Abstract
Purpose of the Review
Osteochondritis dissecans (OCD) is a pathologic condition of subchondral bone most frequently occurring in the medial femoral condyle of the knee in children and adolescents. Salvage techniques are necessary when either nonoperative or typical operative treatments fail, or the OCD presents in an unsalvageable state. The purpose of this review is to describe the evaluation and management of failed OCDs.
Recent Findings
Thorough preoperative planning is essential to the treatment of failed OCDs. Radiographs and advanced imaging such as MRI and CT allow for a detailed assessment of subchondral bone and cartilage. Long-leg alignment radiographs are critical to assess for malalignment which may increase the contact forces on the affected condyle. Malalignment can be corrected with hemiepiphysiodesis or an osteotomy depending on the skeletal maturity of the patient. Osteochondral allografts and autologous chondrocyte implantation treat the defect in both bone and cartilage or solely cartilage and have good short to moderate term outcomes, particularly as compared to the inferior outcomes of microfracture of larger OCDs.
Summary
Osteochondritis dissecans of the knee that fails to heal with initial operative measures can result in a large defect of bone and cartilage in the knee of adolescents. Treatment of the bone and cartilage defect can be accomplished with either osteochondral allograft transplantation or matrix-assisted autologous chondrocyte implantation can be performed with good outcomes. Assessment and correction of lower extremity malalignment is a critical component of treatment. Durable long-term solutions are necessary for the treatment of these difficult lesions.
Keywords: Osteochondritis dissecans, Cartilage, Osteochondral allograft, Autologous chondrocyte implantation
Introduction
Osteochondritis dissecans (OCD) of the knee, most commonly affecting the lateral aspect of the medial femoral condyle, is a pathologic condition of subchondral bone [1, 2]. The incidence of OCD of the knee is 9 to 29 cases per 100,000 populations, occurring more frequently in boys than girls at a rate of 4:1 [1].The ultimate goal of treatment of OCD is to preserve native cartilage and bone. In skeletally immature children, nonoperative treatment with limited weight-bearing, immobilization, and activity restrictions can be successful in 50–67% of cases [3, 4]. The standard treatment of OCD of the knee with intact cartilage that has either failed nonoperative treatment or has risk factors for failure is drilling, with or without fixation [5]. This can be performed in trans-articular or retro-articular fashion. In a systematic review of twelve studies, including 205 OCD lesions, both retro-articular drilling and trans-articular drilling were associated with favorable healing rates, 86 and 91%, respectively [6]. Internal fixation of the OCD with a large progeny fragment can be added to drilling, with highly variable success rates reported in the literature, ranging from 67–100% [7–12]. Multiple fixation methods, ranging from metal screws to bioabsorbable implants have been described. A systematic review of 13 small studies showed no significant differences in outcomes between fixation types [13].
Salvage techniques are necessary when an OCD lesion fails to heal with appropriate treatment (Fig. 1) or that presents in an unsalvageable state with fragmentation of the progeny fragment, a loose body, or with extensive cartilage deterioration. Salvage techniques include debridement, microfracture, osteochondral grafting, or autologous chondrocyte implantation.
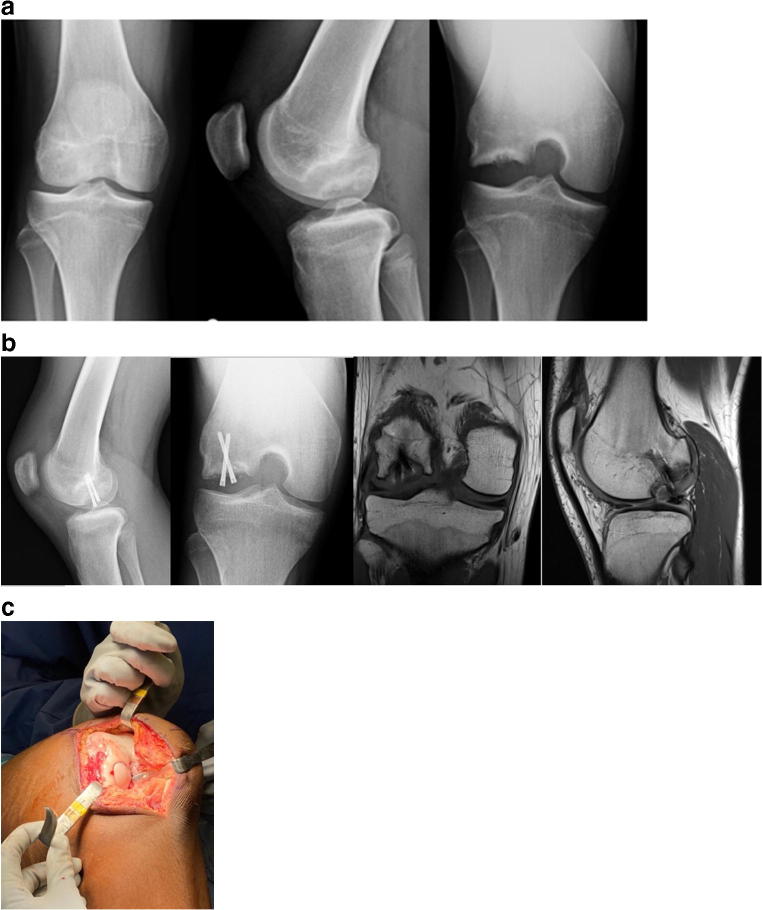
Fig. 1.
