Abstract
The aim of this study was to assess the agreements of both biplane and short-axis Simpson’s (SAX) methods for left atrial ejection fraction (LAEF) calculation utilising cardiovascular magnetic resonance imaging (CMR) in heart failure with preserved ejection fraction (HFpEF) and evaluate their relation to clinical outcomes. One hundred and thirty six subjects (HFpEF n = 97, controls n = 39) underwent CMR, six-minute walk tests and blood sampling in our prospective, observational, single-centre study. Overall, LAEF (%) was lower in HFpEF patients compared to controls (SAX 34 ± 13 vs 47 ± 8, biplane 34 ± 16 vs 51 ± 11; p < 0.0001 for both). Atrial fibrillation (AF) was present in 24% of HFpEF and was associated with higher LA volumes and lower LAEF compared to sinus rhythm (p < 0.0001) with both methods. Biplane LAEF correlated strongly with SAX measurements (overall Pearson’s r = 0.851, sinus rhythm r = 0.651, AF r = 0.882; p < 0.0001). Biplane LAEF did not differ significantly compared to SAX LAEF (overall 34 ± 16 vs 34 ± 13%; p = 0.307) except in AF subjects in whom biplane LAEF was lower (mean difference 2 ± 4%, p = 0.013). There were 44 composite events (25 deaths, 19 HF hospitalizations) in HFpEF during median follow-up of 1429 days. LAEF below the median was associated with increased risk of composite endpoints (Log-Rank biplane p < 0.0001; SAX p = 0.009). In multivariable Cox proportional hazards regression analysis, both biplane LAEF (hazard ratio [HR] 0.604; 95% confidence interval [CI] (0.406–0.900); p = 0.013) and SAX LAEF (HR 0.636; CI 0.441–0.918; p = 0.016) remained independent predictors along with indexed extracellular volume. CMR LAEF, derived from either the short-axis or biplane method is lower in HFpEF compared to healthy controls and remains a strong marker of prognosis.
Keywords: Heart failure with preserved ejection fraction, Prognosis, Left atrial ejection fraction, Biplane, Short-axis
Introduction
In our recently published article [1], we reported that cardiovascular magnetic resonance (CMR) derived left atrial ejection fraction (LAEF) measured from the biplane method was lower in a well characterized cohort of heart failure with preserved ejection fraction (HFpEF) compared to healthy controls and was also independently associated with adverse outcomes. Furthermore, CMR biplane LAEF had excellent reproducibility. However, volumetric assessment of left atrial volumes (and hence function) derived from the short-axis (Simpson’s short-axis [SAX]) method is widely recognised as the imaging gold standard [2, 3]. In a recent study of HFpEF patients, the biplane method was shown to have good correlation and agreements for LA volumetric analysis compared to the SAX method [4]. However, no prior CMR studies in HFpEF have compared LAEF between both methods. Furthermore, the prognostic role of SAX LAEF in HFpEF has not been reported. We aimed to assess the agreements of both methods for LAEF (and LA volumes) utilising CMR in HFpEF. We also evaluated whether SAX LAEF is also related to clinical outcomes, in order to validate and strengthen our previous findings implicating CMR LAEF as a prognostic biomarker in HFpEF.
Methods
From our original study cohorts [1] of HFpEF (n = 140) and healthy controls (n = 48), paired data for image analysis of both CMR biplane and SAX LAEF derivation was available in 136 subjects (HFpEF n = 97, controls n = 39). HFpEF was defined as clinical or radiographic evidence of heart failure and left ventricular ejection fraction > 50%. Exclusion criteria for HFpEF included: myocardial infarction in the preceding 6 months, suspected or confirmed cardiomyopathy or constrictive pericarditis, non-cardiovascular life expectancy < 6 months, severe chronic obstructive pulmonary disease (or forced expiratory volume [FEV1] < 30% predicted or forced vital capacity [FVC] < 50% predicted), severe native valve disease and significant renal impairment (estimated glomerular filtration rate [eGFR] < 30 ml/min. The control population were age- and sex-matched compared to HFpEF and asymptomatic. As previously reported, hypertensive controls were also included since hypertension is highly prevalent in this age group of patients. Furthermore, hypertension is intimately linked with HFpEF development and is also reportedly associated with LA dysfunction [5] and we wanted to account for this potential confounder.
