Abstract
Background
Short periods of fasting and/or low-carbohydrate diet have been proven beneficial for decreasing the myocardial uptake of 18F-fluorodeoxyglucose (18F-FDG) and enhancing the detection of inflammatory heart diseases by 18F-FDG positron emission tomography (PET). This study aimed at determining whether this benefit is increased when a low-carbohydrate ketogenic diet is prolonged up to 7 days.
Methods
Wistar rats underwent serial 18F-FDG-PET imaging after an 18-hour fasting period and after 2, 4 and 7 days of a ketogenic diet (3% carbohydrate) and they were compared to rats submitted to the same protocol but with normal diet (44% carbohydrate). The 18F-FDG-PET/ketogenic protocol was also applied in rats with immune myocarditis (injection of porcine cardiac myosin).
Results
The 7-day ketogenic diet was associated with (1) a sustained increase in circulating ketone bodies at an equivalent level to that reached after 18-hour fasting, (2) a gradual decrease in 18F-FDG uptake within normal myocardium reaching a lower level compared to fasting at the 7th day (myocardium-to-blood ratios: 1.68 ± 1.02 vs 3.25 ± 1.40, P < .05) and (3) a high 18F-FDG-PET detectability of myocarditis areas.
Conclusion
One-week extension of a ketogenic diet provides a further decrease in the 18F-FDG uptake of normal myocardium and a high detectability of inflammatory areas.
Electronic supplementary material
The online version of this article (10.1007/s12350-018-1404-7) contains supplementary material, which is available to authorized users.
Keywords: 18F-fluorodeoxyglucose, positron emission tomography, fasting, low-carbohydrate diet, myocarditis
Introduction
A fasting period of at least 12 hours,1-4 as well as low-carbohydrate diet,5-12 have been proven beneficial for decreasing the normal myocardial uptake of 18F-fluorodeoxyglucose (18F-FDG) and for enhancing the detectability of inflammatory and/or infectious heart diseases by 18F-FDG positron emission tomography (18F-FDG-PET). Unfortunately, none of these previously reported protocols enable the complete and consistent suppression of the cardiac uptake of 18F-FDG.
This study was aimed at determining whether a drastic ketogenic diet provides a further decrease in physiological myocardial 18F-FDG uptake and a high detectability of myocarditis by 18F-FDG-PET in rats, when this diet is prolonged up to 1 week, as compared with a standard 18-hour fasting conditioning. Such diet can be prescribed at a much longer term than fasting,13-15 leading to metabolic changes that are known to progressively suppress the metabolic use of glucose, even in the brain, within at least a 3- to 5-day period.15-18
Methods
Study Design and Experimental Groups
All protocols were approved by the Lorraine Committee No. 68 according to Guidelines of Animal Care and Use (APAFIS # 1755-201509151127522v1).
Twelve adult male Wistar rats underwent cardiac 18F-FDG-PET after an 18-hour fasting period (day-0) and subsequently after 2, 4 and 7 days of a ketogenic diet19,20 (3% carbohydrate, 73% fat, 15% protein, 0% fiber, and 9% vitamins and minerals; ketocal®, SDS, France). This human pharmacological ketogenic product was processed in biscuits by adding water, as already described in previous rat experiments.19,20 The experimental animals were compared to a control group of 7 rats submitted to the same protocol but with a normal diet (44% carbohydrate, 6% fat, 19% protein, 18% fiber, and 13% vitamins and minerals; Envigo, Gannat, France). Food and water were given ad libitum in both ketogenic and control groups and each rat was weighted daily, at a fixed time, all along the experimental protocol and with a dedicated small-animal weighing balance (Mettler Toledo, DeltaRange-PR5002, France).
Blood venous samples of approximately 1 mL were collected in EDTA tubes from the tail vain just before each 18F-FDG injection and thereafter, blood was centrifuged at 3000 g for 15 minutes, the plasma being subsequently frozen at − 80 °C for further ketones bodies measurements (β-Hydroxybutyrate Assay Kit MAK041, Sigma, Saint-Quentin-Fallavier, France). The kit is designed to produce a compound whose colorimetric intensity, determined at a wavelength of 450 nm, is proportional to the concentration of β-hydroxybutyrate.
