Abstract
Improved husbandry and better knowledge of exotic pets have led to a gradual increase in the life span of pets, such as rats, mice, hamsters, and gerbils. Much of the information on these senior patients is derived from the laboratory animal studies and anecdotal practitioner information. Although the small size of some of the patients makes blood collection problematic for hematology and organ function testing, the advent of polymerase chain reaction testing and other molecular diagnostics is allowing practitioners to test for specific etiologies with the small biologic samples available. Radiology and ultrasonography also are valuable diagnostic modalities.
Keywords: Rat, Mouse, Gerbil, Hamster, Geriatric, Nutrition, Euthanasia, Pain
Key points
-
•
Due to improvements in husbandry and increased availability of veterinary specialists, more geriatric rats, mice, hamsters, and gerbils with associated aged pet diseases are being seen by veterinarians.
-
•
Given the short life span of these pets, quality-of-life concerns should be discussed with owners as part of the overall veterinary medical plan.
-
•
Many of the typical dog and cat formulary drugs can be used in these geriatric pets with minimal modifications.
Introduction
Improved husbandry and better knowledge of exotic pets have led to a gradual increase in the life span of pets, such as rats, mice, hamsters, and gerbils. Much of the information on these senior patients is derived from the laboratory animal studies and anecdotal practitioner information.
Although the small size of some of the patients makes blood collection problematic for hematology and biochemical function testing, the advent of polymerase chain reaction testing (Box 1 ) and other molecular diagnostics is allowing practitioners to test for specific etiologies with the very small biologic samples available. Both ultrasonography (Figs. 1 and 2 ) and radiology (Fig. 3, Fig. 4, Fig. 5 ) also are valuable diagnostic modalities.
Box 1. Partial list of polymerase chain reaction tests available for rats, mice, hamsters, and gerbils.
Aspiculuris tetraptera
Coccidia
Ectromelia
Epizootic diarrhea of infant mice
Encephalomyocarditis virus
Fur mites
Hantavirus
Helicobacter species
K virus
Lactate dehydrogenase–elevating virus
Leptospira
Lymphocytic choriomeningitis virus
Mites
Mouse adenoviruses type 1 and type 2
Mouse cytomegaloviruses type 1 and type 2
Mouse hepatitis
Mouse minute virus
Mouse norovirus
Mouse parvovirus
Mouse polyomavirus
Mousepox virus
Mouse rotavirus
Mycoplasma species
Pasteurella multocida
Pinworms
Pneumocystis carinii
Pneumonia virus of mice
Rat-bite fever
Rat coronavirus
Reovirus type 3
Rodent infestation panel
Salmonella
Sendai virus
Seoul virus
Shigella
Sialodacryoadenitis virus
Streptobacillus moniliformis
Streptococcus pneumoniae
Syphacia muris
Syphasia obvelata
Theiler murine encephalomyelitis virus
Tick-borne encephalitis virus
Tyzzer disease
Yersinia pestis
Yersinia pseudotuberculosis
Fig. 1.
Ultrasonography is an invaluable diagnostic tool. The use of a microconvex or linear probe typically is needed for these small patients.
Fig. 2.
Suspected mediastinal mass located on ultrasound.
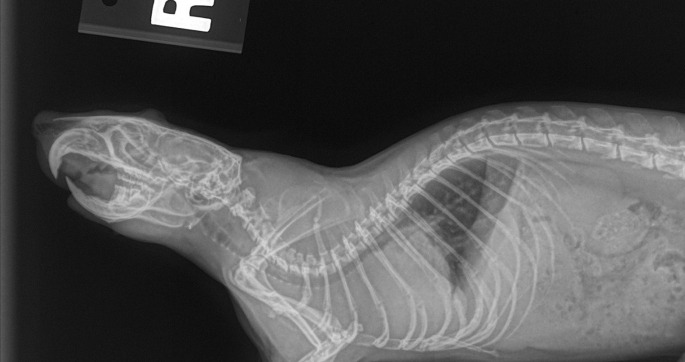
Fig. 3.
Standard 2-view radiographs can be diagnostic. For these smaller patients, a dorsoventral position can be easier to obtain than a ventrodorsal projection.
Fig. 4.
Suspected cranial mediastinal mass, which was confirmed on ultrasound.
Fig. 5.
Dorsoventral radiograph of same rat. In this patient, a mediastinal mass was confirmed on ultrasound.
General concerns for geriatric rats, mice, hamsters, and gerbils
Aging Changes
The species outlined in this article have relatively short lives and reach geriatric status in a matter of a 2 years to 5 years. Although clients intellectually know this, it can be a surprise to them when discussing kidney disease or osteoarthritis, when a pet may be only 18 months old.
The aging changes seen in these species mimic similar changes in the more common species, such as dogs and cats. Changes, such as sleeping more, moving less, decreased interaction with owners, and so forth, are common. Miscellaneous skin masses appear with some regularity and weight gain may occur.
These species have continually growing incisors that may need periodic grinding if malocclusion has occurred (Fig. 6 ).
Fig. 6.
Overgrown incisors on a rat.
Nutrition
There is little information about dietary changes that may be needed for these species. In general, the dietary requirements that are used for adolescence or adulthood seem appropriate for the geriatric patient.
Quality of Life
Knowing when a patient is not experiencing an appropriate quality of life includes weighing many factors. One proposed scale is the HHHHHMM algorithm.1
Hurt
The hurt criterion assesses the pain that a pet is exhibiting. Also important to acknowledge is that dyspnea also can be painful. In humans, dyspnea is ranked high as a painful process. The use of nonsteroidal anti-inflammatory drugs (NSAIDs) is warranted early in the course of pain to minimize the establishment of wind-up pain. Clients should be counseled on the signs of pain and the need to medicate for pain even when a pet may not be exhibiting signs.
Meloxicam commonly is used because it already is in a suspension form. For very small patients, it can be diluted for more appropriate dosing with the preferred diluent being methylcellulose products.
