Abstract
Erysipelothrix spp. comprise a group of small Gram-positive bacteria that can infect a variety of hosts including mammals, fish, birds, reptiles and insects. Among the eight Erysipelothrix species that have been described to date, only Erysipelothrix rhusiopathiae plays a major role in farmed livestock where it is the causative agent of erysipelas. E. rhusiopathiae also has zoonotic potential and can cause erysipeloid in humans with a clear occupational link to meat and fish industries. While there are 28 known Erysipelothrix serovars, over 80% of identified isolates belong to serovars 1 or 2. Vaccines to protect pigs against E. rhusiopathiae first became available in 1883 as a response to an epizootic of swine erysipelas in southern France. The overall vaccine repertoire was notably enlarged between the 1940s and 1960s following major outbreaks of swine erysipelas in the Midwest USA and has changed little since. Traditionally, E. rhusiopathiae serovar 1a or 2 isolates were inactivated (bacterins) or attenuated and these types of vaccines are still used today on a global basis. E. rhusiopathiae vaccines are most commonly used in pigs, poultry, and sheep where the bacterium can cause considerable economic losses. In addition, erysipelas vaccination is also utilized in selected vulnerable susceptible populations, such as marine mammals in aquariums, which are commonly vaccinated at regular intervals. While commercially produced erysipelas vaccines appear to provide good protection against clinical disease, in recent years there has been an increase in perceived vaccine failures in farmed animals, especially in organic outdoor operations. Moreover, clinical erysipelas outbreaks have been reported in animal populations not previously considered at risk. This has raised concerns over a possible lack of vaccine protection across various production species. This review focuses on summarizing the history and the present status of E. rhusiopathiae vaccines, the current knowledge on protection including surface antigens, and also provides an outlook into future directions for vaccine development.
Keywords: Erysipelothrix spp., history, immune protection, vaccines, review
Introduction
Erysipelas in animals and erysipeloid in people are both caused by infection with the Gram positive bacteria Erysipelothrix spp. which belong to the family Erysipelothrichaceae, order Erysipelotrichales, class Erysipelotrichia and phylum Firmicutes (1). Erysipelothrix spp. can be divided into eight different species: Erysipelothrix rhusiopathiae (2, 3), Erysipelothrix tonsillarum (4), Erysipelothrix species 1, Erysipelothrix species 2, and Erysipelothrix species 3 (5), Erysipelothrix inopinata (6), Erysipelothrix larvae (7) and Erysipelothrix piscisicarius sp. nov. (8). Isolates associated with these species were identified in mammals, birds and fish (E. rhusiopathiae, E. tonsillarum and E. species 1, 2, and 3; E. piscisicarius), a vegetable peptone broth (E. inopinata) or insects (E. larvae). Erysipelothrix spp. have a worldwide distribution and are considered ubiquitous with most identified isolates belonging to E. rhusiopathiae (9). The most important reservoir for E. rhusiopathiae are pigs, where an estimated 30-50% of healthy pigs appear to harbor the organisms in tonsils or lymphoid tissues (10).
Erysipelas describes an acute bacterial disease in pigs and other species, often characterized by raised, red skin patches (11). Not surprisingly, the term “erysipelas” is derived from the ancient Greek “ερυσíπελας” which means “rose” or “red skin” (12, 13). While skin discoloration in pigs may occur due to various etiologies and the term “swine erysipelas” is not very specific, it has still become a synonym for Erysipelothrix spp. infection in pigs and is well-known by veterinarians and other occupations connected to the food animal industry (14). Today, erysipelas is also used to describe clinical manifestations associated with this bacterium in other species including mammals, fish, birds and reptiles (15, 16). In humans, where the disease was first described in 1870 (17) since 1909 the term “erysipeloid” is used (18).
Prior to successful bacterial isolation and characterization, erysipelas was thought to be anthrax of swine as it resembled some of the clinical manifestations in cattle (9). During 1877–1878 the “great swine plague” (i.e., classical swine fever) moved through the United States, England and Scandinavian countries before entering and spreading through mainland Europe (14). Losses associated with these epidemics likely prompted early bacteriologists to investigate swine diseases (14). In 1876, Robert Koch successfully isolated a bacterium from a mouse inoculated with putrefying blood (19). Koch initially designated the bacterium “Bacillus of mouse septicemia” (19). Since Koch's discovery, the bacterium changed its name a total of 36 times; Erysipelothrix rhusiopathiae was officially designated as its scientific name only in 1966 (20) and has been in use ever since (Figure 1). This specific name was created from the Greek words “erysipelas” (rose, red skin), “trix” (hair), “rhusius” (reddening), and “pathus” (disease). During a swine erysipelas outbreak in southern France from 1882-1883 Louis Thuillier successfully isolated the bacterium from pigs with “rouget” as erysipelas is called in France (21). At approximately the same time Friedrich Löffler also isolated the bacterium but only published his findings after completing the first accurate description of E. rhusiopathiae and reproducing erysipelas in experimentally infected pigs in 1886 (22) (Figure 1).
Figure 1.
Timeline and milestones in Erysipelothrix spp. nomenclature and research.
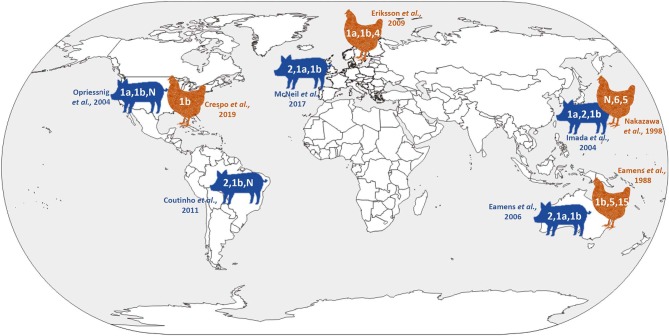
Historically, Erysipelothrix isolates have been divided into at least 28 serovars based on specific polysaccharide complexes that can be demonstrated by double agar-gel precipitation testing using type specific rabbit antiserum (23, 24). Initially there were several serotyping systems, and the most commonly used recognized two serovars, A and B, with a third group (N) for all isolates that did not react with A or B antiserum (25). Because this typing scheme quickly became unpractical with more and more isolates falling in the N group, a new system was created which is used to this day (23, 24). In this system, the serovars are indicated by consecutive Arabic numbers in order of discovery (i.e., 1–26), serovar 1 and sometimes 2 may be further subdivided and indicated by small letters (i.e., 1a, 1b, 2a, 2b). The old A and B serovars correspond to 1 and 2 in the new system. Serovar N, which lacks serovar-specific antigens and is still used in the new serotyping system, has been shown to arise from various other serovars following genetic mutation (26, 27). Certain serovars have historically been associated with particular Erysipelothrix species including serovars 1a, 1b, 2, 4–6, 8, 9, 11, 12, 15–17, 19, 21, 23, and N with E. rhusiopathiae, serovars 3, 7, 10, 14, 20, 22, and 24–26 with E. tonsillarum, serovar 13 with E. species 1 and serovar 18 with E. species 2 (28). However, this is not absolute and certain serovars can be associated with more than one species (5, 29). Serotyping studies have indicated that, with some geographic differences, serovars 1a, 1b, and 2 are most widely distributed in pigs and likely of greatest importance overall (24, 29–36) (Figure 2). Serotyping is not performed routinely, and serovars are not species specific or phylogenetically informative (37). It has been shown that the chromosomal locus responsible for determining antigenicity of serovars 1a and 2 is involved in the virulence (26); however, the overall importance of serovar in pathogenesis and immune protection is largely unknown.
Figure 2.
Current knowledge on global Erysipelothrix spp. serovar distribution in pigs and poultry.
Erysipelas vaccines are commonly used in pigs (38). In this species, most breeding herds in almost all pork producing regions are regularly vaccinated (Source: https://www.nadis.org.uk/disease-a-z/pigs/erysipelas/). In contrast, growing pigs are not commonly vaccinated against erysipelas as they are expected to have passively acquired antibodies when leaving the breeding farm. However, if there is a perceived high erysipelas pressure in grow-finish farms, growing pigs are also vaccinated (Source: https://www.nadis.org.uk/disease-a-z/pigs/erysipelas/). Erysipelas vaccines are less commonly used in poultry (e.g., turkeys or laying hens) (39) or sheep (40). Turkeys used for meat production can be vaccinated but this may be labor intensive (39). Breeder turkeys are vaccinated four weeks before onset of egg production (39). Chickens (almost exclusively layers) are vaccinated at least twice (or more often) 2–4 weeks apart (35). Ewes are not regularly vaccinated outside the large sheep production regions of Australia/New Zealand due to a low cost-benefit ratio of vaccination. However, to prevent erysipelas arthritis in lambs, vaccinating ewes prior to lambing will provide immunity in lambs for up to 8 weeks (Source: https://www.zoetis.com.au/_locale-assets/faq/erysipelas.pdf).
While for many decades Erysipelothrix spp. infections had minimal impacts on livestock production, erysipelas appears to be re-emerging today due to changing environmental conditions, changes in welfare regulations, an increase in outdoor organic farming (41), and changes in antimicrobial usage, with global reductions and eventual bans anticipated in the very near future (42). Sporadic outbreaks are being reported in various settings such as in farmed animals (31, 35, 43–46) and more specifically organic farms (47, 48), in fish (16, 49) as well as globally in wild animal species (50, 51). Climate change could favor increased bacterial loads and persistent environmental contamination. Alternatively, erysipelas outbreaks may be associated with changes in host resistance. Further explanations for re-emergence of E. rhusiopathiae in livestock could include decreasing vaccine efficacies, adaptations in the Erysipelothrix spp. populations allowing certain isolates to better survive in vaccinated populations or both. With the ever-expanding knowledge on erysipelas in different animal species and the perceived increase of clinical cases in livestock and wild animal species in recent years, it appears important to revisit the accumulated background information on protection and vaccination against Erysipelothrix spp. and gain perspectives for the future.
Disease Manifestation
Erysipelas is known to cause three main clinical manifestations in animals: acute, subacute, and a chronic disease (38). In addition, there is also subclinical disease. For pigs, acute disease is characterized by a sudden onset of clinical signs and can include acute death, fever, withdrawal from the herd, squealing, stiff stilted gait, weight shifting, depression, inappetence, diamond skin lesions which may appear 2–3 days after infection and disappear 4–7 days after first appearance, and necrotic lesions on tail, ears, and posterior aspect of the thighs. As a milder variation of the acute form, the subacute disease manifestation has similar but less severe clinical signs with fewer skin lesions; overall the subacute form may remain unnoticed, i.e., subclinical (38). The chronic manifestation of erysipelas, which may follow acute or subacute disease in pigs, is most commonly characterized by signs of arthritis (stiffness, enlargement) as early as three weeks after initial infection, and/or signs of cardiac insufficiency, sometimes with sudden death (38). In breeding sows, abortions or increased pre- and postpartal vulval discharge may be observed, as well as smaller litter sizes and reduced numbers of live born piglets (52). The chronic form of erysipelas may also affect growth rate and increase losses of cuts in meat packing plants (53).
Erysipelas can occur in a wide range of farmed poultry including turkeys, broiler chickens, laying hens, geese, pheasants and quails. Layers may simply suffer from sudden death due to acute septicaemia. Normally few birds may be depressed initially with mortality starting within 24 h. There may also be dramatic decrease of egg production (35) and conjunctival edema (46). Unsteady locomotion and lack of coordinated movement have also been reported (13). The disease can be fatal in young adult turkeys and ducks, with affected birds developing severe hemorrhages in breast and leg muscles (54). Turkeys with vegetative endocarditis usually do not have clinical signs and may die suddenly (13). Outbreaks have been reported in 2–3 day old poults (55) or commercial breeder flocks of quails (56). In sheep, the most common clinical manifestation is polyarthritis, typically presenting in 2- to 6-month-old lambs (57). Chronic ovine erysipelas is often restricted to joints with subchondral bone involvement (57). While slaughter condemnations in farmed animals other than pigs rarely occur, chronic cases that reach the slaughterhouse may pose a zoonotic risk to workers and infection of slaughterhouse employees has been reported (56).
Economic Impact
Erysipelothrix rhusiopathiae is of economic importance in pigs, poultry and lambs where outbreaks can cause high losses. Today the majority of pig breeding herds in Europe, North America, and South America are routinely vaccinated (every 4–6 months) against erysipelas, and erysipelas vaccine usage is increasing in poultry. From October 2018 to September 2019, ~10,683,595 doses of autogenous E. rhusiopathiae vaccines, 72,440,500 doses of attenuated vaccines and 33,257,460 doses of bacterins were released in the USA across species (Source: USDA, Dr. Paul Hauer, 2019). In the UK, during 2018, ~1,359,120 doses of inactivated vaccines including bivalent and trivalent products were sold (Source: Kynetec, UK data, 2019). In pigs, large scale outbreaks are an ongoing problem in some areas including the USA, where documented outbreaks occurred in 1989–1990 (58) and again in 2000–2001 (32). An outbreak is often devastating for affected producers, and for organic chicken productions in particular, where it may lead to permanent closure of the farm. Additionally, subclinical disease in pigs can be of great importance when it leads to abattoir condemnations, as at this point the producers have already incurred the maximal possible cost on the individual pigs. In modern food animal production, the precise economic impact of Erysipelothrix spp. is often multifaceted and likely underestimated.
