Abstract
Purpose
Fibromyalgia syndrome (FMS) is a chronic musculoskeletal pain disorder that is characterized by persistent and widespread pain. FMS has been associated with sleep disturbance, mood disorders and depression. Racial/ethnic minorities are less likely to receive a diagnosis of FMS than White individuals. Although mood disorders and depression are prevalent among racial/ethnic minority groups, researchers have not examined whether there are differences between racial/ethnic minorities and White individuals with FMS.
Participants and Methods
The participants were 600 people who were 18 years of age or older and who had a physician’s diagnosis of FMS, which was confirmed using the 1990 American College of Rheumatology criteria. Most participants were female (95.5%) and White (85.0%). Sleep disturbance was assessed using the Pittsburgh Sleep Quality Index (PSQI), mood disturbance was assessed using the Profile of Mood States (POMS), and depression was assessed via the Center for Epidemiological Studies Depression Scale (CES-D).
Results
Racial/ethnic minorities reported significantly greater levels of sleep disturbance, significantly greater levels of mood disturbance, and had significantly greater levels of depression than White participants. However, racial/ethnic minorities had significantly greater reductions in mood disturbance over the one-year period than White participants.
Conclusion
Overall, the findings from the present study indicated that racial/ethnic minorities had “worse” physical and psychological outcomes than White participants.
Keywords: chronic illness, pain, health disparities, psychosocial impact
Fibromyalgia syndrome (FMS) is a musculoskeletal disorder that is characterized by persistent and widespread pain.1–5 It is most commonly diagnosed among middle-aged White women.3,4 There are no agreed upon biomarkers or known etiology.5,6 However, researchers have suggested that genetics, abnormal nervous system functioning, socioeconomic status, along with other environmental triggers may be involved in the development of the condition.5,6 In 1990, the American College of Rheumatology (ACR) developed what was considered to be the gold standard for the diagnosis of FMS.5,7 The criteria specified a minimum pain severity score of two or greater on at least 11 of the 18 tender points in all four quadrants of the body, and pain existing for at least three months.7
FMS is more prevalent among White individuals than racial/ethnic minorities.5 However, researchers argue that the lower prevalence of FMS among racial/ethnic minority individuals is circumstantial, and proposed that the reason for this may be because physicians are less likely to diagnose racial/ethnic minorities with FMS than White individuals.2 Others have suggested that the lower rates of diagnoses can be attributed to health care professionals distrusting racial/ethnic minority patients’ claims of pain.3 The diagnosis of FMS is exclusionary, often requiring that other possible causes for patients’ symptoms be ruled out prior to a diagnosis being made.3 For this reason, factors that limit or affect health seeking behaviors such as education level, socioeconomic status, and racial/ethnic minority status may play a role in whether the condition is recognized and correctly diagnosed by a healthcare provider.8,9 Because racial/ethnic minorities are less likely to participate in research studies,10 few researchers have examined how the symptoms of FMS vary as a function of racial/ethnic minority status.3
Racial/Ethnic Minority Status
Barker11 hypothesized that African American women may be less likely to be diagnosed with FMS because of racial/ethnic health disparities and/or different cultural dispositions toward stress and suffering. Furthermore, Pryma3 suggested that women of color experienced racialized stigma when reporting their FMS symptoms. She conducted 1-hr interviews with participants asking them to explain their FMS-related experiences. She found that only the Black participants reported being denied a diagnosis of or treatment of their symptoms. However, this finding was not based on a difference in diagnoses between the racial/ethnic groups, but rather the participants’ report. She also reported that hospital staff distrusted racial/ethnic minority patients’ claims of pain more than those of White patients. Fear of this stigma may result in fewer racial/ethnic minority individuals seeking medical care when experiencing FMS-related symptoms.
Although researchers have suggested that the prevalence of FMS is lower among racial/ethnic minorities than White individuals, others have found that the prevalence of FMS may be higher among racial/ethnic minority women than among White women.2,12 Raphael et al2 asked participants whether they had pain in their muscles, bones, or joints lasting at least one week during the three months prior to responding to a survey. They found that reports of experiencing symptoms were more common among racial/ethnic minority women than among White women. However, these findings were inferences based on the symptoms used to diagnose FMS. Participant’s assessments were conducted over the phone, and no physical exams were performed.
More recently, FMS has been diagnosed by a constellation of physical, mental, and cognitive domains (ie, pain, sleep quality, mood disturbance, etc.).13 Given the limitations in knowledge regarding the sources of differential diagnostic rates between White people and racial/ethnic minority persons, it is important to know whether there are differences in the symptom constellations between these groups.
Pain
Researchers have found racial/ethnic differences in pain perception, pain tolerance, pain unpleasantness and chronic pain symptoms.14,15 Some researchers have examined how racial/ethnic differences affect the perception of experimental pain among college students.14 They found that African American participants reported lower thermal pain tolerance and higher ratings of pain unpleasantness than White participants. They also found that African American participants reported greater daily pain symptoms than White participants. Even though this sample did not consist of people with FMS, the findings may provide an explanation of why racial/ethnic minority individuals may be more sensitive to pain and report greater pain levels than White individuals.
Furthermore, the 2002 National Health Interview Survey questionnaire which was administered to over 31,000 adults, asked respondents whether they had ever been told by a doctor or other health professional that they had some form of arthritis, rheumatoid arthritis, gout, lupus, or FMS. The results indicated that Asian adults were less likely to report a diagnosis of arthritis or chronic joint symptoms than all other races. The results also indicated that White and Black or African American adults were more likely to report that they were diagnosed with arthritis and chronic joint symptoms than Hispanic adults.15
Depression and Mood Disturbance
Thirty to 70% of those diagnosed with FMS reported experiencing comorbid depression.16 Roxburgh17 suggested that depression may be moderated by socioeconomic status rather than by race. She found that “resource rich” Black women were less depressed than other groups of Black individuals, but overall Black individuals were significantly more depressed than White individuals. Gansky and Plesh6 also found that depression was moderated by race, such that the association of depressive symptoms and pain were stronger in African American women than White women among those with FMS. However, they only examined these differences between African American participants and White participants with FMS.