For male rats, oral tramadol (40 mg/kg, orally) and oral buprenorphine (0.5 mg/kg and 0.6 mg/kg, orally) have been shown to be effective analgesia. In female rats, oral buprenorphine was not effective but oral tramadol (20 mg/kg, 30 mg/kg, and 40 mg/kg) was effective.2 Anecdotally veterinarians have used tramadol in gerbils and hamsters.
Oral gabapentin is effective in rats primarily for neuropathic pain control.3 , 4 Oral gabapentin (30–100 mg/kg, orally) is effective in mice.5 Anecdotally, veterinarians have used gabapentin in hamsters and gerbils.
Cannabidiol (CBD) is a new pharmaceutical used in veterinary medicine, but little evidence-based information is available. There are several over-the-counter CBD oils and 1 approved human medication (Epidiolex, Greenwich Biosciences, Carlsbad, California). As with many over-the-counter supplements, quality control may be an issue, and owners are advised to pick a brand name with third-party laboratory validation.
One study showed CBD caused liver damage in mice at the allometrically equivalent of the human maximum dose.6 Topically applied 1% CBD gel over arthritic joints appeared to decrease inflammation in a rat arthritis model.7 CBD in a hamster report showed that dosing of 1.25 mg/kg to 20 mg/kg, orally, protected against cerebral ischemia after bilateral carotid occlusion.8
Hunger
Given the continually growing natures of these pets’ teeth, malocclusion or incisor overgrowth (Fig. 7 ) can lead to problems masticating and prehending food, leading to hunger and debilitation. Many times, this manifests as weight loss, more time at feed bowls but little actual consumption of food, or associated pathology, such as abscessation at the dental site. Correction of dental issues and/or force-feeding with appropriate diet items or prepackaged formulas can alleviate hunger in the short term. Some owners are able to supplement a pet’s feeding habits with scheduled force-feeding.
Fig. 7.
Inability to prehend food can lead to hunger and debilitation.
Hydration
The hydration status of a geriatric pet can be impacted by various issues. These include metabolic diseases, such as diabetes mellitus and kidney dysfunction; dental issues, for example, where a patient cannot use a water dripper bottle appropriately; or arthritis, impacting mobility to the water system. Owners may notice apparent weight loss or sunken/closed eyes on their pet. On physical examination, the standard skin turgor parameters used for measuring dehydration in other veterinary species apply to this group of patients (Fig. 8 ).
Fig. 8.
Dehydrated hamster estimated at 7% dehydration.
Hygiene
Pets that are in distress many times stop normal grooming and become unthrifty. Many times, owners notice the poor fur condition and that is a reason for them bringing a pet to the veterinarian. Because a majority cases of poor hygiene can be attributed to another underlying disease, the veterinarian should focus on determining the primary source of a pet’s discomfort (Fig. 9 ).
Fig. 9.
Hamster with unthrifty perianal area. This patient had colitis and ultimately prolapsed 10 mm of the large intestine.
Happiness
Happiness pertains to owner and veterinarian assessment of a pet’s attitude and overall well-being. If a pet seems anxious, fearful, sensitive to touch, and so forth, all are factors that can be considered in this category of happiness.
Mobility
Preemptive use of NSAIDs can prolong the pain-free mobility of these patients. As in other species, the expectation is that an NSAID will decrease in effectiveness as the disease process progresses. Pain also can result in a pet resisting handling or petting. Adding additional pain medications, such as gabapentin and tramadol, at this time may be helpful.
More good days than bad days
Geriatric pets can have waxing and waning of their condition, resulting in good days and bad days. Many owners understand this concept and a discussion about the percentages of good days and bad days can help owners understand the progression of the aging process and help owners set up their own parameters for when quality of life may be compromised. In the author’s experience, many owners feel that 30% bad days is a common area where euthanasia discussion starts to occur with more frequency.
Pharmaceuticals
-
1.
Pain management is paramount in maintaining quality of life and function. Commonly used veterinary medications can be used in rats, mice, hamsters, and gerbils (Table 1 ).9 Opioids may cause sedation in these pets.
-
2.
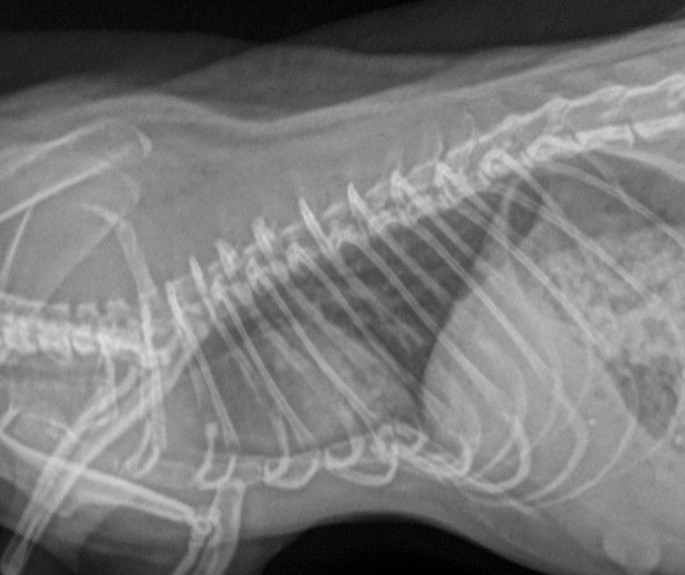
Cardiac medications can be useful for many pets in early to advanced stages of cardiovascular disease. Signs of cardiac disease are similar to other mammals and include cardiomegaly, increased interstitial pattern on radiographs, and possible pleural effusion (Fig. 10 ).
-
3.
Common veterinary cardiac medications can be used in these species (Table 2 ).10
-
4.Supplemental feeding
- Supplemental feeding with several products is manageable for most owners. The feedings can include a pureed version of the normal pelleted food a pet consumes. Packaged products, such as Hills a/d, Oxbow Carnivore Care, and Emeraid products, are options. Depending on a pet’s condition, supplemental feedings may occur daily or less frequently.
