ABSTRACT
Non-shivering thermogenesis can promote negative energy balance and weight loss. In this study, we identified a contextual stimulus that induces rapid and robust thermogenesis in skeletal muscle. Rats exposed to the odor of a natural predator (ferret) showed elevated skeletal muscle temperatures detectable as quickly as 2 min after exposure, reaching maximum thermogenesis of >1.5°C at 10–15 min. Mice exhibited a similar thermogenic response to the same odor. Ferret odor induced a significantly larger and qualitatively different response from that of novel or aversive odors, fox odor or moderate restraint stress. Exposure to predator odor increased energy expenditure, and both the thermogenic and energetic effects persisted when physical activity levels were controlled. Predator odor-induced muscle thermogenesis is subject to associative learning as exposure to a conditioned stimulus provoked a rise in muscle temperature in the absence of the odor. The ability of predator odor to induce thermogenesis is predominantly controlled by sympathetic nervous system activation of β-adrenergic receptors, as unilateral sympathetic lumbar denervation and a peripherally acting β-adrenergic antagonist significantly inhibited predator odor-induced muscle thermogenesis. The potential survival value of predator odor-induced changes in muscle physiology is reflected in an enhanced resistance to running fatigue. Lastly, predator odor-induced muscle thermogenesis imparts a meaningful impact on energy expenditure as daily predator odor exposure significantly enhanced weight loss with mild calorie restriction. This evidence signifies contextually provoked, centrally mediated muscle thermogenesis that meaningfully impacts energy balance.
KEY WORDS: Energy balance, Energy expenditure, Sympathetic nervous system, Weight loss
Summary: Exposure to the odor of a predator (ferret) markedly increases rat skeletal muscle temperature; this persists when physical activity is controlled. This is primarily modulated by sympathetic neural connections.
INTRODUCTION
The increase in obesity rates has been accompanied by intensified interest in thermogenesis, with the goal of exploiting thermogenic mechanisms to combat weight gain (Contreras et al., 2017; Maurya et al., 2015). Increasing attention is turning to the importance of skeletal muscle thermogenesis in body weight homeostasis. With respect to humans, considering the mass of skeletal muscle the human body possesses and its contribution to metabolic rate (Zurlo et al., 1990), muscle thermogenesis represents substantial untapped potential to amplify energy expenditure. In other words, if muscle acts as a thermogenic organ, even a relatively small change in heat generation would substantially increase caloric expenditure. Indeed, evidence supports the ability of muscle thermogenesis and its potential underlying mediators to meaningfully impact energy balance (Maurya et al., 2015; Periasamy et al., 2017). Yet, relatively little is known regarding actual heat generation by muscle (Alekseev et al., 2010) or the physiological modulation of muscle thermogenesis outside of cellular and biochemical interactions at the level of the skeletal myocyte (Bal et al., 2012; Maurya et al., 2015; Pant et al., 2016; Periasamy et al., 2017).
In skeletal muscle, one thermogenic mediator appears to be the uncoupling of sarco/endoplasmic reticulum calcium ATPase (SERCA) by modulators including sarcolipin (Periasamy et al., 2017). The sparse knowledge regarding systemic or neural mediation of muscle thermogenesis stands in stark contrast to the detailed understanding of the neural processes controlling the induction of brown adipose tissue (BAT) thermogenesis (Bartness et al., 2010; Contreras et al., 2017; Morrison and Nakamura, 2019). We can use what is known about parallel thermogenic systems to inform hypotheses regarding central control of muscle thermogenesis. BAT thermogenesis and induction of white adipose browning are strongly provoked by activation of the sympathetic nervous system (SNS) through neural input and adrenergic receptors (Bartness et al., 2010; Contreras et al., 2017). At the level of the organism, both muscle sarcolipin and BAT uncoupling protein (UCP) 1 contribute to cold adaptation and counter diet-induced obesity (Bal et al., 2012; Maurya et al., 2015). Skeletal muscle thermogenesis appears to be even more relevant in larger mammals (e.g. rabbits, dogs), including humans, than in laboratory rodents, with high concentrations of thermogenic mediators like sarcolipin (reviewed in Bal et al., 2018; Maurya and Periasamy, 2015; Rowland et al., 2015).
Centrally, the ventromedial hypothalamus (VMH) is implicated in the modulation of muscle metabolism, including insulin sensitivity (Coutinho et al., 2017; Fujikawa et al., 2016). With respect to thermogenesis, VMH melanocortin receptor activation stimulates muscle thermogenesis and enhances activity energy expenditure following a time course that differs from its induction of BAT thermogenesis (Gavini et al., 2016). Emerging evidence of VMH and steroidogenic factor-1 (SF-1) cell control of peripheral – especially muscle – metabolism (Coutinho et al., 2017; Fujikawa et al., 2016) prompted a closer look at this cell population. In addition to their role in peripheral metabolic regulation (Choi et al., 2013), VMH SF-1 cells are critical in mediating the behavioral response to predator threat, including predator odor (Kunwar et al., 2015; Silva et al., 2013). The VMH may therefore serve as a key interface between competing drives, namely energy balance and predator threat (Viskaitis et al., 2017). Given evidence that predator threat skews energy balance and induces weight loss in multiple species (Genne-Bacon et al., 2016; Monarca et al., 2015; Tidhar et al., 2007), we considered this mechanistic overlap in developing hypotheses regarding the induction of muscle thermogenesis. Using direct measurement of muscle temperature of rats and mice, we explored the possibility that predator odor acts as a contextual stimulus to induce a thermogenic response in skeletal muscle. The ability of predator threat to alter multiple, intertwined aspects of behavior and physiology that impact energy balance (e.g. food intake, physical activity, stress response: Figueiredo et al., 2003; Tidhar et al., 2007) necessitated that these and other confounders be removed, controlled or measured to verify the thermogenic effect of predator odor. In doing this, we identified predator odor as a powerful inducer of thermogenesis and further established the sympathetic nervous system as the dominant regulator of this thermogenic induction.
MATERIALS AND METHODS
Animals and procedure
Muscle thermogenesis was measured in male and female Sprague–Dawley rats (Envigo or bred in-house) and male and female C57/BL6 mice (Jackson Laboratory or bred in-house) in response to predator odor. Ferret odor was selected because of its ability to produce strong, consistent behavioral and physiological responses (Campeau et al., 2008). All testing was conducted during the light phase of the cycle. Food and water were provided ad libitum unless otherwise stated. All procedures were conducted in accordance with the Kent State University Animal Care and Use Committee.
Changes in skeletal muscle temperature in response to predator odor stimuli were measured using temperature transponders (IPTT-300, BioMedic Data Systems, Seaford, DE, USA; calibrated range 32–43°C); transponders were used that had shown high correlation compared with water-bath temperature (r2≥0.97) as well as in vivo with rectal temperature in the physiological range (r2≥0.97) (Wacker et al., 2012). The transponders were 14 mm long and 2 mm in diameter, sufficiently small to surgically implant into the gastrocnemius muscle group bilaterally in rats; in some rats, an additional transponder was implanted adjacent to the BAT. In mice, transponders were implanted unilaterally against the gastrocnemius. The transponders were implanted under surgical anesthesia, with 5% isoflurane for induction followed by 2–3% for maintenance, and analgesia using ketoprofen (5 mg kg−1, s.c., in rats and mice). Transponder readers (DAS-7007R, 7007S or 6007) were used to manually retrieve transponder temperature data. Temperature was measured before and after exposure to a fragment (25–50 mm×50 mm) of a towel that had been used as bedding for ferrets (Mustela putorius furo) for 2 weeks (Marshall BioResources, North Rose, NY, USA). The control stimulus was an identical towel without the ferret odor. Treatments and conditions were counterbalanced to avoid an order effect (with the exception of the study presented in Fig. 1D,E). Exposure to predator odor is also perceived as stressful and aversive for rats (Weinberg et al., 2009). Therefore, other stimuli included Tink's Red Fox-P Cover Scent (500 µl), butyric acid (50 µl) as an aversive odor, 2-methylbenzoxazole (50 µl) as a novel odor, and 1 min of handling and restraint in a clean medical towel. The volatility of these odors precluded counterbalancing the order of odor presentation. Estrous cycle was determined in female rats using vaginal lavage and cytology, with leukocytes present during diestrus, nucleated epithelial cells during proestrus, and cornified epithelial cells during estrous.