Sleep
Sleep disturbance is prevalent among people with FMS, and it can exacerbate other FMS-related symptoms.1 Researchers found that White individuals reported better sleep quality than racial/ethnic minority individuals.1 However, few researchers have examined sleep quality and sleep duration among racial/ethnic minority patients. Some evidence indicates that Black Americans experience a lower sleep duration and quality than White Americans, even after controlling for a variety of socioeconomic and demographic factors.18 Additionally, Hale and Do18 found that racial/ethnic minority individuals were more likely to have shorter sleep durations, and that the shorter sleep durations were associated with higher mortality rates. These researchers suggested that racial/ethnic minorities might have shorter sleep durations because they have a greater number of life stressors than White individuals.
However, other researchers conducted a cross-sectional survey of 9714 individuals to examine sleep quality among different racial/ethnic groups using a self-report measure.19 They found that poor sleep quality was strongly associated with poverty and race. Specifically, they found that African American and Latino groups had worse sleep quality than the non-poor White group. These researchers dichotomized income status as being above or below the Census Bureau poverty threshold. They suggested that income was a dominant factor in predicting sleep quality. Additionally, other researchers found that racial/ethnic minorities and participants with lower socioeconomic status reported having lower sleep durations.20 Thus, there appears to be racial/ethnic disparities in depression and sleep, but researchers have not examined whether these differences exist in people diagnosed with FMS.
The Current Study
Few researchers have examined pain, mood disturbance, depression, and sleep among racial/ethnic minority individuals with FMS. Therefore, the purpose of the present study was to determine whether these symptoms varied as a function of racial/ethnic minority status in a sample of males and females with FMS. In the present study, we examined whether these differences were the result of “other” differences between White individuals and racial/ethnic minorities. Racial/ethnic minority participants in the present study included Native Americans, Blacks, Hispanics/Latinxs, Asian Americans, and Other.
Methods
Participants
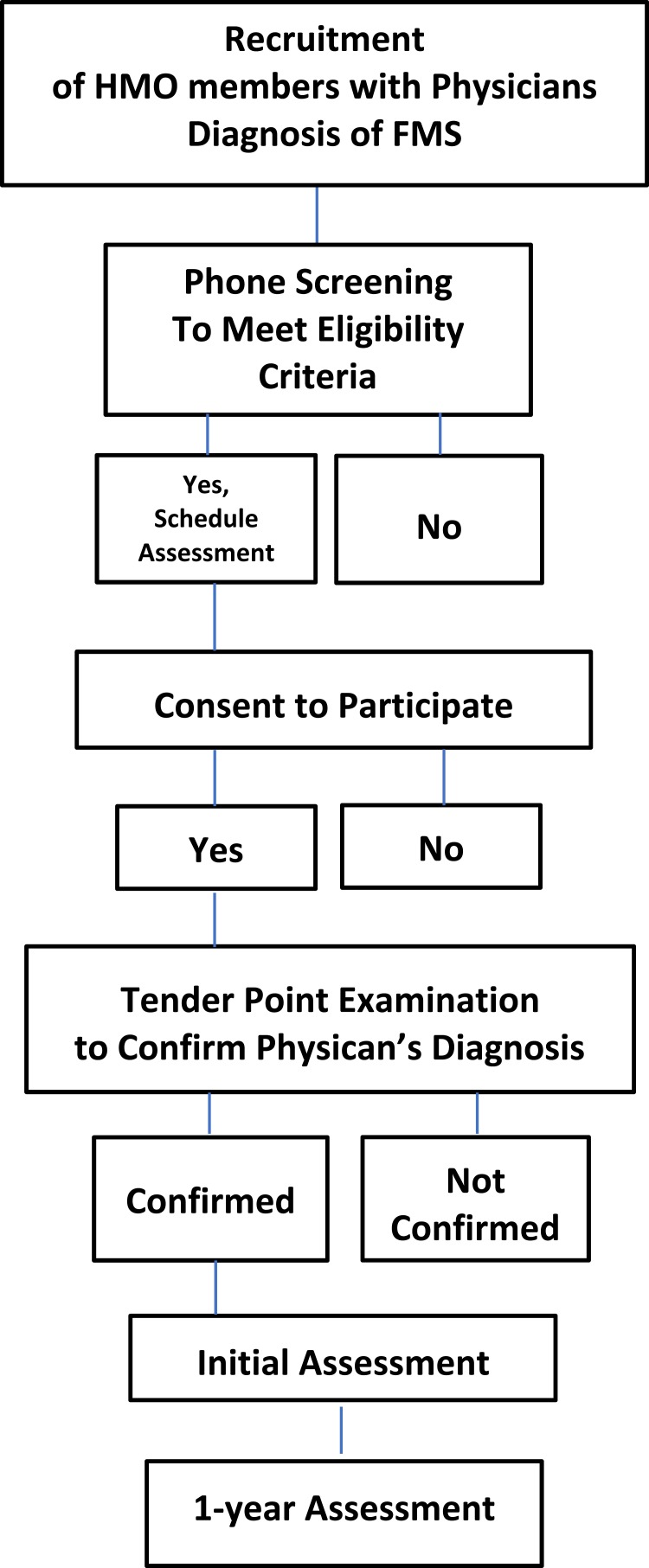
Six hundred participants (573 females) took part in a large randomized control trial intervention study conducted from 1997 to 2002, in which no intervention effects were found. The Institutional Review Boards at both San Diego State University and the Health Maintenance Organization (HMO) reviewed and approved the original study. The sample size for this study was based on a previous randomized clinical trial conducted in the same lab with osteoarthritis participants. Participants were 18 years of age or older, with a mean age of 53.92 (SD = 11.45), fluent in English, and had a physician’s diagnosis of FMS, which was confirmed by trained research assistants using the 1990 American College of Rheumatology diagnostic criteria (see Figure 1). Eighty-five percent were White, 1.7% were Native American, 3.3% were Black, 7.2% were Latinx, 0.5% were Asian, 2.0% were “Other,” and 0.3% declined to state. Furthermore, 62.3% were married, 49.3% were employed either part-time or full-time with 40 hrs being the most common average number of hours worked per week (44.3%) and 77% had completed some college or higher (See Table 1 for further demographic information).
