Abstract
One of the most challenging issues for the large-scale application of nanomaterials, especially nanocarbons, is the lack of industrial synthetic methods. Sonochemistry, which creates an extreme condition of high pressure and temperature, has been thereby applied for synthesizing a wide variety of unusual nanostructured materials. Hydrodynamic cavitation (HC), characterized by high effectiveness, good scalability, and synergistic effect with other physical and chemical methods, has emerged as the promising sonochemistry technology for industrial-scale applications. Recently, it was reported that HC can not only significantly enhance the performance of biochar, but also preserve or improve the respective chemical composition. Moreover, the economic efficiency was found to be at least one order of magnitude higher than that of conventional methods. Due to the great potential of HC in the industrial-scale synthesis of nanomaterials, the present perspective focuses on the mechanism of sonochemistry, advances in HC applications, and development of hydrodynamic cavitation reactors, which is supposed to contribute to the fundamental understanding of this novel technology.
Keywords: sonochemistry, synthesis of nanomaterials, hydrodynamic cavitation, hydrodynamic cavitation reactor, application potentiality
Introduction
The concept of nanoscience and nanotechnology was first proposed by Richard Feynman in 1959 (Feynman, 1992). Until 1974, the term nanotechnology (Taniguchi, 1974) was introduced by Nario Taniguchi during a scientific conference. With the help of the scanning tunneling microscope, which was invented by Gerd Binnig and Heinrich Rohrer in 1981 (Binnig and Rohrer, 1987), the modern nanotechnology has been developing rapidly since then. Recent advances in nanomaterials have significantly influenced various fields, e.g., material science, medical science, environmental science, magnetics, mechanics, and optics. Because the synthesis method largely determines the physical properties and applications of nanomaterials, developing new methods to design appropriate synthetic routes has been the research hotspot (Wang et al., 2019; Xu et al., 2019). Among the diverse physical and chemical synthesis methods, sonochemistry method has been considered one of the most powerful tools for synthesizing nanomaterials (Bang and Suslick, 2010). Sonochemistry can be effectively induced by ultrasounds (i.e., acoustic cavitation, AC) or local pressure drop (i.e., hydrodynamic cavitation, HC). AC has been utilized to achieve a wide variety of unusual nanostructured materials at laboratory scale, e.g., metals, metal oxides, metal chalcogenides and carbides, carbon, protein, and polymer. However, further application in industrial-scale may be considerably difficult as the issues of scale-up and energy efficiency (Gagol et al., 2018). Recently, HC, which has emerged as the promising technology for various industrial-scale applications, was found to be an effective tool for synthesis of nanomaterials through mechanisms similar to that of AC. The present paper aims to discuss the mechanism of sonochemistry, recent advances in the HC technology development, and its application perspective for synthesis of nanomaterials.
Sonochemistry
Unlike traditional energy sources such as heat, light, or ionizing radiation that are required for chemical reactions to proceed, sonochemistry is a unique energy-matter interaction that occurs without direct interaction with molecular species (Thompson and Doraiswamy, 1999). Sonochemistry derives principally from cavitation which is a rapid phase-change phenomenon in liquids, consisting of growth and collapse of cavitation nuclei during an extremely short period (Suslick, 1990). When the bubble collapses, a huge amount of energy, on the order of 1–1018 kW/m3, can be released into surrounding liquids (Gogate et al., 2006). The released energy can be divided into three forms (Sun et al., 2018b).
Mechanical effect: Generation of shock waves with propagation velocities of 2,000 m/s in average (Holzfuss et al., 1998), micro-jets with high water-hammer pressure (450 MPa; Vogel et al., 1989) and velocity (over 120 m/s; Benjamin and Ellis, 1966; Lauterborn and Bolle, 1975; Shima et al., 1981), and high shear stresses (as high as 3.5 kPa; Dijkink and Ohl, 2008).
Thermal effect: Production of local hot spots (2,000–6,000 K, depending on the distance; Hart et al., 1990; Flint and Suslick, 1991; Didenko et al., 1999) with heating/cooling rates >1010 K/s (Suslick et al., 1986).
Chemical effect: Formation of highly active hydroxyl radicals, with an oxidation potential of 2.8 V, by the sonolysis of water molecules (Arrojo et al., 2007; Kuppa and Moholkar, 2010).
The combination of the above three effects creates extreme conditions for synthesis of nanomaterials at ambient conditions (e.g., room temperature and atmospheric pressure) and sometimes even without the utilization of catalysts. Taking synthesizing nanostructured carbon materials as an example, sonochemistry effect can not only enhance the reactions which can lead to exotic carbon nanostructures (Sun et al., 2002), but also induce dramatic morphology changes in pre-synthesized carbon materials (Viculis et al., 2003). However, due to the characteristics of AC, the energy density rapidly attenuates with increasing distance from the ultrasonic horn and disappears at a distance of as low as 2–5 cm (Gagol et al., 2019). Therefore, to achieve the desired energy density, a number of ultrasonic horns have to be closely arranged in an AC reactor. This inherent feature of AC causes the equipment prices and operational costs rise rapidly with the scale (Gagol et al., 2018), which indicates that AC is not suitable for industrial-scale synthesis of nanomaterials. On the other hand, HC, which can effectively induce sonochemistry by utilizing a mechanical approach, has the ability to overcome the inherent defect of AC.
