Abstract
Grape-derived proanthocyanidins could act as a protector against various environmental stresses for Saccharomyces cerevisiae during wine fermentation, resulting in the increased physiological activity, fermentation efficiency and improved wine quality. In order to explore the possible protection mechanism of proanthocyanidins globally, RNA-seq analysis for wine yeast AWRI R2 cultivated with 0 g/L (group A), 0.1 g/L (group B), 1.0 g/L (group C) proanthocyanidins were applied in this study. Differentially expressed genes were enriched into six metabolic pathways including vitamin B6, thiamine, amino acids, aminoacyl-tRNA, carbohydrate and steroid based on KEGG enrichment analysis. Four key genes (SNZ2, THI6, THI21 and THI80), participated in the biosynthesis of vitamin B6 and thiamine, were up-regulated significantly in proanthocyanidins treated yeast cells and the gene expression levels were verified by RT-qPCR. Yeast cells supplemented with proanthocyanidins performed increased intracellular levels of vitamin B6 and thiamine and higher cell viability compared to the control group. In addition, the composition of intracellular fatty acids showed an obvious alternation in proanthocyanidins-treated yeast cells, in which the UFAs content increased whereas the SFA content decreased. In general, we provided an indirect protection effect of proanthocyanidins on the yeast cells to alleviate environmental stresses during wine fermentation.
Subject terms: Applied microbiology, RNA sequencing
Introduction
The widely distributed phenolic substances in wine refer to a large group of chemical compounds that impact the sensory quality of red wine, such as color, flavor and astringency1. A large number of studies have shown that these phenolic substances have also biological activities of interest to the consumers, such as antioxidant properties, vascular protection and immune-modulatory effects2–5. For winemaking, these phenolic compounds are mainly extracted from grape skin, seed, and stem during the maceration and fermentation process6. Oenological practices, such as fermentation temperature, maceration time, storage period and yeast strains, are believed to impact the concentration and structure of these chemical compounds7–9.
As the predominant fraction, proanthocyanidins (PAs) account for more than 50% of the total wine polyphenols, and have several important roles in the quality of red wine, such as the perception of astringency and taste, aroma release, and color stability, throughout the entire period of fermentation and storage of wine10,11.
In addition, PAs could interact with other extracted phenolic compounds and even the fermentation strains, which have been proved to be crucial for the generation of new pigments, color and astringency modifications12,13. It has been reported that the interaction of the polysaccharides released by yeast lees with wine polyphenols during aging were crucial for sensory qualities of wines11. The extracted polyphenols could be adsorbed by yeast cells by forming the Van der Waals bonds and H-bonds during alcoholic fermentations, which might be a reasonable explanation that the yeasts cells could contribute to the modification of the color of the wine13.
Saccharomyces cerevisiae, the preferred microorganism for wine making and bioethanol production, has been exposed simultaneously and sequentially to a variety of environmental stresses during the fermentation process, including high concentration of ethanol, hyperosmotic stress, heat shock, low pH and nutrients deficiency14–16. Cells viability and fermentative behavior will be negatively affected by the stresses that in turn limit the wine production and quality17. In order to carry out wine fermentation properly, yeast cells should possess the capacity of rapid stress perceptions and responses to those stress conditions without substantial viability loss14. Therefore, to solve these problems, a number of previous studies have been performed in the aspects of robust strain isolation, genetic modification and fermentation process control18–21.
It has been reported that phenolic compounds in wine act as a structure-dependent and dose-dependent manner to affect the physiological activities and fermentation performance of yeast and bacterial cells22–24. Our previous work showed that PAs could pose obvious enhancement on the metabolism and fermentation efficiency, which has been elucidated from the metabolic level such as the change of glucose transport, the energy and redox homeostasis as well as the activities of rate-limiting enzymes in glycolysis24,25. However, the possible molecular mechanisms of the effects of PAs on the yeast fermentation performance have not been fully studied. Next-generation sequencing technologies enables the generation of genomic resources more efficient and cost-effective26. Therefore, in this study, we employed RNA-seq as the method to find the differentially expressed key genes and then performed comprehensive and comparative bioinformatics analysis to globally explore the possible protective effects of PAs on the yeast cells during wine fermentation.
Results
Quality evaluation of sequencing reads
Quality evaluation of sequencing reads needed to be evaluated before the following bioinformatics analysis. As shown in Table 1, a total of 524025062 clean data were generated with an average length of 147 bp. The total raw data was deposited in NCBI Gene Expression Omnibus (GEO) public archive database and the accession number is GSE141069. Phred quality score (Q score) was used as an index for the base calling accuracy and calculated by using the FastQC software (v0.10.1) based on Eq. (1):
| 1 |
Table 1.
Quality statistics of clean sequencing data.
| Sample | length | Reads | Bases | Q20 (%) | Q30 (%) |
|---|---|---|---|---|---|
| A1 | 147.32 | 40811558 | 6012389770 | 95.73 | 88.78 |
| A2 | 147.38 | 62030546 | 9141997823 | 95.75 | 89.47 |
| A3 | 147.53 | 60816138 | 8972430801 | 95.93 | 89.89 |
| B1 | 147.45 | 67018594 | 9882123005 | 95.88 | 89.72 |
| B2 | 147.49 | 55217110 | 8143748191 | 96.01 | 90.04 |
| B3 | 147.41 | 66504646 | 9803127605 | 95.89 | 89.78 |
| C1 | 147.18 | 52310564 | 7699225784 | 95.85 | 89.70 |
| C2 | 147.28 | 55806604 | 8219088002 | 95.85 | 89.69 |
| C3 | 147.21 | 63509302 | 9349372301 | 96.02 | 90.10 |
Q20, Q30 refer to the proportion of base calls with Phred scores >20 or 30 in the total bases. Higher scores ensured the base calling accuracy and data quality. Each group has three biological replicates.
About 90% of the clean reads in each sample has Phred quality score >30, indicating that the base calling accuracy for each group was 99.9% and met the requirement for the next analysis (Table 1).
The Pearson correlation coefficients R2 were calculated to assess the reproducibility of gene expression values across biological replicates, as shown in Supplementary Fig. S1 to Supplementary Fig. S3, the average correlation R2 across the replicates for each group (A, B, C) were 0.969, 0.967 and 0.931, respectively, indicating that there were no considerable differences between the biological replicates and the data could be used in following analysis.
Differential gene expression analysis
To identify differentially expressed genes, transcriptome analysis was carried out for the yeast cells supplemented with 0 g/L (group A), 0.1 g/L (group B) and 1.0 g/L (group C) PAs, respectively. As shown in Fig. 1, 389, 1737 and 1566 DEGs were identified by comparative analysis of transcriptome data of group A versus group B, group A versus group C and group B versus group C, respectively, indicating that PAs pose considerable effects on the gene expression level in the yeast cells. Moreover, a dose-dependent increase of DEGs was found in the data comparison (Fig. 2). With the increase of the concentration of PAs from 0.1 g/L to 1.0 g/L in the fermentation broth, more than 4 times of DEGs were identified in the yeast cells. A total of 48 DEGs were found in all the three comparative groups, which might be considered as the key genes related to the PAs protection mechanism.
Figure 1.
