Abstract
Background and purpose
Resource-limited countries face challenges in setting up effective pharmacovigilance systems. This study aimed to monitor the occurrence of adverse events (AEs) after the use of artemisinin-based combination therapies (ACTs), identify potential drivers of reporting suspected adverse drug reactions (ADRs) and monitor AEs among women who were inadvertently exposed to ACTs in the first trimester of pregnancy.
Patients and methods
We conducted a prospective observational study from May 2010 to July 2012 in Nanoro Health and Demographic Surveillance System (HDSS), Burkina Faso. The HDSS area was divided into active and passive surveillance areas to monitor AEs among patients (regardless of age or sex) who received a first-line ACT (artemether–lumefantrine or artesunate–amodiaquine). In the active surveillance area, patients were followed up for 28 days, while in the passive surveillance area, patients were encouraged to return voluntarily to the health facility to report any occurrence of AEs until day 28 after drug intake. We assessed the crude incidence rates of AEs in both cohorts and performed Cox regression with mixed random effects to identify potential drivers of ADR occurrence.
Results
In total, 3170 participants were included in the study. Of these, 40.3% had reported at least one AE, with 39.6% and 44.4% from active and passive surveillance groups, respectively. The types of ADRs were similar in both groups. The most frequent reported ADRs were anorexia, weakness, cough, dizziness and pruritus. One case of abortion and eight cases of death were reported, but none of them was related to the ACT. The variance in random factors showed a high variability of ADR occurrence between patients in both groups, whereas variability between health facilities was low in the active surveillance group and high in passive surveillance group. Taking more than two concomitant medications was associated with high hazard in ADR occurrence, whereas the rainy season was associated with low hazard.
Conclusion
This study showed that both passive and active surveillance approaches were useful tools. The HDSS allowed us to capture a few cases of exposure during the first trimester of pregnancy. The passive surveillance approach, which is more likely to be implemented by malaria control programs, seems to be more relevant in the Sub-Saharan African context.
Keywords: HDSS, artemisinin-based combination therapies, safety, pregnancy, malaria, rural, Burkina Faso
Introduction
Prior to the registration and marketing of a new drug, available data on safety and efficacy are limited to observations from pre-clinical animal studies and pre-registration clinical trials (i.e. Phase I–III). Since the thalidomide case in 1961,1 it has been recognized unanimously that post-licensure safety data on newly registered active substances are crucial for evaluating their risk/benefit profile over time. Pharmacovigilance (PV), which is “the science and activities relating to the detection, assessment, understanding and prevention of adverse effects or any other possible drug-related problem”,2 remains the cornerstone to fully and adequately assess the safety of the drugs when they are used in “real-life” conditions among a large number of people. However, setting up a PV system is difficult, even in developed countries which are usually characterized by effective healthcare systems.3,4 It is unclear how such a system can be efficiently set up and managed in Sub-Saharan African (SSA) countries characterized by poorly performing healthcare systems and limited resources.5–8
Nevertheless, we should recognize that the medical product regulatory framework and the PV landscape in Africa are currently improving.9 In Burkina Faso, a National Pharmacovigilance Monitoring Committee was created in 2011 within the drug regulatory authority, named “Direction Générale de la Pharmacie du Médicament et des Laboratoires (DGPML)”, under the Ministry of Health, although the organization of PV activities was established in 2008 with the traditional spontaneous reporting system. In 2010, the country became officially a full member of the World Health Organization (WHO) Program for International Drug Monitoring. However, as in most SSA countries, the PV system based on spontaneous reporting by health professionals in Burkinabe healthcare settings has not yet reached an appropriate performance level, as the reporting rates are still very low.10,11 In 2015, only 72 reports were referenced in VigiBaseTM (the WHO global individual case safety reports database).12 In SSA countries in general and Burkina Faso in particular, the factors that could explain such low spontaneous reporting are numerous and complex. Some authors have mentioned important health system obstacles to PV growth in Africa, including weak overall national health infrastructure and systems, poor understanding of PV, lack of PV in the formal curriculum of health professionals' training and low interest by healthcare professionals.10,13-16
Over the past decade, Burkina Faso has experienced a large-scale deployment of new essential drugs, especially artemisinin-based combination therapies (ACTs) for the treatment of uncomplicated malaria.17 Although ACTs have been used successfully in Southeast Asia18 and other SSA countries,19–23 where they appear to be safe and well-tolerated, there is relatively limited experience with these medicines in Burkina Faso, where malaria transmission intensity is substantially higher. In addition, the pattern of antimalarial medicine use in real-life is quite different.24–26 Despite this large-scale deployment of ACTs, the National Malaria Control Program (NMCP) had established neither a PV unit nor any staff specifically dedicated to PV of antimalarial drugs.16 Meanwhile, the current malaria treatment guidelines and the training modules on malaria do not take PV into account; therefore, no information on adverse drug reactions (ADRs) was collected by the NMCP. In addition, the safety of ACTs when used in standard conditions in vulnerable populations, including pregnant women during the first trimester of pregnancy and patients with a concomitant chronic illness, is of concern. A study conducted in Burkina Faso revealed that about half of drugs used (including ACTs) with regard to trimester of pregnancy could be considered as potentially risky based on the United States Food and Drug Administration (FDA) or Australian Therapeutic Goods Administration (TGA) drug risk classification.27 These situations are necessarily accompanied by a crucial need to promote and ensure the safety and efficacy of these drugs. In this study, we propose a practical approach for PV through a Health and Demographic Surveillance System (HDSS) to monitor actively and passively the safety of the two first-line antimalarial treatments (artemether–lumefantrine [AL] and artesunate–amodiaquine [ASAQ])28 in real-life conditions. Since developing countries are facing several difficulties in setting up functional PV systems, this platform provided the opportunity to easily monitor and assess public health issues such as PV while significantly reducing the costs of implementation.29,30 The aim of this study was to monitor passively versus actively adverse events (AEs) among the population living in Nanoro HDSS of Burkina Faso, who attended public health facilities and received an ACT (ASAQ or AL), and to assess the factors associated with the time of reporting a suspected ADR occurrence. In addition, through HDSS, the study aimed to monitor AEs in pregnant women who were inadvertently exposed to ACTs in the first trimester of pregnancy.
Ethics
Ethical approval for the study was granted by Centre Muraz Institutional Ethical Committee (ref. no. 03-2010C/E-CM), Burkina Faso National Ethics Committee for Research in Health (no. 2010–27) and WHO research Ethic Review Committee (WHO/TDR-A70283). The study was registered in the ClinicalTrials.gov registry (identifier: NCT01232530). Written informed consent was obtained from all participants prior to their enrolment. Participants who fulfilled the inclusion criteria and agreed to be enrolled in the study were asked to provide written inform consent. For illiterate participants, an independent witness was asked to attend the consent procedures and then attest and sign the consent. A parent or legal guardian provided written informed consent on behalf of any patient under 18 years of age. All procedures followed were carried out in accordance with the Helsinki Declaration as revised in 2013.