Hydrodynamic Cavitation
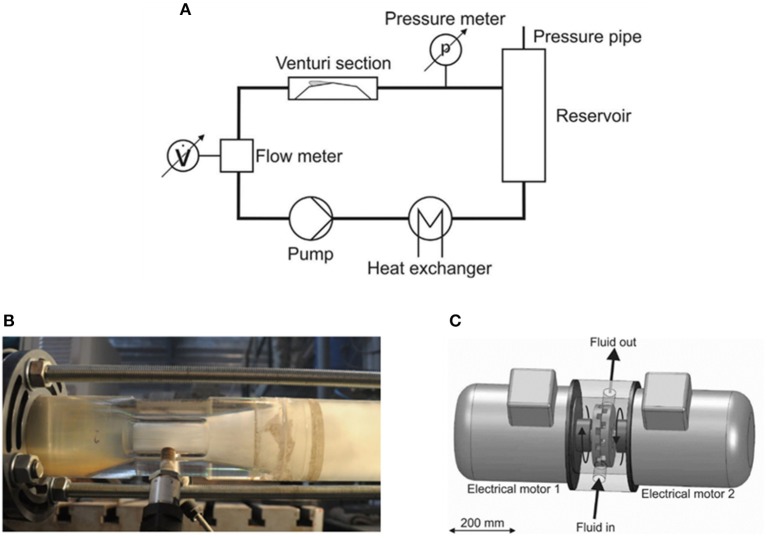
Unlike AC, which is generated by applying ultrasound waves with a cyclic succession of expansion (rarefaction) and compression phases on a liquid (Vajnhandl and Majcen Le Marechal, 2005), HC is induced by static pressure drops of the flowing liquid. When the flow passes through constricted parts or irregular geometries, the flow velocity increases and then, a decrease in static pressure can be caused. Once the pressure falls below the local saturated vapor pressure, cavitation nuclei existing in water begin to grow because their internal pressures become greater than the surface tension. When the flow pressure recovers, the growing nuclei become unstable and collapse (Yan and Thorpe, 1990). The working principle of a typical HC system (Venturi) is shown in Figure 2A (Šarc et al., 2018). The liquids in the reservoir are pumped to the Venturi section, cavitation phenomenon occurs in the diffusion part of the Venturi, and then, the liquids are sent back to the reservoir. The above process will continue for a period of time until the satisfactory treatment effect is obtained.
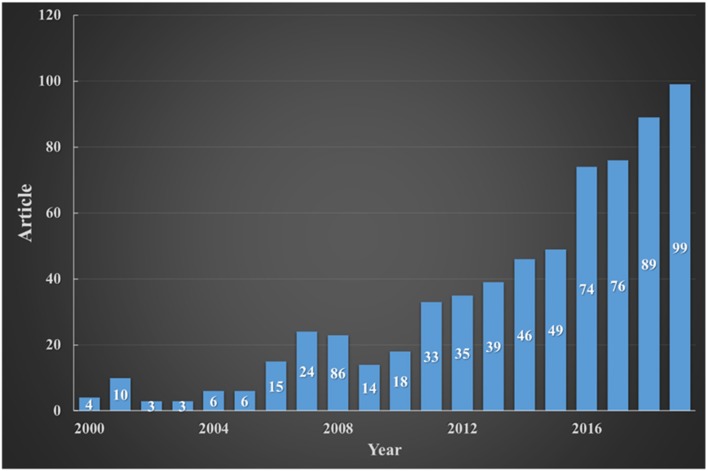
The exploration of HC began at the beginning of the 20th century as a negative consequence of erosion damage. In 1912, Silberrad reported that cavitation was associated with severe destructive damage to the propellers of the great ocean liners Lusitania and Mauretania (Silberrad, 1912). Since then, the researchers have been focusing on the negative effect of cavitation, e.g., performance losses of various fluid machinery, noise, and erosion damage (Rahmeyer, 1981; Sun et al., 2017b). On the other hand, Save et al. (1994) presented the first case study for microbial cell disruption by utilizing HC in 1994. After that, the applications of HC have begun to attract attention in a wide variety of areas, especially in the last few years (Figure 1). Nowadays, researchers have found that HC can be an effective tool for a number of chemical, biological, and other types of applications, e.g., microbial inactivation [bacteria (Mane et al., 2020), algae (Waghmare et al., 2019), virus (Kosel et al., 2017)], the removal of organic compounds (acids Choi et al., 2019, antibiotics (Tao et al., 2018), pesticides (Panda and Manickam, 2019), dyes Yi et al., 2018, pharmaceuticals (Rajoriya et al., 2019), fuel (Torabi Angaji and Ghiaee, 2015), phenols Chakinala et al., 2008, etc.,) decomposition of waste-activated sludge (WAS) (Nabi et al., 2019), depolymerization (Prajapat and Gogate, 2019), denitrification (Song et al., 2019), desulfurization (Gagol et al., 2019), fibrillation (Kosel et al., 2019), intensification of biogas production (Zielinski et al., 2019), biofuel synthesis (Chipurici et al., 2019), liposome destruction (Pandur et al., 2020), catalyst slurry preparation (Kuroki et al., 2019), flotation (Ross et al., 2019), food processing (Terán Hilares et al., 2019), surface finishing (Nagalingam et al., 2019), viscosity reduction (Gregersen et al., 2019), residual stress relief, cleaning, and emulsification (Wu et al., 2019).