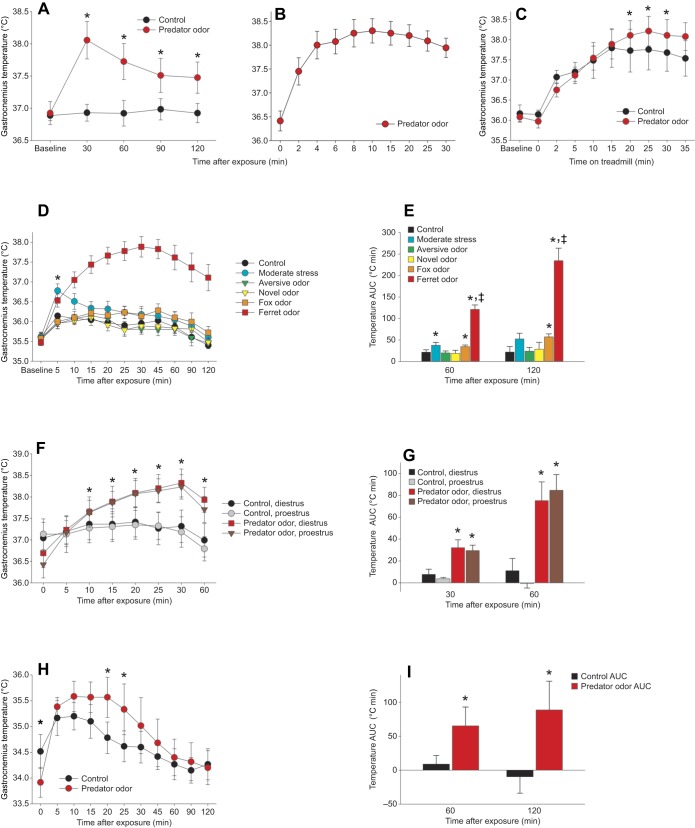
Fig. 1.
Predator odor induces a rapid rise in skeletal muscle thermogenesis. (A) Gastrocnemius temperature was significantly elevated after predator odor compared with control exposure in male rats (n=8). (B) Gastrocnemius temperature elevation was rapid, being detectable at 2 min after exposure (n=4). (C) Predator odor-induced thermogenesis was evident when activity levels were controlled using treadmill walking (n=7). *Predator odor>control. (D,E) Novel or aversive odors did not significantly change muscle temperature (D), and the temperature change induced by fox odor or by handling and restraint stress was significantly less than that induced by ferret odor (E; area under the curve, AUC; n=7). *Effect>control; ‡ferret odor>all other groups. (F,G) Female rats showed similar muscle thermogenic induction, and neither control nor predator odor-stimulated thermogenesis (F) or temperature AUC (G) differed between proestrus and diestrus phases of the estrous cycle (n=5). *Predator odor>control. (H,I) Predator odor-induced increases in muscle temperature (H) and temperature AUC (I) were also seen in mice up to 60 min after exposure (n=3 male+3 female). *Predator odor>control; ‡significant interaction.
For mice, towels were placed inside a small plastic whiffle ball to prevent excessive interaction with the towel. No long-term changes in gait or behavior were seen after transponder implant, and transponder placements were confirmed at the conclusion of the study.
Controlled activity using treadmill walking
As locomotion increases muscle temperature (Gavini et al., 2016), the increased physical activity seen after exposure to predator odor could be one source of muscle heat. To control for altered activity, locomotion was controlled using treadmill walking where muscle temperature was measured in rats exposed to control or predator odors while undergoing the same level of locomotion (i.e. a controlled walk on a treadmill). First, rats (n=7, 3 females and 4 males) were placed on an enclosed treadmill, open on the back to allow for insertion of the reader. A towel, with either control or predator odor, was taped to the internal ceiling of the treadmill approximately level with the rat's head. Exposure condition was counterbalanced on different days and in different orders. The rat was placed into the treadmill, and the treadmill was immediately started at 7 m min−1. Temperature was measured at 2, 5, 10, 15, 20, 25, 30 and 35 min of walking at a constant speed with no incline, adapted from a previous protocol (Gavini et al., 2014).
For rats that underwent surgical unilateral denervation (see below), an established treadmill-walking protocol was used (Gavini et al., 2014) without odor exposure. Gastrocnemius temperature was measured before walking and during a 35 min treadmill test starting at 7 m min−1 speed and 0 deg incline at 2, 5 and 10 min; 9 m min−1 and 0 deg incline at 15 min; 9 m min−1 and 10 deg incline at 20 min; 11 m min−1 and 10 deg incline at 25 min; and 11 m min−1 and 20 deg incline at 30 and 35 min.
Lastly, to more precisely examine the potential survival value of the metabolic sequelae of the response to predator threat, we subjected the same rats to a treadmill protocol designed to induce running fatigue rather than exhaustion (adapted from Sopariwala et al., 2015), with fatigue defined as the inability to maintain locomotion at a given speed while still physically able to run (Soares et al., 2004). To measure running fatigue, treadmill speed started at 7 m min−1 (0 deg incline) for 5 min, then 5 min each at 10 m min−1 (0 deg incline), 10 m min−1 (5 deg incline), 11 m min−1 (5 deg incline), then 11 m min−1 (10 deg incline) for 10 min; then, 13 m min−1 (10 deg incline) was maintained for the remainder of the trial until the rat attained fatigue. Onset of fatigue was reached when a rat was either unable to keep up with the treadmill belt speed or had maintained at least partial contact with the shocker for 10 s.
Calorimetry
To assess changes in caloric expenditure with predator odor exposure, energy expenditure (EE) was measured using a 4-chamber Oxymax FAST system with infrared activity monitors (Columbus Instruments, Columbus, OH, USA); EE and respiratory exchange ratio (RER) were measured in rats exposed to predator odor or control odor, counterbalanced on separate days, as previously described (Gavini et al., 2016), using the equation: EE=CV+V̇O2, where CV (calorific value)=3.815+(1.232×RER), and RER=V̇CO2/V̇O2 (rate of CO2 production divided by rate of O2 consumption). Briefly, rats were enclosed in calorimetry chambers and fresh air was supplied to each chamber at 2.79–3.0 l min−1, depending on the weight of the rat, with the same flow (in l min−1) within rat between tests. Sample air was measured at 0.5 l min−1 with reference air measured after each 30 samples (3.4 min break in experimental measurement). Rats were placed in the chamber and the calorimeter was calibrated with primary gas standards. Gas exchange was measured for 15 min; during the first reference period, the chambers were briefly opened and predator odor or control towels were supplied to the rats before each chamber was re-sealed. Gas exchange and physical activity measurements continued for an additional 3 h.
For EE during treadmill walking, 9 adult female rats were placed on the treadmill after a 5 min predator odor or control exposure, the treadmill was sealed, and rats were allowed to walk for 30 min at 7 m min−1 (Gavini et al., 2016). For V̇O2,max, the treadmill was started at 10 m min−1 at 0 deg incline for 5 min, then at 10 m min−1 at 15 deg incline for 5 min, then 10 m min−1 at 25 deg incline for 5 min. At 15 min, belt speed was increased to 15 m min−1 for 2 min, then increased 2 m min−1 every 2 min thereafter until the rat reached exhaustion (when the rat was unable to continue running or was unable to move off the shocker, or was on the shocker for 5 s). For calorimetry and all treadmill studies, rats received the two treatments in random order on different days.