-
5.Directed therapies
- Depending on the etiology of the geriatric pet’s condition, there may be specific therapeutic interventions that can occur. These include subcutaneous fluid administration, antibiotics for any bacterial infection (such as from dental disease), surgical removal of benign and malignant neoplasia, cardiac medications, blood pressure modulators, and hormonal medications.
Table 1.
Drugs used for pain management
| Drug | Rat | Mouse | Gerbil | Hamster |
|---|---|---|---|---|
| Meloxicam | 0.5–2.0 mg/kg, PO, SQ, q24h | 1–5 mg/kg, PO, SQ, q24 h | 0.5 mg/kg, PO, SQ, q24h | 0.5 mg/kg, PO, SQ q24h |
| Carprofen | 2–5 mg/kg, PO, SQ, IM, q12–24 h | 2–5 mg/kg, PO, SQ, IM, q12–24h | 5 mg/kg, SQ, q24h | 5 mg/kg, SQ, q24h |
| Butorphanol | 1–2 mg/kg, SQ, IM, IV, q2–4h | 1–2 mg/kg, SQ, IP, q2-4h | 1–5 mg/kg, SQ, q4h | 1–5 mg/kg, SQ, q4h |
| Buprenorphine | 0.05–0.1 mg/kg, SQ, IM, q6-12h | 0.05–0.1 mg/kg, SQ, q6-12h | 0.1–0.2 mg/kg, SQ, q8h | 0.5 mg/kg, SQ, q8h |
| Tramadol | 5–20 mg/kg, PO, SQ, IV, IP | 5–40 mg/kg, SQ, IP | 5–10 mg/kg, PO, q12–24h | 5–10 mg/kg, PO, q12–24h |
| Gabapentin | 50 mg/kg, PO, q24h | 10–70 mg/kg, PO, q24h | 50 mg/kg, PO, q24h | 50 mg/kg, PO, q24h |
| Ketoprofen | 2–5 mg/kg, SC, IM, q12–24h | 2–5 mg/kg, SC, IM, q12–24h | 5 mg/kg, SQ, q24h | 5 mg/kg, SQ, q24h |
Fig. 10.
Pleural effusion in a rat secondary to congestive heart failure.
Table 2.
Drugs used for cardiovascular disease
| Therapeutics for Use in Small Exotic Mammals with Cardiovascular Disease | |||
|---|---|---|---|
| Species | Drug | Dosage | Comments, Indication |
| Hamster | Amlodipine | 10 mg/kg/d in food | Calcium antagonist Amlodipine prevents cell death and fibrosis and reduces cardiac dysfunction in cardiomyopathic hamsters. |
| Rat | Atenolol | 5 mg/kg | β-blocker, hypertrophic cardiomyopathy Prolongs filling, decreases myocardial ischemia |
| Hamster | Digoxin | 0.05–0.01 mg/kg, PO, q12–24h | Positive inotrope Right-sided heart failure, nonresponsive cardiomyopathy, dilated cardiomyopathy Also indicated for atrial fibrillation |
| Hamster | Diltiazem | 25 mg/kg/d, PO | Calcium channel blocker Benzodiazepine like calcium antagonist Increases ventricular filling, reduces heart rate and blood pressure; reduces myocardial oxygen consumption |
| Hamster | Enalapril | 0.5 mg/kg PO, q24–48h, 20 mg/kg/d in food | Angiotensin-converting enzyme inhibitor Balanced vasodilator; avoid use in animal with concurrent renal disease. |
| Hamster, mouse, rat | Furosemide | 1–10 mg/kg IM, SQ, PO, q4–12h | Diuretic, reduction of ascites, pleural effusion, pulmonary edema |
| Rodents in general | Isoprenaline | 0.1–1 mg/kg/min IV, IC Total dose 5–10 μg/kg |
Complete heart block, low cardiac output |
| All | Nitroglycerin ointment 2% | 1/16 in/kg Apply to hairless region q12–24h | Initial adjunctive venodilation for emergency use |
| Rodents | Propranolol | 0.1 mg/kg IV, IC | β-blocker, hypertrophic cardiomyopathy Prolongs filling, decreases myocardial ischemia Tachyarrhythmia |
| Rat | Pimobendan | 1 mg/kg | Phosphodiesterase inhibitor (which causes peripheral ateriodilation and venodilation and improved myocardial contractility) |
| All | Omega 3 | 25 mg/d | Generally recommended nutraceuticals for cardiac support in rodents and small exotic mammals |
| All | Oils, including flax oil | 10–30 mg/d | Generally recommended nutraceuticals for cardiac support in rodents and small exotic mammals |
| Coenzyme Q10 | 25 mg/d | Generally recommended nutraceuticals for cardiac support in rodents and small exotic mammals | |
| l-carnitine | 50 mg/d | Generally recommended nutraceuticals for cardiac support in rodents and small exotic mammals | |
| All | Taurine | 50 mg/d | Generally recommended nutraceuticals for cardiac support in rodents and small exotic mammals |
| Hamster, rat | Verapamil | 0.25–0.5 mg/hamster SQ, 5 mg/kg/d IP, 0.75 mg/mL in drinking water | Calcium channel blocker Increases ventricular filling, reduces heart rate and blood pressure, reduces myocardial oxygen consumption |
Dental Issues
Dental issues are common, and special attention needs to be placed on treating concerns, such as overgrown incisors. An owner may note decreased eating habits (and concurrent decrease in fecal matter), and a physical examination usually reveals a dental malocclusion or overgrowth (Figs. 11 and 12 ).
Fig. 11.
The upper incisors are deviated laterally but it is the overgrown lower incisors that are the primary reason this rat cannot eat.
Fig. 12.
Significant reduction in the lower incisors allow this rat to eat normally.
