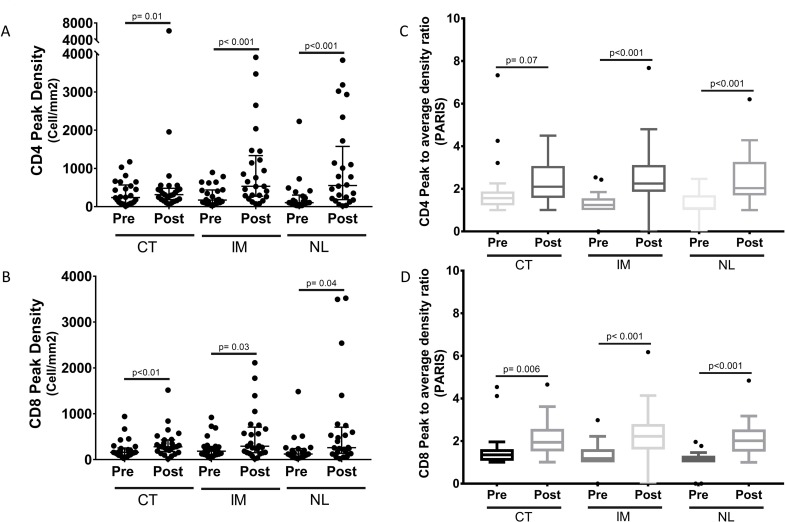
Intratumoral heterogeneity of various immune-cell infiltrate frequencies was more evident in RP specimens than in biopsies. Non-standard peak densities and a newly proposed heterogeneity score (peak to average immune score (PARIS), defined as peak density divided by average density, figure 3) were exploratory and used to assess the heterogeneity of immune infiltrates. The analysis of peak cell densities showed overall increases in CD4 and CD8 T-cell infiltrates in most compartments (p<0.05). This is particularly true for IM CD4 (median 533/mm² vs 174/mm²; IQR 287–1221/mm² vs 100–433/mm²; p=0.0001; median ratio 2.82; IQR 1.08–9.06), and NL CD4 (median 554/mm² vs 106/mm²; IQR 194–1431/mm² vs 67–276/mm²; p<0.0001; median ratio 4.65; IQR 1.99–9.85), as well as CT CD8 (median 281/mm² vs 159/mm²; IQR 186–429/mm² vs 98–243/mm²; p=0.001 (figure 3); median ratio 1.85; IQR 0.69–2.91 (figure 3)). PARIS score means for CD4 T cells postvaccination (2.26, 2.64, 2.43) were compared with prevaccination score means (2.21, 1.3, 1.25) in CT, IM and NL, respectively. PARIS score means for CD8 T cells postvaccination (2.11, 2.37, 2.10) were also compared with prevaccination score means (1.58, 1.33, 1.13) in CT, IM and NL, respectively. These scores were increased in all three compartments for both CD4 and CD8 T cells (p<0.05), except for CD4 T cells in CT (p=0.07) (figure 3).
Figure 3.
Heterogeneity scores of immune-cell infiltrates within prostatic tumor immune microenvironment (TIME) in prevaccination and postvaccination sections. (A, B) CD4 and CD8 T-cell infiltrate peak densities were quantified as previously described using inForm software in each compartment before and after immunotherapy. CD4 and CD8 immune-cell density ratios were assessed by the Wilcoxon signed rank test. Median±IQR is shown with horizontal lines. (C, D) Mean PARIS scores within each compartment in both prevaccination and postvaccination sections are shown. CD4 and CD8 cell density ratios were assessed by the Wilcoxon signed rank test. CT, tumor core; IM, infiltrative margins; NL, benign lymph gland; PARIS, peak to average immune score.