15-year-old male with OCD of the lateral femoral condyle (a) initially treated with curretage, grafting, and fixation. He had incomplete healing on MRI, radiographs (b) and arthroscopy at 5 months post-op. He later underwent osteochondral allograft transplantation as a salvage surgery 6 months after his initial surgery (c)
Preoperative Planning
Preoperative planning requires a complete imaging portfolio for each patient. AP, lateral, tunnel, and sunrise view radiographs should routinely be obtained in all patients with a known OCD. Lesions located in the femoral condyle are more posterior and typically seen better on the tunnel view than the AP view (Fig. 2). Full-length lower extremity alignment radiographs are used to assess standing limb alignment. Genu valgum in the setting of a lateral femoral condyle OCD or genu varum in the setting of a medial femoral condyle OCD should be identified and treated. MRI is most commonly utilized at the time of OCD diagnosis but is also beneficial for monitoring OCD healing and/or progression. This modality allows for a detailed assessment of both cartilage and subchondral bone. Although MRI has nearly 100% diagnostic sensitivity for OCD [14], one limitation of this imaging modality is its low specificity for diagnosing fragment instability [15, 16]. New techniques, such as T1rho sequences, have been described to detect early changes in cartilage integrity in osteoarthritis models and may have future applications in OCD [17, 18]. Lastly, computerized tomography (CT) can be selectively used for quantifying healing after initial surgery. This may be particularly beneficial in patients with prior fixation, in whom the utility of MRI may be limited secondary to artifact created by indwelling implants such as headless compression screws. In patients who have had prior surgery, surgeons must consider factors which may have contributed to failed initial treatment, which may include coronal malalignment, unrecognized fragment instability, or patient noncompliance with activity restrictions.
Fig. 2.
AP, lateral, and notch radiographs of the right knee of a 15-year-old male with a lateral femoral condyle OCD
Treatment Algorithm
Coronal Alignment
In children and adolescents with open physes and growth remaining, guided growth of the distal femur and/or proximal tibia can be performed to obtain gradual correction. A careful assessment of bone age, using the Greulich and Pyle Atlas [19] or shorthand bone age assessment [20] is performed to ensure the patient has adequate growth remaining to enable successful deformity correction. Correction of 0.5–1 degree per month [21] or 5 degrees per remaining year in the tibia and 7 degrees per remaining year in the femur [22] can be anticipated.
Guided growth, described with physeal stapling by Blount in 1949 [23], is more commonly now performed with hemiepiphyseal screws [24–26] or plates [27]. The Heuter and Volkmann principles of mechanical manipulation of bone explain the mechanics of guided growth, with increased and asymmetric pressure parallel to the axis of the epiphysis resulting in asymmetrical growth [28]. Multiple studies have assessed the effectiveness of hemiepiphyseal screws and plates and found no to small differences in the rate of correction [29] or clinical outcomes [30••].
In skeletally mature adolescents with coronal malalignment and an OCD requiring salvage treatment, alignment correction can be corrected with a distal femoral or proximal tibial osteotomy, with the goal of unloading the affected compartment and protecting the salvage surgery. The authors’ ideal correction of the mechanical alignment is to the base of the medial tibial spine for a lateral femoral condyle OCD (Fig. 3) and to the base of the lateral tibial spine for a medial femoral condyle OCD. This corresponds to the Fujisawa point, which is 62.5% of the medial-lateral width of the knee, which effectively unloads the affected compartment [31]. Osteotomy is typically performed at the time of final articular cartilage restoration such as osteochondral allograft transplantation or autologous chondrocyte implantation to limit the patient to one instead of two periods of prolonged altered weight-bearing.