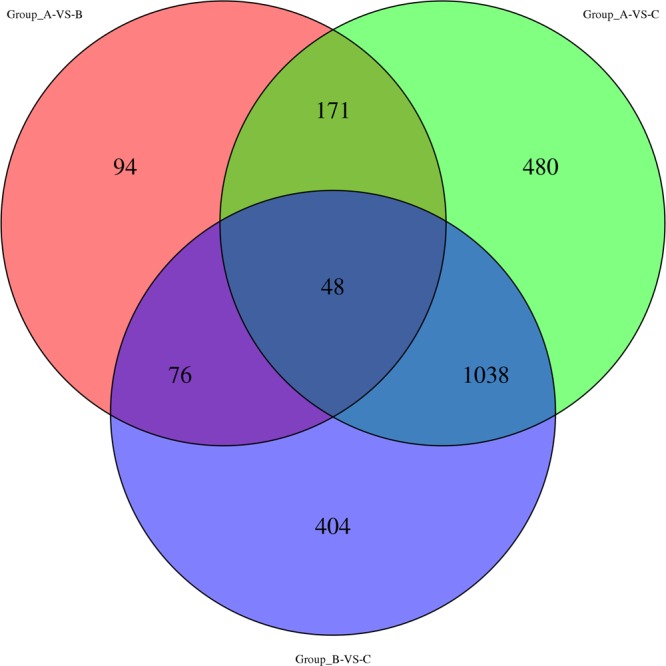
Venn diagrams of transcripts in the three comparative groups marked as group A versus group B, group A versus group C and group B versus group C, respectively.
Figure 2.
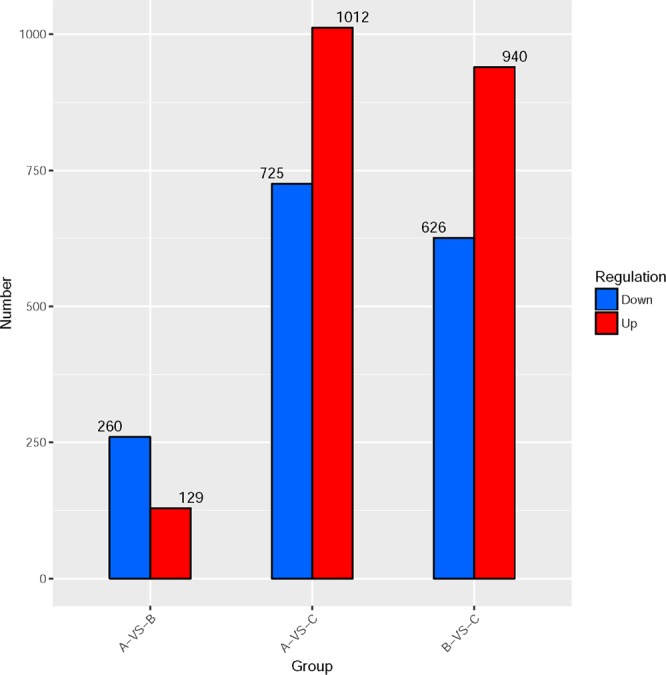
Up or down regulated DEGs in pairwise comparisons marked as group A versus group B, group A versus group C and group B versus group C, respectively.
GO enrichment analysis of the DEGs
To better understand the functions of the DEGs, gene annotation including homologous protein annotation was performed by using the gene ontology (GO) database. As shown in Fig. 3, the identified DEGs could be classified into three categories including biological process, cellular component and molecular function.
Figure 3.
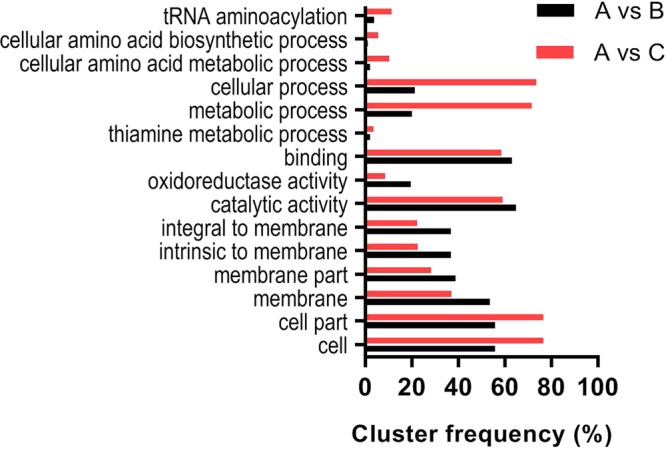
Gene ontology (GO) analysis of the identified DEGs.
In the cellular component category, DEGs were mainly enriched in the terms related with the structure of cell membrane and organelle; In the biological process group, metabolic process and cellular process were the important functions, especially the thiamine and cellular amino acid metabolic pathways and tRNA aminoacylation process; catalytic activity, oxidoreductase activity and binding were the three main molecular functions of the identified DEGs.
In addition, an obvious positive correlation between the number of enriched DGEs and the concentration of PAs were found in the three biological functions. Especially in the thiamine metabolic process and tRNA aminoacylation, as the concentration of PAs was increased to 1.0 g/L, more than 6 times of the numbers of DGEs were enriched compared to that in the 0.1 g/L PAs-treated group. It indicated that PAs could act as an exogenous regulator to perturb more genes expression to protect yeast cells against the stress factors during fermentation.
Metabolic pathways enrichment analysis
To explore the basic protection mechanism of PAs in yeast cells, the main metabolic or signaling pathways were enriched using the KEGG database (Table 2). In the comparison between PAs treated and untreated groups, six pathways, including vitamin B6 metabolism, thiamine metabolism, biosynthesis of amino acids, aminoacyl-tRNA biosynthesis, starch and sucrose metabolism and steroid biosynthesis were significantly enriched (p values were shown in Table 2). Most of the enriched genes within the identified pathways were up-regulated significantly (p values were shown in Table 2), indicating that PAs could be act as an extracellular enhancer to regulate the important metabolic pathways to produce more anabolic products (e.g. vitamins, amino acid, glucan, ergosterol and fatty acid) necessary to survive in wine fermentation process.
Table 2.
The KEGG pathway enrichment analysis.
| Gene name | Description | log2 Fold Change | Corrected p-value |
|---|---|---|---|
| Vitamin B6 metabolism | 4.54×10−4 | ||
| SNO3 | putative pyridoxal 5′-phosphate synthase | +1.01 | 1.08×10−5 |
| SNZ2 | GMP synthase activity | +1.46 | 3.15×10−40 |
| SNZ3 | Pyridoxal-5′-phosphate synthase | +1.48 | 1.34×10−28 |
| SNO2 | putative pyridoxal 5′-phosphate synthase | +1.75 | 2.67×10−40 |
| Thiamine metabolism | 8.15×10 | ||
| THI80 | thiamine diphosphokinase activity | +1.23 | 4.34×10−20 |
| THI21 | phosphomethylpyrimidine kinase activity | +1.54 | 7.4×10−18 |
| THI5 | Protein involved in synthesis of the thiamine precursor HMP | +1.08 | 1.39×10−4 |
| THI6 | thiamine-phosphate diphosphorylase activity | +1.21 | 9.36×10−18 |
| THI20 | phosphomethylpyrimidine kinase activity | +1.14 | 2.12×10−13 |
| THI22 | hydroxymethylpyrimidine phosphate kinases | +1.39 | 1.51×10−15 |
| THI72 | Transporter of thiamine or related compound | +1.12 | 5.67×10−10 |
| Arginine and proline metabolism | 6.43×10 | ||
| CAR1 | arginase | −2.10 | 2.28×10−32 |
| CAR2 | L-ornithine transaminase | +1.04 | 4.91×10−8 |
| PUT2 | δ−1-pyrroline-5-carboxylate dehydrogenase | −1.00 | 1.44×10−10 |
| PUT4 | Proline permease | −5.40 | 1.72×10−147 |
| PRO2 | γ-glutamyl phosphate reductase | −1.26 | 3.12×10−17 |
| GAP1 | Amino acid permease | −2.09 | 3.23×10−18 |
| SPE1 | Ornithine decarboxylase | −1.23 | 3.73×10−15 |
| Aminoacyl-tRNA biosynthesis | 5.09×10 | ||
| MSE1 | glutamate-tRNA ligase activity | +1.84 | 5.04×10−50 |
| MSW1 | tryptophan-tRNA ligase activity | +1.38 | 1.34×10−32 |
| MSM1 | methionine-tRNA ligase activity | +1.69 | 6.71×10−32 |
| MSF1 | phenylalanine-tRNA ligase activity | +1.60 | 1.76×10−30 |
| MSD1 | aminoacyl-tRNA ligase activity | +1.34 | 9.97×10−28 |
| AIM10 | proline-tRNA ligase activity | +1.42 | 3.10×10−25 |
| HER2 | glutaminyl-tRNA synthase activity | +1.12 | 3.00×10−17 |
| DIA4 | serine-tRNA ligase activity | +1.56 | 8.10×10−17 |
| TYS1 | tyrosine-tRNA ligase activity | −1.42 | 8.10×10−17 |
| KRS1 | lysine-tRNA ligase activity | −1.34 | 5.02×10−9 |
| CDC60 | leucine-tRNA ligase activity | −1.42 | 5.11×10−9 |
| THS1 | threonine-tRNA ligase activity | −1.20 | 6.05×10−9 |
| DPS1 | aspartate-tRNA ligase activity | −1.15 | 1.05×10−8 |
| DED81 | asparagine-tRNA ligase activity | −1.20 | 1.17×10−8 |
| ALA1 | alanine-tRNA ligase activity | −1.19 | 6.36×10−8 |
| FRS1 | phenylalanine-tRNA ligase activity | −1.01 | 4.96×10−7 |
| GLN4 | glutamine-tRNA ligase activity | −1.03 | 9.35×10−5 |
| Starch and sucrose metabolism | 4.56×10 | ||
| GSC2 | 1,3-beta-D-glucan synthase activity | +1.89 | 1.43×10−9 |
| DSE4 | glucan endo-1,4-beta-glucanase activity | +1.36 | 8.55×10−21 |
| Steroid biosynthesis | 1.48×10 | ||
| ERG26 | C-3 sterol dehydrogenase | +1.14 | 1.55×10−11 |
| ERG5 | oxidoreductase activity | +1.26 | 7.52×10−8 |
| ERG3 | oxidoreductase activity | +1.25 | 2.11×10−7 |
| ERG4 | oxidoreductase activity | +2.13 | 6.11×10−23 |
| ERG28 | ergosterol biosynthesis | +1.77 | 2.81×10−10 |
The column of “log2 Fold Change” represents the base-two logarithm value of the fold change of the gene expression; “+” means up-regulated genes; “−” means down-regulated genes. Hypergeometric test was used for statistical analysis. The p-values have been corrected for multiple testing by the Benjamini and Hochberg adjustment method. The corrected p value <0.05 plus the absolute log2 Fold Change value >1 were considered statistically significant.