Associative learning and habituation
To determine whether predator odor exposure induces associative learning, we exposed 8 adult male rats to the predator odor stimulus (towel) inside a 7 cm diameter green whiffle ball, serving as the unconditioned stimulus; 8 control rats were exposed to a control stimulus (whiffle ball containing a clean towel). Temperature was measured before and after exposure to the odor or control stimulus. The following day, all rats were exposed to the same stimulus – the clean towel in the whiffle ball – which acted as a conditioned stimulus in the rats that had received the predator odor/whiffle ball stimulus the previous day. Temperature was again measured before and after presentation with the whiffle ball/towel stimulus.
After recognizing the potential for learned associations to induce muscle thermogenesis, studies were designed to minimize the effect of associative conditioning on baseline and predator odor-evoked muscle thermogenesis. Rats and mice were habituated to the experimental conditions before predator odor and control stimulus exposure. Rats or mice were moved to the room where predator odor or control stimulus would be presented, and temperature was measured. This was repeated multiple times before and between experimental conditions.
Surgical denervation
To determine the role of the lumbar sympathetic nerve (LSN) in predator odor-induced muscle thermogenesis, 9 rats received unilateral surgical denervation (n=5 left LSN denervation, 4 right LSN denervation). Rats were anesthetized with isoflurane (5% induction, 2–3% maintenance) with analgesics given before the procedure and during recovery (ketoprofen, 5 mg kg−1, s.c.; buprenorphine, 0.05 mg kg−1, s.c.); the surgical site was prepared with alcohol and betadine scrub before an incision was made on the ventral surface. Using the procedure described by Kerman et al. (2003), the LSN was unilaterally dissected and extirpated. Briefly, a ventral laparotomy was performed, the viscera were gently shifted using sterile gauze and saline, and the LSN was isolated near the lumbar muscles; a ∼1 cm portion of the LSN was removed at the level between the renal vein and iliac bifurcation. Sutures were used to close the abdominal musculature, and outer incisions were closed using subcuticular sutures; rats were allowed to recover for 14 days. There were no noticeable negative consequences of the denervation to rat gait, activity or behavior. Control and predator odor-induced thermogenesis over 120 min was measured for each rat in random order 7 days apart. Walking-induced (treadmill activity) muscle temperature was also measured in these rats using an established protocol described above (Gavini et al., 2014). Care was taken to examine muscle thermogenesis before potential nerve regrowth, predicted to start at 10–12 weeks (Rodionova et al., 2016); all measurements were complete by 70 days after surgical denervation. After terminal anaesthesia, muscles were dissected, catecholamines isolated, and analysis performed using high performance liquid chromatography. As the primary source of noradrenaline (NA; norepinephrine) in skeletal muscle is from sympathetic nerve fibers, altered NA concentration was used as an indicator of successful SNS denervation of muscle. In addition, to consider potential changes in baseline thermogenic capacity with SNS denervation, we used qPCR to compare expression between the control and denervated lateral gastrocnemius of the known muscle thermogenic mediator sarcolipin (reviewed by Periasamy et al., 2017), as well as a mitochondrial metabolic protein uncoupling protein 3 (UCP3; reviewed by Fuller-Jackson and Henry, 2018). Muscle (∼0.05 g) was homogenized by Bullet Blender (Next Advance, Bullet Blender 24 Gold, Troy, NY, USA), and mRNA was isolated using TRIzol™ Reagent (Thermo Fisher Scientific, Waltham, MA, USA); bromochloropropane (BCP) was added and incubated for 5 min at room temperature to facilitate phase separation, then samples were centrifuged at 12,000 RCF for 10 min at 4°C. The aqueous RNA phase (∼250 µl) was pipetted into a new microtube and mixed with 100% ethanol (150 µl) to precipitate mRNA. The mixture was then pipetted on a silica-based column membrane (Invitrogen™ PureLink™ RNA Mini Kit, Thermo Fisher Scientific), and samples were centrifuged at 12,000 RCF for 30 s at room temperature. Samples were washed according to kit instructions, and RNA was eluted from the filter with 100 µl kit elution buffer by centrifuging at 12,000 RCF for 30 s at room temperature. The purity and quantity of mRNA were measured using Cytation 5 Cell Imaging Multi-Mode Reader (BioTek Instruments, Winooski, VT, USA), resulting in A260/280 ratios ranging from 1.8 to 2.1 with 50–110 ng µl−1 mRNA concentration. Approximately 250 ng of isolated RNA was reverse transcribed using a High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Warrington, Cheshire, UK). The target cDNA was amplified by PCR at 37°C for 60 min, and 95°C for 5 min. All qPCR assays were carried out in triplicate using the Brilliant III Ultra-Fast QPCR Master Mix (Agilent Technologies, Santa Clara, CA, USA) and using PrimeTime Gene Expression Probes (IDT DNA Technologies). The assay identification numbers for each gene are: Gapdh (Rn.PT.39a.11180736.g), Sln (Rn.PT.58.8785990) and UCP3 (Rn.PT.58.17938212). Relative expression for each gene was calculated using the housekeeping gene Gapdh as a reference and the −ΔCt method (Schmittgen and Livak, 2008) as described previously (Gavini et al., 2016).
β-Adrenergic receptor inhibition
Because SNS neural activation of β-adrenergic receptors, specifically β2 receptors, mediates centrally induced changes in skeletal muscle glucose metabolism and insulin sensitivity (Miyaki et al., 2011), we hypothesized that β2 adrenergic receptors similarly mediate the thermogenic effect evoked by predator threat. To examine the role of β-adrenergic receptors in predator odor-induced muscle thermogenesis, we opted to use a peripherally active mixed adrenergic antagonist to avoid central effects of β2-adrenergic receptor antagonists that would affect brain regions modulating muscle metabolism (Ibrahim et al., 2019; Miyaki et al., 2011). In random order over 17 days, each rat was exposed to predator odor and control stimulation with either vehicle (sterile saline) or nadolol (8 mg kg−1, i.p.) given 2 h before predator odor or control exposure; temperature was measured for 120 min after exposure. Nadolol is active at β1 and β2, but not β3, adrenergic receptors (Cernecka et al., 2014). We also measured BAT temperature in this study because, in addition to the well-known actions of β3 in BAT, brown adipocytes also have β1 and β2 receptors that also have functional relevance (Collins, 2011; Larson, 2019).
Weight loss
Baseline food intake was measured in 12 male and female Sprague–Dawley rats (n=7 males, 5 females), then a weight-maintenance diet was found for each rat by adjusting daily food allotment. Weight-maintenance food allocation was continued while rats were subjected to 7 days of control odor exposure. The procedure in the subsequent week was identical, except the rats were exposed to a new predator odor stimulus daily (24 h). Stimulus towels were replaced and rats were weighed daily within the hour prior to lights-off. Rats underwent EchoMRI measurement of body composition before and after the week of control odor and after the week of chronic predator odor. Changes in body weight, fat and lean mass, and food intake relative to weight-maintenance feeding were calculated and compared between control and ferret odor exposure conditions.
For calorie restriction, baseline food intake was determined over 5 days in 10 rats. All rats were subjected to calorie restriction with a 25% reduction in kcal from baseline intake for 15 days while housed in thermoneutral conditions. Rats were weight matched and divided into 2 groups (n=5 per group) where half of the rats were exposed to a new predator odor stimulus on each day of food restriction and the remaining rats were given the control stimulus on each day of food restriction. Body weight was measured daily, and EchoMRI measurement of body composition was done 3 days prior to restriction and on days 4 and 15 of restriction. Muscle and BAT thermogenic response to control or predator odor stimuli was assessed on the first day of restriction (day 0), day 7 and day 14. On these days, temperature was measured immediately after the removal of the prior day's stimulus (without replacement), again 1 h later (baseline, immediately after presentation of new predator odor or control stimulus), and over 2 h after predator odor or control exposure.
