Abstract
The BCL-2 family of proteins integrates signals that trigger either cell survival or apoptosis. The balance between pro-survival and pro-apoptotic proteins is important for tissue development and homeostasis, while impaired apoptosis contributes to several pathologies and can be a barrier against effective treatment. BCL-w is an anti-apoptotic protein that shares a sequence similarity with BCL-XL, and exhibits a high conformational flexibility. BCL-w level is controlled by a number of signaling pathways, and the repertoire of transcriptional regulators largely depends on the cellular and developmental context. As only a few disease-relevant genetic alterations of BCL2L2 have been identified, increased levels of BCL-w might be a consequence of abnormal activation of signaling cascades involved in the regulation of BCL-w expression. In addition, BCL-w transcript is a target of a plethora of miRNAs. Besides its originally recognized pro-survival function during spermatogenesis, BCL-w has been envisaged in different types of normal and diseased cells as an anti-apoptotic protein. BCL-w contributes to survival of senescent and drug-resistant cells. Its non-apoptotic role in the promotion of cell migration and invasion has also been elucidated. Growing evidence indicates that a high BCL-w level can be therapeutically relevant in neurodegenerative disorders, neuron dysfunctions and after small intestinal resection, whereas BCL-w inhibition can be beneficial for cancer patients. Although several drugs and natural compounds can bi-directionally affect BCL-w level, agents that selectively target BCL-w are not yet available. This review discusses current knowledge on the role of BCL-w in health, non-cancerous diseases and cancer.
Subject terms: Oncogenes, Apoptosis, Cell invasion, Senescence, Molecular neuroscience
Facts
In addition to its pro-survival function, BCL-w plays a non-apoptotic role in regulation of cell motility and senescence.
The role of BCL-w has been demonstrated in many types of normal cells and diseases, including disorders of nervous system and cancer.
A plethora of regulators involved in the control of BCL2L2 expression determine cellular and developmental contexts of BCL-w level and activity.
Open questions
How unique is the apoptotic and non-apoptotic role of BCL-w compared with other members of the BCL-2 family of proteins?
Can BCL-w level be a prognostic factor in cancer and non-cancerous diseases?
Can BCL-w be selectively targeted by natural and/or synthetic drugs?
Introduction
The balance between pro-survival and pro-apoptotic proteins is important for tissue development and homeostasis, while impaired apoptosis contributes to several pathologies and can be a barrier against effective treatment1,2. Proteins from the B-cell lymphoma-2 (BCL-2) family are essential integrators of signals that trigger cell survival or apoptosis, while cell fate depends on the abundance, localization, and interactions between particular BCL-2-like proteins3. The BCL-2 family members are classified based on the structure and structure-related function. Anti-apoptotic members of this family, BCL-2 itself, B-cell lymphoma-extra-large (BCL-XL), B-cell lymphoma-w (BCL-w), BCL-2-related protein A1/BCL-2-related isolated from fetal liver-11 (A1/BFL-1) and myeloid cell leukemia-1 (MCL-1)4,5 share four BCL-2-homology (BH) domains (BH1-BH4), but A1/BFL-1 and certain isoforms of MCL-1 lack the BH4 domain (Fig. 1a)6. The pro-apoptotic proteins such as BCL-2-associated X protein (BAX) and BCL-2 antagonist/killer (BAK) possess BH1-BH3 motifs4,5,7. In addition to their non-canonical roles8,9, BAX and BAK directly execute mitochondrial outer membrane permeabilization (MOMP), which is usually considered a point of no return in an apoptotic cascade10. The proteins of the third subclass (BH3-only proteins) share exclusively the BH3 domain. BCL-2-interacting mediator of cell death (BIM), p53-upregulated modulator of apoptosis (PUMA) and truncated form of BH3-interacting domain death agonist (tBID) are called ‘activators’ as they can bind to and provoke a conformational change of BAX and/or BAK to induce MOMP. In turn, BH3-only proteins that do not associate with BAX and BAK are named ‘sensitizers’5,11,12. Regulation of apoptosis by the proteins of BCL-2 family relies on the balance between the activity of anti-apoptotic proteins that leash the ‘activators’ and MOMP-initiating molecules, and the ‘sensitizers’ that antagonize the pro-survival members by liberating BAX/BAK and the BAX/BAK-activating BH3-only proteins (Fig. 1b)4,5. This succinct review will address the current understanding of the structure and function of BCL-w and its apoptotic and non-apoptotic role in health and disease.
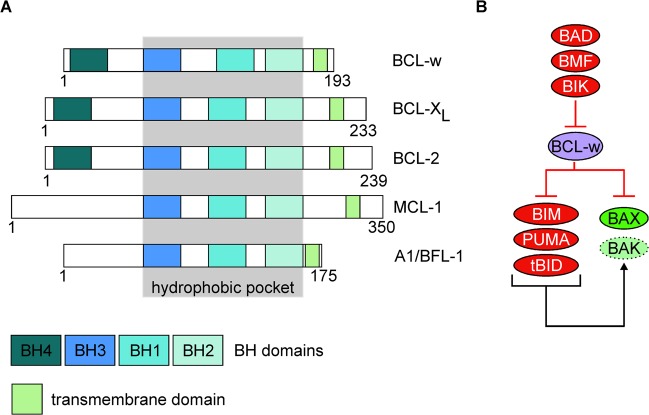
Fig. 1. BCL-w is an anti-apoptotic member of the BCL-2 family of proteins.
a Schematic domain structure of BCL-w and other pro-survival members of the BCL-2 family. Number of amino acid residues in particular human BCL-2-like protein is shown below. BH3 homology (BH) and transmembrane domains are shown in different colors, and BH domains involved in the formation of a hydrophobic pocket are marked in gray background. b The functional relationship between BCL-w and pro-apoptotic members of BCL-2 family.
BCL-w as a pro-survival member of the BCL-2 family of proteins
BCL-w is an anti-apoptotic protein that shares the highest sequence similarity (51%) with BCL-XL in comparison to other pro-survival molecules13. BCL-w interacts with BAX and BAK, and several BH3-only proteins such as BAD, tBID, BIM, PUMA, BMF, and BIK as shown by co-immunoprecipitation14–16. Results of the isothermal titration calorimetry indicate a preferential binding of BCL-w to BAX in comparison to its binding to BAK, with KD = 22.9 nM and KD = 114 nM, respectively17. The precisely regulated interactions between pro- and anti-apoptotic proteins are possible due to the spatial architecture of the BH1-3 domains. They form the hydrophobic groove responsible for the sequestration capability of pro-survival molecules (Fig. 1a), and structure of the binding site dictates the repertoire of interacting proteins4,5. For example, it has been demonstrated that a Gly94 residue within the BH1 domain of BCL-w is critical for BAX inhibition18, and a G94E substitution in BCL-w abolishes its cytoprotective function in response to interleukin-3 (IL-3) deprivation14. In addition, FXXRXR and R/KXV/IXF motifs in BCL-w enables interaction with protein phosphatase 1α (PP1α)19. Consequently, BCL-w forms a complex with PP1α and BAD, which leads to dephosphorylation of BAD upon interleukin-4 (IL-4) deprivation19. The interactions between BCL-w and poorly characterized members of the BCL-2 family, BFK20 and BOP21 have also been reported. Moreover, α1/2 and α5/6 loops of BCL-w can associate with p53 through p53 DNA-binding domain, which contributes to transcription-independent regulation of cell death22. BCL-w also interacts with BH3-like domain of Beclin-1, an autophagy-related protein23. Interactions between anti- and pro-apoptotic proteins can be precisely quantified as recently demonstrated by using a fluorescence resonance energy transfer (FRET) assay24.
The pro-survival BCL-2-like proteins normally associate with the lipid bilayer of mitochondrial, endoplasmic reticulum (ER) and nuclear envelope membranes via their hydrophobic domains (Fig. 1a)25. Accordingly, confocal microscopy and cell fractioning have revealed that BCL-w associates with intracellular membranes26, and these interactions are strengthened under stress27. It has been demonstrated that in unstressed cells the C-terminal domain of BCL-w is folded back within the hydrophobic pocket, and remains only loosely attached to the mitochondrial membrane. When an apoptotic signal is received, C-terminal arm of BCL-w is released by a ligation of pro-apoptotic BH3-only protein, which consequently promotes a tight interaction between BCL-w and mitochondrion28–30. Notably, it has been demonstrated that the membrane-inserted pool of BCL-w interacts with BH3-only proteins, whereas BCL-w molecules loosely attached to the mitochondrial membrane are associated with MOMP-inducing proteins28. Deletion of C-terminal α-helix increased BCL-w binding affinity for BID-derived BH3 peptide, which indicates that this helix modulated interactions of BCL-w with pro-apoptotic partners by competing for peptide binding to the hydrophobic pocket27. More recent study that involved BCL-w in complex with designed ankyrin repeat proteins (DARPins) has revealed, however, greater structural similarity of BCL-w to ligand-free BCL-XL than it was primarily thought31. In addition, the BCL-XL C-terminus has also been shown to interact with a hydrophobic groove in the water-soluble form of the protein, however, the C-terminal tail in BCL-XL did not trigger a conformational change and did not contribute to the formation of a tightly bound structure as observed in BCL-w32. It has been suggested that increased flexibility of the BCL-w groove area is not determined by the hinge regions, but by the weaker interactions between the α3-α4 and the α5-α6 helical hairpins of BCL-w31. Consequently, crucial interactions identified in the ligand-binding area of BCL-XL are weakened or lost in BCL-w31, which is in line with previous observations showing weaker interactions between the BH1 domain of BCL-w and BID or BIM in comparison to BCL-XL/BID and BCL-XL/BIM complexes33. Identification of BCL-w homodimer has further envisaged a high conformational flexibility of BCL-w. The X-ray crystallography structure has revealed that helices α3 and α4 hinge away from the core of one molecule to cross into another BCL-w protomer. This conformation results in the dimerization-specific exposition of helices α5 and α6 while remaining BH3-binding pocket intact. BCL-w homodimer retains selectivity of binding to BH3-only proteins, but the affinity is lower than for monomeric BCL-w as exemplified for BAD binding with KD = 150 nM and KD = 14 nM, respectively34. Further research is necessary to delineate how the conformational flexibility of BCL-w is unique compared with other members of the BCL-2 family of proteins, and how it can be exploited in the development of the BCL-w-selective inhibitors.
Regulation of BCL-w level
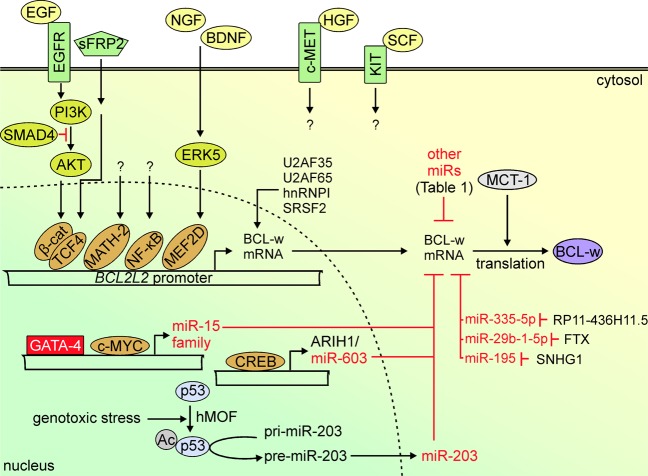
BCL-w protein, 193-amino acid residues in length (Fig. 1a) is encoded by BCL2L2, which is located on human chromosome 14 at band q11.2-q1235. BCL2L2 consists of two coding exons in addition to two non-coding exons located at the 5′-end36. The BCL2L2 promoter is highly conserved between human, mouse and rat, and the minimal promoter region lies within the non-coding exon 1a37. Analysis of the rat Bcl2l2 promoter by using phylogenetic approach has revealed putative binding sites for several transcription factors including myocyte enhancer factor 2 (MEF2), erythroblastosis virus E26 oncogene homolog (ETS-1 and ETS-2), CCAAT/enhancer binding protein (C/EBP) and nuclear factor-kappa B (NF-κB)37. A number of signaling pathways and downstream transcription factors have been experimentally validated as the regulators of BCL2L2 expression (Fig. 2), although the contribution of different transcriptional factors largely depends on the cellular and developmental context. BCL2L2 was identified as a target of p65/NF-κB in chronic lymphocytic leukemia cells, which was confirmed in experiments involving BAY110782, an inhibitor of NF-κB38. In addition, p65/p52 NF-κB dimer was involved in upregulation of BCL-w in glial-cell-line-derived neurotrophic factor (GDNF)-treated dopaminergic neurons39. BCL2L2 transcription was also positively regulated by the β-catenin/transcription factor 4 (TCF4) complex, and overexpression of either dominant-negative TCF4 (TCF4ΔN) or wild-type β-catenin resulted in decreased or increased activity of the BCL2L2 promoter, respectively36. The role of secreted Frizzled-related protein 2 (sFRP2) in β-catenin-dependent expression of BCL2L2 has also been reported40. Increased BCL2L2 transcription was assessed after stimulation of distal axon with nerve growth factor (NGF) and brain-derived neurotrophic factor (BDNF), used either alone or in combination, and engaged ERK5-dependent phosphorylation of MEF2D transcription factor41. In addition, BCL-w was the only anti-apoptotic protein regulated by neuronal differentiation 6 factor (NeuroD6/MATH-2) under non-stress conditions, and NeuroD6/MATH-2 also assisted in a proper subcellular localization of BCL-w upon serum deprivation37. A positive correlation between expression of BCL2L2 and MET was demonstrated, and c-MET downregulation was followed by a decrease in mRNA level of BCL-w, but not other pro-survival members of the BCL-2 family42. Different reports have suggested a cell type-specific contribution of cAMP responsive element binding protein (CREB) to BCL2L2 expression43–45. A temporal increase of CREB activity in adult visual neocortex was concomitant with an upregulation of anti-apoptotic molecules, including BCL-w43. In rat Sertoli cells, CREB was dispensable for 17-beta-estradiol-induced BCL-w expression44. In turn, CREB indirectly reduced the BCL-w level in colorectal cancer cells by binding to the promoter of the gene encoding ariadne RBR E3 ubiquitin protein ligase 1 (ARIH1), which contains microRNA-603 (miR-603) within its exon45. A few other transcription factors can also indirectly control BCL-w level by affecting expression of miRs involved in BCL-w downregulation (Fig. 2). GATA-binding protein 4 (GATA-4) inhibited expression of miRs from the miR-15 family, including miR-15b, miR-16 and miR-195, and consequently promoted BCL-w-dependent survival of mesenchymal stem cells46. On the contrary, c-MYC upregulated the miR-15 family members responsible for the suppression of BCL-w expression, and this effect was independent of p5347. An indirect role of p53 in the control of BCL-w level was, however, demonstrated during genotoxic stress as a human ortholog of males absent on the first (hMOF)-mediated acetylation of Lys120 residue in p53 was essential for p53-dependent processing of miR-203, which downregulated BCL-w level48.