Animals were sacrificed after the last PET recording for subsequent histological and autohistoradiographic studies.
The same PET/ketogenic diet protocol was also applied to five rats with an immune myocarditis, starting 6 weeks after the subcutaneous injection of an emulsion of 1 mg (10 mg·mL−1) of porcine cardiac myosin (Sigma, Saint-Quentin-Fallavier, France).21
Approximately 74 MBq of 18F-FDG were injected in the caudal vein under brief anesthesia (1.5%-2.5% isoflurane inhalation), 60 minutes prior to initiating PET acquisition. This acquisition was obtained under the same isoflurane anesthesia with a dedicated small-animal PET system (Inveon, Siemens, Knoxville, Tennessee, USA), as previously described elsewhere.22-24 The animals were positioned in prone position on a heating pad with a recording time of 30 minutes for 18F emission and 10 minutes for 57Co transmission.
Images were reconstructed in 16 cardiac intervals, and the corresponding 16 sinograms were reconstructed with a 3D OSEM algorithm with attenuation and scatter correction according to the following parameters: 4 iterations, 128 × 128 matrix, 2.0 zoom, and 0.8 mm thickness, leading to a voxel size of 0.8 × 0.4 × 0.4 mm. The study groups were compared according to their myocardial/blood activity ratio (M/B), an index of myocardial 18F-FDG uptake.5,22-24 As previously described,22 this ratio was estimated on a single mid-ventricular end-diastolic short-axis slice with mean myocardial counts being determined using a half-moon-shaped region of interest and mean blood counts, on a sphere of 1.5 mm in diameter positioned at the center of the LV cavity.22
Histological Section Analysis
At the end of the study, animals were sacrificed by sodium pentobarbital overdose (180 mg·kg−1) and their hearts were excised and snap frozen in isopentane cooled with liquid nitrogen. Contiguous 8 μm sections were obtained with a cryostat at − 22 °C for autohistoradiography and histological staining, respectively.
Distribution of 18F-FDG activity was recorded with an autohistoradiography system dedicated to the detection of electrons and positrons (µImager™, Biospace, France).22 For Hematoxylin-Eosin-Safran (HES) staining, the sections were fixed in 95% ethanol, stained with hematoxylin for 1 minute, eosin and safran for 30 seconds each, before being dehydrated in ethanol 100% and xylene. For the Masson trichrome staining, the sections were fixed by immersion in Bouin solution for 15 minutes and picric acid for 5 minutes. The nuclei were stained with Weigert hematoxylin for 10 minutes, cytoplasm and smooth muscle with Biebrich solution and collagen fibers by immersion for 5 minutes in aniline blue.
For further immunohistology analyses, adjacent 5 µm sections were fixed with paraformaldehyde 4% (VWR, Fontenay-sous-Bois, France), incubated with a rabbit polyclonal antibody to determine macrophage infiltrates (anti-Vimentin antibody; 1:500; Dako, Les Ulis, France).
Statistical Analyses
All data are expressed as mean ± standard deviation (SD). Statistical analyses were performed using the SPSS Statistics Software package v. 20 (IBM, NY, USA). Comparisons of quantitative variables were performed with ANOVA-test, after verifying for distribution normality. P values < .05 were considered as statistically significant.
Results
As detailed in Figure 1, mean body weight was equivalent between rats fed with the ketogenic diet and control rats throughout the experimental period (at the 7th day: 261 ± 25 g vs 272 ± 30 g, NS). By contrast, a sustained increase in the level of circulating ketone bodies was documented throughout the ketogenic diet period, contrary to that documented in the standard diet control group during the same period (Figure 2A). The level achieved at the 7th day of ketogenic diet was as high as that observed after the initial 18-hour fasting period (Figure 2A).
Figure 1.