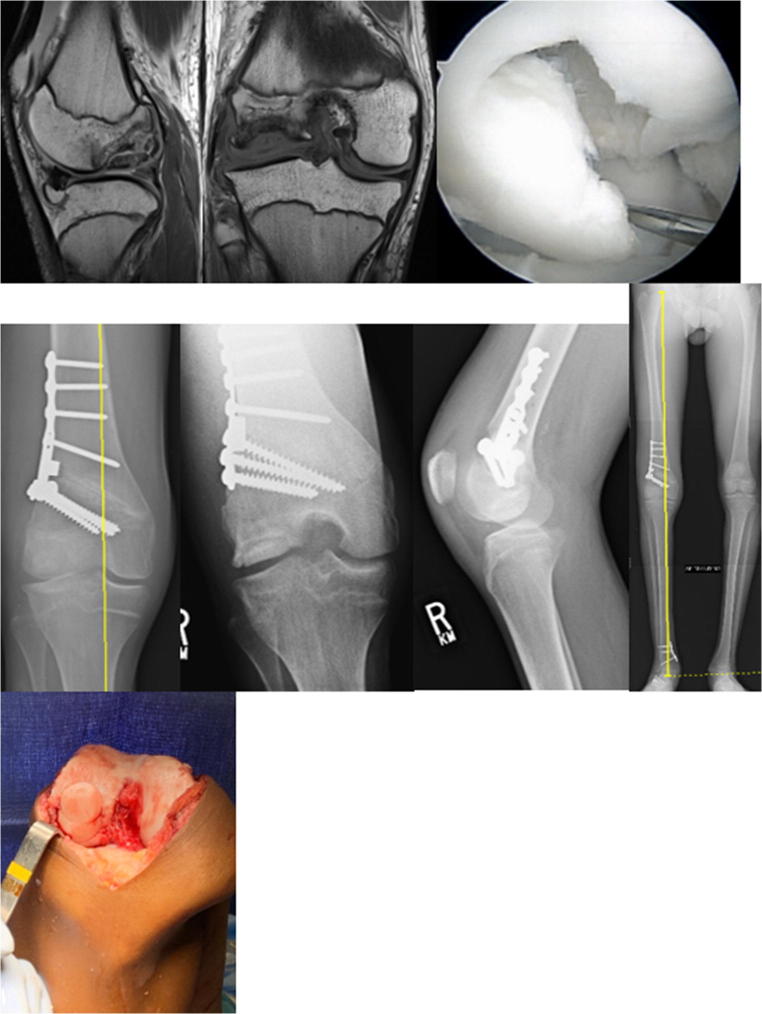
Fig. 3.
16-year-old male with unsalvageable lateral femoral condyle OCD and genu valgum treated with osteochondral allograft transplantation and distal femoral osteotomy
Debridement and Microfracture
Debridement alone is associated with poor long-term outcomes with degenerative radiographic changes and inferior outcome scores although early follow-up shows improvement in 1–2 years but gradual deterioration and increasing symptoms in mid-term follow-up and beyond [32–34]. Marrow stimulation, in the form of microfracture produces fibrocartilage. The decreased durability of fibrocartilage is likely secondary to the relative deficiency of type 2 collagen which predominates in hyaline cartilage. In a prospective randomized controlled trial of debridement and microfracture versus osteochondral autograft for juvenile OCD, similar clinical outcomes were present at 1 year, but microfracture outcomes were far inferior at 4 years, with 41% failure [35]. Recent studies suggest improved outcomes associated with biologic augmentation of microfracture with injectable or scaffold adjuvants [36], but larger long-term outcome studies among pediatric patients are necessary to determine if improved outcomes are sustainable. Although debridement and microfracture may be appropriate for very small OCDs or to address small articular cartilage defects in an OCD which has otherwise healed throughout, we do not recommend this as a standard treatment for the failed OCD.
Osteochondral Transplantation
Osteochondral grafts address both bone and cartilage deficiency in a single graft, which make them attractive for the treatment of osteochondritis dissecans. Autograft transfer using multiple small grafts in a mosaic technique has been described with good outcomes [35]. The noncritical areas available for autograft are limited, therefore, limiting their utility in large OCDs. Additionally, the mosaic technique depends on fibrocartilage fill between plugs, potentially limiting their durability and creating an incongruent articular surface. The use of osteochondral autografts on OCDs of 6 cm2 or larger is associated with poor outcomes [37, 38]. The authors do not utilize more than a single 10 mm osteochondral autograft given the availability and outcomes of osteochondral allograft transplantation and autologous chondrocyte implantation techniques.
Osteochondral allograft transplantation allows for the transfer of larger cores of bone and cartilage from a matching portion of the donor knee, therefore, resulting in a congruent articular surface. These fresh allografts contain viable hyaline cartilage and bone. Ultimately, the donor bone is replaced with host bone through creeping substitution, and the donor chondrocytes remain viable [39]. Unlike solid organ transplants, bone and cartilage transplants are immunoprivileged, requiring no blood or leukocyte antigen matching and producing no host immune response [40]. The grafts are recovered within 24 h of donor expiration, held for 14 days for testing, and then released for implantation, once screening tests are confirmed as negative, no later than 28 days following procurement [41].
