Abstract
The study estimated changes of 5‐hydroxymethyl‐2‐furfuraldehyde (5‐HMF) in different ginseng products with different temperatures and time pretreatment. Heat treatment was performed at various temperatures for 1.50, 2.00, 2.50, and 3.00 hr, respectively. Ultrasonic extraction and reflux extraction were used to evaluate the extraction rate and different solvents (such as 80% methanol, dichloromethane, ethyl acetate, and an extraction with both dichloromethane and ethyl acetate solvents) using two extraction methods (liquid–liquid extraction and solid‐phase extraction) to remove matrix interference. An ultraperformance liquid chromatography–mass spectrometer (UPLC‐MS) method was used for quantitative and changing analysis of 5‐HMF in different ginseng samples. The results indicated that the content of 5‐HMF increased dramatically with heating temperature and time, and the 5‐HMF in the ginseng samples ranged from 0.01 to 112.32 g/kg protein. The highest value was observed in the honey‐added ginseng samples with the highest amount of addition and highest temperature treatment, and the lowest value was found in the fresh ginseng samples. These results implied that 5‐HMF may be as an indicator to estimate the honey addition level and heat treatment degree during the processing of ginseng products, and the content of 5‐HMF is a promising parameter to evaluate the quality of products (ginseng). The production and regulation of potentially harmful Maillard reaction products (PHMRPs)‐5‐HMF in ginseng manufacture will provide an important reference for safe ginseng processing.
Keywords: 5‐hydroxymethyl‐2‐furfural, ginseng processing, ultraperformance liquid chromatography–mass spectrometer
The identification of 5‐hydroxymethyl‐2‐furfural (5‐HMF) in ginsengs was confirmed using an ultraperformance liquid chromatography–mass spectrometer (UPLC‐MS) method. The generation and regulation of potentially harmful Maillard reaction products (MRPs) 5‐HMF in ginseng processing are investigated and made suggestions for considering 5‐HMF as an evaluating indicator for quality of ginseng products.

1. INTRODUCTION
Hydroxymethyl‐2‐furaldehyde or 5‐hydroxymethyl‐2‐furfuraldehyde (5‐HMF, CAS NO. 67‐47‐0) is one kind of the Maillard reaction products (MRPs) (Hellwig, Lennart Kühn, & Henle, 2018; Imahori et al., 2018; Oliver, Melton, & Stanley, 2006) that occurs in many carbohydrate‐rich foods, traditional Chinese medicines (TCM) and injection, bread, corn syrups (de Andrade et al., (2017), cereal products (Ameur, Trystram, & Birlouez‐Aragon, 2006), biscuits (Švecová & Mach, 2017), honey (Kowalski & Lukasiewicz, 2017), Schisandra chinensis Fructus, Rehmannia glutinosa, DangShen, and Shengqifuzheng injection (Kowalski, Lukasiewicz, Duda‐Chodak, & Zięć, 2003). It is mainly produced by acid catalysis and thermal dehydration of hexoses via 1,2‐enolisation, appearing in products where water coexists with saccharides (Puignou, 2006). The scheme for generation of 5‐HMF from the fructose is shown in Figure 1. In the past decades, there have been a few debates concerning the 5‐HMF’s biological activity. It has been reported that 5‐HMF has biological effects such as antioxidant activity (Zhao et al., 2013), antihypoxia (Li et al., 2011), and inhibition of sickling of red blood cells (Janzowski, Glaab, Samimi, Schlatter, & Eisenbrand, 2000). However, the formation of 5‐HMF could reduce the content of sugar and lessen the nutrient composition. However, numerous studies have raised toxicological concern on 5‐HMF, and its derivatives, 5‐sulfooxymethylfurfural and 5‐chloromethylfurfural, have been found to be genotoxic, cytotoxic, carcinogenic, and mutagenic, inducing skin and hepatic cancers (Durling, Busk, & Hellman, 2009; Ito et al., 2013; Pereira, Albuquerque, Ferreira, Cacho, & Marques, 2011). Furthermore, recent reports have shown that 5‐HMF is a mutagen promoter, which is an important biomarker of carcinogenicity and genotoxicity in mouse cell lines (Glatt, Schneider, & Liu, 2005; Janzowski et al., 2000).
Figure 1.