Immune-cell subsets
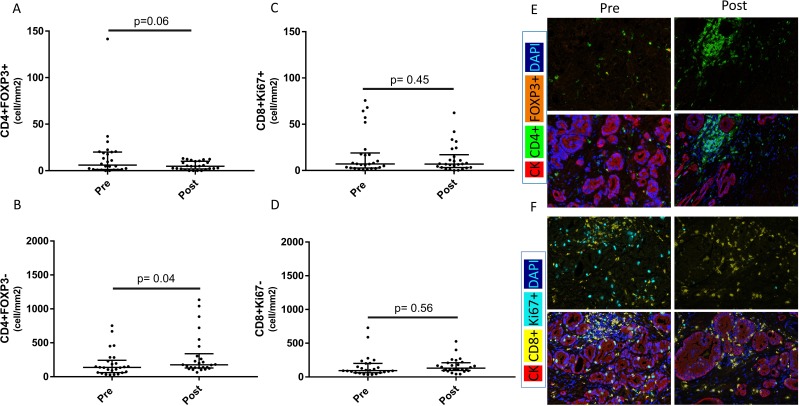
In the NCA, an increase in effector (CD4+FOXP3–) T helper cell infiltrates (mean 307/mm² vs 194/mm²; 95% CI 184–430/mm² vs 117–271/mm²; p=0.03) was seen in postvaccination RP specimens compared with baseline biopsies (figure 4). Tumor-infiltrating T-regulatory cells (Tregs) (CD4+FOXP3+) tended to substantially decrease in postvaccination RP specimens compared with baseline biopsies (mean 5/mm² vs 17/mm²; 95% CI 4–7/mm² vs 5–29/mm²; p=0.06) (figure 4). Similarly, proliferative (Ki67+) effector cytotoxic CD8 T-cell infiltrates tended to have lower density in postvaccination RP specimens compared with baseline biopsies (mean 14/mm² vs 9/mm²; 95% CI 6–23/mm² vs 4–14/mm²; p=0.44). There was no change in CD8+Ki67– T-cell infiltrates (mean 165/mm² vs 156/mm²; 95% CI 121–209/mm² vs 90–222/mm²; p=0.56) in postvaccination versus baseline specimens.
Figure 4.
Changes in immune-cell subsets within prostatic tissue before and after PROSTVAC vaccine therapy. All cell densities were quantified as previously described using inForm software. (A) Frequency of T-regulatory cells (Tregs) (CD4+FOXP3+) before and after PROSTVAC therapy in non-compartmentalized analysis (NCA). (B) Frequency of T helper cells (CD4+FOXP3–) before and after PROSTVAC therapy in NCA. (C, D) Activated (Ki67+) and non-activated (Ki67–) CD8 cytotoxic T lymphocytes were quantified using NCA in both prevaccination and postvaccination sections. For (A–D), Wilcoxon signed rank test was used. Median±IQR is shown with horizontal lines. (E, F) Representative images of all four immune-cell subsets (Tregs, T helper, activated cytotoxic T lymphocytes and non-proliferative cytotoxic T lymphocytes).
PD-L1 expression
Programmed death-ligand 1 (PD-L1) expression was focal (1%–10% on tumor cells) and only seen in 3/26 RP specimens (11.5 %) compared with 0/26 (0%) baseline specimens (online supplementary figure 5). Immune-cell PD-L1 expression was also focal and seemed to be present on macrophage-like cells. These cells were not stained with a dedicated marker or quantitated. On average, although variable by case and ROI, cases with PD-L1+ status had a higher post/pre ratio of immune-cell infiltrates (online supplementary figure 5).