Statistical analyses
Muscle and BAT thermogenesis over time and during treadmill walking were analyzed using a 2-way mixed analysis of variance (ANOVA). Area under the curve (AUC) relative to baseline temperature was calculated using the trapezoidal method and subjected to t-tests or ANOVA. Gas exchange and physical activity variables were measured using 2×3 repeated-measures ANOVA. Analyses of data from rats with unilateral denervation, given nadolol or over the estrous cycle were carried out using 3×3 repeated-measures ANOVA, and 2×2 repeated-measures ANOVA for AUC comparisons. Muscle NA concentration was compared using a paired 1-tailed t-test. Weight loss over time, fat mass and baseline temperature were analyzed using a 2-way mixed ANOVA, and thermogenic responses were analyzed using a 3-way ANOVA followed by a 2-way mixed ANOVA for each day separately. Lastly, we subjected previously published data (Gavini et al., 2016) to a more temporally detailed analysis to compare the time course of changes in physical activity, EE and muscle temperature after intra-VMH microinjections to activate melanocortin receptors. Significance was determined at P<0.05.
RESULTS
Exposure to predator odor evokes a rapid and high-magnitude induction of skeletal muscle temperature in rats and mice
To examine the potential for predator threat to activate skeletal muscle thermogenesis, predator (ferret) odor was presented and muscle temperature was directly measured using transponders implanted in the gastrocnemius muscle group bilaterally as well as in BAT. Predator odor induced a robust increase in gastrocnemius temperature (Fig. 1A). The rise in temperature was rapid, increasing by 0.99°C at 2 min, 1.54°C at 4 min, and peaking at 1.84°C at 10 min after exposure (Fig. 1B). A predator odor-induced rise in BAT temperature was also evident (Fig. S2A), following a less distinct time course than muscle.
Because the locomotion induced by odor presentation could also increase muscle temperature, rats were subjected to a standardized treadmill-walking protocol to equalize activity levels between conditions. The predator odor-induced muscle heat induction persisted when locomotion was held constant at a walking speed, as rats exposed to predator odor showed elevated muscle temperature during locomotion compared with the same rats after control stimulation (Fig. 1C). To examine the potential impact of stressful or aversive aspects of the ferret odor, thermogenesis after ferret and control odor exposure was compared with that after exposure to an aversive odor (butyric acid), a novel odor (2-methylbenzoxazole), fox odor and 1 min of restraint stress. As shown in Fig. 1D,E, neither novel nor aversive odors significantly altered muscle temperature from baseline conditions. Stress induced by handling and moderate restraint induced a different temporal response in muscle temperature, which increased at 5 min after initial handling and decreased thereafter. Interestingly, fox odor induced a prolonged but small increase in muscle temperature (Fig. 1D,E). Overall, ferret odor induced a strong change in muscle temperature distinct from the effect of all other conditions.
We examined whether the thermogenic effect of predator odor generalized across species and sexes. A similar increase in muscle temperature was seen in female rats, with equivalent responses occurring at proestrus and diestrus phases of the estrous cycle (Fig. 1F,G). Lastly, mice also demonstrated a predator odor-induced increase in gastrocnemius muscle temperature relative to control stimulation (Fig. 1H,I), illustrating that the effect was not limited to rats. Demonstrating generalizability is crucial to the argument that the induction of muscle thermogenesis is a fundamental physiological response to predator threat; thus, each of these studies was repeated using separate groups of animals (Fig. S1A–D).
Exposure to predator odor induces an increase in energy expenditure
The heat generated in thermogenesis would be predicted to increase overall EE. Indeed, predator odor exposure in rats significantly increased EE, V̇O2 and physical activity, without significantly changing RER (Fig. 2A–D). As with heat dissipation, elevated EE might also stem in part from predator odor-induced increases in physical activity levels, however (Fig. 2D). To examine the dependence of predator odor-induced changes in EE on physical activity, EE was measured in the same rats after exposure to predator odor and control exposure with physical activity held constant. Using a treadmill-walking protocol to control locomotor-induced muscle temperature (Almundarij et al., 2017; Gavini et al., 2016), gas exchange was measured during prescribed treadmill-walking activity beginning 5 min after predator odor or control exposure. Compared with control, predator odor significantly increased walking-associated EE and oxygen consumption, but not RER, during controlled activity (Fig. 2E–G).
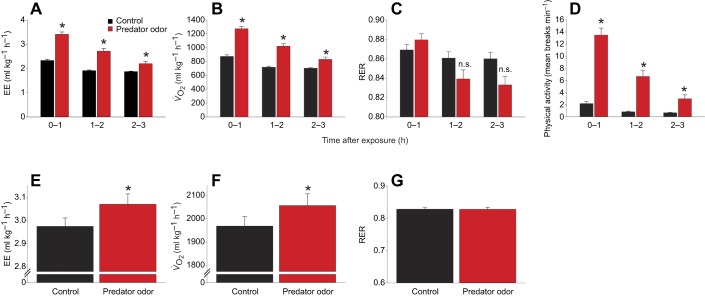
Fig. 2.
Predator odor exposure increases energy expenditure, even after activity levels are controlled. (A–C) Exposure to predator odor increased energy expenditure (EE; A) and oxygen consumption (V̇O2; B), without significantly altering respiratory exchange ratio (RER; C). (D) Physical (horizontal) activity was also significantly increased. (E–G) When physical activity level was held constant (7 m min−1 treadmill walking), exposure to predator odor significantly elevated EE (E) and V̇O2 (F) during treadmill walking, without altering RER (G) (n=8). *Predator odor>control; n.s., not significantly different.
While muscle temperature clearly increased during locomotion (Fig. 1C), the idea that muscle can generate heat apart from, or in addition to, heat generated secondary to muscle contraction is consistent with our past studies measuring muscle temperature and EE after intra-VMH melanocortin receptor activation with melanotan II (MTII; Table S1) (Gavini et al., 2016). We examined these data in more temporal detail than was previously reported to determine whether a stimulus that induces physical activity (in this case, intra-VMH MTII) is sufficient to alter muscle temperature. Physical activity significantly increased in the first hour after intra-VMH MTII, and rats displayed a corresponding 25.5% (±5%) increase in EE in the same hour. Exposure to predator odor for 1 h significantly increased physical activity in rats to a similar level as activity induced by MTII (Fig. 2D; Table S1), but a 46.9% (±4%) increase in EE was evident after predator odor. Conversely, while predator odor exposure increased gastrocnemius temperature in the first hour after exposure (Fig. 1A), intra-VMH MTII injection-induced muscle heat dissipation was not significantly elevated until considerably later – 90 min or more after injection (Gavini et al., 2016). In summary, intra-VMH microinjection of a melanocortin receptor agonist increased physical activity, but not muscle temperature, in the first hour after stimulation, whereas a similarly moderate increase in physical activity in the first hour of predator odor exposure induced a substantial increase in muscle temperature and a faster increase in EE (Fig. 2A; Table S1).
Predator odor-induced muscle thermogenic response is subject to associative learning
In subsequent studies, rats showed more volatile muscle temperature during control and baseline measurements, inconsistent with prior findings of rat muscle thermogenesis (Almundarij et al., 2017), including muscle response to central microinjections (Gavini et al., 2016). This suggested that learned cues might be provoking muscle thermogenesis. To investigate this, rats underwent conditioned learning. On day 1, male rats were presented with either a control stimulus (towel with no odor in a green plastic whiffle ball) or a conditioned stimulus (ball with predator odor; Fig. S3A). The following day, all rats were exposed to the same control stimulus (ball with no odor; Fig. S3B,C). Compared with control rats, conditioned rats showed a significantly greater muscle thermogenic response to an identical stimulus, where the temperature response (20 min AUC) to the conditioned stimulus was significantly higher in rats that had received the predator odor stimulus previously (Fig. S3C).
