Abstract
Beyond its extraordinary genome editing ability, the CRISPR-Cas system has opened a new era of biosensing applications due to its high base resolution and isothermal signal amplification. However, the reported CRISPR-Cas sensors are largely only used for the detection of nucleic acids with limited application for non-nucleic acid targets. To realize the full potential of the CRISPR-Cas sensors and broaden their applications for detection and quantitation of non-nucleic acid targets, we herein report CRISPR-Cas12a sensors that are regulated by functional DNA (fDNA) molecules such as aptamers and DNAzymes that are selective for small organic molecule and metal ion detections. The sensor is based on the Cas12a dependent reporter system consisting of Cas12a, CRISPR RNA (crRNA) and its single stranded DNA substrate labeled with a fluorophore and quencher at each end (ssDNA-FQ), and fDNA molecules that can lock a DNA activator for Cas12a-crRNA, preventing the ssDNA cleavage function of Cas12a in the absence of the fDNA targets. The presence of fDNA targets can trigger the unlocking of the DNA activator, which can then activate the cleavage of ssDNA-FQ by Cas12a, resulting in an increase of the fluorescent signal detectable by commercially available portable fluorimeters. Using this method, ATP and Na+ have been detected quantitatively under ambient temperature (25 °C) using a simple and fast detection workflow (two steps and <15 min), making the fDNA-regulated CRISPR system suitable for field tests or point-of-care diagnostics. Since fDNAs can be obtained to recognize a wide range of targets, the methods demonstrated here can expand this powerful CRISPR-Cas sensor system significantly to many other targets and thus provide a new toolbox to significantly expand the CRISPR-Cas system into many areas of bioanalytical and biomedical applications.
Keywords: Functional DNA, CRISPR-Cas, point of care diagnostic
Graphical Abstract

INTRODUCTION
Medical diagnostics are crucial for identification, prevention, and treatment of many diseases, and therefore developing efficient diagnostic tools is of vital importance.1–4 While much progress has been made with clinical lab tests, there is a need for developing new diagnostic technologies with high sensitivity and specificity, and most importantly, broad applicability under point-of-care (POC) settings. The recent discovery and development of Clustered Regularly Interspaced Short Palindromic Repeats (CRISPR) and CRISPR-associated proteins (Cas), together referred to as the CRISPR-Cas system, has revolutionized not only genome editing, but also other areas, including medical diagnostics,5–8 such as nucleic acid sensors based on SHERLOCK (Specific High-sensitivity Enzymatic Reporter UnLOCKing) and DETECTR (DNA Endonuclease-Targeted CRISPR Trans Reporter).9–16 Among them, CRISPR-Cas12a (Cpf1), a class 2 type V-A CRISPR-associated enzyme that binds to a single stranded guide CRISPR RNA (crRNA), has been reported to not only cleave the target DNA like in many other CRISPR-Cas systems, but also indiscriminately cleaves any non-target ssDNA near the target DNA.11 This collateral cleavage of the non-targeted ssDNA can dramatically increases the sensitivity of DNA detection in many applications. Most of these CRISPR-Cas platforms have advantages of simplicity to fabricate, ultra-high sensitivity down to attomolar concentration limits of detection (LOD), high specificity to single-base variation, and good portability for POC diagnostics. As a result, it has demonstrated enormous potential for nucleic acid-related diagnostics, including human genotyping and pathogen detection, cancer mutation testing, and single nucleotide polymorphism (SNP) identification, especially under POC settings.13, 17–21 Despite recent progress, almost all of the reported CRISPR-Cas sensors are developed to detect nucleic acid-related targets. Thus, there is still a need for more strategies in the design of activatable CRISPR sensors that can be applied to an even wider range of targets beyond nucleic acids.
A major challenge for the development of activatable CRISPR-Cas sensors for non-nucleic acid targets lies in finding a method to transduce the target recognition event into the collateral cleavage activity of Cas effectors, which is often activated through the specific binding of the crRNA to its target DNA (hereafter called the “activator”). To overcome this limitation, a CRISPR-Cas sensor based on competitive binding activities of bacterial allosteric transcription factors (aTFs) for small molecules and double-stranded DNA has been reported recently, but the detection targets are still limited, due to the small number of target-responsive aTFs available compared to the enormous number of POC sensors desired.22 In addition, a CRISPR-responsive hydrogel material has been developed. This material shows great potential in diagnosing various non-nucleic acid targets for POC needs through changing the input crRNA.23–24 To reach the full potential of CRISPR technologies for diagnostic applications, we here report a general strategy for the design of activatable CRISPR-Cas sensors based on target-responsive functional DNAs (fDNAs, e.g. structure-switching aptamer or RNA-cleaving DNAzyme). We chose fDNA because they have been shown to selectively bind a diverse range of non-nucleic acids targets, including organic small molecules, metal ions, proteins, viruses, and even cells and are obtained through a process called in vitro selection from a large DNA library of up to 1015 sequences.25–28 By coupling fDNA with CRISPR-Cas12a, we demonstrated that such a system can detect and quantify small organic molecules using aptamer-activated CRISPR-Cas12a and metal ions using DNAzyme-activated CRISPR-Cas12a under conditions required for POC devices, such as ambient temperatures and a simple and fast detection workflow.