jitc-2020-000655supp009.pdf (2.3MB, pdf)
Secondary end points
Antigen-specific T-cell responses
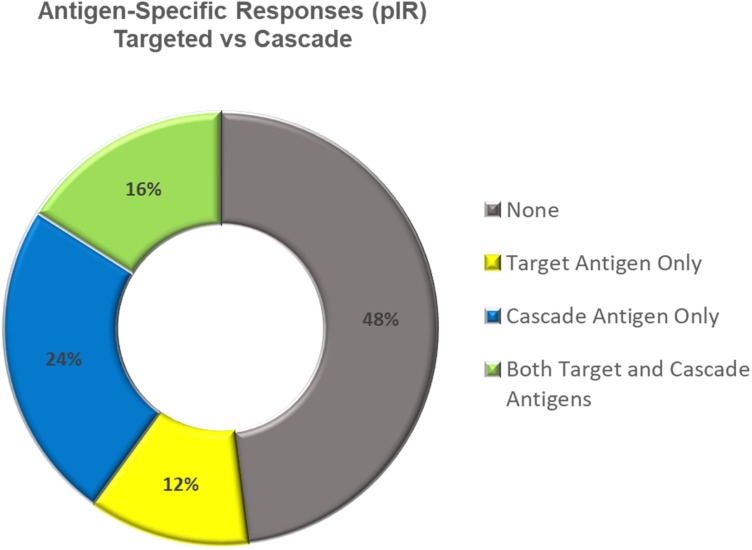
Of 25 patients evaluable for PSA-specific CD4+ and CD8+ T-cell responses, 7 (28%) developed measurable PSA-specific increases in T-cell cytokine production and/or CD107a positivity postvaccination (online supplementary table 4). Further evaluation showed that seven (28%) and six (24%) patients, respectively, developed measurable responses to the cascade antigens MUC-1 and brachyury. A total of 13/25 patients (52%) developed responses to any of the three TAAs tested, and 5 of the 13 had responses to more than one evaluated antigen (figure 5). None of the four patients who experienced early biochemical recurrence (BCR) developed antigen-specific T-cell responses to any of the TAAs tested, compared with the 12/20 responses in the non-recurrence group (p=0.093 by Fisher’s exact test). Polyfunctional TAA-specific responses, defined as CD4+ or CD8+ T cells that express >2 of the markers interferon (IFN)-γ, tumor necrosis factor (TNF)-α, interleukin (IL)-2 or CD107a, were also measured before and after vaccination. Using the criterion of a >threefold increase postvaccination versus prevaccination, or the presence of >100 polyfunctional cells postvaccination per 1×106 peripheral blood mononuclear cells (PBMCs) (if negative at prevaccination), polyfunctional T cells specific for at least one of the TAAs tested were generated in 28% of patients (online supplementary figure 6A). Representative flow plots from a patient (#22) developing multifunctional MUC1-specific CD4+ T cells after vaccination is shown in online supplementary figure 6B.
Figure 5.
Antigen-specific T-cell responses assessed by intracellular cytokine staining of peripheral blood mononuclear cells following in vitro stimulation (pIR). Responses to the vaccine target antigen prostate-specific antigen and the cascade antigens MUC-1 and brachyury were measured by increases in the number of CD4+ or CD8+ lymphocytes producing cytokines (interferon-γ, tumor necrosis factor-α or interleukin-2) or positive for CD107a. An increase of ≥twofold from baseline was considered positive. pIR, peripheral immune response.
jitc-2020-000655supp010.pdf (172.9KB, pdf)
jitc-2020-000655supp011.pdf (1.3MB, pdf)
Discussion
The vaccine was well-tolerated, making it a low-risk intervention in patients with potentially curable cancer in the neoadjuvant setting. In addition, the results of this study show an increase in T-cell infiltrates, particularly CD4 T cells, into the tumor immune microenvironment (TIME) as a direct effect of PROSTVAC vaccine in PC. While cytotoxic CD8 T cells remain the essential effector of tumor-cell killing, intratumoral CD4 T helper cells have been shown to play an essential antitumoral and immunomodulatory role.15 16 Spitzer et al also describe an essential antitumor role for peripheral CD4 T-cell subsets rather than cytotoxic CD8 cells consistent with effective immune responses in a clinical trial with ipilimumab.17 18 In our study, more than a third of patients doubled their average CD4 T-cell densities to a level similar to what is seen with other vaccines,3 but these densities remain low compared with hot tumors such as melanomas where average T-cell densities range from 500 to 2000/mm². Subset analysis showed a significant increase in T helper cells while Tregs constituted a minority of CD4 T cells. A trend to an absolute decrease in Treg density postvaccination is consistent with previously reported effects of PROSTVAC on Tregs peripherally1 19 and intratumorally.20 Since the average density of infiltrating cytotoxic CD8 T cells and their subsets (Ki67+/–) did not change postvaccination, we asked whether this effect on CD4 T cells is accompanied by a more targeted CD8 T-cell response.
