INTRODUCTION
Hepatitis C virus antibody (HCV Ab) testing of patients born between 1945 and 1965 has dramatically improved HCV diagnosis in primary care.1 HCV Ab tests are highly sensitive, widely available, and low cost, but require confirmation with HCV RNA when positive. HCV Ab testing is the most cost-effective strategy for screening birth cohort (1945–1965) patients given their anti-HCV prevalence of 3.25%.2 Abnormal alanine and aspartate aminotransferases (ALT and AST) are another indication for HCV testing, but with higher anti-HCV prevalence, the most cost-effective strategy for testing patients with these lab findings is not clear.3
To evaluate costs associated with different HCV testing strategies, we modeled (1) reflex testing, a positive HCV Ab immediately followed by confirmatory RNA test without a second blood draw; compared with (2) direct HCV RNA testing for all patients with elevated ALT and AST.4 We hypothesized that a direct HCV RNA testing strategy would be more efficient and cost-effective for diagnosing HCV in patients with abnormal transaminases.
METHODS
Using EHR data from a PCMH, we identified all patients with HCV testing results (Ab or RNA) between 2007 and 2018. Patients were the unit of observation, only included once in the sample, and categorized into 2 groups: (1) 1945–1965 birth cohort and (2) elevated transaminases. Patients were allocated to the elevated transaminase group if they possessed at least one elevated ALT (> 45 U/L) or AST (> 34 U/L) result during the study period. We compared testing strategies by cost per patient tested and cost per HCV diagnosis for patients with elevated transaminases and HCV Ab results. Not knowing the RNA status for those patients without follow-up testing, we presumed a similar proportion of positive RNA tests to those with confirmation (75.7% in the birth cohort, 80.0% in the elevated transaminase sample).
We also projected the costs of both strategies for PCMH birth cohort patients and those with elevated transaminases not already tested for HCV. We assumed an anti-HCV prevalence of 3.25% in the untested birth cohort, and considered prevalence estimates of 12%, 8.4%, and 5.5% in the untested patients with elevated transaminases, based on estimates from previously published data.2, 3 Median charges from the 2019 Clinical Laboratory Fee Schedule from the Centers for Medicare and Medicaid Services were used to measure costs.5 This study was approved by MUSC’s IRB.
RESULTS
Of the 36,841 patients visiting the PCMH between 2007 and 2018, 10,973 (29.8%) patients had elevated aminotransferases. Of these, 4873 (44.4%) had HCV Ab tests and 585 (12.0% of those tested) had positive results.
Using the HCV testing results previously obtained, and assuming the completion of confirmatory testing, HCV Ab with reflex RNA testing would cost less overall, less per patient tested, and less per HCV diagnosis (Table 1). When the testing strategies were applied to unassessed PCMH groups, HCV Ab with reflex RNA testing led to lower overall cost, cost per patient tested, and cost per diagnosis.
Table 1.
Costs of HCV Testing Strategies by Patient Group and Anti-HCV Prevalence
| Retrospective | Projected | ||||
|---|---|---|---|---|---|
| Elevated transaminases (HCV Ab + 12.0%) | Birth cohort (HCV Ab + 3.25%) | Elevated transaminases (HCV Ab + 12.0%) | Elevated transaminases (HCV Ab + 8.4%)3 | Elevated transaminases Trans (5.5%)* |
|
| n = 4873 | n = 8698 | n = 6100 | n = 6100 | n = 6100 | |
| HCV Ab with reflex RNA | |||||
| Total cost† | $100,146.68 | $151,851.64 | $125,352.56 | $117,594.09 | $111,344.22 |
| Cost/pt tested | $20.55 | $17.46 | $20.55 | $19.28 | $18.25 |
| Cost/diagnosis‡ | $214.45 | $709.61** | $214.09*** | $286.87*** | $414.84*** |
| Direct RNA testing | |||||
| Total cost | $172,163.09 | $307,300.34 | $215,513.00 | $215,513.00 | $215,513.00 |
| Cost/pt tested | $35.33 | $35.33 | $35.33 | $35.33 | $35.33 |
| Cost/diagnosis‡ | $368.66 | $1436.03 | $368.02 | $525.74 | $802.95 |