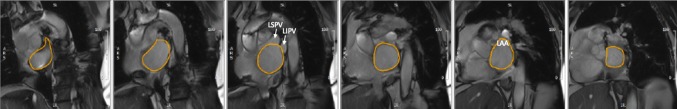
Study recruitment, blood sampling, six-minute walk testing, imaging protocols and analytical methods for CMR have been detailed previously. For the SAX method (see Fig. 1), a contiguous stack of short-axis steady-state-free-precession images were acquired with retrospective ECG gating (or prospective gating in subjects with AF). Both end-diastolic and end-systolic frames were contoured to derive maximal left atrial volume [LAV max] and minimal left atrial volume (LAV min) and LAEF was calculated. Unlike the biplane method (see Fig. 2), SAX volumes were inclusive of the LA appendage but both techniques excluded pulmonary veins. The primary endpoint remained the composite of all-cause mortality or first HF hospitalization. Parameters with univariable association with the composite endpoint at p < 0.1 was entered into Cox proportional hazards regression analysis. In cases of collinearity, variables with historically stronger prognostic importance from published literature were chosen for inclusion in Cox regression analysis and underwent stepwise elimination. Four separate clinically relevant Cox regression models were generated including a final model incorporating the strongest predictors. Cox regression models were limited to no more than 4 parameters (plus LAEF), allowing for approximately one parameter per 10 composite events. To allow hazard ratios (HR) to be compared according to one standard deviation increase, continuous predictor variables were Z-standardized. Event rates were calculated from Kaplan–Meier analysis. The Log-Rank test was used to detect differences in survival curves. In order to further assess the strength of both biplane and SAX LAEF in predicting outcomes, receiver operator characteristics (ROC) analyses were also performed.
Fig. 1.
Biplane method. Cine 2- (a) and 4-chamber (b) images illustrating contoured (yellow) left atrial areas for volume (and ejection fraction) derivation excluding the left atrial appendage and pulmonary veins; LAA left atrial appendage; LSPV left superior pulmonary vein; RIPV right inferior pulmonary vein
Fig. 2.
Simpson’s short-axis method. Short-axis cine stack of images illustrating contoured (yellow) left atrial areas for volume (and ejection fraction) derivation inclusive of the left atrial appendage but excluding the pulmonary veins. LAA left atrial appendage, LSPV left superior pulmonary vein; LIPV left inferior pulmonary vein
Inter-technique comparison of both LAEF derivation methods included paired t-testing, regression and the Bland–Altman method. Ten randomly selected subjects were chosen to undergo intra-observer evaluation for both techniques, a minimum of 4 weeks apart. The two-way mixed-effect intraclass correlation co-efficient (ICC), coefficient of variation (COV) and Bland–Altman analysis were used for test intra-observer agreements.
Results
Baseline clinical and imaging characteristics are shown in Tables 1 and 2. Both HFpEF and controls remained evenly matched for age (73 ± 8) and gender. HFpEF was associated with a high burden of co-morbidities including hypertension, diabetes, anaemia and renal dysfunction. Atrial fibrillation (AF) was noted in 24% of HFpEF. Compared to controls, HFpEF was characterised by lower exercise capacity, worse diastolic function (LA volumes, left ventricular mass, B-type natriuretic peptide [BNP] and N-terminal pro-atrial natriuretic peptide [NT-proANP]), more prevalent focal (late gadolinium enhancement imaging) and diffuse fibrosis (extracellular volume and indexed extracellular volume [iECV]); p < 0.05 for all. Feasibility of image analysis for LA measures by both techniques was 100% in all subjects. With both methods, HFpEF patients had lower LAEF (biplane 34 ± 16; SAX 34 ± 13) compared to controls: overall (biplane 51 ± 11, SAX 47 ± 8), with hypertension (biplane 48 ± 12, SAX 45 ± 9) and without hypertension (biplane 52 ± 9, SAX 49 ± 7); p < 0.0001 for all. No significant differences in LAEF were noted between hypertensive and non-hypertensive controls (biplane p = 0.222, SAX p = 0.119). Irrespective of methodology and cardiac rhythm, HFpEF was characterised by significantly higher LA volumes compared to controls (p < 0.0001). HFpEF patients in AF had higher LA volumes and lower LAEF compared to sinus rhythm (p < 0.0001).
Table 1.