Figure 1.
Process Selection of Participants with Inclusion and Exclusion Criteria.
Abbreviations: HMO, Health Maintenance Organization; FMS, Fibromyalgia Syndrome.
Table 1.
White and Racial/Ethnic Minority Mean Demographic Characteristic (N=600)
| Total N = 600 |
White n = 510 (85) |
Racial/Ethnic Minority n = 88 (14.67) |
|
|---|---|---|---|
| Age, M(SD) | 53.92 (11.45) | 54.63 (11.47) | 49.59 (10.33) |
| Gender, female, N (%) | 573 (95.5) | 486 (95.29) | 85 (96.59) |
| Weight, M(SD) | 174.04 (41.12) | 174.39 (40.79) | 169.88 (40.88) |
| Marital Status, N (%) | |||
| Single | 64 (10.67) | 52 (10.20) | 12 (13.64) |
| Married | 374 (62.33) | 327 (64.12) | 46 (52.27) |
| Widow or Widower | 28 (4.67) | 27 (5.29) | 1 (1.14) |
| Separated | 9 (1.50) | 8 (1.57) | 1 (1.14) |
| Divorced | 114 (19.00) | 86 (16.86) | 27 (30.68) |
| Remarried | 11 (1.83) | 10 (1.96) | 1 (1.14) |
| Employment Status, N (%) | |||
| Employed p/t | 93 (15.50) | 80 (15.69) | 13 (14.77) |
| Employed f/t | 203 (33.83) | 162 (31.76) | 41 (46.59) |
| Unemployed | 45 (7.50) | 39 (7.65) | 6 (6.82) |
| Retired | 137 (22.83) | 123 (24.12) | 12 (13.64) |
| Disabled | 68 (11.33) | 58 (11.37) | 10 (11.36) |
| Homemaker | 52 (8.67) | 46 (9.02) | 6 (6.82) |
| Student | 2 (0.33) | 2 (0.39) | 0 (0.00) |
| Education, N (%) | |||
| Grade school | 2 (0.33) | 1 (0.20) | 1 (1.14) |
| High school | 111 (18.50) | 101 (19.80) | 10 (11.36) |
| Some college | 300 (50.00) | 249 (48.82) | 50 (56.82) |
| Bachelor’s degree | 96 (16.00) | 82 (16.08) | 13 (14.77) |
| Master’s degree | 61 (10.17) | 53 (10.39) | 8 (9.09) |
| Doctorate | 5 (0.83) | 5 (0.98) | 0 (0.00) |
| Other Prof. Cert Decline to State |
24 (4.00) 1 (0.17) |
19 (3.73) 0 (0.00) |
5 (5.68) 1 (1.14) |
| Total Household Income, N (%) | |||
| Below $10,000 | 30 (5.16) | 25 (5.07) | 5 (5.81) |
| $10k - $20k | 63 (10.84) | 53 (10.75) | 9 (10.47) |
| $20,001 - $30k | 93 (16.01) | 79 (16.02) | 14 (16.28) |
| $30,001 - $40k | 127 (21.86) | 109 (22.11) | 18 (20.93) |
| $40,0001 - $50k | 90 (15.49) | 79 (16.02) | 11 (12.79) |
| $50,001 - $60k | 60 (10.33) | 48 (9.74) | 11 (12.79) |
| $60,001 - $70k | 41 (7.06) | 36 (7.30) | 5 (5.81) |
| Above $70k | 77 (13.25) | 64 (12.98) | 13 (15.12) |
| Weekly Hours Worked, M(SD) | 35.57 (12.75) | 34.71 (13.08) | 39.39 (10.42) |
Note: Two participants declined to state their ethnicity.
Abbreviations: M, mean; SD, standard deviation; N, sample size; p/t, part-time; f/t, full-time; Prof, professional; Cert, certification.
Procedure
Participants were recruited from a large Health Maintenance Organization (HMO) in San Diego, California. Researchers recruited participants by listing advertisements in the newspaper, posting flyers in the HMO waiting rooms, sending letters to randomly selected HMO members, and through emails sent to physicians informing them of the study and asking them to refer qualified patients. Interested individuals were asked to call the project coordinator. The project coordinator reviewed the eligibility criteria and scheduled an assessment in the project offices for qualified individuals.
After participants provided their written informed consent, trained research assistants confirmed the individual’s FMS diagnosis by conducting a manual tender point examination using the 1990 ACR criteria for FMS. This diagnostic criteria identifies 18 locations on the human body that have been associated with widespread pain reported by FMS patients.7 To complete the examination in accordance with the ACR criteria, trained research assistants applied digital pressure to 21 tender point locations (3 control locations) and asked participants to rate their pain levels verbally using an 11-point scale ranging from 0 (no pain) to 10 (the worst pain they have ever experienced). Participants were required to demonstrate a minimum pain severity score of two or greater on at least 11 of the 18 tender point locations, for their pain to be located in all four quadrants of the body, and for their pain to have existed for at least three months. Participants who qualified for the study were asked to complete a battery of questionnaires at entry into the study and again 1 year later.
Measures
Demographics
As part of the assessment, participants reported their ethnicity, gender, age in years, marital status, employment status, number of hours worked per week, their highest level of education, and income at baseline.
Fibromyalgia Impact
Health status was assessed using the Fibromyalgia Impact Questionnaire (FIQ). The FIQ is a self-report questionnaire that measures fatigue, physical functioning, anxiety, sleep, depression, well-being, and work status. The FIQ has 10 questions and some of the questions have multiple items. The first question assessed the ability to do tasks (ie, make a bed, vacuum), which had a 4-point Likert scale ranging from 0 (always) to 3 (never). The second two questions asked how many days the participant felt good and how many days FMS impacted work, including housework. Questions four through 10 assessed the symptoms of FMS (ie, energy, sleep), which had a response option of a horizontal linear scale with 10 different increments ranging from zero to 10 with varying response anchors. Each of the questions had their own score and were then summed for the FIQ total.21 Within our sample, the FIQ had good internal consistency at baseline (Cronbach’s α = 0.751).
