Abstract
Based on the results of the IPERGAY study, on-demand HIV pre-exposure prophylaxis (PrEP; also known as “non-daily PrEP,” “event-driven PrEP,” or “2-1-1 PrEP”) is being requested more frequently by patients who have intermittent sexual risk or are unable/unwilling to take daily PrEP; therefore, clinicians will be increasingly required to familiarize themselves with its appropriate use. In this perspective, we summarize data related to on-demand PrEP, describe advantages and disadvantages for this alternative dosing strategy, and provide clinical counseling points.
KEY WORDS: on-demand, 2-1-1, pre-exposure prophylaxis, PrEP, tenofovir/emtricitabine, clinician
INTRODUCTION
On-demand oral pre-exposure prophylaxis (PrEP) with tenofovir disoproxil fumarate (TDF)/emtricitabine (FTC), also known as “non-daily PrEP,” “event-driven PrEP,” or “2-1-1 PrEP,” was studied in the IPERGAY trial.1 In this trial, men who have sex with men (MSM) and transgender women who have sex with men (TGWM), who were hepatitis B surface antigen (HBsAg) negative and had a creatinine clearance above 60 mL/min, were instructed to take a loading dose of two TDF/FTC tablets 2 to 24 h before sex, followed by a third tablet 24 h and a fourth tablet 48 h after the first double dose. The relative reduction of HIV-1 infection in the TDF/FTC group was 86% (95% confidence interval [CI] = 40–98%; p value = 0.002). In the open-label extension of the IPERGAY trial,2 there was a relative reduction of 97% (95% CI = 81–100%) in HIV incidence with on-demand dosing compared to the placebo group. Participants in the IPERGAY study used an average of four TDF/FTC pills per week, which corresponds to an HIV-1 risk reduction of approximately 96%3 seen in the iPrEX study.4 On-demand PrEP is a dosing strategy that is being offered in clinical settings in Paris, Amsterdam, and the USA. Although an off-label use, on-demand PrEP is recommended as an alternative PrEP option by joint International and USA guidelines5 and is being requested more frequently by patients who have intermittent sexual risk or are unable/unwilling to take daily PrEP. Clinicians should therefore familiarize themselves with its appropriate use. In this article, we summarize research to date, describe advantages and disadvantages for this alternative dosing strategy, and provide clinical counseling points.
Advantages and Disadvantages of On-demand PrEP
As a result of the significant reduction in HIV acquisition, increasing PrEP roll-out has been a pillar in several US jurisdictional plans to curb local epidemics (in combination with HIV testing and treatment as prevention). San Francisco, New York, and Seattle have all demonstrated significant declines of new HIV diagnoses as PrEP uptake has increased over the last 4–5 years.6 In addition to patient-centered benefits, PrEP can also reduce HIV anxiety, increase sexual satisfaction and intimacy, and be an incentive for people to connect to healthcare.7–9 However, PrEP uptake has been challenged by several factors including perception of low risk of HIV acquisition, access to care, concerns about cost, and not wanting to take a daily pill. Clinician biases are also limiting PrEP uptake among the most at-risk populations,10, 11 and have been a focus of education and intervention. Additionally, PrEP uptake has been lower among Black and Latino MSM, the two populations most disproportionally impacted by HIV in the US.12–14
On-demand dosing may support increased PrEP uptake among populations where PrEP uptake has been slow and those for whom taking a daily pill is undesirable. It further fosters the sense of control and self-efficacy among individuals who view themselves as having intermittent risk for HIV acquisition or are unable/unwilling to take daily PrEP.15, 16 On-demand dosing may minimize the number of PrEP doses among those who have less frequent sex, reduce pill fatigue, and result in cost savings.17
On the other hand, on-demand dosing is associated with the same short-term adverse events as daily PrEP including nausea, vomiting, diarrhea, abdominal pain, and other gastrointestinal symptoms (i.e., “start-up syndrome”).1 There are no data comparing long-term adverse events (i.e., renal and bone toxicity) of on-demand versus daily PrEP; however, a recent analysis suggests that there is a dose-response relationship between higher TDF/FTC exposure and lower kidney function, and that on-demand PrEP showed low renal toxicity.18 It is unknown how reductions in renal and bone adverse events with on-demand dosing directly compare to daily dosing; however, given the association of reduced renal function with tenofovir plasma concentrations,19 fewer TDF/FTC doses are likely associated with lower levels of renal toxicity.20 Despite this potential, similar to daily PrEP, on-demand PrEP requires quarterly follow-up for laboratory testing, side effect assessment, and adherence evaluation.