Baseline clinical characteristics
| HFpEF n = 97 |
Controls n = 39 |
P value | |
|---|---|---|---|
| Demographics | |||
| Age (years) | 72 ± 10 | 73 ± 5 | 0.486 |
| Male (%) | 52 (54) | 18 (46) | 0.682 |
| Clinical | |||
| Heart rate (beats per minute) | 71 ± 14 | 68 ± 10 | 0.133 |
| Systolic blood pressure (mmHg) | 145 ± 23 | 151 ± 24 | 0.155 |
| Diastolic blood pressure (mmHg) | 74 ± 12 | 79 ± 10 | 0.012 |
| Body mass index (kg/m2) | 34 ± 8 | 25 ± 3 | < 0.0001 |
| Atrial fibrillation (%) | 23 (24) | 0 (0) | < 0.0001 |
| Prior HF hospitalization (%) | 62 (64) | 0 (0) | < 0.0001 |
| Diabetes (%) | 55 (57) | 0 (0) | < 0.0001 |
| Hypertension (%) | 89 (92) | 19 (49) | < 0.0001 |
| Angina (%) | 15 (16) | 0 (0) | 0.004 |
| Known myocardial infarction (%) | 12 (12) | 0 (0) | 0.011 |
| Asthma or COPD (%) | 14 (14) | 3 (8) | 0.149 |
| TIA or CVA (%) | 12 (12) | 0 (0) | 0.011 |
| Functional status | |||
| NYHA III/IV (%) | 23 (24) | NA | – |
| 6MWT distance (m) | 190 (130–275) | 380 (340–440) | < 0.0001 |
| MLHF score | 49 (24–65) | NA | – |
| Medications | |||
| Betablocker (%) | 67 (69) | 2 (5) | < 0.0001 |
| ACEi or ARB (%) | 86 (89) | 9 (23) | < 0.0001 |
| Aldosterone antagonist (%) | 30 (31) | 0 (0) | < 0.0001 |
| Loop diuretic (%) | 77 (79) | 0 (0) | < 0.0001 |
| Laboratory indices | |||
| Urea (mmol/L) | 9 ± 4 | 6 ± 1 | < 0.0001 |
| Creatinine (umol/L) | 92 (74–118) | 67 (56–85) | < 0.0001 |
| Haemoglobin (g/L) | 131 ± 23 | 140 ± 15 | 0.003 |
| BNP (ng/L) | 117 (51–244) | 33 (24–44) | < 0.0001 |
| NTpro-ANP (pg/ml) | 6321 (3874) | 4246 (3402–4532) | < 0.0001 |
Values are mean ± SD, n (%) or median, interquartile range. The p values are for the t test or chi-square test
BNP B-type natriuretic peptide, COPD chronic obstructive pulmonary disease, HF heart failure, HFpEF heart failure with preserved ejection fraction, NA not applicable, NTpro-ANP N-terminal pro-atrial natriuretic peptide, NYHA New York Heart Association class, 6MWT six minute walk test
Table 2.
Baseline imaging characteristics
| HFpEF n = 97 |
Controls n = 39 |
p value | |
|---|---|---|---|
| CMR LV parameters | |||
| LVEF (%) | 56 ± 5 | 58 ± 5 | 0.058 |
| LVEDVI (ml/m2) | 79 ± 18 | 83 ± 14 | 0.237 |
| LVESVI (ml/m2) | 35 ± 10 | 35 ± 8 | 0.950 |
| LV mass indexed (g/m2) | 52 ± 15 | 46 ± 10 | 0.003 |
| LV mass/LVEDV | 0.68 ± 0.16 | 0.57 ± 0.09 | < 0.0001 |
| LV tissue characterisation | |||
| Presence of MI (%) | 13 (13) | 0 (0) | 0.007 |
| MI size (% of LV mass) | 3.0 (0.9–4.9) | NA | – |
| Presence of non-MI focal fibrosis (%) | 38 (39) | 5 (13) | 0.007 |
| Non-MI fibrosis size (% of LV mass) | 2.8 (1.2–6.6) | 2.4 (0.6–3.6) | < 0.0001 |
| aNative myocardial T1 (ms) | 1230 ± 78 | 1191 ± 98 | 0.038 |
| aPost-contrast myocardial T1 (ms) | 457 ± 61 | 489 ± 93 | 0.007 |
| aECV (%) | 28 ± 5 | 26 ± 3 | 0.008 |
| aiECV (ml/m2) | 14 ± 4 | 11 ± 3 | 0.001 |
| CMR LA parameters | |||
| Overall—all subjects including atrial fibrillation | |||
| SAX LAV max (ml) | 120 ± 50 | 88 ± 21 | < 0.0001 |
| SAX LAV min (ml) | 84 ± 49 | 47 ± 14 | < 0.0001 |
| SAX LAEF (%) | 34 ± 13 | 47 ± 8 | < 0.0001 |
| Biplane LAV max (ml) | 102 ± 44 | 62 ± 22 | < 0.0001 |