Pain
Pain was assessed through the McGill Pain Questionnaire (MPQ), which assesses sensory, affective, evaluative, and miscellaneous pain. Participants were presented with 78 adjectives, which were split into four categories. The participants were asked to mark the word that best described their pain in the past week. The adjectives were presented in groups of three to six words and scores ranged from 0 (mild) to 6 (severe). The scores were then summed to total one score.1 The MPQ had good internal consistency in our sample at baseline (Cronbach’s α = 0.830).
Depression
Depression was assessed via the Center for Epidemiological Studies Depression Scale (CES-D). This scale is a self-report scale designed to measure depressive symptoms in the general population.22 This scale contained 20 items rated on a 4-point Likert scale ranging from 0 (rarely or none of the time, < 1 day) to 3 (most or all of the time, 5–7 days), and measured symptoms of depression during the past week. The CES-D had good internal consistency at baseline (Cronbach’s α = 0.718).
Mood Disturbance
Mood disturbance was assessed using the Profile of Mood States (POMS). The POMS is a 65-item self-report questionnaire. Moods were presented on a 5-point Likert Scale from 0 (not at all) to 4 (extremely) and respondents were asked to mark how they had been feeling in the past week. Examples of moods were friendly, tense, lively, and unhappy.23 An individual adjective score and a total mood disturbance score was calculated for each person.Higher scores indicated higher mood disturbance. The depression subscale was excluded because depression was measured with the CES-D. The POMS had very high internal consistency at baseline in our sample (Cronbach’s α = 0.934).
Sleep Quality
Sleep quality was assessed using the Pittsburgh Sleep Quality Index (PSQI). The PSQI is a self-rated questionnaire which assesses sleep quality and disturbances in older adults by measuring seven domains: subjective sleep quality, sleep latency, sleep duration, habitual sleep efficacy, sleep disturbances, use of sleeping medications, and daytime dysfunction in the last month.24 The index used 19 items, grouped into these seven component scores. Each item was weighted equal on a 4-point Likert scale, ranging from 0 (not during the past month) to 3 (≥ 3 times a week).25 These component scores were summed so that the total PSQI score could range from 0–21. Higher scores indicated worse sleep quality.1 The rating scale components of the PSQI had good internal consistency in our sample at baseline (Cronbach’s α =. 0.767).
Statistical Approach
Analyses were performed using Stata/IC 15.1. Given the majority of White participants, all racial/ethnic minority groups were aggregated for analytical purposes. Differences between White participants and racial/ethnic minority participants were examined across a variety of domains, including other demographic characteristics, clinical characteristics, pain and FMS interference, depression and mood, sleep, and self-efficacy. A variety of tests (see Tables 2 and 3), dependent upon the outcome data type, were used to evaluate baseline differences. Scales of measurement for the outcomes were used to select analytic methods. For single items that used ordinal scales with a limited number of discrete categories, ordinal analyses were applied. For nominal data, frequency-based analyses were applied. For the data that were binary, Poisson models were used. Assessing the moments of data and inferential conclusions under robust tests yielded no concerns regarding the application of the conventional tests which are reported. Mixed effects models were used for analyses that included a longitudinal component. Time was treated as categorical because there were only two time-points (baseline and year 1). These models included a random intercept term for participants. Contrasts were performed to evaluate the specific effects within the mixed effects models. All results were evaluated for significance at α = 0.05.
Table 2.
White and Racial/Ethnic Minority Comparisons for Demographic Characteristics at Baseline
| Test | Statistic | p | Effect Size | |
|---|---|---|---|---|
| Gender | Test of Independence | χ2(1) = 0.293 | 0.588 | Cramér’s V = 0.022 |
| Weight | Independent-Samples t-test | t(590) = 0.958 | 0.339 | Cohen’s d = 0.111 |
| Marital Status | Test of Independence | χ2(2) = 5.254 | 0.072 | Cramér’s V = 0.094 |
| Employment Status | Test of Independence | χ2(4) = 6.892 | 0.142 | Cramér’s V = 0.107 |
| Education | Test of Independence | χ2(2) = 3.275 | 0.195 | Cramér’s V = 0.074 |
| Total Household Income | Ordered Logistic Regression | χ2(1) = 0.03 | 0.860 | Pseudo R2 < 0.001 |
| Age | Independent-samples t-test | t(596) = 3.857 | 0.0001* | Cohen’s d = 0.446 |
| Weekly Hours Worked | Independent-samples t-test | t(294) = 2.457 | 0.015* | Cohen’s d = 0.371 |
Note: *p ≤ 0.05.
Table 3.
White and Racial/Ethnic Minority Comparisons for Clinical and Health Outcomes at Baseline
| Test | Statistic | p | Effect Size | |
|---|---|---|---|---|
| Number of TP | Poisson Regression | χ2(1) = 4.47 | 0.034* | IRR REM/W = 1.105 |
| Cumulative TP Pain | Independent-Samples t-test | t(596) = 2.631 | 0.009* | Cohen’s d = 0.304 |
| Number of Words to Describe Pain from MPQ | Poisson Regression | χ2(1) = 19.55 | 0.006* | IRR REM/W = 1.160 |
| PPI from MPQ | Ordered Logistic Regression | χ2(1) = 1.12 | 0.291 | Pseudo R2 < 0.001 |
| Health Rating | Ordered Logistic Regression | χ2(1) = 10.98 | 0.001* | Pseudo R2 = 0.007 |
| Other Rheumatic Disorder(s) | Test of Independence | χ2(1) = 5.26 | 0.02* | ORREM/W = 0.566 |
| Sum of Trauma Severity Ratings | Independent Samples t-test | t(543) = 0.466 | 0.641 | Cohen’s d = 0.057 |
Note: *p ≤ 0.05.
Abbreviations: TP, Tender Points; MPQ, McGill Pain Questionnaire; PPI, Present Pain Index; IRR, Incidence Rate Ratio; OR, Odds Ratio; REM, Racial/Ethnic Minority; W, White.
