Abstract
The single-joint Hybrid Assistive Limb (HAL-SJ) robot is an exoskeleton-type suit developed for the neurorehabilitation of upper limb function. Several studies have addressed the usefulness of the robot; however, the appropriate patient selection remains unclear. In this study, we evaluated the effectiveness of the HAL-SJ exoskeleton in improving upper limb function in the subacute phase after a stroke, as a function of the severity of arm paralysis. Our analysis was based on a retrospective review of 35 patients, treated using the HAL-SJ exoskeleton in the subacute phase after their stroke, between October 2014 and December 2018. The severity of upper limb impairment was quantified using the Brunnstrom recovery stage (BRS) as follows: severe, BRS score 1–2, n = 10; moderate, BRS 3–4, n = 12; and mild, BRS 5–6, n = 13. The primary endpoint was the improvement in upper limb function, from baseline to post-intervention, measured using the Fugl-Meyer assessment upper limb motor score (ΔFMA-UE; range 0–66). The ΔFMA-UE score was significant for all three severity groups (P <0.05). The magnitude of improvement was greater in the moderate group than in the mild group (P <0.05). The greatest improvement was attained for patients with a moderate level of upper limb impairment at baseline. Our findings support the feasibility of the HAL-SJ to improve upper limb function in the subacute phase after a stroke with appropriate patient selection. This study is the first report showing the effect of robot-assisted rehabilitation using the HAL-SJ, according to the severity of paralysis in acute stroke patients with upper extremity motor deficits.
Keywords: stroke, robot-assisted rehabilitation, Fugl-Meyer assessment, Brunnstrom recovery stage
Introduction
Among patients who have sustained a stroke, motor impairment of the upper limb is identified in 66% of cases.1) In the subacute phase of stroke recovery, effective rehabilitation plays an important role in improving the functional prognosis. Robotic technologies have been attracting increasing attention in the field of neurorehabilitation, enabling high-intensity training, supporting appropriate movements of the affected limb and providing motivation to the patient to use their affected limb.2) Recent meta-analyses, however, have reported heterogeneous outcomes of robot-assisted rehabilitation among patients having sustained a hemiplegic stroke.3,4) Sources of variability in the measured effectiveness include the type of robot used, the intervention design, and patient adherence and performance on the exercise intervention. As such, it is necessary to evaluate the effectiveness of each robot.
The Hybrid Assistive Limb robot (HAL; Cyberdyne Inc., Tsukuba, Japan) is an exoskeleton-type suit that is uniquely designed to improve limb function by providing relevant and interactive feedback.5,6) Different versions of the HAL exoskeleton are available, namely a bilateral or single lower limb version and single-joint version (HAL-SJ) for the upper and lower limbs. Previous studies have reported on the safety and effectiveness of HAL-assisted treatment for stroke rehabilitation.7–9) We have also reported on the effectiveness of HAL-assisted gait training, with the magnitude of improvement being a function of the severity of impairment at baseline.10) Although a previous study has reported on the criteria for appropriate patient selection for HAL-assisted training,11) the criteria for the selection of appropriate patients for HAL-SJ-assisted training for upper limb paralysis have not been previously evaluated. Our aim in this study was to clarify the effectiveness of HAL-SJ-assisted training to improve upper limb function after a stroke. We hypothesized that the effectiveness of HAL-SJ-assisted training would depend on the severity of motor dysfunction at baseline.
Materials and Methods
Statement of ethics
The study protocol was approved by our Institutional Review Board (approval number: U19-09-006).