Figure 1.
Articles about HC application from 2000 to 2019 (based on Google Scholar available on 25.2.2020).
Until last year, the first study on the nanomaterial preparation by utilizing HC was reported by Albanese et al. (2019) who utilized HC to enhance the surface area of biochar by as much as 120%, while preserving or improving the respective chemical composition. The increases in functionality and porosity of the biochar were contributed to the effect of the physical impact and oxidation (hydroxyl radicals) of HC. Moreover, the economic efficiency was found to be at least one order of magnitude higher than that of the conventional method, which demonstrates that HC can be an effective alternative approach for synthesis of nanomaterials. HC phenomenon is induced by hydrodynamic cavitation reactor (HCR), i.e., the container for HC reaction, therefore, the HC generation efficiency of HCR determines the treatment effect, economy, and applicability of HC technology. To accelerate the development of HC-based synthetic strategies of industrial-scale production of nanomaterials, the most important thing is to develop new-family HCRs. Therefore, we further make a simple overview of the development of HCR in order to give readership more clearer understandings about this new field.
Development of HCR
HCRs can be generally categorized into two types based on their operational mechanism: non-rotational and rotational. In common conventional HCRs [CHCRs, e.g., Venturi type (Figure 2B; Jančula et al., 2014) and orifice type], low-pressure separation region can be formed when the fluid passes through the contractive portion where the static pressure is converted to kinetic energy (Merzkirch et al., 2015). To understand the mechanisms of HC treatment, CHCRs have been widely utilized in laboratory scale in the previous studies, because of the advantages they offer in simple design, lack of moving parts, and ease of manufacture and use (Dular et al., 2016). Moreover, some researchers found that CHCRs can be applied to real industrial applications (Hirooka et al., 2009).
Figure 2.
Schematic diagram of representative HC working principle (A) Šarc et al. (2018) and HCRs, (B) Venturi, and (C) rotor-stator type (Zupanc et al., 2014).
Recently, a few rotor-stator type HCRs (R-S HCRs) have been introduced in the literature. R-S HCRs utilize a circular disk or cylinder with numerous gaps to generate cavitation (Figure 2C; Zupanc et al., 2014). Due to the viscosity, the fluid is driven by the rotor and the flow direction is identical to the rotational direction. The flow punches the back edge of the gap and forms separation region with low pressure. Cavitation bubbles can occur when the rotational speed reaches the critical value. The results from the corresponding research indicated the effectiveness of the treatment, economic efficiency which is far beyond those of traditional devices in the removal of microorganism (Milly et al., 2007, 2008; Šarc et al., 2018; Sun et al., 2018a,b; Maršálek et al., 2020), WAS treatment (Petkovšek et al., 2015; Kim et al., 2019, 2020; SeŽun et al., 2019), organic wastewater treatment (Badve et al., 2013; Zupanc et al., 2014), biofuel synthesis (Mohod et al., 2017; Chipurici et al., 2019), fibrillation (Kosel et al., 2019), intensification of biogas production (Patil et al., 2016), and delignification (Badve et al., 2014), etc., even without geometrical optimization or in scaled-up application. In addition, due to the extreme conditions and the hydroxyl radicals produced by HC, effective synergic effects between HC and heating, AC (Sun et al., 2018a), various oxidants (Saharan et al., 2011), photocatalyst (Wang et al., 2011), photolysis (Zupanc et al., 2014), and electrochemical (Wang et al., 2010) have been proved. More importantly, it is worth noting that R-S HCRs show promising scalability (Joshi and Gogate, 2019), and their performance can be easily improved by scaling up the dimensions, which was confirmed by Sun et al. (2018a) who found that when the rotor of the HCR doubled in size (290–590 mm), heat generation and thermal efficiency increased from 48 to 200 MJ/h and 82 to 91%, respectively.
So far, HC technology has not been widely utilized in industrial applications around the world, even though it has been investigated and developed for nearly 30 years. Most of the existing research relates to applications, the characteristics of HCRs have been rarely focused, which largely influences the development and application of HC technology. Even though a few researchers have made important contributions in theoretical (Sarvothaman et al., 2019), computational (Badve et al., 2015), and experimental (Zhang et al., 2018) aspects of HCRs, their cavitation generation mechanism, internal flow fields, external characteristics, and scale-up law are not well understood by utilizing experimental flow visualization, particle image velocimetry, and computational fluid dynamic methods, especially for the R-S HCRs. More importantly, the universal research and design methods (e.g., the theoretical and numerical methods for design the rotor, stator, and flow path, scale-up law, and optimization method) for HCRs have not been established yet. The investigations on the internal fluid field, geometrical optimizations, numerical simulation methods, dimensional analyses, and similarity laws for the external characteristics, etc., are required in future.
Conclusion and Perspective
The present paper illustrated the mechanism of sonochemistry, advances in HC applications, and development of hydrodynamic cavitation reactors, with the aim to contribute to the fundamental understanding of this novel technology. With the mechanism similar to that of AC, HC technology appears to be an effective sonochemistry means for synthesizing nanomaterials in industrial-scale due to its good scalability. The development and application of HC synthetic method will be a real challenge because of its highly interdisciplinary (related to sonochemistry, fluid dynamics, material science, and mechanical engineering). However, significant progress in this technology will lead to the considerable promotion of the industrialization of nanomaterials. Several challenges and research directions that can be considered are outlined below:
To understand the HC mechanism, it is necessary to apply HC to the preparation of various types of nanomaterials, including metals, alloys, oxides, sulfides, carbides, carbons, polymers, and biomaterials.
