Abstract
Introduction
It is worldwide accepted that lncRNA PTCSC3 is a tumor suppressor in glioma and thyroid cancer, whereas its role in the recurrence of gastric cancer is unknown.
Patients and Methods
We recruited 80 GC patients (46 males and 34 females, 44 to 68 years, 56.3±6.7 years) in our study. Two human GC cell lines AGS and SNU-1 were transfected with PTCSC3 and HOXA11-AS expression vectors. Then, qPCR was used to detect the level of relative mRNA. Both invasion and migration assays were performed to detect the effect of the lncRNA on gastric cancer cell motility.
Results
In the present study, we showed that PTCSC3 was downregulated in plasma of gastric cancer patients than in plasma of healthy controls. Follow-up study indicated that PTCSC3 was further downregulated in patients with distant-recurrence but not in patients with local recurrence only or non-recurrence. LncRNA HOXA11-AS was upregulated in plasma of gastric cancer cells than in plasma of healthy controls and was inversely correlated with PTCSC3 in plasma of gastric cancer patients. PTCSC3 overexpression mediated the downregulation of HOXA11-AS in gastric cancer cells, while HOXA11-AS overexpression failed to significantly affect PTCSC3. PTCSC3 overexpression led to inhibited, while HOXA11-AS overexpression led to promoted migration and invasion of gastric cancer cells. In addition, HOXA11-AS overexpression reduced the effects of PTCSC3 overexpression.
Discussion
Therefore, lncRNA PTCSC3 alleviates in the postoperative distant recurrence of gastric cancer possible by suppression of HOXA11-AS.
Keywords: PTCSC3, gastric cancer, distant recurrence, HOXA11-AS
Introduction
Gastric cancer (GC) is among the most commonly diagnosed malignancies and a major cause of cancer-related deaths.1 According to the latest GLOBOCAN statistics, GC caused 782,685 deaths in 2018, which accounts for 8.2% of all cancer deaths in that year.2 During the same year, a total of 1,033,701 new cases of GC were diagnosed, accounting for 5.7% of all new cancer cases.2 A considerable number of risk factors, such as alcohol drinking and infections of Helicobacter pylori, have been proved as the major risk factors for GC,3,4 while avoidance of these risk factors requires the intensive intervention of people’s life style, which is not practical. Radical therapies for advanced GC remain lack. Therefore, early diagnosis followed by surgical resection is still the key to patients’ survival. However, postoperative recurrence is common and the survival rate of recurrent GC is extremely low.5,6
Long (>200nt) non-coding RNAs (lncRNAs) are a big group of RNA transcripts that have critical functions in diverse cellular processes.7 LncRNAs encode no proteins and they achieve their functions mainly regulating gene expression at multiple levels.8 In cancer biology, lncRNAs regulate the temporal and spatial expression of oncogenes or tumor suppressors to promote or suppress cancer progression.9 In effect, the regulation of critical lncRNAs involved in cancer biology provided novel targets for the development of targeted therapies.10 However, the function of most lncRNAs remains unclear. PTCSC3 is a recently identified tumor-suppressive lncRNA in thyroid cancer and glioma.11,12 Our preliminary deep sequencing data revealed it downregulated expression pattern in GC and its inverse correlation with HOXA11-AS, an oncogenic lncRNA in GC.13 This study was therefore performed to analyze the interaction between these two lncRNAs in GC.
Patients and Methods
GC Patients and Follow-Up
This study included 80 GC patients (46 males and 34 females, 44 to 68 years, 56.3±6.7 years) who received surgical resection of primary tumors at Changzheng Hospital, Second Military Medical University between March 2013 and July 2016. The control group included 80 healthy controls (46 males and 34 females, 44 to 68 years, 56.2±6.9 years) during the same time period at the same hospital to match the distributions of age and gender of GC group. The aforementioned hospital Ethics Committee approved this study. Patients’ inclusion criteria were the following: 1) newly diagnosed cases; stage I (n=55) and stage II (n=25) cases proper for surgical resection; 3) no therapies were performed within 3 months before this study. Patients’ exclusion criteria were the following: 1) recurrent GC; 2) multiple clinical disorders were diagnosed. All the patients were follow-up for 3 years after surgical resection in a monthly manner through telephone. All patients completed the follow-up. During follow-up, patients’ recurrence was observed and recorded. All participants signed informed consent.