Formation of 5‐HMF from fructose
5‐HMF is an important parameter conferring the quality and freshness of some foods, TCM, and injections; therefore, the analysis and control of 5‐HMF have been used to estimate the processing strategies, quality, and organoleptic characteristics of the products. For instance, the existence of 5‐HMF has been used in different foodstuffs, as a good indicating parameter to estimate the storage time or temperature (Rada‐Mendoza, Sanz, Olano, & Villamiel, 2004). The concentration of 5‐HMF in juice and honey has been limited strictly (Arribas‐Lorenzo & Morales, 2010; Gidamis, Chove, Shayo, Nnko, & Bangu, 2004; Monakhova & Lachenmeier, 2012). For instance, the Codex Alimentarius of the World Health Organization and the European Union Directive 110/2001 have established a maximum 5‐HMF quality standard of 40 mg/kg in honey and 50 mg/kg in apple juice as heat treatment and deterioration indicator (Gaspar & Lopes, 2009).
Ginseng has been used as a precious herbal medicine and dietary supplement for thousands of years in China, Western countries, and Korea (Paik & Lee, 2015; Wan et al., 2015). Processing of ginseng has great influence on the active ingredients and pharmacological effects, so the processing of ginseng is very important for ginseng's usual consumption and medical functions (Lee, Lee, Kim, Hong, & Kim, 2015). Processing ginseng not only produced numerous functional substances, but also generated a small amount of potentially harmful ingredients which cannot be ignored, especially the HPMRPs such as 5‐HMF (Favreaufarhadi, Pecukonis, & Barrett, 2015; Wong, Cheng, & Wang, 2012). Due to the abundant amino and carbonyl compounds (such as ginsenosides, amino acids, proteins, or reducing sugars) in the ginseng, the production of 5‐HMF tends to rise while processing (steaming, drying parameters, and honey addition), and the content of 5‐HMF is a useful indicator/parameter to evaluate the browning reaction extension. 5‐HMF is considered to be an important parameter for the quality of different foods and medicines. However, the presence and changes of 5‐HMF in different ginseng products and suggestions to consider 5‐HMF as a promising indicator for quality of ginseng products have not been reported.
Several methodologies such as classical spectrophotometeric techniques (Jr, 1979), liquid chromatography with ultraviolet detection (UV), and photodiode array (DAD) (de Andrade et al., 2016; Ciulu et al., 2013), capillary zone electrophoresis–tandem ion trap mass spectrometry method (CZE‐MS) (Bignardi, Cavazza, & Corradini, 2014), gas chromatography–mass spectrometry (GC–MS), and liquid chromatography–mass spectrometry (LC–MS) have been developed currently for the analysis of 5‐HMF (Puignou, 2006; Gaspar & Lopes, 2009; Teixidó, Moyano, Santos, & Galceran, 2008). LC–MS is a good tool to ensure the unequivocal identification and quantification of 5‐HMF in pending text samples.
In this study, changes of 5‐HMF in different ginseng samples with different temperatures and time pretreatment were investigated. Heat treatment was performed at different temperatures for 1.50, 2.00, 2.50, and 3.00 hr, respectively; subsequently, reflux extraction and ultrasonic extraction were used to evaluate the extraction rate, and different solvents (such as 80% methanol, dichloromethane, ethyl acetate, and a extraction with both the above solvents) using two extraction methods (liquid–liquid extraction and solid‐phase extraction) were used to remove matrix interference. Consequently, a reliable ultraperformance liquid chromatography–mass spectrometer (UPLC‐MS) method was proposed for qualitative and quantitative analysis of the 5‐HMF in various ginseng samples. At the same time, the production and regulation of 5‐HMF in ginseng processing are also analyzed. To our knowledge, few references have been reported in the literature involving the investigation of 5‐HMF changes in ginseng products. Quality parameters were established, and the proposed methodology was applied to investigate the changes of 5‐HMF in fresh and processed ginseng samples, which will provide an important reference for safe ginseng processing.
2. EXPERIMENTALS
2.1. Materials and methods
Ginseng samples with different growth number of years (3, 4, 5, and 6) were purchased from local markets in Ji'an, China. 5‐HMF standard was purchased from Sigma Company. Solid‐phase extraction (SPE) columns (Oasis HLB 3 cm3/60 mg) were purchased from Waters. Nylon purification kits with pore size of 0.45‐μm cutoff were purchased from Massachusetts, USA. Methanol and acetonitrile with HPLC grade were purchased from Fisher Scientific. Milli‐Q (Millipore) water was used in all experiments. Extract solvents such as methanol, ethyl acetate, dichloromethane, and other chemicals were of HPLC or analytical grade. Different heating temperatures (such as 90°C, 110°C, 120°C, and 130°C) and different heating time (such as 1.50, 2.00, 2.50, and 3.00 hr) were performed, respectively, to prepare different ginseng samples.