Studying the synergistic effects between HC and traditional synthetic methods on the structure and performance of nanomaterials.
Developing appropriate CFD methods to reveal the cavitation generation mechanism and design new HCRs.
Optimizing the geometrical structure of HCRs by advanced algorithms (Sun et al., 2017a; Sun and Yoon, 2018).
Establishing the universal research and design methods for HCRs.
Data Availability Statement
All datasets generated for this study are included in the article/supplementary material.
Author Contributions
XS, JY, and SC contributed conception of the study. XS produced and wrote the article. JL and SZ edited the article.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
Funding. This work was supported by the National Natural Science Foundation of China (No. 51906125), China Postdoctoral Science Foundation (No. 2019M650162), Youth Interdisciplinary Science and Innovative Research Groups of Shandong University (No. 2020QNQT014), Young Scholars Program of Shandong University, Fundamental Research Funds of Shandong University (Nos. 2019HW027 and 2018GN033), Key Laboratory of High-efficiency and Clean Mechanical Manufacture at Shandong University, Ministry of Education, and Ocean Industry Leading Talent Team of Yantai's Double Hundred Plan.
References
- Albanese L., Baronti S., Liguori F., Meneguzzo F., Barbaro P., Vaccari F. P. (2019). Hydrodynamic cavitation as an energy efficient process to increase biochar surface area and porosity: a case study. J. Clean. Prod. 210, 159–169. 10.1016/j.jclepro.2018.10.341 [DOI] [Google Scholar]
- Arrojo S., Nerín C., Benito Y. (2007). Application of salicylic acid dosimetry to evaluate hydrodynamic cavitation as an advanced oxidation process. Ultrason. Sonochem. 14, 343–349. 10.1016/j.ultsonch.2006.06.007 [DOI] [PubMed] [Google Scholar]
- Badve M., Gogate P., Pandit A., Csoka L. (2013). Hydrodynamic cavitation as a novel approach for wastewater treatment in wood finishing industry. Sep. Purif. Technol. 106, 15–21. 10.1016/j.seppur.2012.12.029 [DOI] [Google Scholar]
- Badve M. P., Alpar T., Pandit A. B., Gogate P. R., Csoka L. (2015). Modeling the shear rate and pressure drop in a hydrodynamic cavitation reactor with experimental validation based on KI decomposition studies. Ultrason. Sonochem. 22, 272–277. 10.1016/j.ultsonch.2014.05.017 [DOI] [PubMed] [Google Scholar]
- Badve M. P., Gogate P. R., Pandit A. B., Csoka L. (2014). Hydrodynamic cavitation as a novel approach for delignification of wheat straw for paper manufacturing. Ultrason. Sonochem. 21, 162–168. 10.1016/j.ultsonch.2013.07.006 [DOI] [PubMed] [Google Scholar]
- Bang J. H., Suslick K. S. (2010). Applications of ultrasound to the synthesis of nanostructured materials. Adv. Mater. 22, 1039–1059. 10.1002/adma.200904093 [DOI] [PubMed] [Google Scholar]
- Benjamin T. B., Ellis A. T. (1966). The Collapse of cavitation bubbles and the pressures thereby produced against solid boundaries. Philos. Transact. Royal Soc. London. Series A, Math. Phy. Sci. 260, 221–240. 10.1098/rsta.1966.0046 [DOI] [Google Scholar]
- Binnig G., Rohrer H. (1987). Scanning tunneling microscopy—from birth to adolescence. Rev. Mod. Phys. 59:615 10.1103/RevModPhys.59.615 [DOI] [Google Scholar]
- Chakinala A. G., Bremner D. H., Gogate P. R., Namkung K.-C., Burgess A. E. (2008). Multivariate analysis of phenol mineralisation by combined hydrodynamic cavitation and heterogeneous advanced Fenton processing. Appl. Catal. B: Environ. 78, 11–18. 10.1016/j.apcatb.2007.08.012 [DOI] [Google Scholar]
- Chipurici P., Vlaicu A., Calinescu I., Vinatoru M., Vasilescu M., Ignat N. D., et al. (2019). Ultrasonic, hydrodynamic and microwave biodiesel synthesis – a comparative study for continuous process. Ultrason. Sonochem. 57, 38–47. 10.1016/j.ultsonch.2019.05.011 [DOI] [PubMed] [Google Scholar]
- Choi J., Cui M., Lee Y., Ma J., Kim J., Son Y., et al. (2019). Hybrid reactor based on hydrodynamic cavitation, ozonation, and persulfate oxidation for oxalic acid decomposition during rare-earth extraction processes. Ultrason. Sonochem. 52, 326–335. 10.1016/j.ultsonch.2018.12.004 [DOI] [PubMed] [Google Scholar]
- Didenko Y. T., McNamara W. B., Suslick K. S. (1999). Hot spot conditions during cavitation in water. J. Am. Chem. Soc. 121, 5817–5818. 10.1021/ja9844635 [DOI] [Google Scholar]
