Abstract
Purpose of Review
Orthobiologics, including amniotic products, have been gaining interest in the past decade for the treatment of various orthopedic conditions including osteoarthritis. However, the use of biologics is varied and is currently available with minimal oversight or regulation. This review will assess the current state of research that utilizes amniotic products both in vitro and in vivo.
Recent Findings
Amniotic tissue derivatives have been shown to have positive effects in animal models for a variety of conditions. Clinical trials are limited with mixed outcomes, yet some recent studies suggest the rationale for continued investigation.
Summary
While amniotic products appear promising in numerous animal studies, human clinical trials are still lacking. Future studies are needed to assess whether amniotic products have a role in the treatment of osteoarthritis and other orthopedic pathologies.
Keywords: Cartilage, Osteoarthritis, Amniotic suspension allograft, Amniotic membrane, Amniotic fluid
Introduction
The use of biologics for intra-articular injections has been gaining momentum in the past decade as a possible adjunctive treatment for a myriad of orthopedic conditions. A variety of biologic products have been developed with the goal of altering the cytokine and cellular environment of the joint. Amniotic-derived products, including amniotic membrane and amniotic fluid products, are one subtype of orthobiologic that are being investigated as a potential treatment option through augmentation of joint inflammation and healing. Amniotic membranes (AM) were initially utilized for treatment of skin disorders such as burns, ulcers, and wounds. Their use was first described in 1909, when Davis et al. [1] reported using AM as a biologic dressing for skin defects. The usage of AM has evolved over time and includes a variety of uses for complex tissue regeneration. AM has been used as a treatment for a variety of ophthalmologic conditions such as corneal surface lesions, retinal detachments, and as an agent for limbal stem cell regeneration [2, 3]. Plastic surgeons and wound specialists employ AM for treatment of acute and chronic wounds, while foot and ankle surgeons have noted accelerated healing of diabetic foot ulcers and other slow-healing wounds with AM use [4–7].
Research into amniotic-derived products for tissue regeneration within orthopedics has grown in recent years, including for the treatment of plantar fasciitis, ligament and tendon healing, spinal pathology, cartilage restoration, and osteoarthritis. As of 2018, there were eight commercially available amniotic membrane products that have been studied for treatment of musculoskeletal conditions (Table 1) [8]. This chapter will explore the role of amniotic products in orthopedics from the function of the amniotic membrane in vivo through current clinical studies using this orthobiologic treatment.
Table 1.
Currently available amniotic membrane products
| Product | Manufacturer | Details |
|---|---|---|
| Clarix FLO | Amniox Medical | Umbilical cord and amniotic tissue |
| AmnioFix | MiMedx | Dehydrated human amnion/chorion membrane (dHACM) |
| PX50 | Human Regenerative Technologies | Amnion membrane particulates and products that are cryopreserved |
| PalinGen Flow/ SportFlow | Amnio Technology | Amniotic tissue allografts |
| Allogen | ViVex | Matrix allograft derived from amniotic fluid |
| FloGraft | Applied Biologics | Amniotic tissue allografts |
| NuCel | Organogensis | Cryopreserved, bioactive amniotic suspension allograft |
| Affinity | Organogensis | Fresh amniotic membrane |
Anatomy and Function
The placenta is composed of multiple elements of both fetal and maternal origin. Fetal elements include the amnion, chorion, amniotic fluid, and the umbilical cord; cells from these origins have been investigated for their potential use in regenerative medicine.
The umbilical cord begins to form at 4 weeks and is the main conduit for fetal blood to receive oxygen and to remove byproducts via the maternal circulation. The amnion and chorion come together to form the placenta that encases the amniotic fluid and the fetus. The chorion forms out of cytotrophoblast and syncyotrophoblasts. During the second week of fetal development as the blastocyst begins to implant in the maternal endometrium, the outer layers of the blastocyst form the cytotrophoblast (inner layer) and the syncyotrophoblasts (outer layer). The chorionic villi, which are the main location for gas exchange in utero, are formed by the cytotrophoblasts entering and forming branch-like structures within the syncyotrophoblasts layer, and then the cytotrophoblasts, and eventually mesenchymal tissue, invade and remain in the interior aspect.