Donor grafts are matched based on radiographic, CT, or MRI measurements depending upon the graft supplier. Figure 3 demonstrates a donor hemicondyle that has been matched to the recipient for osteochondral allograft transplantation. An arthrotomy is performed, and the OCD visualized and the appropriately sized allograft are determined. A guide pin is placed perpendicular to the recipient articular surface, the cartilage margin is scored using a size matched tool, and then a reamer is used to debride the selected area of bone and cartilage. Reaming is performed to a depth of bleeding subchondral bone, with ideal bone thickness of 6–9 mm. Donor plug bone thickness less than 5 mm has been associated with an increased odds ratio of cystic changes, while thickness greater than 9 mm is associated with residual osseous clefts, assign of incomplete osseous incorporation [42••]. Next, the donor hemicondyle is assessed to select the area which most closely matches the contour, and a plug of corresponding size is harvested, again ensuring the dowel is reamed perpendicular to the articular surface. Recipient site depths are measured in each quadrant, and the donor graft is likewise marked with these same dimensions and trimmed to appropriate depth. Pulse lavage of the donor dowel has classically been described as a means of removing residual donor marrow elements, although the most recent evidence suggests this is no more effective than no lavage [43••]. The donor graft is then inserted into the recipient site with matching orientation and a press fit. Great care should be taken to dilate the recipient site as forceful insertion of the graft may decrease chondrocyte viability. A moist sponge applied over the graft if light impaction is needed helps dissipate forces on the graft. In large OCDs, additional grafts may be added in a “snowman technique” of interdigitating grafts (Fig. 4) [44].
Fig. 4.
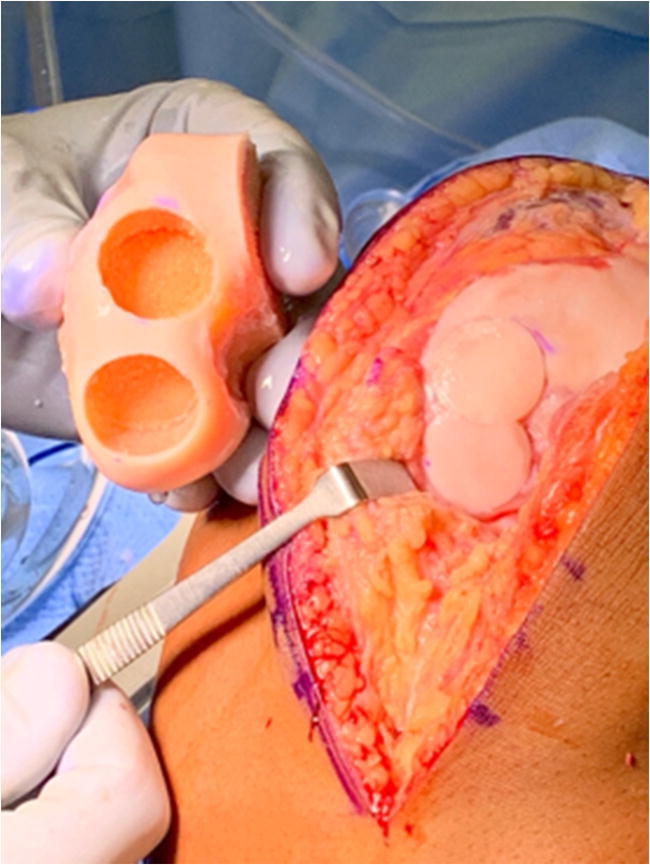
Donor hemicondyle and recipient following osteochondral allograft transplantion to the lateral femoral condyle using a “snowman” technique to interdigitate allograft dowels
The results of osteochondral allograft transplantation for post-traumatic osteochondral defects have been generally favorable [45, 46]. At a mean of 6 years, 135 patients 12–55 years of age with a mean allograft size of 7 cm2 had a survivorship of 95% at 5 years and 93% at 10 years. Ninety-five percent of patients reported being satisfied with the outcome of their procedure [46]. Outcomes specific to pediatric and adolescent patients were published by Bugbee et al. on 2014 [47]. Thirty-nine patients with a mean age of 16.4 years were treated with an osteochondral allograft (mean size 8.5 cm2) for OCD (61%), avascular necrosis (16%), and traumatic chondral injury (14%). Graft survivorship at 10 years was 90%, and of the surviving knees, 89% rated their knees as extremely satisfied or satisfied [47].