To determine the amount of habituation required to prevent a learned association that would increase muscle temperature in the absence of predator odor, gastrocnemius temperature was measured in the housing room, after room transfer, after 60 min of acclimation time and again 10 min after presentation of a control stimulus (towel, no odor). As shown in Fig. S3D, on the first habituation day, gastrocnemius temperature increased after moving rats from the housing room, and again after presentation with the control odor. By the fourth day of habituation, the control stimulus no longer evoked a significant change in temperature. When data from male and female rats were considered separately, the primary factor differentiating the sexes was the muscle temperature at baseline and after transporting the rats (Fig. S3E); there was no sex difference in the response to the control stimulus. Repeated habituation in a separate experimental group confirmed that 4 days of habituation to the experimental procedure decreased baseline temperature and overall measurement volatility (Fig. S1E,F).
Sympathetic blockade inhibits contextual induction of muscle thermogenesis
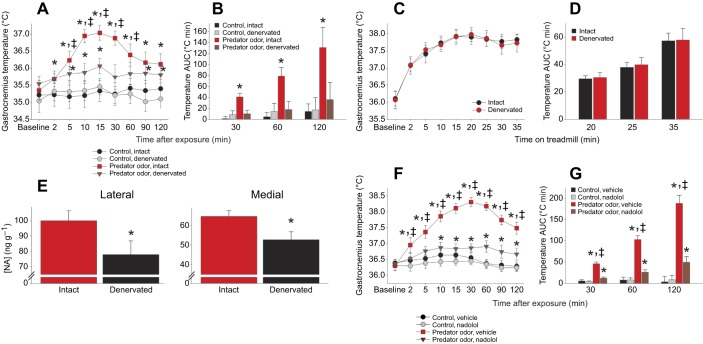
Because of the importance of the SNS in mediating other types of thermogenic response (Contreras et al., 2017), including in muscle (Gavini et al., 2016), we aimed to determine the role of sympathetic nerves in centrally induced muscle thermogenesis. Unilateral surgical denervations were performed, severing the LSN serving the gastrocnemius muscle group in one hindlimb. Predator odor induced a significantly greater increase in temperature in the muscle served by the intact LSN than in the gastrocnemius on the side of the denervated LSN, with the intact gastrocnemius showing 242–574% more induction of thermogenesis than the denervated gastrocnemius in the same rat at 2–30 min after predator odor exposure (Fig. 3A,B). In summary, targeted SNS denervation compromised ipsilateral muscle thermogenic induction by predator odor while sparing the thermogenic response in the contralateral leg. When rats walked on a treadmill, no difference in functionality was detected between the legs on the denervated and intact sides, and the locomotor-associated increase in muscle temperature did not differ between legs, within rat (Fig. 3C,D). When NA was measured in the medial and lateral gastrocnemius to confirm denervation, we found significantly lower NA concentration in muscle on the denervated side compared with that in the contralateral gastrocnemius served by an intact LSN (Fig. 3E). No significant differences were seen within rat between intact and denervated lateral gastrocnemius in expression levels (2−ΔCt) of either sarcolipin (mean±s.d. control 0.01206±0.01065, denervated 0.00541±0.00510) or UCP3 (control 0.00436±0.00268, denervated 0.00402±0.00244).
Fig. 3.
Predator odor-induced muscle thermogenesis is mediated predominantly through sympathetic neural activation. (A) Surgical unilateral sympathetic lumbar nerve (LSN) excision significantly reduced the ability of predator odor exposure to induce thermogenesis in the gastrocnemius on the side of the denervation compared with that in the muscle ipsilateral to the intact LSN concurrently in the same rat, without changing temperature after control stimulation (n=9). *Predator odor>control exposure; ‡intact>denervated within condition. (B) Temperature AUC. *Effect>all other conditions. (C,D) The ability of the rats to walk on a treadmill and the resulting heat generation (C) and temperature AUC (D) were not detectably different between muscles served by denervated compared with intact LSN (n=9). (E) Medial and lateral gastrocnemius noradrenaline (NA; norepinephrine) concentration was significantly higher in the intact than in the denervated muscles. *Intact>denervated. (F,G) Blocking peripheral β-adrenergic receptors with the antagonist nadolol significantly decreased the ability of predator odor to increase gastrocnemius temperature and AUC, without significantly affecting muscle temperature during control stimulus exposure (n=12). *Predator odor>control exposure; ‡vehicle>nadolol within condition.
To assess the contribution of peripheral β1 and β2 adrenergic receptors to predator odor-induced thermogenesis, the mixed β-adrenergic receptor antagonist nadolol was employed to block peripheral but not central receptors. Nadolol significantly inhibited the predator odor-associated increase in muscle temperature (Fig. 3F,G). Muscle temperature during control exposure was not significantly altered by nadolol.
Daily predator odor exposure enhances muscle thermogenesis and weight loss
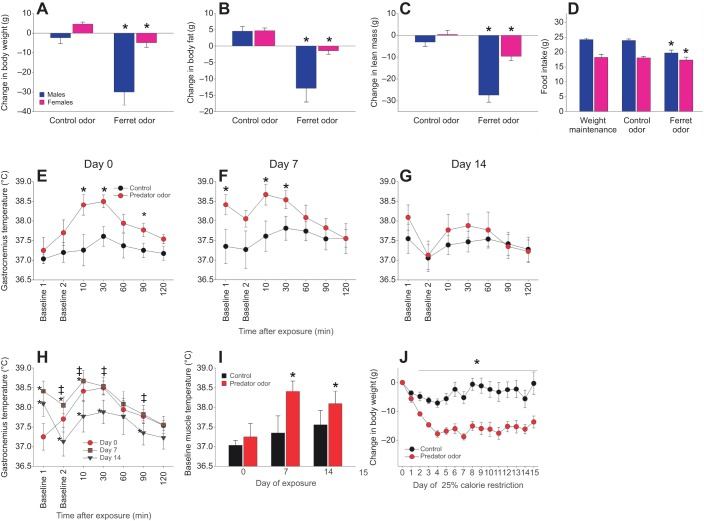
To investigate the impact of chronic predator odor exposure on energy balance, male (n=7) and female (n=5) Sprague–Dawley rats were subjected to successive chronic control and predator odor exposure while on a weight-maintenance diet. Food intake needed to sustain each rat's body weight was established (males, 24.1±0.5 g; females, 18.2±1 g). Body composition was measured before and after rats were fed the weight-maintenance diet; rats were then exposed to a new control stimulus and weighed daily for one week while housed at thermoneutrality. There was no significant decrease in body weight or fat mass over this week (Fig. 4A). As shown in Fig. 4A–C, with no change in food allocation, there were significantly greater decreases in body weight, fat mass and lean mass after one week of chronic exposure to predator odor compared with control odor. Some rats showed decreased food intake with exposure to predator odor (Fig. 4D); this was more pronounced in males than in females, as was loss of body weight, fat and lean mass (Fig. 4A–D). When considering only females, which showed little decrease in food intake (0.85±0.22 g day−1 left uneaten), significant predator odor-induced decreases in body weight and fat mass remained (Fig. 4A–C). The caloric deficit incurred accounted for less than half of the loss of fat and lean mass (Table S2), indicating an additional source of negative energy balance in both males and females.
Fig. 4.