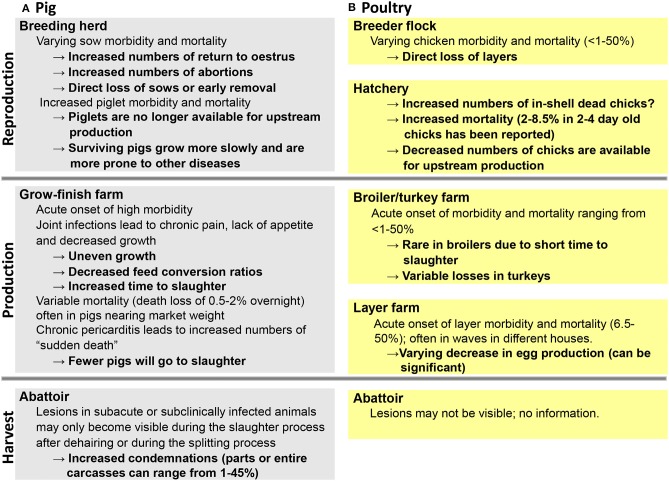
Economic losses can be directly associated with high morbidity and mortality rates during an acute outbreak (Figure 3) resulting in a reduction of animals on a given farm (43, 45), including significant egg drop in layers (47). In addition, as a result of joint pain and lameness due to the chronic form of the disease, there may be substantial decreased weigh gain, subsequently resulting in an increased time to slaughter (57). Finally, there may be increased numbers of condemned carcasses at slaughter due to lesions that may only become visible during scaling and dehairing processes or at the splitting floor (53, 59). In such cases, producers may be completely unaware of any ongoing erysipelas infection on the farm (Figure 3). When 153 arthritic joints from Canadian slaughter pigs were examined for bacteria, E. rhusiopathiae was found most frequently and identified in 45% of the samples (60). However, based on meat inspection rules in the European Union enforced in January 2006, if cutaneous erysipelas is detected prior to slaughter on farm, slaughtering of affected pigs must be deferred for at least 15 days from disappearance of typical lesions to guarantee meat safety (61).
Figure 3.
Impact of erysipelas on different production stages in pigs (A) and poultry (B).
Overall, it is often difficult to get estimates of the direct impact of erysipelas on a population, as E. rhusiopathiae infections are not reportable to health authorities in most countries. In Japan, where swine erysipelas is a reportable disease, ~2,000 pigs are affected annually, which is equivalent to 0.0125% of finishing pigs produced in the country. PCR assays have been developed for improved detection of contaminated pigs at Japanese slaughterhouse (62). Once the infection occurs in farms, pigs often become carriers of the organism and disperse the organism in their feces, resulting in contamination of their environment. Since the disease may also cause abortions in breeding herds, the total economic losses may become intolerable for affected farms. Analysis of erysipelas case trends are rarely published from other countries. However, a recent analysis of epizootic swine erysipelas cases from 2006 to 2017 in the Ukraine found a total of 39,952 cases during this time period, mainly in the Southeast region of the country (63).
Economic losses can be assessed via two routes: (I) by using production and economic models to value the production losses due to disease (presently not available for erysipelas) and (II) by partial budgeting approaches with “rules-of-thumb.” An example of the latter has been published at https://thepigsite.com/articles/erysipelas-why-is-it-still-a-problem-after-100-years (64). Based on the provided example calculations for the United Kingdom, a 400 sow breeding herd with an acute outbreak could lose 388 pigs due to abortions and sow mortality. For 2008, this would have cost GBP 14000. A late/chronic erysipelas outbreak in a 600 head finisher herd could result in ~58 pigs being euthanized and 31 condemned for a total loss of GBP 17451 over a six-month period (64). In a recent study focusing on global trends in infectious diseases in pigs, pathogens were ranked based on overall publication counts from 1966 to 2016 (65). The top 40 pathogens included 16 viruses, 15 bacteria, eight helminth parasites and one protozoan, with E. rhusiopathiae ranking 30th. This relatively low representation in the scientific literature may reflect an overall neglect of investigating long-standing endemic bacterial pathogens.
History of Erysipelas Prevention Methods
Attenuated Vaccines
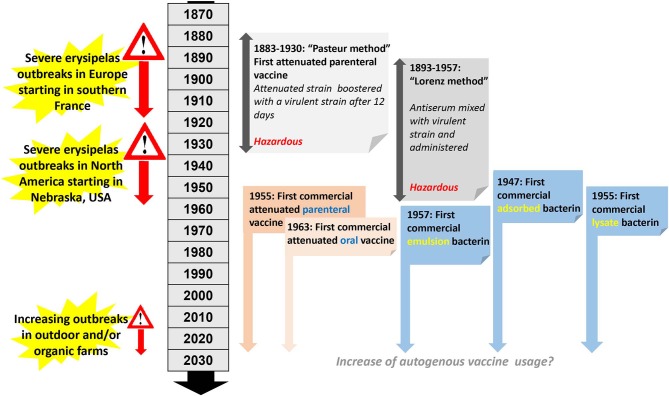
The development of bacterial vaccines for pigs began at the end of the nineteenth century (Figure 4) when the very first erysipelas vaccine became available (66). This initial attempt to produce an attenuated erysipelas vaccine was in direct response to a major swine erysipelas outbreak in southern France (67). After successful isolation of E. rhusiopathiae from affected pigs, the virulence of the bacterium was enhanced by passage through pigeons. This was followed by passages through rabbits which are only weakly susceptible to E. rhusiopathiae infection. Interestingly, this resulted in an increase of virulence of the isolate for rabbits and a decrease of virulence for swine (67). The so derived attenuated vaccine was used in pigs by injecting the attenuated culture, followed by another injection 12 days later using the virulent culture (Figure 4). This method was used from 1886 to 1892 in more than 100,000 pigs in France (68), and was subsequently adapted in other European countries (but never in North America), and used for pig vaccinations until 1930 (69). As this method is hazardous, it has since been abandoned. Subsequently, live-culture erysipelas vaccines for parenteral use in pigs have been available since 1955. The first vaccine of this type was named erysipelas vaccine avirulent or EVA (70). For this particular product, large volumes of the bacteria were injected into mice, pigeons, and pigs without resulting in any clinical signs, hence the vaccine was considered avirulent (70). Oral live erysipelas pig vaccines were developed during the 1960s (71, 72). The idea of orally vaccinating pigs originated by observations made from mass vaccination against human polio, and initial studies using experimentally vaccinated and challenged pigs indicated efficacies of 85–90% (71). Methods for attenuation included air-drying (73), passage in media containing acridine dyes (74), or passage through rabbits or chicken embryos (75). Specifically, oral vaccines were found to be safe in pigs without generating carriers or shedders (76) and vaccines were found to be stable both in the dried and liquid stage (72). Subsequently, swine oral vaccines were also examined for use in turkeys and were considered potentially useful (77). Attenuated vaccines are still in use today, administered via drinking water, intramuscular injection or even air exposure and are useful for vaccinating larger animal populations housed indoors including poultry and pigs. It is recommended to discontinue antibiotic treatment 8–10 days before vaccination (75). A single dose of an attenuated vaccine is sufficient to provide protection which varies across products but in general is guaranteed for up to 6 months. Usage of any attenuated vaccine strain is always associated with certain risks. While reversion to virulence of attenuated vaccines has not been reported in Western countries until now, in Japan attenuated erysipelas vaccines have been associated with chronic outbreaks of erysipelas (78).
Figure 4.
Timeline and milestones of E. rhusiopathiae vaccine development in pigs.
Serum-Culture Co-immunization
In 1891, Emmerich and Mastbaum discovered that when rabbits where hyperimmunized against swine erysipelas, their serum had curative properties (79). In 1893 a simultaneous serum-culture intervention was introduced for immunization, which consisted of concurrently injecting viable culture and a hyperimmune serum (79). This principle evolved into two methods. In the “Leclainche method,” a virulent culture was mixed with antiserum just before inoculation. In contrast, in the “Lorenz method,” named after a German veterinarian and widely used in Germany for more than 50 years (80, 81), antiserum and virulent culture were given at the same time but at different inoculation sites (Figure 4). The Lorenz method was introduced to Nebraska, USA in 1938 and by 1953, 26 U.S states were using this method. Similar to the Pasteur vaccine, ultimately these methods were considered to be hazardous; after other active immunization protocols became available, the usage of these methods eventually ceased (79).
Inactivated Vaccines
The first large-scale swine erysipelas outbreaks in the USA occurred in South Dakota, USA between 1928 and 1930 (82, 83). As a direct consequence, during the late 1940s, shortly after World War II, the first inactivated erysipelas vaccines for usage in pigs were developed (84, 85). Specifically, usage of a bacterin, consisting of formalin-killed whole E. rhusiopathiae culture adsorbed on an aluminum hydroxide gel was first reported in 1947 in East Germany (85) and licensed in the US in 1953 (Figure 4). Bacterins often use selected serovar 2 isolates that produce a soluble immunogenic product when grown in a complex liquid medium containing serum (75). This substance has been described as a glyco-lipoprotein (86) and most of it is released into the medium and is considered the necessary immunogenic ingredient for bacterins (75). Further characterization of the glyco-lipoprotein fraction during the 1990s revealed a 64–66 kDa protein fraction (87, 88) later described as surface protective antigen (Spa) A (89, 90). The main downside of the original bacterin was a protective immunity which only lasted 2-4 months. In an attempt to improve the formalin-killed Traub adsorbate bacterin, an emulsion bacterin was prepared using a water-in-oil emulsion (manide mono-oleate and a light mineral oil; bayol F) (91, 92). Pigs vaccinated with this emulsion bacterin developed immunity lasting at least 7–8 months or 237 days (91, 92). Bacterins developed at this time were also shown to be effective in turkeys (69, 93). A modified bacterin version, the lysate bacterin was developed in the Institut Merieux, Lyon, France in 1953 (94), licensed in the USA in 1955 (Rhusigen, Allied Laboratories, Inc., Indianapolis), and is similar to a regular bacterin with the exception of lysis of bacterial cells. Anecdotal evidence suggested that the bacterin could also be used with anti-swine serum to achieve immediate protection (94). Bacterins are given by subcutaneous or intramuscular routes and a booster vaccination may be required for some products, which typically needs to be administered 2–3 weeks after first vaccination. Manufactured inactivated vaccines have to follow strict quality controls and each batch needs to be tested for relative strength. Early on, this required extensive animal experiments in laboratory mice and pigs for the determination of efficacy (80). The discovery of skin scarification, allowing testing of more than one culture in production on a single animal, was considered a major improvement (95). The World Health Organization (WHO) established International Standards for erysipelas vaccines and antisera concerning potency testing in mice (80). Today, potency testing in Europe is generally performed with enzyme-linked immunosorbent assays (ELISAs), which have largely replaced animal experiments (96, 97). This change has reduced animal usage by over 80% (98, 99).
Passive Immunization
Passive immunity is a way to immediately but temporarily protect an animal for ~2 weeks by administering a commercially available antiserum. This treatment utilizing hyperimmune serum, usually obtained from horses, was introduced in 1899, several years after it had been developed for the serum-culture co-immunization (75). Studies in mice receiving antiserum prepared in rabbits or horses indicated protection even against serologically different isolates (100). Until introduction of antibiotics in pig production in 1949 (101), hyperimmune serum was the only means by which to treat affected pigs (82), but ultimately its usage decreased in later years. In general, the preventive dose is half the therapeutic dose (75). While passive immunization may be useful in protecting pigs during an acute outbreak, it is not very practical as antisera are not readily available and protection is not long-lasting, and therefore this method is not widely used today.
Currently Available Commercial Vaccines
Today several commercial vaccines to protect pigs, lambs and poultry from the negative impacts of Erysipelothrix spp. are available (Table 1). Although they are not very effective in preventing chronic arthritis, its frequency and severity are usually lower in vaccinated pigs compared to non-vaccinated pigs (105). Essentially all commercial vaccines available to date are based on serovar 1a or serovar 2 isolates, where serovar 1a is more commonly used in attenuated vaccines and serovar 2 is more commonly used in bacterins. Since the first introduction of attenuated erysipelas vaccines and bacterins, little has changed in terms of which isolates are being utilized (Table 1). For attenuated vaccines in modern husbandry, care needs to be taken with routine waterline disinfection, anything that may prevent the animals from drinking water (Source: http://www.pig-world.co.uk/features/water-medicating-pigs-what-you-need-to-know.html) and antibiotic administration at vaccination (106), as these may negatively impact vaccine efficacy. For bacterins various adjuvants are added to provide longer immunity. There are notable differences in the geographic distribution of the type of erysipelas vaccines, as live bacterial vaccines are rarely used in Europe (29) while they are common in other swine producing regions including North and South America (32) and Asia (78, 107) (Table 1).