Extraction of fasting blood (5 mL) was performed on both patients and controls before the initiation of therapies. During follow-up, blood extraction was also performed on the day of the diagnosis of recurrence and at the end of follow-up in cases of non-recurrence. Blood was centrifuged in EDTA tubes at room temperature for 10 min at 1200 g to separate plasma samples.
Cell Culture and Transfection
AGS (adenocarcinoma) and SNU-1 (carcinoma) two human GC cell lines were from ATCC (USA). Cell culture conditions were 95% humidity, 37°C and 5% CO2. Cell culture was composed of 90% RPMI-1640 Medium and 10 FBS. Cells were cultivated and harvested at 70–80% confluence. Vectors expressing PTCSC3 or HOXA11-AS and empty vectors were constructed by Sangon Biotech Co., Ltd using the pcDNA3.1 vector as the primary vector. All transient transfections were mediated by Lipofectamine-2000 (Invitrogen, USA) to transfect 10 nM vector (empty vector transfection as NC group) into the aforementioned cell lines at a density of 1x106 cells in 2 mL cell suspension. Control (C) cells were untransfected cells. Cells were used for subsequent experiments when PTCSC3 and HOXA11-AS overexpression rates were >200%. Cells were harvested at 24 h post-transfection to perform the following experiments.
RNA, Reverse Transcription (RT), and qPCR
All RNA extractions in this study were performed using Trizol (Invitrogen, USA). DNA eraser (Sigma-Aldrich) was used to remove genomic DNAs from all RNA samples. After that, 20 μL RT reaction systems were prepared using SSRT IV system (Invitrogen, USA) to transcribe total RNA into cDNA. Reaction thermal conditions were: 30 min at 45°C and 10 at 55°C. To measure the levels of PTCSC3 and HOXA11-AS expression, 2 × SYBR® Premix Ex Taq™ (Takara) was used to prepare qPCR reaction mixtures with cDNA as template and gene-specific primers. Eppendorf Mastercycler (Eppendorf, Germany) was used to carry out all qPCR reactions through following thermal conditions: 95°C for 30 s, followed by 40 cycles of 95°C for 10 s and 55°C for 40 s. Primers for PTCSC3, HOXA11-AS and endogenous control GAPDH were synthesized by Invitrogen (Thermo Fisher Scientific, Inc.). The following primer sequences were used: PTCSC3 forward 5′-TCAAACTCCAGGGCTTGAAC-3′, and reverse 5′-ATT ACGGCTGGGTCTACCT-3′; HOXA11-AS forward, 5ʹ-AGCAACAGATCGT -CACTCGG-3ʹ and reverse 5ʹ-GAGAACGAGGACCCTGCAAT-3ʹ; GAPDH forward, 5′-GGTGATGCTGGTGCTGAGTATGT-3′and reverse, 5′-AAGAATGGGAGTTG -CTGTTGAAGTC-3′. All PCR reactions were repeated 3 times and negative control (C) was the non-template reactions. The 2-ΔΔCT method was used to normalize the fold change of gene expression levels to endogenous control GAPDH.
Cell Invasion and Migration Assay
Transwell chambers (Corning, Cat# 354578) were used to perform both invasion and migration assays. In brief, starvation was performed using non-serum medium. Following trypsinization, 3000 cells in 0.1 mL non-serum medium were transferred to the Transwell chamber. The chambers were placed into a well containing a mixture composed of 20% FBS and 80% cell culture medium. Cells were cultivated under the aforementioned conditions for 12 h. Invading and migrating cells (lower surface) were stained with 0.05% Crystal Violet and were counted under a light microscope. It is worth noting that Matrigel (200mg/mL) was used to stain the membranes before invasion assay but not migration assay.