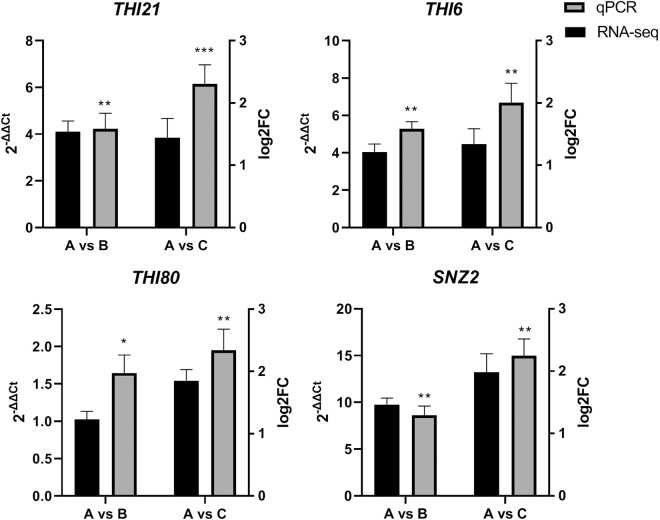
Quantitative Real-time PCR
To further confirm the alterations of gene expressions in PAs-treated yeast cells, we measured the relative expression level of four DEGs related to the metabolic pathway of B-complex vitamins (B1, B6) by RT-qPCR (Fig. 4). The results of RT-qPCR showed a good consistence with that obtained in RNA-seq analysis. The expression levels of the selected genes were up-regulated significantly in PAs-treated groups as relative fold change to the control. More interestingly, a dose-dependent up-regulation appeared as the concentration of PAs increased to 1.0 g/L. Therefore, the RNA-seq could provide reliable data for differential gene expression analysis.
Figure 4.
RT-qPCR validations of the results obtained in RNA-seq analysis. The relative expression level of 4 selected genes compared to the actin gene ACT1 was calculated. Value of the 2−ΔΔCT was calculated and two-way ANOVA with Tukey’s multiple comparisons test were used for the significant analysis of the RT-qPCR at *p < 0.05, **p < 0.01, ***p < 0.001 level. The absolute log2 Fold Change value >1 were considered statistically significant in the RNA-seq analysis. Three biological replicates were prepared and the data were shown as means ± S.D.
Based on the results of transcriptome analysis, the genes related to the metabolic pathway of B-complex vitamins (B1, B6) were up-regulated significantly. In addition, the contents of vitamins B1 and vitamins B6 were measured to verify the impact of PAs from the aspect of yeast cells phenotype (Fig. 5). Calibration curves of peak area-concentration relationship for each vitamin were prepared using a series of mixed solutions at range from 1.0 to 5.0 μg/ mL. As shown in Supplementary Fig. S4, it showed a good linear relation with correlation coefficients (R2) obtained as 0.9947 and 0.9987 for vitamins B1 and B6 at the selected concentration ranges, respectively. This method was suitable for the calculation of the intracellular contents of the vitamins B1 and B6. As shown in Fig. 5, significantly increases of the contents of vitamins B1 and vitamins B6 were detected in PAs-treated yeast cells. For example, vitamins B1 and vitamins B6 contents in yeast cells treated with 0.1 g/L PAs increased significantly from 23.6 μg/g dry weight and 21.6 μg/g dry weight to 28.9 μg/g dry weight (p = 0.015) and 27.4 μg/g dry weight (p = 0.001), respectively. In addition, like the elevation in gene expression levels, obvious dose-dependent increases of intracellular level of vitamins B1 and vitamins B6 in the PAs-treated yeast cells were observed, especially for the vitamins B1 contents which showed a 16.6% increase in the 1.0 g/L PAs-treated yeast cells compared to the 0.1 g/L PAs-treated ones.
Figure 5.
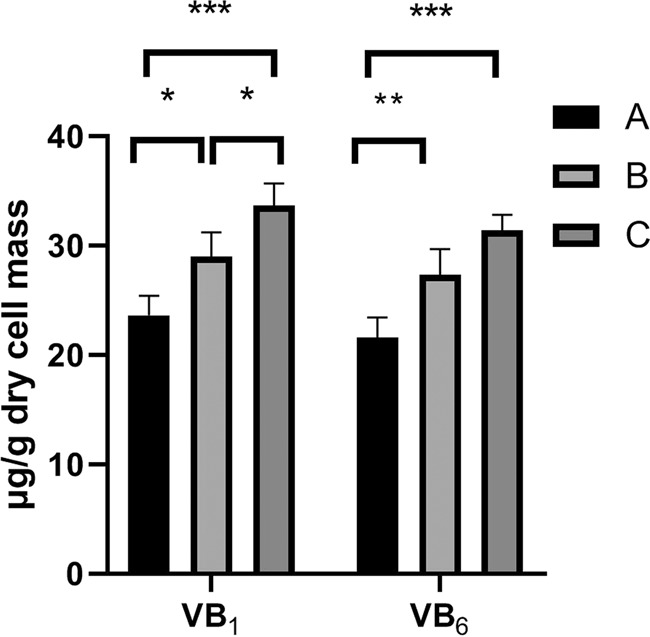
Intracellular contents of vitamins B1 and vitamins B6 in different systems. All data represent the mean values ± S.D. from three independent experiments. Two-way ANOVA with Tukey’s multiple comparisons test were used for the significant analysis at *p < 0.05, **p < 0.01, ***p < 0.001 level.