Therapy usually is the grinding down of the offending overgrown tooth, and treatment of any concurrent skin abscess and management of pain. The use of nail clippers to clip teeth short can lead to fracturing of the enamel and dentin, with extension into the root, leading to pain and infection. For this reason, pets should be anesthetized and teeth ground and shaped to proper length and position with low-speed dental burs. Equipment is readily available from veterinary distributors (Fig. 13 ).
Fig. 13.
Typical dental equipment employed in the author’s practice.
Anesthesia
Contemporary anesthetics and methods minimize, but do not eliminate, anesthetic risk for surgical intervention.
-
1.
Alfaxalone is a neurosteroid anesthetic that is short acting and has the benefit of intramuscular administration. Anesthetic recovery can be rough unless other preanesthetic medications, such as an opioid, are used. It can sting upon injection. Dosing has been reported as 20 mg/kg, intramuscularly, or 120 mg/kg, intraperitoneally (IP), for rodents.11 Anecdotally, practitioners have used this subcutaneously and for pre-euthanasia sedation.
-
2.
Propofol is a γ-aminobutyric acid inhibitory short-acting anesthetic that can be used intravenously (IV) at a dose of 7.5 mg/kg to 10 mg/kg (rats) or 12 mg/kg to 26 mg/kg (mice).11
-
3.
Isoflurane and sevoflurane can be used with an anesthetic cone or induction chamber. These are short-acting gas anesthetics. Recovery can be rough and the use of a preanesthetic medications, such as an opioid, is recommended. If possible, endotracheal intubation should be performed but, given the size of these pets, that may not be doable. Supraglottic devices have been constructed and used successfully.12 Details on construction are available online.13 Commercially available devices are available although they may be too large for smaller pets (Figs. 14 and 15 ; v-gel: http://docsinnovent.com/products/product/rabbit-v-gel).
Fig. 14.
Side profile of #1 v-gel.
(Courtesy of Docsinnovent Ltd., Hempstead, UK.)
Fig. 15.
Top profile of #1 v-gel.
(Courtesy of Docsinnovent Ltd., Hempstead, UK.)
The author finds that a preanesthetic body radiograph can help determine whether overt cardiomegaly is present, indicating overt heart disease. Although the anesthesia procedure still may be necessary, appropriate client communications can occur prior to the anesthesia. A standard orthogonal radiograph of the thorax (and many times the whole body) typically suffices (Figs. 16 and 17 ).
Fig. 16.
Lateral thoracic rat radiograph. Mild cardiomegaly is present.
Fig. 17.
Dorsoventral thoracic radiograph of same rat.
Euthanasia
Unfortunately for many smaller patients, direct venous access is not possible, making IV injection of euthanasia solution improbable. Discussing options with owners about route of administration may be prudent. Options include IV access (in some cases, a small-gauge wing-tip catheter can be used for these small patients), intraosseous administration (although pets should be anesthetized to place an intraosseous infusion catheter [Figs. 18 and 19 ] or needle), intracardiac administration (using an opioid premedication and masking patients under gas anesthesia is preferable before injection), and intrathoracic administration (same caveat as for intracardiac administration). (See End of Life Decisions: Palliative Care, Hospice, and Euthanasia for Exotic Animals, for additional information regarding American Veterinary Medical Association guidelines for humane euthanasia of pets.)
Fig. 18.
Alignment of a 20-g hypodermic needle at the trochanteric fossa of the femur for an intraosseous catheter in a rat. The area is prepped and the needle advanced through the fossa and down the medullary canal.
Fig. 19.
Placement of the intraosseous catheter in same rat. Fluid rates equaling IV rates can be used.
Common issues with selected species
Rats
The fancy rat (Rattus norvegicus domestica) is the most commonly kept pet rat, with most living 2 years to 3 years, although 4 years can occur. Common diseases in the geriatric rat include a variety of skin masses, multifactorial respiratory disease, mammary neoplasia, pituitary adenomas, kidney dysfunction, and neuropathies.
Multifactorial respiratory disease is the norm for rats, with a high number affected by Mycoplasma pulmonis coinfections. Rats usually exhibit chronic sneezing, nasal discharge, epiphora that usually is colored red to brown, and decreased appetite. Infrequently, vestibular signs may be exhibited.
There are several causes for respiratory disease, which are listed in Table 3 .
Table 3.
Rat respiratory disease etiologies and risk factors
| Virus | Sendai virus Coronavirus |
| Bacteria |
Mycoplasma spp Cilia-associated respiratory bacillus Bordetella Corynebacterium kutscheri Streptococcus spp |
| Environment | Poor ventilation Build-up of soiled bedding |
Therapy consists of improving the environment, nutrition, use of antibiotics (enrofloxacin, 10 mg/kg, orally, every 12 h); doxycycline, 5 mg/kg, orally, every 12 h; or azithromycin (20 mg/kg, every 24 h, 7 d), and possible NSAIDs.14 Nebulization can be beneficial. Using a nebulizer that achieves a small particle size of 3 μm is recommended, at a rate of 10 minutes to 30 minutes a session for 1 to 3 sessions a day.15 Anecdotally, just the use of 0.9% NaCl can be beneficial for nebulization. Antibiotics, such as enrofloxacin (2–10 mg/mL saline16) and gentamicin (50 mg in 10 mL saline for 15 min every 8 h to 12 h17), can be used for nebulization.
Neoplasia is common in rats. Mammary tumors (Figs. 20 and 21 ) are easily noted by owner and clinician. Pituitary adenomas also occur commonly, but specialized imaging is required to diagnose this neoplasia.
Fig. 20.
Rat with mammary mass.
Fig. 21.
Excised mammary mass in same rat.