Statistical Analysis
Each experiment was performed in three independent biological replicates. Data are expressed as the mean ± standard deviation. An unpaired t-test was used for comparisons between gastric cancer patients and controls. Comparisons between the pre‑treatment and follow‑up data within the same group were performed using a paired t‑test. Explorations of differences among multiple groups were performed using ANOVA (one-way) and Tukey’s test. We used Pearson’s χ2 tests to determine the correlation between clinicopathological parameters and PTCSC3 lncRNA expression in GC samples Correlation between the expression levels of PTCSC3 and HOXA11-AS were performed using Pearson’s correlation coefficient. p<0.05 was statistically significant.
Results
Follow-Up Data
During the follow-up, there were 23 cases developed distant-recurrence (DR). Twenty-four patients were diagnosed at local-recurrence (LR). Recurrence was not observed in the rest 33 cases (non-recurrence group, NR).
PTCSC3 Was Downregulated in GC Patients
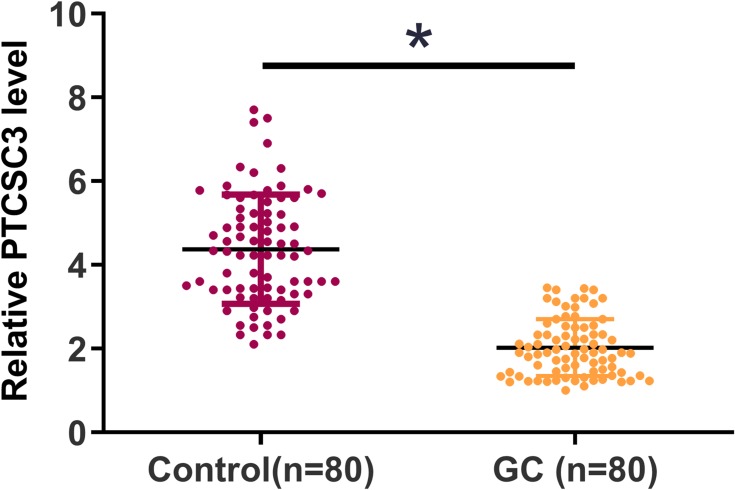
The differential expression of PTCSC3 in GC patients was first analyzed by performing qPCR to measure the expression levels of PTCSC3 in plasma from both GC patients (n=80) and healthy controls (n=80). Unpaired t test analysis showed that plasma levels of PTCSC3 were significantly lower in GC patients than in healthy controls (Figure 1, p<0.05). To further identify the clinicopathological role of PTCSC3 expression in GC patients, we divided 80 patients into a low PTCSC3 expression group (n=39) and a high PTCSC3 expression group (n=41). As presented in Table 1, PTCSC3 level in GC tissues was positively correlated with tumor diameter, differentiation, and metastasis. The results indicated that the abnormal expression of lncRNA PTCSC3 played a crucial role in the progression of GC.
Figure 1.
PTCSC3 was downregulated in GC patients. The differential expression of PTCSC3 in GC patients was first analyzed by performing qPCR to measure the expression levels of PTCSC3 in plasma from both GC patients (n=80) and healthy controls (n=80). PCR was repeated 3 times and the mean values were compared by unpaired t test. *p<0.05.
Table 1.
Association with PTCSC3 and the Clinical Pathological Characteristics of GC Patients
| Num | PTCSC3 | P value | ||
|---|---|---|---|---|
| Low | High | |||
| Gender | ||||
| Male | 46 | 24 | 22 | 0.335 |
| Female | 34 | 15 | 19 | |
| Age (years) | ||||
| >50 | 49 | 23 | 26 | 0.472 |
| <50 | 31 | 16 | 15 | |
| Tumor Diameter | ||||
| ≥4cm | 43 | 29 | 14 | 0.043* |
| <4cm | 37 | 10 | 27 | |
| Differentiation | ||||
| I | 55 | 22 | 33 | 0.049* |
| II | 25 | 17 | 8 | |
| Metastasis | ||||
| NR | 33 | 12 | 21 | |
| DR | 23 | 18 | 5 | 0.032* |
| LR | 24 | 9 | 15 | |
Notes: The mean expression level was used as the threshold. For analysis of the association between PTCSC3 levels and clinical features, Pearson’s χ2 tests were used *P<0.05.