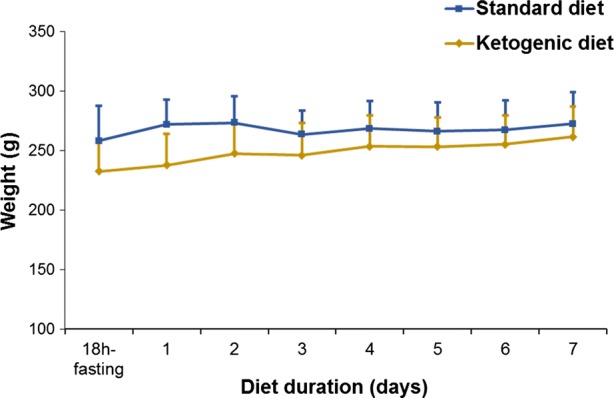
Comparison of mean body weight between rats fed with the ketogenic diet and control rats fed with a normal diet, throughout the experimental period. No significant differences in mean body weight were observed
Figure 2.
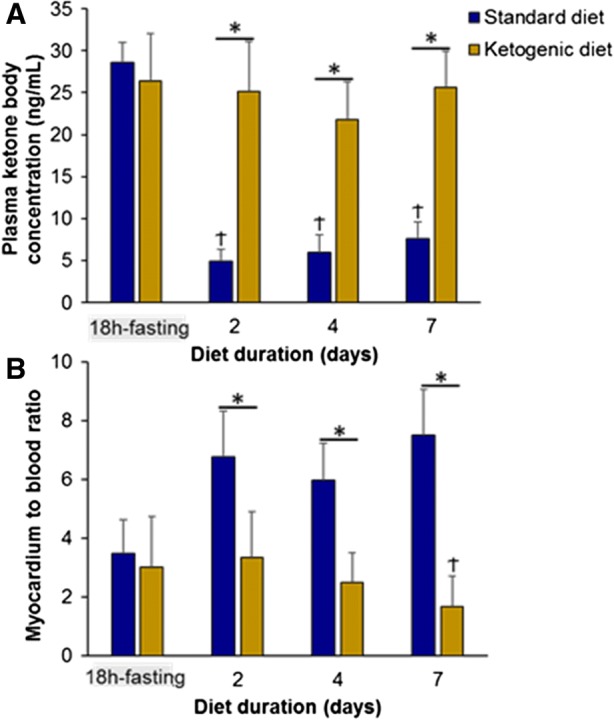
(A) Plasma concentrations of ketone body determined after the initial 18-hour fasting period and thereafter, throughout the standard and ketogenic diet periods (*P < .05 for two-group comparisons and †P < .05 paired comparisons with the 18-hour fasting period). (B) Myocardium-to-blood activity ratio determined in vivo on [18F]-FDG-PET images after the initial 18-hour fasting period and thereafter, throughout the standard and ketogenic diet periods (*P < .05 for two-group comparisons and †P < .05 paired comparisons with the 18-hour fasting period)
In addition, 18F-FDG myocardial uptake, assessed by means of a myocardial-to-blood activity ratio (Figure 2B), exhibited a gradual decline throughout the ketogenic diet period, reaching a lower level at the 7th day than that documented with the initial 18-hour fasting period (1.68 ± 1.02 vs 3.25 ± 1.40, P < .05). Representative 18F-FDG-PET images are displayed in Figure 3A.
Figure 3.
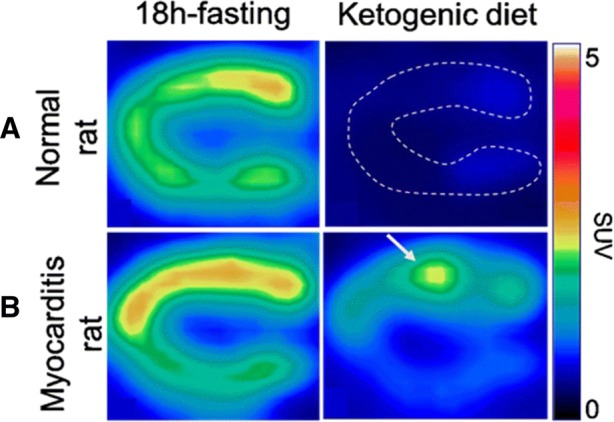
Representative images of the left ventricle obtained with [18F]-FDG-PET in a vertical long-axis orientation in a myocarditis rat (B) and in a normal rat (A) both following the initial 18-hour fasting period and at the end of the 7-day ketogenic diet. Note that the level of [18F]-FDG activity within normal myocardium is much lower after the ketogenic diet than after the 18-hour fasting period, allowing an easy delineation of a myocarditis anterior focus. Demonstrative cine-loop images of the same rats are available in a supplemental file
As shown in Figure 3B and especially on the cine-loop images available in a supplemental (online) file, the delineation of myocarditis areas by 18F-FDG-PET was clearly evident at the 7th day of the ketogenic diet owing to a high contrast with normal myocardium.