RESULTS AND DISCUSSION
Our fDNA-regulated CRISPR-Cas sensor is comprised of two components (Figure 1): a probe system containing a fDNA and DNA activator for CRISPR-Cas12a, and a reporter system containing a pre-assembled Cas12a effector with crRNA and a single-stranded DNA (ssDNA) fluorescence reporter labeled with a fluorophore and quencher pair at the two ends (denoted as ssDNA-FQ). In the absence of the non-nucleic acid target, the fDNA binds to the DNA activator with a melting temperature above ambient temperature, preventing it from binding to the Cas12a-crRNA complex (denoted as “Locked Activator”). As a result, the collateral cleavage activity of Cas12a cannot be activated so the ssDNA-FQ reporter remains intact, resulting in a negligible fluorescence increase of the ssDNA fluorescent reporter, because the quencher is close to the fluorophore. On the other hand, in the presence of the non-nucleic acid target, target binding to the fDNA can decrease the melting temperature of the fDNA/DNA activator to be below ambient temperature. As a result, the DNA activator can dehybridize from the fDNA, becoming an “Open Activator” that binds to and activates the Cas12a. In this case, nearby ssDNA reporters can be cleaved, resulting in separation of the fluorophore from the quencher and thus producing a significant increase of fluorescence signal. Since the output fluorescence signal and its intensity are directly related to the presence and concentration of the activated Cas12a in solution, which in turn depends on the presence and concentration of the released DNA activator by the target, the presence and concentration of the target can be determined by monitoring the fluorescence change in the system. Using this strategy, we can transform the CRISPR-Cas12a system into a general sensor for quantification of a wide range of non-nucleic acid targets.
Figure 1.
Detection strategy and workflow of our fDNAs-regulated CRISPR-Cas12a sensor for detecting non-nucleic acid targets.
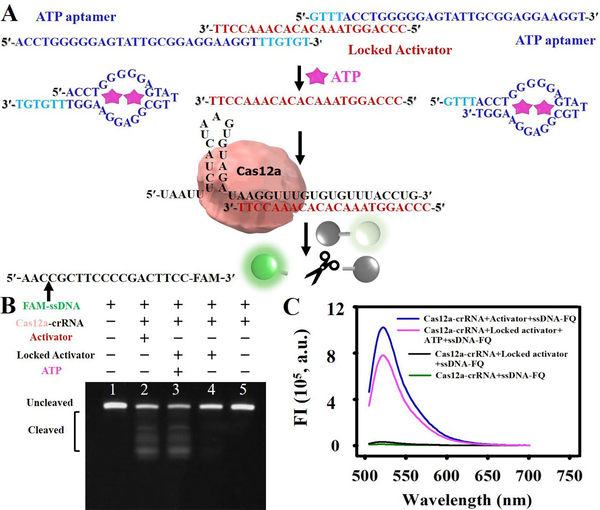
To demonstrate the above fDNA-regulated CRISPR-Cas12a sensor for detecting non-nucleic acid targets, we first chose the most widely used and studied structure-switching aptamer for adenosine 5′-triphosphate (ATP), the primary cellular energy carrier that plays an essential role in many biological processes, as the target. ATP levels can serve as an indicator of metabolic activity in viable cells, can be used to estimate the efficiency of decontamination of medical devices, and can be used to evaluate persistent bacteria after therapy.29–31 Therefore, developing a sensitive and selective strategy for on-site and real-time detection of ATP is very useful. To obtain a good analytical performance for the CRISPR-based ATP sensor, the ATP aptamer should possess two functions: 1) hybridizes with the activator and effectively blocks the activator binding to crRNA, which is essential to minimize the non-specific background signal; 2) effectively releases the activator in response to the ATP target in a structure-switching manner, which is essential to maximize the signal output. In addition, in order to avoid covering the ATP binding sites (highlighted G in 5′-ACCTGGGGGAGTATTGCGGAGGAAGGT-3′) within the ATP aptamer when hybridized with the activator, which could interfere with ATP binding, two ATP aptamers were employed to lock the activator strand (> 20 nucleotide, nt).32–33 As shown in Figure 2A, we further extended one of them at its 5′ end with GTTT and the other one at its 3′ end with TTGTGT in order to hybridize with the activator. In the absence of ATP, these two aptamers are designed to hybridize to the 22 nt DNA activator (shown in red) for CRISPR-Cas12a that is complementary to the guide crRNA, with calculated melting temperatures of these hybridizations of 34.8 and 32.9 °C, respectively. As a result, the DNA activator is locked by the ATP aptamer hybridizations and thus cannot bind the crRNA to activate the collateral cleavage activity of Cas12a-crRNA. In the presence of ATP, however, the binding of ATP by the ATP aptamer results in a conformational change, or structure switch, of the ATP aptamer, which weakens the hybridization between the two ATP aptamers and the DNA activator, due to the decreased melting temperatures of the remaining nucleotides after ATP binding to below room temperature. This allows for release of the DNA activator in an open state that can trigger the collateral cleavage activity of Cas12a-crRNA.