To further prove the protection effects of PAs on the yeast cells, measurements of the cell viability were conducted. The yeast cells were cultivated with 1.0 g/L PAs, 50 mg/L vitamin B1, 50 mg/L vitamin B6 and a 1:1(w/w) mixture of the two vitamins, respectively in MSM medium at 28 °C for 72 h, and then the viable cells were counted on the MSM agar plates. As shown in Fig. 6, cell survival decreased significantly in the negative control group in which no PAs or vitamins were supplemented. As expected, the viable cells on plates containing PAs, vitamin B1, vitamin B6 and mixture of vitamin B1 and B6, respectively, were higher than the control group. Combined with the results in Figs. 4 and 5, we could clearly notice that exogenous PAs could increase the bio-synthetic yields of vitamin B1 and vitamin B6 to relieve the environmental stresses in yeast cells during red wine fermentation.
Figure 6.
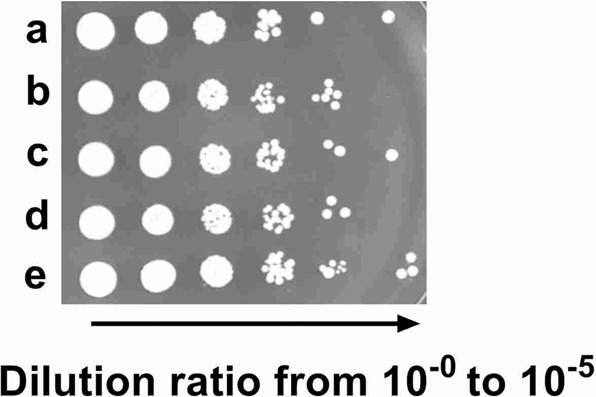
Spot assay of the viability of yeast cells treated with 1.0 g/L PAs (b), 50 mg/L vitamin B1 (c), 50 mg/L vitamin B6 (d) and a 1:1(w/w) mixture of the two vitamins (e), respectively in MSM medium. The negative control (a) was set without the above supplements. After cultivation at 28 °C for 72 h, five microliter of each proper dilution was spotted on the MSM agar plate for viable cell counts. The arrow below the panel indicates a serial dilution of plated cells (1:1; 1:10; 1:102; 1:103; 1:104; 1:105 from left to right).
It has been reported that yeast membrane fatty acid composition and membrane fluidity were linked to the stress tolerance of the cells. Exogenous ergosterol has been proved to be effective for the enhancement of the tolerance of monoterpenes in S. cerevisiae strain by regulating the fatty acid composition27. The intracellular contents of vitamin B1 and vitamin B6 could pose significant effects on the lipid metabolism. To testify whether the PAs show the similar effects on alternation of lipid metabolism and yeast tolerance for environmental stresses, we studied the changes in fatty acid profile in the yeast cells with or without the treatment of PAs. As shown in Fig. 7, lipid profile of PAs-treated cells revealed a 5.36% increase in cellular unsaturated fatty acids (C16:1 and C18:1) with respect to cells grown in the absence of PAs. This comprised a 2.93% increase in C16:1 and a 2.43% increase in C18:1. Meanwhile, the saturated fatty acids (C16:0 and C18:0) in the PAs-treated cells decreased significantly from 25.4% to 19.6% of the total phospholipid fatty acid compared to that in non PAs-treated cells.
Figure 7.
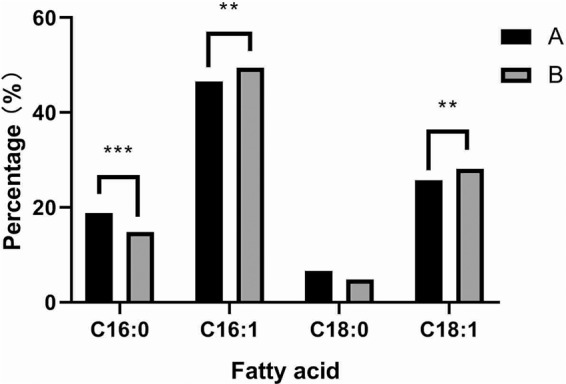
Fatty acid profiles in the membrane of yeast cells. S. cerevisiae yeast strains AWRI R2 were cultivated statically at 28 °C for 72 h with (grey bars) or without (dark bars) the treatment of PAs. And then membrane fatty acid compositions were measured. Data were shown as the mean ± S.D. of three independent experiments. Two-way ANOVA with Tukey’s multiple comparisons test were used for the significant analysis at *p < 0.05, **p < 0.01, ***p < 0.001 level.
Discussion
Generally speaking, red wine fermentation process could be considered as the interaction of yeast cells and the complex composition of the grape, especially the sugar, organic acid and phenolic compounds28. Sensory attributes of wine including color intensity and stability, mouth-feel, astringency and taste mainly come from the phenolic compounds evolution, especially PAs, the principal constitute in the phenolic compounds29. In order to yield high quality red wine with improved sensory and biological properties, thoroughly studies of interactions between yeast cells and PAs should be done. In our previous studies, we have showed that the concentration and structure of PAs evolved progressively in a strain- and time-dependent manner during fermentation1. Moreover, PAs could pose positive effects on the yeast cells that have proved from the aspects of glucose transport, the energy and redox homeostasis as well as the activities of rate-limiting enzymes in glycolysis24. However, to the best of our knowledge, the global regulation posed by PAs from the genetic and metabolic level has never been reported before.
With the rapid development of sequencing technology, RNA-seq has become more efficient and cost-effective in recent years26. Identifying key genes and metabolic pathways related to the stress-tolerance abilities of S. cerevisiae has been considered as the basis of understanding the intracellular global regulation patterns and making the wine fermentation process more controllable30. Therefore, in the present study, we employed the RNA-seq as well as bioinformatics analysis to uncover the possible protection mechanism of PAs for S. cerevisiae during wine fermentation.
We identified a great many of DEGs by measuring the expression levels in the yeast cells cultivated with 0 g/L (group A), 0.1 g/L (group B), 1.0 g/L (group C) PAs, respectively. By GO and KEGG enrichment analysis, the gene functions and participated metabolic pathways have been uncovered. The significantly enriched gene functions were about the structure of cell membrane, thiamine metabolic processes, cellular amino acid metabolic processes, tRNA amino acylation process, oxidoreductase activity and binding activity. The identified DEGs were significantly enriched in to six metabolic pathways including vitamin B6, thiamine, amino acids, aminoacyl-tRNA, carbohydrate and steroid. Most of the enriched genes in the metabolic pathways of vitamin B6, thiamine, starch and sucrose and steroid showed significantly up-regulated expression patterns compared to that of control group, indicating PAs could act as an extracellular activator to enhance the metabolic efficiency of vitamin B6, thiamine, carbohydrates and steroids.
In our study, for the first time we showed that the genes (SNO3, SNZ2, SNZ3, SNO2, THI80, THI21, THI6, THI20, THI72, THI5, THI22) in the PAs-treated yeast cells performed significantly higher expression levels, involving in the sequentially enhanced bio-synthesis of vitamin B1 and B6 for yeasts (Figs. 4 and 5). It has been reported that increased amplification number of these genes related to bio-synthesis of vitamin B1 and vitamin B6 confers the efficient growth of yeast cells in the thiamin and/or pyridoxine-deficient medium with high sugar concentrations31. Interestingly, our results consistent with the previous study that the yeast cells supplemented with PAs and vitamin B1 and B6 compounds showed higher viability compared to the control group (Fig. 6). It deduced there must be problems with the bio-availability of these vitamins in the wine making process and exogenous PAs could cover the deficiency of the two important vitamins for yeast cells against environmental stresses32. It also has reported that decreased intracellular vitamin B6 content could repress many metabolic pathways, including reduced respiration activity and a significant alteration in sterol and unsaturated fatty acids metabolism33. It might resulted in the sequential changes in the composition of lipids, affecting the fluidity and permeability of cell membrane, as wells as the cell growth27. In our study, we also studied the fatty acid profiles in the membrane of yeast cells with or without the treatment of PAs. A significant increase in the quantities of unsaturated fatty acids (C16:1 and C18:1) was found in the presence of PAs compared to the control group, whereas the quantities of palmitic acid (C16:0) and stearic acid (C18:0) decreased significantly, reflecting that an indirect interaction between PAs and the metabolisms of fatty acid and vitamin B6 might exist in yeast cells by which the phenolic compounds in wine could enhance the physiological activities and fermentation performance. Higher contents of unsaturated fatty acids in yeast cells membranes could make the yeast cells less sensitive to heat stress by repressing the expression level of genes involving the heat-shock response (HSR) and general stress response (GSR) pathways34. In other words, there was an obvious positive correlation between the contents of unsaturated fatty acids and stress tolerance capacity of yeast strains. Our findings provided an exciting possibility that it might be possible to make yeast cells more robust by simply modifying the percentage of PAs in the medium.