PTCSC3 Was Further Downregulated in DR Group
Plasma levels of PTCSC3 in DR (n=23), LR (n=24) and NR (n=33) groups were also measured during follow-up by performing qPCR. Paired t test analysis showed that, compared to pre-treatment levels, plasma levels of PTCSC3 were significantly downregulated in patients with DR (Figure 2A, p<0.05), but not in LR (Figure 2B, p>0.05) and NR (Figure 2C, p>0.05) groups during follow-up.
Figure 2.
PTCSC3 was further downregulated in DR group. Plasma levels of PTCSC3 in DR (n=23), (A) LR (n=24), (B) and NR (n=33), (C) groups were also measured during follow-up by performing qPCR. Paired t test was used to compare pretreatment and follow-up levels of PTCSC3. PCR was repeated 3 times and the mean values were compared by unpaired t test. *p<0.05.
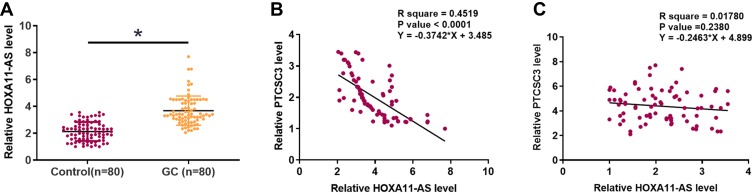
HOXA11-AS Was Upregulated in GC and Inversely Correlated with PTCSC3
The differential expression of HOXA11-AS in GC patients was also analyzed by qPCR to measure the expression levels of HOXA11-AS in plasma from both GC patients (n=80) and healthy controls (n=80). Unpaired t test analysis showed that plasma levels of HOXA11-AS were significantly higher in GC patients than in healthy controls (Figure 3A, p<0.05). Linear regression was performed to analyze the correlation between PTCSC3 and HOXA11-AS. It was observed that plasma levels of PTCSC3 were inversely and significantly correlated with HOXA11-AS across GC samples (Figure 3B) but not healthy control samples (Figure 3C).
Figure 3.
HOXA11-AS was upregulated in GC and inversely correlated with PTCSC3. The differential expression of HOXA11-AS in GC patients was also analyzed by qPCR to measure the expression levels of HOXA11-AS in plasma from both GC patients (n=80) and healthy controls (n=80). Data were compared by unpaired t test (A). Linear regression was performed to analyze the correlation between PTCSC3 and HOXA11-AS across GC samples (B) and healthy control samples (C). PCR was repeated 3 times and the mean values were compared by unpaired t test. *p<0.05.
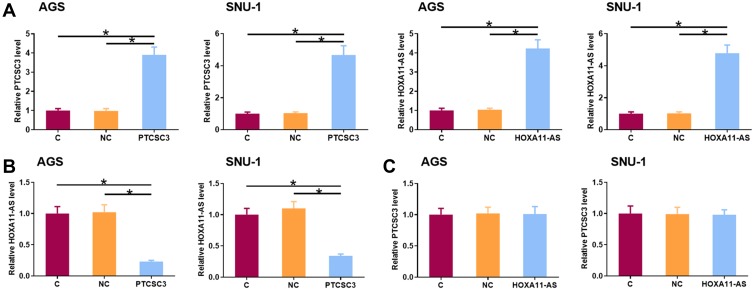
PTCSC3 Overexpression Mediated the Downregulation of HOXA11-AS in AGS and SNU-1 Cells
AGS and SNU-1 cells were transfected with PTCSC3 and HOXA11-AS expression vectors. The overexpression of PTCSC3 and HOXA11-AS was confirmed by qPCR at 24 h post-transfection (Figure 4A, p<0.05). Compared to the C and NC groups, PTCSC3 overexpression led to the significant downregulation of HOXA11-AS in AGS and SNU-1 cells (Figure 4B, p<0.05). In contrast, HOXA11-AS overexpression failed to significantly alter the expression of PTCSC3 in these cells (Figure 4C, p<0.05).