Figure 2.
Design and results of aptamer regulated CRISPR-Cas12a sensor for ATP detection. A. Design of the ATP Aptamer, Locked Activator, and crRNA. B. PAGE analysis of the feasibility of aptamer-regulated CRISPR-Cas12a sensor. FAM-labeled ssDNA without secondary structure was used as the substrate to visualize the results. C. Fluorescence spectra of the aptamer-regulated CRISPR-Cas12a sensor.
The aptamer-regulated activation of Cas12a-crRNA was first verified by PolyAcrylamide Gel Electrophoresis (PAGE) using a 19 nt FAM-labeled ssDNA (FAM-ssDNA) as the substrate for CRISPR-Cas12a. As shown in Figure 2B, the FAM-ssDNA (Lane 1) was digested into random short fragments when incubating Cas12a-crRNA with the DNA activator (Lane 2), but not in its absence (Lane 5), confirming that the DNA activator plays a critical role in activating the DNA cleavage activity of Cas12a-crRNA. More importantly, in the absence of ATP, addition of the two ATP aptamers to the DNA activator (as designed in Figure 2A) resulted in no DNA cleavage (Lane 4), indicating an efficient locking of the DNA activator by the dual ATP aptamers. In contrast, in the presence of 1 mM ATP, the same construct exhibits a similar DNA digestion result (Lane 3) to Lane 2, demonstrating the successful unlocking of the DNA activator to release the open activator through the ATP-triggered disassembly process.
To corroborate the results from PAGE, a fluorescent study was further performed by introducing a 5 nt ssDNA reporter as the substrate for Cas12a-crRNA, with a fluorophore (FAM) and quencher (3IABkFQ) conjugated at the 5′- and 3′ ends, respectively (denoted as ssDNA- FQ). In the absence of the DNA activator, minimal fluorescence signal was observed when the ssDNA-FQ was incubated with Cas12a-crRNA, while a considerable fluorescent signal was observed in the presence of the DNA activator (Figure 2C), and the fluorescence intensity increased as a function of increasing DNA activator concentrations (Figure S1), again confirming the role of the DNA activator in activating the efficient and quantitative cleavage of the fluorescence reporter by the Cas12a-crRNA. However, when dual ATP aptamers were used to hybridize to the DNA activator, the formed “locked” activator showed a significantly lower fluorescence signal (p < 0.001), compared with that of unlocked activator (Figure S2). Moreover, the fluorescence signal decreased with increasing molar ratios of ATP aptamer to activator strand up to 2:1 (Figure S2), at which point the fluorescence spectra displayed a significant overlap with the result of the fluorescence reporter alone (Figure 2C). In contrast, when a single ATP aptamer was used for locking the DNA activator, no significant difference of fluorescence signals was observed in comparison with the unlocked activator (p >0.05), even with a high molar ratio of 2:1 (Figure S3). The above results indicated that the proposed dual aptamer-locking strategy could efficiently prevent the activation of collateral activity of Cas12a by blocking the binding of crRNA with the DNA activator. When 200 μM of ATP was added to the locked activator, a significant increase of fluorescence signal was obtained (Figure 2C), demonstrating the restored collateral activity of Cas12a, likely as a result of binding of ATP that caused dehybridization of the dual aptamers away from the tripartite duplex structure as designed in Figure 2A. Taken together, these results demonstrate the effective regulation of the locked and the open states of the DNA activator using aptamers, forming the basis for a new CRISPR-based diagnostic platform for many non-nucleic acid targets that can be recognized by aptamers.