Fig. 2. Signal transducers and effector transcriptional regulators involved in the control of BCL2L2 expression.
For HGF/c-MET and SCF/KIT signaling, no downstream elements specifically involved in the regulation of BCL-w level were demonstrated. In addition, a few transcription factors were shown to either activate (c-MYC, CREB, p53) or repress (GATA-4) expression of particular miRNAs (miRs) involved in the regulation of BCL-w mRNA level. Other miRs involved in the control of BCL-w level are extensively characterized in Table 1.
A number of other miRs were identified as negative regulators of BCL-w level by binding to the 3’-untranslated region (3’-UTR) of BCL-w transcript (Table 1). In addition, long non-coding RNA (lncRNA) RP11-436H11.5, which functions as a competitive endogenous RNA, was able to sponge miR-335-5p and in turn upregulate BCL-w level49. The sponging activity was also demonstrated for lncRNA FTX, which controlled miR-29b-1-5p-dependent BCL-w transcript level in mouse cardiomyocytes50, while a lncRNA small nucleolar RNA host gene 1 (SNHG1) sponged miR-195 in human cardiomyocytes51. Several splicing- and translation-regulating factors have been involved in the processing of BCL-w mRNA. Upstream sequence element (USE) in the 3’-UTR of BCL-w transcript contributed to 3’-end formation via interaction with splicing factors: U2 small nuclear RNA auxiliary factor 1 (U2AF35), U2 small nuclear RNA auxiliary factor 2 (U2AF65) and heterogeneous nuclear ribonucleoprotein I (hnRNPI)52. In addition, downregulation of serine and arginine-rich splicing factor 2 (SRSF2) was associated with decreased BCL-w transcript level53. It was also demonstrated that multiple copies in T-cell lymphoma-1 (MCT-1) protein interacted with the translation machinery to augment the translation of several mRNAs, including BCL-w transcript54.
Table 1.
miRNAs (miRs) down-regulating BCL-w level. miRs and cell lines involved in experiments that directly evidenced the binding of particular miR to 3’-UTR of BCL-w transcript are included.
| In vitro | |||
|---|---|---|---|
| miR | Cell line | Cell type | Reference |
| let-7a-3p | U251 and A172 | Glioblastoma multiforme | 107 |
| miR-15a | A549 | Non-small cell lung cancer | 162 |
| FaDu | Hypopharyngeal squamous cell carcinoma | 163 | |
| miR-15b | SNU475 | Hepatocellular carcinoma | 164 |
| miR-16 | SCC-25 | Oral squamous cell carcinoma | 165 |
| miR-29a | HEC-1B | Human endometrial carcinoma | 166 |
| miR-29b | HEK293 | Human embryonic kidney epithelial cells | 76 |
| U251 and U87MG | Glioblastoma multiforme | 141 | |
| miR-29b-1-5p | - | Mouse cardiomyocytes | 50 |
| miR-29c | A549 | Non-small cell lung cancer | 167 |
| miR-34a-5p | HEK293 | Human embryonic kidney epithelial cells | 168 |
| miR-92a | H9c2 | Rat cardiomyocytes | 169 |
| miR-93-5p | U251 | Glioblastoma multiforme | 152 |
| H460 | Lung cancer | ||
| miR-107 | A549/Taxol | Paclitaxel-resistant non-small cell lung cancer | 110 |
| HT-22 | Immortalized hippocampal neuron cells | 170 | |
| miR-122 | Hep3B and HepG2 | Hepatocellular carcinoma | 171 |
| Mahlavu | Hepatocellular carcinoma (gefitinib-resistant) | 172 | |
| HeLa | Cervical cancer | 173 | |
| Huh7, Hep3B and HepG2 | Hepatocellular carcinoma | ||
| H460 | Lung cancer | ||
| HepG2 | Hepatocellular carcinoma | 174 | |
| - | Pterygium epithelial cells | 88 | |
| miR-125a-5p | PLC/PRF/5 | Hepatocellular carcinoma | 175 |
| miR-125b | - | Rat hippocampal and cervical neurons | 176 |
| SMMC7721 | Hepatocellular carcinoma | 177 | |
| miR-126-5p | SiHa and HeLa | Cervical cancer | 178 |
| miR-129-2-3p | MDA-MB-231 | Breast cancer | 179 |
| miR-133b | T24 | Bladder cancer | 115 |
| U2OS and MG63 | Osteosarcoma | 180 | |
| H2009 | Non-small cell lung cancer | 181 | |
| miR-146-5p | SKOV3 | Ovarian cancer | 182 |
| miR-150 | SKpac | Paclitaxel-resistant ovarian cancer | 183 |
| oxi-miR-184 | H9c2 | Rat cardiomyocytes | 184 a |
| miR-195 | BEL7402 | Hepatocellular carcinoma | 130 |
| BEL7402/5-FU | Hepatocellular carcinoma (5-FU-resistant) | ||
| HT29 and LOVO | Colon cancer | 101 | |
| HCM | Human cardiomyocytes | 51 | |
| miR-203 | HEK293 | Human embryonic kidney epithelial cells | 114 |
| HCT116 | Colon cancer | 48 | |
| T24 and 5637 | Bladder cancer | 116 | |
| SGC-7901 | Gastric cancer | 185 | |
| miR-204 | HTM1073 and HTM681 | Human trabecular meshwork cells | 186 |
| miR-205 | WPE1-NA22 and WPE1-NB26 | Prostate cancer | 126 |
| H460 | Lung cancer | 103 b | |
| MDA-MB-231 | Breast cancer | ||
| miR-206 | MCF-7 and TY7D | Breast cancer | 108 b |
| miR-214 | HeLa | Cervical cancer | 120 |
| HEK293 | Human embryonic kidney epithelial cells | 187 c | |
| miR-336 | SKOV3 and ES2 | Ovarian cancer | 140 |
| SGC-7901 | Gastric cancer | 143 | |
| A549 and H1299 | Non-small cell lung cancer | 109 | |
| 786-O and CaKi-1 | Clear cell renal carcinoma | 119 | |
| A2780 | Ovarian cancer | 127 b | |
| A2780/DDP | Ovarian cancer (cisplatin-resistant) | ||
| A498 and 786-O | Renal cell carcinoma | 49 b | |
| miR-340-5p | U251 and U87 | Glioblastoma multiforme | 188 |
| miR-378 | SKM-1 | Acute myeloid leukemia | 189 |
| miR-422a | U2OS and MG63 | Osteosarcoma | 123 |
| mmu-miR-466h | CHO | Mammalian Chinese hamster ovary | 190 |
| miR-497 | N2A | Mouse neuroblastoma | 74 |
| MCF-7 | Breast cancer | 191 | |
| miR-509-3p | HEK293T | Human embryonic kidney epithelial cells | 192 |
| miR-630 | JHU-029 | Head and neck squamous cell carcinoma | 133 |
| SW480 and HT29 | Colorectal cancer | 45 | |
| In vivo | |||
|---|---|---|---|
| miR | Cell type | In vivo experimental model | Reference |
| miR-15a | Neuronal cells | miR-15a/16-1null mice | 75 |
| miR-16 | Oral squamous cell carcinoma | BALB/c nude mice | 166 |
| miR-92a | Rat cardiomyocytes | Sprague–Dawley rats | 169 |
| miR-107 | Paclitaxel-resistant non-small cell lung cancer | Nude mice | 110 |
| miR-122 | Gefitinib-resistant hepatocellular carcinoma | BALB/c nude mice | 174 |
| miR-205 | Lung cancer and breast cancer | BALB/c nude mice | 103 b |
| miR-336 | Gastric cancer | BALB/c nude mice | 143 |
| Cisplatin-resistant ovarian cancer | BALB/c nude mice | 127 b | |
| Renal cell carcinoma | Nude mice | 49 | |
| miR-378 | Acute myeloid leukemia | NOD/SCID mice | 189 |
| miR-497 | Neuronal cells | C57/B6 mice | 74 |
aROS-mediated oxidative modification of miR-184 (oxi-miR-184) is indispensable for recognition of BCL-w mRNA.
b5p form of miR was used.
c3p form of miR was used.
Role of BCL-w in normal cells and non-cancer diseases
BCL-w has been already detected in a number of solid tissues, including testes, colon, and brain, as well as in cells of myeloid and lymphoid origin26,35. Mice that lacked BCL-w were viable, exerted normal appearance and most of their tissues exhibited typical histology. However, the males were infertile in contrast to female mice that could efficiently reproduce. It was observed that the seminiferous tubules of BCL-w-deficient male mice contained apoptotic cells, and the numbers of both Sertoli cells and germ cells were reduced55,56. Further studies confirmed the essential contribution of BCL-w to spermatogenesis26, and demonstrated that BCL-w was largely expressed in Sertoli cells57,58, Leydig cells, spermatogonia, and spermatocytes57. Elevated levels of BAX/BCL-w and BAK/BCL-w complexes were found in most of these types of cells57 suggesting a functional significance of BCL-w in their survival. Accordingly, BCL-w promoted survival of mouse post-mitotic Sertoli cells by suppressing BAX-dependent apoptotic activity59. It was demonstrated that BCL-w-dependent survival of germ cells was regulated by stem cell factor (SCF), which simultaneously downregulated expression of pro-apoptotic members of the BCL-2 family, including BAX60, while decreased BCL-w protein levels were assessed in testes from cigarette smoke-exposed rats61. Interestingly, BCL-w overexpression impaired spermatogenesis as it prevented from entering cell cycle62. Testes of transgenic mice that overexpressed BCL-w exhibited degeneration of spermatocytes, vacuolization of Sertoli cells and reduced number of spermatogonia62. This indicates that temporal and spatial expression of BCL2L2 can be essential for normal development and function of testes.
BCL-w has also been shown to contribute to survival of epithelial cells in the gut26. BCL-w protected small intestine- and midcolon-derived epithelial cells from apoptosis induced either by 5-fluorourcil (5-FU) or gamma-irradiation, although spontaneous cell death was not substantial upon loss of BCL-w in these cells63. In addition, BCL-w promoted enterocyte survival after massive small bowel resection, and the role of epidermal growth factor (EGF) was implicated in this process64. The activation of epidermal growth factor receptor (EGFR) decreased BAX/BCL-w ratio, which shifted the balance to cell survival64–66. Accordingly, poor survival and impaired adaptation after the resection of small bowel were observed in either BCL-wnull or EGFR-deficient mice66 suggesting that manipulation of EGF-EGFR-BCL-w pathway might be therapeutically relevant in patients after massive resection of small intestine.