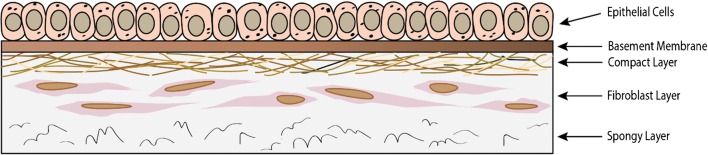
The amnion is the thin inner aspect of the fetal membrane, approximately 0.02 to 0.05 mm thick, and comes into direct contact with the amniotic fluid [2]. It is avascular, aneural, and alymphatic, and receives its nutrients through diffusion. It has three histological layers formed from the trophoblast layer—the epithelial layer, the thick basement membrane, and the avascular mesenchymal tissue—and consists of two cell types: amniotic epithelial cells (AECs) and amniotic mononuclear mesenchymal cells (Fig. 1). The avascular layer lays adjacent to the chorionic layer and can be further subdivided into three components: compact layer, middle fibroblast layer, and a spongy layer. The fibroblast layer contains type I, type III, type V, and type VI collagen, thus increasing the mechanical force this layer can withstand. The outermost spongy layer is named as such due to its characteristic spongy appearance on histology, and due to its high concentration of proteoglycans and glycoproteins. The middle layer of the amniotic membrane is the basement membrane, which serves as a barrier to the amniotic fluid chamber. Its larger thickness and high concentration of type IV, V, and VII collagen along with fibronectin and laminin allow it to perform this function. The inner aspect is the epithelial layer, which is composed of a single cell layer of cuboidal cells with microvilli. These cells function in cell transport and are metabolically active.
Fig. 1.
Structure of the amniotic membrane
The functions of the amniotic membrane include providing a durable membrane for physical protection of the fetus, regulating the pH of the amniotic fluid, and secreting a variety of cell signaling and bioactive molecules. The functions of these molecules range from antimicrobial to anti-inflammatory effects. These molecules include basic fibroblast growth factor (bFGF), epidermal growth factor (EGF), hyaluronic acid (HA), interleukins (IL-1 and IL-10), beta-defensins, transforming growth factor B (TGF-beta), elafin, human leukocyte antigen-G (HLA-G), matrix metalloproteinases (MMPs), tissue inhibitors of metalloproteinases (TIMPs), and platelet-derived growth factor (PDGF) [9, 10]. In addition, amniotic tissues contain anti-inflammatory factors such as IL-1 and IL-10 receptor antagonists and regulators of catabolic enzymes such as TIMPs 1, 2, 3, and 4. Furthermore, it has been shown that AM is a potent downregulator of transforming growth factor beta (TGF-ß) signaling, which stimulates recruitment of fibroblasts and macrophages and upregulates collagen production [11].
Collection and Storage
Amniotic membrane is obtained from donors who undergo an uncomplicated elective cesarean section, as this is an aseptic procedure compared with a vaginal delivery. After collection, the amniotic membrane is stored in aseptic conditions. It is then washed in antibiotics and antifungal agents. It is sectioned and then stored as cryopreserved human amniotic membrane (CHAM) or as dry human amniotic membrane (DHAM) [2]. Differences in preparation allow for DHAM to be stored at room temperature, whereas CHAM must be kept at −80 °C. Both of these methods have been shown to be valid in studies investigating amniotic membrane usage in ophthalmic procedures [12]. In addition, there is an available product that is not dehydrated or frozen (stored at 1 to 10 °C), theoretically providing similar benefits to in vivo AM.
In Vitro Investigations
AM has been studied as a biologic scaffold and as a source for amniotic mesenchymal stem cells for cartilage regeneration in a number of basic science and animal models [13•]. A recent study compared the potential of amniotic and adipose mesenchymal stromal cells to undergo chondrogenic and osteogenic differentiation in cell cultures and produce cartilaginous or mineralized matrix. Topoluk et al. [13•] showed that human amnion contains amniotic epithelial cells and amniotic mesenchymal stromal cells, which both lead to greater type II collagen gene expression and greater production of cartilaginous extracellular matrix than adipose mesenchymal stromal cells.
Cartilage and Bone Studies
The application of AM in injectable form has been tested in preclinical trials in several different animal models. In preliminary studies, this emerging treatment has shown promise in promoting healing of sports-related tendon, ligament, soft tissue, bone, and chondral injury. Wei et al. [14] found that human amniotic mesenchymal cells with a collagen scaffold placed in rat articular cartilage defects underwent morphologic changes and led to deposition of type II collagen after induction of chondrogenesis with BMP-2. In another rat model, Nogami et al. [15] studied the use of a novel human amniotic mesenchymal cell–derived extracellular matrix (ECM)–coated polyactic-co-glycolic acid (PLGA) scaffold to facilitate cartilage repair. ECM-PLGA scaffolds were placed in osteochondral defects in the trochlear groove of rat knees and were examined histologically. The authors found that those treated with this scaffold demonstrated increased type II collagen mRNA expression and induced gradual tissue regeneration that resulted in superior hyaline cartilage repair compared with controls.