| Biplane LAV min (ml) | 71 ± 44 | 31 ± 14 | < 0.0001 |
| Biplane LAEF (%) | 34 ± 16 | 51 ± 11 | < 0.0001 |
| Sinus | |||
| SAX LAV max (ml) | 103 ± 33 | 88 ± 21 | 0.004 |
| SAX LAV min (ml) | 64 ± 26 | 47 ± 14 | < 0.0001 |
| SAX LAEF (%) | 39 ± 10 | 47 ± 8 | < 0.0001 |
| Biplane LAV max (ml) | 88 ± 32 | 62 ± 22 | < 0.0001 |
| Biplane LAV min (ml) | 53 ± 25 | 31 ± 14 | < 0.0001 |
| Biplane LAEF (%) | 41 ± 12 | 51 ± 11 | < 0.0001 |
| Atrial fibrillation | |||
| SAX LAV max (ml) | 175 ± 56 | NA | – |
| SAX LAV min (ml) | 148 ± 51 | NA | – |
| SAX LAEF (%) | 16 ± 8 | NA | – |
| Biplane LAV max (ml) | 147 ± 45 | NA | – |
| Biplane LAV min (ml) | 129 ± 43 | NA | – |
| Biplane LAEF (%) | 14 ± 9 | NA | – |
ECV extracellular volume, iECV indexed to body surface area; extracellular volume, LA left atrium, LAEF left atrial ejection fraction, LAV max maximal left atrial volume, LAV min minimal left atrial volume, LV left ventricle, LVEDVI left ventricular end-diastolic volume indexed to body surface area, LVEF left ventricular ejection fraction, LVESVI left ventricular end-systolic volume indexed to body surface area, MI myocardial infarction
aAvailable in n = 72 HFpEF, n = 35 controls
Biplane LA measures correlated strongly with SAX parameters overall (LAV max Pearson’s r = 0.910, LAV min r = 0.884, LAEF r = 0.851), in sinus rhythm (LAV max 0.926, LAV min r = 0.920, LAEF r = 0.651) and moderate to good in AF (LAV max r = 0.776, LAV min r = 0.788, LAEF r = 0.882); p < 0.0001 for all. LA volumes calculated from the biplane method (excluding the LA appendage) were significantly lower compared to the SAX method overall and irrespective of whether subjects were in sinus rhythm or AF (Table 2). While overall agreements for LA volumes were good (Table 3), the limits of agreement were wider in AF. Biplane LAEF did not differ significantly compared to SAX LAEF except in AF subjects in whom biplane LAEF was comparably lower (mean difference 2 ± 4%, p = 0.013). Intra-observer agreements for both methods were excellent, albeit biplane measures fared slightly worse (Table 4).
Table 3.
Inter-technique agreements for left atrial volumes and ejection fraction between CMR short-axis and biplane methods
| Parameter | CMR SAX mean ± SD | CMR biplane Mean ± SD | Mean dfference ± SD | 95% Limits of agreement | P value |
|---|---|---|---|---|---|
| All patients (n = 97) | |||||
| LAV max (ml) | 120 ± 50 | 102 ± 44 | 18 ± 21 | − 22 to 60 | < 0.0001 |
| LAV min (ml) | 84 ± 49 | 71 ± 44 | 13 ± 18 | − 22 to 48 | < 0.0001 |
| LAEF (%) | 34 ± 13 | 34 ± 16 | − 1 ± 8 | − 17 to 15 | 0.307 |
| Sinus rhythm (n = 74) | |||||
| LAV max (ml) | 103 ± 33 | 88 ± 32 | 15 ± 13 | − 9 to 41 | < 0.0001 |
| LAV min (ml) | 64 ± 26 | 53 ± 25 | 11 ± 10 | − 9 to 31 | < 0.0001 |
| LAEF (%) | 39 ± 10 | 41 ± 12 | − 2 ± 9 | − 20 to 16 | 0.083 |
| Atrial fibrillation (n = 23) | |||||
| LAV max (ml) | 176 ± 56 | 147 ± 45 | 27 ± 35 | − 42 to 97 | 0.001 |
| LAV min (ml) | 148 ± 51 | 129 ± 43 | 19 ± 32 | − 43 to 81 | 0.008 |
| LAEF (%) | 16 ± 8 | 14 ± 9 | 2 ± 4 | − 6 to 10 | 0.013 |
Abbreviations are as for Table 2
Table 4.