Thiamine is another essential molecule for all living organisms. Deficiency in thiamine could decrease NADPH, which affected cellular anabolism and lead to a cessation of glucose consumption35. As a cofactor of transketolase in pentose phosphate pathway, thiamine also could accelerate the metabolism from fructose 6-phosphate and glyceraldehyde 3-phosphate to ribulose-5-phosphate and erythritol 4-phosphate, precursors for the synthesis of amino acids in yeast cells under stress situations36. Our previous study showed that PAs could pose significant enhancement on the activity of H+-ATPase, cell growth, and alcoholic fermentation rate by up-regulating the gene expression levels of PMA1 and HXT7, encoding the plasma membrane ATPase and yeast hexose transporter, respectively24,25. That is, the benefits provided by exogenous PAs to the growth and fermentation performances of yeast cells could be explained from the interaction between the phenolic substances in wine and the metabolisms of thiamine and amino acid.
In our study, the bio-synthesis pathway of ergosterol was also significantly enriched that the expression of five related genes (eg. ERG3, ERG4, ERG5, ERG26, ERG28) were highly induced in the yeast cells with the treatment of PAs. Ergosterols are essential structural and regulatory components involving in the fluidity and permeability of yeast cells membrane. Higher contents of ergosterol could protect yeast against multiple environmental stresses such as oxidative, ethanol, osmotic and so on37. Enhanced amplification numbers of ergosterol synthesis-related genes in yeast cells have been proved to be connected with the PAs supplementation (Table 2). Exogenous PAs might affect the contents of ergosterol and in sequentially contribute for the enhancement of stress tolerance ability in the yeast cells which should be further verified in the future.
It has been reported accumulation of amino acids in plant or microorganisms was critical for the environmental stresses tolerance. The accumulated amino acids could act as osmoprotectants, ROS scavenger and protector against heavy metals38–40. The possibility is that free amino acid could interfere with the side chain bonding and introduce conformational changes in the enzyme protein and thus affect the enzymes synthesis and activity41. Our data demonstrated the pathways of arginine and proline metabolism and aminoacyl-tRNA biosynthesis were significantly enriched in PAs-supplemented yeast cells by the KEGG analysis. Arginine and proline were considered as the important osmoprotectant, playing pivotal role of metabolism in cellular osmoregulation42. The expression level of genes involving in the arginine and proline metabolism showed up or down-regulated patterns (Table 2). CAR2 gene expression level was up-regulated significantly by feeding with PAs, and it might contribute to more synthesis of L-ornithine transaminase which could catalyze glutamate-γ-semialdehyde (GSA) to ornithine in cytosol, the precursor of arginine, more efficiently and alleviate the encountered environmental stress43. The uptake for proline in yeast was largely mediated by a high affinity, specific permease, Put4p, and a low affinity general amino acid permease, Gap1p, encoded by PUT4 and GAP1, respectively44. However, in our study, the gene expression level of PUT4 and GAP1 in the PAs-treated yeast cells showed significant down-regulated. The two inconsistent metabolic patterns of arginine and proline might due to the species-specific alternation or the PAs regulation which should be further studied. Other down-regulated genes (CAR1, SPE1, and PUT2) repressed the degradation pathways of the two kinds of protective amino acids. The gene PRO2 (encoding γ-glutamyl phosphate reductase) were involving in the catalysis of the second step in proline biosynthesis from glutamate. Glutamate and proline were both important amino acid in protecting yeast cells against stress factors. Pyrroline-5-carboxylate (P5C) was an critical reaction intermediate making it possible of the interconversion of glutamate, ornithine, proline and arginine45. In PAs-treated yeast cells the gene expression level of PRO2 was down-regulated significantly, reflecting the cells decreased the consumption of glutamate for the accumulation of proline. It might deduce the phenolic compounds could act as a regulator for the dynamic balance of the intracellular amino acids in yeast cells during wine fermentation.
During wine fermentation, a global regulation is required to maintain the intracellular homeostasis for the survival and growth of the yeast cells. All of the physiological activities could be considered as the enzymatic reactions. Therefore, it was vital that translation reprogramming should be in a correct and controllable way46. In our study, a large amount of genes involving in the aminoacyl-tRNA biosynthesis were differential expressed significantly in PAs-treated yeast cells during fermentation. It indicated that the phenolic compounds in wine could regulate the biosynthesis and activity of aminoacyl-tRNA ligase, and subsequently affect the protein translation globally as the cells responded to the environmental stresses to maintain the intracellular homeostasis.
Conclusions
RNA-seq analysis was employed in our study to systematically explore the protection mechanism of PAs on the yeast cells during wine fermentation. A large amount of DEGs were identified and subsequently enriched into six metabolic pathways including vitamin B6, thiamine, amino acids, aminoacyl-tRNA, carbohydrate and steroid. The intracellular contents of the thiamine and vitamin B6 increased significantly in PAs-treated yeast cells, which was in consistent with the cell viability and the expression pattern of the identified DEGs participating in the metabolic pathways of vitamin B6 and thiamine. In addition, it revealed a significant difference in the fatty acid composition between the PAs-treated yeast cells and the control that the UFA (C16:1 and C18:1) increased whereas SFA (C16:0 and C18:0) decreased. The other enriched metabolic pathways in RNA-seq analysis such as the biosynthesis of amino acids, proteins and steroids should be mainly focused on in further study to fully elucidate the interactions between the phenolic compounds in wine and the yeast cells. In general, data of this study provided insights into the possible molecular mechanisms of PAs protection in S. cerevisiae yeast strains during wine fermentation process and might contribute to the selection and modification of yeast strains with enhanced resistance capacity.
Materials and methods
Yeast strain and culture conditions
AWRI R2, used in this study, was a commercial yeast strain from Marivin (Queensland, Australian) and has good fermentation performance. Yeast cells were maintained on YPD medium slants (1% yeast extract, 2% peptone, 2% glucose, 2% agar). Fresh yeast inoculum was prepared by transferring one loop of yeast colony into 100 mL YPD medium in a 250 mL flask and incubating aerobically overnight in a shaker at 28 °C and 120 rpm.
Fermentation
Model synthetic medium (MSM) was used for fermentation in this study. The MSM is a chemically well-defined medium to simulate a standard grape juice for wine fermentation in laboratory47. The composition of this medium was as follows: glucose (100 g), fructose (100 g), tartaric acid (3 g), citric acid (0.3 g), L-malic acid (0.3 g), MgSO4 (0.2 g), KH2PO4 (2 g), (NH4)2SO4 (0.3 g), asparagine (0.6 g), MnSO4·H2O (4 mg), ZnSO4·7H2O (4 mg), CuSO4·5H2O (1 mg), KI (1 mg), CoCl2·6H2O (0.4 mg), (NH4)6Mo7O24·4H2O (1 mg), H3BO3 (1 mg), meso-inositol (300 mg), biotin (0.04 mg), thiamin (1 mg), pyridoxine (1 mg), nicotinic acid (1 mg), pantothenic acid (1 mg), p-amino benzoic acid (1 mg), palmitic acid (1 mg), palmitoleic acid (0.2 mg), stearic acid (3 mg), oleic acid (0.5 mg), linoleic acid (0.5 mg) and linolenic acid (0.2 mg). The MSM was prepared by mixing thoroughly the above components in 1 L deionized water and the final pH value of the medium was adjusted to 3.3. The crude PAs was firstly subjected to a column-based purification procedure according to our previous research protocol25 to remove the monomers and oligomers. The purified PAs was weighted accurately and dissolved in 10 mL absolute ethyl alcohol thoroughly before use. The obtained PAs solutions were added into the MSM medium to make a series of final concentrations of 0.1 g/L, 1.0 g/L (marked as group B and group C, respectively). The control group marked as group A was prepared by adding 10 mL absolute ethyl alcohol without PAs into the fermentation medium directly. The prepared culture media were sterilized by filtration (nitrate cellulose membrane, 0.45 μm) and supplemented with sulfur dioxide (20 mg/L) to simulate the oenological environment before yeast strain inoculation.