The spatial distribution of certain immune subsets has been shown to be prognostic in various cancer types.21 While drawing the invasive margin for some cancer types such as PC could be challenging due to the intertwining, invasive nature of PC glands, it is clear that such a border is critical.22 This is even more challenging when examining biopsy specimens due to the limited availability of tissue. Our findings show that PROSTVAC vaccine increases CD4 T cells at the invasive margin and cytotoxic CD8 T cells in the tumor core. Such a pattern has been shown to be positively prognostic in other types of cancer. In a recent study of breast cancer using multiplexed ion beam imaging, tumors with compartmentalized immune-cell infiltrates at the IM (rather than mixed) had a better prognosis.23 Nonetheless, up to ~70% and 57.6% of patients in our study showed a >twofold increase in CD4 and CD8 T cells in at least one of the three compartments, respectively. This suggests that PROSTVAC has a modulating effect on the spatial distribution of both CD4 and CD8 T-cell subsets within the prostate TIME.
The frequency of these immune infiltrates varied within each of the three tumor compartments (intracompartmental), suggesting a spatially heterogeneous response in the TIME. Focally diffuse infiltrates (peaks) different from tertiary lymphoid structures were also noted within each compartment. Notably, this heterogeneity can be seen within the same tumor, whether primary or metastatic, as previously described.24 In this study, we proposed a non-standard TIME heterogeneity score that encompasses and compares peak densities (focal) to average density (diffuse) of these immune infiltrates within each compartment. A peak density potentially reflects the maximum activity within the TIME, despite the presence of sampling size bias when comparing a biopsy with a whole section. Increases in both peak densities and PARIS scores of both CD4 and CD8 T-cell infiltrates were seen in the vast majority of the three compartment analyses (figure 3). This highlights a heterogeneous response to PROSTVAC in the TIME compared with baseline not seen with traditional measures. The importance of such scores remains to be proven by further TIME studies with associated clinical outcomes.
The hypothesized benefit of this neoadjuvant vaccine strategy is to induce an inflammatory milieu that eventually leads to a specific immune response (memory) capable of preventing metastases. While the optimal concentration threshold of immune cells needed to elicit a translatable immune response is not known, the odds of recognizing and killing cancer cells increases proportionally with immune-cell infiltration.25 TIME studies historically used similar cutoffs ranging from around a 1.5-fold26 to a ≥3-fold increase in IC. This is particularly important when this vaccine strategy is combined with other antitumor modalities such as checkpoint inhibitors. While PD-L1 expression in prostate cancer is generally low,27 the 11% increase in tumor-cell PD-L1 after PROSTVAC remains low compared with other treatments.28 29 It is hard to compare PD-L1 status across studies in a non-assay setting, but this should not underscore the other limiting factors of this test. In addition, other immune regulatory changes in the TIME might be more relevant, as suggested by other studies.29 30 In this study, 7/25 analyzed patients (28%) developed antigen-specific T-cell responses to PSA, the vaccine’s target antigen. This PSA-targeted response seen primarily in CD4 cells may partially explain the increase in T-cell infiltrates in the NL compartment, given that PSA is expressed in normal and tumor cells. Furthermore, 10/25 patients (40%) mounted postvaccination T-cell responses to other TAAs (MUC-1, brachyury) not present in the vaccine, highlighting the importance of antigen spreading, which is potentially more clinically relevant and effective than the original targeted response.6 Interestingly, the four patients who experienced BCR did not develop any peripheral immune response, a distinction from the recurrence-free group (online supplementary table 5). No correlation with tumor immune response (tIR) or meaningful short-term clinical impacts on PSA or MRI features such as prostate volume, largest lesion size or Prostate Imaging Reporting & Data System (PI-RADS) score were seen.