To achieve good analytical performance for ATP detection, some important detection parameters, including the assay temperature and enzymatic cleavage time were optimized using the fluorescence assay. As shown in Figure S4A, no significant difference of the fluorescence signal was observed when the CRISPR-Cas sensor was performed at 25 °C, 31 °C, and 37 °C (p > 0.05), indicating a good temperature tolerance for POC or field tests. Figure S4B shows the time profile of the substrate cleavage experiments in the presence of unlocked activator or aptamer-locked activator without and with the addition of ATP, and an optimal substrate cleavage time of 35 min was obtained.
To determine if the aptamer-regulated CRISPR-Cas12a sensor could detect ATP quantitatively, we first measured the fluorescence spectra of different concentrations of ATP added in HEPES buffer. Figure 3A shows that the fluorescence signal increased with increasing ATP concentrations from 0.39 μM to 50 μM. The fluorescence signal at 525 nm increased linearly with target ATP concentrations ranging from 0.78 μM to 25 μM (Figure S5), and a limit of detection (LOD) of 0.21 μM was obtained based on a 3σb/slope, where σb is the standard deviation of three blank samples. This is comparable to that of commercial colorimetric/fluorometric ATP kits (LOD ≈1 μM).
Figure 3.
Performance of the aptamer regulated CRISPR-Cas12a sensor for ATP detection. A. Fluorescence signal increase of the sensor in buffer over different concentration of ATP, ranging from 0, 0.39, 0.78, 1.56, 3.13, 6.25, 12.50, 25, 50, 100, 200 μM. Inset: dynamic curve for ATP detection in buffer. B. Selectivity of ATP detection. ATP: 200 μM; UTP, CTP, and GTP: 1 mM. C. Fluorescence signal increased of the sensor in 10% plasma over different concentration of ATP, ranging from 0, 0.10, 0.39, 1.56, 3.13, 6.25, 12.50, 25, 50, 100, 200, 400, 800 μM. Inset: dynamic curve for ATP detection in 10% plasma. D. Comparison of aptamer regulated CRISPR-Cas12a sensor with a commercial ATP kit in 10% plasma for ATP detection. The plasma samples were diluted with buffer A, and the concentration of ATP in each sample were detected both using aptamer regulated CRISPR-Cas12a sensor and ATP kit. The calibration curve in Figure S7 and Figure S8 (ATP kit) were used to calculate the ATP concentration in each plasma sample. ★★★: P < 0.001. ns: no significant difference.
To demonstrate the selectivity of the aptamer-regulated CRISPR-Cas12a sensor, we performed a fluorescence assay using different competing nucleotide-triphosphate analogs, including GTP, CTP, and UTP. As shown in Figure 3B, compared with the blank samples, no significant difference of fluorescence signals was observed for competing nucleotide-triphosphate analogs at a concentration of 1 mM (p > 0.05), while the fluorescence signal in response to 200 μM of ATP showed a more than 10-fold increase (p < 0.001), suggesting that the high selectivity of the ATP aptamer was maintained for the aptamer-regulated CRISPR-Cas12a sensor.
To test the feasibility of applying the aptamer-regulated CRISPR-Cas12a sensor in real samples, we explored the detection of ATP in human plasma, one of the most challenging matrices that contains many interfering molecules. To investigate if there is any non-specific digestion of crRNA by endogenous RNase in human plasma, we first challenged the CRISPR-Cas sensor with 2.5 nM of DNA activator in the absence and presence of 5 U/μL RNase inhibitor added in the assay buffer. As shown in Figure S6, the addition of RNase inhibitor produced a 1.5-fold increase of fluorescence signal in comparison with no RNase inhibitor (P < 0.001), indicating an effective inhibition of endogenous RNase. Then, by testing 30 human plasma samples spiked with different concentrations of ATP with our aptamer-regulated CRISPR-Cas12a sensor, we found that the calibration curve in the presence of 10% human plasma (Figure 3C) was similar with that in HEPES buffer. The fluorescence signal was proportional to the ATP concentrations from 1.56 μM to 100 μM, and LOD was calculated to be 0.44 μM (Figure S7), which is comparable with that obtained in the buffer (0.21 μM), suggesting that our aptamer-regulated CRISPR-Cas12a sensor can overcome interferences from other components in human plasma.