For the fermentation, one percent of the prepared yeast inoculum was inoculated into 400 mL MSM medium supplemented with 0, 0.1 and 1.0 g/L PAs in 500 mL flasks equipped with glass capillary stoppers to maintain the anaerobic conditions and cultivated statically at 28 °C. Samples were taken at the 72 h that the yeast cells were staying in the stationary phase. Ten milliliter of the fermentation broth was centrifuged at 6000 r/min for 5 min to collect the yeast cell pellets. The obtained yeast cells were washed 3 times with 0.9% sodium chloride solution and rapidly frozen in liquid nitrogen before stored at −80 °C for the following experiments.
RNA extraction and sequencing
Yeast cells were grinded in liquid nitrogen. Total RNA of each sample was extracted using RNeasy Mini Kit (Qiagen, Germany). The obtained RNA products were quantified and qualified by Agilent 2100 Bioanalyzer (Agilent Technologies, Palo Alto, CA, USA), NanoDrop (Thermo Fisher Scientific Inc.) and 1% agarose gel electrophoresis before next generation sequencing library preparations. One microgram of total RNA passing the quality test was used for library preparation based on the manufacturer’s protocol of the NEBNext Ultra RNA Library Prep kit for Illumina.
Illumina HiSeq X10 sequencing platform was employed for the library sequencing using a 2×150 bp paired-end (PE) configuration; image analysis and base calling were conducted by the HiSeq Control Software (HCS) + OLB + GAPipeline-1.6 (Illumina) on the HiSeq instrument. The sequences were processed and analyzed by Genewiz (Suzhou, China).
Differential gene expression analysis
Quality control for the sequencing reads were conducted by removing low quality reads containing adapter, primers and poly-N to generate the clean data by Trimmomatic (v0.30). The reference genome sequences and gene model annotation files of S. cerevisiae S288C were downloaded from NCBI https://www.ncbi.nlm.nih.gov/genome/genomes/15 for the reads mapping. Paired-end clean reads were aligned to the reference genome via Hisat2 software (v2.0.1). RPKM (reads per kilo bases per million reads) values were calculated as the indicators of gene expression abundance. DESeq Bioconductor package, a model based on the negative binomial distribution, was used for the differential expression analysis. After adjusted by Benjamini and Hochberg’s approach for controlling the false discovery rate, p-value <0.05 and the absolute value of log2fold change >1were set together to detect differential expressed ones48.
Enrichment analysis
GO-TermFinder was used for identifying Gene Ontology (GO) terms that annotate a list of enriched genes with a significant p-value <0.05. To identify the key metabolic pathways, KOBAS software 2.0 was employed to enrich significant differential expression gene in KEGG pathways.
RT-qPCR validation
Four candidates of the differentially expressed genes associated with the vitamin B complex biosynthesis pathway were selected to validate the transcriptome data using quantitative real-time PCR (RT-qPCR) and the list of the genes and primers were shown in Table 3. Amplification and detection were undertaken with a Thermal Cycler Dice Real-Time System (Takara Bio., Kyoto, Japan). The reaction mixtures were consisted with 12.5 μL of SYBR Premix Ex Taq II (Takara Bio.), 0.5 μL of each primer (10 μM) and 2 μL of prepared cDNA made up to a total volume of 25 μL. Forty cycles at 95 °C for 10 s and at 55 °C for 10 s and at 72 °C for 10 s after the primary denaturation at 95 °C for 10 s were run for the PCR program. The β-Actin gene ACT1 was used as an internal gene expression control. The whole experiment was repeated for three times. The value of 2−ΔΔCT was calculated and used as the indicator for the relative expression level of the target genes49.
Table 3.
Primer lists used in RT-qPCR reactions.
| Gene name | Gene description | Forward primer (5′-3′) | Reverse primer (5′-3′) | Product (bp) |
|---|---|---|---|---|
| SNZ2 | pyridoxal 5′-phosphate synthase | CAAGGATCTAGGTGAGGCTTTG | CTGGATCTCCGCCTTAATCTTG | 130 |
| THI21 | hydroxymethylpyrimidine kinase | ATTTGGCTCGCGGTTATTCC | GCCCGTTGTCCTTAACAGTC | 114 |
| THI6 | thiamine-phosphate diphosphorylase | TATGGCAATTGACGCCGATG | GCCAATGTCTCTACCTCCGA | 132 |
| THI80 | Thiamine pyrophosphokinase | ATCCATCCAAACGAGGATGA | CTATTGGCTGCACCATCTGC | 131 |
| ACT1 | Actin | ACATCGTTATGTCCGGTGGT | CCACCAATCCAGACGGAGTA | 142 |
Measurements of B-complex vitamins (B1, B6)
In order to verify the up-regulated genes participating into the metabolic pathway of vitamin B1 and B6 from the aspect of yeast phenotype, the intracellular contents of the two vitamins were measured by using reverse phase high performance liquid chromatographic (RP-HPLC) method50. The prepared yeast cells were re-suspend in 2 mL deionized water and almost complete cell breakage was obtained using a mechanical bead beater (Biospec Products) set at 6000 rpm for four 30 s periods alternating with 30 s intervals on ice bath. Supernatant were collected by centrifugation at 10,000 rpm for 2 min for the chromatographic measurement. About 50 mg each of thiamine hydrochloride (VB1) and pyridoxine hydrochloride (VB6) standard chemicals was weighed accurately and dissolved in 50 mL 1:1 (v/v) distilled water/ acetonitrile solution thoroughly for the preparation of mixed stock standard solutions. The stock standard solutions were filtered through 0.45 µm membrane filter and diluted with distilled water in the test tubes to yield five series of mixed standard solutions.
The separations and quantitative determination were performed on a Waters 2996 HPLC system (Waters Corp., Milford, MA, USA) consisting of an C18 reverse phase column (Waters X-Terra RP 18 250 × 4.6 mm id., 5 µm particle size), a guard column containing the same material and Waters series 2487 spectrophotometric detector. The wavelength was set at 320 nm to separate all the two metabolites in a single injection. The degassed binary mobile phase consisted of 30% ortho phosphoric acid (Solvent A) and 70% acetonitrile (Solvent B). The flow rate was 1.2 mL/min and the volume injected was 20 µL. Sample peaks were identified by comparing the retention times with those of the standard chemicals. Integration of separated peak area was calculated and the content of each vitamin was determined by using the calibration curve of peak area-concentration relationship. The vitamin contents (μg/ g dry cell weight) in each sample were expressed as the normalized values by dry cell mass weight.
Measurement of cellular fatty acid composition
Freeze dried yeast cells were weighted and subjected to the lipid extraction according to the literature protocol with slightly modification51. Briefly, the cells were disrupted in a MiniBeadbeater-16 (Biospec Products Inc., Bartlesville, OK, USA) according to our previous study52. Lipids were extracted from the cell homogenates with the mixed solution of chloroform and methanol (2:1, v/v). Fatty acid methyl esters (FAMEs) were prepared by catalytic reaction of 2% H2SO4 in anhydrous methanol at 70 °C for 2 h and then the FAMEs solutions were analyzed by gas chromatography (Shimadzu GC-2010 plus), with a QP-5000 mass spectrometer, on an Agilent DB-17 capillary column (30 m length, 0.25 mm inner diameter, 0.25 μm film thickness). FAMEs were identified by comparing the corresponding mass spectra with a spectrum database. The fatty acid composition of each sample was determined based on the integrated peak area for each FAMEs shown as a percentage of the total peak area.