Materials and Methods
Study Site
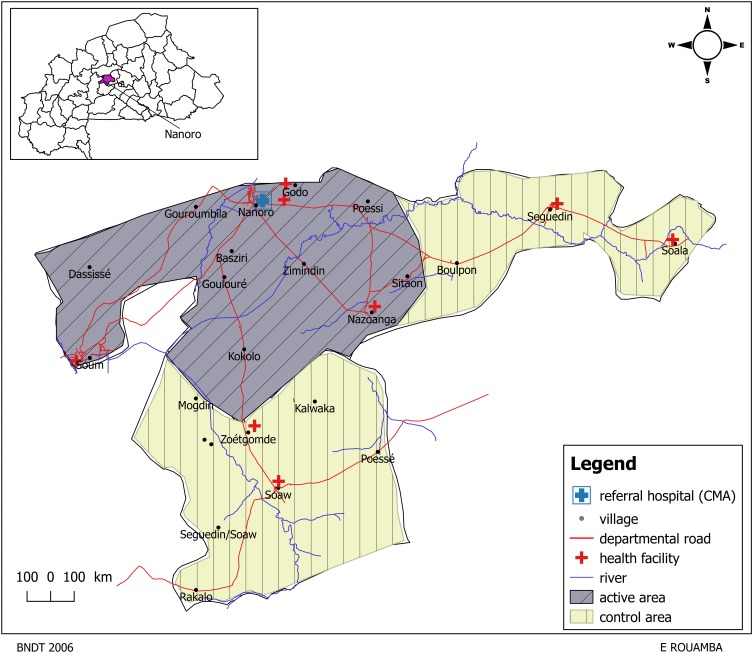
The study was carried out in the Nanoro HDSS catchment area, which is located in a rural setting, in the centre-west region of Burkina Faso, approximately 85 km from Ouagadougou, the capital city. The HDSS area (594.3 km2) lies between longitudes 1°892537 and 2°83146 west and latitudes 12°857955 and 12°872863 north, and includes a total of 24 villages and six peripheral health facilities (Figure 1). Details of the study area and population have been described elsewhere.31,32 The area has hot weather throughout the year (mean temperature >30°C) and two seasons. The rainy season occurs from June to November (average rainfall: 700 mm/year) while the dry season lasts from December to May. In the baseline of the initial census carried out in 2009, 54,781 individuals were recorded (52,502 residents and 2,279 visitors), of whom 56.1% were female. Vital events such as pregnancies, births, migrations and deaths are monitored on a weekly basis. Data on individuals and household characteristics are updated during regular 4-monthly household visits. Malaria incidence is seasonal (three periods of incidence)33 and remains the main disease in this area, accounting for about 45.0% of all consultations and 52.1% of hospitalizations. In this area, the level of adherence to first-line antimalarial drugs seems to be good (86.0%).34
Figure 1.
Location of the study area showing active area (active surveillance) and control area (passive surveillance).
Study Design, Study Population and Sample Size Justification
This was a prospective observational study conducted between May 2010 and July 2012 among the population living in the Nanoro HDSS catchment area, Burkina Faso. The study population consisted of inhabitants living in the health facilities catchment area of Godo, Nanoro, Nazoanga, Seguedin, Soum and Soaw, regardless of age and gender. For routine PV, there is no sample size calculation because when a new drug policy is implemented, the whole population is followed up. Nevertheless, it was essential to provide a clue about the expected number of ACT treatments. In Nanoro health district, according to the health information system, the rate of health facility attendance was about 40%. Considering that about 60% of patients were treated for malaria, we assumed there were about 12,600 treatments per year overall. From previous experience, we expected that approximately 80% of them would agree to participate in the study. Therefore, we had to observe approximately 10,080 treatments per year or 20,160 treatments for 2 years, which was adequate to detect “rare” adverse events (incidence occurring at a frequency of 1 out of 1000 patients or more) with a 95% probability.
Study Participants' Enrolment, Follow-Up and Data Collection
Enrolment and follow-up of participants were defined according to two approaches of surveillance : active surveillance and passive surveillance. To do this, the HDSS area was subdivided into two parts (ratio 1:1) by taking into account the size of the population and the number of health facilities (Table 1). In both areas, we also implemented surveillance of women who were inadvertently exposed to ACTs in the first trimester of pregnancy. Alongside these surveillances, a nested study which had assessed the efficacy of AL versus ASAQ in real-life conditions of use for treatment of uncomplicated malaria among patients of all age groups was set up. The results relating to this study have been reported elsewhere.35 Patients' enrolment and follow-up, data collection, as well as the assessment of the relationship between ACT and AE occurrence, were performed by trained healthcare workers (study staff: physicians, nurses, lab technicians and village reporters).
Table 1.
Resident Population Size by Village, According to the Two Approaches of Surveillance
| Active Surveillance | Passive Surveillance | ||||
|---|---|---|---|---|---|
| HF Area | Village | Population | HF Area | Village | Population |
| Nanoro | Nanoroa,b | 5127 | Seguedin | Seguedina | 3488 |
| Poéssi | 2146 | Soala | 2258 | ||
| Gouroubila | 603 | Boulpon | 3570 | ||
| Basziri | 1655 | Godo | Godoa | 1268 | |
| Goulouré | 2331 | Soaw | Rakalo | 1982 | |
| Soum | Souma | 4167 | Seguedin Soaw | 984 | |
| Dassisse | 1471 | Kolokom | 431 | ||
| Nazoanga | Nazoangaa,b | 4292 | Bokin | 229 | |
| Sitaon | 1041 | Mogdin | 888 | ||
| Zimidin | 1507 | Mogdin | 888 | ||
| Kokolo | 1164 | Zoetgomde | 763 | ||
| Poesse | 3457 | ||||
| Kalwaka | 1770 | ||||
| Soawa | 5910 | ||||
| Total | 25,504 | Total | 26,998 | ||
Notes: aVillage where the health facility is located. bHealth facility where the efficacy of artemether–lumefantrine versus artesunate–amodiaquine in real conditions of use for treatment of uncomplicated malaria was assessed.
Abbreviation: HF, health facility.
In the Passive Surveillance Area
This surveillance included the resident population of the health facilities' catchment areas of Godo, Seguedin and Soaw. Patients attending the health facilities in these areas, diagnosed with malaria (presumptively or rapid diagnostic test [RDT]/microscopically confirmed) and treated with an ACT were identified. The treatment administered was recorded in a drug exposure log book and the patients were encouraged to report passively any AEs/ADRs occurring within 28 days after drug intake. When a patient voluntarily returned to report the occurrence of events, his or her informed consent was required prior to the collection of clinical symptoms and concomitant medications.
In the Active Surveillance Area
This surveillance included the resident population of the health facilities' catchment areas of Nanoro, Nazoanga and Soum. All patients with a diagnosis of malaria (presumptive or RDT/microscopically confirmed) and treated with an ACT were included and actively followed up (after obtaining their informed consent) at days 7, 14 and 28. At each visit, data on medical history, clinical condition and concomitant treatments were collected. In addition, patients were encouraged to return to the health facility at any time outside the scheduled visits if they experienced any health problem.
Surveillance of Pregnant Women Inadvertently Exposed to ACTs in the First Trimester of Pregnancy
Pregnant women were identified during the regular 4-monthly household visits and during a continuous weekly vital event monitoring by HDSS village reporters. The list of pregnant women was matched with the drug exposure log book in order to detect possible exposure to ACTs during the first trimester. All suspected cases of exposure to an ACT during the first trimester were included (if informed consent was obtained) in the pregnancy cohort after exposure confirmation and followed up until delivery to record all medical events and pregnancy outcomes.36
For this study, no study drug was administered directly to the patients by the study staff. Patients had obtained their prescriptions from the health facility staff as routinely done, and then obtained their drugs from the public health facility drug store. At enrolment, sociodemographic data, such as the age, gender and educational level, as well as the weight of each patient, were collected; information regarding ACTs administered, such as brand and generic name, whether the medicine was prescribed or over the counter (OTC), manufacturing and expiry dates, batch number, daily dose (see Table S1 in supplementary material), concomitant medication including traditional or herbal medicine and concomitant illness was also collected (if available). During the surveillance (active or passive group and pregnant women), at each contact, data on clinical condition and concomitant treatments were collected by the study staff.
Identification, Reporting of Adverse Events (Individual Case Safety Reports) and Explanatory Variables
An AE was defined as any untoward medical occurrence, irrespective of its suspected relationship to the study medications, as per International Conference on Harmonization (ICH) guidelines for Good Clinical Practice (GCP).37 The primary endpoint of interest for this study was clinical safety in standard conditions of use, assessed through the incidence rate of AEs occurring in both cohorts within 28 days after the first dose of ACT was taken.
The patients were assessed by the study staff according to a standardized checklist and the information was recorded on an individual case report form. AEs were documented as described by the participant or caregiver, reviewed and coded using the Medical Dictionary for Regulatory Activities (MedDRA®) system organ class (SOC). A severity grading scale, based on the toxicity grading scales developed by the WHO (Toxicity Grading Scale for Determining the Severity of Adverse Events) and the National Institutes of Health, Division of Microbiology and Infectious Diseases,38 was used to grade the severity of all symptoms.
The relationship between ACT and AEs, regardless of their severity, was assessed by the local investigator (study physicians) on the basis of clinical judgment, possible alternative causes (e.g. concomitant therapy, concomitant disease), time of occurrence relative to the treatment and available information on the antimalarial drug. Therefore, AEs were classified into two categories: unsuspected (when the event relationship met the classification of “definitely unrelated” or “unlikely”) or suspected (when the event relationship met the classification of “possible”, “probable” or “certain”).