To verify the accuracy and reliability of our aptamer-regulated CRISPR-Cas12a sensor, we further compared it with a commercial bioluminescence kit for ATP detection by analyzing human plasma samples spiked with different concentrations of ATP. Based on the calibration curves from our fluorescence assay (Figure S7) and the kit’s bioluminescence assay (Figure S8), the ATP concentration in each plasma sample was calculated, and both of these two methods demonstrated a strong positive correlation between the detected and added ATP concentrations (Figure 3D), with a slope of 0.94 ± 0.043 (R2 = 0.9987) and 1.00 ± 0.044 (R2 = 0.9929), respectively. These results confirm that the accuracy of our aptamer-regulated CRISPR-Cas12a sensor is as good as that of the commercial ATP kit, thus confirming the suitability and reliability of the proposed sensor as an alternative test method for POC detection.
Encouraged by the above successful ATP sensing using the aptamer-regulated CRISPR-Cas12a sensor, we further conducted similar tests in human plasma samples using a commercially available portable fluorimeter for field or POC tests. A similar operation workflow is given in Figure 4A, and a fast fluorescence increase of the sensor in response to different concentrations of the DNA activator was obtained (Figure 4B). In addition, taking advantage of the fast fluorescence kinetic measurement of the portable fluorimeter, the enzymatic cleavage time was further shortened to 5 min. Figure 4C shows the relationship between the slope of the fluorescence signal at the time point of 5 min and different ATP concentrations. Moreover, the slope was proportional to the ATP concentrations from 0.78 μM to 50 μM, and LOD was calculated to be 4.75 μM (Figure S9). Although the analytical performance of the portable fluorimeter-based method is slightly decreased, the whole workflow could be finished within 15 min, thus providing a promising alternative approach for portable, fast quantification of ATP using a portable fluorimeter.
Figure 4.
Adapting the aptamer regulated CRISPR-Cas12a sensor to a portable fluorimeter platform. A. Workflow for ATP detection with portable fluorimeter. The whole detection workflow can be completed in less than 15 min with two steps. B. Fluorescence signal increase of Cas12a-crRNA and ssDNA-FQ with different concentrations of unlocked activator over time. C. The slope versus different ATP concentration at 5 min. ★★★: P < 0.001. ns: no significant difference.
Finally, to demonstrate the generality of the fDNA-regulated CRISPR-Cas12a sensor, we extended our methodology from aptamers to DNAzymes for the detection of metal ions. Sodium (Na+) is one of the most important metal ions in biology and is involved in many biological processes. Detection of Na+ can be used in conjunction with disease diagnostics: for instance, Na+ concentration can be used to evaluate the pathophysiological conditions hyponatremia (Na+ level <135 mm) and hypernatremia (Na+ level >145 mm).34–38 To achieve the detection of Na+, taking advantage of the fact that most of the DNAzymes exhibit high flexibility for the design of DNA sequences in the binding arms, we designed the substrate strand (NaA43S′) of a Na+-specific DNAzyme with an embedded DNA activator sequence at the 5′ end (shown in red) and a biotin label at the 3′ end (shown in blue) so that the NaA43S′ can hybridize to its enzyme strand (NaA43E′) via 18-base-pair hybridization with a melting temperature (59.8 °C) above room temperature (Figure 5A). This construct was immobilized onto a microplate with each well containing streptavidin. In the absence of Na+, the construct displayed a minimal fluorescent signal, because the DNA activator was locked by the DNAzyme construct. In the presence of Na+, the NaA43S′was cleaved into two pieces at the rA position by the NaA43E′. Since the binding arm that hybridizes to the DNA activator has a melting temperature of 9.5 °C after the Na+-dependent DNAzyme cleavage, the DNA activator can be dissociated from the binding arm under room temperature. In this case, the released activator could further bind to the Cas12a-crRNA complex to activate the collateral cleavage activity of the Cas12a, resulting in the digestion of the fluorescence reporter and thus a turn-on fluorescence signal. Figure 5B shows that the fluorescence signal increased with increasing Na+ concentrations from 0.024 mM to 100 mM. A linear range of 0.049 mM to 50 mM (Figure S10), and a LOD of 0.10 mM was obtained, which is much lower than the normal Na+ level in human serum. Furthermore, the sensor exhibited excellent selectivity for Na+ over other competing metal ions at physiologically relevant concentrations (Figure 5C). Before verifying the accuracy and reliability of the DNAzyme-regulated CRISPR-Cas12a sensor in plasma, we tested NaA43E′ stability in 50-fold diluted plasma. No obvious fluorescence signal increase was observed with inactive NaA43E′ format unless with active NaA43E′, which suggests the NaA43 DNAzyme remains intact in the presence of RNases and other components from plasma, suggesting that the activation of Cas12a only depends on the Na+-specific cleavage. (Figure S11) Then, we further compared it with a commercial Na+ meter in 50-fold diluted human plasma samples. A strong positive correlation between these two methods was obtained (Figure S12), indicating the good accuracy of the sensor for metal ion detection.