jitc-2020-000655supp012.pdf (93.1KB, pdf)
PROSTVAC failed to demonstrate improved overall survival in a randomized phase III study. However, this study suggests that PROSTVAC induces systemic immune responses, and concomitant increases in immune-cell infiltrates in and around the tumor. Further studies will focus on expanding the cells and promoting their function in the TIME.31
Conclusions
This study shows that neoadjuvant PROSTVAC vaccination can induce both tumor immune response and peripheral immune response. However, the heterogeneity of the immune infiltrate in both cell subtypes and compartmental distribution highlights the complexity of analyzing the clinically relevant impact of immunotherapy in tissue. It is far from a binary process where an immune infiltrate is simply increased or not, and beneficially inflammatory or detrimentally suppressive. A T-cell infiltrate at the IM may indicate a reactive suppressive mechanism of effector cells by exclusion or a positive prognostic sign of increased T helper-cell activity. Further study of various subset markers of exhaustion, activation and checkpoint expression by multiplex immunofluorescence, along with RNA and T-cell receptor sequencing, will help to characterize these infiltrates more thoroughly, providing a better understanding of the benefits and limitations of these immune responses.
jitc-2020-000655supp013.pdf (90.3KB, pdf)
Acknowledgments
The authors would like to thank the patients involved in this study and their families. The authors would like to thank the nurses, medical/surgical oncology fellows and consultation services at the National Institutes of Health Clinical Center for their excellent patient care. The authors would also like to thank the study team at the Laboratory of Tumor Immunology and Biology, Center for Cancer Research, National Cancer Institute for technical assistance with immune assays (Angie Schwab).
Footnotes
Twitter: @chrisheery, @mbilusic, @gulleyj1
HAS and JLM contributed equally.
PAP and JLG contributed equally.
Funding: The control group of untreated RP specimens used in this study is supported by the Department of Defense Prostate Cancer Research Program, Award Nos. W81XWH-18-2-0013, W81XWH-18-2-0015, W81XWH-18-2-0016, W81XWH-18-2-0017, W81XWH-18-2-0018 and W81XWH-18-2-0019, Prostate Cancer Biorepository Network. This work was supported by the Intramural Research Program of the Center for Cancer Research, National Cancer Institute, National Institutes of Health.
Competing interests: The NCI has a collaborative research and development agreement (CRADA) with Bavarian Nordic, the manufacturer of the vaccine used in this study. Under this CRADA, resources to develop agents are provided, including the vaccine.
Patient consent for publication: Not required.
Ethics approval: The study protocol was approved by the National Cancer Institute’s Institutional Review Board, and all patients gave written informed consent according to institutional and federal guidelines.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: Data are available in a public, open-access repository (https://clinicaltrials.gov/ct2/show/NCT02153918).
References
- 1.Gulley JL, Arlen PM, Madan RA, et al. Immunologic and prognostic factors associated with overall survival employing a poxviral-based PSA vaccine in metastatic castrate-resistant prostate cancer. Cancer Immunol Immunother 2010;59:663–74. 10.1007/s00262-009-0782-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.GuhaThakurta D, Sheikh NA, Fan L-Q, et al. Humoral immune response against nontargeted tumor antigens after treatment with Sipuleucel-T and its association with improved clinical outcome. Clin Cancer Res 2015;21:3619–30. 10.1158/1078-0432.CCR-14-2334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fong L, Carroll P, Weinberg V, et al. Activated lymphocyte recruitment into the tumor microenvironment following preoperative sipuleucel-T for localized prostate cancer. J Natl Cancer Inst 2014;106:1. 10.1093/jnci/dju268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Madan RA, Arlen PM, Mohebtash M, et al. Prostvac-VF: a vector-based vaccine targeting PSA in prostate cancer. Expert Opin Investig Drugs 2009;18:1001–11. 10.1517/13543780902997928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gulley J, Chen AP, Dahut W, et al. Phase I study of a vaccine using recombinant vaccinia virus expressing PSA (rV-PSA) in patients with metastatic androgen-independent prostate cancer. Prostate 2002;53:109–17. 10.1002/pros.10130 [DOI] [PubMed] [Google Scholar]