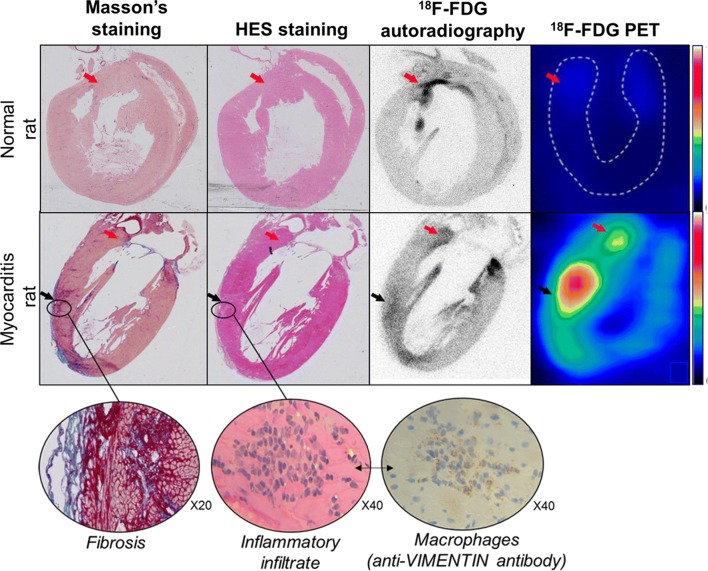
Moreover, as illustrated in Figure 4, the histological sections from myocarditis rats demonstrated that the myocardial areas showing an increase in 18F-FDG uptake mostly corresponded to a sub-acute myocarditis, with evidence of an increased fibrosis and of an inflammatory infiltrate at the corresponding sites. The cardiac uptake of 18F-FDG was additionally found somewhat higher around the mitral annulus in the ketogenic rats with or without myocarditis (Figures 3, 4).
Figure 4.
Images of the left ventricle obtained through a vertical long-axis orientation in the normal rat and myocarditis rat and at the end of the 7 days of the ketogenic diet (1) with the distribution of [18F]-FDG activity obtained in vivo with the PET camera and thereafter, ex vivo at autohistoradiography, both showing the anterior and apical myocarditis areas (black arrows), as well as small areas of increased [18F]-FDG uptake in contact with the mitral annulus (red arrows), and (2) with the colocalization of fibrosis (red color with Masson’s staining) and inflammatory infiltrates (blue color with HES staining) and macrophages (brown color with antibody anti-VIMENTIN staining) on contiguous histological slices
Discussion
This study shows that, when compared with a rather long fasting period of almost 18 hours,8,25 a 1-week extension of a drastic ketogenic diet provides a further decrease in myocardial 18F-FDG uptake and consequently, a high detectability of myocarditis by 18F-FDG-PET.
Current recommendations for an overnight fast after a last meal with low-carbohydrate intake are likely to prove inadequate in a significant proportion of patients for whom detection of inflammatory and/or infectious heart diseases is attempted by 18F-FDG-PET.1,5-7 More prolonged periods of fasting up to 18 hours could potentially constitute a more efficient method to switch the myocardial metabolism to a preferential use of ketone bodies and free fatty acids, thereby leading to a decrease in the cardiac uptake of glucose and 18F-FDG.1-3,7,26 Fasting duration is a key point in this setting, with a marked impact on the ability of 18F-FDG-PET to diagnose inflammatory heart diseases, as shown in a recent meta-analysis performed in cardiac sarcoidosis patients.7 Unfortunately, prolonged fasting of more than 12 or 18 hours may still provide a significant proportion of suboptimal results 1-3,7,26 and is not easily applied in certain severely ill patients with suspected endocarditis or myocarditis.