Figure 5.
Design and performance of DNAzyme-regulated CRISPR-Cas12a sensor for Na+ detection. A. Design of NaA43 DNAzyme for Na+ detection. B. Fluorescence signal increase of the sensor in buffer over different concentrations of Na+, ranging from 0, 0.024, 0.049, 0.098, 0.20, 0.39, 0.78, 1.56, 3.13, 6.25, 12.50, 25, 50, 100 mM. Insert: dynamic curve for Na+ detection. C. Selectivity of Na+ detection. Monovalents: 100 mM; divalents: 2 mM; trivalents: 0.20 mM. SA-Bio: streptavidin-biotin.
CONCLUSION
In summary, we have demonstrated a general and versatile fDNA-regulated CRISPR-Cas sensor for the quantitative detection of two non-nucleic acid targets. Using this system, ATP and Na+ have been detected quantitatively under ambient temperature using a simple and fast detection workflow (two steps and <15 min), making the fDNA-regulated CRISPR system suitable for field tests or POC diagnostics. Since fDNA can be obtained from a large DNA library to recognize a wide range of targets, from metal ions and small organic molecules to proteins and even viruses and cells, using in vitro selection,39–46 the methods demonstrated here can expand this powerful CRISPR-Cas sensor system significantly to many other targets and thus provide a new toolbox to broaden the bioanalytical and biomedical applications of CRISPR-Cas systems.
Supplementary Material
ACKNOWLEDGMENT
We thank the U.S. National Institutes of Health (GM124316 and MH110975) for financial support. Yonghua Xiong acknowledges the National Key Research and Development Program of China (2018YFC1602505). Ying Xiong acknowledges support from the China Scholarship Council. We would like to thank Jennifer Cui for her help with the portable fluorimeter experiments and Ryan Lake for proofreading.
Footnotes
REFERENCES
- (1).Zhang J; Lan T; Lu Y, Molecular engineering of functional nucleic acid nanomaterials toward in vivo applications. Adv. Healthc. Mater. 2019, 8, 1801158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Li L; Xing H; Zhang J; Lu Y, Functional DNA molecules enable selective and stimuli-responsive nanoparticles for biomedical applications. Acc. Chem. Res. 2019, 52, 2415–2426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Somasundaram S; Easley CJ, A nucleic acid nanostructure built through on-electrode ligation for electrochemical detection of a broad range of analytes. J. Am. Chem. Soc. 2019, 141, 11721–11726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Zhang J; Xing H; Lu Y, Translating molecular detections into a simple temperature test using a target-responsive smart thermometer. Chem. Sci. 2018, 9, 3906–3910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Cong L; Ran FA; Cox D; Lin S; Barretto R; Habib N; Hsu PD; Wu X; Jiang W; Marraffini LA, Multiplex genome engineering using CRISPR/Cas systems. Science 2013, 339, 819–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Gilbert LA; Larson MH; Morsut L; Liu Z; Brar GA; Torres SE; Stern-Ginossar N; Brandman O; Whitehead EH; Doudna JA, CRISPR-mediated modular RNA-guided regulation of transcription in eukaryotes. Cell 2013, 154, 442–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Pardee K; Green AA; Takahashi MK; Braff D; Lambert G; Lee JW; Ferrante T; Ma D; Donghia N; Fan M, Rapid, low-cost detection of Zika virus using programmable biomolecular components. Cell 2016, 165, 1255–1266. [DOI] [PubMed] [Google Scholar]
- (8).Bruch R; Urban GA; Dincer C, Unamplified gene sensing via Cas9 on graphene. Nat. Biomed. Eng. 2019, 3, 419–420. [DOI] [PubMed] [Google Scholar]