- 6.Gulley JL, Madan RA, Tsang KY, et al. Immune impact induced by PROSTVAC (PSA-TRICOM), a therapeutic vaccine for prostate cancer. Cancer Immunol Res 2014;2:133–41. 10.1158/2326-6066.CIR-13-0108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kantoff PW, Schuetz TJ, Blumenstein BA, et al. Overall survival analysis of a phase II randomized controlled trial of a Poxviral-based PSA-targeted immunotherapy in metastatic castration-resistant prostate cancer. J Clin Oncol 2010;28:1099–105. 10.1200/JCO.2009.25.0597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gulley JL, Borre M, Vogelzang NJ, et al. Phase III trial of PROSTVAC in asymptomatic or minimally symptomatic metastatic castration-resistant prostate cancer. J Clin Oncol 2019;37:1051–61. 10.1200/JCO.18.02031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Terasawa H, Tsang K-Y, Gulley J, et al. Identification and characterization of a human agonist cytotoxic T-lymphocyte epitope of human prostate-specific antigen. Clin Cancer Res 2002;8:41–53. [PubMed] [Google Scholar]
- 10.Heery CR, Singh BH, Rauckhorst M, et al. Phase I trial of a yeast-based therapeutic cancer vaccine (GI-6301) targeting the transcription factor Brachyury. Cancer Immunol Res 2015;3:1248–56. 10.1158/2326-6066.CIR-15-0119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jochems C, Tucker JA, Vergati M, et al. Identification and characterization of agonist epitopes of the MUC1-C oncoprotein. Cancer Immunol Immunother 2014;63:161–74. 10.1007/s00262-013-1494-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tucker JA, Jochems C, Boyerinas B, et al. Identification and characterization of a cytotoxic T-lymphocyte agonist epitope of Brachyury, a transcription factor involved in epithelial to mesenchymal transition and metastasis. Cancer Immunol Immunother 2014;63:1307–17. 10.1007/s00262-014-1603-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hamoen EHJ, de Rooij M, Witjes JA, et al. Use of the prostate imaging reporting and data system (PI-RADS) for prostate cancer detection with multiparametric magnetic resonance imaging: a diagnostic meta-analysis. Eur Urol 2015;67:1112–21. 10.1016/j.eururo.2014.10.033 [DOI] [PubMed] [Google Scholar]
- 14.Nakamura K, Joja I, Nagasaka T, et al. The mean apparent diffusion coefficient value (ADCmean) on primary cervical cancer is a predictive marker for disease recurrence. Gynecol Oncol 2012;127:478–83. 10.1016/j.ygyno.2012.07.123 [DOI] [PubMed] [Google Scholar]
- 15.Antony PA, Piccirillo CA, Akpinarli A, et al. Cd8+ T cell immunity against a tumor/self-antigen is augmented by CD4+ T helper cells and hindered by naturally occurring T regulatory cells. J Immunol 2005;174:2591–601. 10.4049/jimmunol.174.5.2591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Janssen EM, Lemmens EE, Wolfe T, et al. CD4+ T cells are required for secondary expansion and memory in CD8+ T lymphocytes. Nature 2003;421:852–6. 10.1038/nature01441 [DOI] [PubMed] [Google Scholar]