Low-carbohydrate diet protocols constitute a much more secure alternative for decreasing the myocardial uptake of 18F-FDG.
Such protocols have previously been shown to be well tolerated, even when prolonged several weeks or months in various diseases, including with an established efficacy in pediatric pharmaco-resistant epilepsy.13-15 Ketogenic diet may also be prescribed in diabetic patients without significant risk and at the condition of adapting the antidiabetic treatment to the improvement in blood glucose levels and to the reduction of the need for insulin, which are currently induced by such diets.30 In addition, low-carbohydrate diets have shown a significant albeit variable effectiveness for decreasing myocardial 18F-FDG uptake in a number of 18F-FDG-PET studies conducted in humans 4,8-10 or animals.11,12 The variability of this effectiveness is likely attributable to differences in diet protocols and particularly in the duration and in the degree of carbohydrate reduction. Sustained periods of dietary carbohydrate restriction lasting several weeks in animals12 or at least several days in humans8 have been shown to provide a relatively stable and marked reduction in cardiac 18F-FDG uptake. By contrast, the impact of uncontrolled short diet periods lasting no more than 24 hours did not enhance the results provided by fasting in a large previous meta-analysis.7 These observations are in agreement with the previous knowledge that drastic carbohydrate reduction, prolonged at least 3-5 days, are required to definitely enter into a state of ketosis.15 After this delay-time, the glucose reserves become insufficient, both for normal fat oxidation via the supply of oxaloacetate in the Krebs cycle and for the supply of glucose, even in the central nervous system.15,16
The present experimental study is the first in which the impact of such a drastic diet, leading to an increase in circulating ketone bodies at a very high level and equivalent to that reached by a prolonged 18-hour fasting period, could be monitored by serial 18F-FDG-PET during a 7-day period. In these conditions, cardiac 18F-FDG uptake exhibited a gradual decrease over time up to a very low level on the 7th day, in agreement with the progressive development of the ketosis state, as stated above.
This prolonged diet was additionally found i) to be well tolerated, as evidenced by the absence of any significant loss in body weight, and ii) to provide a normal cardiac 18F-FDG uptake more than twofold lower on the 7th day of the diet than that achieved with the 18-hours fasting. In these conditions, areas of sub-acute myocarditis could be easily delineated because of a high contrast from normal myocardial areas, as evidenced by the comprehensive analysis of PET images and histopathological sections (Figure 4). Accordingly, areas of high 18F-FDG activity in these sections were shown to be associated with increased fibrosis, as well as with a high density of inflammatory infiltrate and macrophages. The anti-vimentin antibodies used in this study are likely to mainly label macrophages in this particular setting of myocarditis, even if it must be recognized that this antibody is not highly specific for this purpose. It should be pointed out that the areas of increased fibrosis were not only those corresponding to the evolving sub-acute myocarditis, but also those physiologically documented at the LV base, in the vicinity of the mitral annulus. This was associated with a ring-like uptake at the LV base, a pattern previously documented by 18F-FDG-PET in normal healthy volunteers after low-carbohydrate and fasting diets.9,27 This may be explained by the fact that these fibrotic regions are rich in fibroblasts, expressing GLUT-1 and GLUT-3 receptors for glucose intake similarly to inflammatory cells; these receptors are known to be insensitive to insulin, fasting, and carbohydrate diet.7,28,29 By contrast, this sensitivity is very high for the GLUT-4 receptors, which are expressed by cardiomyocytes.29
The different patterns of cardiac 18F-FDG uptake documented herein at the 7th day of diet in both myocarditis and normal rats are best illustrated in the movies provided in a supplemental file and where the ring-like and myocarditis foci are shown to follow the left ventricular contraction motions.
It remains to be determined whether these results may be extrapolated to humans and also, whether such ketogenic diets might be even more effective and moreover, if they could be shortened when preceded and/or followed by short fasting periods, such as an overnight fast. A 7-day ketogenic diet is indeed too long to be routinely prescribed in all patients in this setting.