Notification of the occurrence of serious adverse events (SAEs), regardless of their relationship to the ACT, was transmitted to the national ethics committee, the DGPML (according to their standard AE forms for PV) and the study sponsor within 24 hours.
Data Management and Statistical Analysis
Data were captured using individual paper case report forms and then double-entered and verified using a database designed under MySQL 5. Statistical analyses were performed using R® software (R Foundation for Statistical Computing, Vienna, Austria). Descriptive analyses were implemented as required in each cohort. Numerical variables were summarized into median and interquartile range (IQR). Categorical variables were summarized using cross-tabulation. The Wilcoxon test or chi-squared test was used the compare the populations’ characteristics at baseline. The estimates of the incidence of each AE in both cohorts were performed based on crude rates. A reported AE with a relationship classified as “possible”, “probable” or “certain” was classified as a suspected ADR and considered as a dependent variable. The independent variables or covariates included sex, age, symptoms reported at day 0, concomitant drug prescribed at day 0, history of drug use and malaria transmission season. To account for patient-specific random effects and within-health-facility a possible homogeneity in ADR occurrence and reporting, a Cox regression model with mixed random effects (frailty model) was used to assess the effect (association) separately in both cohorts. We stated that the data have two levels, and the binary response variable (ADR occurrence) for the patient is nested within the health facility. Therefore, the hazard function of ADR occurrence for patient i in health facility j at time t is specified as follows:
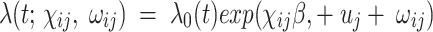 |
where  are the observed values for the covariates, whereas β is the model fixed effect coefficient associated with the covariates. In this formula,
are the observed values for the covariates, whereas β is the model fixed effect coefficient associated with the covariates. In this formula, 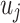 represents the health facility-specific random effect and measures the difference between the average hazard at health facility j and the average hazard in the entire Nanoro HDSS area. The term
represents the health facility-specific random effect and measures the difference between the average hazard at health facility j and the average hazard in the entire Nanoro HDSS area. The term 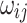 is the patient-specific random effect, i.e. the deviation of the
is the patient-specific random effect, i.e. the deviation of the 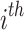 patient from the average for the
patient from the average for the 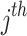 health facility.
health facility.
We performed a frailty model to determine the strength of association between the occurrence of suspected ADR and each independent variable listed above. All independent variables which exhibited a p-value <0.2 in the univariate regression were included in a Cox multivariable analysis.
The analysis was performed by excluding the patients (included in the study) with missing data on the outcome variable. Then, sensitivity analysis including patients by assuming that they experienced an ADR at the average time (estimated through Kaplan–Meier analysis) for a patient to report an AE after drug administration was performed. Likewise, sensitivity analysis including patients with missing data regarding the outcome by assuming that they did not experience any ADR during the 28 days of follow-up was performed. The estimates were presented using the mean of hazard ratios (HRs) and their 95% CI (confidence interval).
Results
Trial Profile and Baseline Characteristics of the Study Participants
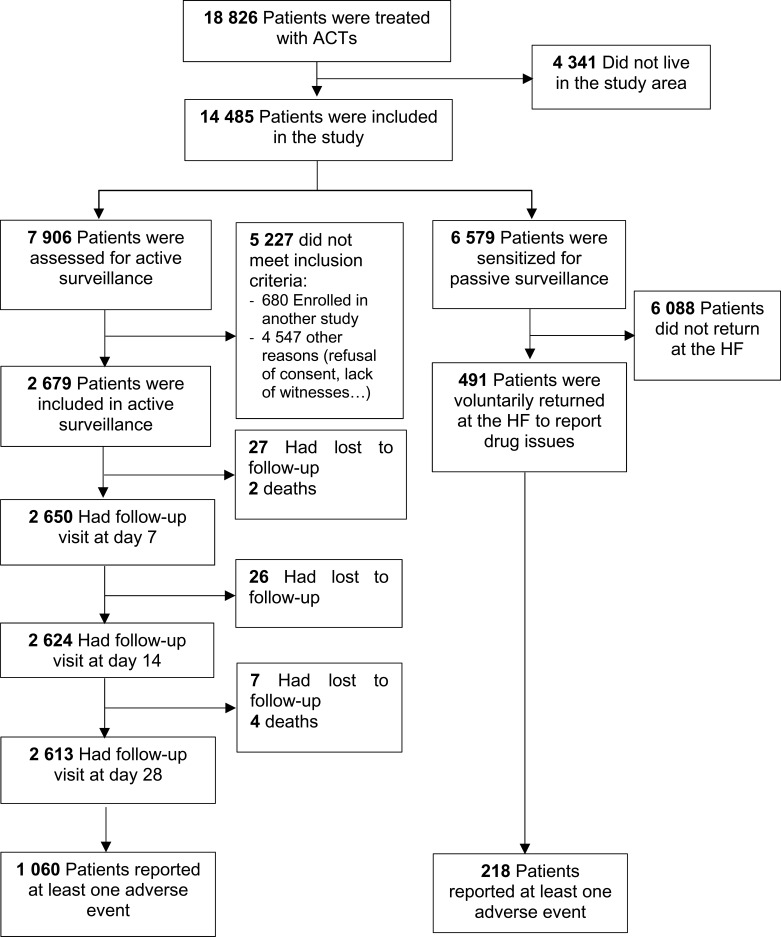
During the period of the study, ASAQ Winthrop® was the only available ACT in the public health facilities, despite the adoption of two first-line treatments. Figure 2 describes the trial profile. A total of 18,826 participants with uncomplicated malaria received a first-line antimalarial drug in the health facilities within the HDSS catchment area. Of these, 76.9% (14,485/18,826) who were permanent residents in the study area were screened to participate in the study. Among them, 7906 and 6579 were residents in the active and passive surveillance areas, respectively. A total of 2679 patients was enrolled inthe active surveillance cohort (because 680 were enrolled in another study and 4547 met other reasons for exclusion [refusal to consent, lack of witnesses to attend the consent process, etc.]); among these, 2613 (97.5%) completed the follow-up of 28 days. However, in the passive surveillance area, only 7.5% (491/6579) returned spontaneously to the health facility to report the treatment outcome. Therefore, in both cohorts, a total of 3170 patients was included in the analysis.
Figure 2.
Trial profile.
The distribution of baseline characteristics of the participants at enrolment was similar in the two surveillance groups, except for the history of fever and axillary temperature (Table 2).
Table 2.
Demographic and Clinical Characteristics of Study Participants at the Time of Enrolment
| Parameters | Total (N=3170) | Cohort | ||
|---|---|---|---|---|
| Active (n=2679) | Passive (n=491) | p value | ||
| Age (years) | ||||
| Median (25;75% IQR) | 3.5 (1.4–9.9) | 3.3 (1.4–8.1) | 3.7 (1.4–11.6) | 0.19 |
| Age class (years), n (%) | ||||
| 0–5 | 2017 (63.6) | 1727 (64.5) | 290 (59.1) | 0.007 |
| 6–13 | 575 (18.1) | 491 (18.3) | 84 (17.1) | |
| 14–17 | 127 (4.1) | 102 (3.8) | 25 (5.1) | |
| ≥18 | 451 (14.2) | 359 (13.4) | 92 (18.7) | |
| Gender, n (%) | 0.15 | |||
| Female | 1543 (48.7) | 1289 (48.1) | 254 (51.7) | |
| Male | 1627 (51.3) | 1390 (51.9) | 237 (48.3) | |
| Symptoms, n (%) | ||||
| Fever in previous 24 hours | 2634 (83.1) | 2554 (95.3) | 80 (16.3) | <0.001 |
| Temperature ≥37.5°C | 2208 (69.6) | 2107 (78.6) | 101 (20.6) | <0.001 |
| Headache | 627 (23.4) | – | ||
| Asthenia | 49 (1.8) | – | ||
| Muscle joint pain | 211 (7.9) | – | ||
| Anorexia | 134 (5.0) | – | ||
| Vomiting | 440 (16.4) | – | ||
| Nausea | 24 (0.9) | – | ||
| Dizziness | 43 (1.6) | – | ||
| ≥3 symptoms | 825 (30.8) | – | ||
| Quantity of concomitant medicationa | 2 (1–3) | 2 (1–3) | 2 (1–2) | 0.21 |
| Prior history of medicine use, n (%) | 796 (25.2) | 671 (25.0) | 125 (25.5) | 0.89 |
| Season, n (%) | 0.23 | |||
| Low | 1260 (45.7) | 960 (45.1) | 300 (47.9) | |
| High | 1495 (54.3) | 1169 (54.9) | 326 (52.1) | |
| Level of educationb | 0.79 | |||
| No formal education | 453 (60.2) | 386 (60.5) | 67 (58.3) | |
| Primary | 262 (34.8) | 219 (34.3) | 43 (37.4) | |
| Secondary | 38 (5.0) | 33 (5.2) | 5 (4.3) | |
Notes: aExcluding antimalarial drugs (artesunate–amodiaquine) taken at day 0. (See Table S2 in supplementary material for more details.) bThe denominator included only subjects above 7 years old.