Autologous Chondrocyte Implantation
The indication for autologous chondrocyte implantation (ACI) has been contained, irreparable lesions 2–16 cm2 from osteochondritis dissecans or osteochondral injury. ACI requires two surgeries; the first of requires harvesting of a cartilage biopsy, and the second being implantation. ACI is approved for treatment of cartilage defects with bone loss of 6 mm or less. If the OCD is associated with bone loss greater than 6 mm, then bone grafting of the defect is performed in the first stage. Similar to osteochondral transplantation, an arthrotomy is performed, and the OCD is visualized. Debridement of the lesion of all nonviable cartilage and bone is completed, with careful attention to create vertical walls. Classically, ACI required that the chondrocytes be implanted free-floating deep to a periosteal patch which was sewn to the bordering intact articular cartilage. More recently, this technique has evolved to autologous cultured chondrocytes impregnated into a porcine collagen membrane (MACI), simplifying the implantation process. A template of the defect is created, the MACI implant is then trimmed using the created template, a thin layer of fibrin glue is placed in the base of the defect, the MACI implant is applied with the cell-side facing down, and then an additional layer of fibrin glue is applied. It is critical with MACI technique that the chondrocyte laden membrane fits within the defect in contrast to ACI technique in which the patch is sewn flush with the intact cartilage border.
A prospective randomized study of ACI versus MACI found similar clinical, arthroscopic, and histological outcomes [48]. The results of MACI as compared to microfracture are far superior in the treatment of symptomatic isolated chondral defects 3–10 cm2 in size with respect to Tegner, Lysholm, KOOS, and ICRS scores [49, 50••]. Further research, including long-term outcomes and studies specific to the pediatric and adolescent population treated with MACI for OCD of the knee, will be necessary.
Rehabilitation
Both osteochondral allograft transplantation and autologous chondrocyte implantation require protected weight-bearing for a total of 8 weeks. Protective knee bracing is also utilized the first 6 weeks. Range of motion is advanced as tolerated for osteochondral grafts, while ROM is restricted to 0–90 degrees for the first weeks following MACI. Serial radiographs are used to monitor incorporation of osteochondral allografts. Postoperative CT or MRI are not routinely obtained, but reserved for patients whose radiographs do not show complete healing or those with persistent pain or effusion. Full return to regular activities is approximately 6 months.
Conclusions
Osteochondritis dissecans of the knee that fails to heal with initial operative measures can result in a large defect of bone and cartilage in the knee of adolescents. Durable long-term solutions are necessary for the treatment of these difficult lesions. Thoughtful preoperative planning with the assessment of coronal alignment and bone and cartilage deficiency are necessary. Correction of coronal alignment, by means of guided growth or osteotomy, should be an essential component of the treatment. Treatment of the combined bone and cartilage defect can be accomplished with either osteochondral allograft transplantation or matrix-assisted autologous chondrocyte implantation with good outcomes.
Compliance with Ethical Standards
Conflict of Interest
Crystal A. Perkins declares that she has no conflict of interest pertaining to this chapter.
S. Clifton Willimon declares that he has no conflict of interest pertaining to this chapter.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Footnotes
This article is part of the Topical Collection on Pediatric Orthopedics
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Papers of particular interest, published recently, have been highlighted as: •• Of major importance
- 1.Kessler JI, Nikizad H, Shea KG, Jacobs JC Jr, Bebchuk JD, Weiss JM. The demographics and epidemiology of osteochondritis dissecans of the knee in children and adolescents. Am J Sports Med. 2014;42(2):320–326. doi: 10.1177/0363546513510390. [DOI] [PubMed] [Google Scholar]
- 2.Hefti F, Beguiristain J, Krauspe R, Möller-Madsen B, Riccio V, Tschauner C, Wetzel R, Zeller R. Osteochondritis dissecans: a multicenter study of the European pediatric orthopedic society. J Pediatr Orthop B. 1999;8(4):231–245. [PubMed] [Google Scholar]