Results
Baseline Analyses
Demographics
Racial/ethnic minority participants did not differ from White participants with respect to gender, weight, marital status (Single, Married/Remarried, Widowed/Separated/Divorced), employment status (Working, Unemployed, Retired, Disabled, Homemaker), education (HS or Less, Some College, 4-year degree or more), and total household income (1 = Below $10,000/year; 8 = Above $70,000/year; see Table 2). Racial/ethnic minority participants (M = 49.59, SD = 10.33, 95% CI[47.40, 51.78]) were significantly younger than White participants (M = 54.63, SD = 11.47, 95% CI[53.63, 55.63]; see Table 2). Racial/ethnic minority participants (M = 39.39, SD = 10.42, 95% CI[36.54, 42.23]) also worked significantly more hours per week than White participants (M = 34.71, SD = 13.08, 95% CI[33.06, 36.37]; see Table 2) among those who reported working outside of the home.
Clinical and Health Outcomes
Racial/ethnic minority participants exhibited significantly more tender points (centered at the minimum requirement of 11; M = 6.24, SE = 0.27) and higher cumulative tender point pain ratings (M = 91.27, SD = 33.29, 95% CI[84.22, 98.33]) than White participants (M = 5.65, SE = 0.11 and M = 81.58, SD = 31.66, 95% CI[78.83, 84.34], respectively; see Table 3). Racial/ethnic minority participants (M = 12.52, SE = 0.38) also reported a significantly greater number of words to describe their pain (centered at the minimum of 2) than White participants (M = 10.80, SE = 0.15; see Table 3). However, there were no differences between White and racial/ethnic minority participants on the McGill Present Pain Intensity (PPI) Index (see Table 3). Racial/ethnic minority participants were more likely to report very poor, poor, and fair health and less likely to report good or excellent health than White participants (see Table 3). However, racial/ethnic minority participants (Odds = 0.42) were significantly less likely to be diagnosed with additional rheumatic conditions than White participants (Odds = 0.74; see Table 3). Lastly, there were no differences between White (M = 28.89, SD = 18.31, 95% CI[27.22, 30.56]) and racial/ethnic minority (M = 27.86, SD = 17.92, 95% CI[23.87, 31.85]) participants in the sum of trauma severity ratings (see Table 3).
Longitudinal Analyses
Fibromyalgia Impact
Racial/ethnic minority participants (M = 64.94, SE = 1.60; M = 1.51, SE = 0.07, respectively) reported significantly greater levels of FMS impact globally and on the physical functioning subcomponent based on the FIQ aggregated over both time points than White participants (M = 58.08, SE = 0.66; M = 1.32, SE = 0.03), p < 0.001, respectively. There was also a significant main effect of time for global impact, p < 0.001, but there was not for physical functioning specifically, p = 0.110. Global FMS impact was higher at baseline (M = 61.19, SE = 0.67) than at Year 1 (M = 56.46, SE = 0.72). There were no interactions, indicating that the change in global FMS impact and physical functioning over the 1-year period did not depend on racial/ethnic minority status, ps = 0.595, 0.848, respectively.
Sensory, Affective, and Evaluative Pain
Racial/ethnic minority participants (M = 19.46, SE = 0.66; M = 4.05, SE = 0.24; M = 5.88, SE = 0.28, respectively) reported significantly greater levels of sensory, affective, and evaluative pain on the MPQ aggregated over both time points than White participants (M = 17.87, SE = 0.27; M = 3.06, SE = 0.10; M = 5.15, SE = 0.12, respectively), ps = 0.027, 0.0003, 0.025, respectively. There was not a significant main effect of time for sensory pain, p = 0.077, but there were significant main effects of time for affective, p < 0.001, and evaluative pain, p < 0.001. Both affective and evaluative pain were higher at baseline (M = 3.52, SE = 0.11; M = 5.54, SE = 0.13, respectively) than at Year 1 (M = 2.79, SE = 0.12; M = 4.89, SE = 0.14, respectively). There were no interactions for any of the pain types, indicating that the change in pain components over the 1-year period did not depend on racial/ethnic minority status, ps = 0.727, 0.148, 0.433, respectively.
Depression and Mood
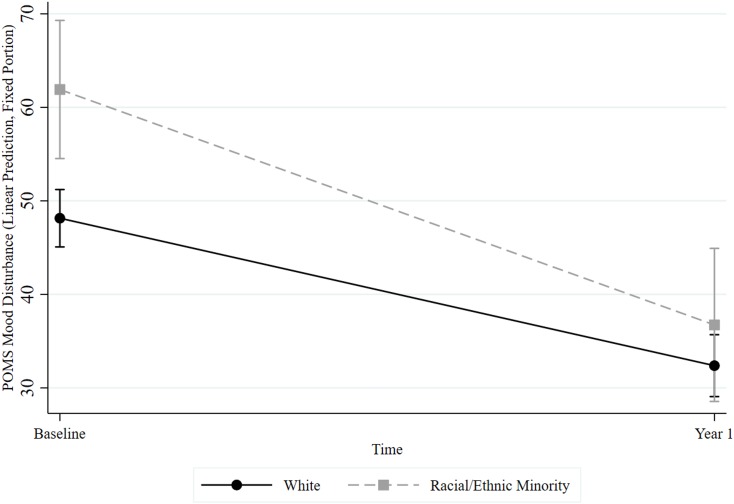
Racial/ethnic minority participants (M = 20.18, SE = 1.02) reported significantly greater levels of depression on the CES-D aggregated over both time points than White participants (M = 17.08, SE = 0.42), p = 0.009. There was a significant main effect of time for depression, p < 0.001. Depression scores were higher at baseline (M = 19.77, SE = 0.43) than at Year 1 (M = 14.75, SE = 0.17). There was no interaction, indicating that changes in depression over the 1-year period did not depend on racial/ethnic minority status, p = 0.120. Racial/ethnic minority participants (M = 50.57, SE = 3.34) reported significantly greater levels of mood disturbance on the POMS over both time points than White participants (M = 41.04, SE = 1.38), p = 0.013. There was a significant main effect of time for mood disturbance, p < 0.001. Mood distribution was higher at baseline (M = 50.12, SE = 1.45) than at Year 1 (M = 33.00, SE = 1.56). There was an interaction, indicating that changes in mood disturbance over the 1-year period did depend on racial/ethnic minority status, p = 0.040 (see Figure 2). Although racial/ethnic minority participants (M = 61.91, SE = 3.77) were significantly higher in mood disturbance than White participants (M = 48.14, SE = 1.57) at baseline, there were no longer significant differences in mood disturbance between racial/ethnic minorities (M = 36.73, SE = 4.18) and White (M = 32.38, SE = 1.69) participants at year 1.