Study group
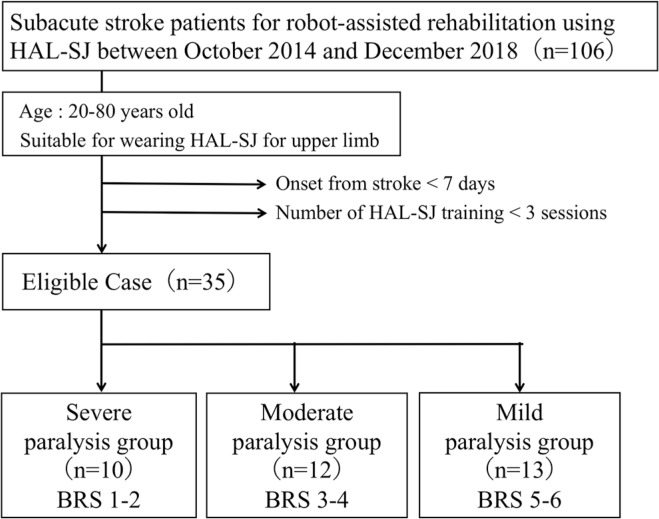
Prospective patients were those admitted to our facility for an acute stroke, between October 2014 and December 2018. Included were hemiplegic patients, 20–80 years old, for whom wearing of the HAL-SJ exoskeleton suit was possible. Patients who complete <3 HAL-SJ-assisted training sessions and initiated HAL-SJ assisted training in <7 days after stroke onset were excluded as the neurological conditions during acute stroke phase are likely to be unstable and intravenous lines and vital sign monitor may have interfered with the rehabilitation procedures. In total, we screened 106 patients who underwent robot-assisted rehabilitation, with 35, meeting our inclusion criteria, enrolled (Fig. 1): 12 men and 23 women, with a mean age of 60.29 ± 11.97 years. All patients were right-hand dominant. Patients completed, on average 5.51 ± 2.19 training sessions, over a period of 13.31 ± 7.73 days. On average, training was initiated 13.29 ± 6.38 days after stroke onset. To investigate the relationship between outcomes of HAL-SJ-assisted training and the severity of hemiparesis at baseline, we subclassified patients based on their Brunnstrom recovery stage (BRS) score of upper limb function, as follows: severe, BRS score 1–2; moderate, BRS score 3–4; and mild, BRS score 5–6.
Fig. 1.
Flow diagram of patient selection.
HAL-SJ-assisted training protocol
The feature of HAL-SJ is that it wears easier than the conventional HAL. In addition, as it is smaller and lighter, it can be used for bedside rehabilitation the patient or by the therapist to move the joint by visual feedback displayed on the monitor screen and the power unit (Fig. 2). We have previously reported on the general parameters of an HAL-SJ-assisted rehabilitation protocol.6,8,12) Briefly, HAL-SJ-assisted rehabilitation can be introduced at the bedside or in the rehabilitation room, as soon as vital signs have stabilized after the stroke. HAL-SJ training was provided in the supine or sitting position depending on the patient’s condition. In the sitting posture, the direction of movement was facilitated by a fixed or hanging device. We have implemented 3–5 sessions per week, with a 20–30 min duration for each session. Conventional occupational therapy was provided in addition to the robot-assisted training, including stretching, upper limb functional training, and task-specific training for activities of daily living.
Fig. 2.
Overview of single-joint Hybrid Assistive Limb (HAL-SJ). (A) HAL-SJ attached to upper limb. (B) The location of electrode detecting Bioelectrical signals (BES) from the biceps and triceps muscles. (C) The controller showing the BES. Red and green waves on the monitor indicate flexor and extensor muscles, respectively. Adapted from Saita et al.6)
Measured outcomes
The primary endpoint was the change in the Fugl-Meyer assessment upper limb motor score (ΔFMA-UE), from baseline to post-intervention.13) The secondary outcome was the change in the action research arm test (ΔARAT) score, again from baseline to post-intervention. All outcome measurements were performed by occupational therapists (K.S and K.H.).
Statistical analysis
Our analysis was based on a retrospective chart review. The normality of the distribution of the data was evaluated using the Shapiro–Wilk test. Continuous variables are reported as the mean ± standard deviation (SD), with categorical variables reported as a frequency and percentage. The ΔFMA-UE and ΔARAT were evaluated using a paired t-test or Wilcoxon signed-rank test, as appropriate for the data type. Between-group comparisons were evaluated using a one-way analysis of variance (ANOVA), Kruskal–Wallis test, or Chi-squared test, as appropriate for the data type and distribution. Comparisons after a one-way ANOVA were performed using the Games-Howell test. All statistical analysis performed using SPSS (version 21.0; IBM Corp., Armonk, NY, USA). Significance was set at a P-value <0.05.
Results
Baseline characteristics for each group are shown in Table 1. The distribution of patient characteristics across the three BRS score severity groups was as follows: severe, 10 patients (nine men and one woman), 61.50 ± 10.30 years of age, including three cases of hemorrhagic stroke and seven of ischemic stroke; moderate, 12 patients (six men and six women), 59.08 ± 11.08 years of age, including three cases of hemorrhagic stroke and nine of ischemic stroke; and mild, 13 patients (eight men and five women), 60.46 ± 14.53 years of age, including nine cases of hemorrhagic stroke and four of ischemic stroke. No between-group differences were identified with regard to the following variables: age (P = 0.770); sex (P = 0.133); stroke type (P = 0.052); affected limb side (P = 0.271); initiation of robot-assisted rehabilitation after the stroke (P = 0.357); duration (P = 0.872), and number of session (P = 0.547) using robot-assisted rehabilitation. baseline BRS-UE, FMA-UE motor score and ARAT score were significantly different between the three groups (P <0.001).