Table 1.
Basic information on selected commercial Erysipelothrix rhusiopathiae vaccines.
| Vaccinea | Manufacterer | Isolate ID | Serovar | Year of initial strain description | Type | Availability | Species |
|---|---|---|---|---|---|---|---|
| ERY VAC FD | ARKO Laboratories, Ltd. | Not disclosed | 1a | Attenuated | USA | Turkeys | |
| ERY VAC 100 | ARKO Laboratories, Ltd. | Not disclosed | 1a | Attenuated | USA | Pigs | |
| Ingelvac® ERY ALC | Boehringer-Ingelheim Vetmedica | R-9 | 1a | 1944 (102) | Attenuated | USA | Pigs |
| Suvaxyn® E-Oral | Zoetis | 31 | 1a | 1963 | Attenuated | USA, Canada | Pigs |
| Swine erysipelas live (seed lot vaccine) | National Veterinary Assay Laboratory, Ministry of Agriculture, Forestry and Fisheries | Koganei 65-0.15 | 1a | 1971 (103) | Attenuated | Japan | Pigs |
| Ruvax® | Boehringer-Ingelheim Vetmedica | Unknown | 2 | Unknown | Lysate bacterin | EU | Pigs |
| Parvoruvax® | Ceva Animal Health, Ltd | Lysate bacterin | EU (not Malta), Brazil, Caucasus, Mexico, Moldova, Switzerland, Russia, Middle East | Pigs | |||
| Coopers® ERYGUARD® | Coopers Animal Health (Intervet Australia Pty Ltd/MSD Animal Health Australia) | Unknown | 2 | Unknown | Bacterin | Australia | Pigs, sheep/lambs |
| ERYSENG® | Hipra | R32/E11 | 2 | 1968 (104) | Bacterin | EU, UK, Brazil, Argentina, Mexico, Taiwan, Thailand, Republic of Belarus, Ukraine, Russia, Georgia, Peru | Pigs |
| MaGESTic® 7 | Merck Animal Health | SE-9 | 2 | 1948 (84) | Bacterin | USA | Pigs |
| Nobilis® Erysipelas | MSD Animal Health | M2 | 2 | 1946 | Bacterin | EU, UK | Turkeys |
| Porcilis®Ery | EU, UK, South Africa | Pigs | |||||
| ER Bac®Plus | Zoetis | CN3342 | 2 | 1963 | Bacterin | USA | Pigs |
| Farrowsure Gold | USA, Canada, South Africa | ||||||
| Eryvac® | Zoetis | Unknown | 1963 (Seed Source: Medical Research Council, National Institute for Medical Research, London) | Bacterin | Australia, New Zealand | Pigs, sheep/lambs | |
| Suvaxyn® Parvo/E | Zoetis | B-7 | 2 | 1989 (Spain) | Bacterin | USA, Canada, EU | Pigs |
| Swivac ERA | Kyoritsu Seiyaku Corp. | SpaA only | 1a | 2011 | Subunit | Japan | Pigs |
| SUIMMUGEN®rART2/ER | KM Biologics Co., Ltd. | SpaA only | 2 | Subunit | Japan | Pigs |
Erysipelas vaccines for usage in pig breeding herds are often available monovalent, or bi- or trivalent in combination with porcine parvovirus and/or Leptospira spp. As monovalent, bivalent or trivalent products from the same company often contain the same Erysipelothrix rhusiopathiae isolate, only one product per isolate is listed.
In breeding herds, vaccination has been shown to increase the numbers of liveborn pigs per litter and to decrease farrowing intervals (52). Breeding herd vaccination has also been shown to decrease the incidence of periparturient vulval discharge (52). Consequently, vaccination against E. rhusiopathiae at every breeding cycle is standard in most pig breeding herds.
Protective immunity after vaccination is generally thought to range between 4 and 6 months. Thus, pig breeding herds are typically re-vaccinated in every cycle or at least twice a year. It has been suggested that in-feed antibiotics could negatively impact vaccine responses, especially when using attenuated erysipelas vaccines (108). However, a study using selected antibiotics could not find major differences between treatment groups, and vaccinated pigs were protected from pathogenic challenge (108). In a similar study by a different group, using 105 8-week old pigs, it was found that vaccination of pigs against erysipelas in the presence of antibiotics may result in a decrease (ceftiofur, doxycycline, tiamulin) or enhancement (amoxillin, tulathromycin) in the production of specific antibodies against Erysipelothrix spp. (106). In contrast, the use of ginseng as a co-adjuvant has been shown to improve the antibody response of vaccination (109). Ginseng contains immunomodulators named ginsenosides, which in pigs enhance the antibody response to viral and bacterial antigens found in vaccines. Two different erysipelas bacterins were tested and the addition of ginseng improved the less immunogenic vaccine so that it became more immunogenic compared to the vaccine without ginseng. The authors concluded that the use of ginseng as a co-adjuvant provides a simple, safe and inexpensive alternative for improving the potency of aluminum hydroxide adjuvanted vaccines (109). Vaccination against other pathogens has not been shown to affect the efficacy of erysipelas vaccines. When pigs were vaccinated with an attenuated porcine reproductive and respiratory syndrome virus (PRRSV) vaccine and later with an attenuated erysipelas vaccine followed by erysipelas challenge, a negative impact of PRRSV vaccine on the erysipelas vaccination was ruled out (110). Besides their usage in farmed animals, Erysipelothrix spp. vaccines are occasionally used off-label in other species, including marine mammals, with encouraging results (111, 112). Marine mammals are highly susceptible to Erysipelothrix spp. infections, the disease course is almost always acute and is commonly fatal (113). Protection against Erysipelothrix spp. isolates recovered from dolphins has been demonstrated in mice vaccinated with a commercial pig bacterin (114). The off-label use of an attenuated swine vaccine in laying hens (35) or turkeys (77) has also been reported.
Autogenous Vaccines
For veterinary purposes, autogenous vaccines refer to “any immunological veterinary medicinal products manufactured (by a qualified person) for the purpose of producing active immunity from pathogenic organisms obtained from an animal or animals from the same herd that have been inactivated and used for the treatment of this animal or of animals from this herd” (Article L 5141-2 of the Public Health code; https://www.biovac.ceva.com/en/Autogenous-vaccines/Autogenous-Vaccines accessed 09-Mar-2020). Autogenous vaccines are popular in the veterinary field, especially in pigs and poultry. They are commonly used in pig or poultry erysipelas outbreaks, usually when there is a perceived lack of commercial vaccine efficacy or when there are no suitable products on the local market. Such vaccines, as outlined above, are limited in their usage to the farm the microorganism originated from. Advantages include the availability of farm-specific isolates to protect the affected herd, while the main disadvantage is the absence of vaccine validation work including dose determination, adjuvant selection and antigen load, which are instead based on estimates from other vaccines or experience. In addition, autogenous vaccines may be accidentally contaminated with other pathogens. In 2004, autogenous vaccines accounted for 15–20% of the US pig market (115). Specifically for erysipelas, of all licensed erysipelas vaccines in the USA during 2019 62.2% were attenuated, 28.6% were inactivated and 9.2% were autogenous (Source: USDA, Dr Paul Hauer).
Cross-Protection Among Erysipelothrix spp. Isolates
Research trials investigating cross-protection among Erysipelothrix spp. isolates are still limited due to the large number of strains corresponding to most serovars and a lack of in vitro models that could be used. Vaccine trials have been conducted in pigs, mice and turkeys. For each trial appropriate positive and negative controls need to be included, and with 3R (replacement, reduction, refinement) regulations it is not always feasible to test more than one isolate per serovar and more than the most commonly occurring serovars. A summary of published trials (77, 105, 116–123) is provided in Table 2.
Table 2.
Cross-protection trials for Erysipelothrix spp., including host species the trial was conducted in (mouse, pig, or turkey), and serovars and Spa details in the vaccines and challenge isolates.
| Vaccine Type | Isolate | Serovar | Spa type | Routea | Dose | Species | Number of animals per serovar (Total) | Number of isolates within a serovar | Serovar used for the challengeb | References | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Protected | Not protected (% mortality) | ||||||||||
| Inactivated | AN-4, SE-9, CN3342, CN3461 | 2 | A | SC | 1× | Mouse | 30-60 (180) | 1,2,4,11 | 9,10 (57–77%) | (117) | |
| 2× | Pig | 3-6 (33) | 1,2,4,11 | 9,10 (70–83%) | |||||||
| Inactivated | AN-4, SE-9, CN3342, CN3461 | 2 | A | SC | 1× | Mouse | 10 (1200) | 10 serovar 1, 2, 4, 9, 10 and 11 isolates | 1,2 | 4,9,10,11 (9–51%) | (105) |
| 2× | Pig | 8 (64) | 1,2 | 9,10 (37.5–100%) | |||||||
| Inactivated | Kyoto | 2 | A | IM | 2× | Pig | 2 (6) | 1a,2 | (124) | ||
| Attenuated | Koganei 65-0.15 | 1a | A | SC | 1× | Pig | 1 (1) | 1a | |||
| Attenuated | Koganei 65-0.15 | 2b | A | SC | 1× | Mouse | 10 (790) | 1a,1b,2,3,5,6,7,8,11, 12,15,16,18,19,21,N | 10,14,20,22 (20–30%) | (116) | |
| ID | 1× | Pig | 2 (78) | 1a,1b,2,5,8,11,12,18, 19,21 | 9,10 (100%) | ||||||
| Attenuated | Koganei 65-0.15 | 2b | A | SC | 1× | Mouse | 10 (200) | 4,6,7,8,9,10,15,16,N | 20 (30%) | (121) | |
| Pig | 2 (40) | 4,6,7,9,15,16,N | 8,10,20 (50%) | ||||||||
| Attenuated | Koganei 65-0.15 | 2b | A | SC | 1× | Mouse | 10 (400) | Two serovar 8 isolates | 1b,2,8,N | 1a,4,5,6,7,8,11,12, 15,16,21 (20–50%) 9,10,18,19,20 (60–100%) | (123) |
| Pig | 2 (40) | Two serovar 8 isolates | 1a,1b,2,4,5,6,7,8,9,10, 11,12,15,16,18,19,21,N | 20 (50%) | |||||||
| Attenuated | EW-2 | 1a | A | Oral | 1 × 2× |
Turkey | 10–13 (82) 10–13 (82) |
1a (15–54%) 1a (10–70%) |
(77) | ||
| Inactivated | Ersipelin, Fort Dodge Lab | Unknown | SC | 1× | 4-8 (64) | 1a | |||||
| Subunit | Not applicable | A | SC | 2× | Mouse Pig |
Not indicated 2(10) | 1a 1a,2 |
(122) | |||
| Subunit | A | SC | 2× | Mouse | 40 | 1a, 2 | 6,18 (30–50%) | (120) | |||
| Not applicable | B | 2× | 40 | 6 | 1a,2,18 (40–50%) | ||||||
| C | 2× | 40 | 6,18 | 1a,2 (10%) | |||||||
| Inactivated | SE-9 | 2 | A | SC | 1× | Mouse | 10–12 (10) | 1a,2,N | 2/15e,N,5,6 (30–100%) | (118) | |
| Subunit | Cc | SC | 2× | Mouse | 10 (200) | 1a,18,19 | (119) | ||||
| Cd | IM | 2× | Pig | 3–6 (9) | 1a | ||||||
Administration route: SC, subcutaneous; IM, intramuscular; ID, intradermal.
This strain is indicated as serovar 2 in these publications.
Full length or N terminal-half region.
N terminal-half region.
Isolate cross-reacted with antisera against serovars 2 and 15.
Protective Antigens
Already in 1970 a protective antigen was described from an E. rhusiopathiae culture (86). In 1990, a 64 to 66-kDa protein in cell wall extracts was shown to be protective (125). However, the genetic fragment, which was cloned in a lambda phage vector and encoded the protein, had not been sequenced at the time. In 1998, the major protective antigen of E. rhusiopathiae, designated as SpaA, was identified and cloned in Escherichia coli and SpaA has since been fully characterized (89, 90). The SpaA protein, whose presence is thought to be highly conserved across E. rhusiopathiae isolates, is not present in E. tonsillarum (30, 31, 37, 120, 126). In 2007, the Erysipelothrix spp. Spa protein was classified into three types, designated as SpaA, SpaB, and SpaC (120). The amino acid sequence similarity within each Spa type was found to be high (96–99%) but among different Spa types it was rather low (~60%). The greatest diversity in Spa proteins was found in the N-terminal half of the molecule (50–57% similarity) (120). Most identified Erysipelothrix spp. isolates from farmed animals contain SpaA. The protective domain of SpaA, cloned from isolate Fujisawa (serovar 1a), was demonstrated to be between amino acid residues 12 to 195 of the protein based on a mouse challenge study (90). In recent years Erysipelothrix spp. isolates containing SpaC have been associated with increased fish mortality (16) in the USA and very recently SpaC has also been identified in Erysipelothrix sp. 2 isolates from turkeys with increased mortality in Brazil (127).