New Knowledge Gained
One-week extension of a ketogenic diet:
Provides a gradual decrease in 18F-FDG uptake within normal myocardium of rats, reaching a lower level compared to a conventional 18-hour fasting protocol, but only at the 7th day of ketogenic diet.
Provides a high detectability of inflammatory areas by 18F-FDG-PET in rats.
Conclusion
This experimental study shows that 1-week extension of a ketogenic diet provides a further decrease in the 18F-FDG uptake of normal myocardium and thus, a high detectability of inflammatory areas. Thereby, clinical trials, assessing prolonged ketogenic diets alone or in association with tolerable fasting periods, are warranted in this setting.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Cine-loop images recorded with 18F-FDG-TEP at the end of the 7 days of ketogenic diet in a myocarditis rat (A: vertical long-axis and B: horizontal long-axis) and in a normal rat (C: vertical long-axis and D: horizontal long-axis). The myocarditis area may be observed in the anterior wall of the myocarditis rat (white shadow in slice A) and areas of increased 18F-FDG uptake are observed in contact with the mitral annulus in all slices from both rats (dark shadows) (GIF 205 kb)
Acknowledgments
Disclosure
The authors have nothing to disclose.
Abbreviations
- 18F-FDG
18F-fluorodeoxyglucose
- PET
Positron emission tomography
- LV
Left ventricle
- SD
Standard deviation
- NS
No significant
- HES
Hematoxylin eosin safan
- M/B
Myocardium to blood ratio
Footnotes
The authors of this article have provided a PowerPoint file, available for download at SpringerLink, which summarises the contents of the paper and is free for re-use at meetings and presentations. Search for the article DOI on SpringerLink.com.
Contributor Information
Alexandra Clément, Email: a.clement@nancyclotep.com.
Fatiha Maskali, Email: f.maskali@nancyclotep.com.
References
- 1.Ishida Y, Yoshinaga K, Miyagawa M, Moroi M, Kondoh C, Kiso K, et al. Recommendations for 18F-fluorodeoxyglucose positron emission tomography imaging for cardiac sarcoidosis: Japanese Society of Nuclear Cardiology recommendations. Ann Nucl Med. 2014;28:393–403. doi: 10.1007/s12149-014-0806-0. [DOI] [PubMed] [Google Scholar]
- 2.Okumura W, Iwasaki T, Toyama T, Iso T, Arai M, Oriuchi N, et al. Usefulness of fasting 18F-FDG PET in identification of cardiac sarcoidosis. J Nucl Med. 2004;45:1989–1998. [PubMed] [Google Scholar]
- 3.Langah R, Spicer K, Gebregziabher M, Gordon L. Effectiveness of prolonged fasting 18f-FDG PET-CT in the detection of cardiac sarcoidosis. J Nucl Cardiol. 2009;16:801–810. doi: 10.1007/s12350-009-9110-0. [DOI] [PubMed] [Google Scholar]
- 4.Shao D, Tian XW, Gao Q, Liang CH, Wang SX. Preparation methods prior to PET/CT scanning that decrease uptake of 18F-FDG by myocardium, brown adipose tissue, and skeletal muscle. Acta Radiol. 2017;58:10–18. doi: 10.1177/0284185116633917. [DOI] [PubMed] [Google Scholar]
- 5.Williams G, Kolodny GM. Suppression of myocardial 18F-FDG uptake by preparing patients with a high-fat, low carbohydrate diet. AJR Am J Roentgenol. 2008;190:W151–W156. doi: 10.2214/AJR.07.2409. [DOI] [PubMed] [Google Scholar]
- 6.Cheng VY, Slomka PJ, Ahlen M, Thomson LE, Waxman AD, Berman DS. Impact of carbohydrate restriction with and without fatty acid loading on myocardial 18F-FDG uptake during PET: A randomized controlled trial. J Nucl Cardiol. 2010;17:286–291. doi: 10.1007/s12350-009-9179-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tang R, Wang JT, Wang L, Le K, Huang Y, Hickey AJ, Emmett L. Impact of patient preparation on the diagnostic performance of 18F-FDG PET in cardiac sarcoidosis: A systematic review and meta-analysis. Clin Nucl Med. 2016;41:e327–e339. doi: 10.1097/RLU.0000000000001063. [DOI] [PubMed] [Google Scholar]