- 3.Cahill BR, Phillips MR, Navarro R. The results of conservative management of juvenile osteochondritis dissecans using joint scintigraphy. A prospective study. Am J Sports Med. 1989;17(5):601–5. doi: 10.1177/036354658901700502. [DOI] [PubMed] [Google Scholar]
- 4.Krause M, Hapfelmeier A, Möller M, Amling M, Bohndorf K, Meenen NM. Healing predictors of stable juvenile osteochondritis dissecans knee lesions after 6 and 12 months of nonoperative treatment. Am J Sports Med. 2013;41(10):2384–2391. doi: 10.1177/0363546513496049. [DOI] [PubMed] [Google Scholar]
- 5.Chambers HG, Shea KG, Anderson AF, Jojo Brunelle TJ, Carey JL, Ganley TJ, et al. American Academy of Orthopaedic surgeons clinical practice guideline on: the diagnosis and treatment of osteochondritis dissecans. J Bone Joint Surg Am. 2012;94(14):1322–4. [DOI] [PubMed]
- 6.Gunton MJ, Carey JL, Shaw CR, Murnaghan ML. Drilling juvenile osteochondritis dissecans: retro- or transarticular? Clin Orthop Relat Res. 2013;471(4):1144–1151. doi: 10.1007/s11999-011-2237-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Adachi N, Deie M, Nakamae A, Okuhara A, Kamei G, Ochi M. Functional and radiographic outcomes of unstable juvenile osteochondritis dissecans of the knee treated with lesion fixation using bioabsorbable pins. J Pediatr Orthop. 2015;35(1):82–88. doi: 10.1097/BPO.0000000000000226. [DOI] [PubMed] [Google Scholar]
- 8.Chun KC, Kim KM, Jeong KJ, Lee YC, Kim JW, Chun CH. Arthroscopic bioabsorbable screw fixation of unstable Osteochondritis Dissecans in adolescents: clinical results, magnetic resonance imaging, and second-look arthroscopic findings. Clin Orthop Surg. 2016;8(1):57–64. doi: 10.4055/cios.2016.8.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Camathias C, Gögüs U, Hirschmann MT, Rutz E, Brunner R, Haeni D, Vavken P. Implant failure after biodegradable screw fixation in osteochondritis dissecans of the knee in skeletally immature patients. Arthroscopy. 2015;31(3):410–415. doi: 10.1016/j.arthro.2014.08.032. [DOI] [PubMed] [Google Scholar]
- 10.Kocher MS, et al. Internal fixation of juvenile osteochondritis dissecans lesions of the knee. Am J Sports Med. 2007;35(5):712–718. doi: 10.1177/0363546506296608. [DOI] [PubMed] [Google Scholar]
- 11.Tabaddor RR, Banffy MB, Andersen JS, McFeely E, Ogunwole O, Micheli LJ, Kocher MS. Fixation of juvenile osteochondritis dissecans lesions of the knee using poly 96L/4D-lactide copolymer bioabsorbable implants. J Pediatr Orthop. 2010;30(1):14–20. doi: 10.1097/BPO.0b013e3181c6318c. [DOI] [PubMed] [Google Scholar]
- 12.Wu IT, et al. Internal fixation of unstable Osteochondritis Dissecans: do open growth plates improve healing rate? Am J Sports Med. 2018;46(10):2394–2401. doi: 10.1177/0363546518783737. [DOI] [PubMed] [Google Scholar]
- 13.Leland DP, Bernard CD, Camp CL, Nakamura N, Saris DBF, Krych AJ. Does internal fixation for unstable Osteochondritis Dissecans of the skeletally mature knee work? A Systematic Review. Arthroscopy. 2019;35(8):2512–2522. doi: 10.1016/j.arthro.2019.03.020. [DOI] [PubMed] [Google Scholar]
- 14.Quatman CE, Quatman-Yates CC, Schmitt LC, Paterno MV. The clinical utility and diagnostic performance of MRI for identification and classification of knee osteochondritis dissecans. J Bone Joint Surg Am. 2012;94(11):1036–1044. doi: 10.2106/JBJS.K.00275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heywood CS, Benke MT, Brindle K, Fine KM. Correlation of magnetic resonance imaging to arthroscopic findings of stability in juvenile osteochondritis dissecans. Arthroscopy. 2011;27(2):194–199. doi: 10.1016/j.arthro.2010.07.009. [DOI] [PubMed] [Google Scholar]
- 16.Rossbach BP, et al. Discrepancy between morphological findings in juvenile osteochondritis dissecans (OCD): a comparison of magnetic resonance imaging (MRI) and arthroscopy. Knee Surg Sports Traumatol Arthrosc. 2016;24(4):1259–1264. doi: 10.1007/s00167-015-3724-3. [DOI] [PubMed] [Google Scholar]
- 17.Ithurburn MP, et al. Lower patient-reported function at 2 years is associated with elevated knee cartilage T1rho and T2 relaxation times at 5 years in young athletes after ACL reconstruction. Knee Surg Sports Traumatol Arthrosc. 2019;27(8):2643–2652. doi: 10.1007/s00167-018-5291-x. [DOI] [PubMed] [Google Scholar]
- 18.Nozaki T, Kaneko Y, Yu HJ, Kaneshiro K, Schwarzkopf R, Hara T, Yoshioka H. T1rho mapping of entire femoral cartilage using depth- and angle-dependent analysis. Eur Radiol. 2016;26(6):1952–1962. doi: 10.1007/s00330-015-3988-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Greulich WW, Pyle SI. Radiographic atlas of skeletal development of the hand and wrist: Standford University Press; 1959.