2.2. Sample preparation
Each ginseng sample about 5.0 g was prepared for the next 5‐HMF detection. Add about 50 ml 80% methanol to reflux extract and ultrasonic extract individually. Different solvents (such as dichloromethane, ethyl acetate, and extraction with both) using two extraction methods (liquid–liquid extraction and solid‐phase extraction) were used to remove the interference. The UPLC‐MS method was explored to study the changes of 5‐HMF for each ginseng samples.
2.3. Protein‐level analysis
Protein level in ginseng samples was measured on a Dumas Nitrogen Analyzer (Velp NDA 701‐Monza) by the previously reported method (Li, Liu, Men, & Wang, 2018).
2.4. Qualitative analysis of 5‐HMF
To identify the presence of 5‐HMF in different ginseng samples, UPLC‐MS analysis was carried on a UPLC/XEVO TQ with electrospray ionization (EI) source (Waters), and a 2 ml aliquot of the prepared sample solution was used for the UPLC‐MS analysis. The separation was achieved on a T3 column (2.1 × 100 mm, 1.7 mm, Waters) with a mobile phase consisting of acetonitrile solution as solvent A and water as solvent B. An equivalent elution system was 10% A and 90% B at a total flow rate of 0.3 ml/min. The column temperature was kept constant at 35°C.
2.5. Changes of 5‐HMF in ginsengs
The sample extractions were subjected to quantitative analysis, and the quantitative analysis was performed in an external standard method. The UPLC‐MS worked in an EI mode under atmospheric pressure and positive polarity (API‐ES). Other UPLC‐MS conditions were as same as the qualitative analysis of 5‐HMF. The ion mode at selective monitoring was set as m/z 127.0317, corresponding to 5‐HMF “[M + H]+.” Every sample run for 3.5 min, and it returned to the initial conditions by setting a balance time of about 1.0 min before each measurement for balancing the system, which ensures a good repeatability of the methodologies. Results were used as g/kg protein, and all the measurements were operated in triplicates.
3. RESULTS AND DISCUSSION
3.1. Optimization of the extraction and clean‐up procedure
Several experiments were carried out to develop an optimized extraction and clean‐up strategy to gain clean ginseng extracts prior to UPLC‐MS measurement. 80% methanol was used for reflux extraction and ultrasonic extraction; subsequently, dichloromethane, ethyl acetate, and an extraction with both solvents were used to select the suitable clean‐up procedure. In all conditions, 80% methanol reflux extraction was for 3 hr, and the best extraction yield was received when using dichloromethane by liquid–liquid extraction.
3.2. Quantitative analysis of 5‐HMF
3.2.1. Identification of 5‐HMF by UPLC‐MS
The contrast of the mass fragments and retention times for the extraction solution of the ginseng products with those of the reference 5‐HMF indicated the existence of 5‐HMF in the ginseng samples. MS detection used positive multiple reactions monitoring (MRM) mode. The detection conditions were optimized as follows: source temperature, 56°C; drying gas flow, 1,000 L/hr; drying gas temperature, 450°C; collision gas flow, 0.16 ml/min; and nebulizer gas flow, 50 L/hr; Figure 2 shows the UPLC‐MS chromatographs of the reference 5‐HMF and a typical red ginseng sample (a the standard and b red ginseng). The selective ion monitoring fraction of mass spectral analysis for the 5‐HMF standard and the ginseng samples had the same parent/daughter ion patterns of 127.0317, 80.9525, and 108.8046 (Figure 2c and d), and the assignment of the potential MS/MS fragmentation of the constituent was shown in Figure 2e; these results were in good consistency with the previous report for wines (Serra‐Cayuela et al., 2013), certifying the existence of the 5‐HMF in the ginseng samples.
Figure 2.
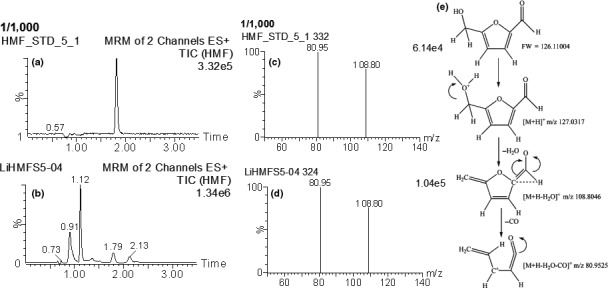
Selective ion monitoring of 5‐HMF by UPLC‐MS. Standard (a); red ginseng at 100°C (b); MS/MS (+) ESI spectra of fragmentation pattern in a solution of 5‐HMF standard (c); and red ginseng at 100°C (d); and the potential MS/MS fragmentation of the compound (e)
3.2.2. Confirmation of 5‐HMF by ultraviolet scanning
The red ginseng has the same UV absorption as the commercial 5‐HMF standard at about 1.8 min in the UV spectrum (Figure 3a and b), and the other ginseng samples have the same absorption. The results further confirmed that the 5‐HMF was existed in the different ginseng samples.