A stage-dependent increase in BCL-w transcript level has been reported during the development of rat brain67. The high levels of BCL-w were assessed in several regions of the mature brain, including cerebellum, hippocampus, and sensory neurons, whereas BCL-XL was abundantly expressed during early stages of development67,68. At the molecular level, both serine and glycine could selectively upregulate BCL2L2 expression in neuronal cells, while retaining BCL-XL level unaltered69. BCL-w also controlled the mitochondria morphogenesis and dendrite development in Purkinje cells, and was involved in synapse formation in mouse cerebellum. In this context, BCL-w did not determine number of cells in the brain, but promoted mitochondrial fission in Purkinje dendrites, which was also shown in vivo as BCL-wnull mice displayed a marked increase in mitochondrial length70. The role of BCL-w has also been demonstrated in several disorders of neuron functions and neurodegenerative diseases. Increased expression of BCL-w was found in ischemic brain suggesting a neuroprotectant role of this protein71,72. Accordingly, study on the rat model revealed that overexpression of BCL-w significantly improved neurological functions after focal cerebral ischemia in up to 40% animals73. In this respect, also indirect manipulation of BCL-w level could attenuate ischemic damage of the brain as exemplified by inhibition of miR-497 (Table 1), which was involved in downregulation of BCL-w and neuronal death after ischemia74. In addition, upregulation of BCL-w as a result of intravenous delivery of miR-15a/16-1 antagomir or miR-15a/16-1 knockout, reduced size of cerebral infarct and improved sensorimotor deficits in a middle cerebral artery occlusion (MCAO) mice75. Using a rat model of transient MCAO and oxygen-glucose deprivation in neurons, it was demonstrated that miR-29b contributed to cell death following ischemic injury as it inhibited BCL-w76. Protein level of BCL-w was also affected in the hippocampus after seizures77. BCL-w was upregulated following brief electroshock seizures, whilst it was bound to BIM and integrated in the mitochondrial membrane in damaged subfields after status epilepticus77. Moreover, epileptic seizures induced more significant nuclear fragmentation and hippocampal damage in BCL-w-deficient mice compared with wild-type controls77. In addition, an increased BCL-w protein level associated with punctate intracytoplasmic structures was found in a model of Alzheimer’s disease, in contrast to low level and diffuse distribution of BCL-w in control cases78. Mechanistically, it was shown that overexpression of BCL-w protected neurons from β-amyloid-induced cell death by blocking mitochondrial release of Smac, as accumulation of β-amyloid has been proposed as a key factor of neuron loss in Alzheimer’s disease78,79. In turn, β-amyloid reduced BCL-w protein level via c-JUN N-terminal kinase (JNK)-dependent mechanism79, whilst hyperactivation of AKT could counteract β-amyloid-mediated downregulation of BCL-w and cytotoxicity80. Neurotoxicity of β-amyloid was substantially attenuated through manipulation of the BCL-w level by β-asarone, a natural compound isolated from Acorus tatarinowii Schott (Table 2)81,82. BCL2L2 expression was also significantly higher in Parkinson’s disease patient-derived dopaminergic neurons harboring mutant PARK283. In turn, BCL2L2 was hypermethylated and expressed at lower levels in multiple sclerosis-affected brain samples than in controls84. The role of BCL-w was also implicated in the viability of nociceptors as BCL-w knockout mice developed the symptoms of small fiber sensory neuropathy, including a decline in sensitivity to thermal stimuli and reduced innervation within the epidermis85. BCL-w level was increased in axons of sensory neurons, and cells deprived of BCL-w exerted mitochondrial dysfunctions such as abnormal size and membrane potential, and low level of intracellular ATP85. A forkhead box O3 (FOXO3a)/c-JUN-dependent upregulation of PUMA followed by inhibition of BCL-w was necessary to initiate axon degeneration86. More recently, it was shown that BH4 domain of BCL-w interacted with inositol 1,4,5-trisphosphate receptor 1 (IP3R1) and protected axons from degeneration87. This cytoprotective mechanism could be impaired by chemotherapeutics used in the treatment of cancer patients as shown for paclitaxel. Paclitaxel diminished the level of RNA-binding protein splicing factor proline and glutamine rich (SFPQ) and reduced translation of BCL-w transcript. As a consequence, deregulation of IP3R1 triggered neuronal degeneration associated with mitochondrial dysfunction and calpain-dependent proteolysis, which largely contributed to the chemotherapy-induced peripheral neuropathy87.
Table 2.
Drugs and natural compounds (N) that affect BCL-w level.
| Drug or compound | Disease model (cell line) | Effective concentration | Effect on BCL-w level | Level of regulation | Reference |
|---|---|---|---|---|---|
| β-asaroneN | β-amyloid-induced rat model of Alzheimer’s disease | 12.5 mg/kg | Up | mRNA protein | 81 |
| 25 mg/kg | |||||
| 50 mg/kg | |||||
| β-amyloid-treated rat pheochromocytoma (PC12) | 15 µg/ml | Up | mRNA protein | 82 | |
| CurcuminN | Breast cancer (MCF-7) | 25 µg/ml | Up | mRNA | 193 |
| 50 µg/ml | |||||
| Breast cancer (MCF-7 and MDA-MB-231) | 5 µM | No effect | protein | 194 | |
| GenisteinN | β-amyloid-treated rat pheochromocytoma (PC12) | 25 µM | Up | mRNA | 195 |
| Phenethyl isothiocyanate (PEITC)N | Sprague–Dawley rats (hepatic cells) | 150 µmol/kg | Up | mRNA | 196 |
| Cisplatin | HNSCC (JHU-029) | 10 µg/ml | Down | protein | 133 |
| Coptidis Rhizoma extractN | Melanoma (A2058, UACC257 and UACC62) | 100 µg/ml | Down | mRNA protein | 197 |
| Cyramza (ramucirumab) | NSCLC (HCC4006) | nd. | Down | protein | 111 |
| Dihydromyricetin N | NSCLC (A549 and H1975) | 75 µM | Down | protein | 198 |
| FisetinN | HCC (Huh-7) | 60 µM | Down | protein | 199 |
| IsoledeneN | Colorectal cancer (HCT-116) | 8–28 µg/ml | Down | mRNA | 200 |
| Phenazine-1-carboxamide (PCN)N | Breast cancer (MDA-MB-231) NSCLC (A549) | nd. | Down | mRNA protein | 201 |
| SanguinarineN | N-Myc-negative neuroblastoma (SH-SY5Y) | 5 µM | Down | mRNA | 202 |
| Tanshinone IIAN | Ovarian cancer (A2780) | 150 µM | Down | protein | 203 |
| QuercetinN | Mouse neuroblastoma (N2a) | 20 µM | Down | mRNA | 204 |
| 40 µM |
HCC hepatocellular carcinoma, HNSCC head and neck squamous cell carcinoma, NSCLC non-small cell lung cancer, nd. not determined.
Several reports have revealed the putative contribution of BCL-w in other types of cells. Abundant expression of BCL2L2 was found within the whole epithelium and blood vessels of pterygium, in contrast to the presence of BCL-w protein predominantly in the basal layer of epithelium in normal conjunctiva88. Recently, it was also shown that BCL2L2 overexpression contributed to the survival of megakaryocytes and increased formation of platelets87. A positive correlation between platelet numbers and BCL-w transcript levels in platelets was assessed in 154 healthy donors89. A fundamental role of BCL-w was also reported in the survival of B lymphocytes, as a loss of BCL-w substantially accelerated cell death upon deprivation of growth factors47. It was also demonstrated that BCL-w prevented from osteogenic differentiation of human mesenchymal stem cells90.
Contribution of BCL-w to survival of cancer cells and their response to anti-cancer drugs
Elevated level of BCL-w has been assessed in various types of cancers91, but survival of different types of cancer cells does not predominantly rely on BCL-w as exemplified for acute myeloid leukemia92 and melanoma93. Only few genetic alterations of BCL2L2 have been detected in cancers, including copy-number variations in small94 and non-small95 cell lung cancer, and a 3’-UTR variant (rs1950252) that was significantly associated with the risk of oral cancer96. In a large-scale analysis of somatic copy-number alterations, BCL2L2 has been, however, classified as neither deleted nor amplified across different types of human cancers97. This suggests that increased level of BCL-w is rather a consequence of abnormal activation of cancer-related signaling pathways, and BCL-w cooperates with oncogene activation in development and progression of cancer.
A significantly higher level of BCL-w was assessed in gastric adenocarcinomas compared with normal neighboring mucosa, and BCL-w was associated with infiltrative morphotype of the tumor98. BCL-w level was also associated with poor survival of patients with colorectal cancer99. BCL-w was expressed at low levels in colorectal adenomas, while the majority (92%) of adenocarcinomas showed positive staining for BCL-w100 suggesting the contribution of BCL-w to cancer progression. This was also supported by higher BCL-w level in samples with node involvement, and in TNM stage III tumors compared with TNM stage II specimens100. BCL-w inhibited cell apoptosis by precluding activation of stress-activated protein kinase (SAPK)/JNK in gastric cancer cells98. A high level of BCL-w in colorectal cancer cells was related to a loss of SMAD family member 4 (SMAD4)99. Downregulation of BCL-w increased ionizing radiation (IR)-induced cytotoxicity in human colorectal cancer cell lines45. BCL-w conferred resistance to 5-FU99. BCL-w protein level was also increased in doxorubicin-resistant colon cancer cells, while the BCL-w inhibition partly reversed resistant phenotype101.
Hypomethylation status of BCL2L2 was frequently observed in patients with glioblastoma multiforme (GBM), which exerted a high proliferation index and low sensitivity to apoptosis102. Consequently, expression of BCL2L2 was significantly higher in GBM than in low-grade gliomas103,104, and BCL-w was involved in an aggressive phenotype of glioblastoma cells associated with specificity protein 1 (Sp1)-dependent expression of stem cell-related markers104. In addition, conditioned medium from the culture of BCL-w-overexpressing cells promoted tumorigenicity of GBM, which was associated with elevated levels of SRY-box 2 (SOX-2), NANOG, octamer-binding transcription factor 4 (OCT4), Nestin, NOTCH2, Musashi and CD133103. Increased BCL-w level was accompanied with upregulation of platelet-derived growth factor alpha (PDGFα)103. BCL-w overexpression also promoted formation of neurospheres105. BCL-w was required for tumor necrosis factor-like weak inducer of apoptosis (TWEAK)-dependent protection of glioblastoma cells against TRAIL and camptothecin106. Downregulation of BCL-w accompanied neurotensin receptor-1 (NTSR1) inhibition-induced mitochondrial apoptosis in glioblastoma cells, while restoration of BCL-w expression rescued these cells to certain extent107.
BCL-w mRNA level was also significantly higher in breast cancer specimens than in adjacent normal cells103,108. In addition, the level of BCL-w transcript was higher in plasma of patients with metastatic disease compared to that of patients with primary tumors103. BCL-w facilitated proliferation of breast cancer cells through a mechanism involving lncRNA HOX transcript antisense RNA (HOTAR)-dependent sequestration of miR-206, which downregulated BCL-w108. Moreover, BCL-w was implicated in resistance of breast cancer cells to radiotherapy. BCL-w was induced in response to IR via a mechanism involving hypermethylation of CpG islands within miR-205-5p promoter, which resulted in the upregulation of BCL2L2 in both in vitro and in vivo models103. IR-induced BCL-w contributed to mesenchymal traits of cancer cells, and supported different phenotypes, including angiogenic, migratory, and stem cell-like phenotype103.
BCL-w promoted survival of non-small cell lung cancer cells109, and its overexpression was significantly associated with advanced tumor stage95. BCL-w level was higher in paclitaxel-resistant than in paclitaxel-sensitive non-small cell lung cancer cells, and miR-107-dependent downregulation of BCL-w sensitized resistant cells to the drug110. BCL-w level also determined the extent of lung cancer cell response to cyramza, a drug used for inhibition of vessel formation111. This might be related to the role of BCL-w in tumor angiogenesis. It was demonstrated in a mouse model of melanoma that blood vessel formation was enhanced upon interactions between endothelial cells (ECs) and pericytes as pericytes promoted EC survival via paracrine integrin αV- and NF-κB-dependent regulation of gene expression in endothelial cells, including BCL-w112. In addition, knockdown of BCL-w increased sensitivity of melanoma cells to tetrathiomolybdate (TTM), which is a copper chelator113.
BCL-w has been associated with malignancy of urinary system. BCL-w protein level was substantially higher in bladder tumor cells than in adjacent normal cells114, which was also confirmed in a cohort of 41 bladder cancer samples115. High level of BCL-w accompanied bladder cancer progression, and downregulation of BCL-w sensitized cells to cisplatin116. Notably, BCL2L2-PABPN1 chimeric RNA, which was generated by cis-splicing of adjacent genes, was detected at significantly higher level in bladder cancer specimens than in normal cells. Additionally, BCL2L2-PABPN1 RNA was preferentially detected in the nuclear fraction suggesting the role as a lncRNA117. BCL-w also showed significantly higher expression in metastatic clear cell renal cell carcinoma than in primary tumor cells118, which is consistent with the study demonstrating that overexpression of BCL-w increased the proliferation rate and invasion of these cancer cells119.