Chronic exposure to predator odor induces weight and fat loss. (A–D) Rats were subjected to weight-maintenance feeding during 7 days of continual exposure to control odor followed by 7 days of ferret odor. Compared with the pre-exposure weight-maintenance condition, significant loss of weight (A), adiposity (B) and lean mass (C) was seen after predator odor exposure; (D) the decrease in food intake associated with predator odor exposure was more pronounced in male (n=7) than in female (n=5) rats. *Change from weight-maintenance feeding, different from control exposure. (E–J) Muscle thermogenic response to chronic exposure to predator odor (n=5) or control odor (n=5) in rats undergoing 25% calorie restriction for 15 days. Relative to day 0 (E), habituation of the muscle thermogenic response was not seen on day 7 (F) but was evident on day 14 of exposure (G). *Predator odor>control. (H) While 14 days of predator odor exposure led to lower acute muscle heat induction (thermogenesis), (I) baseline muscle temperature remained significantly elevated in predator odor-exposed rats after 7 and 14 days of continuous exposure. *Different from day 0; ‡different from day 14. (J) Rats chronically exposed to predator odor lost significantly more weight during 25% food restriction compared with control rats. *Predator odor>control.
In order to examine the effects of chronic predator odor exposure without individual differences in food intake, mild calorie restriction was used to standardize intake. A separate group of male rats was divided into two weight-matched groups and subjected to 25% calorie restriction based on their baseline caloric intake. Starting on the first day of food restriction, half of the rats were exposed to a new predator odor towel daily, and the other half received a new control stimulus daily. Compared with controls, predator odor-exposed rats lost significantly more weight, with a significant difference in weight loss starting on day 3 and continuing through the last day of restriction and exposure on day 15 (Fig. 4J). While exposure to the thermogenic stimulus resulted in sustained weight loss, the calorie restriction alone induced significant loss of fat mass (Table S3).
To assess the potential adaptation (e.g. habituation) to the odor, predator odor-induced thermogenesis was measured on days 0, 7 and 14 of food restriction (25% calorie restriction described above); the thermogenic response to predator odor in the predator odor-exposed rats was compared with the muscle thermogenic response to control odor in the control rats. Predator odor-exposed rats experienced a significant increase in muscle temperature compared with control rats (Fig. 4E–G). Significantly lower predator odor-induced muscle temperature was seen after 2 weeks of daily predator odor exposure, but not after 1 week (Fig. 4H). Interestingly, muscle temperature measured at the time of removal of the previous day's predator odor towel was significantly elevated on days 7 and 14 relative to day 0 (Fig. 4I).
Predator threat imparts resistance to running fatigue
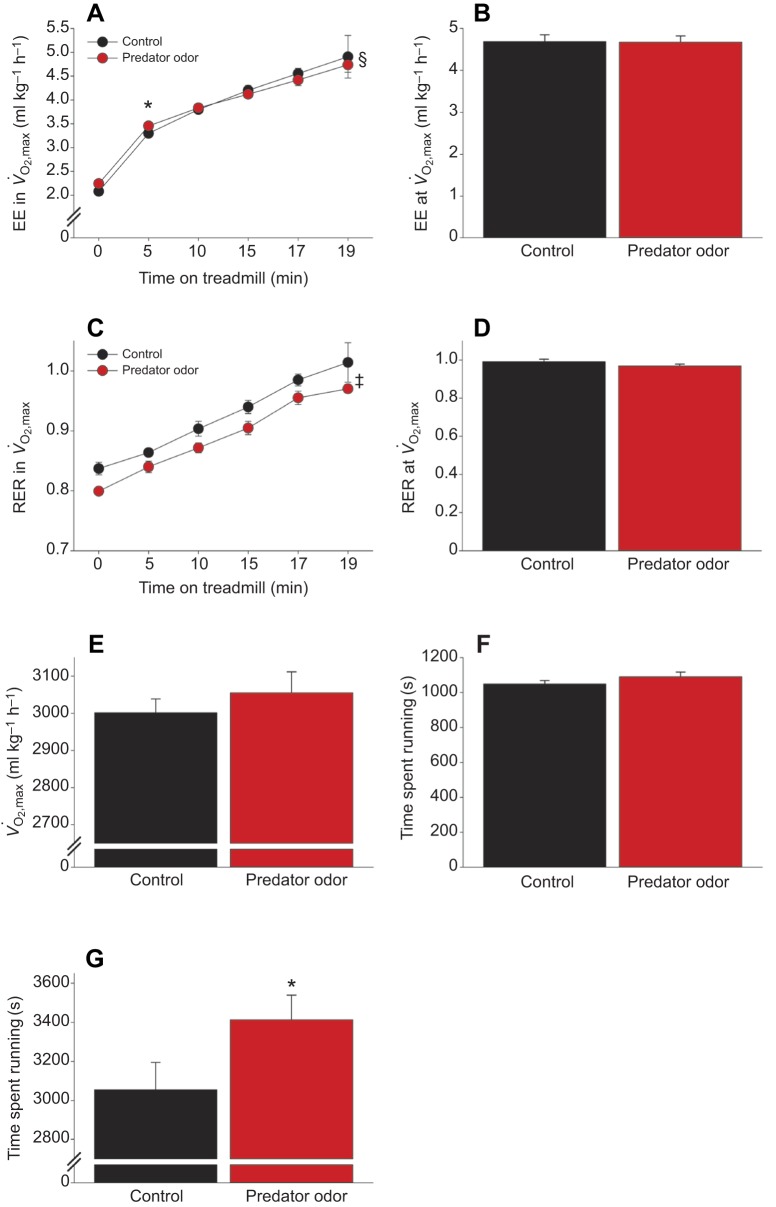
We tested the hypothesis that the innate response to predator threat has adaptive significance through suppressing running fatigue using two separate treadmill-running protocols. First, maximum O2 consumption (V̇O2,max) was assessed in rats after control or predator odor exposure, in random order. At maximal capacity (i.e. V̇O2,max), there were no significant differences between control and predator odor exposure conditions; as shown in Fig. 5, predator odor exposure did not significantly change the maximum time spent running (Fig. 5F), V̇O2,max (Fig. 5E), RER (Fig. 5D) or EE (Fig. 5B) at maximal exertion. However, predator odor-associated changes were seen in RER and EE over the course of treadmill running (Fig. 5A). Throughout treadmill running, RER was significantly lower in rats after predator odor exposure compared with control exposure (Fig. 5C), indicating enhanced fat oxidation during running, but not at maximal exertion (Fig. 5D). There was a significant interaction where predator odor and control exposure differentially affected treadmill-running EE; predator odor induced a small but significant increase in EE at the beginning, but not the end, of the V̇O2,max procedure (Fig. 5A,B). This contrasted to the larger significant effect of predator odor on running fatigue. Rats were considered to have reached fatigue when they could no longer maintain the maximal running speed (13 m min−1 at 10 deg incline). Rats were able to run significantly longer after predator odor exposure, a difference of >5 min (Fig. 5G).
Fig. 5.
Acute predator odor exposure alters fatigue and fuel utilization without changing V̇O2,max. (A–F) During a V̇O2,max treadmill test, exposure to predator odor increased EE in the first 5 min of running (A) and decreased RER (C) without significantly altering maximal EE (B), RER (D), V̇O2 (E) or time spent running (F). (G) During a lower-speed running fatigue treadmill test, rats exposed to predator odor ran significantly (>5 min) longer than after control exposure (n=9). *Predator odor>control; ‡main effect of predator odor; §significant interaction.
DISCUSSION
Here, we report that a contextual stimulus – the odor of a natural predator – produces a rapid and robust induction of skeletal muscle thermogenesis. The induction of muscle temperature was sizeable, increasing as much as 2°C by 10–30 min after odor exposure (Fig. 1B,D). In contrast, the relative temperature increase subsequent to VMH melanocortin receptor activation peaked at 0.4°C after 3–4 h (Gavini et al., 2016). Unilateral denervation and peripheral β-adrenergic receptor blockade each significantly inhibited predator odor-induced thermogenesis in the affected muscles, supporting the assertion that the SNS is the dominant mediator of this effect, specifically through neural release of catecholamines. Although predator odor exposure increases physical activity, enhanced thermogenesis and EE were evident when activity levels were matched between predator odor and control conditions (Figs 1C and 2E). Our finding that chronic exposure to predator odor induces or augments negative energy balance, resulting in weight and fat loss (Fig. 4), is consistent with established evidence regarding predator threat and energy balance; calorie use from muscle thermogenesis is a likely potent contributor. Altogether, these findings support the conclusion that predator threat evokes an innate response that includes the physiological induction of skeletal muscle thermogenesis, and is the first report of a discrete stimulus to rapidly and markedly induce muscle thermogenesis.