In a rabbit model of medial femoral articular defects, researchers found that application of a human acellular amniotic membrane to defects significantly improved histologic grading compared with controls, which included measures of cartilage, surface integrity, and analysis of cell types [16•]. In another study using a rabbit model, authors used human acellular AM to load bone marrow mesenchymal stem cells (BM-MSC) onto focal femoral condyle articular cartilage defects 4 mm in diameter [17]. This group was compared with articular cartilage defects treated with AM alone and controls. At 8 and 12 weeks post-operatively, histologic examination of the rabbits treated with BM-MSCs + AM contained dense cartilage-like cells that stained positive for type II collagen, which were not present in controls or those treated with AM alone. This suggests the need for further research into combining different orthobiologics for maximum clinical benefit.
Recently Tang et al. [18] examined the effect of human amniotic acellular membrane in bone regeneration of femoral defects using a Sprague-Dawley rat model. Human amniotic membranes were collected and processed at the time of delivery. They were then introduced to bone marrow stromal cells (BMSCs), with subsequent analysis demonstrating significant proliferation and osteogenic differentiation of the BMSCs. Bilateral femoral defects were created, with one defect covered with amniotic membrane and the other left uncovered. Specimens were harvested at 15 and 30 days for histologic and PCR analysis. They found that the defects with amniotic membrane augmentation had significantly improved bone regeneration with an associated upregulation in gene expression of C-X-C Chemokine Receptor 4 (CXCR-4), Monocyte Chemoattractant Protein-1 (MCP-1), and Cathepsin K (CatK), all of which induced cell recruitment and bone remodeling.
Clinical trials investigating the effect of amniotic-derived products in patients with articular cartilage defects are lacking. In the only human study identified, Anderson and Swayzee studied use of human amniotic allograft during ankle arthroscopy and microfracture of patients with talar dome osteochondritis dissecans lesions less than 2 cm2 [19]. The study included 101 patients and 54 had augmentation with AM suspension allograft based on surgeon preference; patients were not blinded to their treatment. They found that compared with controls, those treated with AM had greater improvements in VAS-pain scores at 24-month follow-up compared with controls. In addition, the American College of Foot and Ankle Surgeons (ACFAS) scores were higher in the graft group at 3 months (p < 0.001), 12 months (p < 0.001), and 24 months (p < 0.001) post-operative.
Osteoarthritis Studies
Osteoarthritis (OA) results from gradual mechanical degeneration of articular cartilage and is the leading cause of chronic disability in the United States [20–22]. Increased concentrations of several synovial fluid cytokines and pathways including IL-1α, IL-1β, IL-18, TNF-α, and complement have been associated with worsening severity of OA [23, 24]. These cytokines and prostaglandins play a significant role as they increase chondrocyte production of matrix metalloproteinases, which propagate the remodeling process and contribute to cartilage degradation. As our understanding of the pathophysiology of OA expands, there is a greater interest in the therapeutic use of AM due to its cellular properties. AM contains numerous anti-inflammatory and antifibrotic compounds such as interleukin 1 receptor antagonist (IL-1RA) and IL-10 that can interfere in the progression and inhibit the formation of inflammation, attenuating articular cartilage degeneration. In addition, AM promotes the expression of TIMP, an inhibitor of matrix metalloprotease [11]. Matrix metalloproteases are a subtype of endoproteases, which degrade various substrates such as proteoglycans and collagens. Inhibition of this pathway with TIMPs could prevent cartilage degradation. Through all of these properties, AM has the potential to be a treatment option for osteoarthritis.
Willett and colleagues investigated the use of an injectable formulation of dehydrated human amniotic/chorionic membrane (dHACM) for the treatment of OA in rats [25]. The formulation is produced by devitalizing and dehydrating donated placental tissue which retains the epithelial and chorion layers as well as platelet-derived growth factor, fibroblast growth factor, TGF-ß, and TIMPs. Rats underwent medial meniscal transection to induce OA and were injected with dHACM or saline post-operatively. At 21 days, there were no differences in synovial cytokine levels, and most cytokines examined were below the limit of detection. However, equilibrium partitioning of an ionic contrast agent micro-CT (EPIC-μCT), a validated method to evaluate cartilage degeneration, demonstrated that those treated with dHACM showed lower cartilage attenuation, indicating higher proteoglycan content, in the medial tibial plateau compared with controls. Controls also showed statistically significant increase in number of cartilage erosions (mean 2.8 ± 0.2) and lesions (2.4 ± 0.4) compared with the experimental group (1.2 ± 0.37 erosions, no lesions). The authors concluded that intra-articular injection of dHACM may have a therapeutic effect on OA development.