Abbreviation: IQR, interquartile range.
Reporting of Adverse Events Through Active and Passive Surveillance
By the end of the follow-up, at least one AE was reported in 40.3% (n/N = 1278/3170 [95% CI 38.6–42.0]) of the whole study patients, 39.6% (n/N = 1060/2679 [95% CI 37.7–41.4]) and 44.4% (n/N = 218/491 [95% CI 40.0–48.8]) in the active and passive surveillance groups, respectively. Out of the 2758 AEs (2129 and 629 in active and passive groups, respectively) reported, 76 (52 and 24 in active and passive groups, respectively) were classified as SAEs. The most frequently reported events, classified by SOC, were general disorders (22.2%), gastrointestinal disorders (17.5%), and respiratory, thoracic and mediastinal disorders (11.0%). The incidence rates of AEs reported by SOC are summarized in Table 3.
Table 3.
Incidence Rate of Events Reported by System Organ Class (Grouped by MedDRA Coding) in the Two Cohorts
| MedDRA System Organ Class | Total (N=3170) | Active Cohort (n=2679) | Passive Cohort (n=491) | |||
|---|---|---|---|---|---|---|
| na | IRb | na | IRb | na | IRb | |
| General disorders | 703 | 221.8 | 568 | 212.0 | 135 | 274.9 |
| Gastrointestinal disorders | 551 | 173.8 | 415 | 154.9 | 136 | 277.0 |
| Respiratory, thoracic and mediastinal disorders | 348 | 109.8 | 290 | 108.2 | 58 | 118.1 |
| Ear and labyrinth disorders | 252 | 79.5 | 208 | 77.6 | 44 | 89.6 |
| Psychiatric disorders | 207 | 65.3 | 134 | 50.0 | 73 | 148.7 |
| Skin and subcutaneous tissue disorders | 66 | 20.8 | 59 | 22.0 | 7 | 14.3 |
| Musculoskeletal and connective tissue disorders | 61 | 19.2 | 49 | 18.3 | 12 | 24.4 |
| Blood and lymphatic system disorders | 32 | 10.1 | 26 | 9.7 | 6 | 12.2 |
| Nervous system disorders | 30 | 9.5 | 19 | 7.1 | 11 | 22.4 |
| Eye disorders | 21 | 6.6 | 15 | 5.6 | 6 | 6.1 |
| Renal and urinary disorders | 9 | 2.8 | 8 | 3.0 | 1 | 2.0 |
| Pregnancy, puerperium and perineal conditions | 2 | 0.6 | 2 | 0.7 | 0 | 0 |
Notes: aIndicates the number of events; a patient could have one or more events at a time. bIndicates the incidence rate per 1000 (n/N).
During the follow-up through the study period in both groups, 60.2% of AEs reported were classified as mild in term of intensity (severity grading) of AE. The different SAEs were mainly represented by hospitalization (1.9%), death (0.2%) and life-threatening events (0.1%). However, all of the deaths were considered as unrelated to ASAQ. The safety outcomes reported in both cohorts are summarized in Table 4.
Table 4.
Safety Outcomes
| Event | Total (N=3170) | Active Cohort (n=2679) | Passive Cohort (n=491) | ||||
|---|---|---|---|---|---|---|---|
| na | IRb | na | IRb | na | IRb | ||
| Reason to consider event as serious | Death | 8 | 2.5 | 6 | 2.2 | 2 | 4.1 |
| Life-threatening | 6 | 1.9 | 6 | 2.2 | 0 | 0.0 | |
| Hospitalization | 61 | 19.2 | 39 | 14.6 | 22 | 44.8 | |
| Disability/incapacityc | 1 | 0.3 | 1 | 0.4 | 0 | 0.0 | |
| Any SAE during 28 days of follow-up | 76 | 24.0 | 52 | 19.4 | 24 | 48.9 | |
| SAE intensity | Moderate | 12 | 3.8 | 9 | 3.4 | 3 | 6.1 |
| Severe | 52 | 16.4 | 31 | 11.6 | 21 | 42.8 | |
| Life-threatening | 12 | 3.8 | 12 | 4.5 | 0 | 0 | |
| Number of persons who had SAE | 62 | 19.6 | 42 | 15.7 | 20 | 40.7 | |
| Specific SAE during 28 days of follow-up | Severe anemia | 1 | 0.3 | 1 | 0.4 | 0 | 0 |
| Lethargy | 1 | 0.3 | 0 | 0 | 1 | 2.0 | |
| Severe convulsions | 1 | 0.3 | 1 | 0.4 | 0 | 0 | |
| Severe somnolence | 1 | 0.3 | 1 | 0.4 | 0 | 0 | |
| Severe dizziness | 2 | 0.6 | 0 | 0 | 2 | 4.1 | |
| Spontaneous abortion | 1 | 0.3 | 1 | 0.4 | 0 | 0 | |
| Time of event occurrenced | During first 3 days | 903 | 284.9 | 564 | 210.5 | 339 | 690.4 |
| Between 4 and 7 days | 447 | 141.0 | 377 | 140.7 | 70 | 142.6 | |
| After 7 days | 1345 | 424.3 | 1188 | 443.4 | 157 | 319.8 | |
Notes: aIndicates the number of events; a patient could have one or more events at a time. bIndicates the incidence rate per 1000 (n/N). cOne case of rheumatoid arthritis. dNumbers may not add up to total number due to missing values.
Abbreviation: SAE, serious adverse event.
Reporting of Adverse Events Among Women Who Had Been Inadvertently Exposed to ACT During the First Trimester
In the surveillance of pregnant women, only 13 pregnant women were identified as having been inadvertently exposed to ACT during the first trimester of pregnancy. Of the 13 pregnant women, 10 were exposed in their third month of pregnancy, two were exposed in their second month of pregnancy and only one in her first month of pregnancy. During the follow-up of these pregnant women, 12 women had experienced deliveries of live newborns (including one with twins, raising the total to 13). No congenital malformations were observed during surface examination of these 13 live newborns. One woman in the active group had experienced a spontaneous abortion with a birth defect (a type of cervical agenesis and defect of the dome of the skull). After investigation, this abortion (with birth defect) was judged not to be related to ASAQ, because the event occurred just 4 days after a malaria treatment episode. Therefore, this abortion was probably caused by placental malaria or fetal loss due to the major birth defect.
Serious Adverse Events and Suspected Adverse Drug Reactions
Of the AEs reported, none was evaluated as definitively drug related. Reported AEs that were “possibly” or “probably” related to ASAQ, as established by the local investigators, occurred mostly within 3 days after treatment and were significantly more frequent in the passive group. A total of 584 ADRs was documented during the entire course of the study period. The most commonly reported ADRs among patients in both groups were anorexia, general weakness, vomiting, dizziness, nausea and cough.
A total of 62 patients (42 and 20 in the active and passive groups, respectively) experienced 76 SAEs during the 28 days of follow-up (see Table S3 in supplementary material). Of the 76 SAEs reported, four (5.3%) were considered by the local investigators as being probably related to the study medication. The different types of SAE reported during the active and passive surveillance are summarized in Table 5.
Table 5.