Figure 2.
The Effects of Racial/Ethnic Minority Status and Time on Mood Disturbance.
Abbreviation: POMS, Profile of Mood States.
Sleep Quality
Racial/ethnic minority participants (M = 12.50, SE = 0.38) reported significantly greater levels of sleep disturbance on the Global Sleep Quality Index of the PSQI aggregated over both time points than White participants (M = 10.76, SE = 0.16), p < 0.001. There was a significant main effect of time, p = 0.004. Sleep disturbance was higher at baseline (M = 11.23, SE = 0.16) than at Year 1 (M = 10.74, SE = 0.17). There was no interaction, indicating that changes in sleep disturbance over the 1-year period did not depend on racial/ethnic minority status, p = 0.241.
Self-Efficacy
Racial/ethnic minority participants (M = 54.99, SE = 1.66) reported significantly lower levels of disease-specific self-efficacy aggregated over both time points than White participants (M = 59.03, SE = 0.68), p < 0.001. There was a significant main effect of time, p < 0.001. Self-efficacy was lower at baseline (M = 55.63, SE = 0.70) than at Year 1 (M = 61.91, SE = 0.75). There was no interaction, indicating that changes in self-efficacy over the 1-year period did not depend on racial/ethnic minority status, p = 0.639.
Discussion
The purpose of the present study was to determine whether sleep, mood disturbance, and depression varied as a function of racial/ethnic minority status in people with FMS. Both White and racial/ethnic minority individuals improved over time on all but one of the outcome measures. Both groups had reductions in physical (FMS impact) and psychological well-being from baseline to the 1-year follow-up. Thus, with one exception, the degree of change over the 1-year period did not differ between the two groups. However, the results indicated that the experiences of White individuals and racial/ethnic minority individuals with FMS differed significantly across a wide array of domains—with racial/ethnic minorities having “worse” outcomes in general.
The racial/ethnic minorities in the present study had a significantly greater number of tender points and higher cumulative tender point ratings than White participants. Researchers have not assessed whether the number of tender points differed between White individuals and racial/ethnic minority individuals. However, some researchers found racial/ethnic minority individuals had greater daily pain than White individuals in a non-FMS sample.14 In the present study, racial/ethnic minorities reported higher levels of sensory, affective, and evaluative pain as measured by the MPQ than White individuals. This finding is consistent with other reports of racial/ethnic differences in both clinical and experimental pain.26 Furthermore, Fabian et al27 found that African American individuals and Asian/Pacific Islanders had higher intensity scores in affective and sensory pain than White individuals. This finding is interesting given that other researchers found that Asian adults were less likely to report arthritis or chronic joint symptoms than all other races/ethnicities as mentioned previously.15 Thus, the findings from the present study and those of others indicate that racial/ethnic minorities report higher levels of pain than White individuals.
One possible explanation for the mixed results in racial/ethnic minority pain differences could be that there are intra-racial and ethnic differences in pain perception and to the sensitivity of pain. For instance, in one study, Black individuals generally showed lower pain tolerance and lower pain thresholds than White individuals.28 These researchers also suggested that Black individuals may experience greater negative affect than White individuals. Negative affect is a general measure of negative mood. It reflects emotions such as anger, fear, anxiety, shame and disgust.29 Thus, Black individuals may have lower pain thresholds because of higher levels of negative affect. Another explanation may be that Black individuals have higher levels of pain that lead to higher levels of negative affect. However, in the present study, there were no differences in the global measures of pain intensity between White participants and racial/ethnic minority participants; the differences were only in specific aspects of the pain experience.
In the present study, racial/ethnic minorities had significantly lower health status, and FMS impacted them significantly more than White individuals. Researchers have suggested that the reason racial/ethnic minorities were more likely to suffer from severe pain and pain related disabilities than White individuals, was because they were less likely to have access to primary care.30 This finding is supported by the findings of Bonham31 who found that among Americans, Black and Hispanic individuals were more likely to be undertreated for pain than White persons. However, all the participants in the present study were a part of the same HMO, so access to care may not explain differences between our sample. However, some researchers have found that racial/ethnic minorities were less likely to explain the pain they were experiencing when there was race-discordance with the physician. The explanation for this was because they did not feel respected when communicating with a physician who was not of the same racial/ethnic group.28,32
The findings from the present study indicated that racial/ethnic minorities were less likely to have been diagnosed with other rheumatic conditions than White individuals. However, few researchers have examined the reasons for these differences. Some have suggested that the differences in the rates of FMS may be because physicians underestimate the pain of racial/ethnic minorities,28,33 or because health care providers do not perform accurate pain assessments on racial/ethnic minorities.34
The results of the present study indicated that racial/ethnic minority participants also experienced greater mood disturbance and depression, greater levels of sleep disturbance, and lower levels of disease specific self-efficacy than White participants. Previous researchers have not examined differences in psychosocial functioning between racial/ethnic minorities with FMS. However, Green et al35 examined psychosocial differences between White and African American individuals in the general population. They found that the African American participants reported higher levels of depression, greater sleep disturbances, greater irritability, and were affected by their chronic pain more than the White participants. In addition, Gagnon et al36 found that both the Latinx and African American participants had greater emotional distress than White participants. They found that African American individuals had significantly higher levels of depression than the White individuals, but the Latinx participants did not. One possible explanation for these findings may be that untreated pain causes more psychological distress. Thus, racial/ethnic minority individuals may experience a variety of negative psychosocial outcomes because of their increased levels of distress.