Intra-observer assessments for left atrial volumes and ejection fraction
| Parameter | Observer 1 Mean ± SD |
Observer 2 Mean ± SD |
Mean difference ± SD | ICC | Variability (1—ICC) | Co-efficient of variation | 95% Limits of agreement |
|---|---|---|---|---|---|---|---|
| Intra-observer | |||||||
| SAX | |||||||
| LAV max (ml) | 123 ± 40 | 122 ± 39 | 1 ± 3 | 0.99 | 0.01 | 3 | − 7 to 5 |
| LAV min (ml) | 85 ± 44 | 84 ± 43 | 1 ± 2 | 0.99 | 0.01 | 2 | − 4 to 3 |
| LAEF (%) | 34 ± 14 | 34 ± 14 | 0 ± 2 | 0.99 | 0.01 | 6 | − 4 to 4 |
| Biplane | |||||||
| LAV max (ml) | 99 ± 48 | 101 ± 49 | 2 ± 5 | 0.99 | 0.01 | 4.8 | − 7 to 12 |
| LAV min (ml) | 70 ± 45 | 71 ± 44 | 1 ± 4 | 0.99 | 0.01 | 5.4 | − 7 to 8 |
| LAEF (%) | 33 ± 13 | 33 ± 13 | 0.1 ± 3 | 0.98 | 0.02 | 9.4 | − 6 to 6 |
Abbreviations are as for Table 2
ICC intra-class correlation coefficient
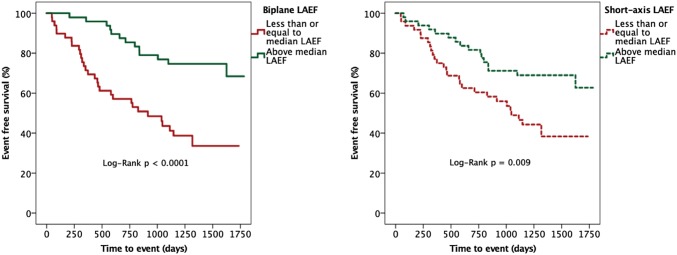
Forty four composite events (45%, 25 deaths, 19 HF hospitalizations) were observed in the HFpEF group during median follow-up of 1429 days (1157–1657). Sixteen parameters were associated with outcomes during Cox regression analysis, including both biplane and SAX LAEF (Table 5). Urea and extracellular volume (ECV) were excluded from regression analysis due to collinearity. On Cox regression analysis (Table 6), both biplane and SAX LAEF remained significantly associated with outcome in 3 separate models incorporating clinical factors, biochemical markers and imaging parameters. In a final model comprising the strongest predictors overall, biplane LAEF (HR 0.604; 95% confidence interval [CI] (0.406–0.900); p = 0.013) and SAX LAEF (HR 0.636; CI 0.441–0.918; p = 0.016) remained independent predictors along with indexed extracellular volume (iECV). Irrespective of methodology, a lower LAEF group (below median) was associated with increased risk of the composite endpoint (biplane Log-Rank p < 0.0001; SAX Log-Rank p = 0.009); see Fig. 3. The area under curve for predicting outcomes was higher for SAX LAEF (0.709, p < 0.0001), albeit not significantly different compared to biplane LAEF (0.690, p = 0.001).
Table 5.