- Dijkink R., Ohl C.-D. (2008). Measurement of cavitation induced wall shear stress. Appl. Phys. Lett. 93, 254107 10.1063/1.3046735 [DOI] [Google Scholar]
- Dular M., Griessler-Bulc T., Gutierrez-Aguirre I., Heath E., Kosjek T., Krivograd Klemenčič A., et al. (2016). Use of hydrodynamic cavitation in (waste)water treatment. Ultrason. Sonochem. 29, 577–588. 10.1016/j.ultsonch.2015.10.010 [DOI] [PubMed] [Google Scholar]
- Feynman R. P. (1992). There's plenty of room at the bottom. J. Microelectromech. Systems 1, 60–66. 10.1109/84.128057 [DOI] [Google Scholar]
- Flint E. B., Suslick K. S. (1991). The temperature of cavitation. Science 253, 1397–1399. 10.1126/science.253.5026.1397 [DOI] [PubMed] [Google Scholar]
- Gagol M., Przyjazny A., Boczkaj G. (2018). Wastewater treatment by means of advanced oxidation processes based on cavitation – a review. Chem. Eng. J. 338, 599–627. 10.1016/j.cej.2018.01.049 [DOI] [Google Scholar]
- Gagol M. R., Soltani D. C., Przyjazny A., Boczkaj G. (2019). Effective degradation of sulfide ions and organic sulfides in cavitation-based advanced oxidation processes (AOPs). Ultrason. Sonochem. 58:104610. 10.1016/j.ultsonch.2019.05.027 [DOI] [PubMed] [Google Scholar]
- Gogate P. R., Tayal R. K., Pandit A. B. (2006). Cavitation: a technology on the horizon. Curr. Sci. 91, 35–46. Available online at: https://www.currentscience.ac.in/php/toc.php?vol=091&issue=01 [Google Scholar]
- Gregersen S. B., Wiking L., Bertelsen K. B., Tangsanthatkun J., Pedersen B., Poulsen K. R., et al. (2019). Viscosity reduction in concentrated protein solutions by hydrodynamic cavitation. Int. Dairy J. 97, 1–4. 10.1016/j.idairyj.2019.04.015 [DOI] [Google Scholar]
- Hart E. J., Fischer C.-H., Henglein A. (1990). Sonolysis of hydrocarbons in aqueous solution. Int. J. Radiat. Appl. Instrum. Part Radiat. Phy. C Chem. 36, 511–516. 10.1016/1359-0197(90)90198-Q [DOI] [Google Scholar]
- Hirooka K., Asano R., Yokoyama A., Okazaki M., Sakamoto A., Nakai Y. (2009). Reduction in excess sludge production in a dairy wastewater treatment plant via nozzle-cavitation treatment: Case study of an on-farm wastewater treatment plant. Bioresour. Technol. 100, 3161–3166. 10.1016/j.biortech.2009.01.011 [DOI] [PubMed] [Google Scholar]
- Holzfuss J., Rüggeberg M., Billo A. (1998). Shock wave emissions of a sonoluminescing bubble. Phys. Rev. Lett. 81, 5434–5437. 10.1103/PhysRevLett.81.5434 [DOI] [Google Scholar]
- Jančula D., Mikula P., Maršálek B., Rudolf P., Pochylý F. (2014). Selective method for cyanobacterial bloom removal: hydraulic jet cavitation experience. Aquacult. Int. 22, 509–521. 10.1007/s10499-013-9660-7 [DOI] [Google Scholar]
- Joshi S. M., Gogate P. R. (2019). Intensification of industrial wastewater treatment using hydrodynamic cavitation combined with advanced oxidation at operating capacity of 70 L. Ultrason. Sonochem. 52, 375–381. 10.1016/j.ultsonch.2018.12.016 [DOI] [PubMed] [Google Scholar]
- Kim H., Koo B., Sun X., Yong Yoon J. (2020). Investigation of sludge disintegration using rotor-stator type hydrodynamic cavitation reactor. Sep. Purif. Technol. 240:116636 10.1016/j.seppur.2020.116636 [DOI] [Google Scholar]
- Kim H., Sun X., Koo B., Yoon J. Y. (2019). Experimental investigation of sludge treatment using a rotor-stator type hydrodynamic cavitation reactor and an ultrasonic bath. Processes 7:790 10.3390/pr7110790 [DOI] [Google Scholar]
- Kosel J., Gutiérrez-Aguirre I., Rački N., Dreo T., Ravnikar M., Dular M. (2017). Efficient inactivation of MS-2 virus in water by hydrodynamic cavitation. Water Res. 124, 465–471. 10.1016/j.watres.2017.07.077 [DOI] [PubMed] [Google Scholar]
- Kosel J., Šinkovec A., Dular M. (2019). A novel rotation generator of hydrodynamic cavitation for the fibrillation of long conifer fibers in paper production. Ultrason. Sonochem. 59:104721. 10.1016/j.ultsonch.2019.104721 [DOI] [PubMed] [Google Scholar]
- Kuppa R., Moholkar V. S. (2010). Physical features of ultrasound-enhanced heterogeneous permanganate oxidation. Ultrason. Sonochem. 17, 123–131. 10.1016/j.ultsonch.2009.05.011 [DOI] [PubMed] [Google Scholar]
- Kuroki H., Onishi K., Asami K., Yamaguchi T. (2019). Catalyst slurry preparation using a hydrodynamic cavitation dispersion method for polymer electrolyte fuel cells. Ind. Eng. Chem. Res. 58, 19545–19550. 10.1021/acs.iecr.9b02111 [DOI] [Google Scholar]