Additionally, on-demand PrEP requires planned sex and some level of organization to adhere to the more complex dosing strategy that has less “forgiveness” for missed dosing than daily PrEP. One study showed that over half of MSM participants did not plan sex in advance,21 and in another study, the on-demand post-sex doses were more likely to be missed compared to daily or intermittent dosing.22 Even though on-demand PrEP has only been studied among MSM and TGWM at high risk for HIV acquisition, given the small sample size of TGWM in on-demand studies precluding sub-analyses and the presence of data regarding reductions in tenofovir and FTC levels when used with feminizing hormones in TGWM,23 more clinical data are needed on the use of this dosing strategy in TGWM. Finally, while daily PrEP can be recommended for heterosexual women and men and people who inject drugs, on-demand PrEP has not been studied in these populations.
Counseling Points
Laboratory Screening
Before starting any PrEP regimen, it is essential to ensure that the patient has completed all initial laboratory tests (including HIV antibody, serum creatinine, hepatitis B and C serologies, and sexually transmitted infection screening), and that there are no contraindications to initiating 2-1-1 PrEP (such as positive HBsAg or creatinine clearance > 60 mL/min). Unlike daily PrEP, chronic active Hepatitis B infection is a contraindication with intermittent dosing given the risk of triggering a potentially fatal hepatic flare.24
Who Can Use On-demand PrEP?
We recommend asking patients about their frequency of anal sex, and ability to plan or delay sex by 2–24 h. If patients are unable to anticipate sex or adhere to the pre- and post-sex dosing, daily PrEP is likely preferable. Therefore, asking patients about their ability to plan and negotiate the timing of sex and their self-efficacy around this issue is critical. Patients may move between on-demand and daily PrEP, matching their PrEP regimen to their sexual patterns.
It is important to counsel patients to use on-demand dosing for each sexual encounter and with each sexual partner, instead of choosing when and with whom to use on-demand dosing. If patients miss any of the doses, they should contact their healthcare team or go to urgent care for possible initiation of post-exposure prophylaxis. Finally, patients should be notified of the on-demand dosing adverse events and the need for quarterly laboratory testing.
If on-demand PrEP is an appropriate option, we recommend prescribing Truvada, no. 30, 2 refills, “take as directed.”. Providing the full 30-day supply will ensure that the patient has adequate pills to cover daily dosing in a month, if needed. For documentation, use ICD-10 code Z20.6 (i.e., “Exposure to HIV”) in the patient’s chart to minimize stigmatizing language on problem lists, and involve the multidisciplinary care team, if possible, to follow up with the patient regarding prescription pick-up, PrEP initiation, barriers to use, questions or concerns, adherence, and monitoring and follow-up at 1 month and quarterly visits.
Managing the Gaps in Sex
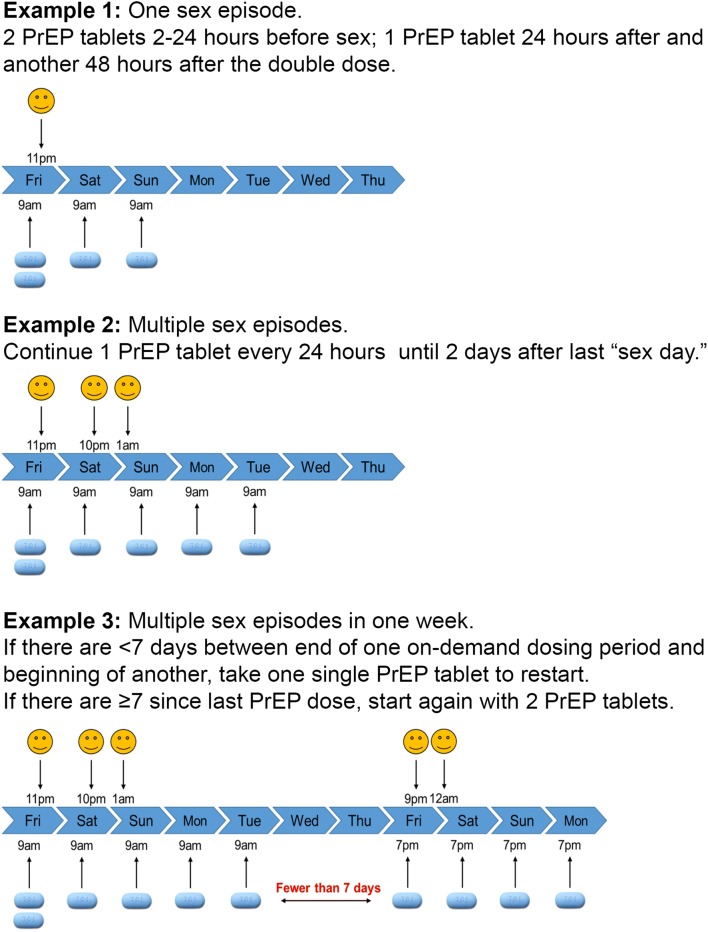
Patients may find the management of gaps in sexual encounters confusing. If it has been more than 7 days since the last dose, it is recommended that patients take a double dose of TDF/FTC and the third and fourth single doses 24 and 48 h after the double dose. However, if the last tablet intake was fewer than 7 days ago, it is recommended that patients take a single tablet and continue taking TDF/FTC single doses 24 and 48 h after the last sexual encounter. Reviewing various examples based on a patient’s last several sexual encounters can help them better understand how they would manage the dosing schedule. Providing a descriptive schematic (Fig. 1) is helpful (figure note: to minimize confusion with timing and number of PrEP doses, we have elected to use the concept of “sex day” during clinical consultations. Therefore, the patient is counseled to continue PrEP every 24 h until 2 days after last “sex day.”). Alarm or other reminder tools can also be useful to support adherence, especially for the post-sex doses at 24 and 48 h.]-->
Figure 1.