Unadjusted predictors for the composite endpoint of death and/or hospitalization with heart failure
| Unadjusted predictors of outcome | ||
|---|---|---|
| Hazard ratio (95%CI) | P value | |
| Clinical | ||
| Age (years) | 1.630 (1.203–2.210) | 0.002 |
| Average diastolic BP (mmHg) | 0.562 (0.388–0.814) | 0.002 |
| Prior HF hospitalization | 2.236 (1.104–4.529) | 0.025 |
| 6MWT distance (m) | 0.545 (0.339–0.876) | 0.012 |
| Clinical blood samples | ||
| aUrea (mmol/L) | 1.284 (1.002–1.644) | 0.048 |
| Log creatinine (umol/L) | 1.352 (1.024–1.784) | 0.033 |
| Haemoglobin (g/L) | 0.766 (0.576–1.019) | 0.067 |
| Log BNP (ng/L) | 1.542 (1.086–2.189) | 0.016 |
| NTpro-ANP (pg/ml) | 1.551 (1.113–2.160) | 0.009 |
| Imaging (CMR) | ||
| LV mass index (g/m2) | 1.432 (1.020–2.010) | 0.038 |
| LAVI max (ml/m2) | 1.595 (1.202–2.115) | 0.001 |
| LGE MI (%) | 1.687 (0.784–3.632) | 0.181 |
| aECV (%) | 1.822 (1.173–2.831) | 0.008 |
| iECV (ml/m2) | 1.558 (1.097–2.213) | 0.013 |
| Biplane LAEF (%) | 0.575 (0.419–0.788) | 0.001 |
| SAX LAEF (%) | 0.596 (0.447–0.794) | 0.0001 |
Abbreviations are as for Tables 1 and 2; Hazard ratios are based upon one standard deviation increase in the predictor variable for continuous variables which are Z-standardized
CI confidence interval; LAVI max left atrial maximal volume indexed to body surface area
aParameters not entered into multivariable analysis
Table 6.
Multiple Cox regression models inclusive of biplane and SAX LAEF for the composite endpoint of death and/or hospitalization with heart failure
| Cox proportional hazard regression predictors of outcome | |||||
|---|---|---|---|---|---|
| Including biplane LAEF | Including SAX LAEF | ||||
| Hazard ratio (95%CI) | P value | Hazard ratio (95%CI) | P value | ||
| Clinical | |||||
| Age | 1.126 (0.771–1.645) | 0.538 | Age | 1.049 (0.708–1.555) | 0.811 |
| Average diastolic BP | 0.606 (0.407–0.904) | 0.014 | Average diastolic BP | 0.580 (0.390–0.863) | 0.007 |
| Prior HF hospitalization | 1.693 (0.810–3.540) | 0.162 | Prior HF hospitalization | 1.588 (0.754–3.347) | 0.224 |
| 6MWT distance | 0.596 (0.372–0.956) | 0.032 | 6MWT distance | 0.613 (0.387–0.972) | 0.038 |
| + Biplane LAEF | 0.535 (0.385–0.744) | < 0.0001 | + SAX LAEF | 0.532 (0.391–0.724) | < 0.0001 |
| Clinical blood samples | |||||
| Log creatinine (umol/L) | 1.035 (0.731–1.466) | 0.0847 | Log creatinine (umol/L) | 1.033 (0.734–1.454) | 0.853 |
| Haemoglobin (g/L) | 0.805 (0.584–1.109) | 0.155 | Haemoglobin (g/L) | 0.677 (0.496–0.923) | 0.114 |
| Log BNP (ng/L) | 1.126 (0.712–1.782) | 0.611 | Log BNP (ng/L) | 1.128 (0.712–1.787) | 0.607 |
| Log NTpro-ANP | 1.373 (0.990–1.906) | 0.058 | NTpro-ANP | 1.244 (0.890–1.739) | 0.202 |
| + Biplane LAEF | 0.649 (0.456–0.924) | 0.016 | + SAX LAEF | 0.552 (0.410–0.741) | < 0.0001 |
| Imaging | |||||
| LV mass index | 0.582 (0.217–1.560) | 0.282 | LV mass index | 0.529 (0.205–1.366) | 0.188 |
| LAVI max | 0.961 (0.599–1.541) | 0.869 | LAVI max | 0.997 (0.630–1.577) | 0.988 |
| iECV | 1.558 (1.097–2.213) | 0.013 | iECV | 1.564 (1.106–2.211) | 0.011 |
| + Biplane LAEF | 0.575 (0.419–0.788) | 0.001 | + SAX LAEF | 0.668 (0.472–0.944) | 0.022 |
| Strongest markers combined | |||||
| Average diastolic BP | 0.723 (0.461–1.134) | 0.158 | Average diastolic BP | 0.725 (0.461–1.139) | 0.163 |
| 6MWT distance | 0.611 (0.354–1.053) | 0.076 | 6MWT distance | 0.592 (0.346–1.011) | 0.055 |
| iECV | 1.491 (1.038–2.143) | 0.031 | iECV | 1.584 (1.110–2.260) | 0.011 |
| + Biplane LAEF | 0.604 (0.406–0.900) | 0.013 | + SAX LAEF | 0.636 (0.441–0.918) | 0.016 |
Fig. 3.