Spot assay
In order to verify the physiological functions of the increased intracellular contents of vitamin B1 and B6 as well as the up-regulated expression level of the encoding genes, spot assay of the viable cell counts were conducted. The harvested fresh cells were re-suspended in the MSM medium supplemented with 1.0 g/L PAs, 50 mg/L vitamin B1, 50 mg/L vitamin B6 and a 1:1(w/w) mixture of the two vitamins, respectively. The negative control without the above supplements was set for the comparison of cell viability. All the samples were cultivated statically at 28 °C for 72 h and then serially diluted. Five microliter of proper dilutions was spotted on the MSM agar plate for viable cell counts.
Supplementary information
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant number 31601439; 31501458). We thank GENEWIZ Suzhou for the part of high throughput sequencing and bioinformatics analysis in this work.
Author contributions
H.W.Z. and J.Y.L. designed the study and K.L.Z. conducted the whole expriments and data analysis. J.Y.L. interpreted the RNA-seq data, and wrote the draft manuscript. H.W.Z. participated in the research coordination and revised this manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
is available for this paper at 10.1038/s41598-020-63631-2.
References
- 1.Li J, Zhao H, Huang W. Yeast-induced changes in the concentration and structure of oligomeric proanthocyanidins during simulated wine fermentation. Aust. J. Grape Wine R. 2015;21:404–410. doi: 10.1111/ajgw.12140. [DOI] [Google Scholar]
- 2.Aron PM, Kennedy JA. Flavan-3-ols: Nature, occurrence and biological activity. Mol. Nutr. Food Res. 2008;52:79–104. doi: 10.1002/mnfr.200700137. [DOI] [PubMed] [Google Scholar]
- 3.Ghosh D, Scheepens A. Vascular action of polyphenols. Mol. Nutr. Food Res. 2009;53:322–331. doi: 10.1002/mnfr.200800182. [DOI] [PubMed] [Google Scholar]
- 4.Serrano J, Puupponen-Pimiä R, Dauer A, Aura A-M, Saura-Calixto F. Tannins: Current knowledge of food sources, intake, bioavailability and biological effects. Mol. Nutr. Food Res. 2009;53:S310–S329. doi: 10.1002/mnfr.200900039. [DOI] [PubMed] [Google Scholar]
- 5.Terra X, et al. Grape-seed procyanidins prevent low-grade inflammation by modulating cytokine expression in rats fed a high-fat diet. J. Nutr. Biochem. 2009;20:210–218. doi: 10.1016/j.jnutbio.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 6.Kennedy JA, Matthews MA, Waterhouse AL. Effect of maturity and vine water status on grape skin and wine flavonoids. Am. J. Enol. Viticult. 2002;53:268–274. [Google Scholar]
- 7.Kovac V, Alonso E, Bourzeix M, Revilla E. Effect of several enological practices on the content of catechins and proanthocyanidins of red wines. J. Agr. Food Chem. 1992;40:1953–1957. doi: 10.1021/jf00022a045. [DOI] [Google Scholar]
- 8.Scudamore-Smith P, Hooper R, McLaran E. Color and phenolic changes of Cabernet Sauvignon wine made by simultaneous yeast/bacterial fermentation and extended pomace contact. Am. J. Enol. Viticult. 1990;41:57–67. [Google Scholar]
- 9.Recamales ÁF, Sayago A, González-Miret ML, Hernanz D. The effect of time and storage conditions on the phenolic composition and colour of white wine. Food Res. Int. 2006;39:220–229. doi: 10.1016/j.foodres.2005.07.009. [DOI] [Google Scholar]
- 10.Vivar-Quintana A, Santos-Buelga C, Francia-Aricha E, Rivas-Gonzalo J. Formation of anthocyanin-derived pigments in experimental red wines. Food Sci. Technol. Int. 1999;5:347–352. doi: 10.1177/108201329900500407. [DOI] [Google Scholar]
- 11.Mazauric J-P, Salmon J-M. Interactions between yeast lees and wine polyphenols during simulation of wine aging: I. Analysis of remnant polyphenolic compounds in the resulting wines. J. Agr. Food Chem. 2005;53:5647–5653. doi: 10.1021/jf050308f. [DOI] [PubMed] [Google Scholar]
- 12.Gawel R. Red wine astringency: a review. Aust. J. Grape Wine R. 1998;4:74–95. doi: 10.1111/j.1755-0238.1998.tb00137.x. [DOI] [Google Scholar]
- 13.Morata A, et al. Adsorption of anthocyanins by yeast cell walls during the fermentation of red wines. J. Agr. Food Chem. 2003;51:4084–4088. doi: 10.1021/jf021134u. [DOI] [PubMed] [Google Scholar]
- 14.Bauer F, Pretorius IS. Yeast stress response and fermentation efficiency: how to survive the making of wine-a review. S. Afr. J. Enol. Vitic. 2000;21:27–51. [Google Scholar]
- 15.Olofsson K, Bertilsson M, Lidén G. A short review on SSF-an interesting process option for ethanol production from lignocellulosic feedstocks. Biotechnol. biofuels. 2008;1:7. doi: 10.1186/1754-6834-1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carrasco P, Querol A. Analysis of the stress resistance of commercial wine yeast strains. Arch. Microbiol. 2001;175:450–457. doi: 10.1007/s002030100289. [DOI] [PubMed] [Google Scholar]
- 17.Erasmus DJ, Cliff M, van Vuuren HJ. Impact of yeast strain on the production of acetic acid, glycerol, and the sensory attributes of icewine. Am. J. Enol. Viticult. 2004;55:371–378. [Google Scholar]
- 18.Querol A, Fernández-Espinar MT, lí del Olmo M, Barrio E. Adaptive evolution of wine yeast. Int. J. Food Microbiol. 2003;86:3–10. doi: 10.1016/S0168-1605(03)00244-7. [DOI] [PubMed] [Google Scholar]
- 19.Zaky AS, Greetham D, Tucker GA, Du C. The establishment of a marine focused biorefinery for bioethanol production using seawater and a novel marine yeast strain. Sci. Rep. 2018;8:1–14. doi: 10.1038/s41598-018-30660-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cardona F, Carrasco P, Pérez-Ortín JE, lí del Olmo M, Aranda A. A novel approach for the improvement of stress resistance in wine yeasts. Int. J. Food Microbiol. 2007;114:83–91. doi: 10.1016/j.ijfoodmicro.2006.10.043. [DOI] [PubMed] [Google Scholar]
- 21.Sablayrolles J. Control of alcoholic fermentation in winemaking: Current situation and prospect. Food Res. Int. 2009;42:418–424. doi: 10.1016/j.foodres.2008.12.016. [DOI] [Google Scholar]
- 22.Reguant C, Bordons A, Arola L, Rozes N. Influence of phenolic compounds on the physiology of Oenococcus oeni from wine. J. Appl. Microbiol. 2000;88:1065–1071. doi: 10.1046/j.1365-2672.2000.01075.x. [DOI] [PubMed] [Google Scholar]
- 23.Vivas N, Lonvaud-Funel A, Glories Y. Effect of phenolic acids and anthocyanins on growth, viability and malolactic activity of a lactic acid bacterium. Food Microbiol. 1997;14:291–299. doi: 10.1006/fmic.1996.0086. [DOI] [Google Scholar]
- 24.Li J, Zhao H, Huang W. Mechanism of proanthocyanidins-induced alcoholic fermentation enhancement in Saccharomyces cerevisiae. J. Ind. Microbiol. Biot. 2014;41:1793–1802. doi: 10.1007/s10295-014-1517-1. [DOI] [PubMed] [Google Scholar]