Examples for the use of on-demand PrEP. Note refers to “sex day.”
DISCUSSION AND CONCLUSIONS
Future PrEP dosing strategies and delivery methods that would minimize pill fatigue, improve adherence, and potentially reduce adverse events such as long-acting injectables, vaginal rings, and implants may be on the horizon. Until then, on-demand PrEP provides an evidence-based alternative that clinicians can prescribe in specific situations. Qualitative data from the IPERGAY study highlight the importance of providers adopting a trusting, non-judgmental, patient-centered approach guiding patients towards self-care and allowing the patient to have an active role in their care pathway.25 Therefore, clinicians should familiarize themselves with the on-demand dosing strategy to provide patient choice in PrEP options and ultimately help reduce HIV transmissions.
Acknowledgments
The authors would like to thank Kristin Ming for her assistance with the figure.
Funding Information
The authors’ work was supported by the National Institute of Nursing Research (award number R01NR017573).
Compliance with Ethical Standards
Disclaimer
The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. The funders had no role in (1) the writing of the report, or (2) the decision to submit the manuscript for publication.
Conflict of Interest
The authors declare that they do not have a conflict of interest.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Molina JM, Capitant C, Spire B, et al. On-Demand Preexposure Prophylaxis in Men at High Risk for HIV-1 Infection. New Engl J Med. 2015;373(23):2237–2246. doi: 10.1056/NEJMoa1506273. [DOI] [PubMed] [Google Scholar]
- 2.Molina JM, Charreau I, Spire B, et al. Efficacy, safety, and effect on sexual behaviour of on-demand pre-exposure prophylaxis for HIV in men who have sex with men: an observational cohort study. Lancet Hiv. 2017;4(9):E402–E410. doi: 10.1016/S2352-3018(17)30089-9. [DOI] [PubMed] [Google Scholar]
- 3.Anderson PL, Glidden DV, Liu A, et al. Emtricitabine-Tenofovir Concentrations and Pre-Exposure Prophylaxis Efficacy in Men Who Have Sex with Men. Sci Transl Med. 2012;4(151). [DOI] [PMC free article] [PubMed]
- 4.Grant RM, Lama JR, Anderson PL, et al. Preexposure Chemoprophylaxis for HIV Prevention in Men Who Have Sex with Men. New Engl J Med. 2010;363(27):2587–2599. doi: 10.1056/NEJMoa1011205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Saag MS, Benson CA, Gandhi RT, et al. Antiretroviral Drugs for Treatment and Prevention of HIV Infection in Adults: 2018 Recommendations of the International Antiviral Society-USA Panel. JAMA. 2018;320(4):379–396. doi: 10.1001/jama.2018.8431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fauci A. Ending the HIV Epidemic: a plan for the united states (Abstract #2010). Conference on Retroviruses and Opportunistic Infections (CROI); 2019.