Suspected Adverse Drug Reactions During the Study Period
| Adverse Drug Reaction | Total (N=3170) | Active Cohort (n=2679) | Passive Cohort (n=491) | |||
|---|---|---|---|---|---|---|
| na | IRb | na | IRb | na | IRb | |
| Total | 548 | 173.0 | 384 | 143.0 | 164 | 334.0 |
| No serious (top 20) | 544 | 172.0 | 383 | 143.0 | 161 | 328.0 |
| Anorexia | 109 | 34.4 | 76 | 28.4 | 33 | 67.2 |
| General weakness | 87 | 27.4 | 58 | 21.6 | 29 | 59.1 |
| Cough | 76 | 24.0 | 50 | 18.7 | 26 | 53.0 |
| Vomiting | 47 | 14.8 | 24 | 9.0 | 6 | 12.2 |
| Dizziness | 37 | 11.7 | 26 | 9.7 | 11 | 22.4 |
| Diarrhea | 36 | 11.4 | 30 | 11.2 | 23 | 46.8 |
| Abdominal pain | 36 | 11.4 | 22 | 8.2 | 14 | 28.5 |
| Pruritus | 14 | 4.4 | 12 | 4.5 | 2 | 4.1 |
| Nausea | 14 | 4.4 | 7 | 2.6 | 2 | 4.1 |
| Fever | 13 | 4.1 | 11 | 4.1 | 0 | 0.0 |
| Rhinitis | 9 | 2.8 | 9 | 3.4 | 0 | 0.0 |
| Somnolence | 9 | 2.8 | 5 | 1.9 | 7 | 14.3 |
| Insomnia | 7 | 2.2 | 7 | 2.6 | 0 | 0.0 |
| Muscle/joint pain | 6 | 1.9 | 6 | 2.2 | 4 | 8.1 |
| Headache | 6 | 1.9 | 4 | 1.5 | 0 | 0.0 |
| Tinnitus | 5 | 1.6 | 3 | 1.1 | 0 | 0.0 |
| Anemia | 4 | 1.3 | 4 | 1.5 | 2 | 4.1 |
| Epigastric pain | 4 | 1.3 | 4 | 1.5 | 0 | 0.0 |
| Dermatosis | 3 | 0.9 | 3 | 1.1 | 2 | 4.1 |
| Chest pain | 2 | 0.6 | 2 | 0.7 | 0 | 0.0 |
| Serious | 4 | 1.3 | 1 | 0.4 | 3 | 6.11 |
| Gastroenteritis | 1 | 0.3 | 0 | 0 | 1 | 2.0 |
| Convulsion | 1 | 0.3 | 1 | 0.4 | 0 | 0 |
| Dizziness | 1 | 0.3 | 0 | 0 | 1 | 2.0 |
| General weakness | 1 | 0.3 | 0 | 0 | 1 | 2.0 |
Notes: aIndicates the number of events; a patient could have one or more events at a time. bIndicates the incidence rate per 1000 (n/N).
Factors Associated with the Time to Report Suspected ADR Occurrence
As presented in Table 6, concomitant medications used by patients at day 0 (most frequently metronidazole, cotrimoxazole, quinine, chlorpheniramine, amoxicillin, etc.) had a significant effect on ADR occurrence in both cohorts. This result was a 2.18- and 4.70-fold increase in risk (adjusted) of ADR occurrence in the active and passive surveillance cohorts, respectively. In the active surveillance group, the season of malaria transmission was significantly associated with a decreased risk of ADR reporting (adj. HR: 0.45, 95% CI: 0.20–0.92). This significant association also existed in the passive surveillance group (adj. HR: 0.45, 95% CI: 0.38–0.53). In the active surveillance group, the data indicate that when the patient’s age is relatively low, the risk of reporting an ADR decreased. However, this finding regarding the significant association with the age group was not observable in the passive surveillance group.
Table 6.
Determinants of Adverse Drug Reaction Occurrence After Taking Amodiaquine–Artesunate During 28 Days of Follow-Up in Both Active and Passive Surveillance
| Active Surveillance | Passive Surveillance | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Univariate | Multivariable | Univariate | Multivariable | ||||||
| HR (95% CI) | pvalue | Adj. HR (95% CI) | p value | HR (95% CI) | p value | Adj. HR (95% CI) | p value | ||
| Fixed Effect | |||||||||
| Sex | Male | 1 | 1 | 1 | |||||
| Female | 0.76 (0.68; 0.86) | <0.001 | 0.95 (0.80; 1.12) | 0.38 | 1.07 (0.6; 1.89) | 0.18 | 1.41 (0.63; 3.15) | 0.25 | |
| Age (years) | ≥18 | 1 | 1 | 1 | |||||
| 14–17 | 0.56 (0.36; 0.88) | 0.001 | 0.53 (0.29; 0.95) | <0.001 | 1.03 (0.19; 5.53) | 0.97 | 1.12 (0.19; 6.75) | 0.86 | |
| 6–13 | 0.44 (0.35; 0.55) | <0.001 | 0.44 (0.24; 0.82) | <0.001 | 0.49 (0.14; 1.65) | 0.16 | 0.33 (0.02; 5.03) | 0.27 | |
| 0–5 | 0.15 (0.12; 0.17) | <0.001 | 0.18 (0.08; 0.39) | <0.001 | 0.35 (0.14; 0.88) | 0.007 | 0.30 (0.01; 8; 04) | 0.32 | |
| Weight | 1.04 (1.03; 1.04) | <0.001 | 1.00 (0.99; 1.02) | 0.82 | 1.02 (1.01; 1.04) | 0.006 | 0.99 (0.92; 1.07) | 0.82 | |
| Symptoms at day 0 | ≤2 | 1 | 1 | – | – | ||||
| >2 | 1.47 (1.26; 1.71) | <0.001 | 0.93 (0.78; 1.12) | 0.31 | – | – | |||
| Concomitant medication | <2 | 1 | 1 | 1 | 1 | ||||
| ≥2 | 1.27 (0.98; 1.63) | 0.07 | 2.18 (1.54; 3.09) | <0.001 | 5.10 (2.91; 8.93) | <0.001 | 4.70 (2.11; 10.46) | <0.001 | |
| Temperature at day 0 | <37.5 | 1 | 1 | 1 | |||||
| ≥37.5 | 0.61 (0.53; 0.69) | <0.001 | 0.92 (0.76; 1.12) | 0.24 | 1.52 (0.76; 3.05) | 0.20 | 1.05 (0.40; 2.77) | 0.90 | |
| Drug use before day 0 | No | 1 | 1 | 1 | 1 | ||||
| Yes | 1.49 (1.31; 1.69) | <0.001 | 1.60 (1.34; 1.91) | <0.001 | 1.53 (0.75; 3.13) | 0.14 | 1.54 (0.65; 3.63) | 0.17 | |
| Transmission season | Low | 1 | 1 | 1 | 1 | ||||
| High | 0.37 (0.33; 0.41) | <0.001 | 0.45 (0.38; 0.53) | <0.001 | 0.49 (0.27; 0.88) | 0.017 | 0.45 (0.20; 0.92) | 0.005 | |
| Random effect | SD | SD | |||||||
| Patient specific | 0.385 | 1.892 | |||||||
| HF specific | 0.006 | 1.378 | |||||||
Abbreviations: HF, health facility; HR, hazard ratio; adj. HR, adjusted hazard ratio; SD, standard deviation.
The variance in random factors showed a high variability of ADR occurrence between patients in both groups (standard deviation [SD] equal to 0.385 and 1.892 for active and passive surveillance, respectively), whereas variability between health facilities was low in active surveillance (SD 0.006) and high in passive surveillance (SD 1.378).
The sensitivity analysis performed did not produce differences in the results from the main analysis.
Discussion
The results of our study show that a PV system based on an HDSS platform could be a useful tool for effective assessment and monitoring of the safety of newly registered drugs in resource-limited settings, especially in rural areas. Currently, over 4121 drugs have been registered by the drug regulatory authority in Burkina Faso.39 A robust PV is needed to monitor the possible AEs in the population. However, despite the government's effort to build a sustainable and functional PV system, the reporting of ADRs remains scarce and several obstacles to such poor reporting have been identified previously.15,16 In order to improve and increase the reporting rate of ADRs of medicinal products by patients and healthcare professionals, the WHO launched a new web application (VigiAccessTM) in April 2015.40 In addition, in June 2017 the Ministry of Health of Burkina Faso formally launched a web-RADR application (Recognizing Adverse Drug Reactions), which can be used on tablets and smartphones. Nevertheless, such applications are difficult to implement in rural areas owing to the lack of internet and poor telephone telecommunication services, combined with an absence or low level of education of the population. Our study was carried out with the objectives to support and strengthen the national PV activities for effective identification of potential ADRs, and to enhance patient care and patient safety in relation to the use of medicines. Therefore, the study focused on the feasibility of a PV system using the HDSS platform, as suggested elsewhere.29 The encouraging results reported here could be used as the basis to improve the national PV system in Burkina Faso by setting up similar approaches in the other four HDSS sites in the country.31,41-43 This approach can be also replicated in any other limited resource setting where an HDSS platform is available.