In another study on rats with OA induced through medial meniscus transection, the authors evaluated whether intra-articular injection of particulate amniotic membrane and umbilical cord matrix (AM/UC) could slow progression of OA [26]. Two weeks following meniscectomy, rats received 50 μg/μL AM/UC injection, 100 μg/μL AM/UC injection, or saline injection. EPIC-μCT at 1 week showed overall attenuation of cartilage destruction and increase in cartilage volume and thickness in both groups treated with AM/UC compared with the saline group. This difference persisted for only the higher dose AM/UC group at 4 weeks. In addition, histological analysis using the Osteoarthritis Research Society International (OARSI) histology scores demonstrated similar findings. OARSI scores at 1 week showed significantly less cartilage degeneration, calcified cartilage, and subchondral bone damage for both doses of AM/UC administered compared with controls, whereas only the group receiving the higher dose showed significant differences at 4 weeks.
A recent study on rabbits investigated use of intra-articular injection of lypophilized AM for treatment of OA [27•]. OA was induced by injection of collagenase, and after OA was established, the subjects were injected with the AM formulation on one knee and saline solution on the contralateral knee. Significant differences were noted at 3 and 6 weeks, as those injected with lypophilized AM showed cell clusters without disruption in articular cartilage integrity, while control knees exhibited greater fibrillations, erosions, cracks, and decreased matrix staining based on validated scores for morphological and histological analysis. Unpublished data of treatment with amniotic suspension allograft (ASA) in a rat model of OA (treatment with monosodium iodoacetate [MIA]) showed that ASA treatment for 7 days led to improvement in pain thresholds and decreased swelling and weight bearing aversion compared with saline and triamcinolone injection. In addition, the ASA injection group had elevated IL-10 levels compared with controls.
There are only two human studies published on amniotic membrane use for treatment of osteoarthritis. These were performed using a cryogenically preserved human amniotic suspension allograft containing human amniotic membrane and human amniotic fluid–derived cells [28]. The first was open-label prospective feasibility pilot study and was performed on six patients with Kellgren-Lawrence grades 3 and 4 knee OA with 1 year follow-up. Authors noted no injection site reactions and no significant changes in serum white blood cells, hematocrit, platelets, erythrocyte sedimentation rate, or C-reactive protein. At 1-year follow-up, there was a mean increase in patient-reported outcome measures, including Knee Injury and Osteoarthritis Outcome Score (KOOS) (43.4 to 70.2), International Knee Documentation Committee scale (IKDC) (41.7 to 63.4), and Single Assessment Numeric Evaluation (SANE) score (51.3 to 85.8). However, no statistical analysis was performed due to the limited sample size, no comparison group was available, and follow-up was limited to 1 year. Therefore, no meaningful conclusions can be drawn from these outcome measures.
The second human study was a randomized control study with 200 patients with KL grade 2–3 osteoarthritis who were randomized to saline (n = 68), hyaluronic acid (HA (n = 64)), or ASA) (n = 68) injections. Outcome measures, including patient-reported outcomes and unacceptable pain levels, were evaluated at 3 months and 6 months post-injection. Regarding patient-reported outcome measures, those who underwent ASA injection had significantly larger deltas on VAS, KOOS pain subscore, and KOOS daily living subscore compared with HA at 3 months and HA and saline at 6 months. In addition, at 3 months, those who underwent ASA injection had a lower rate of unacceptable pain (13.2%) compared with HA (68.8%) and saline (75%). This randomized control trial suggests that ASA could be beneficial in the non-operative treatment of osteoarthritis. Future, additional randomized trials are warranted to further our understanding of the clinical use of amniotic tissue on OA [29••].
Tendon Injury and Tendinopathy Studies
Several studies have investigated the use of amniotic membranes as an adjunct in tendon repair. In a rat model of Achilles tendon repair, augmentation of the repair with DHAM showed a 0% re-rupture rate at 28 days compared with 20% in controls. Those augmented with DHAM showed increased cell migration to repair sites and improved tendon fiber organization [30]. Demirkan et al. [31] compared tendon adhesions in a chicken model simulating zone II flexor tendon injuries. Recovery from these injuries is often complicated by peritendinous adhesions which leads to restricted tendon gliding and poor range of motion. They found that application of amniotic membrane to repair sites prevented tendon adhesions to surrounding tissues at 12 weeks compared with sheath excision and repair alone. Similarly, AM decreased severity of peritendonous adhesion formation macroscopically and histologically and resulted in greater active and passive range of motion in a similar chicken model [32]. These differences were more pronounced when augmented with hyaluronic acid to protect against AM breakdown.