The role of BCL-w has been also implicated in survival of other types of cancers and their response to drugs. Expression of BCL2L2 was significantly higher in cervical tumor samples compared with normal cervix tissue120. Downregulation of BCL-w reduced cell survival and attenuated resistance of cervical cancer cells to cisplatin120, and accelerated paclitaxel-induced mitotic cell death in vitro121. BCL2L2 was selectively upregulated in samples of endometrial cancer representing G2 histological stage122. Downregulation of BCL-w enhanced serum deprivation-induced apoptosis in osteosarcoma cells123, while increased level of BCL-w protein accompanying overexpression of miR-196a promoted survival of osteosarcoma cells in vitro124. A significantly higher BCL-w protein level was also assessed in leiomyosarcomas in comparison to benign uterine smooth muscle tumors, and BCL-w expression reversely correlated with overall patient survival125. BCL-w protein level was also increased in advanced prostate cancer cell lines, which might result from epigenetic silencing of miR-205 expression, and conferred resistance to cisplatin and docetaxel126. In addition, a high expression of BCL-w rendered resistance of ovarian cancer cells to cisplatin, and BCL-w knockdown significantly reduced size of tumor derived from cisplatin-resistant cells127. Downregulation of BCL2L2 re-sensitized ovarian cancer cells resistant to etoposide (VP-16)128. Recently, BCL2L2 has been correlated with drug resistance of high-grade serous ovarian cancer (HGSOC) cells129. BCL-w protein level was also markedly higher in hepatocellular carcinoma (HCC) cells resistant to 5-FU compared with matched drug-sensitive cells130. miR-122-dependent downregulation of BCL-w rendered HCC cells sensitive to adriamycin and vincristine131, while inhibition of BCL-w and BCL-2 as a result of cyclooxygenase-2 (COX-2) silencing potentiated TRAIL-mediated apoptosis in HCC cells132. In head and neck squamous cell carcinoma cells, cisplatin-induced miR-630-dependent downregulation of BCL-w was reported133. A high expression of BCL2L2 was assessed in diffuse large B-cell lymphoma (DLBCL) and in almost 90% of patients with Burkitt lymphoma (BL). BCL-w knockdown induced apoptosis in Burkitt lymphoma cells whilst BCL-w overexpression conferred resistance to ABT-737 and ABT-263, BH3 mimetics targeting BCL-2-like proteins47. Downregulation of BCL-w markedly delayed MYC-mediated development of B-cell lymphoma47. In another report, however, BCL-w was expressed at high level only in a subset of BL and DLBCL cell lines. Moreover, CRISPR/CAS9 gene editing or RNA interference leading to downregulation of BCL2L2 expression did not sensitize lymphoma cells to apoptosis, even when these cells were exposed to BH3 mimetics134. It has been also demonstrated that BCL-w, in addition to BCL-2 and BCL-XL, played a minor role in the development of sarcoma and thymic lymphoma in p53-deficient mice135. BCL-w was highly expressed in patient-derived B-cell chronic lymphocytic leukemia (B-CLL) cells in comparison to normal peripheral blood lymphocytes136. BCL-w was also involved in autocrine exosome-mediated regulation of chronic myeloid leukemia cell survival137.
Role of BCL-w in migratory and invasive potentials of cancer cells
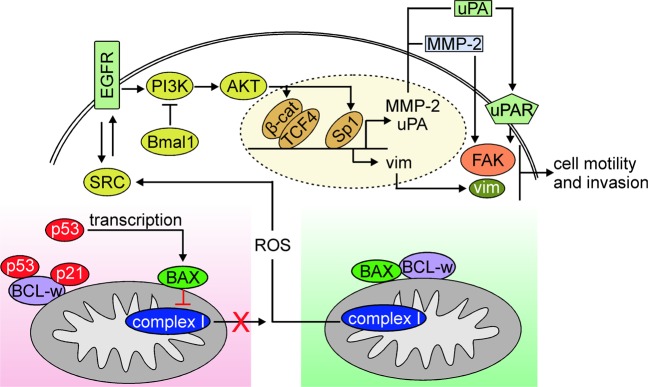
Pro-survival proteins from the BCL-2 family have been shown to contribute to migratory and invasive capabilities of normal and cancer cells138,139, and the role of BCL-w to this process has been delineated. It was reported that ectopic BCL2L2 expression almost fully nullified the inhibitory effect of miR-335 on migration and invasion of ovarian cancer cells140. BCL-w potentiated mesenchymal phenotype of GBM cells141,142, and regulated the invasion capability of human gastric cancer cells143. BCL-w enhances the migratory and invasive potentials of gastric cancer cells by facilitating the production of several types of extracellular matrix (ECM)-degrading proteinases126. Secreted matrix metallopeptidase-2 (MMP-2) and urokinase plasminogen activator surface receptor (uPAR) have been demonstrated to activate focal adhesion kinase (FAK), which acts as an executioner of BCL-w-dependent invasive phenotype of gastric cancer cells144. Mechanistically, BCL-w increases the level of mitochondria-derived reactive oxygen species (ROS), which is followed by SRC-mediated phosphorylation of EGFR145, and the activation of PI3K/AKT/Sp1 signaling pathway to increase MMP2 expression in GBM and gastric cancer cells18,104,146. BCL-w promotes activation of MMP-2 and FAK via PI3K/AKT/β-catenin signaling pathway in GBM cells105,142, while BCL-w-induced nuclear accumulation of β-catenin contributes to the upregulation of vimentin (Fig. 3)141,142. Notably, BCL-w-mediated BAX inhibition is essential for cell invasion as a variant of BCL-w (BCL-wG94A) that does not bind to BAX failed to stimulate ROS production and cell invasion18 as well as cancer cell intravasation in an in vivo model of lung cancer147.
Fig. 3. BCL-w controls cell motility.
Cell motility and invasion are promoted by BCL-w-mediated inhibition of BAX that enables the mitochondrial complex I to produce ROS (green background). ROS activate signaling cascade involving SRC-EGFR-PI3K/AKT, which engages transcriptional regulators, β-catenin/TCF4 and Sp1, to upregulate expression of genes encoding pro-invasive and pro-migratory molecules, including MMP-2, uPAR, and vimentin (vim). On the contrary, cell motility and invasion are attenuated upon substitution of BAX for cytosolic p53 and p21 in the BAX/BCL-w complex (red background). BAX resides in the outer mitochondrial membrane, and the topology allows C-terminal four residues of BAX (KKMG) to interact with a subunit of complex I in the inner membrane of mitochondria, which is followed by inhibition of ROS production. BAX-dependent attenuation of ROS synthesis is potentiated by nuclear p53-driven transcription of BAX that contributes to increased BAX protein abundance.
On the contrary, several mechanisms to counteract BCL-w-dependent cell invasion and motility have been evidenced. The PI3K/AKT/MMP-2 signaling pathway involved in cell invasion-promoting activity of BCL-w is inhibited by brain and muscle aryl hydrocarbon receptor nuclear translocator (ARNT)-like (Bmal1) in GBM and lung cancer cells148. In addition, cytosolic p53 liberates BAX from BCL-w and suppresses non-small cell lung cancer cell invasion by attenuation of ROS production147. This is driven by BAX-dependent inhibition of NADH:ubiquinone oxidoreductase core subunit 5 (ND5), a subunit of respiratory complex I147. Simultaneously, nuclear p53 augments the pool of BAX molecules via executing transcription of BAX147. The inhibitory role of p21 in the regulation of BCL-w-dependent lung cancer, colon cancer, and neuroblastoma cell invasion has been demonstrated in addition to p53149. Although p53 and p21 can bind to BCL-w independently, the triple p53/p21/BCL-w complex is required for BAX release from BCL-w and suppression of cell invasion (Fig. 3)149.
Role of BCL-w in cellular senescence
Cellular senescence is a form of cell cycle arrest that can develop in response to DNA damage, nutrient deficiency, telomere shortening, oxidative stress, and oncogene activation. Senescence induction is often executed as a barrier against tumorigenesis, but senescent cells can produce growth factors and cytokines, collectively named as the senescent-associated secretory phenotype (SASP), which can promote tumor development150. It was demonstrated that co-inhibition of BCL-w and BCL-XL by specific siRNAs or by a BH3 mimetic (ABT-737) induced apoptosis in senescent human fibroblasts in vitro151. This observation was further validated in an in vivo model, and ABT-737 efficiently eliminated epidermal cells exhibiting senescent features triggered by DNA damage or p14ARF-p53 activation151. More recently, BCL-w contribution to senescent phenotype has also been evidenced in GBM and lung cancer cells152. BCL-w promotes senescence-associated β-galactosidase (SA-β-gal) activity and trimethylation of histone H3, as well as expression of genes encoding senescence-related proteins including p53, p21, and p16152. It has also been shown that overexpression of miR-93-5p in GBM and lung cancer cells is sufficient to prevent from premature senescence through downregulation of BCL-w and p21152.
Concluding remarks and future perspectives
BCL-w with diverse functions in development, health, and disease, can play both positive and negative roles in the particular process or cellular context. BCL-w is an attractive therapeutic target as its inhibition might be relatively well-tolerated in patients. This is supported by studies showing that loss of BCL-w was associated with defects in spermatogenesis and small intestine cells in mice but had no deleterious effects in the majority of other tissues56,58,63. The contribution of BCL-w to differentiation of lymphocytes has appeared questionable as BCL2L2-knockout mice exhibited unaffected lymphoid development55, probably as a result of low level of BCL-w in normal and malignant lymphoid cells26. Further research is necessary to determine an unequivocal role of BCL-w in these cells in the light of conflicting results of more recent reports47,134. Notably, the redundant role of BCL-w is in sharp contrast to other pro-survival members of the BCL-2 family that have been shown essential during embryogenesis, development of nervous system and hematopoiesis as exemplified especially by BCL-2, MCL-1 and BCL-XL153–155. Thus, observations from experiments using knockout mice have provided an overview of the loss-of-function phenotypes that may have an impact on prediction of clinical applications of the drugs that inhibit activity of specific pro-survival proteins. Consequently, while tissue-specific BCL-w inhibition can be beneficial to overcome therapy resistance of cancer patients, increasing BCL-w level might be therapeutically relevant in a number of neurological disorders and after small intestinal resection (Fig. 4). In addition, the role of BCL-w in sustaining the survival of senescent cells suggests that manipulating BCL-w can be an useful approach in age-related disorders. To not disturb overall organismal homeostasis and limit unwanted drug cytotoxicity, it is essential to define actual cell dependence on specific anti-apoptotic protein eg., BCL-w. In this respect, BH3 profiling can be used to identify protein(s) that must be inhibited to efficiently execute MOMP156 while Dynamic BH3 profiling, which has been established more recently as an alternative functional approach, allows to measure cell dependence that can be altered in response to drugs157. For the time being, there are no drugs that selectively affect BCL-w level, which might be associated with a high conformational flexibility of this protein. Several drugs and natural compounds have been shown to affect BCL-w level in in vitro and in vivo models of different diseases (Table 2), however, BCL-w is not their exclusive target. Two BH3 mimetics, ABT-737 and its orally bioavailable derivative ABT-263, represent agents that inhibit BCL-w activity5,158. As both compounds mimic BAD, they neutralize BCL-2 and BCL-XL in addition to BCL-w5,159. Moreover, it has been demonstrated that ABT-737 displaces BIM from BCL-w with much lower efficiency than from other pro-survival proteins160,161 suggesting that cellular effects induced by ABT-737/ABT-263 could predominantly result from BCL-2 and BCL-XL inhibition. For that reason, further research directed to the development of selective drugs either upregulating or inhibiting BCL-w is still needed.
Fig. 4. An overview of cell type-specific roles of BCL-w.

BCL-w is broadly expressed in many types of normal cells as well as diseased cells, in which either increased (red background) or decreased (green background) BCL-w levels are assessed. In addition to the pro-survival role exerted in health and disease, BCL-w regulates additional cell programs and functions (color frames). Consequently, drugs that either decrease or increase BCL-w level and activity can exhibit therapeutic relevance against different disorders.
Acknowledgements
The work was supported by the National Science Centre (Poland), Grant number 2016/23/D/NZ7/03904.