Although core temperature was not measured here, predator odor-induced muscle thermogenesis is likely to translate into increased body temperature considering reports of an elevation in core temperature in response to predator threat in rats and mice (Campeau et al., 2008; Lecorps et al., 2019). Ferret odor also increased the temperature of interscapular BAT, albeit less robustly than in muscle (Fig. S2). Predator odor-induced BAT and muscle thermogenesis is consistent with the phenomenon of stress-induced hyperthermia (Kataoka et al., 2014; Nakamura, 2015). This raises the question as to how much of the thermogenesis seen here is specific to predator threat versus secondary to a general stress response. Predator threat provokes characteristic behavioral and endocrine responses, including corticosterone release and a concomitant defensive emotional state (Kunwar et al., 2015; Masini et al., 2005). The central pathways mediating the response to predator odor largely overlap with, but also diverge from, pathways activated by other stressors (Baisley et al., 2011; Figueiredo et al., 2003), suggesting that other stressors may affect muscle thermogenesis. This is consistent with our finding that brief restraint stress produced only a transient change in muscle temperature (Fig. 1D,E). Aversive or novel odors produced no significant change in muscle temperature (Fig. 1D,E), and fox odor, known to induce a fear-like stress response (Staples, 2010), provoked only a small change in muscle temperature (Fig. 1D,E). Taken together, this evidence suggests that the muscle thermogenesis induced by ferret odor is innate and stems from activation of a pathway at least partly distinct from a general stress response (Canteras, 2018). The precise odors or other sensory stimuli to which this response is tuned likely differ among species. While predator threat may activate muscle thermogenesis more effectively than other challenges, any survival value conferred by the muscle metabolic response would also apply to challenging situations other than predator threat.
Although physical activity (i.e. locomotion, treadmill walking) predictably increases muscle temperature (Almundarij et al., 2017; Gavini et al., 2018, 2016) (Figs 1C and 3C), the locomotion induced by predator odor exposure does not account for the majority of the heat generation evidenced here. First, to get an idea of the amount of physical activity sufficient to detectably change muscle temperature, we performed a more temporally detailed analysis of prior measurement of physical activity after activation of brain melanocortin receptors (Gavini et al., 2016). Intra-VMH melanocortin receptor activation increased activity to levels comparable to those seen after predator odor exposure in the first hour after stimulation (Table S1). In contrast to the rapid increase in muscle temperature seen after exposure to ferret odor (Fig. 1A,B), no detectable muscle thermogenesis was seen until at least 90 min after central receptor activation, despite the elevated activity levels (Gavini et al., 2016). This demonstrates that this magnitude of physical activity in the absence of predator odor is insufficient to significantly increase muscle temperature. Second, subjecting rats to forced treadmill walking revealed that predator odor elevates both muscle temperature (Fig. 1C) and EE (Fig. 2E) above levels seen during comparable locomotion without predator odor; this mitigates the confound of differences in locomotor activity. Altogether, this reinforces the assertion that significantly elevated physical activity can occur in the absence of significantly increased gastrocnemius temperature (i.e. after intra-VMH MTII), and that predator odor exposure increases muscle thermogenesis and EE beyond what would be predicted based on elevated physical activity alone. These data do not rule out the possibility that the muscle thermogenesis evidenced here is dependent on some baseline level of contraction, or that predator threat enhances existing muscle contractile thermogenesis. For example, predator odor could induce alertness and thereby increase muscle tension, which could then augment heat generated from muscle contraction (Cattarelli and Chanel, 1979; Meigal et al., 1998; Steenland and Zhuo, 2009).
Examining the neural control of other thermogenic systems, the response of BAT and the induction of a brown fat-like thermogenic phenotype in white adipose tissue are both activated by SNS neural stimulation (Contreras et al., 2017). Not surprisingly, muscle thermogenesis is similarly reliant on SNS outflow. Blocking peripheral β-adrenergic receptors significantly suppressed the predator odor-induced muscle thermogenic response (Fig. 3F,G). As nadolol does not cross the blood–brain barrier, this reinforces the role of the SNS and peripheral adrenergic receptors in centrally induced muscle thermogenesis. This is also consistent with the importance of muscle β2 adrenergic receptors in modulating muscle metabolism, including insulin sensitivity and glucose uptake (Shiuchi et al., 2009), and muscle activity thermogenesis (Gavini et al., 2016). The rapid time course of the thermogenic response (Fig. 1B) suggests that adrenergic receptor activation results from the neural release of NA rather than from circulating adrenal medullary hormones. Accordingly, unilateral extirpation of the LSN resulted in significant suppression of ipsilateral predator odor-induced muscle thermogenesis, where robust thermogenesis was evident in the contralateral muscle served by the intact LSN in the same rat at the same time (Fig. 3A,B). In addition to strongly supporting the importance of intact neural SNS input to muscle for full expression of predator odor-induced thermogenesis, it also rules out other factors as potential dominant influences on muscle thermogenesis. Locomotor activity, systemic hormones (e.g. stress-related adrenal cortical or medullary hormones) and other sources of heat (e.g. BAT) would be expected to affect the innervated and denervated legs equally. Shivering occurs through a separate pathway ultimately mediated by somatic motoneurons unlikely to be affected by unilateral LSN denervation (Morrison and Nakamura, 2019); in other words, shivering thermogenesis should be intact in the denervated leg. Locomotion in the absence of predator odor increased muscle temperature similarly in the innervated and denervated muscles (Fig. 3C,D), demonstrating that the denervated muscles were not deficient in their ability to produce heat. The small increase in temperature of the denervated leg (0.58°C at 15 min; Fig. 3A) could stem from these factors, alterations of local muscle blood flow secondary to lower SNS tone (Clark et al., 2000) or a minor contribution of SNS fibers in somatic nerves or a contralateral SNS projection. Altogether, these data suggest that activation of β-adrenergic receptors by neural catecholamines is the predominant mechanism through which central activation by predator threat triggers heat generation by skeletal muscle.
Using different methods and odors to model predator threat, most interest has been directed at behavioral and endocrine mechanisms rather than metabolic effects (Kunwar et al., 2015; Perez-Gomez et al., 2015; Silva et al., 2013). Here, we demonstrate that exposure to ferret odor increased EE in freely moving rats, including EE required to walk on a treadmill (Fig. 2). Comparison of gas exchange data at different activity demands suggests that predator odor increases EE by decreasing locomotor efficiency at low and moderate workloads (Figs 2E and 5A) without affecting V̇O2,max or gas exchange at maximal exertion (Fig. 5B,D–F). In contrast, comparison of RER data revealed a predator odor-induced decrease in RER during the highly demanding V̇O2,max test (Fig. 5C) with no significant change in RER during low workloads (Fig. 2G) or when freely moving (Fig. 2C), suggesting that fuel selection is altered preferentially at more intense workloads.
The elevated EE accompanying muscle thermogenesis may contribute to the negative energy balance and weight loss seen in response to chronic predator threat demonstrated here (Fig. 4). This is consistent with reports documenting that chronic exposure to a fear-inducing odorant attenuates weight gain in mice (Genne-Bacon et al., 2016) and suppresses weight gain in other species (Tidhar et al., 2007), and that daily exposure to ferret odor reduces weight gain over time in rats (Campeau et al., 2008). Here, chronic exposure to ferret odor decreased food intake, notably in male rats; this is not surprising given that exposure to predator threat decreases food intake along with body weight in multiple species (Liesenjohann and Eccard, 2008; Tidhar et al., 2007). Loss of lean mass was more pronounced when food intake was suppressed either experimentally (Table S3) or in response to predator odor (Fig. 4C,D). Even with minimal change in food intake, chronic exposure to predator odor induced significant loss of weight and fat mass, which was amplified in rats that also reduced their caloric intake.