Figure 3.

UV spectrum of the compound corresponding to the peak at 1.80 min on UPLC fingerprint of the 5‐HMF standard (a) and red ginseng at 100°C (b)
3.3. Quantitative analysis of 5‐HMF
3.3.1. UPLC‐MS analysis of 5‐HMF
A series of 5‐HMF standard solutions (0.05–5.0 mg/ml) were filtered and then analyzed by the UPLC‐MS system. A good linearity was achieved at concentration range from 0.10 to 3.5 mg/ml with an equation of Y = 1.45561 × 106X + 100.91407 (R 2 = 0.99999). The detection limit (DL) of 5‐HMF (three signal‐to‐noise ratio, S/N = 3) was 0.03 mg/ml, and the quantitation limit (QL) was 0.10 mg/ml.
3.3.2. Precision and recoveries
The 5‐HMF concentrations in fresh ginseng and red ginseng samples were observed by the peak area calculation strategy, and RSD values of the 5‐HMF concentrations were 2.28%, 2.30%, 1.97%, 1.83%, 2.65%, and 3.06% by six parallel measurements. The results showing the established method had good precision for 5‐HMF analysis. To evaluate accuracy of the method, the recovery of 5‐HMF was studied by spiking a mixture of standard 5‐HMF (1 to 3 times of the sample's concentrate) into the red ginseng sample. As shown in Table 1, the standard deviations for each spiked sample were less than 3%, thus approving the absence of matrix effects and the accuracy of the detection.
Table 1.
Standard deviations for three replicates of each spiked sample of 5‐HMF in red ginseng
| Samples | Original (mg/ml) | 3 replicates | Spiked (mg/ml) | Found (mg/ml) | Standard deviation (%) |
|---|---|---|---|---|---|
| Red ginseng | 0.10 | A1 | 0.10 | 0.20 | 2.08 |
| A2 | 0.10 | 0.23 | |||
| A3 | 0.10 | 0.19 | |||
| B1 | 0.15 | 0.26 | 3.00 | ||
| B2 | 0.15 | 0.23 | |||
| B3 | 0.15 | 0.28 | |||
| C1 | 0.20 | 0.30 | 2.52 | ||
| C2 | 0.20 | 0.33 | |||
| C3 | 0.20 | 0.28 | |||
| D1 | 0.25 | 0.38 | 2.08 | ||
| D2 | 0.25 | 0.37 | |||
| D3 | 0.25 | 0.34 | |||
| E1 | 0.30 | 0.43 | 2.65 | ||
| E2 | 0.30 | 0.38 | |||
| E3 | 0.30 | 0.42 |
3.3.3. Changes of 5‐HMF in ginsengs
Quantitative detection results of 5‐HMF in heated 5‐year‐old ginseng at different temperatures for 2.5 hr under the given UPLC‐MS conditions are shown in Figure 4 and Table 2. It was found that the content of 5‐HMF in processed ginsengs increased significantly when the temperature exceeds about 100°C. It indicates that temperature should be set lower than 100°C when we fabricate the red ginseng products. Chromatograms of reference 5‐HMF and ginseng samples with different growth years heated at 100°C for 2.5 hr indicated that the content of 5‐HMF was increasing with the age of ginseng (Figure 5). Monitoring the content of 5‐HMF during the processed red ginseng indicated that the generation of 5‐HMF was slow during the steaming treatment, obtaining values of 3.09 to 12.24 g/kg protein (Table 3).
Figure 4.

Contents of the 5‐HMF for heated ginseng with 5 years of age at various temperatures
Table 2.
5‐HMF value of heated 5‐year‐old ginseng at various temperatures
| Samples | Treatment | HMF content (mg/kg) |
|---|---|---|
| 5‐year‐old ginseng | 40°C, 2.5 hr | 0.015 |
| 5‐year‐old ginseng | 70°C, 2.5 hr | 0.02 |
| 5‐year‐old ginseng | 90°C, 2.5 hr | 1.82 |
| 5‐year‐old ginseng | 110°C, 2.5 hr | 8.46 |
| 5‐year‐old ginseng | 120°C, 2.5 hr | 12.25 |
| 5‐year‐old ginseng | 130°C, 2.5 hr | 15.85 |
Figure 5.

Chromatograms of standard HMF (a), 3 years (b), 4 years (c), 5 years (d), and 6 years (e) ginseng heated at 100°C for 2.5 hr
Table 3.