- 17.Kwek SS, Kahn J, Greaney SK, et al. GM-CSF and ipilimumab therapy in metastatic melanoma: clinical outcomes and immunologic responses. Oncoimmunology 2016;5:e1101204. 10.1080/2162402X.2015.1101204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Spitzer MH, Carmi Y, Reticker-Flynn NE, et al. Systemic immunity is required for effective cancer immunotherapy. Cell 2017;168:487–502. 10.1016/j.cell.2016.12.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vergati M, Cereda V, Madan RA, et al. Analysis of circulating regulatory T cells in patients with metastatic prostate cancer pre- versus post-vaccination. Cancer Immunol Immunother 2011;60:197–206. 10.1007/s00262-010-0927-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Farsaci B, Jochems C, Grenga I, et al. Identification by digital immunohistochemistry of intratumoral changes of immune infiltrates after vaccine in the absence of modifications of PBMC immune cell subsets. Int J Cancer 2014;135:862–70. 10.1002/ijc.28743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jochems C, Schlom J. Tumor-infiltrating immune cells and prognosis: the potential link between conventional cancer therapy and immunity. Exp Biol Med 2011;236:567–79. 10.1258/ebm.2011.011007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Berthel A, Zoernig I, Valous NA, et al. Detailed resolution analysis reveals spatial T cell heterogeneity in the invasive margin of colorectal cancer liver metastases associated with improved survival. Oncoimmunology 2017;6:e1286436. 10.1080/2162402X.2017.1286436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Keren L, Bosse M, Marquez D, et al. A structured Tumor-Immune microenvironment in triple negative breast cancer revealed by multiplexed ion beam imaging. Cell 2018;174:1373–87. 10.1016/j.cell.2018.08.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Angelova M, Mlecnik B, Vasaturo A, et al. Evolution of metastases in space and time under immune selection. Cell 2018;175:751–65. 10.1016/j.cell.2018.09.018 [DOI] [PubMed] [Google Scholar]
- 25.Fridman WH, Galon J, Pagès F, et al. Prognostic and predictive impact of intra- and peritumoral immune infiltrates. Cancer Res 2011;71:5601–5. 10.1158/0008-5472.CAN-11-1316 [DOI] [PubMed] [Google Scholar]
- 26.Tarhini AA, Edington H, Butterfield LH, et al. Immune monitoring of the circulation and the tumor microenvironment in patients with regionally advanced melanoma receiving neoadjuvant ipilimumab. PLoS One 2014;9:e87705. 10.1371/journal.pone.0087705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Martin AM, Nirschl TR, Nirschl CJ, et al. Paucity of PD-L1 expression in prostate cancer: innate and adaptive immune resistance. Prostate Cancer Prostatic Dis 2015;18:325–32. 10.1038/pcan.2015.39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Calagua C, Russo J, Sun Y, et al. Expression of PD-L1 in Hormone-naïve and treated prostate cancer patients receiving neoadjuvant abiraterone acetate plus prednisone and leuprolide. Clin Cancer Res 2017;23:6812–22. 10.1158/1078-0432.CCR-17-0807 [DOI] [PubMed] [Google Scholar]
- 29.Gao J, Ward JF, Pettaway CA, et al. VISTA is an inhibitory immune checkpoint that is increased after ipilimumab therapy in patients with prostate cancer. Nat Med 2017;23:551–5. 10.1038/nm.4308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhao SG, Lehrer J, Chang SL, et al. The immune landscape of prostate cancer and nomination of PD-L2 as a potential therapeutic target. J Natl Cancer Inst 2019;111:301–10. 10.1093/jnci/djy141 [DOI] [PubMed] [Google Scholar]
- 31.Redman JM, Steinberg SM, Gulley JL. Quick efficacy seeking trial (QuEST1): a novel combination immunotherapy study designed for rapid clinical signal assessment metastatic castration-resistant prostate cancer. J Immunother Cancer 2018;6:91. 10.1186/s40425-018-0409-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
jitc-2020-000655supp001.pdf (137.2KB, pdf)
jitc-2020-000655supp002.pdf (212.5KB, pdf)
jitc-2020-000655supp003.pdf (808.6KB, pdf)
jitc-2020-000655supp004.pdf (179.6KB, pdf)
jitc-2020-000655supp005.pdf (889.4KB, pdf)
jitc-2020-000655supp006.pdf (884.4KB, pdf)
jitc-2020-000655supp007.pdf (211.5KB, pdf)
jitc-2020-000655supp008.pdf (825.9KB, pdf)
jitc-2020-000655supp009.pdf (2.3MB, pdf)
jitc-2020-000655supp010.pdf (172.9KB, pdf)
jitc-2020-000655supp011.pdf (1.3MB, pdf)
jitc-2020-000655supp012.pdf (93.1KB, pdf)
jitc-2020-000655supp013.pdf (90.3KB, pdf)