Using a Sprague-Dawley rat model, Kueckelhaus et al. [23] investigated the efficacy of amnion-derived cellular cytokine solution (ACCS) developed from amnion-derived multipotent progenitor cells of the placenta in carboxy-methyl cellulose (CMC) gel in healing Achilles tendon ruptures. Tendons were ruptured and repaired in a control group by Kessler suture alone or in an experimental group with ACCS in CMC-augmented Kessler suture repair. Mechanical and immunohistochemical analysis was performed on tendons harvested at 1, 2, 4, 6, and 8 weeks after surgery. They found that at early time points of 2 and 4 weeks, the augmented repairs had greater tensile strength, breaking strength, and yield strength, suggesting a positive effect of ACCS in early healing. However, at 8 weeks after repair, the control groups had significantly improved tensile and load-to-failure strength compared with the ACCS-treated group, bringing into question the long-term benefits of ACCS in CMC augmentation.
In 2013, Lange-Consiglio et al. [24, 33] used a horse model in two trials to assess the effectiveness of expanded equine amniotic membrane–derived mesenchymal stem cells (AMSCs) in horse tendon and ligament injuries. The first trial compared the efficacy of ultrasound-guided injections of AMSCs with BM-MSCs at the time of injury and followed the horses clinically for 2 years. They found that compared with BM-MSCs, AMSC-treated injuries had significantly fewer re-injury rates (4% vs. 23%) and more rapid return to activity 4–5 months vs. 4–12 months). In a subsequent investigation, they again identified improved re-injury and return to activity rates with no adverse events, suggesting efficacy of AMSCs in treating tendon and ligament injury.
In a pilot study of ten patients with various lower extremity injuries (including posterior tibial tendonitis, peroneal tendonitis, anterior tibial tendonitis, foot extensor and plantar musculature injury, and Achilles tendonitis), Lullove et al. [34] tested a commercially available flowable tissue matrix allograft derived from human placental connective tissue (PX50, Human Regenerative Technologies) in a case series of 10 patients. Notably, this investigation did not include a detailed description of the composition of the product. The results demonstrated a benefit of PX50, with eight out of ten participants reporting no pain on visual analog scale at 4 weeks after injection and all patients reporting no pain by 5 weeks. These results are limited by a small number of patients from one single physician, heterogeneity of pathology, and a lack of control group undergoing standard of care as a comparison.
The effect of amniotic-derived products on other tendons has also been investigated. A recent controlled, multicenter trial on zone II flexor tendon outcomes in humans was performed using amniotic membrane as a biologic barrier to encase flexor tendons following primary repair [35]. Results showed significantly increased proximal interphalangeal (PIP) and distal interphalangeal (DIP) range of motion and increased rates of “excellent to good” functional scores in the AM group compared with control (p < 0.01) with a mean of 140.8° versus 123° and 84% versus 43% based on Strickland and Glogovac grading. There were also higher rates of total complications in the control group (p = 0.007), which included re-rupture, erythema, itching, and exudate, which led the authors to conclude that AM is a safe and effective absorbable material that reduces adhesion formation.
Plantar Fasciitis Studies
There are a few clinical trials investigating the effect of amniotic-derived products in the treatment of plantar fascitis. A randomized, controlled, double-blind pilot study was performed by Hanselman and colleagues comparing cryopreserved human amniotic membrane (c-hAM) to corticosteroid injection for the treatment of plantar fasciitis [36]. Patients received an injection at baseline and were offered a repeat injection of the same drug at their first 6-week follow-up. Twenty-three patients were followed for 18-week follow-up, which included VAS pain scale and the Foot Health Status Questionnaire (FHSQ), a validated instrument that evaluates foot pain and function, physical activity, and general health. They found no significant differences between groups in patients receiving one injection, and in patients that received 2 injections, there was greater improvement in FHSQ foot pain scores in patients treated with c-hAM compared with controls (66.3 vs. 32.5, respectively; p = 0.011). However, the corticosteroid group showed significantly better shoe fit (p = 0.024) and general health (p = 0.013) at 6 weeks and better subjective improvement at 12 weeks (p = 0.041) compared with c-hAM.
In another prospective, randomized clinical trial of 45 patients, authors compared saline injection (controls) with 0.5 cc and 1.25 cc mDHACM injections for plantar fasciitis [37]. At 8-week follow-up, American Orthopaedic Foot and Ankle Society (AOFAS) Hindfoot scores increased for those receiving mDHACM (mean improvement in AOFAS Hindfoot score of 12.9 ± 16.9 points for controls versus 51.6 ± 10.1 and 53.3 ± 9.4 for 0.5 cc and 1.25 cc of mDHACM, respectively, both p < 0.001). Sample sizes and follow-up for these studies are limited, and results should be interpreted with caution. However, no adverse outcomes were seen in either study and they present early evidence that amniotic-derived products may be beneficial in treatment of this challenging condition.