With additional molecular tools becoming available and also affordable, several research groups have investigated Erysipelothrix spp. for the presence of different protective antigens and many of those have also been further characterized. Protection against Erysipelothrix spp. is generally mediated by antibodies against antigens located at its surface and certain antigens present in the culture supernatant (66). The first genomic sequence of E. rhusiopathiae became available in 2011 and indicated presence of a complete set of peptidoglycan biosynthesis genes, two-component regulatory systems, and various cell wall-associated virulence factors, including a capsule and adhesins (1). The capsule above all plays an important role in immune evasion; however, the capsule of E. rhusiopathiae is poorly immunogenic and the antibodies against the capsular antigen are not protective (128, 129).
Other E. rhusiopathiae protective antigens reported so far include RspA (rhusiopathiae surface protein A) (130), CbpB (choline-binding protein) (131, 132), and GAPDH (glyceraldehyde-3-phosphate dehydrogenase) (133). These protective antigens play important roles in biofilm formation (RspA) (130) and adhesion to porcine endothelial cells (SpaA and GAPDH) (133, 134), and to extracellular matrix proteins, including fibronectin (RspA and GAPDH) (130, 133), collagens (RspA) (130), and plasminogen (GAPDH) (133). All of these protective antigens are surface exposed and the protective roles of these proteins are conferred by opsonophagocytic killing by macrophages. While all the protective antigens that have been proposed appear widespread within E. rhusiopathiae (135), it is unknown whether differences in these antigens result in varying degrees of cross-protection.
Immunological Basis or Correlates of Protection for Existing and Novel Vaccine Candidates
By far the best way to assess vaccine efficacy is to do a live pathogen challenge. However, this is not always possible due to cost, animal species assessed (pathogen challenges would be impossible in cetaceans), the high numbers of Erysipelothrix spp. isolates circulating in animals, animal welfare reasons, and lack of availability of appropriate research facilities or relevant permits or both. Therefore, when assessing vaccines and vaccine efficacy one of the first correlates of immunity measured is antibody levels in vaccinated animals, most commonly IgG but also IgM, to confirm that a B-cell response took place. Several assays including agglutination assays, indirect immunofluorescence assays (IFA), ELISAs, and fluorescent microbead immunoassays (FMIAs) have been developed for E. rhusiopathiae (38). In experimentally vaccinated pigs, IgM and IgG responses can be demonstrated between 7 and 21 days post vaccination (136–140). A study using conventional pigs found that vaccination of pigs with a commercial inactivated vaccine in the presence of antibiotics may result in a decrease (ceftiofur, doxycycline, tiamulin) or enhancement (amoxicillin, tulathromycin) in the production of specific antibodies as measured by a commercially available E. rhusiopathiae ELISA (106). During assessment of a large vaccination program in various marine mammals by a modified FMIA, a mean 311-fold increase in the IgG antibody index was detected 14 days after the first booster vaccination (112). In that study serum IgG antibody titers were influenced by the number of vaccinations received but not by age, sex, history of natural infection, adverse vaccine reaction, vaccination interval or time since last vaccination (112). When investigating vaccine responses in endangered species, it may be necessary to develop a novel assay. An ELISA was developed to assess IgY antibodies in kakapos before and after vaccination (141). The results indicated a possible transfer of maternal IgY molecules to fledglings via the yolk. Evidence that vaccination increased the kakapo population's mean anti-E. rhusiopathiae IgY levels was lacking. The authors concluded that vaccination may only raise the IgY levels of birds with relatively low pre-vaccination IgY levels (141). In New Zealand, an existing swine erysipelas ELISA was modified to assess swine erysipelas vaccination in layer birds (142). The domestic poultry were vaccinated twice with an inactivated pig vaccine at low (2 ml) or high (4 ml) dose on days 0 and 21. Optical density readings were higher on days 21, 42, and 63 than day 0 in both groups suggesting that vaccination using either dose induced detectable levels of antibody. The authors concluded that the ELISA will be useful for monitoring responses to vaccination in future (142).
Another possibility to assess whether an animal has been vaccinated and responded appropriately to the vaccination is to measure cell mediated immunity (CMI). This is typically done by enzyme-linked immune absorbent spot (ELIspot) assays, lymphocyte proliferation assays (140, 143), or by assessing presence and amount of cytokines by molecular methods (144). These assays are labor intensive, rely on fresh blood samples that are processed within hours of collection, and are currently only used in selected research laboratories. A study in cetaceans vaccinated off-label with a commercial swine erysipelas vaccine found a vaccine-induced interferon γ response consistent with a T helper 1 (TH1) response which was correlated with lack of clinical erysipelas in that group (144). Furthermore, the same study also compared 6 and 12 month booster vaccinations. While a superior memory response was found in the group re-vaccinated 6 months later, anamnestic responses were only identified in the group re-vaccinated every 12 months (144).
Some studies compared or correlated both humoral immunity and CMI. Under field conditions, most growing pigs will have maternally derived antibodies (MDAs) due to regular breeding herd vaccination, and vaccine efficacy may be difficult to assess using serological assays unless paired serum samples (pre-vaccination and post-vaccination) are available. Previously it was determined that presence of MDAs may interfere with vaccination and subsequent protection (138, 139). The effect of MDAs on the immune response to an oral live E. rhusiopathiae vaccine given at 6, 8, or 10 weeks of age was investigated in conventional pigs (140). A clear seroconversion was only detected in pigs vaccinated at 8 or 10 weeks of age. The CMI response was assessed by a lymphocyte proliferation assay and the investigators found a response in 25% of piglets vaccinated at 6 weeks of age and in 100% of piglets vaccinated at 8 or 10 weeks of age (140). In an Australian study, isolates derived from six herds affected by erysipelas vaccine breakdowns were utilized and responses to commercial and experimental bacterins were assessed (143). The investigators found significantly different humoral and CMI responses (determined by a lymphoproliferation assay) among treatments. While a similar antibody response against a serovar 2 lysate was induced by all vaccines, only those providing significant protection against serovar 1 produced significantly elevated antibodies against the serovar 1 lysate. Vaccination in general significantly reduced CMI responses to the mitogens concanavalin A and phytohaemagglutinin. The results were confirmed in an experimental pig challenge system. The most effective vaccine response was associated with the highest mean serovar 1 antibody response and the highest CMI response to serovar 2 (143).
Evidence of Vaccine Failures, Vaccine Mismatches, and Inability to Provide Cross-Protection
Erysipelas vaccine failures are not always reported and case reports are rare. Over the last few decades, E. rhusiopathiae outbreaks were commonly observed whenever established erysipelas vaccination programs were discontinued in an effort to reduce production costs. Failure to properly vaccinated pigs has been documented (145). This was likely also the case for the last large erysipelas outbreak in the USA during 2000–2001 (32). However, in recent years evidence has grown to support that current vaccines may not always be effective.
In Australia between 1995 and 1998, vaccine failures were reported in four different states (30). The majority of the outbreaks were due to serovar 2 isolates but further characterization with available methods at that time could not identify any single clonal population responsible for the outbreaks (30). As stated in the previous section, challenge studies including CMI and antibody analysis indicated a lack of protection against serovar 1 isolates (143).
More recently, during 2015-2016, a continuous grow-finish farm located in the UK experienced recurring outbreaks of erysipelas in 18–22 week-old pigs (43). Clinically, there was delayed average daily gain, a high incidence of lameness and ear discolorations, with an average morbidity rate of 8–12%. E. rhusiopathiae serovar 15 was isolated from lesions on three different occasions. The source breeding herd was routinely vaccinated with a commercial serovar 2 bacterin which was switched to another bacterin during the investigation without much improvement. Cross-sectional serological assessment of the outbreak farm revealed a lack of anti-Erysipelothrix SpaA antibodies up to ~14 weeks of age. As treatments were not effective, the herd was eventually depopulated (43).
The first documented outbreak of erysipelas in chickens dates back to 1958 (146). Outbreaks of erysipelas in layers have been reported since (35). Commonly the layers are not being vaccinated at the time of the erysipelas outbreak, and subsequent introduction of vaccination programs resolves these situations (35). In Europe, where poultry-dominant countries have relied on erysipelas vaccines for many years, increasing outbreaks in chickens are being observed in various countries including Denmark (48), Germany (46), Sweden (147), the United Kingdom, and the Netherlands (TO, unpublished observations). Erysipelas outbreaks were also described in commercial geese in Poland (44). Affected flocks are often outdoor and organic operations and they are almost always layers. A Swedish study previously confirmed an association of erysipelas in laying hens with housing system (148). Specifically, the risk for an outbreak was higher in free-range systems than in indoor litter-based systems, and lowest for flocks housed in cages (148). Vaccines currently used in European poultry are inactivated and based on serovar 2. Interestingly, the dominant serovars from chicken flocks with outbreaks include 1b and 5 (TO, unpublished observation).
In a more molecular approach to assessing the relationship between field and vaccine strains, the genomic and immunogenic protein diversity of E. rhusiopathiae isolates from pigs obtained between 1987 and 2014 was analyzed and compared to the currently predominant vaccine strain in the UK, an inactivated serovar 2 vaccine (149). While all British pig isolates had one amino acid difference in the 385-amino acid immunoprotective domain of the SpaA protein compared to the vaccine strain, in silico structural protein analyses suggested that this difference is unlikely to compromise vaccine protection. The authors hypothesized that the observed sequence variants in surface proteins could be responsible for differences in the efficacy of the immune response (149). This work was solely based on serovars 1a, 1b and 2 from pigs and should perhaps be expanded to other commonly found serovars and species affected including chickens and serovar 5.
Future Directions in Vaccine Development
Today, commercial erysipelas vaccines, either attenuated or inactivated, are based on a small number of E. rhusiopathiae strains isolated several decades ago. Regardless of the vaccine type, E. rhusiopathiae vaccines are generally considered effective in preventing erysipelas. However, if vaccines are not fully effective or retain residual virulence, chronic forms of the disease, which tend to follow the acute form, may develop. In Australia, outbreaks due to vaccine breakdowns of multivalent and bivalent vaccines have been reported (30). Recently, it was reported that the acriflavine-resistant E. rhusiopathiae vaccine (Koganei 65-0.15 strain), developed from serial passage in the presence of acriflavine mutagen, may have reverted to a virulent strain in vivo and is now associated with clinical disease in Japan (150). Thus, erysipelas vaccines developed using empirical approaches may not represent the best vaccines and require improvement.
Many vaccine platforms have become available over the last decades, including purified microbial components, subunit vaccines based on polysaccharide-carrier protein conjugates or recombinant proteins, DNA vaccines, nanovaccines, and others. New approaches and strategies for vaccine development against swine erysipelas have been achieved and have reached the pre-clinical stage. These include subunit vaccine candidates (Table 3) and attenuated vaccine candidates (Table 4). Microcrystalline cellulose (Avicel PH-101) as a delivery carrier of recombinant protein-based antigen has been assessed by fusing SpaA to a cellulose-binding domain from the fungus Trichoderma harzianum endoglucanase II through a S3N10 peptide (153). The fusion protein was expressed in E. coli and subsequently bound to Avicel PH-101. The vaccine was tested in the mouse model and provided 100% protection in mice against challenge with a serovar 15 isolate (153). While this particular vaccine candidate appears promising it needs to be verified using pigs.
Table 3.
Novel approaches and strategies for subunit erysipelas vaccines.
| Vaccine type | Antigena | Expression vector | Adjuvant | Challenge serovar | Species | Number (route)b | Survival | References |
|---|---|---|---|---|---|---|---|---|
| Subunit | SpaA | E. coli | Whole E. coli cells | 2 (Tama-96) | Mice | 10 (IP) | 100% | (89) |
| Subunit | SpaA | E. coli | 1a (Fujisawa) | Mice | 5 (SC) | 100% | (90) | |
| 5 (IP) | 100% | |||||||
| Subunit | SpaA | E. coli | Freund's adjuvant | 1a (Fujisawa) | Pigs | 4 (IM) | 100% | (122) |
| 2b (82–875) | 2 (IM) | 100% | ||||||
| Subunit | SpaA | Bacillus brevis | E. coli heat-labile enterotoxin | 1a (Fujisawa) | Pigs | 6 (IN) | 100% | (151) |
| Subunit | CbpB | E. coli | Freund's adjuvant | 1a (Fujisawa) | Mice | 10 (IM) | 80% | (131) |
| Pigs | 7 (IM) | 86% | ||||||
| Subunit | GAPDH | E. coli | Freund's adjuvant | 1a (SE38) | Mice (C57BL/6) | 10 (IP) | 100% | (152) |
| HP0728 | 10 (IP) | 0% | ||||||
| HP1472 | 10 (IP) | 0% | ||||||
| CbpB-N | 10 (IP) | 50% | ||||||
| SpaA | 10 (IP) | 100% | ||||||
| None (PBS) | 10 (IP) | 0% | ||||||
| GAPDH | E. coli | Montanide ISA 206 | 1a (SE38) | Pigs | 5 (SC) | 80% | ||
| SpaA | 5 (SC) | 100% | ||||||
| None (PBS) | 5 (SC) | 0% | ||||||
| Subunit | Soluble CBD-SpaA | E. coli | None | 15 | Mice | 8 (SC) | 75% | (153) |
| Coated CBD-SpaA | Avicelc | 8 (SC) | 100% | |||||
| ERT2T-A containing whole bacterin | None | 8 (SC) | 62.5% | |||||
| None (PBS) | None | 8 (SC) | 0% |
SpaA, surface protective antigen A; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; CbpB, choline-binding protein B; PBS, Phosphate-buffered saline; CBD, cellulose-binding domain.