Survival analysis stratified according to median left atrial ejection fraction. Kaplan–Meier analysis stratified according to median left atrial ejection fraction for the composite endpoint of death and/or hospitalization with heart failure using the biplane method (left panel) and the short-axis method (right panel)
Discussion
Our study is the first to compare the agreements of both CMR biplane and SAX LAEF in HFpEF and assess their prognostic capabilities in the same setting. Our results confirm LAEF as an independent marker of prognosis in HFpEF, irrespective of CMR technique and validate our earlier findings of biplane LAEF as a strong prognostic biomarker [1]. The ability of CMR LAEF to be measured via 2 separate methods with a high degree of precision and reproducibility in addition to its prognostic capabilities are important strengths for consideration when being proposed as an imaging biomarker [6]. While CMR SAX LAEF is considered the more accurate of both measures [2, 3], the CMR biplane method represents a potentially useful alternative for both research and clinical settings in HFpEF and has been recently proposed as the preferred method of LA volumetric analysis in an expert consensus document by the European Association of Cardiovascular Imaging [7]. Biplane LAEF derivation does not necessitate additional, multiple short axis image acquisitions which can prolong scan times in predominantly elderly subjects typical of HFpEF, who may struggle with multiple breath-holds. Furthermore, image analysis of the biplane method is also significantly shorter. On the other hand, SAX LAEF is an alternative if long axis images are degraded by artefact. Our findings are also important since LAEF represents a potential therapeutic target in HFpEF [8] where there are currently no effective therapies [9] and may also act as a trial endpoint [10].
While LA volumes were underestimated by the biplane method compared to SAX evaluation, LAEF did not differ significantly except in AF. Since LAEF is a fraction derived from volumetric analysis, systematic bias of similar magnitudes in the same direction i.e. underestimation of both LAV max and LAV min likely explains this lack of LAEF difference between both the techniques. The difference in LA volumes between both methods however is unsurprising given that the LA appendage is typically excluded from the biplane method but included in the SAX method [2, 3]. These volumetric differences are likely further exaggerated in HFpEF where LA dilation is typical [2, 4] and associated with a higher prevalence of AF. Furthermore, in AF we employed prospective gating which can lower the margins for erroneous measurements. As addressed in our recent publication, we also recognise that having some subjects with hypertension in our control group is a limitation given that these subjects were not totally free from cardiovascular disease. However, LAEF was again lower in HFpEF with either methodology, irrespective of controls’ hypertensive status, reinforcing altered LA contractile function in the pathophysiology of HFpEF.
Conclusions
CMR LAEF calculated from either the short-axis or biplane method has excellent reproducibility and remains a strong marker of prognosis in HFpEF.
Acknowledgements
The authors would like to thank the CMR radiographers at Glenfield Hospital for image acquisition and Bristol Myers Squibb, in particular Jing Yang and Lei Zhao for facilitating plasma biomarker (NTpro-ANP) analysis.
Author contributions
PK recruited the patients, supervised the study visits and CMR scans (with AS and JNK), analysed the data, performed the statistical analysis and drafted the initial manuscript along with JRA. GGS undertook follow-up outcome data collection. BNP and other serum sampling were undertaken in the hospital pathology laboratory under the supervision of PG. PK, IBS, LLN and GPM conceived the study. All authors critically revised the manuscript for important intellectual content, approved the final version for submission and agree to be accountable for all aspects of the work in ensuring that questions relating to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Funding
This work was supported by the National Institute for Health Research (NIHR) Leicester Cardiovascular Biomedical Research Centre overall project Grant: IRS_BRU_0211_20033 and the John and Lucille Van Geest Foundation. Professor GPM was supported by NIHR Research Fellowships (PDF-2011-0451 and CDF 2014-07-045).