- Lauterborn W., Bolle H. (1975). Experimental investigations of cavitation-bubble collapse in the neighbourhood of a solid boundary. J. Fluid Mech. 72, 391–399. 10.1017/S0022112075003448 [DOI] [Google Scholar]
- Mane M. B., Bhandari V. M., Balapure K., Ranade V. V. (2020). A novel hybrid cavitation process for enhancing and altering rate of disinfection by use of natural oils derived from plants. Ultrason. Sonochem. 61:104820. 10.1016/j.ultsonch.2019.104820 [DOI] [PubMed] [Google Scholar]
- Maršálek B., Zezulka Š., Maršálková E., Pochylý F., Rudolf P. (2020). Synergistic effects of trace concentrations of hydrogen peroxide used in a novel hydrodynamic cavitation device allows for selective removal of cyanobacteria. Chem. Eng. J. 382:122383 10.1016/j.cej.2019.122383 [DOI] [Google Scholar]
- Merzkirch W., Rockwell D., Tropea C. (2015). Orifice Plates and Venturi Tubes. Cham; Heidelberg; New York, NY; Dordrecht; London: Springer International Publishing. Available online at: https://link.springer.com/content/pdf/bfm%3A978-3-319-16880-7%2F1.pdf
- Milly P. J., Toledo R. T., Harrison M. A., Armstead D. (2007). Inactivation of food spoilage microorganisms by hydrodynamic cavitation to achieve pasteurization and sterilization of fluid foods. J. Food Sci. 72, M414–M422. 10.1111/j.1750-3841.2007.00543.x [DOI] [PubMed] [Google Scholar]
- Milly P. J., Toledo R. T., Kerr W. L., Armstead D. (2008). Hydrodynamic cavitation: characterization of a novel design with energy considerations for the inactivation of saccharomyces cerevisiae in apple juice. J. Food Sci. 73, M298–M303. 10.1111/j.1750-3841.2008.00827.x [DOI] [PubMed] [Google Scholar]
- Mohod A. V., Gogate P. R., Viel G., Firmino P., Giudici R. (2017). Intensification of biodiesel production using hydrodynamic cavitation based on high speed homogenizer. Chem. Eng. J. 316, 751–757. 10.1016/j.cej.2017.02.011 [DOI] [Google Scholar]
- Nabi M., Zhang G., Zhang P., Tao X., Wang S., Ye J., et al. (2019). Contribution of solid and liquid fractions of sewage sludge pretreated by high pressure homogenization to biogas production. Bioresour. Technol. 286:121378. 10.1016/j.biortech.2019.121378 [DOI] [PubMed] [Google Scholar]
- Nagalingam A. P., Thiruchelvam V. C., Yeo S. H. (2019). A novel hydrodynamic cavitation abrasive technique for internal surface finishing. J. Manuf. Process. 46, 44–58. 10.1016/j.jmapro.2019.08.014 [DOI] [Google Scholar]
- Panda D., Manickam S. (2019). Hydrodynamic cavitation assisted degradation of persistent endocrine-disrupting organochlorine pesticide dicofol: optimization of operating parameters and investigations on the mechanism of intensification. Ultrason. Sonochem. 51, 526–532. 10.1016/j.ultsonch.2018.04.003 [DOI] [PubMed] [Google Scholar]
- Pandur Z., Dogsa I., Dular M., Stopar D. (2020). Liposome destruction by hydrodynamic cavitation in comparison to chemical, physical and mechanical treatments. Ultrason. Sonochem. 61:104826. 10.1016/j.ultsonch.2019.104826 [DOI] [PubMed] [Google Scholar]
- Patil P. N., Gogate P. R., Csoka L., Dregelyi-Kiss A., Horvath M. (2016). Intensification of biogas production using pretreatment based on hydrodynamic cavitation. Ultrason. Sonochem. 30, 79–86. 10.1016/j.ultsonch.2015.11.009 [DOI] [PubMed] [Google Scholar]
- Petkovšek M., Mlakar M., Levstek M. M., StraŽar Širok B., Dular M. (2015). A novel rotation generator of hydrodynamic cavitation for waste-activated sludge disintegration. Ultrason. Sonochem. 26, 408–414. 10.1016/j.ultsonch.2015.01.006 [DOI] [PubMed] [Google Scholar]
- Prajapat A. L., Gogate P. R. (2019). Depolymerization of carboxymethyl cellulose using hydrodynamic cavitation combined with ultraviolet irradiation and potassium persulfate. Ultrason. Sonochem. 51, 258–263. 10.1016/j.ultsonch.2018.10.009 [DOI] [PubMed] [Google Scholar]
- Rahmeyer W. J. (1981). Cavitation damage to hydraulic structures. J. Am. Water Works Assoc. 73, 270–274. 10.1002/j.1551-8833.1981.tb04703.x [DOI] [Google Scholar]
- Rajoriya S., Bargole S., George S., Saharan V. K., Gogate P. R., Pandit A. B. (2019). Synthesis and characterization of samarium and nitrogen doped TiO2 photocatalysts for photo-degradation of 4-acetamidophenol in combination with hydrodynamic and acoustic cavitation. Sep. Purif. Technol. 209, 254–269. 10.1016/j.seppur.2018.07.036 [DOI] [Google Scholar]