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Edited by K. Rajalingam
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Baig S, et al. Potential of apoptotic pathway-targeted cancer therapeutic research: where do we stand? Cell Death Dis. 2016;7:e2058. doi: 10.1038/cddis.2015.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Elkholi R, Renault TT, Serasinghe MN, Chipuk JE. Putting the pieces together: How is the mitochondrial pathway of apoptosis regulated in cancer and chemotherapy? Cancer Metab. 2014;2:16. doi: 10.1186/2049-3002-2-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kale J, Osterlund EJ, Andrews DW. BCL-2 family proteins: changing partners in the dance towards death. Cell Death Differ. 2018;25:65–80. doi: 10.1038/cdd.2017.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Singh R, Letai A, Sarosiek K. Regulation of apoptosis in health and disease: the balancing act of BCL-2 family proteins. Nat. Rev. Mol. Cell Biol. 2019;20:175–193. doi: 10.1038/s41580-018-0089-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hartman ML, Czyz M. Pro-apoptotic activity of BH3-only proteins and BH3 mimetics: from theory to potential cancer therapy. Anticancer Agents Med. Chem. 2012;12:966–981. doi: 10.2174/187152012802650084. [DOI] [PubMed] [Google Scholar]
- 6.Warren CFA, Wong-Brown MW, Bowden NA. BCL-2 family isoforms in apoptosis and cancer. Cell Death Dis. 2019;10:177. doi: 10.1038/s41419-019-1407-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hartman ML, Czyz M. Anti-apoptotic proteins on guard of melanoma cell survival. Cancer Lett. 2013;331:24–34. doi: 10.1016/j.canlet.2013.01.010. [DOI] [PubMed] [Google Scholar]
- 8.Lindenboim L, Ferrando-May E, Borner C, Stein R. Non-canonical function of Bax in stress-induced nuclear protein redistribution. Cell. Mol. Life Sci. 2013;70:3013–3027. doi: 10.1007/s00018-013-1306-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Autret A, Martin SJ. Bcl-2 family proteins and mitochondrial fission/fusion dynamics. Cell. Mol. Life Sci. 2010;67:1599–1606. doi: 10.1007/s00018-010-0286-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gama V, Deshmukh M. Life after MOMP. Mol. Cell. 2015;58:199–201. doi: 10.1016/j.molcel.2015.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Knight T, Luedtke D, Edwards H, Taub JW, Ge Y. A delicate balance - The BCL-2 family and its role in apoptosis, oncogenesis, and cancer therapeutics. Biochem. Pharmacol. 2019;162:250–261. doi: 10.1016/j.bcp.2019.01.015. [DOI] [PubMed] [Google Scholar]
- 12.Shamas-Din A, et al. Multiple partners can kiss-and-run: Bax transfers between multiple membranes and permeabilizes those primed by tBid. Cell Death Dis. 2014;5:e1277. doi: 10.1038/cddis.2014.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee EF, Fairlie WD. The Structural Biology of Bcl-xL. Int. J. Mol. Sci. 2019;20:E2234. doi: 10.3390/ijms20092234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Holmgreen SP, Huang DC, Adams JM, Cory S. Survival activity of Bcl-2 homologs Bcl-w and A1 only partially correlates with their ability to bind pro-apoptotic family members. Cell Death Differ. 1999;6:525–532. doi: 10.1038/sj.cdd.4400519. [DOI] [PubMed] [Google Scholar]
- 15.Rooswinkel RW, et al. Antiapoptotic potency of Bcl-2 proteins primarily relies on their stability, not binding selectivity. Blood. 2014;123:2806–2815. doi: 10.1182/blood-2013-08-519470. [DOI] [PubMed] [Google Scholar]
- 16.Lee EF, et al. The functional differences between pro-survival and pro-apoptotic B cell lymphoma 2 (Bcl-2) proteins depend on structural differences in their Bcl-2 homology 3 (BH3) domains. J. Biol. Chem. 2014;289:36001–36017. doi: 10.1074/jbc.M114.610758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ku B, Liang C, Jung JU, Oh BH. Evidence that inhibition of BAX activation by BCL-2 involves its tight and preferential interaction with the BH3 domain of BAX. Cell Res. 2011;21:627–641. doi: 10.1038/cr.2010.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim EM, et al. Bcl-w promotes cell invasion by blocking the invasion-suppressing action of Bax. Cell. Signal. 2012;24:1163–1172. doi: 10.1016/j.cellsig.2012.01.019. [DOI] [PubMed] [Google Scholar]
- 19.Ayllón V, Cayla X, García A, Fleischer A, Rebollo A. The anti-apoptotic molecules Bcl-xL and Bcl-w target protein phosphatase 1alpha to Bad. Eur. J. Immunol. 2002;32:1847–1855. doi: 10.1002/1521-4141(200207)32:7<1847::AID-IMMU1847>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 20.Ozören N, Inohara N, Núñez G. A putative role for human BFK in DNA damage-induced apoptosis. Biotechnol. J. 2009;4:1046–1054. doi: 10.1002/biot.200900091. [DOI] [PubMed] [Google Scholar]
- 21.Zhang X, et al. Human Bop is a novel BH3-only member of the Bcl-2 protein family. Protein Cell. 2012;3:790–801. doi: 10.1007/s13238-012-2069-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee DH, et al. A conserved mechanism for binding of p53 DNA-binding domain and anti-apoptotic Bcl-2 family proteins. Mol. Cells. 2014;37:264–269. doi: 10.14348/molcells.2014.0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Erlich S, et al. Differential interactions between Beclin 1 and Bcl-2 family members. Autophagy. 2007;3:561–568. doi: 10.4161/auto.4713. [DOI] [PubMed] [Google Scholar]
- 24.Yang, F. et al. Stoichiometry and regulation network of Bcl-2 family complexes quantified by live-cell FRET assay. Preprint at https://link.springer.com/article/10.1007%2Fs00018-019-03286-z (2019). [DOI] [PMC free article] [PubMed]
- 25.Kaufmann T, et al. Characterization of the signal that directs Bcl-x(L), but not Bcl-2, to the mitochondrial outer membrane. J. Cell Biol. 2003;160:53–64. doi: 10.1083/jcb.200210084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.O’Reilly LA, et al. Tissue expression and subcellular localization of the pro-survival molecule Bcl-w. Cell Death Differ. 2001;8:486–494. doi: 10.1038/sj.cdd.4400835. [DOI] [PubMed] [Google Scholar]
- 27.Denisov AY, et al. Solution structure of human BCL-w: modulation of ligand binding by the C-terminal helix. J. Biol. Chem. 2003;278:21124–21128. doi: 10.1074/jbc.M301798200. [DOI] [PubMed] [Google Scholar]
- 28.Wilson-Annan J, et al. Proapoptotic BH3-only proteins trigger membrane integration of prosurvival Bcl-w and neutralize its activity. J. Cell. Biol. 2003;162:877–887. doi: 10.1083/jcb.200302144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hinds MG, et al. The structure of Bcl-w reveals a role for the C-terminal residues in modulating biological activity. EMBO J. 2003;22:1497–1507. doi: 10.1093/emboj/cdg144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Denisov AY, et al. Structural model of the BCL-w-BID peptide complex and its interactions with phospholipid micelles. Biochemistry. 2006;45:2250–2256. doi: 10.1021/bi052332s. [DOI] [PubMed] [Google Scholar]
- 31.Schilling J, Schöppe J, Sauer E, Plückthun A. Co-crystallization with conformation-specific designed ankyrin repeat proteins explains the conformational flexibility of BCL-W. J. Mol. Biol. 2014;426:2346–2362. doi: 10.1016/j.jmb.2014.04.010. [DOI] [PubMed] [Google Scholar]
- 32.Yao Y, et al. Conformation of BCL-XL upon Membrane Integration. J. Mol. Biol. 2015;427:2262–2270. doi: 10.1016/j.jmb.2015.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moroy G, Martin E, Dejaegere A, Stote RH. Molecular basis for Bcl-2 homology 3 domain recognition in the Bcl-2 protein family: identification of conserved hot spot interactions. J. Biol. Chem. 2009;284:17499–17511. doi: 10.1074/jbc.M805542200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee EF, et al. Crystal structure of a BCL-W domain-swapped dimer: implications for the function of BCL-2 family proteins. Structure. 2011;19:1467–1476. doi: 10.1016/j.str.2011.07.015. [DOI] [PubMed] [Google Scholar]
- 35.Gibson L, et al. bcl-w, a novel member of the bcl-2 family, promotes cell survival. Oncogene. 1996;13:665–675. [PubMed] [Google Scholar]
- 36.Lapham A, et al. The Bcl-w promoter is activated by beta-catenin/TCF4 in human colorectal carcinoma cells. Gene. 2009;432:112–117. doi: 10.1016/j.gene.2008.12.002. [DOI] [PubMed] [Google Scholar]
- 37.Uittenbogaard M, Baxter KK, Chiaramello A. Cloning and characterization of the 5’UTR of the rat anti-apoptotic Bcl-w gene. Biochem. Biophys. Res. Commun. 2009;389:657–662. doi: 10.1016/j.bbrc.2009.09.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pickering BM, et al. Pharmacological inhibitors of NF-kappaB accelerate apoptosis in chronic lymphocytic leukaemia cells. Oncogene. 2007;26:1166–1177. doi: 10.1038/sj.onc.1209897. [DOI] [PubMed] [Google Scholar]
- 39.Cao JP, Niu HY, Wang HJ, Huang XG, Gao DS. NF-κB p65/p52 plays a role in GDNF up-regulating Bcl-2 and Bcl-w expression in 6-OHDA-induced apoptosis of MN9D cell. Int. J. Neurosci. 2013;123:705–710. doi: 10.3109/00207454.2013.795149. [DOI] [PubMed] [Google Scholar]
- 40.Yamamura S, et al. Oncogenic functions of secreted Frizzled-related protein 2 in human renal cancer. Mol. Cancer Ther. 2010;9:1680–1687. doi: 10.1158/1535-7163.MCT-10-0012. [DOI] [PubMed] [Google Scholar]
- 41.Pazyra-Murphy MF, et al. A retrograde neuronal survival response: target-derived neurotrophins regulate MEF2D and bcl-w. J. Neurosci. 2009;29:6700–6709. doi: 10.1523/JNEUROSCI.0233-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kitamura S, et al. Met/HGF receptor modulates bcl-w expression and inhibits apoptosis in human colorectal cancers. Br. J. Cancer. 2000;83:668–673. doi: 10.1054/bjoc.2000.1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lalonde J, Chaudhuri A. Dynamic changes in CREB phosphorylation and neuroadaptive gene expression in area V1 of adult monkeys after monocular enucleation. Mol. Cell. Neurosci. 2007;35:24–37. doi: 10.1016/j.mcn.2007.01.015. [DOI] [PubMed] [Google Scholar]
- 44.Royer C, Lucas TF, Lazari MF, Porto CS. 17Beta-estradiol signaling and regulation of proliferation and apoptosis of rat Sertoli cells. Biol. Reprod. 2012;86:108. doi: 10.1095/biolreprod.111.096891. [DOI] [PubMed] [Google Scholar]
- 45.Zhang Y, et al. Novel epigenetic CREB-miR-630 signaling axis regulates radiosensitivity in colorectal cancer. PLoS One. 2015;10:e0133870. doi: 10.1371/journal.pone.0133870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yu B, et al. Enhanced mesenchymal stem cell survival induced by GATA-4 overexpression is partially mediated by regulation of the miR-15 family. Int. J. Biochem. Cell Biol. 2013;45:2724–2735. doi: 10.1016/j.biocel.2013.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Adams CM, et al. BCL-W has a fundamental role in B cell survival and lymphomagenesis. J. Clin. Invest. 2017;127:635–650. doi: 10.1172/JCI89486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chang J, et al. Acetylation of p53 stimulates miRNA processing and determines cell survival following genotoxic stress. EMBO J. 2013;32:3192–3205. doi: 10.1038/emboj.2013.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang K, Jin W, Song Y, Fei X. LncRNA RP11-436H11.5, functioning as a competitive endogenous RNA, upregulates BCL-W expression by sponging miR-335-5p and promotes proliferation and invasion in renal cell carcinoma. Mol. Cancer. 2017;16:166. doi: 10.1186/s12943-017-0735-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Long B, et al. Long noncoding RNA FTX regulates cardiomyocyte apoptosis by targeting miR-29b-1-5p and Bcl2l2. Biochem. Biophys. Res. Commun. 2018;495:312–318. doi: 10.1016/j.bbrc.2017.11.030. [DOI] [PubMed] [Google Scholar]
- 51.Zhang N, et al. The long non-coding RNA SNHG1 attenuates cell apoptosis by regulating miR-195 and BCL2-like protein 2 in human cardiomyocytes. Cell. Physiol. Biochem. 2018;50:1029–1040. doi: 10.1159/000494514. [DOI] [PubMed] [Google Scholar]