Conclusion
Amniotic products have been well explored for a variety of conditions in animal models with many of these studies illustrating superior healing and regenerative effects. Given the differences in formulation and content in amniotic-derived products, it is vital to understand and report these specifics in animal studies and clinical trials. However, clinical trials remain extremely limited for both osteoarthritis and other orthopedic pathologies. Future studies including large, randomized clinical trials are needed to validate the preliminary animal model findings.
Compliance with Ethical Standards
Conflict of Interest
Hailey P. Huddleston, Matthew Cohn, Eric Haunschild, and Stephanie E. Wong declare no conflicts of interest.
Jack Farr reports research support from Active Implants, Arthrex, Inc., Episurf, Fidia Pharma, JRF Ortho, Moximed, Inc., Novartis, Inc., Organogenesis, Samumed, Vericel, and ZimmerBiomet; paid consultant for Aesculap/B.Braun, Arthrex, Inc., Cartiheal, Cook Biotech, Inc., Exactech, Moximed, Inc., Organogenesis, Regentis, Samumed, Inc., Vericel, and ZKR Orthopedics, Inc.; royalties from Arthrex, Inc., Biopoly, LLC, Organogenesis, Springer, and Thieme Medical Publishers, Inc.; stock/stock options for MedShape, Inc. and Ortho Regenerative Tech, Inc.; and editorial board appointments to the American Journal of Orthopedics and Cartilage outside the submitted work.
Adam B. Yanke reports research support from Arthrex, Inc., Organogenesis, and Vericel; and paid consultant for JRF Ortho, Olympus, Patient IQ, Smith & Nephew, and Sparta Biomedical.
Human and Animal Rights and Informed Consent
All reported studies/experiments with human or animal subjects performed by the authors have been previously published and complied with all applicable ethical standards (including the Helsinki Declaration and its amendments, institutional/national research committee standards, and international/national/institutional guidelines).
Footnotes
This article is part of the Topical Collection on Stem Cells in Orthopaedic Surgery
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
- 1.Davis J. Skin grafting at the Johns Hopkins Hospital. Ann Surg. 1909;50:542–549. doi: 10.1097/00000658-190909000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Malhotra C, Jain AK. Human amniotic membrane transplantation: different modalities of its use in ophthalmology. World J Transplant. 2014;4:111–121. doi: 10.5500/wjt.v4.i2.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Caporossi T, De Angelis L, Pacini B, Tartaro R, Finocchio L, Barca F, et al. A human amniotic membrane plug to manage high myopic macular hole associated with retinal detachment. Acta Ophthalmol. 2019. 10.1111/aos.14174. [DOI] [PubMed]
- 4.Shah AP. Using amniotic membrane allografts in the treatment of neuropathic foot ulcers. J Am Podiatr Med Assoc. 2014;104:198–202. doi: 10.7547/0003-0538-104.2.198. [DOI] [PubMed] [Google Scholar]
- 5.Swan J. Use of cryopreserved, particulate human amniotic membrane and umbilical cord (AM/UC) tissue: a case series study for application in the healing of chronic wounds. Surg Technol Int. 2014;25:73–78. [PubMed] [Google Scholar]
- 6.DiDomenico LA, Orgill DP, Galiano RD, Serena TE, Carter MJ, Kaufman JP, Young NJ, Jacobs AM, Zelen CM. Use of an aseptically processed, dehydrated human amnion and chorion membrane improves likelihood and rate of healing in chronic diabetic foot ulcers: a prospective, randomised, multi-centre clinical trial in 80 patients. Int Wound J. 2018;15:950–957. doi: 10.1111/iwj.12954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.DiDomenico LA, Orgill DP, Galiano RD, Serena TE, Carter MJ, Kaufman JP, Young NJ, Zelen CM. Aseptically processed placental membrane improves healing of diabetic foot ulcerations. Plastic Reconstr Surg Glob Open. 2016;4:e1095. doi: 10.1097/GOX.0000000000001095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sultan AA, Piuzzi NS, Mont MA. Nonoperative applications of placental tissue matrix in orthopaedic sports injuries. Clin J Sport Med. 2018. 10.1097/JSM.0000000000000684. [DOI] [PubMed]