- (9).Gootenberg JS; Abudayyeh OO; Lee JW; Essletzbichler P; Dy AJ; Joung J; Verdine V; Donghia N; Daringer NM; Freije CA; Myhrvold C; Bhattacharyya RP; Livny J; Regev A; Koonin EV; Hung DT; Sabeti PC; Collins JJ; Zhang F, Nucleic acid detection with CRISPR-Cas13a/C2c2. Science 2017, 356, 438–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Gootenberg JS; Abudayyeh OO; Kellner MJ; Joung J; Collins JJ; Zhang F, Multiplexed and portable nucleic acid detection platform with Cas13, Cas12a, and Csm6. Science 2018, 360, 439–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Chen JS; Ma E; Harrington LB; Da Costa M; Tian X; Palefsky JM; Doudna JA, CRISPR-Cas12a target binding unleashes indiscriminate single-stranded DNase activity. Science 2018, 360, 436–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).East-Seletsky A; O’Connell MR; Knight SC; Burstein D; Cate JH; Tjian R; Doudna JA, Two distinct RNase activities of CRISPR-C2c2 enable guide-RNA processing and RNA detection. Nature 2016, 538, 270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Myhrvold C; Freije CA; Gootenberg JS; Abudayyeh OO; Metsky HC; Durbin AF; Kellner MJ; Tan AL; Paul LM; Parham LA, Field-deployable viral diagnostics using CRISPR-Cas13. Science 2018, 360, 444–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Shan Y; Zhou X; Huang R; Xing D, High-fidelity and rapid quantification of miRNA combining crRNA programmability and CRISPR/Cas13a trans-cleavage activity. Anal. Chem. 2019, 91, 5278–5285. [DOI] [PubMed] [Google Scholar]
- (15).Chen Y; Yang S; Peng S; Li W; Wu F; Yao Q; Wang F; Weng X; Zhou X, N1-methyladenosine detection with CRISPR-Cas13a/C2c2. Chem. Sci. 2019, 10, 2975–2979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Kundert K; Lucas JE; Watters KE; Fellmann C; Ng AH; Heineike BM; Fitzsimmons CM; Oakes BL; Qu J; Prasad N, Controlling CRISPR-Cas9 with ligand-activated and ligand-deactivated sgRNAs. Nat. Commun. 2019, 10, 2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Yang X; Tang Q; Jiang Y; Zhang M; Wang M; Mao L, Nanoscale ATP-responsive zeolitic imidazole framework-90 as a general platform for cytosolic protein delivery and genome editing. J. Am. Chem. Soc. 2019, 141, 3782–3786. [DOI] [PubMed] [Google Scholar]
- (18).Raper AT; Stephenson AA; Suo Z, Sharpening the Scissors: mechanistic details of CRISPR/Cas9 improve functional understanding and inspire future research. J. Am. Chem. Soc. 2018, 140, 11142–11152. [DOI] [PubMed] [Google Scholar]
- (19).Zhang K; Deng R; Teng X; Li Y; Sun Y; Ren X; Li J, Direct visualization of single-nucleotide variation in mtDNA using a CRISPR/Cas9-mediated proximity ligation assay. J. Am. Chem. Soc. 2018, 140, 11293–11301. [DOI] [PubMed] [Google Scholar]
- (20).Hemphill J; Borchardt EK; Brown K; Asokan A; Deiters A, Optical control of CRISPR/Cas9 gene editing. J. Am. Chem. Soc. 2015, 137, 5642–5645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Palermo G; Ricci CG; Fernando A; Basak R; Jinek M; Rivalta I; Batista VS; McCammon JA, Protospacer adjacent motifinduced allostery activates CRISPR-Cas9. J. Am. Chem. Soc. 2017, 139, 16028–16031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Liang M; Li Z; Wang W; Liu J; Liu L; Zhu G; Karthik L; Wang M; Wang K-F; Wang Z, A CRISPR-Cas12a-derived biosensing platform for the highly sensitive detection of diverse small molecules. Nat. Commun. 2019, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).English MA; Soenksen LR; Gayet RV; de Puig H; Angenent-Mari NM; Mao AS; Nguyen PQ; Collins JJ, Programmable CRISPR-responsive smart materials. Science 2019, 365, 780–785. [DOI] [PubMed] [Google Scholar]
- (24).Han D; Li J; Tan W, CRISPR propels a smart hydrogel. Science 2019, 365, 754–755. [DOI] [PubMed] [Google Scholar]
- (25).Xiang Y; Lu Y, Using personal glucose meters and functional DNA sensors to quantify a variety of analytical targets. Nat. chem. 2011, 3, 697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Wang W; Yu S; Huang S; Bi S; Han H; Zhang J-R; Lu Y; Zhu J-J, Bioapplications of DNA nanotechnology at the solid-liquid interface. Chem. Soc. Rev. 2019, 48, 4892–4920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Li J; Mo L; Lu C-H; Fu T; Yang H-H; Tan W, Functional nucleic acid-based hydrogels for bioanalytical and biomedical applications. Chem. Soc. Rev. 2016, 45, 1410–1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Liu J; Cao Z; Lu Y, Functional nucleic acid sensors. Chem. Rev. 2009, 109, 1948–1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Wang L; Yuan L; Zeng X; Peng J; Ni Y; Er JC; Xu W; Agrawalla BK; Su D; Kim B; Chang YT, A multisite-binding switchable fluorescent probe for monitoring mitochondrial ATP level fluctuation in live cells. Angew. Chem., Int. 2016, 55, 1773–6. [DOI] [PubMed] [Google Scholar]