Compliance with ethical standards
Conflicts of interest
Lei Zhao and Jing Yang are employees of Bristol Myers Squibb which facilitated plasma NTpro-ANP analysis. All authors declare that they have no competing interests relevant to this study. All authors also state that they have full control of all primary data and that they agree to allow the journal to review their data if requested.
Ethical approval
The study was approved by the United Kingdom National Research Ethics Service Committee East Midlands—Nottingham (reference: 12/EM/0222). Informed consent was obtained from all individual participants included in the study. The study was performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Prathap Kanagala, Email: pkk12@leicester.ac.uk.
Jayanth R. Arnold, Email: jra14@leicester.ac.uk
Anvesha Singh, Email: as707@leicester.ac.uk.
Jamal N. Khan, Email: mally777@hotmail.com
Gaurav S. Gulsin, Email: gg149@leicester.ac.uk
Pankaj Gupta, Email: pankaj_gupta54@hotmail.com.
Iain B. Squire, Email: is11@leicester.ac.uk
Leong L. Ng, Email: lln1@leicester.ac.uk
Gerry P. McCann, Email: gpm12@leicester.ac.uk
References
- 1.Kanagala P, Arnold JR, Cheng ASH, et al. Left atrial ejection fraction and outcomes in heart failure with preserved ejection fraction. Int J Cardiovasc Imaging. 2019 doi: 10.1007/s10554-019-01684-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hudsmith LE, Cheng AS, Tyler DJ, et al. Assessment of left atrial volumes at 15 Tesla and 3 Tesla using FLASH and SSFP cine imaging. J Cardiovasc Magn Reson. 2007;9(4):673–679. doi: 10.1080/10976640601138805. [DOI] [PubMed] [Google Scholar]
- 3.Maceira AM, Cosin-Sales J, Roughton M, et al. Reference left atrial dimensions and volumes by steady state free precession cardiovascular magnetic resonance. J Cardiovasc Magn Reson. 2010;12:65. doi: 10.1186/1532-429X-12-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vassiliou VS, Patel HC, Rosen SD, et al. Left atrial dilation in patients with heart failure and preserved ejection fraction: Insights from cardiovascular magnetic resonance. Int J Cardiol. 2016;210:158–160. doi: 10.1016/j.ijcard.2016.02.101. [DOI] [PubMed] [Google Scholar]
- 5.Mondillo S, Cameli M, Caputo ML, et al. Early detection of left atrial strain abnormalities by speckle-tracking in hypertensive and diabetic patients with normal left atrial size. J Am Soc Echocardiogr. 2011;24(8):898–908. doi: 10.1016/j.echo.2011.04.014. [DOI] [PubMed] [Google Scholar]
- 6.Vasan RS. Biomarkers of cardiovascular disease: molecular basis and practical considerations. Circulation. 2006;113(19):2335–2362. doi: 10.1161/CIRCULATIONAHA.104.482570. [DOI] [PubMed] [Google Scholar]
- 7.Petersen SE, Khanji MY, Plein S, et al. European Association of Cardiovascular Imaging expert consensus paper: a comprehensive review of cardiovascular magnetic resonance normal values of cardiac chamber size and aortic root in adults and recommendations for grading severity. Eur Heart J Cardiovasc Imaging. 2019 doi: 10.1093/ehjci/jez232. [DOI] [PubMed] [Google Scholar]
- 8.Shah SJ, Feldman T, Ricciardi MJ, et al. One-year safety and clinical outcomes of a transcatheter interatrial shunt device for the treatment of heart failure with preserved ejection fraction in the reduce elevated left atrial pressure in patients with heart failure (REDUCE LAP-HF I) Trial: a randomized clinical trial. JAMA Cardiol. 2018;3(10):968–977. doi: 10.1001/jamacardio.2018.2936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ponikowski P, Voors AA, Anker SD, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: the task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016;37(27):2129–2200. doi: 10.1093/eurheartj/ehw128. [DOI] [PubMed] [Google Scholar]
- 10.Lewis GA, Schelbert EB, Naish JH, et al. Pirfenidone in heart failure with preserved ejection fraction-rationale and design of the PIROUETTE trial. Cardiovasc Drugs Ther. 2019 doi: 10.1007/s10557-019-06876-y. [DOI] [PMC free article] [PubMed] [Google Scholar]