- 25.Li J, Du G, Yang X, Huang W. Effect of proanthocyanidins on yeast metabolism, H+ -ATPase activity, and wine fermentation. Am. J. Enol. Viticult. 2011;62:512. doi: 10.5344/ajev.2011.11021. [DOI] [Google Scholar]
- 26.Metzker ML. Sequencing technologies-the next generation. Nat. Rev. Genet. 2010;11:31–46. doi: 10.1038/nrg2626. [DOI] [PubMed] [Google Scholar]
- 27.Liu J, Zhu Y, Du G, Zhou J, Chen J. Exogenous ergosterol protects Saccharomyces cerevisiae from D-limonene stress. J. Appl. Microbiol. 2013;114:482–491. doi: 10.1111/jam.12046. [DOI] [PubMed] [Google Scholar]
- 28.Fleet GH. Yeast interactions and wine flavour. Int. J. Food Microbiol. 2003;86:11–22. doi: 10.1016/S0168-1605(03)00245-9. [DOI] [PubMed] [Google Scholar]
- 29.Soares S, Brandão E, Mateus N, de Freitas V. Sensorial properties of red wine polyphenols: Astringency and bitterness. Crit. Rev. Food Sci. 2017;57:937–948. doi: 10.1080/10408398.2014.946468. [DOI] [PubMed] [Google Scholar]
- 30.Walker ME, et al. Genome-wide identification of the Fermentome; genes required for successful and timely completion of wine-like fermentation by Saccharomyces cerevisiae. BMC Genomics. 2014;15:552. doi: 10.1186/1471-2164-15-552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stambuk BU, Dunn B, Alves SL, Duval EH, Sherlock G. Industrial fuel ethanol yeasts contain adaptive copy number changes in genes involved in vitamin B1 and B6 biosynthesis. Genome Res. 2009;19:2271–2278. doi: 10.1101/gr.094276.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Matxain JM, Padro D, Ristilä M, Strid Å, Eriksson LA. Evidence of high •OH radical quenching efficiency by vitamin B6. J. Phys. Chem. B. 2009;113:9629–9632. doi: 10.1021/jp903023c. [DOI] [PubMed] [Google Scholar]
- 33.Kamihara, T. & Nakamura, Regulation of respiration and its related metabolism by vitamin B1 and vitamin B6 in Saccharomyces yeasts in Immobilized biocatalysts Saccharomyces Yeasts Wastewater Treament (ed. Scheper, T.) 35–82 (Springer Verlag Berlin Heidelberg, 1984). [DOI] [PubMed]
- 34.Chatterjee MT, Khalawan SA, Curran BPG. Cellular lipid composition influences stress activation of the yeast general stress response element (STRE) Microbiology. 2000;146:877–884. doi: 10.1099/00221287-146-4-877. [DOI] [PubMed] [Google Scholar]
- 35.Leite, H. P. & de Lima, L. F. P. Thiamine (Vitamin B1) deficiency in intensive care: Physiology, risk factors, diagnosis, and treatment in Diet and nutrition in critical care (eds. Rajkumar Rajendram, Victor R. Preedy, & Vinood B. Patel) 959–972 (Springer New York, 2015).
- 36.Stincone A, et al. The return of metabolism: Biochemistry and physiology of the pentose phosphate pathway. Biol. Rev. 2015;90:927–963. doi: 10.1111/brv.12140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Landolfo S, et al. Oleic acid and ergosterol supplementation mitigates oxidative stress in wine strains of Saccharomyces cerevisiae. Int. J. Food Microbiol. 2010;141:229–235. doi: 10.1016/j.ijfoodmicro.2010.05.020. [DOI] [PubMed] [Google Scholar]
- 38.Tsolmonbaatar A, et al. Isolation of baker’s yeast mutants with proline accumulation that showed enhanced tolerance to baking-associated stresses. Int. J. Food Microbiol. 2016;238:233–240. doi: 10.1016/j.ijfoodmicro.2016.09.015. [DOI] [PubMed] [Google Scholar]
- 39.Nuccio ML, Rhodes D, McNeil SD, Hanson AD. Metabolic engineering of plants for osmotic stress resistance. Curr. Opin. Plant Biol. 1999;2:128–134. doi: 10.1016/S1369-5266(99)80026-0. [DOI] [PubMed] [Google Scholar]
- 40.Matysik, J., Alia, Bhalu, B. & Mohanty, P. Molecular mechanisms of quenching of reactive oxygen species by proline under stress in plants. Curr. Sci., 525–532 (2002).
- 41.Hoque MA, et al. Exogenous proline and glycinebetaine increase NaCl-induced ascorbate-glutathione cycle enzyme activities, and proline improves salt tolerance more than glycinebetaine in tobacco Bright Yellow-2 suspension-cultured cells. J. Plant Physiol. 2007;164:1457–1468. doi: 10.1016/j.jplph.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 42.Long D, Wilkinson KL, Taylor DK, Jiranek V. Novel wine yeast for improved utilisation of proline during fermentation. Fermentation. 2018;4:10. doi: 10.3390/fermentation4010010. [DOI] [Google Scholar]
- 43.Nishimura A, Kotani T, Sasano Y, Takagi H. An antioxidative mechanism mediated by the yeast N-acetyltransferase Mpr1: oxidative stress-induced arginine synthesis and its physiological role. FEMS Yeast Res. 2010;10:687–698. doi: 10.1111/j.1567-1364.2010.00650.x. [DOI] [PubMed] [Google Scholar]
- 44.Poole K, et al. Proline transport and stress tolerance of ammonia-insensitive mutants of the PUT4-encoded proline-specific permease in yeast. J. Gen. Appl. Microbiol. 2009;55:427–439. doi: 10.2323/jgam.55.427. [DOI] [PubMed] [Google Scholar]
- 45.Takagi H, Taguchi J, Kaino T. Proline accumulation protects Saccharomyces cerevisiae cells in stationary phase from ethanol stress by reducing reactive oxygen species levels. Yeast. 2016;33:355–363. doi: 10.1002/yea.3154. [DOI] [PubMed] [Google Scholar]
- 46.Shenton D, et al. Global Translational responses to oxidative stress impact upon multiple levels of protein synthesis. J. Biol. Chem. 2006;281:29011–29021. doi: 10.1074/jbc.M601545200. [DOI] [PubMed] [Google Scholar]
- 47.Marullo P, Bely M, Masneuf-Pomarede I, Aigle M, Dubourdieu D. Inheritable nature of enological quantitative traits is demonstrated by meiotic segregation of industrial wine yeast strains. FEMS Yeast Res. 2004;4:711–719. doi: 10.1016/j.femsyr.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 48.Gao D, et al. A survey of statistical software for analysing RNA-seq data. Hum. Genomics. 2010;5:56. doi: 10.1186/1479-7364-5-1-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Livak, K. J. & Schmittgen, T. D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods25, 402–408 (2001). [DOI] [PubMed]
- 50.León-Ruiz V, Vera S, González-Porto AV, San Andrés MP. Analysis of water-soluble vitamins in honey by isocratic RP-HPLC. Food Anal. Method. 2013;6:488–496. doi: 10.1007/s12161-012-9477-4. [DOI] [Google Scholar]
- 51.Redón M, Guillamón JM, Mas A, Rozès N. Effect of lipid supplementation upon Saccharomyces cerevisiae lipid composition and fermentation performance at low temperature. Eur. Food Res. Technol. 2009;228:833–840. doi: 10.1007/s00217-008-0996-6. [DOI] [Google Scholar]
- 52.Zhao H, Li J, Han B, Li X, Chen J. Improvement of oxidative stress tolerance in Saccharomyces cerevisiae through global transcription machinery engineering. J. Ind. Microbiol. Biotechnol. 2014;41:869–878. doi: 10.1007/s10295-014-1421-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.