- 9.Hao Y, Ma DH, Hwang DG, Kim WS, Zhang F. Identification of antiangiogenic and antiinflammatory proteins in human amniotic membrane. Cornea. 2000;19:348–352. doi: 10.1097/00003226-200005000-00018. [DOI] [PubMed] [Google Scholar]
- 10.Koh JW, Shin YJ, Oh JY, Kim MK, Ko JH, Hwang JM, Wee WR, Lee JH. The expression of TIMPs in cryo-preserved and freeze-dried amniotic membrane. Curr Eye Res. 2007;32:611–616. doi: 10.1080/02713680701459441. [DOI] [PubMed] [Google Scholar]
- 11.Bennett NT, Schultz GS. Growth factors and wound healing: biochemical properties of growth factors and their receptors. Am J Surg. 1993;165:728–737. doi: 10.1016/s0002-9610(05)80797-4. [DOI] [PubMed] [Google Scholar]
- 12.Dua HS, Gomes JA, King AJ, Maharajan VS. The amniotic membrane in ophthalmology. Surv Ophthalmol. 2004;49:51–77. doi: 10.1016/j.survophthal.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 13.Topoluk N, Hawkins R, Tokish J, Mercuri J. Amniotic mesenchymal stromal cells exhibit preferential osteogenic and chondrogenic differentiation and enhanced matrix production compared with adipose mesenchymal stromal cells. Am J Sports Med. 2017;45:2637–2646. doi: 10.1177/0363546517706138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wei JP, Nawata M, Wakitani S, Kametani K, Ota M, Toda A, et al. Human amniotic mesenchymal cells differentiate into chondrocytes. Cloning Stem Cells. 2009;11:19–26. doi: 10.1089/clo.2008.0027. [DOI] [PubMed] [Google Scholar]
- 15.Nogami M, Kimura T, Seki S, Matsui Y, Yoshida T, Koike-Soko C, Okabe M, Motomura H, Gejo R, Nikaido T. A human amnion-derived extracellular matrix-coated cell-free scaffold for cartilage repair: in vitro and in vivo studies. Tissue Eng Part A. 2016;22:680–688. doi: 10.1089/ten.TEA.2015.0285. [DOI] [PubMed] [Google Scholar]
- 16.Zhang Z, Zeng L, Yang J, Guo L, Hou Q, Zhu F. Amniotic membrane-derived stem cells help repair osteochondral defect in a weight-bearing area in rabbits. Exp Ther Med. 2017;14:187–192. doi: 10.3892/etm.2017.4497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu PF, Guo L, Zhao DW, Zhang ZJ, Kang K, Zhu RP, Yuan XL. Study of human acellular amniotic membrane loading bone marrow mesenchymal stem cells in repair of articular cartilage defect in rabbits. Genet Mol Res. 2014;13:7992–8001. doi: 10.4238/2014.September.29.12. [DOI] [PubMed] [Google Scholar]
- 18.Tang K, Wu J, Xiong Z, Ji Y, Sun T, Guo X. Human acellular amniotic membrane: a potential osteoinductive biomaterial for bone regeneration. J Biomater Appl. 2018;32:754–764. doi: 10.1177/0885328217739753. [DOI] [PubMed] [Google Scholar]
- 19.Anderson JJ, Swayzee Z. The use of human amniotic allograft on osteochondritis dissecans of the talar dome: a comparison with and without allografts in arthroscopically treated ankles. Surg Sci. 2015;6:412–417. doi: 10.4236/ss.2015.69059. [DOI] [Google Scholar]
- 20.Gerwin N, Hops C, Lucke A. Intraarticular drug delivery in osteoarthritis. Adv Drug Deliv Rev. 2006;58:226–242. doi: 10.1016/j.addr.2006.01.018. [DOI] [PubMed] [Google Scholar]
- 21.Berenbaum F. Osteoarthritis as an inflammatory disease (osteoarthritis is not osteoarthrosis!) Osteoarthr Cartil. 2013;21:16–21. doi: 10.1016/j.joca.2012.11.012. [DOI] [PubMed] [Google Scholar]
- 22.Wardle F. Involving adolescents in head start. Child Today. 1988;17:14–15. [PubMed] [Google Scholar]
- 23.Kueckelhaus M, Philip J, Kamel RA, Canseco JA, Hackl F, Kiwanuka E, et al. Sustained release of amnion-derived cellular cytokine solution facilitates Achilles tendon healing in rats. Eplasty. 2014;14:e29. [PMC free article] [PubMed] [Google Scholar]
- 24.Lange-Consiglio A, Rossi D, Tassan S, Perego R, Cremonesi F, Parolini O. Conditioned medium from horse amniotic membrane-derived multipotent progenitor cells: immunomodulatory activity in vitro and first clinical application in tendon and ligament injuries in vivo. Stem Cells Dev. 2013;22:3015–3024. doi: 10.1089/scd.2013.0214. [DOI] [PubMed] [Google Scholar]