- 20.Heyworth BE, Osei DA, Fabricant PD, Schneider R, Doyle SM, Green DW, Widmann RF, Lyman S, Burke SW, Scher DM. The shorthand bone age assessment: a simpler alternative to current methods. J Pediatr Orthop. 2013;33(5):569–574. doi: 10.1097/BPO.0b013e318293e5f2. [DOI] [PubMed] [Google Scholar]
- 21.Ballal MS, Bruce CE, Nayagam S. Correcting genu varum and genu valgum in children by guided growth: temporary hemiepiphysiodesis using tension band plates. J Bone Joint Surg (Br) 2010;92(2):273–276. doi: 10.1302/0301-620X.92B2.22937. [DOI] [PubMed] [Google Scholar]
- 22.Bowen JR, et al. Partial epiphysiodesis at the knee to correct angular deformity. Clin Orthop Relat Res. 1985;198:184–190. [PubMed] [Google Scholar]
- 23.Blount WP, Clarke GR. Control of bone growth by epiphyseal stapling; a preliminary report. J Bone Joint Surg Am. 1949;31A(3):464–478. doi: 10.2106/00004623-194931030-00002. [DOI] [PubMed] [Google Scholar]
- 24.De Brauwer V, Moens P. Temporary hemiepiphysiodesis for idiopathic genua Valga in adolescents: percutaneous transphyseal screws (PETS) versus stapling. J Pediatr Orthop. 2008;28(5):549–554. doi: 10.1097/BPO.0b013e31817baab2. [DOI] [PubMed] [Google Scholar]
- 25.Mesa PA, Yamhure FH. Percutaneous hemi-epiphysiodesis using transphyseal cannulated screws for genu valgum in adolescents. J Child Orthop. 2009;3(5):397–403. doi: 10.1007/s11832-009-0203-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nouth F, Kuo LA. Percutaneous epiphysiodesis using transphyseal screws (PETS): prospective case study and review. J Pediatr Orthop. 2004;24(6):721–725. doi: 10.1097/01241398-200411000-00023. [DOI] [PubMed] [Google Scholar]
- 27.Stevens PM. Guided growth for angular correction: a preliminary series using a tension band plate. J Pediatr Orthop. 2007;27(3):253–259. doi: 10.1097/BPO.0b013e31803433a1. [DOI] [PubMed] [Google Scholar]
- 28.Eastwood DM, Sanghrajka AP. Guided growth: recent advances in a deep-rooted concept. J Bone Joint Surg (Br) 2011;93(1):12–18. doi: 10.1302/0301-620X.93B1.25181. [DOI] [PubMed] [Google Scholar]
- 29.Lykissas MG, Jain VV, Manickam V, Nathan S, Eismann EA, McCarthy J. Guided growth for the treatment of limb length discrepancy: a comparative study of the three most commonly used surgical techniques. J Pediatr Orthop B. 2013;22(4):311–317. doi: 10.1097/BPB.0b013e32836132f0. [DOI] [PubMed] [Google Scholar]
- 30.Funk SS, et al. Hemiepiphysiodesis Implants for Late-onset Tibia Vara: A Comparison of Cost, Surgical Success, and Implant Failure. J Pediatr Orthop. 2016;36(1):29–35. doi: 10.1097/BPO.0000000000000388. [DOI] [PubMed] [Google Scholar]
- 31.Fujisawa Y, Masuhara K, Shiomi S. The effect of high tibial osteotomy on osteoarthritis of the knee. An arthroscopic study of 54 knee joints. Orthop Clin North Am. 1979;10(3):585–608. [PubMed] [Google Scholar]
- 32.Anderson AF, Pagnani MJ. Osteochondritis dissecans of the femoral condyles. Long-term results of excision of the fragment. Am J Sports Med. 1997;25(6):830–834. doi: 10.1177/036354659702500617. [DOI] [PubMed] [Google Scholar]
- 33.Murray JR, Chitnavis J, Dixon P, Hogan NA, Parker G, Parish EN, Cross MJ. Osteochondritis dissecans of the knee; long-term clinical outcome following arthroscopic debridement. Knee. 2007;14(2):94–98. doi: 10.1016/j.knee.2006.11.011. [DOI] [PubMed] [Google Scholar]
- 34.Wright RW, et al. Osteochondritis dissecans of the knee: long-term results of excision of the fragment. Clin Orthop Relat Res. 2004;424:239–243. doi: 10.1097/01.blo.0000128216.10732.d8. [DOI] [PubMed] [Google Scholar]
- 35.Gudas R, Simonaityte R, Cekanauskas E, Tamosiūnas R. A prospective, randomized clinical study of osteochondral autologous transplantation versus microfracture for the treatment of osteochondritis dissecans in the knee joint in children. J Pediatr Orthop. 2009;29(7):741–748. doi: 10.1097/BPO.0b013e3181b8f6c7. [DOI] [PubMed] [Google Scholar]