Table 1.
Between-group comparison of baseline demographics and clinical characteristics
| Mild paralysis group (n = 13) | Moderate paralysis group (n = 12) | Severe paralysis group (n = 10) | P-value | ||
|---|---|---|---|---|---|
| Age (years) | 60.46 ± 14.53 | 59.08 ± 11.08 | 61.50 ± 10.30 | 0.770† | |
| Sex (%) | Female | 5 (38.4) | 6 (50.0) | 1 (10.0) | 0.133§ |
| Affected limb side (%) | Right | 6 (46.2) | 2 (16.7) | 4 (40.0) | 0.271§ |
| Stroke type (%) | Infarction | 4 (30.8) | 9 (75.0) | 7 (70.0) | 0.052§ |
| Hemorrhagic | 9 (69.2) | 3 (25.0) | 3 (30.0) | ||
| Initiation of robot-assisted rehabilitation after the stroke, days | 10.92 ± 3.75 | 15.67 ± 9.17 | 13.5 ± 4.06 | 0.357‡ | |
| Duration of robot-assisted rehabilitation, days | 13.62 ± 7.33 | 11.92 ± 5.12 | 14.6 ± 10.83 | 0.872‡ | |
| Number of sessions using robot-assisted rehabilitation, times | 5.08 ± 0.95 | 5.08 ± 1.83 | 6.6 ± 3.31 | 0.547‡ | |
| Brunnstrom stage for upper extremity (%) | 1 | 0 | 0 | 0 | <0.001‡ |
| 2 | 0 | 0 | 10 (100.0) | ||
| 3 | 0 | 6 (50.0) | 0 | ||
| 4 | 0 | 6 (50.0) | 0 | ||
| 5 | 13 (100.0) | 0 | 0 | ||
| 6 | 0 | 0 | 0 | ||
| Fugl-Meyer assessment upper limb, motor scores (range: 0–66) | 56.62 ± 5.84 | 24.92 ± 14.39 | 7.3 ± 2.50 | <0.001† | |
| Action research arm test, total scores (range: 0–57) | 42.23 ± 13.32 | 8.83 ± 14.71 | 0.1 ± 0.32 | <0.001† |
One-way analysis of variance.
Kruskal–Wallis test.
Square test.
There was a significant difference in the ΔFMA-UE among the three groups (P <0.05), as follows: severe group, ΔFMA-UE: 7.30 ± 4.72; moderate group, ΔFMA-UE: 12.42 ± 9.42; and mild group ΔFMA-UE: 4.31 ± 2.18. The magnitude of improvement was greater in the moderate group than in the mild group (P <0.05). On the other hand, there was no significant difference in the change in the ARAT score (P = 0.126). These clinical outcomes are summarized in Table 2.
Table 2.
Between-group comparison of the primary outcome measure
| Outcome measure | Mild paralysis group (n = 13) | Moderate paralysis group (n = 12) | Severe paralysis group (n = 10) | P-value | Post-hoc test | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Pre | Post | Difference | Pre | Post | Difference | Pre | Post | Difference | |||
| Fugl-Meyer assessment upper limb motor score | 56.62 ± 5.84 | 60.92 ± 4.59 | 4.31 ± 2.18 | 24.92 ± 14.39 | 37.33 ± 16.38 | 12.42 ± 9.42 | 7.30 ± 2.50 | 14.6 ± 5.42 | 7.30 ± 4.72 | <0.05† | P1 = 0.745 P2 < 0.05 P3 = 0.621 |
| Action research arm test | 42.23 ± 13.32 | 48.92 ± 10.79 | 6.69 ± 6.98 | 8.33 ± 14.71 | 15.08 ± 19.05 | 6.25 ± 8.81 | 0.1 ± 0.32 | 2.1 ± 2.08 | 2.0 ± 2.05 | 0.126‡ | |
One-way analysis of variance.
Kruskal–Wallis test. P1: severe – mild, P2: moderate – mild, P3: severe – moderate.
Discussion
Our findings support the use of HAL-SJ-assisted training for the neurorehabilitation of upper limb function in the subacute phase of a stroke, with the effectiveness being modulated by the severity of the hemiparesis at baseline. In particular, a greater magnitude of improvement was identified in the moderate group (BRS score, 3–4) than in the mild group (BRS score, 5–6). Our findings were not consistent with those of Fukuda et al.10) who reported a positive effect of HAL-assisted training in improving gait in patients with a mild paresis. Therefore, it appears that even when using the same robot, the effectiveness of training might be influenced by the body part and the joint movement affected. In order to clarify the clinical effects, the minimal clinically important difference score should be referred. Based on previous research14) a change in the ΔFMA-UE of nine points was considered to be of clinical significance among patients with a subacute stroke. In our study, the ΔFMA-UE of 12.42 points in the moderate BRS score group was considered to be a clinically meaningful effect. We consider that the ΔFMA-UE of 4.31 points in the mild BRS score group was limited by the ceiling effect of the FMA-UE evaluation.