- 25.Willett NJ, Thote T, Lin AS, Moran S, Raji Y, Sridaran S, Stevens HY, Guldberg RE. Intra-articular injection of micronized dehydrated human amnion/chorion membrane attenuates osteoarthritis development. Arthritis Res Ther. 2014;16:R47. doi: 10.1186/ar4476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Raines AL, Shih MS, Chua L, Su CW, Tseng SC, O’Connell J. Efficacy of particulate amniotic membrane and umbilical cord tissues in attenuating cartilage destruction in an osteoarthritis model. Tissue Eng Part A. 2017;23:12–19. doi: 10.1089/ten.TEA.2016.0088. [DOI] [PubMed] [Google Scholar]
- 27.Marino-Martínez IA, Martínez-Castro AG, Peña-Martínez VM, Acosta-Olivo CA, Vílchez-Cavazos F, Guzmán-López A, et al. Human amniotic membrane intra-articular injection prevents cartilage damage in an osteoarthritis model. Exp Ther Med. 2019;17:11–16. doi: 10.3892/etm.2018.6924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vines J, Aliprantis AO, Gomoll AH, Farr J. Cryopreserved amniotic suspension for the treatment of knee osteoarthritis. J Knee Surg. 2015;29:443–450. doi: 10.1055/s-0035-1569481. [DOI] [PubMed] [Google Scholar]
- 29.•• Farr J, Gomoll AH, Yanke AB, Strauss EJ, Mowry KC, ASA Study Group. A randomized controlled single-blind study demonstrating superiority of amniotic suspension allograft injection over hyaluronic acid and saline control for modification of knee osteoarthritis symptoms. J Knee Surgery. 2019. 10.1055/s-0039-1696672This single-blind randomized control trial investigated the effect of amniotic suspension injections in patients with knee osteoarthritis and found that patients who underwent this treatment had significant improvement in patient reported outcomes and pain compared with those who underwent saline or hyaluronic acid injection.
- 30.McQuilling JP, Sanders M, Poland L, Sanders M, Basadonna G, Waldrop NE, Mowry KC. Dehydrated amnion/chorion improves Achilles tendon repair in a diabetic animal model. Wounds. 2019;31:19–25. [PMC free article] [PubMed] [Google Scholar]
- 31.Demirkan F, Colakoglu N, Herek O, Erkula G. The use of amniotic membrane in flexor tendon repair: an experimental model. Arch Orthop Trauma Surg. 2002;122:396–399. doi: 10.1007/s00402-002-0418-3. [DOI] [PubMed] [Google Scholar]
- 32.Özgenel GY. The effects of a combination of hyaluronic and amniotic membrane on the formation of peritendinous adhesions after flexor tendon surgery in chickens. J Bone Joint Surg Br. 2004;86:301–307. doi: 10.1302/0301-620x.86b2.14435. [DOI] [PubMed] [Google Scholar]
- 33.Lange-Consiglio A, Tassan S, Corradetti B, Meucci A, Perego R, Bizzaro D, Cremonesi F. Investigating the efficacy of amnion-derived compared with bone marrow–derived mesenchymal stromal cells in equine tendon and ligament injuries. Cytotherapy. 2013;15:1011–1020. doi: 10.1016/j.jcyt.2013.03.002. [DOI] [PubMed] [Google Scholar]
- 34.Lullove E. A flowable placental tissue matrix allograft in lower extremity injuries: a pilot study. Cureus. 2015;7:e275. doi: 10.7759/cureus.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu C, Bai J, Yu K, Liu G, Tian S, Tian D. Biological amnion prevents flexor tendon adhesion in zone II: a controlled, multicentre clinical trial. Biomed Res Int. 2019;2354325. 10.1155/2019/2354325. [DOI] [PMC free article] [PubMed]
- 36.Hanselman AE, Tidwell JE, Santrock RD. Cryopreserved human amniotic membrane injection for plantar fasciitis. Foot Ankle Int. 2015;36:151–158. doi: 10.1177/1071100714552824. [DOI] [PubMed] [Google Scholar]
- 37.Zelen CM, Poka A, Andrews J. Prospective, randomized, blinded, comparative study of injectable micronized dehydrated amniotic/chorionic membrane allograft for plantar fasciitis—a feasibility study. Foot Ankle Int. 2013;34:1332–1339. doi: 10.1177/1071100713502179. [DOI] [PubMed] [Google Scholar]