Figure 4.
PTCSC3 overexpression mediated the downregulation of HOXA11-AS in AGS and SNU-1 cells. AGS and SNU-1 cells were transfected with PTCSC3 and HOXA11-AS expression vectors. The overexpression of PTCSC3 and HOXA11-AS was confirmed by qPCR at 24 h post-transfection (A, p<0.05). The effects of PTCSC3 overexpression on HOXA11-AS (B) and the effects of HOXA11-AS overexpression on PTCSC3 (C) in AGS and SNU-1 cells were also analyzed by qPCR. Experiments were repeated 3 times and mean values were presented. *p<0.05.
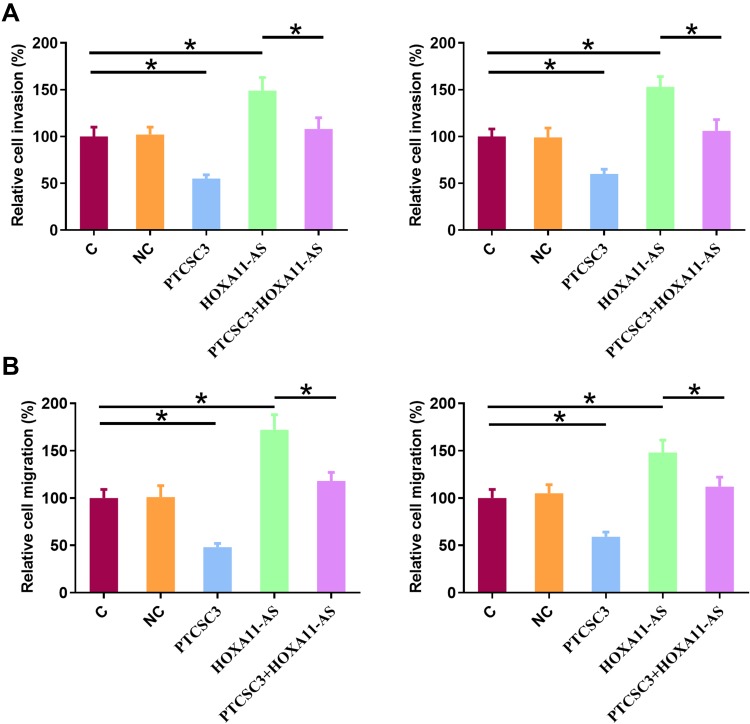
PTCSC3 Overexpression Inhibited the Invasion and Migration of AGS and SNU-1 Cells Through HOXA11-AS
Transwell migration and invasion assays were performed to explore the effects of the interactions between PTCSC3 and HOXA11-AS on the invasion (Figure 5A) and migration (Figure 5B) of both AGS and SNU-1 cells. Comparing to C and NC groups, PTCSC3 overexpression led to inhibited, while HOXA11-AS overexpression led to promoted migration and invasion of gastric cancer cells. In addition, HOXA11-AS overexpression reduced the effects of PTCSC3 overexpression (p<0.05).
Figure 5.
PTCSC3 overexpression inhibited the invasion and migration of AGS and SNU-1 cells through HOXA11-AS. Transwell migration and invasion assays were performed to explore the effects of the interactions between PTCSC3 and HOXA11-AS on the invasion (A) and migration (B) of both AGS and SNU-1 cells. Experiments were repeated 3 times and mean values were presented. *p<0.05.