Contents of 5‐HMF in red ginsengs of 5 years when heating at 100°C for 1.0, 1.5, 2.0, 2.5, and 3.0 hr
| Samples | Treatment | HMF content (mg/kg) |
|---|---|---|
| 5‐year‐old ginseng | 100°C, 1.0 hr | 3.09 |
| 5‐year‐old ginseng | 100°C, 1.5 hr | 5.34 |
| 5‐year‐old ginseng | 100°C, 2.0 hr | 6.80 |
| 5‐year‐old ginseng | 100°C, 2.5 hr | 8.29 |
| 5‐year‐old ginseng | 100°C, 3.0 hr | 12.24 |
Table 4 shows the values of 5‐HMF content in red ginsengs of 5 years old which were dried at different temperatures after steaming at 100°C for 2.5 hr. The value of 5‐HMF varied from 13.29 to 28.16 g/kg protein. The content of 5‐HMF increased significantly when the drying temperature was higher than 70°C. The 5‐HMF content increased during the heating process, reaching the values of 12.15, 15.99, 22.52, 28.36, and 42.55 g/kg protein after 6, 12, 18, 24, and 30 hr at 70°C, respectively (Table 5). The formation of 5‐HMF was mainly in the air‐heating (drying) process from the data. In addition, the addition of honey to the ginseng increased the 5‐HMF content to 112.32 g/kg protein. Although 5‐HMF was detected in both fresh and processed ginseng samples, its value ranged from 0.01 to 112.32 g/kg protein. These results indicated that ginseng protein was glycosylated such as UHT milk (310–603 g/kg protein) or processed cheese (3.5–366.6 g/kg protein) (Bosch, Alegrı´a, Farré, & Clemente, 2008). The content of 5‐HMF in fresh ginseng is low, but relatively high in honey‐added red ginsengs. The content of 5‐HMF in processed ginseng products depends on many factors such as processing strategies, heating levels, and the excipients, but fresh ginseng is not affected on any of the cases. 5‐HMF is one of the most important PHMRPs from MRs in ginseng, and the processing conditions and the addition of honey to the red ginsengs are more feasible to MRs; thus, the more 5‐HMF was generated. The highest value of 5‐HMF in honey‐added red ginseng shows that honey addition to red ginseng decreased nutritional and medical values, although it improved its organoleptic properties (flavor and taste).
Table 4.
Contents of 5‐HMF in ginseng of 5 years when steaming at 100°C for 2.5 hr and heating at 40°C, 50°C, 60°C, 70°C, and 80°C, respectively
| Samples | Treatment | HMF content (mg/kg) |
|---|---|---|
| 5‐year‐old ginseng | Steaming at 100°C for 2.5 hr, drying at 40°C for 12 hr | 13.29 |
| 5‐year‐old ginseng | Steaming at 100°C for 2.5 hr, drying at 50°C for 12 hr | 14.35 |
| 5‐year‐old ginseng | Steaming at 100°C for 2.5 hr, drying at 60°C for 12 hr | 14.52 |
| 5‐year‐old ginseng | Steaming at 100°C for 2.5 hr, drying at 70°C for 12 hr | 15.99 |
| 5‐year‐old ginseng | Steaming at 100°C for 2.5 hr, drying at 80°C for 12 hr | 28.16 |
Table 5.
Contents of 5‐HMF in ginseng of 5 years when steaming at 100°C for 2.5 hr and heating at 70°C with 6, 12, 18, 24, and 30 hr, respectively
| Samples | Treatment | HMF content (mg/kg) |
|---|---|---|
| 5‐year‐old ginseng | Heating at 70°C, 6 hr | 12.15 |
| 5‐year‐old ginseng | Heating at 70°C, 12 hr | 15.99 |
| 5‐year‐old ginseng | Heating at 70°C, 18 hr | 22.52 |
| 5‐year‐old ginseng | Heating at 70°C, 24 hr | 28.36 |
| 5‐year‐old ginseng | Heating at 70°C, 30 hr | 42.55 |
4. CONCLUSIONS
The study estimated the changes of 5‐HMF in fresh and manufactured ginseng samples. The results indicated that the lowest content of 5‐HMF is found in the fresh ginseng sample, and the highest is found in honey adding red ginsengs with the highest amount of honey addition and temperature treatment. In conclusion, the heat treatment and honey addition dramatically increased the 5‐HMF content in ginsengs. These results implied that the concentration of 5‐HMF may be used as a promising indicator to estimate the honey addition and heating level during ginseng processing. Lower level honey addition and lower temperature should be used to avoid forming the potentially harmful MRPs 5‐HMF during processing of ginseng and other high polysaccharides‐contained foods and herbal medicines. This research provided valuable information for the formation and regulation of 5‐HMF in ginseng processing and also provided a useful reference for safe approach of ginseng processing. In addition, the existence of different values of 5‐HMF in different ginseng samples for this study can serve as a foundation for the standard doses of the ginseng available doses per day.