In addition, recent studies have indicated that setting appropriate task difficulty is important to improve functional recovery.15,16) In the present study, moderately paretic patients with subacute stroke may have the greatest effect on the improvement in upper limb function owing to the task difficulty of the HAL-SJ. On the other hand, the task difficulty is considered to be lower for patients with mild paralysis and higher for those with severe paralysis to gain additional effect of HAL-SJ. In this context, the improvements shown in mild and severe paralysis groups may have represented the natural course of neurological improvement in the stroke survivors without the beneficial effect of robot-assisted rehabilitation.
Regarding the results of the examination of the upper extremity function, the difference in the FMA score was significant among the three groups, but the ARAT score showed no significant differences. One of the reasons is the difference in the evaluation characteristics of the FMA and ARAT scale. According to the International Classification of Functioning, Disability and Health (ICF) for upper limb function evaluation, the FMA scale is considered to belong to the ICF body function, and the ARAT scale to the ICF activities.17) Therefore, these results provide evidence that the effect of HAL-SJ rehabilitation in subacute stroke phase is more likely to contribute to improvement in physical functions rather than activities of daily living.
A recent multicenter randomized controlled trial indicated that the rehabilitation effect of robot-assisted training was equivalent to conventional rehabilitation.18) This study, however, used an end-effector type robot, and not an exoskeleton robot as we used in our study. The severity of hemiparesis has also been identified as an important feature to consider when evaluating the effectiveness of an intervention. In our study group, overall, the baseline FMA-UE score was approximately 18 points, corresponding to severe upper limb paralysis. A previous study failed to identify a beneficial effect of robot-assisted rehabilitation, compared to conventional therapy, in improving upper limb function among patients with a moderate severity of upper limb impairment (baseline FMA-UE: about 30 points).19) A meta-analysis of the limited number of studies available regarding single-joint robot-assisted training failed to identify an effect of robot-assisted training in the acute and subacute phases after a stroke.3) Currently, there is an ongoing clinical trial regarding the effectiveness of robot-assisted rehabilitation, using the HAL-SJ, to improve upper limb functional outcomes after an acute stroke patients (JRCT number: jRCTs052180010).
Despite the favorable results we report, it is important to note the limitations of our study. The intensity of robot-assisted rehabilitation was not uniform in our cohort. In our view, there might be a benefit of increasing the intensity of the training, in terms of the number and duration of sessions, considering the findings of a recent meta-analysis of the beneficial effect of a robot-assisted training of 6–8 weeks, at a frequency of 2–3 h per week, for a total volume of training of 12 or more hours.4) We note that achieving this intensity of robot-assisted rehabilitation would not be feasible in our acute care setting. Therefore, it might be important to collaborate with home-based rehabilitation to provide robot-assisted training to improve upper limb function after a stroke.20) Additionally, our study cannot demonstrate the superiority of the robot-assisted rehabilitation to conventional methods as the present study lacks control cohort. Further studies are warranted to clarify the evidence for HAL-SJ-assisted training to improve upper limb function in the acute/subacute phase after a stroke.
In conclusion, the results of the present study support the feasibility of HAL-SJ-assisted neurorehabilitation selection in improving upper limb function in the subacute phase after a stroke. The largest effect was identified among patients with a moderate paralysis of the upper limb at the baseline, and this finding addresses the importance of appropriate patient for the procedure. Therefore, there is a need for appropriate patient selection, to maximize the effectiveness of robot-assisted rehabilitation.
Acknowledgment
This study was supported, in part, by the Japan Agency for Medical Research and Development (AMED).
Footnotes
Conflicts of Interest Disclosure
The authors declare no conflicts of interest.