Discussion
Post-operative recurrence is a major cause of deaths of all cancer patients GC cancer patients. In this study, we showed that the downregulation of PTCSC3 is correlated with the distance recurrence of GC after surgical resection. In addition, PTCSC3 may play its tumor-suppressive roles in cancer cell invasion and migration by downregulating HOXA11-AS.
The functions of PTCSC3 have only been reported in thyroid cancer and glioma.11,12 In thyroid cancer, PTCSC3 is downregulated and can regulate multiple pathways, such as Wnt/β‐catenin signaling,13 S100A4 gene14 and STAT3/INO80 pathway to regulate cancer cell behaviors, such as proliferation, invasion and migration as well as the sensitivity of cancer cells to drug treatment.13–16 PTCSC3 is also downregulated in glioma and can interact with Wnt/β-catenin signaling pathway to suppress the invasion and proliferation of cancer cells.12 Recent studies suggested that PTCSC3 was reported to be a potential biomarker17 and inhibited GC growth by interacting with lncRNA Linc-pint.18 However, the role of PTCSC3 in gastric cancer recurrence remains unknown. Our study is the first to observe that patients with DR have a significant decrease level of PTCSC3. Similarly, the invasion and migration rates of GC cells were reduced after PTCSC3 overexpression. Our data suggested that PTCSC3 is likely a tumor-suppressive lncRNA in GC, which has strong potential clinical values.
We also provide evidence that the roles of PTCSC3 in GC are likely mediated by another lncRNA named HOXA11-AS, which is overexpression in GC and promotes GC cell invasion. Recently, Guo et al observed that HOXA11-AS could promote the migration and invasion in gastric cancer through miR-148a/WNT1/beta-catenin pathway,18 which is consistent with our result. However, the mechanism that mediates the interactions between PTCSC3 and HOXA11-AS in GC remains unclear. In the previous research, we found that HOXA11-AS was mediated by PTCSC3 as a downstream target in gastric cancer cell migration and invasion, which seems relatively novel in the mechanism. However, more studies are needed to verify the truly functional effects of PTCSC3 on HOXA11-AS. We observed a significant correlation between HOXA11-AS and PTCSC3 only in GC patients but not in healthy controls. Therefore, certain pathological factors may mediate the interaction between HOXA11-AS and PTCSC3 in GC.
The present experiments only conducted in two cancer cell lines. Future investigations should include additional cancer cell lines in order to perform an in‑depth analysis of the correlation between PTCSC3 expression and cancer cell behavior.
In conclusion, PTCSC3 was downregulated in GC and reduced the expression of HOXA11-AS to promote cancer cell invasion and migration, thereby promoting distance recurrence of GC after surgical resection.
Funding Statement
This work was supported by Shanghai Municipal Health and Family Planning Commission Scientific Research Project (Grant No.20174Y0222); the Natural Science Foundation of Shanghai (Grant No. 17ZR1439300); Shanghai Municipal Health and Family Planning Commission Scientific Research Project (Grant No.201640269).