Mammary tumors in female rats typically are fibroadenomas. These subcutaneous fibroadenomas can grow to 8 cm to 10 cm in diameter and the overlying skin can be traumatized. Mammary tumors can occur in male rats but at a lower incidence.18 Surgical removal is recommended but other neoplasia may occur in the remaining mammary tissue. The incidence of mammary tumors, in addition to pituitary tumors, can be reduced in rats that are ovariectomized at 90 days of age compared with those that were not ovariectomized.19 Current studies do not show a reduction in neoplasia recurrence if the rat is ovariectomized once mammary tumors have occurred. Tamoxifen, an antiestrogen compound, has been tried but liver toxicity issues have limited its use.20
Anecdotally, it has been used successfully to prevent further mammary fibroadenomas in rats, especially those unable to undergo ovariectomy due to comorbid disease.
In several studies, food restriction to approximately 65% of ad libitum consumed food showed a reduced incidence of mammary neoplasia.21
Pituitary adenomas are common in aging rats, leading to lactotroph hyperplasia and hyperprolactinemia. Increasing prolactin levels in the aged rat may play a role in mammary neoplasia development. Cabergoline, a prolactin inhibitor that suppresses pituitary prolactin secretion, can be given orally. It has been used successfully in the reducing the size of a pituitary adenoma in a rat at a dose of 0.6 mg/kg, orally, every 72 hours.22
As in mammary tumors, rats fed on a restricted diet had the lowest incidence of pituitary adenomas and focal pituitary hyperplasia.23
Chronic progressive nephrosis/nephropathy (CPN) is one of the more common causes of death in aged rats and the incidence has been reported as high as 75% in some strains (Sprague-Dawley).24 The disease occurs more frequently in male rate and generally is of greater severity than in female rats. In CPN, lesions consist of a chronic glomerulosclerosis and interstitial disease involving the convoluted proximal tubules. The kidneys can be enlarged to twice normal size or more and are pale and mottled.25 Signs are those seen in a renal failure state and include weight loss, lethargy, azotemia, and proteinuria.26
Treatment is palliative. A lower protein diet (10%–14%) is recommended and, in more severe cases, supplemental subcutaneous fluids may be necessary, dosed at 50 mL/kg, for 24 hours, to 100 mL/kg, for 24 hours, and warmed to body temperature.27
Posterior weakness (Fig. 22 ) or tail dragging may indicate radiculoneuropathy, with resulting disturbances in motor function. This is a degenerative disease of the spinal roots accompanied by atrophy of skeletal muscle in the lumbar region and hind limbs.28 The incidence in rats over 24 months of age may be as high as 75% to 90%. Demyelination and vacuolation are seen in the lumbosacral roots, notably in the ventral spinal regions. Treatment with B complex, at a dose of 2 mg/kg, subcutaneously/intramuscularly, appears to decrease symptoms along with NSAIDs, such as meloxicam.29 , 30
Fig. 22.
This rat had progressive posterior weakness that was suspected to be radiculoneuropathy.
Mice
Mammary gland adenocarcinoma is more common in the geriatric mouse, and different mouse strains have different incidences of neoplasia. Probably all mammary tumors in mice are influenced by the mammary mouse tumor virus transmitted either in germ cells (endogenous form) or in the milk and saliva (exogenous form). The endogenous virus can be incorporated in the mouse genome and be passed in a mendelian fashion.31 Hormones, stress, and chemical carcinogens also may influence in the development of these tumors. The neoplasms can involve 1 or more glands along the chain, which extends from the axillary to inguinal region. The exogenous form also can cause lymphoma.
Mammary neoplasia commonly infiltrates surrounding tissues.
Spontaneous mammary tumors metastasize with high frequency, but this property is somewhat mouse strain dependent. Metastases primarily go to the lung.
Mammary tumors vary in the types of cell receptors they contain. Ovary-dependent tumors contain estrogen and progesterone receptors, whereas pregnancy-dependent tumors have prolactin receptors. Ovariectomy dramatically reduces the incidence of mammary tumors in certain genetic strains (such as C3H) of mice. If surgery is done in adult mice 2 months to 5 months of age, mammary tumors will develop, but at a later age than normal.
Several other diseases can present in the older mouse. Depending on the organs affected, clinical signs may be noted32:
-
1.
Alveolar and/or bronchial epithelium hyperplasia, which can be confused with pulmonary tumors on radiographs. Hyperplasia may be a coincidental finding and no signs are manifested by the pet.
-
2.
Age-associated liver lesions are common and can include biliary hyperplasia, hepatitis, amyloid deposition, fibrosis, hepatomas, and carcinomas. In many cases, therapy is palliative in nature only. The use of liver protectants may benefit mice. One study reported that a silymarin dose of 500 mg/kg, every 24 hours, decreased fatty liver–associated damage.33
-
3.
Nearly all strains of mice develop some form of osteoarthrosis. The use of NSAIDs is indicated.
-
4.
Kidney lesions, such as glomerulonephritis. It is associated more often with persistent viral infections or immune disorders rather than with bacterial infections. Signs include muscle wasting, weight loss, and proteinuria. It is progressive. Force-feeding a liquid gruel and/or subcutaneous fluids may prolong quality of life.
Hamsters
Hamsters as pets include the Syrian (also called teddy bear) hamster, Campbell dwarf hamster, Roborovski dwarf hamster, Russian winter white hamster, and Chinese hamster. The life span typically is no more than 2 years.
Diseases affecting the geriatric hamster include dilated cardiomyopathy, atrial thrombosis, amyloidosis of various organs, hyperadrenocorticism, and neoplasia.10 , 34 , 35
Dilated cardiomyopathy and atrial thrombosis have common nonspecific signs, such as lethargy, anorexia, and tachypnea. There is a correlation with atrial thrombosis. Radiography and ultrasonography may aid in the diagnosis. Therapy is symptomatic and can include diuretics and other cardiac medications, as shown in above table.
The incidence of atrial thrombosis is influenced by the endocrine status of the animal, especially by the amount of circulating androgens. As a result, castration of male Syrian hamsters is linked to an increase in the prevalence of atrial thrombosis.36 Disseminated intravascular coagulation has been found in conjunction with cases of atrial thrombosis.