References
- 1).Nijland RH, van Wegen EE, Harmeling-van der Wel BC, Kwakkel G, EPOS investigators : Presence of finger extension and shoulder abduction within 72 hours after stroke predicts functional recovery: early prediction of functional outcome after stroke: the EPOS cohort study. Stroke 41: 745–750, 2010 [DOI] [PubMed] [Google Scholar]
- 2).Turner DL, Ramos-Murguialday A, Birbaumer N, Hoffmann U, Luft A: Neurophysiology of robot-mediated training and therapy: a perspective for future use in clinical populations. Front Neurol 4: 184, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3).Veerbeek JM, Langbroek-Amersfoort AC, van Wegen EE, Meskers CG, Kwakkel G: Effects of robot-assisted therapy for the upper limb after stroke. Neurorehabil Neural Repair 31: 107–121, 2017 [DOI] [PubMed] [Google Scholar]
- 4).Mehrholz J, Pohl M, Platz T, Kugler J, Elsner B: Electromechanical and robot-assisted arm training for improving activities of daily living, arm function, and arm muscle strength after stroke. Cochrane Database Syst Rev 9: CD006876, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5).Morishita T, Inoue T: Interactive bio-feedback therapy using hybrid assistive limbs for motor recovery after stroke: current practice and future perspectives. Neurol Med Chir (Tokyo) 56: 605–612, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6).Saita K, Morishita T, Arima H, et al. : Biofeedback effect of hybrid assistive limb in stroke rehabilitation: a proof of concept study using functional near infrared spectroscopy. PLoS One 13: e0191361, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7).Watanabe H, Tanaka N, Inuta N, Saitou H, Yanagi H: Locomotion improvement using a hybrid assistive limb in recovery phase stroke patients: a randomized controlled pilot study. Arch Phys Med Rehabil 95: 2006–2012, 2014 [DOI] [PubMed] [Google Scholar]
- 8).Fukuda H, Morishita T, Ogata T, et al. : Tailor-made rehabilitation approach using multiple types of hybrid assistive limb robots for acute stroke patients: a pilot study. Assist Technol 28: 53–56, 2016 [DOI] [PubMed] [Google Scholar]
- 9).Saita K, Morishita T, Hyakutake K, et al. : Combined therapy using botulinum toxin A and single-joint hybrid assistive limb for upper-limb disability due to spastic hemiplegia. J Neurol Sci 373: 182–187, 2017 [DOI] [PubMed] [Google Scholar]
- 10).Fukuda H, Samura K, Hamada O, et al. : Effectiveness of acute phase hybrid assistive limb rehabilitation in stroke patients classified by paralysis severity. Neurol Med Chir (Tokyo) 55: 487–492, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11).Chihara H, Takagi Y, Nishino K, et al. : Factors predicting the effects of hybrid assistive limb robot suit during the acute phase of central nervous system injury. Neurol Med Chir (Tokyo) 56: 33–37, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12).Ogata T, Abe H, Samura K, et al. : Hybrid assitive limb (HAL) rehabilitation in patiens with acute hemorrhagic stroke. Neurol Med Chir (Tokyo) 55: 901–906, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13).Fugl-Meyer AR, Jääskö L, Leyman I, Olsson S, Steglind S: The post-stroke hemiplegic patient. 1. a method for evaluation of physical performance. Scand J Rehabil Med 7: 13–31, 1975 [PubMed] [Google Scholar]
- 14).Arya KN, Verma R, Garg RK: Estimating the minimal clinically important difference of an upper extremity recovery measure in subacute stroke patients. Top Stroke Rehabil 18: 599–610, 2011 [DOI] [PubMed] [Google Scholar]
- 15).Bauer R, Vukelić M, Gharabaghi A: What is the optimal task difficulty for reinforcement learning of brain self-regulation? Clin Neurophysiol 127: 3033–3041, 2016 [DOI] [PubMed] [Google Scholar]
- 16).Woodbury ML, Anderson K, Finetto C, et al. : Matching task difficulty to patient ability during task practice improves upper extremity motor skill after stroke: a proof-of-concept study. Arch Phys Med Rehabil 97: 1863–1871, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17).Santisteban L, Térémetz M, Bleton JP, Baron JC, Maier MA, Lindberg PG: Upper limb outcome measures used in stroke rehabilitation studies: a systematic literature review. PLoS One 11: e0154792, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18).Rodgers H, Bosomworth H, Krebs HI, et al. : Robot assisted training for the upper limb after stroke (RATULS): a multicentre randomised controlled trial. Lancet 394: 51–62, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19).Takahashi K, Domen K, Sakamoto T, et al. : Efficacy of upper extremity robotic therapy in subacute poststroke hemiplegia: an exploratory randomized trial. Stroke 47: 1385–1388, 2016 [DOI] [PubMed] [Google Scholar]
- 20).Hyakutake K, Morishita T, Saita K, et al. : Effects of home-based robotic therapy involving the single-joint hybrid assistive limb robotic suit in the chronic phase of stroke: a pilot study. Biomed Res Int 2019: 5462694, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]