Administration route: IP, intraperitoneal; SC, subcutaneous; IM, intramuscular; IN, intranasal.
Micro-crystalline cellulose.
Table 4.
Novel approaches and strategies for attenuated erysipelas vaccines.
| Vaccine type | E. rhusiopathiae strain | Specifics | Dose (route)a | Challenge serovar | Species | No. of animals used | Survival | References |
|---|---|---|---|---|---|---|---|---|
| Attenuated (vectored) | YS-1 | R1b and R2 regions of the P97 adhesin of M. hyopneumoniae strain E-1 fused with SpaA | 2 (IN) 1 (IN) |
1a | Pigs | 3 3 |
100% 0% |
(154) |
| Koganei 65-0.15 | 7 (oral) | 1a | Pigs | 8 | 100% | (155) | ||
| Attenuated | Δ432 | 651 mutants were screened in mice to determine attenuation and protective immunity | 2 (oral) 1 (oral) |
1a | Pigs | 10 10 |
100% 90% |
(150) |
Administration route: IN, intranasal.
R1 and R2 are repeat regions.
In the swine industry, where cost effective vaccines are strongly required, one of the most challenging strategies is the development of vectored vaccines which can prevent different diseases simultaneously and are inexpensive to produce. Due to the ability of E. rhusiopathiae to effectively induce both humoral and cell-mediated immune responses, attenuated E. rhusiopathiae isolates have been used for this purpose. It was shown that attenuated E. rhusiopathiae vaccines expressing the recombinant protein of the P97 adhesin of Mycoplasma hyopneumoniae could induce protective immunity against a lethal E. rhusiopathiae challenge and also reduce mycoplasmal lesions following experimental infection with a virulent M. hyopneumoniae when administered intranasally (154) and orally (155). It was also found that a single intradermal injection with a needle-free injector of a vectored vaccine was effective against the mycoplasmal infection (YS, unpublished observation). Thus, attenuated E. rhusiopathiae isolates may be used as platform vectors for live delivery of heterologous antigens through the oral and parenteral routes. Very recently, based on genome-wide screening for E. rhusiopathiae virulence-related genes, an E. rhusiopathiae mutant deficient in a tagF homolog (encoding a putative CDP-glycerol glycerophosphotransferase) proved to be a safe and effective vaccine candidate that can be administered via the oral and subcutaneous routes (150). The tagF mutant may be the best choice for the development of vectored vaccines.
Another innovative strategy to protect against Erysipelothrix spp. is utilization of the protective epitope of SpaA protein. It has been shown that SpaA protein is a major protective antigen and the antibodies against the N-terminal one third of the protein play an important role in protection (90). If the protective epitope within this region is identified, the epitope sequences can be included into subunit or DNA vaccines against other pathogens. With the expansion of next generation sequencing, several draft genome sequences of Erysipelothrix spp. isolates have become publicly available (37). Capitalizing on this genome sequence data, bioinformatics approaches may enable identification of novel protective epitopes and/or antigens for possible future vaccine candidates (156).
Changes in vaccine administration may also need to be considered. With more and more outdoor poultry affected by erysipelas, parenteral vaccination may be less suitable, as outdoor poultry can be difficult to catch and vaccinate, especially in larger flocks. Alternatives include in ovo vaccination, nasal vaccination via dust or drop, oral vaccination via the feed, or vaccination via the drinking water similar to pigs or via spray using atomizers. While the ease of vaccination is important, cost is even more important in poultry and needs to be addressed with any new vaccine administration route.
A final necessary consideration into the vaccine efficacy of Erysipelothrix spp. is the compatibility between vaccine and field isolates. Current vaccines are mostly based on E. rhusiopathiae strains isolated several decades ago. Whether these remain effective in providing protection against globally circulating isolates is an important area for investigation. With increasing whole genome sequences of E. rhusiopathiae from various sources available within the public domain, comparative genomic studies are expected to provide valuable insights into these questions.
Thus, exciting studies have been published on novel E. rhusiopathiae vaccine concepts and more research will likely become available in the near future. The importance of testing novel vaccine candidates on a regular basis, side-by-side with trials performed by independent and unbiased institutions to confirm promising candidates and discard the others at a very early stage, should be underscored.
Summary and Conclusions
The history of erysipelas is probably as old as pig domestication, and, for many centuries, this devastating disease resulted in high morbidity and mortality in livestock species. Since the first isolation of Erysipelothrix spp. in 1876 and its link to swine erysipelas in 1882, several important milestones have led to the development of safe and effective vaccines. These are nowadays widely used in farmed pigs, poultry and lambs, as well as in highly susceptible individuals such as marine mammals in commercial aquarium settings. However, a major persistent knowledge gap is the limited to non-existent understanding of the factor(s) that confer protective immunity (i.e., whether a similar serovar, genotype, Spa type or other protective antigen is needed to confer cross-protection, or any combination of these). For example, if an erysipelas outbreak in a vaccinated population occurs, it is currently unknown if serovar is of any importance for cross-protection. Yet, in the field, serotyping of erysipelas isolates in outbreak scenarios is commonly requested by practitioners and results are used to make vaccine decisions, i.e., whether to switch from a serovar 1 vaccine to a serovar 2 or vice versa or to have an autogenous vaccine produced. As outlined, Spa type could also be of importance. However, most erysipelas isolates from farmed animals contain SpaA so excellent cross-protection should be expected. This is based on the assumption that spaA genes are similar across Erysipelothrix spp., which is presently poorly characterized. With vaccine failures increasingly reported in pigs and poultry, and erysipelas also emerging in various wild animal reservoirs, investigations into factors associated with protective immunity are warranted, ultimately leading to novel, updated vaccine candidates for improved protection against erysipelas.
Author Contributions
TO drafted the original manuscript. YS and TF corrected the draft and added relevant information. All authors read and approved the final manuscript version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors thank Ann Chen for assistance in locating literature resources and Ashley Mattei for critical review of this manuscript.
Footnotes
Funding. We acknowledge Biotechnology and Biological Sciences Research Council (BBSRC) support of the Roslin Institute Strategic Programme Control of Infectious Diseases (BBS/E/D/20002173 and BBS/E/D/20002174). TF was supported by a BBSRC Future Leader Fellowship (FORDE/BB/R012075/1).
References
- 1.Ogawa Y, Ooka T, Shi F, Ogura Y, Nakayama K, Hayashi T, et al. The genome of Erysipelothrix rhusiopathiae, the causative agent of swine erysipelas, reveals new insights into the evolution of firmicutes and the organism's intracellular adaptations. J Bacteriol. (2011) 193:2959–71. 10.1128/JB.01500-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Skerman VBD, McGowan V, Sneath PHA. Approved lists of bacterial names. Int J Syst Bacteriol. (1980) 30:225–420. 10.1099/00207713-30-1-225 [DOI] [PubMed] [Google Scholar]
- 3.Buchanan RE. Studies in the nomenclature and classification of the bacteria. J Bacteriol. (1918) 3:27–61. 10.1128/JB.3.1.27-61.1918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Takahashi T, Fujisawa T, Benno Y, Tamura Y, Sawada T, Suzuki S, et al. Erysipelothrix tonsillarum sp. nov. isolated from tonsils of apparently healthy pigs. Int J Syst Bacteriol. (1987) 37:166–8. 10.1099/00207713-37-2-166 [DOI] [Google Scholar]
- 5.Takahashi T, Fujisawa T, Umeno A, Kozasa T, Yamamoto K, Sawada T. A taxonomic study on erysipelothrix by DNA-DNA hybridization experiments with numerous strains isolated from extensive origins. Microbiol Immunol. (2008) 52:469–78. 10.1111/j.1348-0421.2008.00061.x [DOI] [PubMed] [Google Scholar]
- 6.Verbarg S, Rheims H, Emus S, Frühling A, Kroppenstedt RM, Stackebrandt E, et al. Erysipelothrix inopinata sp. nov., isolated in the course of sterile filtration of vegetable peptone broth, and description of Erysipelotrichaceae fam. nov. Int J Syst Evol Microbiol. (2004) 54:221–5. 10.1099/ijs.0.02898-0 [DOI] [PubMed] [Google Scholar]
- 7.Bang BH, Rhee MS, Chang DH, Park DS, Kim BC. Erysipelothrix larvae sp. nov., isolated from the larval gut of the rhinoceros beetle, Trypoxylus dichotomus (Coleoptera: Scarabaeidae). Antonie Van Leeuwenhoek. (2015) 107:443–51. 10.1007/s10482-014-0342-x [DOI] [PubMed] [Google Scholar]
- 8.Pomaranski EK, Griffin MJ, Camus AC, Armwood AR, Shelley J, Waldbieser GC, et al. Description of Erysipelothrix piscisicarius sp. nov., an emergent fish pathogen, and assessment of virulence using a tiger barb (Puntigrus tetrazona) infection model. Int J Syst Evol Microbiol. (2019) 70:857–67. 10.1099/ijsem.0.003838 [DOI] [PubMed] [Google Scholar]
- 9.Wood RL. Swine erysipelas–a review of prevalence and research. J Am Vet Med Assoc. (1984) 184:944–9. [PubMed] [Google Scholar]
- 10.Stephenson EH, Berman DT. Isolation of Erysipelothrix rhusiopathiae from tonsils of apparently normal swine by two methods. Am J Vet Res. (1978) 39:187–8. [PubMed] [Google Scholar]
- 11.Wang Q, Chang BJ, Riley TV. Erysipelothrix rhusiopathiae. Vet Microbiol. (2010) 140:405–17. 10.1016/j.vetmic.2009.08.012 [DOI] [PubMed] [Google Scholar]
- 12.Woodbine M. Erysipelothrix rhusiopathiae. Bacteriology and chemotherapy. Bacteriol Rev. (1950) 14:161–78. 10.1128/MMBR.14.2.161-178.1950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bobrek K, Gawel A, Mazurkiewicz M. Infections with Erysipelothrix rhusiopathiae in poultry flocks. Worlds Poultry Sci J. (2013) 69:803–12. 10.1017/S0043933913000822 [DOI] [Google Scholar]
- 14.Connell R, Moynihan IW, Frank JF. Studies of swine erysipelas. Literature review I, and survey of Erysipelothrix rhusiopathiae infection in Canada. Can J Comp Med Vet Sci. (1952) 16:104–28. [PMC free article] [PubMed] [Google Scholar]
- 15.Eamens GJ, Turner MJ, Catt RE. Serotypes of Erysipelothrix rhusiopathiae in Australian pigs, small ruminants, poultry, and captive wild birds and animals. Aust Vet J. (1988) 65:249–52. 10.1111/j.1751-0813.1988.tb14311.x [DOI] [PubMed] [Google Scholar]
- 16.Pomaranski EK, Reichley SR, Yanong R, Shelley J, Pouder DB, Wolf JC, et al. Characterization of spaC-type Erysipelothrix sp. isolates causing systemic disease in ornamental fish. J Fish Dis. (2018) 41:49–60. 10.1111/jfd.12673 [DOI] [PubMed] [Google Scholar]