Amyloidosis is common in the geriatric hamster and may be a coincidental finding. Amyloid is an insoluble pathologic proteinaceous substance, deposited between cells in various tissues and organs of the body. Amyloid deposits are more common in female hamsters greater than 1.5 years of age. Deposits can be seen in the liver, kidney, spleen, and adrenal glands as well as occasionally in almost any other organ. Diagnosis is made on histopathology. Signs depend on the organ affected. Depending on the organ affected, therapy is directed at supporting that organ function (such as subcutaneous fluids if the kidneys are affected).
Neoplasia can affect a variety of organs. Treatment usually is palliative, with some neoplasia amenable to surgery.
Approximately 30% of Syrian hamsters have neoplasms, with no appreciable sex difference in the overall tumor incidence. The most frequent tumor types were those of the adrenal cortex (13.5%), the lymphoreticular system (3%), and the endometrium (3%). Small intestinal adenocarcinomas occurred in 0.8% of the animals.37
In older Syrian hamsters, lymphoma is the most frequently observed neoplasm of the hematopoietic system. It is multicentric and commonly affects lymphatic organs.36 It is speculated that some of these adult-onset lymphomas may be transmissible tumors that capitalize on the homozygosity of Syrian hamsters.
Cutaneous lymphoma (or epitheliotropic lymphoma) also can occur. Diagnosis is made based on histopathology. Depending on the location, some may be removed surgically.
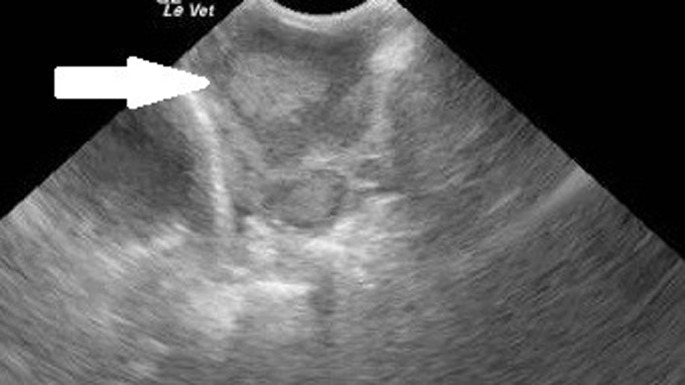
Hyperadrenocorticism occurs commonly, usually in male hamsters, and is associated with bilateral symmetric alopecia of flanks and lateral thighs, thinning and hyperpigmentation of the skin, polydipsia, polyuria, and polyphagia (Fig. 23 ). Research has suggested that hamsters may secrete both cortisol and corticosterone, making confirmation with dynamic assays difficult.38 Enlarged adrenal glands may be located on ultrasonography in some cases (Fig. 24 ). A consistent successful therapy has not been reported to date.
Fig. 23.
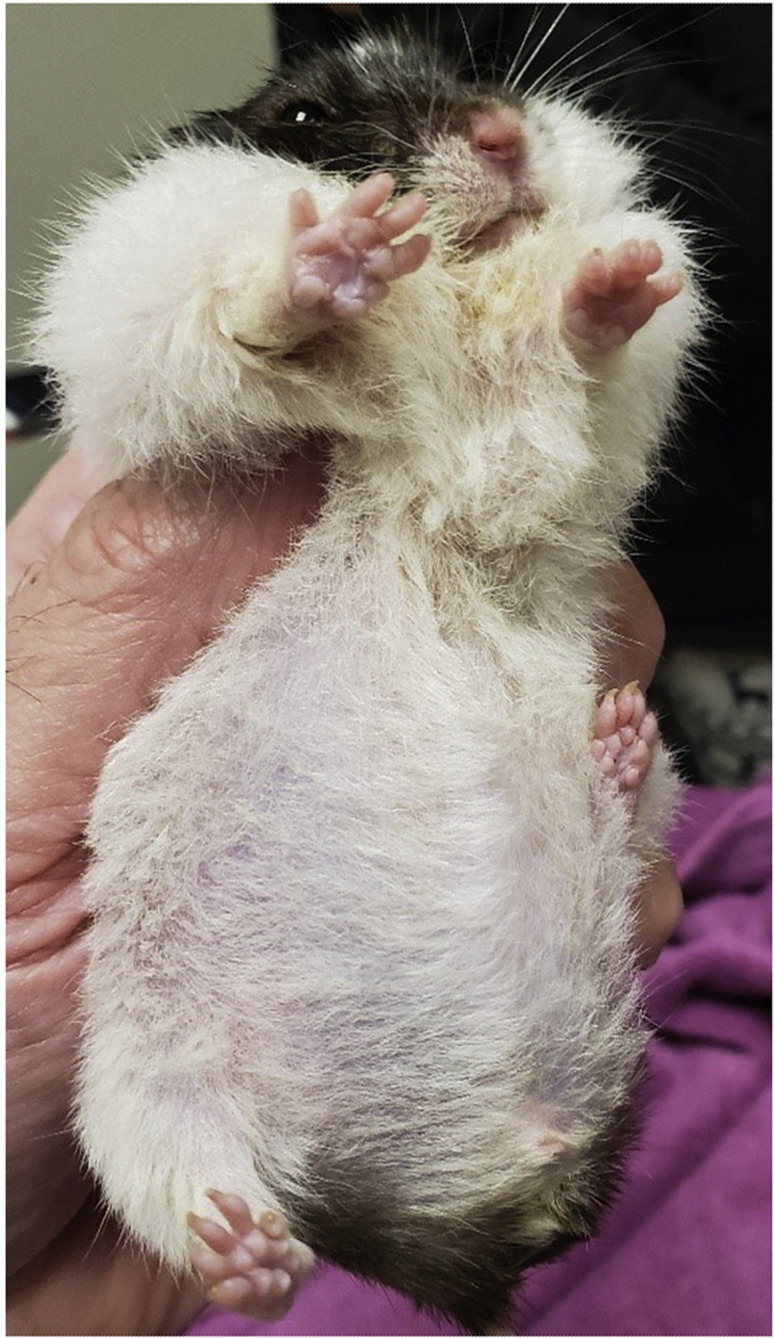
Thinning fur on gerbil with hyperadrenocorticism.