Ethics and Consent Statements
This study was approved by the ethics committee of Changzheng Hospital. All procedures performed in participants were in accordance with the ethical standards of the Declaration of Helsinki. Informed consent was written by all participants.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Sitarz R, Skierucha M, Mielko J, et al. Gastric cancer: epidemiology, prevention, classification, and treatment. Cancer Manag Res. 2018;10:239–248. doi: 10.2147/CMAR.S149619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi: 10.3322/caac.21492 [DOI] [PubMed] [Google Scholar]
- 3.Ferro A, Morais S, Rota M, et al. Alcohol intake and gastric cancer: meta-analyses of published data versus individual participant data pooled analyses (StoP Project). Cancer Epidemiol. 2018;54:125–132. doi: 10.1016/j.canep.2018.04.009 [DOI] [PubMed] [Google Scholar]
- 4.Plummer M, Franceschi S, Vignat J, et al. Global burden of gastric cancer attributable to Helicobacter pylori. Int J Cancer. 2015;136(2):487–490. doi: 10.1002/ijc.28999 [DOI] [PubMed] [Google Scholar]
- 5.Maehara Y, Hasuda S, Koga T, et al. Postoperative outcome and sites of recurrence in patients following curative resection of gastric cancer. Br J Surg. 2000;87(3):353–357. doi: 10.1046/j.1365-2168.2000.01358.x [DOI] [PubMed] [Google Scholar]
- 6.Roviello F, Marrelli D, De Manzoni G, et al. Prospective study of peritoneal recurrence after curative surgery for gastric cancer. Br J Surg. 2003;90(9):1113–1119. doi: 10.1002/bjs.4164 [DOI] [PubMed] [Google Scholar]
- 7.Mercer TR, Dinger ME, Mattick JS. Long non-coding RNAs: insights into functions. Nat Rev Genet. 2009;10(3):155–159. doi: 10.1038/nrg2521 [DOI] [PubMed] [Google Scholar]
- 8.Kornienko AE, Guenzl PM, Barlow DP, et al. Gene regulation by the act of long non-coding RNA transcription. BMC Biol. 2013;11(1):59. doi: 10.1186/1741-7007-11-59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gutschner T, Diederichs S. The hallmarks of cancer: a long non-coding RNA point of view. RNA Biol. 2012;9(6):703–719. doi: 10.4161/rna.20481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Spizzo R, Almeida MI, Colombatti A, et al. Long non-coding RNAs and cancer: a new frontier of translational research? Oncogene. 2012;31(43):4577–4587. doi: 10.1038/onc.2011.621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fan M, Li X, Jiang W, et al. A long non-coding RNA, PTCSC3, as a tumor suppressor and a target of miRNAs in thyroid cancer cells. Exp Ther Med. 2013;5(4):1143–1146. doi: 10.3892/etm.2013.933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xia S, Ji R, Zhan W. Long noncoding RNA papillary thyroid carcinoma susceptibility candidate 3 (PTCSC3) inhibits proliferation and invasion of glioma cells by suppressing the Wnt/β-catenin signaling pathway. BMC Neurol. 2017;17(1):30. doi: 10.1186/s12883-017-0813-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang X, Lu X, Geng Z, et al. LncRNA PTCSC3/miR‐574‐5p governs cell proliferation and migration of papillary thyroid carcinoma via Wnt/β‐catenin signaling. J Cell Biochem. 2017;118(12):4745–4752. doi: 10.1002/jcb.26142 [DOI] [PubMed] [Google Scholar]
- 14.Jendrzejewski J, Thomas A, Liyanarachchi S, et al. PTCSC3 is involved in papillary thyroid carcinoma development by modulating S100A4 gene expression. J Clin Endocrinol Metab. 2015;100(10):E1370–E1377. doi: 10.1210/jc.2015-2247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang X, Liu Y, Fan Y, et al. LncRNA PTCSC3 affects drug resistance of anaplastic thyroid cancer through STAT3/INO80 pathway. Cancer Biol Ther. 2018;19(7):590–597. doi: 10.1080/15384047.2018.1449610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang G, Chi N, Lu Q, et al. LncRNA PTCSC3 is a biomarker for the treatment and prognosis of gastric cancer. Cancer Biother Radiopharm. 2020;35:77–81. doi: 10.1089/cbr.2019.2991 [DOI] [PubMed] [Google Scholar]
- 17.Lei H, Haijuan W, Junyan W, et al. LncRNA PTCSC3 inhibits tumor growth and cancer cell stemness in gastric cancer by interacting with lncRNA Linc-pint. Cancer Manag Res. 2019;11:10393–10399. doi: 10.2147/CMAR.S231369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guo T, Yuan X, Liu DF, et al. LncRNA HOXA11-AS promotes migration and invasion through modulating miR-148a/WNT1/β-catenin pathway in gastric cancer. Neoplasma. 2020. doi: 10.4149/neo_2020_190722N653 [DOI] [PubMed] [Google Scholar]