- 17.Barber M, Gledhill AW. Discussion on swine erysipelas infection, Erysipelothrix rhusiopathiae, in man and animals. Proc R Soc Med. (1948) 41:328–32. 10.1177/003591574804100543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rosenbach FJ. Experimentelle morphologische und klinische Studie über die krankheitserregenden Mikroorganismen des Schweinerotlaufs, des Erysipeloid und der Mäusesepsis. Z. Hyg. Infektionskr. (1909) 63:343–69. 10.1007/BF02227897 [DOI] [Google Scholar]
- 19.Koch R. Untersuchungen über die Ätiologie der Wundinfektionskrankheiten. In: Schwalbe J, editor. Gesammelte Werke von Robert Koch. Leipzig: Robert Koch-Institut; (1878). p. 61–108. [Google Scholar]
- 20.Shuman RD, Wellman G. Status of the speices name Erysipelothrix rhusiopathiae with request for an opinion. Int J Syst Bacteriol. (1966) 16:195 10.1099/00207713-16-2-195 [DOI] [Google Scholar]
- 21.Pasteur L, Dumas M. Sur le rouget ou mal rouge des porcs. C R Acad Sci. (1882) 95:1120–21. [Google Scholar]
- 22.Löffler D. Experimentelle Untersuchung über Schweine-Rotlauf. Arb. Reichsgesundh. (1886) 1:46. [Google Scholar]
- 23.Kucsera G. Proposal for standardization of the designations used for serotypes of Erysipelothrix rhusiopathiae (Migula) Buchanan. Int J Syst Bacteriol. (1973) 23:184–8. 10.1099/00207713-23-2-184 [DOI] [Google Scholar]
- 24.Imada Y, Takase A, Kikuma R, Iwamaru Y, Akachi S, Hayakawa Y. Serotyping of 800 strains of Erysipelothrix isolated from pigs affected with erysipelas and discrimination of attenuated live vaccine strain by genotyping. J Clin Microbiol. (2004) 42:2121–6. 10.1128/JCM.42.5.2121-2126.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dedié K. Die säurelöslichen Antigene von Erysipelothrix rhusiopathiae. Monatshefte fur Veterinärmedizin. (1949) 4:7. [Google Scholar]
- 26.Ogawa Y, Shiraiwa K, Nishikawa S, Eguchi M, Shimoji Y. Identification of the chromosomal region essential for serovar-specific antigen and virulence of serovar 1 and 2 strains of Erysipelothrix rhusiopathiae. Infect Immun. (2018) 86:e00324-18. 10.1128/IAI.00324-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shiraiwa K, Ogawa Y, Nishikawa S, Eguchi M, Shimoji Y. Identification of serovar 1a, 1b, 2, and 5 strains of Erysipelothrix rhusiopathiae by a conventional gel-based PCR. Vet Microbiol. (2018) 225:101–4. 10.1016/j.vetmic.2018.09.014 [DOI] [PubMed] [Google Scholar]
- 28.Pal N, Bender JS, Opriessnig T. Rapid detection and differentiation of Erysipelothrix spp. by a novel multiplex real-time PCR assay. J Appl Microbiol. (2010) 108:1083–93. 10.1111/j.1365-2672.2009.04560.x [DOI] [PubMed] [Google Scholar]
- 29.McNeil M, Gerber PF, Thomson J, Williamson S, Opriessnig T. Serotypes and Spa types of Erysipelothrix rhusiopathiae isolates from British pigs (1987 to 2015). Vet J. (2017) 225:13–5. 10.1016/j.tvjl.2017.04.012 [DOI] [PubMed] [Google Scholar]
- 30.Eamens GJ, Forbes WA, Djordjevic SP. Characterisation of Erysipelothrix rhusiopathiae isolates from pigs associated with vaccine breakdowns. Vet Microbiol. (2006) 115:329–38. 10.1016/j.vetmic.2006.02.015 [DOI] [PubMed] [Google Scholar]
- 31.To H, Sato H, Tazumi A, Tsutsumi N, Nagai S, Iwata A, et al. Characterization of Erysipelothrix rhusiopathiae strains isolated from recent swine erysipelas outbreaks in Japan. J Vet Med Sci. (2012) 74:949–53. 10.1292/jvms.11-0533 [DOI] [PubMed] [Google Scholar]
- 32.Opriessnig T, Hoffman LJ, Harris DL, Gaul SB, Halbur PG. Erysipelothrix rhusiopathiae: genetic characterization of midwest US isolates and live commercial vaccines using pulsed-field gel electrophoresis. J Vet Diagn Invest. (2004) 16:101–7. 10.1177/104063870401600202 [DOI] [PubMed] [Google Scholar]
- 33.Nakazawa H, Hayashidani H, Higashi J, Kaneko K, Takahashi T, Ogawa M. Occurrence of Erysipelothrix spp. in broiler chickens at an abattoir. J Food Prot. (1998) 61:907–9. 10.4315/0362-028X-61.7.907 [DOI] [PubMed] [Google Scholar]
- 34.Eriksson H, Jansson DS, Johansson KE, Baverud V, Chirico J, Aspan A. Characterization of Erysipelothrix rhusiopathiae isolates from poultry, pigs, emus, the poultry red mite and other animals. Vet Microbiol. (2009) 137:98–104. 10.1016/j.vetmic.2008.12.016 [DOI] [PubMed] [Google Scholar]
- 35.Crespo R, Bland M, Opriessnig T. Use of commercial swine live attenuated vaccine to control an Erysipelothrix rhusiopathiae outbreak in commercial cage-free layer chickens. Avian Dis. (2019) 63:520–4. 10.1637/12004-112118-Case.1 [DOI] [PubMed] [Google Scholar]
- 36.Coutinho TA, Imada Y, Barcellos DE, Oliveira SJ, Moreno AM. Phenotypic and molecular characterization of recent and archived Erysipelothrix spp. isolated from Brazilian swine. Diagn Microbiol Infect Dis. (2011) 69:123–9. 10.1016/j.diagmicrobio.2010.09.012 [DOI] [PubMed] [Google Scholar]
- 37.Forde T, Biek R, Zadoks R, Workentine ML, De Buck J, Kutz S, et al. Genomic analysis of the multi-host pathogen Erysipelothrix rhusiopathiae reveals extensive recombination as well as the existence of three generalist clades with wide geographic distribution. BMC Genomics. (2016) 17:461. 10.1186/s12864-016-2643-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Opriessnig T, Coutinho TA. 53: Erysipelas. In: Zimmerman JJ, Karriker LA, Ramirez A, Schwartz K, Stevenson GW, Zhang J, editors. Diseases of Swine. Hobokon, NJ: Wiley Blackwell; (2019). p. 835–43. 10.1002/9781119350927.ch53 [DOI] [Google Scholar]
- 39.Bricker JM, Saif YM. Erysipelas. In: Saif YM, Barnes HJ, Glisson JR, Fadly AM, McDougald LR, Swayne DE, editor. Diseases of Poultry. Ames, IA: Iowa State Press; (2003). p. 812–26. [Google Scholar]
- 40.Angus K. Arthritis in lambs and sheep. In Practice. (1991) 13:204–7. 10.1136/inpract.13.5.204 [DOI] [Google Scholar]
- 41.Reganold JP, Wachter JM. Organic agriculture in the twenty-first century. Nat Plants. (2016) 2:15221. 10.1038/nplants.2015.221 [DOI] [PubMed] [Google Scholar]
- 42.Tellez G, Laukova A, Latorre JD, Hernandez-Velasco X, Hargis BM, Callaway T. Food-producing animals and their health in relation to human health. Microb Ecol Health Dis. (2015) 26:25876. 10.3402/mehd.v26.25876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gerber PF, MacLeod A, Opriessnig T. Erysipelothrix rhusiopathiae serotype 15 associated with recurring pig erysipelas outbreaks. Vet Rec. (2018) 182:635. 10.1136/vr.104421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bobrek K, Gawel A. Erysipelas outbreaks in flocks of geese in Poland–biochemical and genetic analyses of the isolates. Avian Dis. (2015) 59:436–9. 10.1637/11050-030115-Case.1 [DOI] [PubMed] [Google Scholar]
- 45.Zou Y, Zhu X, Muhammad HM, Jiang P, Li Y. Characterization of Erysipelothrix rhusiopathiae strains isolated from acute swine erysipelas outbreaks in Eastern China. J Vet Med Sci. (2015) 77:653–60. 10.1292/jvms.14-0589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schmitt F, Schade B, Böhm B, Shimoji Y, Pfahler C. Erysipelas in a free-range layer flock with conjunctival oedema as an unusual clinical sign. Berl Munch Tierarztl Wochenschr. (2014) 127:183–7. 10.2376/0005-9366-127-183 [DOI] [PubMed] [Google Scholar]
- 47.Eriksson H, Bagge E, Baverud V, Fellstrom C, Jansson DS. Erysipelothrix rhusiopathiae contamination in the poultry house environment during erysipelas outbreaks in organic laying hen flocks. Avian Pathol. (2014) 43:231–7. 10.1080/03079457.2014.907485 [DOI] [PubMed] [Google Scholar]
- 48.Stokholm NM, Permin A, Bisgaard M, Christensen JP. Causes of mortality in commercial organic layers in Denmark. Avian Dis. (2010) 54:1241–50. 10.1637/9375-041910-Reg.1 [DOI] [PubMed] [Google Scholar]
- 49.Stilwell JM, Griffin MJ, Rosser TG, Leary J, Hagen-Frei K, Mischke CC, et al. First detection of Erysipelothrix sp. infection in western mosquitofish Gambusia affinis inhabiting catfish aquaculture ponds in Mississippi, USA. Dis Aquat Organ. (2019) 133:39–46. 10.3354/dao03332 [DOI] [PubMed] [Google Scholar]
- 50.Spraker TR, White PA. Shaggy Lame Fox Syndrome in Pribilof Island Arctic Foxes (Alopex lagopus pribilofensis), Alaska. Vet Pathol. (2017) 54:258–68. 10.1177/0300985816660745 [DOI] [PubMed] [Google Scholar]
- 51.Forde TL, Orsel K, Zadoks RN, Biek R, Adams LG, Checkley SL, et al. Bacterial genomics reveal the complex epidemiology of an emerging pathogen in arctic and boreal ungulates. Front Microbiol. (2016) 7:1759. 10.3389/fmicb.2016.01759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hoffmann CW, Bilkei G. Case study: chronic erysipelas of the sow–a subclinical manifestation of reproductive problems. Reprod Domest Anim. (2002) 37:119–20. 10.1046/j.1439-0531.2002.00339.x [DOI] [PubMed] [Google Scholar]
- 53.Bender JS, Irwin CK, Shen HG, Schwartz KJ, Opriessnig T. Erysipelothrix spp. genotypes, serotypes, and surface protective antigen types associated with abattoir condemnations. J Vet Diagn Invest. (2011) 23:139–42. 10.1177/104063871102300126 [DOI] [PubMed] [Google Scholar]
- 54.Bickford AA, Corstvet RE, Rosenwald AS. Pathology of experimental erysipelas in turkeys. Avian Dis. (1978) 22:503–18. 10.2307/1589306 [DOI] [PubMed] [Google Scholar]
- 55.Hollifield JL, Cooper GL, Charlton BR. An outbreak of erysipelas in 2-day-old poults. Avian Dis. (2000) 44:721–4. 10.2307/1593119 [DOI] [PubMed] [Google Scholar]
- 56.Mutalib A, Keirs R, Austin F. Erysipelas in quail and suspected erysipeloid in processing plant employees. Avian Dis. (1995) 39:191–3. 10.2307/1592002 [DOI] [PubMed] [Google Scholar]
- 57.Ersdal C, Jorgensen HJ, Lie KI. Acute and chronic Erysipelothrix rhusiopathiae infection in lambs. Vet Pathol. (2015) 52:635–43. 10.1177/0300985814556187 [DOI] [PubMed] [Google Scholar]
- 58.Armbrecht P. Veterinarians on call. National Hog Farmer: (1990). 52-5. [Google Scholar]
- 59.Wang Q, Fidalgo S, Chang BJ, Mee BJ, Riley TV. The detection and recovery of Erysipelothrix spp. in meat and abattoir samples in Western Australia. J Appl Microbiol. (2002) 92:844–50. 10.1046/j.1365-2672.2002.01578.x [DOI] [PubMed] [Google Scholar]
- 60.Hariharan H, MacDonald J, Carnat B, Bryenton J, Heaney S. An investigation of bacterial causes of arthritis in slaughter hogs. J Vet Diagn Invest. (1992) 4:28–30. 10.1177/104063879200400107 [DOI] [PubMed] [Google Scholar]
- 61.Colavita G, Vergara A, Ianieri A. Deferment of slaughtering in swine affected by cutaneous erysipelas. Meat Sci. (2006) 72:203–5. 10.1016/j.meatsci.2005.07.001 [DOI] [PubMed] [Google Scholar]
- 62.Makino S, Okada Y, Maruyama T, Ishikawa K, Takahashi T, Nakamura M, et al. Direct and rapid detection of Erysipelothrix rhusiopathiae DNA in animals by PCR. J Clin Microbiol. (1994) 32:1526–31. 10.1128/JCM.32.6.1526-1531.1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pinchuk NG. Analysis of the epizootic situation of the swine erysipelas on the territory of Ukraine for 2006-2017. Vet Biotechnology. (2019) 34:108–18. 10.31073/vet_biotech34-13 [DOI] [Google Scholar]
- 64.White M. Erysipelas: Why is it still a problem after 100 years? The Pig Site (2008). Available online at: https://thepigsite.com/articles/erysipelas-why-is-it-still-a-problem-after-100-years (accessed March 28, 2020).