- 8.Lu Y, Grant C, Xie K, Sweiss NJ. Suppression of myocardial 18F-FDG uptake through prolonged high-fat, high-protein, and very-low-carbohydrate diet before FDG-PET/CT for evaluation of patients with suspected cardiac sarcoidosis. Clin Nucl Med. 2017;42:88–94. doi: 10.1097/RLU.0000000000001465. [DOI] [PubMed] [Google Scholar]
- 9.Harisankar CN, Mittal BR, Agrawal KL, Abrar ML, Bhattacharya A. Utility of high fat and low carbohydrate diet in suppressing myocardial FDG uptake. J Nucl Cardiol. 2011;18:926–936. doi: 10.1007/s12350-011-9422-8. [DOI] [PubMed] [Google Scholar]
- 10.Soussan M, Brillet PY, Nunes H, Pop G, Ouvrier MJ, Naggara N, et al. Clinical value of a high-fat and low-carbohydrate diet before FDG-PET/CT for evaluation of patients with suspected cardiac sarcoidosis. J Nucl Cardiol. 2013;20:120–127. doi: 10.1007/s12350-012-9653-3. [DOI] [PubMed] [Google Scholar]
- 11.Cussó L, Vaquero JJ, Bacharach S, Desco M. Comparison of methods to reduce myocardial 18F-FDG uptake in mice: Calcium channel blockers versus high-fat diets. PLoS ONE. 2014;9:e107999. doi: 10.1371/journal.pone.0107999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fine EJ, Miao W, Koba W, Volek JS, Blaufox MD. Chronic effects of dietary carbohydrate variation on [18F]-2-fluoro-2-deoxyglucose uptake in rodent heart. Nucl Med Commun. 2009;30:675–680. doi: 10.1097/MNM.0b013e32832aa6e8. [DOI] [PubMed] [Google Scholar]
- 13.Branco AF, Ferreira A, Simoes RF, Magalhaes-Novais S, Zehowski C, Cope E, et al. Ketogenic diets: From cancer to mitochondrial diseases and beyond. Eur J Clin Invest. 2016;46:285–298. doi: 10.1111/eci.12591. [DOI] [PubMed] [Google Scholar]
- 14.Paoli A, Rubini A, Volek JS, Grimaldi KA. Beyond weight loss: A review of the therapeutic uses of very-low-carbohydrate (ketogenic) diets. Eur J Clin Nutr. 2013;67:789–796. doi: 10.1038/ejcn.2013.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Paoli A. Ketogenic diet for obesity: Friend or foe? Int J Environ Res Public Health. 2014;11:2092–2107. doi: 10.3390/ijerph110202092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Owen OE. Ketone bodies as a fuel for the brain during starvation. Biochem Mol Biol Educ. 2005;33:246–251. doi: 10.1002/bmb.2005.49403304246. [DOI] [Google Scholar]
- 17.Wentz AE, André d’Avignon D, Weber ML, Cotter DG, Doherty JM, Kerns R, et al. Adaptation of myocardial substrate metabolism to a ketogenic nutrient environment. J Biol Chem. 2010;285:24447–24456. doi: 10.1074/jbc.M110.100651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aubert G, Martin OJ, Horton JL, Lai L, Vega RB, Leone TC, et al. The failing heart relies on ketone bodies as a fuel. Circulation. 2016;23:698–705. doi: 10.1161/CIRCULATIONAHA.115.017355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Raffo E, François J, Ferrandon A, Koning E, Nehlig A. Calorie-restricted ketogenic diet increases thresholds to all patterns of pentylenetetrazol-induced seizures: Critical importance of electroclinical assessment. Epilepsia. 2008;49:320–328. doi: 10.1111/j.1528-1167.2007.01380.x. [DOI] [PubMed] [Google Scholar]
- 20.Linard B, Ferrandon A, Koning E, Nehlig A, Raffo E. Ketogenic diet exhibits neuroprotective effects in hippocampus but fails to prevent epileptogenesis in the lithium-pilocarpine model of mesial temporal lobe epilepsy in adult rats. Epilepsia. 2010;51:1829–1836. doi: 10.1111/j.1528-1167.2010.02667.x. [DOI] [PubMed] [Google Scholar]