HCV Ab testing was $15.85 (CPT 86803) and HCV RNA testing was $31.22 (CPT 87520).5 Specimen collection tubes cost $0.46 and $4.11 for the HCV Ab and RNA tests respectively (https://www.fishersci.com). Phlebotomy costs were assumed equal. Total cost for HCV Ab negative patients was $16.31, $51.64 for HCV Ab positive patients (Ab + RNA), and $35.33 for patients with direct RNA testing
*This prevalence that would be expected in the remaining, untested individuals if we assume an overall anti-HCV prevalence of 8.4% for all patients with elevated aminotransferases and account for the 585 already testing positive
†Cost = 16.31n +35.33(anti-HCV prevalence × n)
‡= 76 pts with missing RNA. Assuming an additional 0.8 × 76 = 60 HCV RNA diagnoses. Total HCV diagnosis now 467
**Assuming 75.7% of HCV Ab–positive birth cohort patients were HCV RNA positive (8698 × .0325 × 0.757)
***Assuming 80.0% of HCV Ab–positive patients with HCV RNA testing were HCV RNA positive (6100 × prevalence × 0.8)
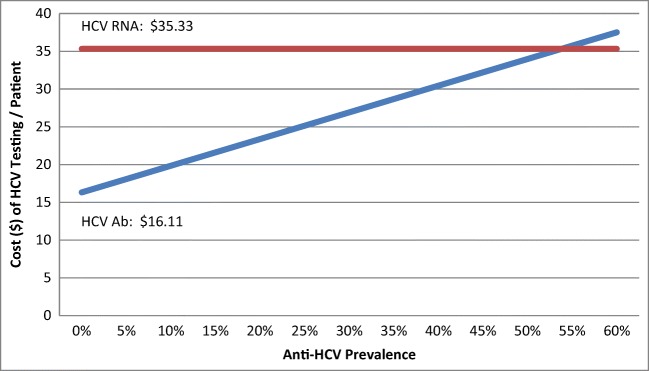
With costs of testing remaining the same, it would require a patient sample prevalence of 53.8% for direct RNA testing to equal the cost of HCV Ab with reflex RNA (Fig. 1).
Figure 1.
HCV testing cost per patient by anti-HCV prevalence for (1) Ab with reflex RNA and (2) direct RNA strategies. Figure 1 contains poor-quality text inside the artwork. Please do not re-use the file that we have rejected or attempt to increase its resolution and re-save. It is originally poor; therefore, increasing the resolution will not solve the quality problem. We suggest that you provide us the original format. We prefer replacement figures containing vector/editable objects rather than embedded images. Preferred file formats are .eps,. ai, .tiff, and .pdf.Sorry about that. I have attached the original figure, created in word (directly in the word document), as well as the figure taken directly from word in .pdf form.
DISCUSSION
HCV Ab testing with reflex RNA was more cost-effective than direct RNA testing for all anti-HCV prevalence projections in PCMH patients with abnormal transaminases. With current cost estimates, direct RNA testing would be more costly in most settings with the possible exceptions of hepatology and needle exchange clinics. This study does not incorporate the psychological harm possibly associated with falsely positive HCV Ab tests, a consideration deserving attention in future work. Analyzing the costs of different testing strategies is necessary as HCV testing expands beyond the birth cohort in primary care.
Author Contribution
All authors made significant contributions to the completed manuscript.
Funding
This work is financially supported by a K23 Award from the National Institute of Diabetes and Digestive and Kidney Diseases (NIH/NIDDK), 1K23DK118200-01 (PI: Schreiner).
Compliance with Ethical Standards
This study was approved by MUSC’s IRB.
Conflict of Interest
The authors declare that they do not have a conflict of interest.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Barocas JA, Wang J, White LF, et al. Hepatitis C Testing Increased Among Baby Boomers Following The 2012 Change To CDC Testing Recommendations. Health Aff (Millwood). 2017;36(12):2142–2150. doi: 10.1377/hlthaff.2017.0684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Smith BD, Morgan RL, Beckett GA, Falck-Ytter Y, Holtzman D, Ward JW. Hepatitis C virus testing of persons born during 1945-1965: recommendations from the Centers for Disease Control and Prevention. Ann Intern Med. 2012;157(11):817–822. doi: 10.7326/0003-4819-157-9-201211060-00529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Smith BD, Yartel AK. Comparison of hepatitis C virus testing strategies: birth cohort versus elevated alanine aminotransferase levels. Am J Prev Med. 2014;47(3):233–241. doi: 10.1016/j.amepre.2014.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chapko MK, Dufour DR, Hatia RI, Drobeniuc J, Ward JW, Teo CG. Cost-effectiveness of strategies for testing current hepatitis C virus infection. Hepatology (Baltimore, Md) 2015;62(5):1396–1404. doi: 10.1002/hep.27966. [DOI] [PubMed] [Google Scholar]
- 5.Services CfMM. CY 2019 Q1 Release: Revised for January 2019. 2019.