CONFLICTS OF INTEREST
The authors have no conflicts of interest.
AUTHORS’ CONTRIBUTIONS
Yali Li designed the experiments and contributed to the statistical analyses and the writing of the manuscript. Yufang Wang and Xiangmin Piao contributed to the processing of ginsengs. Peihe Zheng and Hao Zhang contributed to the pretreatment of samples. Shifeng Pang and Zhengyi Qu contributed to UPLC‐MS experiments. Yingping Wang put forward the ideas and approved the manuscript. All the authors read and proofed the final manuscript.
ETHICAL APPROVAL
This study does not involve any human or animal testing.
ACKNOWLEDGMENTS
This work was supported by the National Science Foundation for Youths of China (No. 31401606), The Innovation Project of Medicinal Plant Germplasm Resources and Breeding Innovation Team, The Chinese Academy of Agricultural Sciences (No. CAAS‐ASTIP‐2016‐ISAPS), The Project of the Jilin Province Department of Science and Technology, China (No. 20190201160JC and 20170101011JC), and Jilin Province Development and Reform Commission 2019C052‐10).
Li Y, Wang Y, Piao X, et al. Changes of 5‐hydroxymethyl‐2‐furfural in fresh and processed ginsengs. Food Sci Nutr. 2020;8:2068–2075. 10.1002/fsn3.1496
Contributor Information
Yali Li, Email: yalilee@126.com.
Yingping Wang, Email: yingpingw@126.com.
REFERENCES
- Ameur, L. A. , Trystram, G. , & Birlouez‐Aragon, I. (2006). Accumulation of 5‐hydroxymethyl‐2‐furfural in cookies during the backing process: Validation of an extraction method. Food Chemistry, 98(4), 790–796. 10.1016/j.foodchem.2005.07.038 [DOI] [Google Scholar]
- Armour, D. G. , van den Berg, J. A. , & Verheij, L. K. (1979). The structural and compositional analysis of single crystal surfaces using low energy ion scattering. J. Radioanal. Chem, 48, 359–378. 10.1007/BF02519799 [DOI] [Google Scholar]
- Arribas‐Lorenzo, G. , & Morales, F. J. (2010). Estimation of dietary intake of 5‐hydroxymethylfurfural and related substances from coffee to Spanish population. Food & Chemical Toxicology, 48(2), 644–649. 10.1016/j.fct.2009.11.046 [DOI] [PubMed] [Google Scholar]
- Bignardi, C. , Cavazza, A. , & Corradini, C. (2014). Selected product ion monitoring for quantification of 5‐hydroxymethylfurfural in food products by capillary zone electrophoresis‐tandem ion trap mass spectrometry. Food Control, 46(46), 41–48. 10.1016/j.foodcont.2014.04.049 [DOI] [Google Scholar]
- Bosch, L. , Alegrı´a, A. , Farré, R. , & Clemente, G. (2008). Effect of storage conditions on furosine formation in milk‐cereal based baby foods. Food Chemistry, 107(4), 1681–1686. 10.1016/j.foodchem.2007.09.051 [DOI] [Google Scholar]
- Ciulu, M. , Farre, R. , Floris, I. , Nurchi, V. M. , Panzanelli, A. , Pilo, M. I. , … Sanna, G. (2013). Determination of 5‐hydroxymethyl‐2‐furaldehyde in royal jelly by a rapid reversed phase HPLC method. Analytical Methods, 5(19), 5010–5013. 10.1039/c3ay40634b [DOI] [Google Scholar]
- de Andrade, J. K. , de Andrade, C. K. , Komatsu, E. , Perreault, H. , Torres, Y. R. , da Rosa, M. R. , & Felsner, M. L. (2017). A validated fast difference spectrophotometric method for 5‐hydroxymethyl‐2‐furfural (HMF) determination in corn syrups. Food Chemistry, 228, 197–203. 10.1016/j.foodchem.2017.01.158 [DOI] [PubMed] [Google Scholar]
- de Andrade, J. K. , Emy, K. , Hélène, P. , Torres, Y. R. , Rosa, M. R. D. , & Felsner, M. L. (2016). In house validation from direct determination of 5‐hydroxymethyl‐2‐furfural (HMF) in Brazilian corn and cane syrups samples by HPLC‐UV. Food Chemistry, 190, 481–486. 10.1016/j.foodchem.2015.05.131 [DOI] [PubMed] [Google Scholar]