- 7.Marcus JL, Levine K, Grasso C, et al. HIV Preexposure Prophylaxis as a Gateway to Primary Care. Am J Public Health. 2018;108(10):1418–1420. doi: 10.2105/AJPH.2018.304561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Whitfield THF, Jones SS, Wachman M, Grov C, Parsons JT, Rendina HJ. The Impact of Pre-Exposure Prophylaxis (PrEP) Use on Sexual Anxiety, Satisfaction, and Esteem Among Gay and Bisexual Men. J Sex Res. 2019:1–8. [DOI] [PMC free article] [PubMed]
- 9.Mabire X, Puppo C, Morel S, et al. Pleasure and PrEP: Pleasure-Seeking Plays a Role in Prevention Choices and Could Lead to PrEP Initiation. Am J Mens Health. 2019;13(1). [DOI] [PMC free article] [PubMed]
- 10.Calabrese SK, Earnshaw VA, Underhill K, Hansen NB, Dovidio JF. The impact of patient race on clinical decisions related to prescribing HIV pre-exposure prophylaxis (PrEP): assumptions about sexual risk compensation and implications for access. Aids Behav. 2014;18(2):226–240. doi: 10.1007/s10461-013-0675-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Calabrese SK, Krakower DS, Mayer KH. Integrating HIV Preexposure Prophylaxis (PrEP) Into Routine Preventive Health Care to Avoid Exacerbating Disparities. Am J Public Health. 2017;107(12):1883–1889. doi: 10.2105/AJPH.2017.304061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Siegler AJ, Mouhanna F, Giler RM, et al. The prevalence of pre-exposure prophylaxis use and the pre-exposure prophylaxis-to-need ratio in the fourth quarter of 2017, United States. Ann Epidemiol. 2018;28(12):841–849. doi: 10.1016/j.annepidem.2018.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Finlayson T, Cha SS, Xia M, et al. Changes in HIV Preexposure Prophylaxis Awareness and Use Among Men Who Have Sex with Men-20 Urban Areas, 2014 and 2017. Mmwr-Morbid Mortal W. 2019;68(27):597–603. doi: 10.15585/mmwr.mm6827a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang YLA, Zhu WM, Smith DK, Harris N, Hoover KW. HIV Preexposure Prophylaxis, by Race and Ethnicity - United States, 2014-2016. Mmwr-Morbid Mortal W. 2018;67(41):1147–1150. doi: 10.15585/mmwr.mm6741a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Auerbach JD, Hoppe TA. Beyond "getting drugs into bodies": social science perspectives on pre-exposure prophylaxis for HIV. J Int Aids Soc. 2015;18(4 Suppl 3):19983. doi: 10.7448/IAS.18.4.19983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zimmermann HM, Eekman SW, Achterbergh RC, et al. Motives for choosing, switching and stopping daily or event-driven pre-exposure prophylaxis - a qualitative analysis. J Int Aids Soc. 2019;22(10):e25389. doi: 10.1002/jia2.25389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Durand-Zaleski I, Mutuon P, Charreau I, et al. Costs and benefits of on-demand HIV preexposure prophylaxis in MSM. Aids. 2018;32(1):95–102. doi: 10.1097/QAD.0000000000001658. [DOI] [PubMed] [Google Scholar]
- 18.Liegeon G, Antoni G, Pialoux G, et al. Changes in kidney function among MSM initiating on-demand TDF/FTC for HIV PrEP (Abstract #960). Paper presented at: Conference on Retrovirusis and Opportunistic Infections2019; Seattle, WA.
- 19.Rodriguez-Novoa S, Labarga P, D'Avolio A, et al. Impairment in kidney tubular function in patients receiving tenofovir is associated with higher tenofovir plasma concentrations. Aids. 2010;24(7):1064–1066. doi: 10.1097/QAD.0b013e32833202e2. [DOI] [PubMed] [Google Scholar]
- 20.Anderson PL, Garcia-Lerma JG, Heneine W. Nondaily preexposure prophylaxis for HIV prevention. Current opinion in HIV and AIDS. 2016;11(1):94–101. doi: 10.1097/COH.0000000000000213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Volk JE, Liu A, Vittinghoff E, et al. Sexual Frequency and Planning Among At-Risk Men Who Have Sex With Men in the United States: Implications for Event-Based Intermittent Pre-Exposure Prophylaxis. Jaids-J Acq Imm Def. 2012;61(1):112–115. doi: 10.1097/QAI.0b013e31825bd87d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kibengo FM, Ruzagira E, Katende D, et al. Safety, Adherence and Acceptability of Intermittent Tenofovir/Emtricitabine as HIV Pre-Exposure Prophylaxis (PrEP) among HIV-Uninfected Ugandan Volunteers Living in HIV-Serodiscordant Relationships: A Randomized, Clinical Trial. Plos One. 2013;8(9). [DOI] [PMC free article] [PubMed]
- 23.Hiransuthikul A., Himmad K., Kerr S, et al. Drug-drug interactions between the use of feminizing hormone therapy and pre-exposure prophylaxis among transgender women: The iFACT study. Paper presented at: AIDS 20182018; Amsterdam, The Netherlands. [DOI] [PMC free article] [PubMed]
- 24.Fung J, Lai CL, Yuen MF. Management of chronic hepatitis B in severe liver disease. World J Gastroenterol. 2014;20(43):16053–16061. doi: 10.3748/wjg.v20.i43.16053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Di Ciaccio M, Protiere C, Castro DR, et al. The ANRS-Ipergay trial, an opportunity to use qualitative research to understand the perception of the "participant"-physician relationship. Aids Care. 2018;30:41–47. doi: 10.1080/09540121.2018.1468013. [DOI] [PubMed] [Google Scholar]