Both systems of surveillance (active and passive) appeared to be useful tools for monitoring the safety of widely available and utilized medicines. Indeed, about 39.6% and 44.4% of study participants reported at least one AE during the 28 days of follow-up, respectively, in the active and passive surveillance systems. These rates were higher than those reported in another study carried out in Ghana, a neighboring country of Burkina Faso, where only 29.4% of patients reported at least one event.44 Similarly to what has been reported previously with ASAQ, we found that general disorders (anorexia and asthenia), gastrointestinal disorders (vomiting, diarrhea, abdominal pain) and skin disorders (pruritus) comprised the majority of reported ADRs.35,45-48 Our study did not report any new ADRs and the types of events reported reflected the types of events expected in patients treated with ASAQ, as nearly all events reported are listed in the WHO Summary of Product Characteristics.49
In the passive surveillance system, we noted a relative underreporting of AEs when we compared the number of people sensitized to take part in this group (N=6579) and the absolute number of patients who actually reported events. In contrast to this relative underreporting, a remarkable finding in this study is the fact that spontaneous ADR reports in the passive surveillance group were more relevant in terms of crude incidence rate and severity than in active surveillance. This finding shows that a PV system based on spontaneous reporting is feasible and could be effective in Burkina Faso's rural resource-limited settings. However, with the low rate (7.5%) of patients who voluntarily returned to report AEs (probably due to the lack of interest of the population in the study area), such a notification system may be accompanied by regular community sensitization, training of health workers and implementation of PV focal points with personnel to promote ADR reporting.10,13,50 Moreover, to build a functional and sustainable PV system based on routine spontaneous reporting, the National Pharmacovigilance Monitoring Committee needs to liaise with the nursing/medical schools to introduce training on PV, especially spontaneous reporting, for students prior to graduation.10,50
The active surveillance seems to be relevant in term of numbers of AEs reported, but it requires resources that are difficult to mobilize and sustain in resource-limited settings. In such contexts, the use of the HDSS platform combined with existing resources (such as personnel and an established database which is constantly updated) could be an alternative to reduce the cost of this surveillance. In addition, the introduction of newer technologies such as cell phones to report and investigate AEs to any medical products or certain specific therapeutic classes could provide added value.44,51-53 The latter could facilitate rapid identification of drug safety signals, provided that connectivity conditions are good.
The approach used to monitor pregnancy exposure to ASAQ in this study allowed us to identify 13 women who had been inadvertently exposed to ASAQ during the first trimester of pregnancy. To improve the information on the safety of drugs used during pregnancy, the WHO recommends the implementation of a pregnancy registry with the objective to obtain reliable information on obstetric, medical and drug history during pregnancy, and to diagnose, assess, monitor and manage pregnancy and the outcomes of pregnancy, including congenital malformations, stillbirths and prematurity.54 The HDSS platform will make the pregnancy exposure registry more effective in providing reliable PV data during pregnancy.29
In both surveillance systems, during the malaria transmission season (which coincides with the rainy season), we noticed that the number of ADRs reported decreased significantly. In addition, with the increase in the number of concomitant medication prescriptions, the risk of occurrence of ADRs increased. This could be explained partly by the fact that during the period of high malaria transmission, the diagnosis of malaria episodes (with malaria RDTs) is more accurate55 and therefore would improve the accuracy in the drug prescription for illness management (less prescription of concomitant therapy). At the same time, the rainy season coincides with the beginning of farming activities, and families usually move from their usual homeland to hamlets with limited geographical accessibility. Therefore, it is possible that patients (especially caregivers) did not report ADRs of mild to moderate intensity.
Although the study has achieved its aims, we faced several constraints during the conduct of the project. Firstly, information about AEs was mostly self-reported and, thus, dependent on the patients’ reporting and recall. To partly solve this psychological constraint, to enhance the recall of AEs, data collection tools have been itemized by including a list of symptoms, especially those documented as ADRs related to ASAQ. Data collection tools also included symptoms of acute diseases commonly encountered in the study area, as well as drug-related AEs for drugs commonly used to treat these diseases. However, AEs recorded in this study following the prescription of ASAQ were similar to malaria symptoms. This could lead to a bias in the evaluation of causal links with the study drug.
Secondly, the frequent stockouts of ACT drugs observed during the study period did not optimize recruitment to reach the desired sample size. However, we were not able to take any corrective action because these stockouts were beyond the control of the research staff.
Thirdly, estimation of the incidence of AEs did not systematically take into consideration anomalies in biological parameters, because the study was designed to monitor mainly the occurrence of clinical AEs in peripheral health facilities in the rural setting, where there is no access to laboratory equipment and expertise.
Finally, the enrolment procedure (based on study inclusion and non-inclusion) resulted in the non-inclusion of many participants. This highlights the difficulties of implementing PV studies in African countries, and could be partly explained in our context mainly by the mobility of the population for their daily activities, the lack of interest of the population in reporting an ADR and the fact that our study was conducted in a rural community where the literacy rate is low (difficulty in finding an impartial witness for consent). This non-inclusion of certain participants could result in underestimation or overestimation of the incidence of ADRs. Continuous sensitization would certainly have improved this rate, but that was beyond the scope of our study. However, the final sample size was adequate to detect “rare” AEs (incidence occurring at a frequency of 1 out of 1000 patients or more) with a 95% probability.
Conclusion
Active surveillance through an HDSS platform seems to be an achievable approach to collect safety data in rural areas without major loss to follow-up, but this requires extra resources that are difficult to afford in resource-limited settings. The two surveillance systems were able to report many ADRs over a period of 2 years, although they did not report any new ADRs related to ASAQ. This shows that the HDSS platform could be a useful tool for the national PV system to monitor the safety of medicines, particularly in a rural environment where the use of newer technologies, such as web applications on tablets and smartphones, to report AEs may be very difficult to integrate. However, it is important to notice that the passive surveillance approach, which is more likely to be implemented by malaria control programs, seems to be more relevant in the SSA context, where health facilities have scarce human resources and insufficient funds to operate an active PV system. A few cases of exposure in the first trimester of pregnancy were reported, emphasizing the crucial need to develop a specific maternal and newborn health surveillance system (pregnancy exposure registry), which could contribute toward improving the assessment of maternal and neonatal outcomes.