- Durling, L. J. K. , Busk, L. , & Hellman, B. E. (2009). Evaluation of the dna damaging effect of the heat‐induced food toxicant 5‐hydroxymethylfurfural (HMF) in various cell lines with different activities of sulfotransferases. Food and Chemical Toxicology,, 47(4), 880–884. 10.1016/j.fct.2009.01.022 [DOI] [PubMed] [Google Scholar]
- Favreaufarhadi, N. , Pecukonis, L. , & Barrett, A. (2015). The inhibition of maillard browning by different concentrations of rosmarinic acid and epigallocatechin‐3‐gallate in model, bakery, and fruit systems. Journal of Food Science, 80(10), C2140–C2146. 10.1111/1750-3841.13014 [DOI] [PubMed] [Google Scholar]
- Gaspar, E. M. S. M. , & Lopes, J. F. (2009). Simple gas chromatographic method for furfural analysis. Journal of Chromatography A, 1216(14), 2762–2767. 10.1016/j.chroma.2008.10.049 [DOI] [PubMed] [Google Scholar]
- Gidamis, A. B. , Chove, B. E. , Shayo, N. B. , Nnko, S. A. , & Bangu, N. T. (2004). Quality evaluation of honey harvested from selected areas in Tanzania with special emphasis on hydroxymethyl furfural (HMF) levels. Plant Foods for Human Nutrition, 59(3), 129–132. 10.1007/s11130-004-0020-7 [DOI] [PubMed] [Google Scholar]
- Glatt, H. , Schneider, H. , & Liu, Y. (2005). V79‐hcyp2e1‐hsult1a1, a cell line for the sensitive detection of genotoxic effects induced by carbohydrate pyrolysis products and other food‐borne chemicals. Mutation Research, 580(1), 41–52. 10.1016/j.mrgentox.2004.11.005 [DOI] [PubMed] [Google Scholar]
- Hellwig, M. , Kühn, L. , & Henle, T. (2018). Individual maillard reaction products as indicators of heat treatment of pasta‐a survey of commercial products. Journal of Food Composition & Analysis, 72, 83–92. 10.1016/j.jfca.2018.06.009 [DOI] [Google Scholar]
- Imahori, D. , Matsumoto, T. , Kojima, N. , Hasei, T. , Sumii, M. , Sumida, T. , … Watanabe, T. (2018). Chemical structures of novel maillard reaction products under hyperglycemic conditions. Chemical & Pharmaceutical Bulletin, 66(4), 363–367. 10.1248/cpb.c17-00809 [DOI] [PubMed] [Google Scholar]
- Ito, T. , Sato, A. , Ono, T. , Goto, K. , Maeda, T. , Takanari, J. , … Matsuura, H. (2013). Isolation, structural elucidation, and biological evaluation of a 5‐hydroxymethyl‐2‐furfural derivative, asfural, from enzyme‐treated asparagus extract. Journal of Agriculture and Food Chemistry, 61(38), 9155–9159. 10.1021/jf402010c [DOI] [PubMed] [Google Scholar]
- Janzowski, C. , Glaab, V. , Samimi, E. , Schlatter, J. , & Eisenbrand, G. (2000). 5‐hydroxymethylfurfural: Assessment of mutagenicity, dna‐damaging potential and reactivity towards cellular glutathione. Food & Chemical Toxicology, 38(9), 801–809. 10.1016/S0278-6915(00)00070-3 [DOI] [PubMed] [Google Scholar]
- Kowalski, S. , & Lukasiewicz, M. (2017). Invertase activity changes and 5‐Hydroxymethyl 2‐Furfural formation in honeys under influence of microwave irradiation. Journal of Food Process Engineering, 40(2), e12410 10.1111/jfpe.12410 [DOI] [Google Scholar]
- Kowalski, S. , Lukasiewicz, M. , Duda‐Chodak, A. , & Zięć, G. (2003). 5‐Hydroxymethyl‐2‐furfural (HMF) ‐ heat‐induced formation, occurrence in food and biotransformation‐a review. Polish Journal of Food and Nutrition Sciences, 63(4), 207–225. 10.2478/v10222-012-0082-4 [DOI] [Google Scholar]