- Ross V., Singh A., Pillay K. (2019). Improved flotation of PGM tailings with a high-shear hydrodynamic cavitation device. Miner. Eng. 137, 133–139. 10.1016/j.mineng.2019.04.005 [DOI] [Google Scholar]
- Saharan V. K., Badve M. P., Pandit A. B. (2011). Degradation of reactive red 120 dye using hydrodynamic cavitation. Chem. Eng. J. 178, 100–107. 10.1016/j.cej.2011.10.018 [DOI] [Google Scholar]
- Šarc A., Kosel J., Stopar D., Oder M., Dular M. (2018). Removal of bacteria Legionella pneumophila, Escherichia coli, and Bacillus subtilis by (super)cavitation. Ultrason. Sonochem. 42, 228–236. 10.1016/j.ultsonch.2017.11.004 [DOI] [PubMed] [Google Scholar]
- Sarvothaman V. P., Simpson A. T., Ranade V. V. (2019). Modelling of vortex based hydrodynamic cavitation reactors. Chem. Eng. J. 377:119639 10.1016/j.cej.2018.08.025 [DOI] [Google Scholar]
- Save S. S., Pandit A. B., Joshi J. B. (1994). Microbial cell disruption: role of cavitation. Chem. Eng. J. Biochem. Eng. J. 55, B67–B72. 10.1016/0923-0467(94)06062-2 [DOI] [Google Scholar]
- SeŽun M., Kosel J., Zupanc M., Hočevar M., Vrtovšek J., Petkovšek M., et al. (2019). Cavitation as a potential technology for wastewater management – an example of enhanced nutrient release from secondary pulp and paper mill sludge. Stroj. Vestn. J. Mech. Eng. 65:9 10.5545/sv-jme.2019.6328 [DOI] [Google Scholar]
- Shima A., Takayama K., Tomita Y., Miura N. (1981). An Experimental study on effects of a solid wall on the motion of bubbles and shock waves in bubble collapse. Acta Acust. United Ac. 48, 293–301. [Google Scholar]
- Silberrad D. (1912). Propeller erosion. Engineering 93, 33–35. [Google Scholar]
- Song L., Yang J., Yu S., Xu M., Liang Y., Pan X., et al. (2019). Ultra-high efficient hydrodynamic cavitation enhanced oxidation of nitric oxide with chlorine dioxide. Chem. Eng. J. 373, 767–779. 10.1016/j.cej.2019.05.094 [DOI] [Google Scholar]
- Sun X., Kang C. H., Park J. J., Kim H. S., Om A. S., Yoon J. Y. (2018a). An experimental study on the thermal performance of a novel hydrodynamic cavitation reactor. Exp. Therm Fluid Sci. 99, 200–210. 10.1016/j.expthermflusci.2018.02.034 [DOI] [Google Scholar]
- Sun X., Kim H. S., Yang S. D., Kim C. K., Yoon J. Y. (2017b). Numerical investigation of the effect of surface roughness on the flow coefficient of an eccentric butterfly valve. J. Mech. Sci. Technol. 31, 2839–2848. 10.1007/s12206-017-0527-0 [DOI] [Google Scholar]
- Sun X., Kim S., Yang S. D., Kim H. S., Yoon J. Y. (2017a). Multi-objective optimization of a Stairmand cyclone separator using response surface methodology and computational fluid dynamics. Powder Technol. 320, 51–65. 10.1016/j.powtec.2017.06.065 [DOI] [Google Scholar]
- Sun X., Park J. J., Kim H. S., Lee S. H., Seong S. J., Om A. S., et al. (2018b). Experimental investigation of the thermal and disinfection performances of a novel hydrodynamic cavitation reactor. Ultrason. Sonochem. 49, 13–23. 10.1016/j.ultsonch.2018.02.039 [DOI] [PubMed] [Google Scholar]
- Sun X., Yoon J. Y. (2018). Multi-objective optimization of a gas cyclone separator using genetic algorithm and computational fluid dynamics. Powder Technol. 325, 347–360. 10.1016/j.powtec.2017.11.012 [DOI] [Google Scholar]
- Sun X.-H., Li C.-P., Wong N.-B., Lee C.-S., Lee S.-T., Teo B.-K. (2002). Templating effect of hydrogen-passivated silicon nanowires in the production of hydrocarbon nanotubes and nanoonions via sonochemical reactions with common organic solvents under ambient conditions. J. Am. Chem. Soc. 124, 14856–14857. 10.1021/ja0283706 [DOI] [PubMed] [Google Scholar]
- Suslick K. S. (1990). Sonochemistry. Science 247, 1439–1445. 10.1126/science.247.4949.1439 [DOI] [PubMed] [Google Scholar]
- Suslick K. S., Hammerton D. A., Cline R. E. (1986). Sonochemical hot spot. J. Am. Chem. Soc. 108, 5641–5642. 10.1021/ja00278a055 [DOI] [Google Scholar]
- Taniguchi N. (1974). “On the basic concept of nanotechnology,” in Proceeding of the ICPE (Tokyo: ), 18–23. [Google Scholar]
- Tao Y., Cai J., Huai X., Liu B. (2018). A novel antibiotic wastewater degradation technique combining cavitating jets impingement with multiple synergetic methods. Ultrason. Sonochem. 44, 36–44. 10.1016/j.ultsonch.2018.02.008 [DOI] [PubMed] [Google Scholar]