- 21.Shichao L, Meifang W, Li Meng, Qiang W, Ling X, Xiaojing W, et al. Effect and mechanism of QiShenYiQi pill on experimental autoimmune myocarditis rats. Med Sci Monit. 2016;22:752–760. doi: 10.12659/MSM.895655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maskali F, Poussier S, Louis H, Boutley H, Lhuillier M, Thornton SN, et al. Assessment of the early stage of cardiac remodeling of spontaneously hypertensive heart failure rats using the quantitative 3-dimensional analysis provided by acipimox-enhanced FDG-PET. Int J Cardiovasc Imaging. 2014;30:449–456. doi: 10.1007/s10554-013-0350-3. [DOI] [PubMed] [Google Scholar]
- 23.Bousquenaud M, Maskali F, Poussier S, Zangrando J, Marie PY, Boutley H, et al. Cardioprotective effects of adenosine within the border and remote areas of myocardial infarction. EJNMMI Res. 2013;3:65. doi: 10.1186/2191-219X-3-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Poussier S, Maskali F, Tran N, Person C, Maureira P, Boutley H, et al. ECG-triggered 18F-fluorodeoxyglucose positron emission tomography imaging of the rat heart is dramatically enhanced by acipimox. Eur J Nucl Med Mol Imaging. 2010;37:1745–1750. doi: 10.1007/s00259-010-1418-0. [DOI] [PubMed] [Google Scholar]
- 25.Manabe O, Yoshinaga K, Ohira H, Masuda A, Sato T, Tsujino I, et al. The effects of 18-h fasting with low-carbohydrate diet preparation on suppressed physiological myocardial (18)F-fluorodeoxyglucose (FDG) uptake and possible minimal effects of unfractionated heparin use in patients with suspected cardiac involvement sarcoidosis. J Nucl Cardiol. 2016;23:244–252. doi: 10.1007/s12350-015-0226-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morooka M, Moroi M, Uno K, Ito K, Wu J, Nakagawa T, et al. Long fasting is effective in inhibiting physiological myocardial 18F-FDG uptake and for evaluating active lesions of cardiac sarcoidosis. EJNMMI Res. 2014;4:1. doi: 10.1186/2191-219X-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ito K, Okazaki O, Morooka M, Kubota K, Minamimoto R, Hiroe M. Visual findings of 18F-fluorodeoxyglucose positron emission tomography/computed tomography in patients with cardiac sarcoidosis. Intern Med. 2014;53:2041–2049. doi: 10.2169/internalmedicine.53.2491. [DOI] [PubMed] [Google Scholar]
- 28.Vom Dahl J, Herman WH, Hicks RJ, Ortiz-Alonso FJ, Lee KS, Allman KC, et al. Myocardial glucose uptake in patients with insulin-dependent diabetes mellitus assessed quantitatively by dynamic positron emission tomography. Circulation. 1993;88:395–404. doi: 10.1161/01.CIR.88.2.395. [DOI] [PubMed] [Google Scholar]
- 29.Mueckler M, Thorens B. The SLC2 (GLUT) family of membrane transporters. Mol Aspects Med. 2013;34:121–138. doi: 10.1016/j.mam.2012.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mobbs CV, Mastaitis J, Isoda F, Poplawski M. Treatment of diabetes and diabetic complications with a ketogenic diet. J Child Neurol. 2013;28:1009–1014. doi: 10.1177/0883073813487596. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Cine-loop images recorded with 18F-FDG-TEP at the end of the 7 days of ketogenic diet in a myocarditis rat (A: vertical long-axis and B: horizontal long-axis) and in a normal rat (C: vertical long-axis and D: horizontal long-axis). The myocarditis area may be observed in the anterior wall of the myocarditis rat (white shadow in slice A) and areas of increased 18F-FDG uptake are observed in contact with the mitral annulus in all slices from both rats (dark shadows) (GIF 205 kb)