- 65.VanderWaal K, Deen J. Global trends in infectious diseases of swine. Proc Natl Acad Sci USA. (2018) 115:11495–500. 10.1073/pnas.1806068115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Haesebrouck F, Pasmans F, Chiers K, Maes D, Ducatelle R, Decostere A. Efficacy of vaccines against bacterial diseases in swine: what can we expect? Vet Microbiol. (2004) 100:255–68. 10.1016/j.vetmic.2004.03.002 [DOI] [PubMed] [Google Scholar]
- 67.Pasteur L, Thuillier L. La vaccination du rouget des porcs à l'aide due virus mortel atténué de cete maladie. C R Acad Sci. (1883) 95:1163–71. [Google Scholar]
- 68.Berche P. Louis Pasteur, from crystals of life to vaccination. Clin Microbiol Infect. (2012) 5:1–6. 10.1111/j.1469-0691.2012.03945.x [DOI] [PubMed] [Google Scholar]
- 69.Cooper MS, Personeus GR, Choman BR. Laboratory studies on the vaccination of mice and turkeys with an Erysipelothrix rhusiopathiae vaccine. Can J Comp Med Vet Sci. (1954) 18:83–92. [PMC free article] [PubMed] [Google Scholar]
- 70.Gray CW, Norden CJ, Jr. Erysipelas vaccine avirulent (EVA) a new agent for erysipelas control. J Am Vet Med Assoc. (1955) 127:506–10. [PubMed] [Google Scholar]
- 71.Ose EE, Barnes LE, Berkman RN. Experimental evaluation of an avirulent oral erysipelas vaccine. J Am Vet Med Assoc. (1963) 143:1084–9. [PubMed] [Google Scholar]
- 72.Lawson KF, Pepevnak F, Walker VCR, Crawley JF. Vaccination of swine with erysipelas vaccine (live culture-modified) by the oral route. Can Vet J. (1966) 7:13–7. [PMC free article] [PubMed] [Google Scholar]
- 73.Staub A. Sur la vaccination contre le rouget du porc. C R Acad Sci Paris. (1939) 208:775–6. [Google Scholar]
- 74.Kondo S, Sugimura K. Experimental studies regarding living swine erysipelas vacine. II. The pathogenicity and immunizing property for swine of aviulrent swine erysipelas bacilli obtained by treating with trypaflavin. J Jpn Soc Vet Sci. (1935) 14:322–39. 10.1292/jvms1922.14.322 [DOI] [Google Scholar]
- 75.Wood RL. Erysipelas. In: Straw EB, D'Allaire S, Mengeling WL, Taylor DJ, editors. Diseases of Swine. Ames, IA: Iowa State University Press; (1999). p. 419–30. [Google Scholar]
- 76.Neumann EJ, Grinberg A, Bonistalli KN, Mack HJ, Lehrbach PR, Gibson N. Safety of a live attenuated Erysipelothrix rhusiopathiae vaccine for swine. Vet Microbiol. (2009) 135:297–303. 10.1016/j.vetmic.2008.09.059 [DOI] [PubMed] [Google Scholar]
- 77.Bricker JM, Saif YM. Use of a live oral vaccine to immunize turkeys against erysipelas. Avian Dis. (1988) 32:668–73. 10.2307/1590982 [DOI] [PubMed] [Google Scholar]
- 78.Shiraiwa K, Ogawa Y, Nishikawa S, Kusumoto M, Eguchi M, Shimoji Y. Single nucleotide polymorphism genotyping of Erysipelothrix rhusiopathiae isolates from pigs affected with chronic erysipelas in Japan. J Vet Med Sci. (2017) 79:699–701. 10.1292/jvms.17-0040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Van Es L, McGrath CB. Historical research bulletins of the nebraska agricultural experiment station. Swine erysipelas, Revised edition of Research Bulletin 84 (1942). Available online at: http://digitalcommons.unl.edu/ardhistrb/205
- 80.Cussler K, Balks E. [100 years of erysipelas prophylaxis: significance and reduction of animal experiments]. Altex. (2001) 18:29–33. Available online at: https://www.altex.org/index.php/altex/article/view/1370 (accessed March 28, 2020). [PubMed] [Google Scholar]
- 81.Lorenz Ein Schutzimpfungsverfahren gegen Schweinerotlauf. Zentralbl f. Bakteriol. (1893) 13:357. [Google Scholar]
- 82.Fosterman AA, Utica SD. Personal observations and experiences with swine erysipelas. Iowa Veterinarian. (1934) 5:5–7, 20–2. [Google Scholar]
- 83.Ray JD. Transmissible diseases of swine. Vet Med. (1952) 47:9. [Google Scholar]
- 84.Dinter Z. Zur Schutzimpfung gegen Schweinerotlauf mit einer Adsorbatvakzine. Tierärztliche Umschau. (1948). 3(17-18):279–81. [PubMed] [Google Scholar]
- 85.Traub E. Immunisierung gegen Schweinerotlauf mit konzentrierten adsorbat. Impfstoffen. Monatsh. Veterinaermed. (1947) 2:165–72. [Google Scholar]
- 86.White RR, Verwey WF. Solubilization and characterization of a protective antigen of Erysipelothrix rhusiopathiae. Infect Immun. (1970) 1:387–93. 10.1128/IAI.1.4.387-393.1970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Timoney JF, Groschup MM. Properties of a protective protein antigen of Erysipelothrix rhusiopathiae. Vet Microbiol. (1993) 37:381–7. 10.1016/0378-1135(93)90036-7 [DOI] [PubMed] [Google Scholar]
- 88.Sato H, Hirose K, Saito H. Protective activity and antigenic analysis of fractions of culture filtrates of Erysipelothrix rhusiopathiae. Vet Microbiol. (1995) 43:173–82. 10.1016/0378-1135(95)92533-H [DOI] [PubMed] [Google Scholar]
- 89.Makino S, Yamamoto K, Murakami S, Shirahata T, Uemura K, Sawada T, et al. Properties of repeat domain found in a novel protective antigen, SpaA, of Erysipelothrix rhusiopathiae. Microb Pathog. (1998) 25:101–9. 10.1006/mpat.1998.0216 [DOI] [PubMed] [Google Scholar]
- 90.Shimoji Y, Mori Y, Fischetti VA. Immunological characterization of a protective antigen of Erysipelothrix rhusiopathiae: identification of the region responsible for protective immunity. Infect Immun. (1999) 67:1646–51. 10.1128/.67.4.1646-1651.1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Jungk NK, Murdock FM. An emulsion-type erysipelas bacterin. I. Duration of immunity in pigs vaccinated at weaning. Am J Vet Res. (1957) 18:121–5. [PubMed] [Google Scholar]
- 92.Jungk NK, Murdock FM. An emulsion-type erysipelas bacterin. II. Duration of immunity following vaccination of newborn pigs. Am J Vet Res. (1957) 18:126–32. [PubMed] [Google Scholar]
- 93.Adler HE, Nilson MA. Immunization of turkeys against swine erysipelas with several types of bacterins. Can J Comp Med Vet Sci. (1952) 16:390–3. [PMC free article] [PubMed] [Google Scholar]
- 94.Delpy D-P, Hars E. Observations sure le mode d'action des vaccins tués Vaccin solubilisé (Immunigène) contre le rouget du porc. Bull Acad Vet Fr. (1953) 26:539–46. 10.4267/2042/69144 [DOI] [Google Scholar]
- 95.Gouge HE, Bolton R, Brown R. Laboratory studies on erysipelas. II. Use of various cultures in production of infection in pigs by skin scarification. Am J Vet Res. (1956) 17:132–4. [PubMed] [Google Scholar]
- 96.Balks E, Wolf C, Loessner H, Werner E. Towards in vitro potency testing of inactivated erysipelas vaccines. Dev Biol. (2012) 134:37–44. [PubMed] [Google Scholar]
- 97.Schutte K, Szczepanska A, Halder M, Cussler K, Sauer UG, Stirling C, et al. Modern science for better quality control of medicinal products “Towards global harmonization of 3Rs in biologicals”: The report of an EPAA workshop. Biologicals. (2017) 48:55–65. 10.1016/j.biologicals.2017.05.006 [DOI] [PubMed] [Google Scholar]
- 98.Rosskopf-Streicher U, Johannes S, Wilhelm M, Cussler K. Quality control of inactivated erysipelas vaccines: results of an international collaborative study to establish a new regulatory test. Vaccine. (2001) 19:1477–83. 10.1016/S0264-410X(00)00346-7 [DOI] [PubMed] [Google Scholar]
- 99.Rosskopf-Streicher U, Johannes S, Gyra H, Beckmann R, Cussler K. Batch potency testing of inactivated erysipelas vaccines by ELISA–development, validation and implementation. Dev Biol. (2002) 111:153–8. [PubMed] [Google Scholar]
- 100.Gledhill AW. The passive protection of mice against infection with E. rhusiopathiae. J Comp Pathol. (1945) 55:98–108. 10.1016/S0368-1742(45)80010-3 [DOI] [Google Scholar]
- 101.Cromwell GL. Why and how antibiotics are used in swine production. Anim Biotechnol. (2002) 13:7–27. 10.1081/ABIO-120005767 [DOI] [PubMed] [Google Scholar]
- 102.Sandstedt H, Lehrert E. Experience of swine erysipelas immunization in (1943). Sven Vet Tidskr. (1944) 56:189–92. [Google Scholar]
- 103.Seto K, Nishimura Y, Fujiki M, Azechi H, Suzuki K. [Attenuated acriflavin-fast Erysipelothrix insidiosa. Relationship between its capability to cause arthritis in mice and immunogenicity in swine]. Nihon Juigaku Zasshi. (1971) 33:161–71. 10.1292/jvms1939.33.161 [DOI] [PubMed] [Google Scholar]
- 104.Erler W. [Serological, chemical and immunochemical studies of the erysipelas bacteria. II. Purification of the type-specific hydrochloric acid extract of the erysipelas bacteria of the strain R32 E11, type B1]. Zentralbl Bakteriol Orig. (1968) 207:340–53. [PubMed] [Google Scholar]
- 105.Wood RL, Booth GD, Cutlip RC. Susceptibility of vaccinated swine and mice to generalized infection with specific serotypes of Erysipelothrix rhusiopathiae. Am J Vet Res. (1981) 42:608–14. [PubMed] [Google Scholar]
- 106.Pomorska-Mol M, Kwit K, Wierzchoslawski K, Dors A, Pejsak Z. Effects of amoxicillin, ceftiofur, doxycycline, tiamulin and tulathromycin on pig humoral immune responses induced by erysipelas vaccination. Vet Rec. (2016) 178:559. 10.1136/vr.103533 [DOI] [PubMed] [Google Scholar]
- 107.Ogawa Y, Shiraiwa K, Ogura Y, Ooka T, Nishikawa S, Eguchi M, et al. Clonal lineages of Erysipelothrix rhusiopathiae responsible for acute swine erysipelas in Japan identified by using genome-wide single-nucleotide polymorphism analysis. Appl Environ Microbiol. (2017) 83:e00130-17. 10.1128/AEM.00130-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Yamamoto K, Takagi M, Endoh YS, Kijima M, Takahashi T. Influence of antibiotics used as feed additives on the immune effect of erysipelas live vaccine in swine. J Vet Med B Infect Dis Vet Public Health. (2000) 47:453–60. 10.1046/j.1439-0450.2000.00366.x [DOI] [PubMed] [Google Scholar]
- 109.Rivera E, Daggfeldt A, Hu S. Ginseng extract in aluminium hydroxide adjuvanted vaccines improves the antibody response of pigs to porcine parvovirus and Erysipelothrix rhusiopathiae. Vet Immunol Immunopathol. (2003) 91:19–27. 10.1016/S0165-2427(02)00269-6 [DOI] [PubMed] [Google Scholar]
- 110.Sakano T, Shibata I, Namimatsu T, Mori M, Ono M, Uruno K, et al. Effect of attenuated Erysipelothrix rhusiopathiae vaccine in pigs infected with porcine reproductive respiratory syndrome virus. J Vet Med Sci. (1997) 59:977–81. 10.1292/jvms.59.977 [DOI] [PubMed] [Google Scholar]
- 111.Lacave G, Cui Y, Salbany A, Flanagan C, Grande F, Cox E. Erysipelas vaccination protocols in dolphins Tursiops truncatus evaluated by antibody responses over twenty continuous years. Dis Aquat Organ. (2019) 134:237–55. 10.3354/dao03367 [DOI] [PubMed] [Google Scholar]
- 112.Nollens HH, Gimenéz-Lirola LG, Robeck TR, Schmitt TL, DiRocco S, Opriessnig T. Evaluation of anti-Erysipelothrix rhusiopathiae IgG response in bottlenose dolphins Tursiops truncatus to a commercial pig vaccine. Dis Aquat Organ. (2016) 121:249–56. 10.3354/dao03061 [DOI] [PubMed] [Google Scholar]
- 113.Kinsel MJ, Boehm JR, Harris B, Murnane RD. Fatal Erysipelothrix rhusiopathiae septicemia in a captive Pacific white-sided dolphin (Lagenorhyncus obliquidens). J Zoo Wildl Med. (1997) 28:494–7. [PubMed] [Google Scholar]
- 114.Lacave G, Cox E, Hermans J, Devriese L, Goddeeris BM. Induction of cross-protection in mice against dolphin Erysipelothrix rhusiopathiae isolates with a swine commercial vaccine. Vet Microbiol. (2001) 80:247–53. 10.1016/S0378-1135(01)00311-X [DOI] [PubMed] [Google Scholar]
- 115.Chase CC. Autogenous vaccines: current use in the field in the U.S. cattle and hog industry. Dev Biol. (2004) 117:69–71. [PubMed] [Google Scholar]