Fig. 24.
Ultrasound of same gerbil with adrenal gland enlargement (white arrow) confirmed by histopathology.
Gerbils
Gerbils live 2 years to 5 years, with the occasional reported pet living to 8 years of age.
Commonly reported diseases of geriatric gerbils include scent gland neoplasms, cystic ovaries, chronic interstitial nephritis, and cerebral vascular ischemia (stroke).
Ventral marking, gland hyperplasia, and carcinomas (Fig. 25 ) are common in older gerbils (>1.5 years). They present as small, possible reddish, waxy skin masses over the umbilicus. Diagnosis is made by histopathology and treatment is surgical removal. If neoplastic, they may recur.
Fig. 25.
Histopathology confirmed scent gland carcinoma in a gerbil.
Cystic ovaries are common in gerbils over 1 year of age and clinical signs include symmetric alopecia, abdominal swelling, lethargy, anorexia, dyspnea, and reduced fertility. Diagnosis can be made on physical examination and ultrasonography. Therapy is an ovariectomy or ovariohysterectomy.
Chronic interstitial nephritis is a common finding. If the gerbil is clinical for this disease, signs include polyuria, proteinuria, lethargy, and proteinuria. Treatment is supportive in nature.
In mammals, the circle of Willis is composed of a communication of arteries at the bottom of the brain, consisting of the internal carotid arteries, anterior cerebral arteries, anterior communicating arteries, posterior communicating arteries, posterior cerebral arteries, and basilar arteries. This structure provides for alternate blood flow to the brain in case 1 artery becomes occluded.
Some gerbils do not have an anatomically complete circle of Willis and may be prone to cerebral ischemia. Gerbils are used as a human model for cerebral ischemia, and research has shown several pharmaceuticals may help in protecting neuronal tissue, especially in the acute phase. From a clinical perspective, most of these pharmaceuticals are not commonly found in the veterinary pharmacy. Steroids do not appear to be beneficial in return to function.39
Cerebral ischemia signs include paralysis, inability to open 1 or both eyes, head tilt, and/or incoordination. Therapy includes supportive treatment of subcutaneous fluids, force-feeding, and maintenance of normal body temperature. Anecdotally, gerbils may benefit from NSAIDs. Many gerbils recover with a residual head tilt.
Acknowledgments
Disclosure
The authors have nothing to disclose.
References
- 1.Johnson-Delaney CA. Geriatric Care of Rabbits and Rodents. Wild West Veterinary Conference 2011.
- 2.Taylor B.F., Ramirez H.E., Battles A.H. Analgesic activity of tramadol and buprenorphine after voluntary ingestion by rats (Rattus norvegicus) J Am Assoc Lab Anim Sci. 2016;55(1):74–82. [PMC free article] [PubMed] [Google Scholar]
- 3.Wodarrski R., Clark A.K., Grist J. Gabapentin reverses microglial activation in the spinal cord of streptozotocin-induced diabetic rats. Eur J Pain. 2009;13(8):807–811. doi: 10.1016/j.ejpain.2008.09.010. [DOI] [PubMed] [Google Scholar]
- 4.Vollmer K.O., von Hodenberg A., Kölle E.U. Pharmacokinetics and metabolism of gabapentin in rat, dog and man. Arzneimittelforschung. 1986;36(5):830–839. [PubMed] [Google Scholar]
- 5.Gauchan P., Andoh T., Ikeda K. Mechanical allodynia induced by paclitaxel, oxaliplatin and vincristine: different effectiveness of gabapentin and different expression of voltage-dependent calcium channel alpha(2)delta-1 subunit. Biol Pharm Bull. 2009;32(4):732–734. doi: 10.1248/bpb.32.732. [DOI] [PubMed] [Google Scholar]
- 6.Ewing L.E., Skinner C.M., Quick C.M. Hepatotoxicity of a cannabidiol-rich cannabis extract in the mouse model. Molecules. 2019;24(9) doi: 10.3390/molecules24091694. [pii:E1694] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hammell D.C., Zhang L.P., Ma F. Transdermal cannabidiol reduces inflammation and pain-related behaviours in a rat model of arthritis. Eur J Pain. 2016;20(6):936–948. doi: 10.1002/ejp.818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Braida D., Pegorini S., Arcidiacono M.V. Post-ischemic treatment with cannabidiol prevents electroencephalographic flattening, hyperlocomotion and neuronal injury in gerbils. Neurosci Lett. 2003;346(1–2):61–64. doi: 10.1016/s0304-3940(03)00569-x. [DOI] [PubMed] [Google Scholar]
- 9.Quesenberry K., Carpenter J. 3rd edition. 2011. Ferrets, rabbits, and rodents. Clinical medicine and surgery. [Google Scholar]
- 10.Heatley J.J. Cardiovascular anatomy, physiology, and disease of rodents and small exotic mammals. Vet Clin Exot Anim Pract. 2009;12(1):99–113. doi: 10.1016/j.cvex.2008.08.006. [DOI] [PubMed] [Google Scholar]
- 11.BSAVA Small Animal Formulary 7th edition.
- 12.Jin H., Nishino T., Aoe T. A simple and safe method for tracheal intubation using a supraglottic intubation-aid device in mice. Respir Physiol Neurobiol. 2019;263:9–13. doi: 10.1016/j.resp.2019.02.004. [DOI] [PubMed] [Google Scholar]
- 13.Cheong S.H., Lee J.H., Kim M.H. Airway management using a supraglottic airway device without endotracheal intubation for positive ventilation of anaesthetized rats. Lab Anim. 2013;47:89–93. doi: 10.1177/0023677212473919. [DOI] [PubMed] [Google Scholar]
- 14.Tamura Y. Current approach to rodents as patients. J Exot Pet Med. 2010;19(1):36–55. doi: 10.1053/j.jepm.2010.01.014. Standards of Care. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Coutant T., Vergneau-Grosset C., Langlois I. Overview of drug delivery methods in exotics, including their anatomic and physiologic considerations. Vet Clin Exot Anim Pract. 2018;21(2):215–259. doi: 10.1016/j.cvex.2018.01.006. [DOI] [PubMed] [Google Scholar]
- 16.VIN Formulary for Exotic Animals.