- 52.Danckwardt S, et al. Splicing factors stimulate polyadenylation via USEs at non-canonical 3’ end formation signals. EMBO J. 2007;26:2658–2669. doi: 10.1038/sj.emboj.7601699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kedzierska H, et al. Decreased expression of SRSF2 splicing factor inhibits apoptotic pathways in renal cancer. Int. J. Mol. Sci. 2016;17:E1598. doi: 10.3390/ijms17101598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Reinert LS, et al. MCT-1 protein interacts with the cap complex and modulates messenger RNA translational profiles. Cancer Res. 2006;66:8994–9001. doi: 10.1158/0008-5472.CAN-06-1999. [DOI] [PubMed] [Google Scholar]
- 55.Print CG, et al. Apoptosis regulator bcl-w is essential for spermatogenesis but appears otherwise redundant. Proc. Natl Acad. Sci. USA. 1998;95:12424–12431. doi: 10.1073/pnas.95.21.12424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ross AJ, et al. Testicular degeneration in Bclw-deficient mice. Nat. Genet. 1998;18:251–256. doi: 10.1038/ng0398-251. [DOI] [PubMed] [Google Scholar]
- 57.Yan W, Samson M, Jégou B, Toppari J. Bcl-w forms complexes with Bax and Bak, and elevated ratios of Bax/Bcl-w and Bak/Bcl-w correspond to spermatogonial and spermatocyte apoptosis in the testis. Mol. Endocrinol. 2000;14:682–699. doi: 10.1210/mend.14.5.0443. [DOI] [PubMed] [Google Scholar]
- 58.Russell LD, et al. Spermatogenesis in Bclw-deficient mice. Biol. Reprod. 2001;65:318–332. doi: 10.1095/biolreprod65.1.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ross AJ, et al. BCLW mediates survival of postmitotic Sertoli cells by regulating BAX activity. Dev. Biol. 2001;239:295–308. doi: 10.1006/dbio.2001.0445. [DOI] [PubMed] [Google Scholar]
- 60.Yan W, Suominen J, Samson M, Jégou B, Toppari J. Involvement of Bcl-2 family proteins in germ cell apoptosis during testicular development in the rat and pro-survival effect of stem cell factor on germ cells in vitro. Mol. Cell. Endocrinol. 2000;165:115–129. doi: 10.1016/S0303-7207(00)00257-4. [DOI] [PubMed] [Google Scholar]
- 61.He L, et al. Cigarette smoke induces rat testicular injury via mitochondrial apoptotic pathway. Mol. Reprod. Dev. 2017;84:1053–1065. doi: 10.1002/mrd.22863. [DOI] [PubMed] [Google Scholar]
- 62.Yan W, et al. Overexpression of Bcl-W in the testis disrupts spermatogenesis: revelation of a role of BCL-W in male germ cell cycle control. Mol. Endocrinol. 2003;17:1868–1879. doi: 10.1210/me.2002-0389. [DOI] [PubMed] [Google Scholar]
- 63.Pritchard DM, et al. Bcl-w is an important determinant of damage-induced apoptosis in epithelia of small and large intestine. Oncogene. 2000;19:3955–3959. doi: 10.1038/sj.onc.1203729. [DOI] [PubMed] [Google Scholar]
- 64.Stern LE, et al. Epidermal growth factor alters the bax:bcl-w ratio following massive small bowel resection. J. Surg. Res. 2000;91:38–42. doi: 10.1006/jsre.2000.5897. [DOI] [PubMed] [Google Scholar]
- 65.Knott AW, et al. EGF receptor signaling affects bcl-2 family gene expression and apoptosis after massive small bowel resection. J. Pediatr. Surg. 2003;3:875–880. doi: 10.1016/S0022-3468(03)00114-3. [DOI] [PubMed] [Google Scholar]
- 66.Bernal NP, Stehr W, Coyle R, Erwin CR, Warner BW. Epidermal growth factor receptor signaling regulates Bax and Bcl-w expression and apoptotic responses during intestinal adaptation in mice. Gastroenterology. 2006;130:412–423. doi: 10.1053/j.gastro.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 67.Hamnér S, Skoglösa Y, Lindholm D. Differential expression of bcl-w and bcl-x messenger RNA in the developing and adult rat nervous system. Neuroscience. 1999;91:673–684. doi: 10.1016/S0306-4522(98)00642-3. [DOI] [PubMed] [Google Scholar]
- 68.Middleton G, Wyatt S, Ninkina N, Davies AM. Reciprocal developmental changes in the roles of Bcl-w and Bcl-x(L) in regulating sensory neuron survival. Development. 2001;128:447–457. doi: 10.1242/dev.128.3.447. [DOI] [PubMed] [Google Scholar]
- 69.Yang L, et al. Improvement of the viability of cultured rat neurons by the non-essential amino acids L-serine and glycine that upregulates expression of the anti-apoptotic gene product Bcl-w. Neurosci. Lett. 2000;295:97–100. doi: 10.1016/S0304-3940(00)01597-4. [DOI] [PubMed] [Google Scholar]
- 70.Liu QA, Shio H. Mitochondrial morphogenesis, dendrite development, and synapse formation in cerebellum require both Bcl-w and the glutamate receptor delta2. PLoS Genet. 2008;4:e1000097. doi: 10.1371/journal.pgen.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yan C, et al. Overexpression of the cell death suppressor Bcl-w in ischemic brain: implications for a neuroprotective role via the mitochondrial pathway. J. Cereb. Blood Flow. Metab. 2000;20:620–630. doi: 10.1097/00004647-200003000-00020. [DOI] [PubMed] [Google Scholar]
- 72.Minami M, et al. Bcl-w expression is increased in brain regions affected by focal cerebral ischemia in the rat. Neurosci. Lett. 2000;279:193–195. doi: 10.1016/S0304-3940(99)00987-8. [DOI] [PubMed] [Google Scholar]
- 73.Sun Y, et al. Adeno-associated virus-mediated delivery of BCL-w gene improves outcome after transient focal cerebral ischemia. Gene Ther. 2003;10:115–122. doi: 10.1038/sj.gt.3301868. [DOI] [PubMed] [Google Scholar]
- 74.Yin KJ, et al. miR-497 regulates neuronal death in mouse brain after transient focal cerebral ischemia. Neurobiol. Dis. 2010;38:17–26. doi: 10.1016/j.nbd.2009.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yang X, et al. MicroRNA-15a/16-1 antagomir ameliorates ischemic brain injury in experimental stroke. Stroke. 2017;48:1941–1947. doi: 10.1161/STROKEAHA.117.017284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Shi G, et al. Upregulated miR-29b promotes neuronal cell death by inhibiting Bcl2L2 after ischemic brain injury. Exp. Brain Res. 2012;216:225–230. doi: 10.1007/s00221-011-2925-3. [DOI] [PubMed] [Google Scholar]
- 77.Murphy B, et al. Bcl-w protects hippocampus during experimental status epilepticus. Am. J. Pathol. 2007;171:1258–1268. doi: 10.2353/ajpath.2007.070269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhu X, et al. Neuroprotective properties of Bcl-w in Alzheimer disease. J. Neurochem. 2004;89:1233–1240. doi: 10.1111/j.1471-4159.2004.02416.x. [DOI] [PubMed] [Google Scholar]
- 79.Yao M, Nguyen TV, Pike CJ. Beta-amyloid-induced neuronal apoptosis involves c-Jun N-terminal kinase-dependent downregulation of Bcl-w. J. Neurosci. 2005;25:1149–1158. doi: 10.1523/JNEUROSCI.4736-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yin G, et al. Upregulation of AKT attenuates amyloid-β-induced cell apoptosis. J. Alzheimers Dis. 2011;25:337–345. doi: 10.3233/JAD-2011-110104. [DOI] [PubMed] [Google Scholar]
- 81.Geng Y, et al. Beta-asarone improves cognitive function by suppressing neuronal apoptosis in the beta-amyloid hippocampus injection rats. Biol. Pharm. Bull. 2010;33:836–843. doi: 10.1248/bpb.33.836. [DOI] [PubMed] [Google Scholar]
- 82.Li C, et al. Beta-asarone protection against beta-amyloid-induced neurotoxicity in PC12 cells via JNK signaling and modulation of Bcl-2 family proteins. Eur. J. Pharmacol. 2010;635:96–102. doi: 10.1016/j.ejphar.2010.03.013. [DOI] [PubMed] [Google Scholar]
- 83.Konovalova EV, et al. Mutations in the Parkinson’s disease-associated PARK2 gene are accompanied by imbalance in programmed cell death systems. Acta Nat. 2015;7:146–149. doi: 10.32607/20758251-2015-7-4-146-149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Huynh JL, et al. Epigenome-wide differences in pathology-free regions of multiple sclerosis-affected brains. Nat. Neurosci. 2014;17:121–130. doi: 10.1038/nn.3588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Courchesne SL, Karch C, Pazyra-Murphy MF, Segal RA. Sensory neuropathy attributable to loss of Bcl-w. J. Neurosci. 2011;31:1624–1634. doi: 10.1523/JNEUROSCI.3347-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Simon DJ, et al. Axon degeneration gated by retrograde activation of somatic pro-apoptotic signaling. Cell. 2016;164:1031–1045. doi: 10.1016/j.cell.2016.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Pease-Raissi SE, et al. Paclitaxel reduces axonal Bclw to initiate IP3R1-dependent axon degeneration. Neuron. 2017;96:373–386.e6. doi: 10.1016/j.neuron.2017.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Cui YH, et al. Regulation of apoptosis by miR-122 in pterygium via targeting Bcl-w. Invest. Ophthalmol. Vis. Sci. 2016;57:3723–3730. doi: 10.1167/iovs.16-19402. [DOI] [PubMed] [Google Scholar]
- 89.Bhatlekar S, et al. Anti-apoptotic BCL2L2 increases megakaryocyte proplatelet formation in cultures of human cord blood. Haematologica. 2019;104:2075–2083. doi: 10.3324/haematol.2018.204685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Tzeng SY, Hung BP, Grayson WL, Green JJ. Cystamine-terminated poly(beta-amino ester)s for siRNA delivery to human mesenchymal stem cells and enhancement of osteogenic differentiation. Biomaterials. 2012;33:8142–8151. doi: 10.1016/j.biomaterials.2012.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Beverly LJ, Varmus HE. MYC-induced myeloid leukemogenesis is accelerated by all six members of the antiapoptotic BCL family. Oncogene. 2009;28:1274–1279. doi: 10.1038/onc.2008.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Glaser SP, et al. Anti-apoptotic Mcl-1 is essential for the development and sustained growth of acute myeloid leukemia. Genes Dev. 2012;26:120–125. doi: 10.1101/gad.182980.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Senft D, et al. Selective induction of cell death in melanoma cell lines through targeting of Mcl-1 and A1. PLoS One. 2012;7:e30821. doi: 10.1371/journal.pone.0030821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kim YH, et al. Combined microarray analysis of small cell lung cancer reveals altered apoptotic balance and distinct expression signatures of MYC family gene amplification. Oncogene. 2006;25:130–138. doi: 10.1038/sj.onc.1208997. [DOI] [PubMed] [Google Scholar]
- 95.Kawasaki T, et al. BCL2L2 is a probable target for novel 14q11.2 amplification detected in a non-small cell lung cancer cell line. Cancer Sci. 2007;98:1070–1077. doi: 10.1111/j.1349-7006.2007.00491.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Datta S, et al. Sequence and expression variations in 23 genes involved in mitochondrial and non-mitochondrial apoptotic pathways and risk of oral leukoplakia and cancer. Mitochondrion. 2015;25:28–33. doi: 10.1016/j.mito.2015.09.001. [DOI] [PubMed] [Google Scholar]
- 97.Beroukhim R, et al. The landscape of somatic copy-number alteration across human cancers. Nature. 2010;18:899–905. doi: 10.1038/nature08822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lee HW, Lee SS, Lee SJ, Um HD. Bcl-w is expressed in a majority of infiltrative gastric adenocarcinomas and suppresses the cancer cell death by blocking stress-activated protein kinase/c-Jun NH2-terminal kinase activation. Cancer Res. 2003;63:1093–1100. [PubMed] [Google Scholar]
- 99.Zhang B, et al. Loss of Smad4 in colorectal cancer induces resistance to 5-fluorouracil through activating Akt pathway. Br. J. Cancer. 2014;110:946–957. doi: 10.1038/bjc.2013.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wilson JW, et al. Bcl-w expression in colorectal adenocarcinoma. Br. J. Cancer. 2000;82:178–185. doi: 10.1054/bjoc.1999.0897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Qu J, et al. MicroRNA-195 chemosensitizes colon cancer cells to the chemotherapeutic drug doxorubicin by targeting the first binding site of BCL2L2 mRNA. J. Cell. Physiol. 2015;230:535–545. doi: 10.1002/jcp.24366. [DOI] [PubMed] [Google Scholar]
- 102.Hervouet E, et al. Folate supplementation limits the aggressiveness of glioma via the remethylation of DNA repeats element and genes governing apoptosis and proliferation. Clin. Cancer Res. 2009;15:3519–3529. doi: 10.1158/1078-0432.CCR-08-2062. [DOI] [PubMed] [Google Scholar]