- (30).Rechmann P; Chaffee BW; Rechmann BM; Featherstone JD, Efficacy of an adenosine triphosphate meter for evaluating caries risk in clinical dental practice. J. Am. Dent. Assoc. 2019, 150, 873–882. [DOI] [PubMed] [Google Scholar]
- (31).Labib M; Sargent EH; Kelley SO, Electrochemical methods for the analysis of clinically relevant biomolecules. Chem. Rev. 2016, 116, 9001–9090. [DOI] [PubMed] [Google Scholar]
- (32).Lin CH; Patei DJ, Structural basis of DNA folding and recognition in an AMP-DNA aptamer complex: distinct architectures but common recognition motifs for DNA and RNA aptamers complexed to AMP. Chem. Biol. 1997, 4, 817–832. [DOI] [PubMed] [Google Scholar]
- (33).Nutiu R; Li Y, Structure-switching signaling aptamers. J. Am. Chem. Soc. 2003, 125, 4771–4778. [DOI] [PubMed] [Google Scholar]
- (34).Torabi S-F; Wu P; McGhee CE; Chen L; Hwang K; Zheng N; Cheng J; Lu Y, In vitro selection of a sodium-specific DNAzyme and its application in intracellular sensing. Proc. Natl. Acad. Sci. U. S. A. 2015, 112, 5903–5908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (35).Wu Z; Fan H; Satyavolu NSR; Wang W; Lake R; Jiang JH; Lu Y, Imaging endogenous metal ions in living cells using a DNAzyme-catalytic hairpin assembly probe. Angew. Chem., Int. 2017, 56, 8721–8725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (36).Zhang J; Lu Y, Biocomputing for portable, resettable, and quantitative point-of-care diagnostics: making the glucose meter a logic-gate responsive device for measuring many clinically relevant targets. Angew. Chem., Int. 2018, 57, 9702–9706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (37).Ma L; Liu J, An in vitro-selected DNAzyme mutant highly specific for Na+ under slightly acidic conditions. Chem. Bio. Chem. 2019, 20, 537–542. [DOI] [PubMed] [Google Scholar]
- (38).Agrawal V; Agarwal M; Joshi SR; Ghosh A, Hyponatremia and hypernatremia: disorders of water balance. JAPI 2008, 56, 956–64. [PubMed] [Google Scholar]
- (39).Liu M; Yin Q; Chang Y; Zhang Q; Brennan JD; Li Y, In vitro selection of circular DNA aptamers for biosensing applications. Angew. Chem., Int. 2019, 58, 8013–8017. [DOI] [PubMed] [Google Scholar]
- (40).Yang Z; Loh KY; Chu Y-T; Feng R; Satyavolu NSR; Xiong M; Nakamata Huynh SM; Hwang K; Li L; Xing H, Optical control of metal ion probes in cells and zebrafish using highly selective DNAzymes conjugated to upconversion nanoparticles. J. Am. Chem. Soc. 2018, 140, 17656–17665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (41).Yang W; Yu H; Alkhamis O; Liu Y; Canoura J; Fu F; Xiao Y, In vitro isolation of class-specific oligonucleotide-based small-molecule receptors. Nucleic Acids Res. 2019, 47, e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (42).Tang Y; Ge B; Sen D; Yu H-Z, Functional DNA switches: rational design and electrochemical signaling. Chem. Soc. Rev. 2014, 43, 518–529. [DOI] [PubMed] [Google Scholar]
- (43).Sczepanski JT; Joyce GF, Specific inhibition of microRNA processing using L-RNA aptamers. J. Am. Chem. Soc. 2015, 137, 16032–16037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (44).Wang L; Liang H; Sun J; Liu Y; Li J; Li J; Li J; Yang H, Bispecific aptamer induced artificial protein-pairing: A strategy for selective inhibition of receptor function. J. Am. Chem. Soc. 2019, 141, 12673–12681. [DOI] [PubMed] [Google Scholar]
- (45).Chang X; Zhang C; Lv C; Sun Y; Zhang M; Zhao Y; Yang L; Han D; Tan W, Construction of a multiple-aptamer-based DNA logic device on live cell membranes via associative toehold activation for accurate cancer cell identification. J. Am. Chem. Soc. 2019, 141, 12738–12743. [DOI] [PubMed] [Google Scholar]
- (46).Ge Z; Gu H; Li Q; Fan C, Concept and development of framework nucleic acids. J. Am. Chem. Soc. 2018, 140, 17808–17819. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.