- 116.Takahashi T, Takagi M, Sawada T, Seto K. Cross protection in mice and swine immunized with live erysipelas vaccine to challenge exposure with strains of Erysipelothrix rhusiopathiae of various serotypes. Am J Vet Res. (1984) 45:2115–8. [PubMed] [Google Scholar]
- 117.Wood RL. Specificity in response of vaccinated swine and mice to challenge exposure with strains of Erysipelothrix rhusiopathiae of various serotypes. Am J Vet Res. (1979) 40:795–801. [PubMed] [Google Scholar]
- 118.Ingebritson AL, Roth JA, Hauer PJ. Erysipelothrix rhusiopathiae: association of Spa-type with serotype and role in protective immunity. Vaccine. (2010) 28:2490–6. 10.1016/j.vaccine.2010.01.041 [DOI] [PubMed] [Google Scholar]
- 119.To H, Someno S, Nagai S, Koyama T, Nagano T. Immunization with truncated recombinant protein SpaC of Erysipelothrix rhusiopathiae strain 715 serovar 18 confers protective immunity against challenge with various serovars. Clin Vaccine Immunol. (2010) 17:1991–7. 10.1128/CVI.00213-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.To H, Nagai S. Genetic and antigenic diversity of the surface protective antigen proteins of Erysipelothrix rhusiopathiae. Clin Vaccine Immunol. (2007) 14:813–20. 10.1128/CVI.00099-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Sawada T, Takahashi T. Cross protection of mice and swine given live-organism vaccine against challenge exposure with strains of Erysipelothrix rhusiopathiae representing ten serovars. Am J Vet Res. (1987) 48:81–4. [PubMed] [Google Scholar]
- 122.Imada Y, Goji N, Ishikawa H, Kishima M, Sekizaki T. Truncated surface protective antigen (SpaA) of Erysipelothrix rhusiopathiae serotype 1a elicits protection against challenge with serotypes 1a and 2b in pigs. Infect Immun. (1999) 67:4376–82. 10.1128/IAI.67.9.4376-4382.1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Sawada T, Takahashi T. Cross protection of mice and swine inoculated with culture filtrate of attenuated Erysipelothrix rhusiopathiae and challenge exposed to strains of various serovars. Am J Vet Res. (1987) 48:239–42. [PubMed] [Google Scholar]
- 124.Kitajima T, Oishi E, Amimoto K, Ui S, Nakamura H, Okada N, et al. Protective effect of NaOH-extracted Erysipelothrix rhusiopathiae vaccine in pigs. J Vet Med Sci. (1998) 60:9–14. 10.1292/jvms.60.9 [DOI] [PubMed] [Google Scholar]
- 125.Galan JE, Timoney JF. Cloning and expression in Escherichia coli of a protective antigen of Erysipelothrix rhusiopathiae. Infect Immun. (1990) 58:3116–21. 10.1128/IAI.58.9.3116-3121.1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Janssen T, Voss M, Kuhl M, Semmler T, Philipp HC, Ewers C. A combinational approach of multilocus sequence typing and other molecular typing methods in unravelling the epidemiology of Erysipelothrix rhusiopathiae strains from poultry and mammals. Vet Res. (2015) 46:84. 10.1186/s13567-015-0216-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Hoepers PG, dos Reis TFM, Mendonça EP, Rossi DA, Koerich PK, França TVJ, et al. First outbreak reported caused by Erysipelothrix species strain 2 in turkeys from poultry-producing farms in Brazil. Ann Microbiol. (2019) 69:1211–5. 10.1007/s13213-019-01505-3 [DOI] [Google Scholar]
- 128.Shimoji Y, Yokomizo Y, Sekizaki T, Mori Y, Kubo M. Presence of a capsule in Erysipelothrix rhusiopathiae and its relationship to virulence for mice. Infect Immun. (1994) 62:2806–10. 10.1128/IAI.62.7.2806-2810.1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Shi F, Harada T, Ogawa Y, Ono H, Ohnishi-Kameyama M, Miyamoto T, et al. Capsular polysaccharide of Erysipelothrix rhusiopathiae, the causative agent of swine erysipelas, and its modification with phosphorylcholine. Infect Immun. (2012) 80:3993–4003. 10.1128/IAI.00635-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Shimoji Y, Ogawa Y, Osaki M, Kabeya H, Maruyama S, Mikami T, et al. Adhesive surface proteins of Erysipelothrix rhusiopathiae bind to polystyrene, fibronectin, and type I and IV collagens. J Bacteriol. (2003) 185:2739–48. 10.1128/JB.185.9.2739-2748.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Shi F, Ogawa Y, Sano A, Harada T, Hirota J, Eguchi M, et al. Characterization and identification of a novel candidate vaccine protein through systematic analysis of extracellular proteins of Erysipelothrix rhusiopathiae. Infect Immun. (2013) 81:4333–40. 10.1128/IAI.00549-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Zhu W, Cai C, Huang J, Liu L, Xu Z, Sun X, et al. Characterization of pathogenic roles of two Erysipelothrix rhusiopathiae surface proteins. Microb Pathog. (2018) 114:166–8. 10.1016/j.micpath.2017.11.057 [DOI] [PubMed] [Google Scholar]
- 133.Zhu W, Zhang Q, Li J, Wei Y, Cai C, Liu L, et al. Glyceraldehyde-3-phosphate dehydrogenase acts as an adhesin in Erysipelothrix rhusiopathiae adhesion to porcine endothelial cells and as a receptor in recruitment of host fibronectin and plasminogen. Vet Res. (2017) 48:16. 10.1186/s13567-017-0421-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Harada T, Ogawa Y, Eguchi M, Shi F, Sato M, Uchida K, et al. Phosphorylcholine and SpaA, a choline-binding protein, are involved in the adherence of Erysipelothrix rhusiopathiae to porcine endothelial cells, but this adherence is not mediated by the PAF receptor. Vet Microbiol. (2014) 172:216–22. 10.1016/j.vetmic.2014.04.012 [DOI] [PubMed] [Google Scholar]
- 135.Shimoji Y, Bito M, Shiraiwa K, Ogawa Y, Nishikawa S, Eguchi M. Disassociation of Spa type and serovar of an Erysipelothrix rhusiopathiae serovar 6 strain isolated from a diseased pig. J Vet Diagn Invest. (2019) 31:488–91. 10.1177/1040638719835883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Gimenéz-Lirola LG, Xiao CT, Halbur PG, Opriessnig T. Development of a novel fluorescent microbead-based immunoassay and comparison with three enzyme-linked immunoassays for detection of anti-Erysipelothrix spp. IgG antibodies in pigs with known and unknown exposure. J Microbiol Methods. (2012) 91:73–9. 10.1016/j.mimet.2012.07.014 [DOI] [PubMed] [Google Scholar]
- 137.Gimenéz-Lirola LG, Xiao CT, Halbur PG, Opriessnig T. Development and evaluation of an enzyme-linked immunosorbent assay based on a recombinant SpaA protein (rSpaA415) for detection of anti-Erysipelothrix spp. IgG antibodies in pigs. J Microbiol Methods. (2012) 91:191–7. 10.1016/j.mimet.2012.06.011 [DOI] [PubMed] [Google Scholar]
- 138.Imada Y, Mori Y, Daizoh M, Kudoh K, Sakano T. Enzyme-linked immunosorbent assay employing a recombinant antigen for detection of protective antibody against swine erysipelas. J Clin Microbiol. (2003) 41:5015–21. 10.1128/JCM.41.11.5015-5021.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Sawada T, Takahashi T, Takagi M, Seto K. Effect of live Erysipelothrix vaccine in piglets with maternally acquired antibody. Annu Rep Natl Vet Assay Lab. (1989) 26:3–19. [Google Scholar]
- 140.Pomorska-Mol M, Markowska-Daniel I, Pejsak Z. Effect of age and maternally-derived antibody status on humoral and cellular immune responses to vaccination of pigs against Erysipelothrix rhusiopathiae. Vet J. (2012) 194:128–30. 10.1016/j.tvjl.2012.03.009 [DOI] [PubMed] [Google Scholar]
- 141.Livingston M, Fidler A, Mellor D, de Kloet S, Eason D, Elliott G, et al. Prevalence of IgY antibodies against Erysipelothrix rhusiopathiae in a critically endangered parrot (kakapo, Strigops habroptilus) and associated responses to vaccination. Avian Pathol. (2013) 42:502–7. 10.1080/03079457.2013.832146 [DOI] [PubMed] [Google Scholar]
- 142.Kurian A, Neumann EJ, Hall WF, Christensen N. Development of an enzyme-linked immunosorbent assay for the serological detection of exposure of poultry in New Zealand to Erysipelothrix rhusiopathiae and their serological response to vaccination. N Z Vet J. (2012) 60:100–5. 10.1080/00480169.2011.639057 [DOI] [PubMed] [Google Scholar]
- 143.Eamens GJ, Chin JC, Turner B, Barchia I. Evaluation of Erysipelothrix rhusiopathiae vaccines in pigs by intradermal challenge and immune responses. Vet Microbiol. (2006) 116:138–48. 10.1016/j.vetmic.2006.03.018 [DOI] [PubMed] [Google Scholar]
- 144.Sitt T, Bowen L, Blanchard MT, Gershwin LJ, Byrne BA, Dold C, et al. Cellular immune responses in cetaceans immunized with a porcine erysipelas vaccine. Vet Immunol Immunopathol. (2010) 137:181–9. 10.1016/j.vetimm.2010.05.003 [DOI] [PubMed] [Google Scholar]
- 145.Amass SF, Scholz DA. Acute nonfatal erysipelas in sows in a commercial farrow-to-finish operation. J Am Vet Med Assoc. (1998) 212:708–9. [PubMed] [Google Scholar]
- 146.Vance HN, Whenham GR. An erysipelas outbreak in chickens. Can J Comp Med Vet Sci. (1958) 22:86–7. [PMC free article] [PubMed] [Google Scholar]
- 147.Eriksson H, Brannstrom S, Skarin H, Chirico J. Characterization of Erysipelothrix rhusiopathiae isolates from laying hens and poultry red mites (Dermanyssus gallinae) from an outbreak of erysipelas. Avian Pathol. (2010) 39:505–9. 10.1080/03079457.2010.518313 [DOI] [PubMed] [Google Scholar]
- 148.Eriksson H, Nyman AK, Fellstrom C, Wallgren P. Erysipelas in laying hens is associated with housing system. Vet Rec. (2013) 173:18. 10.1136/vr.101388 [DOI] [PubMed] [Google Scholar]
- 149.Forde TL, Kollanandi Ratheesh N, Harvey WT, Thomson JR, Williamson S, Biek R, et al. Genomic and immunogenic protein diversity of Erysipelothrix rhusiopathiae isolated from pigs in Great Britain: implications for vaccine protection. Front Microbiol. (2020) 11:418. 10.3389/fmicb.2020.00418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Shimoji Y, Ogawa Y, Tsukio M, Shiraiwa K, Nishikawa S, Eguchi M. Genome-wide identification of virulence genes in Erysipelothrix rhusiopathiae: use of a mutant deficient in a tagF homolog as a safe oral vaccine against swine erysipelas. Infect Immun. (2019) 87:e00673-19. 10.1128/IAI.00673-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Yokomizo Y, Watanabe F, Imada Y, Inumaru S, Yanaka T, Tsuji T. Mucosal immunoadjuvant activity of the low toxic recombinant Escherichia coli heat-labile enterotoxin produced by Bacillus brevis for the bacterial subunit or component vaccine in pigs and cattle. Vet Immunol Immunopathol. (2002) 87:291–300. 10.1016/S0165-2427(02)00055-7 [DOI] [PubMed] [Google Scholar]
- 152.Zhu W, Wu C, Kang C, Cai C, Wang Y, Li J, et al. Evaluation of the protective efficacy of four newly identified surface proteins of Erysipelothrix rhusiopathiae. Vaccine. (2018) 36:8079–83. 10.1016/j.vaccine.2018.10.071 [DOI] [PubMed] [Google Scholar]
- 153.Jeon W, Kim YC, Hong M, Rejinold S, Park K, Yoon I, et al. Microcrystalline cellulose for delivery of recombinant protein-based antigen against erysipelas in mice. Biomed Res Int. (2018) 2018:7670505. 10.1155/2018/7670505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Shimoji Y, Oishi E, Kitajima T, Muneta Y, Shimizu S, Mori Y. Erysipelothrix rhusiopathiae YS-1 as a live vaccine vehicle for heterologous protein expression and intranasal immunization of pigs. Infect Immun. (2002) 70:226–32. 10.1128/IAI.70.1.226-232.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Ogawa Y, Oishi E, Muneta Y, Sano A, Hikono H, Shibahara T, et al. Oral vaccination against mycoplasmal pneumonia of swine using a live Erysipelothrix rhusiopathiae vaccine strain as a vector. Vaccine. (2009) 27:4543–50. 10.1016/j.vaccine.2009.04.081 [DOI] [PubMed] [Google Scholar]
- 156.Adu-Bobie J, Capecchi B, Serruto D, Rappuoli R, Pizza M. Two years into reverse vaccinology. Vaccine. (2003) 21:605–10. 10.1016/S0264-410X(02)00566-2 [DOI] [PubMed] [Google Scholar]