- 103.Kim ES, Choi JY, Hwang SJ, Bae IH. Hypermethylation of miR-205-5p by IR governs aggressiveness and metastasis via regulating Bcl-w and Src. Mol. Ther. Nucleic Acids. 2019;14:450–464. doi: 10.1016/j.omtn.2018.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lee WS, et al. Specificity protein 1 expression contributes to Bcl-w-induced aggressiveness in glioblastoma multiforme. Mol. Cells. 2014;37:17–23. doi: 10.14348/molcells.2014.2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Bae IH, Lee WS, Yun DH, Han YH, Lee JS. 3-Hydroxy-3’,4’-dimethoxyflavone suppresses Bcl-w-induced invasive potentials and stemness in glioblastoma multiforme. Biochem. Biophys. Res. Commun. 2014;450:704–710. doi: 10.1016/j.bbrc.2014.06.038. [DOI] [PubMed] [Google Scholar]
- 106.Tran NL, et al. The tumor necrosis factor-like weak inducer of apoptosis (TWEAK)-fibroblast growth factor-inducible 14 (Fn14) signaling system regulates glioma cell survival via NFkappaB pathway activation and BCL-XL/BCL-W expression. J. Biol. Chem. 2005;280:3483–3492. doi: 10.1074/jbc.M409906200. [DOI] [PubMed] [Google Scholar]
- 107.Dong Z, et al. Inhibition of neurotensin receptor 1 induces intrinsic apoptosis via let-7a-3p/Bcl-w axis in glioblastoma. Br. J. Cancer. 2017;116:1572–1584. doi: 10.1038/bjc.2017.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ding W, Ren J, Ren H, Wang D. Long noncoding RNA HOTAIR modulates MiR-206-mediated Bcl-w signaling to facilitate cell proliferation in breast cancer. Sci. Rep. 2017;7:17261. doi: 10.1038/s41598-017-17492-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Wang H, et al. Effect of miR-335 upregulation on the apoptosis and invasion of lung cancer cell A549 and H1299. Tumour Biol. 2013;34:3101–3109. doi: 10.1007/s13277-013-0878-9. [DOI] [PubMed] [Google Scholar]
- 110.Lu C, Xie Z, Peng Q. MiRNA-107 enhances chemosensitivity to paclitaxel by targeting antiapoptotic factor Bcl-w in non-small cell lung cancer. Am. J. Cancer Res. 2017;7:1863–1873. [PMC free article] [PubMed] [Google Scholar]
- 111.Fang L, Li Z, Chen YJ, Xiao GM. Cyramza induces apoptosis of HCC4006 cell by affecting the level of Bcl-w. Eur. Rev. Med. Pharmacol. Sci. 2017;21:3069–3074. [PubMed] [Google Scholar]
- 112.Franco M, Roswall P, Cortez E, Hanahan D, Pietras K. Pericytes promote endothelial cell survival through induction of autocrine VEGF-A signaling and Bcl-w expression. Blood. 2011;118:2906–2917. doi: 10.1182/blood-2011-01-331694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kim YJ, Tsang T, Anderson GR, Posimo JM, Brady DC. Inhibition of BCL2 family members increases the efficacy of copper chelation in BRAFV600E-driven melanoma. Cancer Res. 2020;80:1387–1400. doi: 10.1158/0008-5472.CAN-19-1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Bo J, et al. microRNA-203 suppresses bladder cancer development by repressing bcl-w expression. FEBS J. 2011;278:786–792. doi: 10.1111/j.1742-4658.2010.07997.x. [DOI] [PubMed] [Google Scholar]
- 115.Chen XN, et al. MiR-133b regulates bladder cancer cell proliferation and apoptosis by targeting Bcl-w and Akt1. Cancer Cell Int. 2014;14:70. doi: 10.1186/s12935-014-0070-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Zhang X, et al. MicroRNA-203 is a prognostic indicator in bladder cancer and enhances chemosensitivity to cisplatin via apoptosis by targeting Bcl-w and survivin. PLoS ONE. 2015;10:e0143441. doi: 10.1371/journal.pone.0143441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Zhu D, et al. The landscape of chimeric RNAs in bladder urothelial carcinoma. Int. J. Biochem. Cell. Biol. 2019;110:50–58. doi: 10.1016/j.biocel.2019.02.007. [DOI] [PubMed] [Google Scholar]
- 118.Sanjmyatav J, et al. A specific gene expression signature characterizes metastatic potential in clear cell renal cell carcinoma. J. Urol. 2011;186:289–294. doi: 10.1016/j.juro.2011.03.033. [DOI] [PubMed] [Google Scholar]
- 119.Wang K, et al. miR-335 inhibits the proliferation and invasion of clear cell renal cell carcinoma cells through direct suppression of BCL-W. Tumour Biol. 2015;36:6875–6882. doi: 10.1007/s13277-015-3382-6. [DOI] [PubMed] [Google Scholar]
- 120.Wang F, Liu M, Li X, Tang H. MiR-214 reduces cell survival and enhances cisplatin-induced cytotoxicity via down-regulation of Bcl2l2 in cervical cancer cells. FEBS Lett. 2013;587:488–495. doi: 10.1016/j.febslet.2013.01.016. [DOI] [PubMed] [Google Scholar]
- 121.Huang S, Tang R, Poon RY. BCL-W is a regulator of microtubule inhibitor-induced mitotic cell death. Oncotarget. 2016;7:38718–38730. doi: 10.18632/oncotarget.9586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Witek L, Janikowski T, Bodzek P, Olejek A, Mazurek U. Expression of tumor suppressor genes related to the cell cycle in endometrial cancer patients. Adv. Med. Sci. 2016;61:317–324. doi: 10.1016/j.advms.2016.04.001. [DOI] [PubMed] [Google Scholar]
- 123.Zhang H, et al. miR-422a inhibits osteosarcoma proliferation by targeting BCL2L2 and KRAS. Biosci. Rep. 2018;38:BSR20170339. doi: 10.1042/BSR20170339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Shang Y, Wang LQ, Guo QY, Shi TL. MicroRNA-196a overexpression promotes cell proliferation and inhibits cell apoptosis through PTEN/Akt/FOXO1 pathway. Int. J. Clin. Exp. Pathol. 2015;8:2461–2472. [PMC free article] [PubMed] [Google Scholar]
- 125.de Graaff MA, et al. Inhibition of Bcl-2 family members sensitises soft tissue leiomyosarcomas to chemotherapy. Br. J. Cancer. 2016;114:1219–1226. doi: 10.1038/bjc.2016.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Bhatnagar N, et al. Downregulation of miR-205 and miR-31 confers resistance to chemotherapy-induced apoptosis in prostate cancer cells. Cell Death Dis. 2010;1:e105. doi: 10.1038/cddis.2010.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Liu R, Guo H, Lu S. MiR-335-5p restores cisplatin sensitivity in ovarian cancer cells through targeting BCL2L2. Cancer Med. 2018;7:4598–4609. doi: 10.1002/cam4.1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Yasui K, et al. Alteration in copy numbers of genes as a mechanism for acquired drug resistance. Cancer Res. 2004;64:1403–1410. doi: 10.1158/0008-5472.CAN-3263-2. [DOI] [PubMed] [Google Scholar]
- 129.Stover EH, et al. Pooled genomic screens identify anti-apoptotic genes as targetable mediators of chemotherapy resistance in ovarian cancer. Mol. Cancer Res. 2019;17:2281–2293. doi: 10.1158/1541-7786.MCR-18-1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Yang X, et al. miRNA-195 sensitizes human hepatocellular carcinoma cells to 5-FU by targeting BCL-w. Oncol. Rep. 2012;27:250–257. doi: 10.3892/or.2011.1606. [DOI] [PubMed] [Google Scholar]
- 131.Xu Y, et al. MicroRNA-122 sensitizes HCC cancer cells to adriamycin and vincristine through modulating expression of MDR and inducing cell cycle arrest. Cancer Lett. 2011;310:160–169. doi: 10.1016/j.canlet.2011.06.027. [DOI] [PubMed] [Google Scholar]
- 132.Chen Q, et al. Potent antitumor activity in experimental hepatocellular carcinoma by adenovirus-mediated coexpression of TRAIL and shRNA against COX-2. Clin. Cancer Res. 2010;16:3696–3705. doi: 10.1158/1078-0432.CCR-09-3097. [DOI] [PubMed] [Google Scholar]
- 133.Huang Y, et al. Phospho-ΔNp63α is a key regulator of the cisplatin-induced microRNAome in cancer cells. Cell Death Differ. 2011;18:1220–1230. doi: 10.1038/cdd.2010.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Diepstraten ST, et al. BCL-W is dispensable for the sustained survival of select Burkitt lymphoma and diffuse large B-cell lymphoma cell lines. Blood Adv. 2020;4:356–366. doi: 10.1182/bloodadvances.2019000541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Grabow S, et al. Pharmacological blockade of Bcl-2, Bcl-x(L) and Bcl-w by the BH3 mimetic ABT-737 has only minor impact on tumour development in p53-deficient mice. Cell Death Differ. 2012;19:623–632. doi: 10.1038/cdd.2011.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Sanz L, et al. Bcl-2 family gene modulation during spontaneous apoptosis of B-chronic lymphocytic leukemia cells. Biochem. Biophys. Res. Commun. 2004;315:562–567. doi: 10.1016/j.bbrc.2004.01.095. [DOI] [PubMed] [Google Scholar]
- 137.Raimondo S, et al. Chronic myeloid leukemia-derived exosomes promote tumor growth through an autocrine mechanism. Cell Commun. Signal. 2015;13:8. doi: 10.1186/s12964-015-0086-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Um HD. Bcl-2 family proteins as regulators of cancer cell invasion and metastasis: a review focusing on mitochondrial respiration and reactive oxygen species. Oncotarget. 2016;7:5193–5203. doi: 10.18632/oncotarget.6405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Gross A, Katz SG. Non-apoptotic function of BCL-2 family proteins. Cell Death Differ. 2017;24:1348–1358. doi: 10.1038/cdd.2017.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Cao J, et al. miR-335 represents an invasion suppressor gene in ovarian cancer by targeting Bcl-w. Oncol. Rep. 2013;30:701–706. doi: 10.3892/or.2013.2482. [DOI] [PubMed] [Google Scholar]
- 141.Chung HJ, et al. miR-29b attenuates tumorigenicity and stemness maintenance in human glioblastoma multiforme by directly targeting BCL2L2. Oncotarget. 2015;6:18429–18444. doi: 10.18632/oncotarget.4384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Lee WS, et al. Bcl-w Enhances mesenchymal changes and invasiveness of glioblastoma cells by inducing nuclear accumulation of beta-catenin. PLoS ONE. 2013;8:e68030. doi: 10.1371/journal.pone.0068030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Xu Y, et al. MicroRNA-335 acts as a metastasis suppressor in gastric cancer by targeting Bcl-w and specificity protein 1. Oncogene. 2012;31:1398–1407. doi: 10.1038/onc.2011.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Bae IH, et al. Signaling components involved in Bcl-w-induced migration of gastric cancer cells. Cancer Lett. 2009;277:22–28. doi: 10.1016/j.canlet.2008.11.022. [DOI] [PubMed] [Google Scholar]
- 145.Kim EM, Park JK, Hwang SG, Um HD. Src and epidermal growth factor receptor mediate the pro-invasive activity of Bcl-w. Tumour Biol. 2016;37:1245–1252. doi: 10.1007/s13277-015-3917-x. [DOI] [PubMed] [Google Scholar]
- 146.Bae IH, et al. Bcl-w promotes gastric cancer cell invasion by inducing matrix metalloproteinase-2 expression via phosphoinositide 3-kinase, Akt, and Sp1. Cancer Res. 2006;66:4991–4995. doi: 10.1158/0008-5472.CAN-05-4254. [DOI] [PubMed] [Google Scholar]
- 147.Kim EM, et al. Nuclear and cytoplasmic p53 suppress cell invasion by inhibiting respiratory complex-I activity via Bcl-2 family proteins. Oncotarget. 2014;5:8452–8465. doi: 10.18632/oncotarget.2320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Jung CH, et al. Bmal1 suppresses cancer cell invasion by blocking the phosphoinositide 3-kinase-Akt-MMP-2 signaling pathway. Oncol. Rep. 2013;29:2109–2113. doi: 10.3892/or.2013.2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Kim EM, et al. The p53/p21 complex regulates cancer cell invasion and apoptosis by targeting Bcl-2 family proteins. Cancer Res. 2017;77:3092–3100. doi: 10.1158/0008-5472.CAN-16-2098. [DOI] [PubMed] [Google Scholar]
- 150.Janikiewicz J, et al. Mitochondria-associated membranes in aging and senescence: structure, function, and dynamics. Cell Death Dis. 2018;9:332. doi: 10.1038/s41419-017-0105-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Yosef R, et al. Directed elimination of senescent cells by inhibition of BCL-W and BCL-XL. Nat. Commun. 2016;7:11190. doi: 10.1038/ncomms11190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Choi JY, Shin HJ, Bae IH. miR-93-5p suppresses cellular senescence by directly targeting Bcl-w and p21. Biochem. Biophys. Res. Commun. 2018;505:1134–1140. doi: 10.1016/j.bbrc.2018.10.010. [DOI] [PubMed] [Google Scholar]
- 153.Opferman JT, Kothari A. Anti-apoptotic BCL-2 family members in development. Cell Death Differ. 2018;25:37–45. doi: 10.1038/cdd.2017.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Sochalska M, Tuzlak S, Egle A, Villunger A. Lessons from gain- and loss-of-function models of pro-survival Bcl2 family proteins: implications for targeted therapy. FEBS J. 2015;282:834–849. doi: 10.1111/febs.13188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Roset R, Ortet L, Gil-Gomez G. Role of Bcl-2 family members on apoptosis: what we have learned from knock-out mice. Front. Biosci. 2007;12:4722–4730. doi: 10.2741/2421. [DOI] [PubMed] [Google Scholar]