- Terán Hilares R., dos Santos J. G., Shiguematsu N. B., Ahmed M. A., da Silva S. S., Santos J. C. (2019). Low-pressure homogenization of tomato juice using hydrodynamic cavitation technology: effects on physical properties and stability of bioactive compounds. Ultrason. Sonochem. 54, 192–197. 10.1016/j.ultsonch.2019.01.039 [DOI] [PubMed] [Google Scholar]
- Thompson L. H., Doraiswamy L. K. (1999). Sonochemistry: science and engineering. Ind. Eng. Chem. Res. 38, 1215–1249. 10.1021/ie9804172 [DOI] [Google Scholar]
- Torabi Angaji M., Ghiaee R. (2015). Decontamination of unsymmetrical dimethylhydrazine waste water by hydrodynamic cavitation-induced advanced fenton process. Ultrason. Sonochem. 23, 257–265. 10.1016/j.ultsonch.2014.09.007 [DOI] [PubMed] [Google Scholar]
- Vajnhandl S., Majcen Le Marechal A. (2005). Ultrasound in textile dyeing and the decolouration/mineralization of textile dyes. Dyes Pigments 65, 89–101. 10.1016/j.dyepig.2004.06.012 [DOI] [Google Scholar]
- Viculis L. M., Mack J. J., Kaner R. B. (2003). A chemical route to carbon nanoscrolls. Science 299:1361. 10.1126/science.1078842 [DOI] [PubMed] [Google Scholar]
- Vogel A., Lauterborn W., Timm R. (1989). Optical and acoustic investigations of the dynamics of laser-produced cavitation bubbles near a solid boundary. J. Fluid Mech. 206, 299–338. 10.1017/S0022112089002314 [DOI] [Google Scholar]
- Waghmare A., Nagula K., Pandit A., Arya S. (2019). Hydrodynamic cavitation for energy efficient and scalable process of microalgae cell disruption. Algal Res. 40:101496 10.1016/j.algal.2019.101496 [DOI] [Google Scholar]
- Wang X., Jia J., Wang Y. (2010). Electrochemical degradation of reactive dye in the presence of water jet cavitation. Ultrason. Sonochem. 17, 515–520. 10.1016/j.ultsonch.2009.10.023 [DOI] [PubMed] [Google Scholar]
- Wang X., Jia J., Wang Y. (2011). Degradation of C.I. reactive red 2 through photocatalysis coupled with water jet cavitation. J. Hazard. Mater. 185, 315–321. 10.1016/j.jhazmat.2010.09.036 [DOI] [PubMed] [Google Scholar]
- Wang Z., Xu X., Kim J., Malgras V., Mo R., Li C., et al. (2019). Nanoarchitectured metal–organic framework/polypyrrole hybrids for brackish water desalination using capacitive deionization. Mater. Horiz. 6, 1433–1437. 10.1039/C9MH00306A [DOI] [Google Scholar]
- Wu Z., Tagliapietra S., Giraudo A., Martina K., Cravotto G. (2019). Harnessing cavitational effects for green process intensification. Ultrason. Sonochem. 52, 530–546. 10.1016/j.ultsonch.2018.12.032 [DOI] [PubMed] [Google Scholar]
- Xu X., Tan H., Wang Z., Wang C., Pan L., Kaneti Y. V., et al. (2019). Extraordinary capacitive deionization performance of highly-ordered mesoporous carbon nano-polyhedra for brackish water desalination. Environ. Sci. Nano 6, 981–989. 10.1039/C9EN00017H [DOI] [Google Scholar]
- Yan Y., Thorpe R. B. (1990). Flow regime transitions due to cavitation in the flow through an orifice. Int. J. Multiphase Flow 16, 1023–1045. 10.1016/0301-9322(90)90105-R [DOI] [Google Scholar]
- Yi C., Lu Q., Wang Y., Wang Y., Yang B. (2018). Degradation of organic wastewater by hydrodynamic cavitation combined with acoustic cavitation. Ultrason. Sonochem. 43, 156–165. 10.1016/j.ultsonch.2018.01.013 [DOI] [PubMed] [Google Scholar]
- Zhang Y., Tian Y., Zhang Z., Lin S. (2018). Experimental and numerical study of cavitating flow with suction in a mixing reactor for water treatment. Chem. Eng. J. 353, 796–804. 10.1016/j.cej.2018.07.183 [DOI] [Google Scholar]
- Zielinski M., Debowski M., Kisielewska M., Nowicka A., Rokicka M., Szwarc K. (2019). Cavitation-based pretreatment strategies to enhance biogas production in a small-scale agricultural biogas plant. Energy Sust. Dev. 49, 21–26. 10.1016/j.esd.2018.12.007 [DOI] [Google Scholar]
- Zupanc M., Kosjek T., Petkovšek M., Dular M., Kompare B., Širok B., et al. (2014). Shear-induced hydrodynamic cavitation as a tool for pharmaceutical micropollutants removal from urban wastewater. Ultrason. Sonochem. 21, 1213–1221. 10.1016/j.ultsonch.2013.10.025 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All datasets generated for this study are included in the article/supplementary material.