- 36.Arshi A, et al. Can biologic augmentation improve clinical outcomes following microfracture for symptomatic cartilage defects of the knee? A Systematic Review. Cartilage. 2018;9(2):146–155. doi: 10.1177/1947603517746722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Horas U, Pelinkovic D, Herr G, Aigner T, Schnettler R. Autologous chondrocyte implantation and osteochondral cylinder transplantation in cartilage repair of the knee joint. A prospective, comparative trial. J Bone Joint Surg Am. 2003;85(2):185–192. doi: 10.2106/00004623-200302000-00001. [DOI] [PubMed] [Google Scholar]
- 38.Wang CJ. Treatment of focal articular cartilage lesions of the knee with autogenous osteochondral graftsA 2- to 4-year follow-up study. Arch Orthop Trauma Surg. 2002;122(3):169–172. doi: 10.1007/s004020100343. [DOI] [PubMed] [Google Scholar]
- 39.Williams SK, et al. Analysis of cartilage tissue on a cellular level in fresh osteochondral allograft retrievals. Am J Sports Med. 2007;35(12):2022–2032. doi: 10.1177/0363546507305017. [DOI] [PubMed] [Google Scholar]
- 40.Langer F, Gross AE. Immunogenicity of allograft articular cartilage. J Bone Joint Surg Am. 1974;56(2):297–304. doi: 10.2106/00004623-197456020-00007. [DOI] [PubMed] [Google Scholar]
- 41.American Association of Tissue Banks. Available from: www.aatb.org. Accessed 2 Nov 2019
- 42.Ackermann J, et al. Decreased Graft Thickness Is Associated With Subchondral Cyst Formation After Osteochondral Allograft Transplantation in the Knee. Am J Sports Med. 2019;47(9):2123–2129. doi: 10.1177/0363546519851098. [DOI] [PubMed] [Google Scholar]
- 43.Ambra LF, de Girolamo L, Gomoll AH. Pulse Lavage Fails to Significantly Reduce Bone Marrow Content in Osteochondral Allografts: A Histological and DNA Quantification Study. Am J Sports Med. 2019;47(11):2723–2728. doi: 10.1177/0363546519864716. [DOI] [PubMed] [Google Scholar]
- 44.Cotter EJ, Hannon CP, Christian DR, Wang KC, Lansdown DA, Waterman BR, Frank RM, Cole BJ. Clinical outcomes of multifocal Osteochondral allograft transplantation of the knee: an analysis of overlapping grafts and multifocal lesions. Am J Sports Med. 2018;46(12):2884–2893. doi: 10.1177/0363546518793405. [DOI] [PubMed] [Google Scholar]
- 45.Emmerson BC, Görtz S, Jamali AA, Chung C, Amiel D, Bugbee WD. Fresh osteochondral allografting in the treatment of osteochondritis dissecans of the femoral condyle. Am J Sports Med. 2007;35(6):907–914. doi: 10.1177/0363546507299932. [DOI] [PubMed] [Google Scholar]
- 46.Sadr KN, Pulido PA, McCauley J, Bugbee WD. Osteochondral allograft transplantation in patients with Osteochondritis Dissecans of the knee. Am J Sports Med. 2016;44(11):2870–2875. doi: 10.1177/0363546516657526. [DOI] [PubMed] [Google Scholar]
- 47.Murphy RT, Pennock AT, Bugbee WD. Osteochondral allograft transplantation of the knee in the pediatric and adolescent population. Am J Sports Med. 2014;42(3):635–640. doi: 10.1177/0363546513516747. [DOI] [PubMed] [Google Scholar]
- 48.Bartlett W, et al. Autologous chondrocyte implantation versus matrix-induced autologous chondrocyte implantation for osteochondral defects of the knee: a prospective, randomised study. J Bone Joint Surg (Br) 2005;87(5):640–5. doi: 10.1302/0301-620X.87B5.15905. [DOI] [PubMed] [Google Scholar]
- 49.Basad E, et al. Matrix-induced autologous chondrocyte implantation versus microfracture in the treatment of cartilage defects of the knee: a 2-year randomised study. Knee Surg Sports Traumatol Arthrosc. 2010;18(4):519–527. doi: 10.1007/s00167-009-1028-1. [DOI] [PubMed] [Google Scholar]
- 50.Brittberg M, et al. Matrix-Applied Characterized Autologous Cultured Chondrocytes Versus Microfracture: Five-Year Follow-up of a Prospective Randomized Trial. Am J Sports Med. 2018;46(6):1343–1351. doi: 10.1177/0363546518756976. [DOI] [PubMed] [Google Scholar]