Acknowledgments
We are very grateful to all participants in the study. We also thank the entire medical and field staff team who conducted this study. The medical staff in the health facilities and the CMA de Nanoro are also acknowledged. We would like to acknowledge Prof Umberto d’Alessandro and Dr Ambrose Talisuna, who contributed to finalizing the study protocol. The field work of this project was financially supported by the Tropical Diseases Research of the World Health Organization (WHO-TDR) [grant no. 2010/63792-0] (project HQTDR1003871) and the Sanofi Aventis Group [grant no. DJO-R-D/112066 – Avenant 112066], both awarded to Institut de Recherche en Sciences de la santé -IRSS (Principal Investigator: Halidou Tinto). The laboratory work was financially supported by the Institute of Tropical Medicine (ITM) of Antwerp (Belgium) [grant no. 930600] (project FA3-II). The Data analysis and manuscript drafting was conducted thanks to a travel grant (awarded to Rouamba Toussaint) from Beligium Academy for Research and Higher Education – Commission for Development Cooperation (ARES-CCD)
Data Sharing Statement
All data generated or analyzed during this study are available at Clinical Research Unit of Nanoro data repository and shareable upon request addressed to the corresponding author.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.McBride W. Thalidomide and congenital malformations. Lancet. 1961;2:1358. doi: 10.1016/S0140-6736(61)90927-8 [DOI] [Google Scholar]
- 2.World Health Organization. The Importance of Pharmacovigilance - Safety Monitoring of Medicinal Products. Geneva; 2002. Available from: http://apps.who.int/medicinedocs/pdf/s4893e/s4893e.pdf. Accessed April11, 2018. [Google Scholar]
- 3.Wiktorowicz M, Lexchin J, Moscou K. Pharmacovigilance in Europe and North America: divergent approaches. Soc Sci Med. 2012;75:165–170. doi: 10.1016/j.socscimed.2011.11.046 [DOI] [PubMed] [Google Scholar]
- 4.Wood SM, Coulson RA. Adverse drug reactions on-line information tracking (ADROIT). Pharmaceut Med. 1993;7:203–213. [Google Scholar]
- 5.Kuemmerle A, Dodoo ANO, Olsson S, Van Erps J, Burri C, Lalvani PS. Assessment of global reporting of adverse drug reactions for anti-malarials, including artemisinin-based combination therapy, to the WHO programme for international drug monitoring. Malar J. 2011;10:57. doi: 10.1186/1475-2875-10-57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Olsson S, Pal SN, Stergachis A, Couper M. Pharmacovigilance activities in 55 low- and middle-income countries. Drug Saf. 2010;33:689–703. doi: 10.2165/11536390-000000000-00000 [DOI] [PubMed] [Google Scholar]
- 7.Olsson S, Pal SN, Dodoo A. Pharmacovigilance in resource-limited countries. Expert Rev Clin Pharmacol. 2015;8:449–460. doi: 10.1586/17512433.2015.1053391 [DOI] [PubMed] [Google Scholar]
- 8.Elshafie S, Zaghloul I, Roberti AM. Pharmacovigilance in developing countries (part I): importance and challenges. Int J Clin Pharm. 2017;1–6. [DOI] [PubMed] [Google Scholar]
- 9.Ndomondo-sigonda M, Miot J, Naidoo S. Medicines regulation in Africa: current state and opportunities. Pharmaceut Med. 2017;31:383–397. doi: 10.1007/s40290-017-0210-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sabblah GT, Akweongo P, Darko DM, Dodoo ANO, Sulley AM. Adverse drug reaction reporting by doctors in a developing country: a case study from Ghana. Ghana Med J. 2014;48:189–193. doi: 10.4314/gmj.v48i4.4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ministère de la Santé/Direction Générale de la Pharmacie du Médicament et des Laboratoires. Manuel De Formation En Pharmacovigilance – Version 01 [Pharmacovigilance Training Manual-Version 01]. Ouagadougou, Burkina Faso: Ministère de la Santé/Direction Générale de la Pharmacie du Médicament et des Laboratoires; 2012. [Google Scholar]
- 12.Ampadu HH, Hoekman J, de Bruin ML, et al. Adverse drug reaction reporting in Africa and a comparison of individual case safety report characteristics between Africa and the rest of the world: analyses of spontaneous reports in VigiBase®. Drug Saf. 2016;39:335–345. doi: 10.1007/s40264-015-0387-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sevene E, Mariano A, Mehta U, et al. Spontaneous adverse drug reaction reporting in rural districts of Mozambique. Drug Saf. 2008;31:867–876. doi: 10.2165/00002018-200831100-00005 [DOI] [PubMed] [Google Scholar]
- 14.Kiguba R, Karamagi C, Waako P, Ndagije HB, Bird SM. Recognition and reporting of suspected adverse drug reactions by surveyed healthcare professionals in Uganda: key determinants. BMJ Open. 2014;4:e005869. doi: 10.1136/bmjopen-2014-005869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Strengthening Pharmaceutical Systems (SPS). Soutien aux programmes de pharmacovigilance dans les pays en développement: une approche systémique Soumis À l’Agence Des États-Unis Pour Le Développement International Par Le Programme SPS [Support for pharmacovigilance programs in developing countries: a systems approach. Submitted to the United States Agency for International Development by the SPS Program]. Arlington, VA: Management Sciences for Health; 2009. http://apps.who.int/medicinedocs/documents/s21530fr/s21530fr.pdf. Accessed April11, 2018. [Google Scholar]
- 16.Kabore L, Millet P, Fofana S, Berdai D, Adam C, Haramburu F. Pharmacovigilance systems in developing countries: an Evaluative Case Study in Burkina Faso. Drug Saf. 2013;36:349–358. doi: 10.1007/s40264-013-0043-9 [DOI] [PubMed] [Google Scholar]
- 17.World Health Organization. Malaria Control Improves for Vulnerable in Africa, but Global Progress Off-Track. WHO; 2016. Available from: http://www.who.int/mediacentre/news/releases/2016/malaria-control-africa/en/. Accessed September28, 2017. [Google Scholar]
- 18.Nosten F, van Vugt M, Price R, et al. Effects of artesunate-mefloquine combination on incidence of Plasmodium falciparum malaria and mefloquine resistance in western Thailand: a prospective study. Lancet (London, England). 2000;356:297–302. doi: 10.1016/S0140-6736(00)02505-8 [DOI] [PubMed] [Google Scholar]
- 19.Baiden R, Oduro A, Halidou T, et al. Prospective observational study to evaluate the clinical safety of the fixed-dose artemisinin-based combination Eurartesim® (dihydroartemisinin/piperaquine), in public health facilities in Burkina Faso, Mozambique, Ghana, and Tanzania. Malar J. 2015;14:1–7. doi: 10.1186/s12936-015-0664-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.White NJ, Olliaro P. Artemisinin and derivatives in the treatment of uncomplicated malaria. Med Trop (Mars). 1998;58:54–56. [PubMed] [Google Scholar]
- 21.Njau JD, Kabanywanyi AM, Goodman CA, et al. Adverse drug events resulting from use of drugs with sulphonamide-containing anti-malarials and artemisinin-based ingredients: findings on incidence and household costs from three districts with routine demographic surveillance systems in rural Tanzania. Malar J. 2013;12:1–12. doi: 10.1186/1475-2875-12-236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Adjuik M, Babiker A, Garner P, et al. Artesunate combinations for treatment of malaria: meta-analysis. Lancet (London, England). 2004;363:9–17. [DOI] [PubMed] [Google Scholar]
- 23.Ndagije HB, Nambasa V, Manirakiza L, et al. The burden of adverse drug reactions due to artemisinin-based antimalarial treatment in selected ugandan health facilities: an Active Follow-Up Study. Drug Saf. 2018;41:753–765. doi: 10.1007/s40264-018-0659-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tipke M, Diallo S, Coulibaly B, et al. Substandard anti-malarial drugs in Burkina Faso. Malar J. 2008;7:95. doi: 10.1186/1475-2875-7-95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tipke M, Louis VR, Yé M, et al. Access to malaria treatment in young children of rural Burkina Faso. Malar J. 2009;8:266. doi: 10.1186/1475-2875-8-266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yaméogo TM, Kyelem CG, Bamba S, et al. Chemin de soin des cas présomptifs de paludisme admis dans un hôpital de district au Burkina Faso. Med Sante Trop. 2014;24:301–306. doi: 10.1684/mst.2014.0368 [DOI] [PubMed] [Google Scholar]
- 27.Rouamba T, Valea I, Bognini JD, et al. Safety profile of drug use during pregnancy at peripheral health centres in Burkina Faso: a Prospective Observational Cohort Study. Drugs Real World Outcomes. 2018;5:193–206. doi: 10.1007/s40801-018-0141-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ministère de la Santé/Programme National de Lutte contre le Paludisme. Directives Nationales Pour La Prise En Charge Du Paludisme Au Burkina Faso [National Guidelines for Malaria Treatment in Burkina Faso]. Ouagadougou, Burkina Faso: Ministère de la Santé/Programme National de Lutte contre le Paludisme; 2017. [Google Scholar]