The results from the present study also indicated that racial/ethnic minority participants showed significantly greater reductions in mood disturbance over the 1-year period than did White participants. Both groups had reductions in pain and global impact of FMS, but the reductions were greater for racial/ethnic minorities. One explanation for this reduction may be because of the reductions in pain and the global impact of FMS that occurred over the 1-year period. Racial/ethnic minorities levels of pain and the global impact on FMS were higher at baseline than the 1-year assessment, which could have resulted in greater improvements in mood than in the White participants.
The findings from the present study indicated that although most of the participants had experienced at least one traumatic event (91%) before they were diagnosed with FMS, there were no significant differences between White individuals and racial/ethnic minorities in their ratings of the severity of their traumatic event(s). Traumatic events have been reported to precede the onset of FMS regardless of their racial/ethnic group.5 However, more research is needed in this area to determine whether the type of trauma experienced results in different symptoms of FMS.
Few researchers have examined the effects of interventions designed to reduce pain among racial/ethnic individuals with FMS. However, Meghani30 reported that the poor, racial/ethnic minorities, and the uninsured and underinsured were more likely to experience disparities than those who were not affected by such circumstances. Of course, there is tremendous overlap in these three groups. Other researchers have reported that White individuals and racial/ethnic minorities have similar enrollments in HMOs (Hispanics 51.6%, Blacks 47.2%, and Whites and other 40.1%),37 and in the present study, as mentioned previously, all participants were enrolled in the same HMO. However, there may have been differences in the treatment provided to racial/ethnic minorities within the HMO for their chronic pain or factors that limited individuals’ help seeking behaviors as previously mentioned (ie education, socioeconomic status, and race-discordance).8,9,28,32
Limitations
Although all of the participants were members of the same HMO, a large percentage of the population belongs to HMOs, and these findings may be generalized to members of other HMOs but may not be applicable to non-HMO populations. Also, because the majority of the sample were White participants, intra-racial/ethnic group differences were not able to be examined. Having a sample comprised of more racial/ethnic minority individuals would have allowed for the examination of within-group differences. Finally, most of the participants were females, which is consistent with the reported prevalence of FMS. However, this may limit the findings from this study to males with FMS.
Conclusion
The findings from the present study indicated that among FMS patients, racial/ethnic minority individuals had “worse” physical and psychological outcomes than White individuals. Our findings provide evidence for health disparities in FMS, but we do not know the reasons for or causal interrelations of these differences. More attention needs to be given to the recruitment of racial/ethnic minorities in FMS research studies because there may be differences in the presentation of symptoms as a function of racial/ethnic groups. Health disparities merit special consideration in the context of treatment studies and options. Physicians and other health care providers may need additional training in racial/ethnic differences to ensure that all patients receive appropriate care.
Funding Statement
This study was supported by NIH grant AR-44020.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Bigatti SM, Hernandez AM, Cronan TA, Rand KL. Sleep disturbances in fibromyalgia syndrome: relationship to pain and depression. Arthritis Rheum. 2008;59(7):961–967. doi: 10.1002/art.v59:7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Raphael KG, Janal MN, Nayak S, Schwartz JE, Gallagher RM. Psychiatric comorbidities in a community sample of women with fibromyalgia. Pain. 2006;124(1–2):117–125. doi: 10.1016/j.pain.2006.04.004 [DOI] [PubMed] [Google Scholar]
- 3.Pryma J. Even my sister says I’m acting like a crazy to get a check: race, gender, and moral boundary-work in women’s claims of disabling chronic pain. Soc Sci Med. 2017;181:66–73. doi: 10.1016/j.socscimed.2017.03.048 [DOI] [PubMed] [Google Scholar]
- 4.Van Liew C, Brown KC, Cronan TA, Bigatti SM. The effects of self-efficacy on depression and pain in fibromyalgia syndrome: does initial depression matter? J Musculoskelet Pain. 2013;21(2):113–125. doi: 10.3109/10582452.2013.797536 [DOI] [Google Scholar]
- 5.Haviland MG, Morton KR, Oda K, Fraser GE. Traumatic experiences, major life stressors, and self-reporting a physician-given fibromyalgia diagnosis. Psychiatry Res. 2010;177(3):335–341. doi: 10.1016/j.psychres.2009.08.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gansky SA, Plesh O. Widespread pain and fibromyalgia in a biracial cohort of young women. J Rheumatol. 2007;34(4):810–817. [PubMed] [Google Scholar]
- 7.Wolfe F, Smythe HA, Yunus MB, et al. The American College of Rheumatology 1990 criteria for the classification of fibromyalgia. Report of the multicenter criteria committee. Arthritis Rheum. 1990;33(2):160–172. doi: 10.1002/(ISSN)1529-0131 [DOI] [PubMed] [Google Scholar]
- 8.Begley C, Basu R, Lairson D, et al. Socioeconomic status, health care use, and outcomes: persistence of disparities over time. Epilepsia. 2011;52(5):957–964. doi: 10.1111/epi.2011.52.issue-5 [DOI] [PubMed] [Google Scholar]
- 9.Vickrey BG, Shapiro MF. Disparities research in neurology: an urgent need. Nat Rev Neurol. 2009;5(4):184–185. doi: 10.1038/nrneurol.2009.30 [DOI] [PubMed] [Google Scholar]