- Lee, M. H. , Lee, Y. C. , Kim, S. S. , Hong, H. D. , & Kim, K. T. (2015). Quality and antioxidant activity of ginseng seed processed by fermentation strains. Journal of Ginseng Research, 39(2), 178–182. 10.1016/j.jgr.2014.10.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, M.‐M. , Wu, L.‐Y. , Zhao, T. , Xiong, L. , Huang, X. , Liu, Z.‐H. , … Fan, M. (2011). The protective role of 5‐HMF against hypoxic injury. Cell Stress & Chaperones, 16(3), 267–273. 10.1007/s12192-010-0238-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, Y. , Liu, X. , Men, L. , & Wang, Y. (2018). Qualitative and quantitative analysis of furosine in fresh and processed ginsengs. Journal of Ginseng Research, 42(1), 21–26. 10.1016/j.jgr.2016.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monakhova, Y. B. , & Lachenmeier, D. W. (2012). The margin of exposure of 5‐hydroxymethylfurfural (HMF) in, alcoholic beverages. Environmental Health & Toxicology, 27, e2012016 10.5620/eht.2012.27.e2012016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver, C. M. , Melton, L. D. , & Stanley, R. A. (2006). Creating proteins with novel functionality via the maillard reaction: A review. Critical Reviews in Food Science & Nutrition, 46(4), 337–350. 10.1080/10408690590957250 [DOI] [PubMed] [Google Scholar]
- Paik, D. J. , & Lee, C. H. (2015). Review of cases of patient risk associated with ginseng abuse and misuse. Journal of Ginseng Research, 39(2), 89–93. 10.1016/j.jgr.2014.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira, V. , Albuquerque, F. M. , Ferreira, A. C. , Cacho, J. , & Marques, J. C. (2011). Evolution of 5‐hydroxymethylfurfural (HMF) and furfural (F) in fortified wines submitted to overheating conditions. Food Research International, 44(1), 71–76. 10.1016/j.foodres.2010.11.011 [DOI] [Google Scholar]
- Rada‐Mendoza, M. , Sanz, M. L. , Olano, A. , & Villamiel, M. (2004). Formation of hydroxymethylfurfural and furosine during the storage of jams and fruit‐based infant foods. Food Chemistry, 85(4), 605–609. 10.1016/j.foodchem.2003.07.002 [DOI] [Google Scholar]
- Serra‐Cayuela, A. , Castellari, M. , Bosch‐Fusté, J. , Riu‐Aumatell, M. , Buxaderas, S. , & López‐Tamames, E. (2013). Identification of 5‐hydroxymethyl‐2‐furfural (5‐HMF) in cava sparkling wines by LC‐DAD‐MS/MS and NMR spectrometry. Food Chemistry, 141(4), 3373–3380. 10.1016/j.foodchem.2013.05.158 [DOI] [PubMed] [Google Scholar]
- Švecová, B. , & Mach, M. (2017). Content of 5‐hydroxymethyl‐2‐furfural in biscuits for kids. Interdisciplinary Toxicology, 10(2), 66–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teixidó, E. , Moyano, E. , Santos, F. J. , & Galceran, M. T. (2008). Liquid chromatography multi‐stage mass spectrometry for the analysis of 5‐hydroxymethylfurfural in foods. Journal of Chromatography A, 1185(1), 102–108. 10.1016/j.chroma.2008.01.057 [DOI] [PubMed] [Google Scholar]
- Teixidó, E. , Santos, F. J. , Puignou, L. , & Galceran, M. T. (2006). Analysis of 5‐hydroxymethylfurfural in foods by gas chromatography‐mass spectrometry. Journal of Chromatography A, 1135(1), 85–90. 10.1016/j.chroma.2006.09.023 [DOI] [PubMed] [Google Scholar]
- Wan, J.‐Y. , Fan, Y. , Yu, Q.‐T. , Ge, Y.‐Z. , Yan, C.‐P. , Alolga, R. N. , … Qi, L.‐W. (2015). Integrated evaluation of malonyl ginsenosides, amino acids and polysaccharides in fresh and processed ginseng. Journal of Pharmaceutical and Biomedical Analysis, 107, 89–97. 10.1016/j.jpba.2014.11.014 [DOI] [PubMed] [Google Scholar]
- Wong, D. , Cheng, K. W. , & Wang, M. (2012). Inhibition of heterocyclic amine formation by water‐soluble vitamins in maillard reaction model systems and beef patties. Food Chemistry, 133(3), 760–766. 10.1016/j.foodchem.2012.01.089 [DOI] [Google Scholar]
- Zhao, L. , Chen, J. , Su, J. , Li, L. , Hu, S. , Li, B. , … Chen, T. (2013). In vitro antioxidant and antiproliferative activities of 5‐hydroxymethylfurfural. Journal of Agriculture and Food Chemistry, 61(44), 10604–10611. 10.1021/jf403098y [DOI] [PubMed] [Google Scholar]