- 29.Kirakoya-Samadoulougou F, Sombié I, Ogutu B, et al. Using health and demographic surveillance systems for teratovigilance in Africa. Lancet Glob Heal. 2016;4:e906. doi: 10.1016/S2214-109X(16)30252-2 [DOI] [PubMed] [Google Scholar]
- 30.Mosha D, Mazuguni F, Mrema S, Abdulla S, Genton B. Medication exposure during pregnancy: a pilot pharmacovigilance system using health and demographic surveillance platform. BMC Pregnancy Childbirth. 2014;14:1–10. doi: 10.1186/1471-2393-14-322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Derra K, Rouamba E, Kazienga A, et al. Profile: Nanoro health and demographic surveillance system. Int J Epidemiol. 2012;41:1293–1301. doi: 10.1093/ije/dys159 [DOI] [PubMed] [Google Scholar]
- 32.Ministère De La Santé. Direction Générale Des Etudes Et Des Statistiques Sectorielles. Annuaire Statistique 2016 [Statistical Yearbook 2016]. Burkina Faso: Ministère De La Santé; Direction Générale Des Etudes Et Des Statistiques Sectorielles; 2017. Available from: http://www.sante.gov.bf/index.php?option=com_edocman&task=document.viewdoc&id=363&Itemid=1123. Accessed September8, 2017. French. [Google Scholar]
- 33.Rouamba T, Nakanabo-Diallo S, Derra K, et al. Socioeconomic and environmental factors associated with malaria hotspots in the Nanoro demographic surveillance area, Burkina Faso. BMC Public Health. 2019;19:249. doi: 10.1186/s12889-019-6565-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rouamba T, Sondo P, Yerbanga IW, et al. High adherence level to artemisinin-based combination therapies in rural settlement 11 years after their introduction in the health system, Nanoro, Burkina Faso. Patient Prefer Adherence. 2019;13:371–380. doi: 10.2147/PPA.S190927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sondo P, Derra K, Diallo-Nakanabo S, et al. Effectiveness and safety of artemether-lumefantrine versus artesunate-amodiaquine for unsupervised treatment of uncomplicated falciparum malaria in patients of all age groups in Nanoro, Burkina Faso: a randomized open label trial. Malar J. 2015;14:325. doi: 10.1186/s12936-015-0843-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tinto H, Sevene E, Dellicour S, et al. Assessment of the safety of antimalarial drug use during early pregnancy (ASAP): protocol for a multicenter prospective cohort study in Burkina Faso, Kenya and Mozambique. Reprod Health. 2015;12:112. doi: 10.1186/s12978-015-0101-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.International conference on harmonisation of technical requirements for registration of pharmaceuticals for human use.ICH harmonised tripartite guideline. Post-Approval Safety Data Management: Definitions and Standards for Expedited Reporting; E2D. Rockville, MD: ICH Expert Working Group; 2003. Available from: https://database.ich.org/sites/default/files/E2D_Guideline.pdf. Accessed February 18, 2020. [Google Scholar]
- 38.Safety Reporting and Pharmacovigilance. NIH: national institute of allergy and infectious diseases. Available from: https://www.niaid.nih.gov/research/dmid-safety-reporting-pharmacovigilance. Accessed July15, 2018.
- 39.Ministère de la Santé, Organisation mondiale de la Santé. Burkina Faso: Profil Pharmaceutique Du Pays [Burkina Faso: Country Pharmaceutical Profile. Ouagadougou, Burkina Faso; 2011.]. Ouagadougou, Burkina Faso: Ministry of Health and World Health Organization; 2011. Available from: https://www.who.int/medicines/areas/coordination/Burkina_Faso_PSCPNarrativeQuestionnaire_FR_16062011.pdf?ua=1. Accessed April28, 2019. [Google Scholar]
- 40.WHO. The WHO Programme for International Drug Monitoring. WHO; 2016. Available from: http://www.who.int/medicines/areas/quality_safety/safety_efficacy/National_PV_Centres_Map/en/. Accessed May11, 2018. [Google Scholar]
- 41.Kouanda S, Bado A, Yaméogo M, et al. Health and demographic surveillance system profile. The Kaya HDSS, Burkina Faso: a platform for epidemiological studies and health programme evaluation. Int J Epidemiol. 2013;42:741–749. doi: 10.1093/ije/dyt076 [DOI] [PubMed] [Google Scholar]
- 42.Sié A, Louis VR, Gbangou A, et al. The Health and Demographic Surveillance System (HDSS) in Nouna, Burkina Faso, 1993–2007. Glob Health Action. 2010;3:5284. doi: 10.3402/gha.v3i0.5284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rossier C, Soura A, Baya B, et al. Profile: the ouagadougou health and demographic surveillance system. Int J Epidemiol. 2012;41:658–666. doi: 10.1093/ije/dys090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dodoo ANO, Fogg C, Nartey ET, Malik A, Augustina S, David K. Profile of adverse events in patients receiving treatment for malaria in Urban Ghana: a Cohort-Event Monitoring Study. Drug Saf. 2014;37:433–448. doi: 10.1007/s40264-014-0164-9 [DOI] [PubMed] [Google Scholar]
- 45.Ndiaye J, Randrianarivelojosia M, Sagara I, et al. Randomized, multicentre assessment of the efficacy and safety of ASAQ – a fixed-dose artesunate-amodiaquine combination therapy in the treatment of uncomplicated plasmodium falciparum malaria. Malar J. 2009;8:125. doi: 10.1186/1475-2875-8-125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tinto H, Diallo S, Zongo I, et al. Effectiveness of artesunate-amodiaquine vs. artemether-lumefantrine for the treatment of uncomplicated falciparum malaria in Nanoro, Burkina Faso: a non-inferiority randomised trial. Trop Med Int Heal. 2014;19:469–475. doi: 10.1111/tmi.12274 [DOI] [PubMed] [Google Scholar]
- 47.Price R, Van Vugt M, Phaipun L, Luxemburger C, Simpson J. Adverse effects in patients with acute falciparum malaria treated with artemisinin derivatives. Am J Trop Med Hyg. 1999;60:547–555. doi: 10.4269/ajtmh.1999.60.547 [DOI] [PubMed] [Google Scholar]
- 48.Bassi PU, Osakwe AI, Isah A, et al. Safety of artemisinin-based combination therapies in Nigeria: a Cohort Event Monitoring Study. Drug Saf. 2013;36:747–756. doi: 10.1007/s40264-013-0044-8 [DOI] [PubMed] [Google Scholar]
- 49.World Health Organization. Artesunate/amodiaquine tablets: summary of product characteristics. 2014. Available from: http://whqlibdoc.who.int/publications/2010/9789241547925_eng.pdf. Accessed January2, 2020.
- 50.Kaboré L, Yaméogo TM, Sombié I, et al. Plaidoyer pour un renforcement du système de pharmacovigilance au Burkina Faso [A call to strengthen the Burkina Faso pharmacovigilance system]. Paris: Santé Publique; 2017;29:921–925. doi: 10.3917/spub.176.0921 [DOI] [PubMed] [Google Scholar]
- 51.Kukula VA, Dodoo AAN, Akpakli J, et al. Feasibility and cost of using mobile phones for capturing drug safety information in peri - urban settlement in Ghana: a prospective cohort study of patients with uncomplicated malaria. Malar J. 2015;14(1):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kabanywanyi AM, Baiden R, Ali AM, et al. Multi-country evaluation of safety of dihydroartemisinin/piperaquine post-licensure in African Public Hospitals with electrocardiograms. PLoS One. 2016;11:e0164851. doi: 10.1371/journal.pone.0164851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Masenyetse LJ, Manda SO, Mwambi HG. An assessment of adverse drug reactions among HIV positive patients receiving antiretroviral treatment in South Africa. AIDS Res Ther. 2015;12:6. doi: 10.1186/s12981-015-0044-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mehta U, Clerk C, Allen E, et al. Protocol for a drugs exposure pregnancy registry for implementation in resource-limited settings. BMC Pregnancy Childbirth. 2012;12:89. doi: 10.1186/1471-2393-12-89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kiemde F, Bonko MDA, Tahita MC, et al. Accuracy of a Plasmodium falciparum specific histidine-rich protein 2 rapid diagnostic test in the context of the presence of non-malaria fevers, prior anti-malarial use and seasonal malaria transmission. Malar J. 2017;16:1–11. doi: 10.1186/s12936-017-1941-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ministère De La Santé/Programme National De Lutte Contre Le Paludisme: Directives Nationales Pour La Prise En Charge Du Paludisme Au Burkina Faso. Ouagadougou, Burkina Faso: Ministère De La Santé; 2014. French. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Safety Reporting and Pharmacovigilance. NIH: national institute of allergy and infectious diseases. Available from: https://www.niaid.nih.gov/research/dmid-safety-reporting-pharmacovigilance. Accessed July15, 2018.