- 17.BSAVA Small Animal Formulary 8th edition.
- 18.Hocker S.E., Eshar D., Wouda R.M. Rodent oncology. Vet Clin Exot Anim Pract. 2017;20(1):111–134. doi: 10.1016/j.cvex.2016.07.006. [DOI] [PubMed] [Google Scholar]
- 19.Hotchkiss C.E. Effect of surgical removal of subcutaneous tumors on survival of rats. J Am Vet Med Assoc. 1995;206:1575–1579. [PubMed] [Google Scholar]
- 20.Dragan Y.P., Fahey S., Street K. Studies of tamoxifen as a promoter of hepatocarcinogenesis in female Fischer F344 rats. Breast Cancer Res Treat. 1994;31:11–25. doi: 10.1007/BF00689673. [DOI] [PubMed] [Google Scholar]
- 21.Keenan K., Smith P.F., Hertzog P. The effects of overfeeding and dietary restriction on Sprague-Dawley rat survival and early pathology biomarkers of aging. Toxicol Pathol. 1994;22(3):300–315. doi: 10.1177/019262339402200308. [DOI] [PubMed] [Google Scholar]
- 22.Mayer J., Sato A., Kiupel M. Extralabel use of cabergoline in the treatment of a pituitary adenoma in a rat. J Am Vet Med Assoc. 2011;239:656–660. doi: 10.2460/javma.239.5.656. [DOI] [PubMed] [Google Scholar]
- 23.Tucker M.J. The effect of long-term food restriction on tumours in rodents. Int J Cancer. 1979;23:803–807. doi: 10.1002/ijc.2910230611. [DOI] [PubMed] [Google Scholar]
- 24.Percy D.H., Barthold S.W. Pathology of laboratory rodents & rabbits. 2nd edition. Iowa State University Press; Ames (IA): 2001. Rat; pp. 107–158. [Google Scholar]
- 25.Donnelly T. Disease problems of small rodents. In: Quesenberry K.E., Carpenter J.W., editors. Ferrets, rabbits and rodents: clinical medicine and surgery. 2nd edition. WB Saunders; Philadelphia: 2004. pp. 305–308. [Google Scholar]
- 26.Anver M.R., Cohen B.J. Lesions associated with aging. In: Baker H.J., Lindsey J.R., Weisbroth S.H., editors. 1st edition. vol. 1. Academic Press; New York: 1979. pp. 378–399. (The laboratory rat). [Google Scholar]
- 27.Haines V. The ancient rat. Vet Clin North Am Exot Anim Pract. 2010;13(1):15–25. doi: 10.1016/j.cvex.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 28.Krinke G.J. Spontaneous radioneuropathology, aged rats. In: Jones T.C., Mohr U., Hunt R.D., editors. Monographs on pathology of laboratory animals: nervous system. SpringerVerlag; New York: 1988. pp. 203–208. [Google Scholar]
- 29.Jolivalt C.G., Mizisin L.M., Nelson A. B vitamins alleviate indices of neuropathic pain in diabetic rats. Eur J Pharmacol. 2009;10(612):41–47. doi: 10.1016/j.ejphar.2009.04.028. [DOI] [PubMed] [Google Scholar]
- 30.Sun H., Yang T., Li Q. Dexamethasone and vitamin B12 synergistically promote peripheral nerve regeneration in rats by upregulating the expression of brain-derived neurotrophic factor. Arch Med Sci. 2012;8(5):924–930. doi: 10.5114/aoms.2012.31623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.MacLachlan N.J., Dubovi E.J., editors. Retroviridae. Fenner’s veterinary virology. 5th edition. Elsevier; 2016. [Google Scholar]
- 32.Whary M.T., Anderson L., Otto G. American College of Laboratory Animal Medicine; 2012. Biology and diseases of mice. Lab animal medicine. The laboratory rabbit, Guinea pig, Hamster, and other rodents. [Google Scholar]
- 33.Pais P., D’Amato M. In vivo efficacy study of milk thistle extract (ETHIS-094™) in STAM™ model of nonalcoholic steatohepatitis. Drugs R D. 2014;14(4):291–299. doi: 10.1007/s40268-014-0068-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Karolewski B., Mayer T.W., Ruble G. American College of Laboratory Animal Medicine; 2012. Non-infectious diseases. Lab animal medicine. The laboratory rabbit, Guinea pig, Hamster, and other rodents. [Google Scholar]
- 35.Schmidt R.E., Reavill D.R. Cardiovascular disease in hamsters: review and retrospective study. J Exot Pet Med. 2007;16(1):49–51. [Google Scholar]
- 36.Merck Veterinary Manual. Accessed October 18, 2019.
- 37.Fabry A. The incidence of neoplasms in Syrian hamsters with particular emphasis on intestinal neoplasia. Arch Toxicol Suppl. 1985;8:124–127. doi: 10.1007/978-3-642-69928-3_18. [DOI] [PubMed] [Google Scholar]
- 38.Brown C., Donnelly T.M. Disease problems of small rodents. In: Quesenberry K., Ferrets C.J., editors. Rabbits, and rodents. Clinical medicine and surgery. 3rd edition. 2011. [Google Scholar]
- 39.McGraw C.P., Fleming D.F., Spruil J.H. Effect of methylprednisolone on experimental cerebral infarction in the Mongolian Gerbil. Stroke. 1974;5:444–445. doi: 10.1161/01.str.5.4.444. [DOI] [PubMed] [Google Scholar]