- 156.Certo M, et al. Mitochondria primed by death signals determine cellular addiction to antiapoptotic BCL-2 family members. Cancer Cell. 2006;9:351–365. doi: 10.1016/j.ccr.2006.03.027. [DOI] [PubMed] [Google Scholar]
- 157.Montero J, et al. Drug-induced death signaling strategy rapidly predicts cancer response to chemotherapy. Cell. 2015;26:977–989. doi: 10.1016/j.cell.2015.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Opferman JT. Attacking cancer’s Achilles heel: antagonism of anti-apoptotic BCL-2 family members. FEBS J. 2016;283:2661–2675. doi: 10.1111/febs.13472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Oltersdorf T, et al. An inhibitor of Bcl-2 family proteins induces regression of solid tumours. Nature. 2005;435:677–681. doi: 10.1038/nature03579. [DOI] [PubMed] [Google Scholar]
- 160.Rooswinkel RW, van de Kooij B, Verheij M, Borst J. Bcl-2 is a better ABT-737 target than Bcl-xL or Bcl-w and only Noxa overcomes resistance mediated by Mcl-1, Bfl-1, or Bcl-B. Cell Death Dis. 2012;3:e366. doi: 10.1038/cddis.2012.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Mérino D, et al. Bcl-2, Bcl-x(L), and Bcl-w are not equivalent targets of ABT-737 and navitoclax (ABT-263) in lymphoid and leukemic cells. Blood. 2012;119:5807–5816. doi: 10.1182/blood-2011-12-400929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Yang T, et al. MicroRNA-15a induces cell apoptosis and inhibits metastasis by targeting BCL2L2 in non-small cell lung cancer. Tumour Biol. 2015;36:4357–4565. doi: 10.1007/s13277-015-3075-1. [DOI] [PubMed] [Google Scholar]
- 163.Lu W, et al. miR-15a induces cell apoptosis by targeting BCL2L2 and BCL2 in HPV-positive hypopharyngeal squamous cell carcinoma. Oncol. Rep. 2016;36:2169–2176. doi: 10.3892/or.2016.5049. [DOI] [PubMed] [Google Scholar]
- 164.Chung GE, et al. High expression of microRNA-15b predicts a low risk of tumor recurrence following curative resection of hepatocellular carcinoma. Oncol. Rep. 2010;23:113–119. doi: 10.3892/or_00000682. [DOI] [PubMed] [Google Scholar]
- 165.Wang X, Li GH. MicroRNA-16 functions as a tumor-suppressor gene in oral squamous cell carcinoma by targeting AKT3 and BCL2L2. J. Cell. Physiol. 2018;233:9447–9457. doi: 10.1002/jcp.26833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Xia HF, Jin XH, Cao ZF, Hu Y, Ma X. MicroRNA expression and regulation in the uterus during embryo implantation in rat. FEBS J. 2014;281:1872–1891. doi: 10.1111/febs.12751. [DOI] [PubMed] [Google Scholar]
- 167.Guan Z, et al. Induction of the cellular microRNA-29c by influenza virus contributes to virus-mediated apoptosis through repression of antiapoptotic factors BCL2L2. Biochem. Biophys. Res. Commun. 2012;425:662–667. doi: 10.1016/j.bbrc.2012.07.114. [DOI] [PubMed] [Google Scholar]
- 168.Lu H, et al. Elevated circulating stearic acid leads to a major lipotoxic effect on mouse pancreatic beta cells in hyperlipidaemia via a miR-34a-5p-mediated PERK/p53-dependent pathway. Diabetologia. 2016;59:1247–1257. doi: 10.1007/s00125-016-3900-0. [DOI] [PubMed] [Google Scholar]
- 169.Gong L, et al. Astragaloside IV protects rat cardiomyocytes from hypoxia-induced injury by down-regulation of miR-23a and miR-92a. Cell. Physiol. Biochem. 2018;49:240–2253. doi: 10.1159/000493827. [DOI] [PubMed] [Google Scholar]
- 170.Cheng H, et al. LncRNA RMST-mediated miR-107 transcription promotes OGD-induced neuronal apoptosis via interacting with hnRNPK. Neurochem. Int. 2020;133:104644. doi: 10.1016/j.neuint.2019.104644. [DOI] [PubMed] [Google Scholar]
- 171.Lin CJ, Gong HY, Tseng HC, Wang WL, Wu JL. miR-122 targets an anti-apoptotic gene, Bcl-w, in human hepatocellular carcinoma cell lines. Biochem. Biophys. Res. Commun. 2008;375:315–320. doi: 10.1016/j.bbrc.2008.07.154. [DOI] [PubMed] [Google Scholar]
- 172.Chen CL, et al. Baculovirus-mediated miRNA regulation to suppress hepatocellular carcinoma tumorigenicity and metastasis. Mol. Ther. 2015;23:79–88. doi: 10.1038/mt.2014.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Ma L, et al. Expression of miR-122 mediated by adenoviral vector induces apoptosis and cell cycle arrest of cancer cells. Cancer Biol. Ther. 2010;9:554–561. doi: 10.4161/cbt.9.7.11267. [DOI] [PubMed] [Google Scholar]
- 174.Li S, et al. Hepato-specific microRNA-122 facilitates accumulation of newly synthesized miRNA through regulating PRKRA. Nucleic Acids Res. 2012;40:884–891. doi: 10.1093/nar/gkr715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Ming M, Ying M, Ling M. miRNA-125a-5p inhibits hepatocellular carcinoma cell proliferation and induces apoptosis by targeting TP53 regulated inhibitor of apoptosis 1 and Bcl-2-like-2 protein. Exp. Ther. Med. 2019;18:1196–1202. doi: 10.3892/etm.2019.7674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Banzhaf-Strathmann J, et al. MicroRNA-125b induces tau hyperphosphorylation and cognitive deficits in Alzheimer’s disease. EMBO J. 2014;33:1667–1680. doi: 10.15252/embj.201387576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Gong J, et al. MicroRNA-125b promotes apoptosis by regulating the expression of Mcl-1, Bcl-w and IL-6R. Oncogene. 2013;32:3071–3079. doi: 10.1038/onc.2012.318. [DOI] [PubMed] [Google Scholar]
- 178.Wang C, Zhou B, Liu M, Liu Y, Gao R. miR-126-5p restoration promotes cell apoptosis in cervical cancer by targeting Bcl2l2. Oncol. Res. 2017;25:463–470. doi: 10.3727/096504016X14685034103879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Tang X, et al. Downregulation of miR-129-2 by promoter hypermethylation regulates breast cancer cell proliferation and apoptosis. Oncol. Rep. 2016;35:2963–2969. doi: 10.3892/or.2016.4647. [DOI] [PubMed] [Google Scholar]
- 180.Zhao H, et al. MiR-133b is down-regulated in human osteosarcoma and inhibits osteosarcoma cells proliferation, migration and invasion, and promotes apoptosis. PLoS One. 2013;8:e83571. doi: 10.1371/journal.pone.0083571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Crawford M, et al. MicroRNA 133B targets pro-survival molecules MCL-1 and BCL2L2 in lung cancer. Biochem. Biophys. Res. Commun. 2009;388:483–489. doi: 10.1016/j.bbrc.2009.07.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Li X, Jin Y, Mu Z, Chen W, Jiang S. MicroRNA-146a-5p enhances cisplatin-induced apoptosis in ovarian cancer cells by targeting multiple anti-apoptotic genes. Int. J. Oncol. 2017;51:327–335. doi: 10.3892/ijo.2017.4023. [DOI] [PubMed] [Google Scholar]
- 183.Kim TH, et al. miR-150 enhances apoptotic and anti-tumor effects of paclitaxel in paclitaxel-resistant ovarian cancer cells by targeting Notch3. Oncotarget. 2017;8:72788–72800. doi: 10.18632/oncotarget.20348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Wang JX, et al. Oxidative Modification of miR-184 Enables It to Target Bcl-xL and Bcl-w. Mol. Cell. 2015;59:50–61. doi: 10.1016/j.molcel.2015.05.003. [DOI] [PubMed] [Google Scholar]
- 185.You HY, Xie XM, Zhang WJ, Zhu HL, Jiang FZ. Berberine modulates cisplatin sensitivity of human gastric cancer cells by upregulation of miR-203. Vitr. Cell Dev. Biol. Anim. 2016;52:857–863. doi: 10.1007/s11626-016-0044-y. [DOI] [PubMed] [Google Scholar]
- 186.Li G, Luna C, Qiu J, Epstein DL, Gonzalez P. Role of miR-204 in the regulation of apoptosis, endoplasmic reticulum stress response, and inflammation in human trabecular meshwork cells. Invest. Ophthalmol. Vis. Sci. 2011;52:2999–3007. doi: 10.1167/iovs.10-6708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Fan Y, Wu Y. Tetramethylpyrazine alleviates neural apoptosis in injured spinal cord via the downregulation of miR-214-3p. Biomed. Pharmacother. 2017;94:827–833. doi: 10.1016/j.biopha.2017.07.162. [DOI] [PubMed] [Google Scholar]
- 188.Kim S, et al. miR-340-5p suppresses aggressiveness in glioblastoma multiforme by targeting Bcl-w and Sox2. Mol. Ther. Nucleic Acids. 2019;17:245–255. doi: 10.1016/j.omtn.2019.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Kuang X, et al. miR-378 inhibits cell growth and enhances apoptosis in human myelodysplastic syndromes. Int. J. Oncol. 2016;49:1921–1930. doi: 10.3892/ijo.2016.3689. [DOI] [PubMed] [Google Scholar]
- 190.Druz A, et al. A novel microRNA mmu-miR-466h affects apoptosis regulation in mammalian cells. Biotechnol. Bioeng. 2011;108:1651–1661. doi: 10.1002/bit.23092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Shen L, et al. miR-497 induces apoptosis of breast cancer cells by targeting Bcl-w. Exp. Ther. Med. 2012;3:475–480. doi: 10.3892/etm.2011.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Chen W, et al. miR-509-3p promotes cisplatin-induced apoptosis in ovarian cancer cells through the regulation of anti-apoptotic genes. Pharmacogenomics. 2017;18:1671–1682. doi: 10.2217/pgs-2017-0115. [DOI] [PubMed] [Google Scholar]
- 193.Ramachandran C, et al. Expression profiles of apoptotic genes induced by curcumin in human breast cancer and mammary epithelial cell lines. Anticancer Res. 2005;25:3293–3302. [PubMed] [Google Scholar]
- 194.Zhou QM, et al. Curcumin reduces mitomycin C resistance in breast cancer stem cells by regulating Bcl-2 family-mediated apoptosis. Cancer Cell Int. 2017;17:84. doi: 10.1186/s12935-017-0453-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.You F, et al. Genistein protects against Aβ25-35 induced apoptosis of PC12 cells through JNK signaling and modulation of Bcl-2 family messengers. BMC Neurosci. 2017;18:12. doi: 10.1186/s12868-016-0329-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Telang U, Morris ME. Effect of orally administered phenethyl isothiocyanate on hepatic gene expression in rats. Mol. Nutr. Food Res. 2010;54:1802–1806. doi: 10.1002/mnfr.200900607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Xu X, Yokoyama S, Hayakawa Y, Saiki I. Coptidis Rhizoma induces intrinsic apoptosis through BAX and BAK activation in human melanoma. Oncol. Rep. 2017;38:538–544. doi: 10.3892/or.2017.5672. [DOI] [PubMed] [Google Scholar]
- 198.Kao SJ, et al. Suppression of reactive oxygen species-mediated ERK and JNK activation sensitizes dihydromyricetin-induced mitochondrial apoptosis in human non-small cell lung cancer. Environ. Toxicol. 2017;32:1426–1438. doi: 10.1002/tox.22336. [DOI] [PubMed] [Google Scholar]
- 199.Kim JY, Jeon YK, Jeon W, Nam MJ. Fisetin induces apoptosis in Huh-7 cells via downregulation of BIRC8 and Bcl2L2. Food Chem. Toxicol. 2010;48:2259–2264. doi: 10.1016/j.fct.2010.05.058. [DOI] [PubMed] [Google Scholar]
- 200.Asif M, et al. Isoledene from Mesua ferrea oleo-gum resin induces apoptosis in HCT 116 cells through ROS-mediated modulation of multiple proteins in the apoptotic pathways: a mechanistic study. Toxicol. Lett. 2016;257:84–96. doi: 10.1016/j.toxlet.2016.05.027. [DOI] [PubMed] [Google Scholar]
- 201.Kennedy RK, et al. Phenazine-1-carboxamide (PCN) from Pseudomonas sp. strain PUP6 selectively induced apoptosis in lung (A549) and breast (MDA MB-231) cancer cells by inhibition of antiapoptotic Bcl-2 family proteins. Apoptosis. 2015;20:858–868. doi: 10.1007/s10495-015-1118-0. [DOI] [PubMed] [Google Scholar]
- 202.Cecen E, Altun Z, Ercetin P, Aktas S, Olgun N. Promoting effects of sanguinarine on apoptotic gene expression in human neuroblastoma cells. Asian Pac. J. Cancer Prev. 2014;15:9445–9451. doi: 10.7314/APJCP.2014.15.21.9445. [DOI] [PubMed] [Google Scholar]
- 203.Zhang X, Zhou Y, Gu YE. Tanshinone IIA induces apoptosis of ovarian cancer cells in vitro and in vivo through attenuation of PI3K/AKT/JNK signaling pathways. Oncol. Lett. 2019;17:1896–1902. doi: 10.3892/ol.2018.9744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Sugantha Priya E, et al. Anti-cancer activity of quercetin in neuroblastoma: an in vitro approach. Neurol. Sci. 2014;35:163–170. doi: 10.1007/s10072-013-1462-1. [DOI] [PubMed] [Google Scholar]