- 10.Fisher JA, Kalbaugh CA. Challenging assumptions about minority participation in US clinical research. Am J Public Health. 2011;101(12):2217–2222. doi: 10.2105/AJPH.2011.300279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barker KK. The Fibromyalgia Story: Medical Authority and Women’s Worlds of Pain. Philadelphia: Temple University Press; 2005. [Google Scholar]
- 12.Menzies V, Sunny K. Relaxation and guided imagery in Hispanic persons diagnosed with fibromyalgia: A pilot study. Fam Community Health. 2008;31(3):204–212. doi: 10.1097/01.FCH.0000324477.48083.08 [DOI] [PubMed] [Google Scholar]
- 13.Wolfe F, Clauw DJ, Fitzcharles MA, et al. The American College of Rheumatology preliminary diagnostic criteria for fibromyalgia and measurement of symptom severity. Arthritis Care Res. 2010;62(5):600–610. doi: 10.1002/acr.20140 [DOI] [PubMed] [Google Scholar]
- 14.Edwards RR, Fillingim RB. Ethnic differences in thermal pain responses. Psychosom Med. 1999;61(3):346–354. doi: 10.1097/00006842-199905000-00014 [DOI] [PubMed] [Google Scholar]
- 15.Lethbridge-Cejku M, Schiller J, Bernadel L. Summary health statistics for U.S. adults: national health interview survey, 2002. Vital Health Stat. 2004;10(222):1–151. [PubMed] [Google Scholar]
- 16.Thieme K, Turk DC, Flor H. Comorbid depression and anxiety in fibromyalgia syndrome: relationship to somatic and psychosocial variables. Psychosom Med. 2004;66(6):837–844. doi: 10.1097/01.psy.0000146329.63158.40 [DOI] [PubMed] [Google Scholar]
- 17.Roxburgh S. Untangling inequalities: gender, race, and socioeconomic differences in depression. Sociol Forum. 2009;24(2):357–381. doi: 10.1111/j.1573-7861.2009.01103.x [DOI] [Google Scholar]
- 18.Hale L, Do P. Racial differences in self-reports of sleep duration in a population-based study. Sleep. 2007;30(9):1096–1103. doi: 10.1093/sleep/30.9.1096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Patel NP, Grandner MA, Xie D, Branas CC, Gooneratne N. “Sleep disparity” in the population: poor sleep quality is strongly associated with poverty and ethnicity. BMC Public Health. 2010;10(475):1–11. doi: 10.1186/1471-2458-10-475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Whinnery J, Jackson N, Rattanaumpawan P, Grandner MA. Short and long sleep duration associated with race/ethnicity, sociodemographics, and socioeconomic position. Sleep. 2014;37(3):601–611. doi: 10.5665/sleep.3508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bennett R. The Fibromyalgia Impact Questionnaire (FIQ): a review of its development, current version, operating characteristics and uses. Clin Exp Rheumatol. 2005;23(5 Suppl 39):S154–S162. [PubMed] [Google Scholar]
- 22.Radloff LS. The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1(3):385–401. doi: 10.1177/014662167700100306 [DOI] [Google Scholar]
- 23.Bigatti SM, Cronan TA. An examination of the physical health, health care use, and psychological well-being of spouses of people with fibromyalgia syndrome. Health Psychol. 2002;21(2):157–166. doi: 10.1037/0278-6133.21.2.157 [DOI] [PubMed] [Google Scholar]
- 24.Smyth C. The Pittsburgh Sleep Quality Index (PSQI). J Gerontol Nurs. 1999;25(12):10–11. doi: 10.3928/0098-9134-19991201-10 [DOI] [PubMed] [Google Scholar]
- 25.Buysse DJ, Reynolds CF III, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28(2):193–213. doi: 10.1016/0165-1781(89)90047-4 [DOI] [PubMed] [Google Scholar]
- 26.Edwards RR, Doleys DM, Fillingim RB, Lowery D. Ethnic differences in pain tolerance: clinical implications in a chronic pain population. Psychosom Med. 2001;63(2):316–323. doi: 10.1097/00006842-200103000-00018 [DOI] [PubMed] [Google Scholar]
- 27.Fabian LA, McGuire L, Goodin BR, Edwards RR. Ethnicity, catastrophizing, and qualities of the pain experience. Pain Med. 2011;12(2):314–321. doi: 10.1111/j.1526-4637.2010.01015.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tait RC, Chibnall JT. Racial/ethnic disparities in the assessment and treatment of pain. Am Psychol. 2014;69(2):131–141. doi: 10.1037/a0035204 [DOI] [PubMed] [Google Scholar]
- 29.Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: the PANAS scales. J Pers Soc Psychol. 1988;54(6):1063–1070. doi: 10.1037/0022-3514.54.6.1063 [DOI] [PubMed] [Google Scholar]
- 30.Meghani SH. Corporatization of pain medicine: implications for widening pain care disparities. Pain Med. 2011;12(4):634–644. doi: 10.1111/j.1526-4637.2011.01074.x [DOI] [PubMed] [Google Scholar]
- 31.Bonham VL. Race, ethnicity, and pain treatment: striving to understand the causes and solutions to the disparities in pain treatment. J Law Med Ethics. 2001;28(s4):52–68. doi: 10.1111/j.1748-720X.2001.tb00039.x [DOI] [PubMed] [Google Scholar]
- 32.Cooper-Patrick L, Gallo JJ, Gonzales JJ, et al. Race, gender, and partnership in the patient-physician relationship. JAMA. 1999;282(6):583–589. doi: 10.1001/jama.282.6.583 [DOI] [PubMed] [Google Scholar]
- 33.Staton LJ, Panda M, Chen I, et al. When race matters: disagreement in pain perception between patients and their physicians in primary care. J Nati Med Assoc. 2007;99(5):532–538. [PMC free article] [PubMed] [Google Scholar]
- 34.Mailis-Gagnon A, Yegneswaran B, Nicholson K, et al. Ethnocultural and sex characteristics of patients attending a tertiary care pain clinic in Toronto, Ontario. Pain Res Manag. 2007;12(2):100–106. doi: 10.1155/2007/425318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Green CR, Anderson KO, Baker TA, et al. The unequal burden of pain: confronting racial and ethnic disparities in pain. Pain Med. 2003;4(3):277–294. doi: 10.1046/j.1526-4637.2003.03034.x [DOI] [PubMed] [Google Scholar]
- 36.Gagnon CM, Matsuura JT, Smith CC, Stanos SP. Ethnicity and interdisciplinary pain treatment. Pain Pract. 2014;14(6):532–540. doi: 10.1111/papr.2014.14.issue-6 [DOI] [PubMed] [Google Scholar]
- 37.Banthin JS, Taylor AK. HMO Enrollment in the United States: Estimates Based on Household Reports. No 15. Rockville: Agency for Healthcare Research and Quality; 1996. [Google Scholar]