Because behavioral and physiological responses to predator odors in laboratory rodent models are subject to both habituation and sensitization (Weinberg et al., 2009), we questioned whether the thermogenic response to ferret odor would abate over time with daily exposure. Over 2 weeks of nearly continuous exposure to ferret odor, rats showed a muscle thermogenic response after 7 days, but this was not evident the subsequent week (Fig. 4E–G). This implies that some habituation occurred between 1 and 2 weeks of nearly constant predator odor exposure, while weight loss was maintained (Fig. 4J). Even after 2 weeks of exposure, however, daytime muscle temperature of rats housed with the ferret odor remained significantly elevated relative to that of control rats (Fig. 4H,I), implying that their muscle thermogenesis was chronically elevated for the duration of the exposure period, likely contributing to the maintenance of the enhanced weight loss.
Rats also showed a conditioned response to predator odor that was sufficient to alter muscle temperature in the absence of the odor (Fig. S3C). This demonstrates that the rats learned to associate the odor with the conditioned stimulus after a single trial. This is consistent with the ability to produce one-trial learning using some predator odors, but not others (Staples, 2010), and with evidence of activation of overlapping brain regions in response to cat odor and odor-associated cues after a single exposure in rats (Staples et al., 2005). As the experimental procedure and testing environment likely also acted as conditioned stimuli, we incorporated habituation (Fig. S3D,E) to personnel, the towel stimulus, transport and measurement into our experimental procedure. This minimized volatility in the thermogenic response to the control odor (Fig. S3A,B versus Fig. 1D,F) and was critical for accurate detection of predator odor-induced thermogenesis. Additionally, these results have implications regarding the context in which metabolic studies are conducted. Housing rats or mice in close proximity to predators (e.g. ferrets, cats) or exposure to their odors can skew energy balance data, for example by altering physical activity, EE, appetite or weight loss (Figs 2A,D and 5F). Laboratory personnel carrying pet odor could conceivably distort experimental outcomes relating to energy balance and, moreover, rodents exposed to those personnel may be subject to learned associations wherein behavioral and physiological responses are elicited even in the absence of the acute odor. Once learned, conditioned responses to predator threat can be resistant to habituation (File et al., 1993).
Insights gained investigating the behavioral antipredator response can be applied to metabolic outcomes as well (Canteras, 2018; Genne-Bacon et al., 2016). Differential behavioral and metabolic effects have been identified among different predator odors. House mice exposed to TMT, a component of fox odor, showed a peripheral thermal response in the absence of behavioral changes indicative of a defense response (Lecorps et al., 2019). Interestingly, these mice also exhibited an increase in tail skin temperature that is not typically suggestive of a fear response, but consistent with thermoregulatory heat dissipation (Gordon et al., 2002; Vianna and Carrive, 2005). The muscle thermogenic response to fox odor was prolonged like the response to ferret odor, but of lower magnitude (Fig. 1D,E). The most potent stimulus we identified to induce muscle thermogenesis was ferret odor. This provides an excellent system to investigate the neural mechanisms modulating muscle thermogenesis, especially given the thoroughly described mechanisms underlying behavioral responses to predator threat (Canteras, 2002) including the importance of VMH SF-1 cells (Silva et al., 2013).
At the level of the skeletal myocyte, emerging evidence suggests the importance of SERCA and its modulators, especially sarcolipin, in heat generation (Periasamy et al., 2017). Another potential source of thermogenesis is ATP-gated K+ channels (Alekseev et al., 2010); heat generation by muscle UCPs is less likely (reviewed in Bézaire et al., 2007; Giralt and Villarroya, 2017). Investigation into muscle thermogenic mechanisms at the level of the organism has focused primarily on cold adaptation or obesity resistance (Bal et al., 2012, 2017; Maurya et al., 2015), rather than on identifying central or physiological processes controlling acute induction. Here, we demonstrate predator odor-induced generation of muscle thermogenesis and EE that occurs rapidly – within minutes of predator odor exposure (Fig. 1B). The speed of this induction implicates rapid (e.g. neural) communication to muscle coupled with channel-based intracellular mechanisms in skeletal myocytes. While muscle thermogenesis and accompanying cellular mechanisms are evidenced in smaller mammals, including laboratory rodent models, muscle thermogenesis is likely to be even more relevant to energy balance in larger mammals (e.g. rabbits, dogs), including humans, because of the proportionally larger skeletal muscle volume and higher abundance of proteins implicated in muscle thermogenesis (reviewed in Fuller-Jackson and Henry, 2018; Rowland et al., 2015). Given the relatively high expression of key muscle thermogenic proteins in human skeletal muscle (Fajardo et al., 2013), the prospective ability to harness an inducer of muscle thermogenesis has considerable appeal with respect to modulating metabolic rate.
Predator odor-induced muscle thermogenesis occurred in the absence of a thermal challenge, implying that the heat generated may be a secondary consequence of a mechanistic process serving a purpose other than body temperature homeostasis. Along with muscle heat dissipation, predator odor also induced a significant increase in the distance run before the onset of fatigue (Fig. 5G). This supports the hypothesis that predator threat leads to neural and physiological sequelae that promote resistance to running fatigue, presumably allowing the animal to more effectively outrun predators. This reduced fatigue could stem from one or more potential sources, including overall SNS activation, direct effects of temperature on muscle function (Binkhorst et al., 1977), skeletal myocyte metabolic alterations or central nervous system changes related to motivation to run. Although substrate utilization was not measured in the context of running fatigue, increased fat utilization could also promote running performance; however, altered substrate utilization did not enhance running endurance in the V̇O2,max test (Fig. 5C,E,F). Our data do not speak to the mechanisms that confer resistance to fatigue; existing evidence supports muscle (Sopariwala et al., 2015) and central (Zaretsky et al., 2018) mechanisms that promote fatigue resistance or endurance capacity, some of which overlap with central mechanisms of predator threat and SNS outflow (Fujikawa et al., 2016; Guimarães et al., 2013; Kunwar et al., 2015; Lindberg et al., 2013; Perez-Gomez et al., 2015; Silva et al., 2013). The mediating role of the SNS is consistent with the known ability of β-adrenergic receptor agonists to facilitate skeletal muscle force production and delay fatigue (Blackwood and Katz, 2019). The overlap between processes modulating thermogenesis or cold adaptation, muscle performance and cost of transport (Schaeffer et al., 2001, 2005) prompts us to speculate that the underlying thermogenic mechanisms may have been co-opted depending on the selective forces at play (i.e. cold, predation, energy conservation). In other words, despite the ability of predator odor exposure to induce muscles to generate heat, the selective advantage conferred may not be thermoregulatory in nature (Campbell and Dicke, 2018).
Overall, these findings set the initial framework for the contextual induction of thermogenesis wherein the SNS is engaged to activate muscle thermogenesis through β, likely β2, adrenergic receptors. The central and muscle mechanisms underlying thermogenesis evoked by predator threat remain to be elucidated, though promising candidates exist (Coutinho et al., 2017; Fujikawa et al., 2016; Gavini et al., 2016; Kunwar et al., 2015; Pant et al., 2016; Perez-Gomez et al., 2015; Silva et al., 2013). Given the potential magnitude of heat generated by skeletal muscle upon predator threat, more detailed knowledge of this physiological system is warranted.
Supplementary Material
Acknowledgements
We thank Dr Ilan Kerman for advice and guidance with denervation surgery, and Dr Walter Horne for training on subcuticular sutures. We also thank Drs Tony Nunez, Paul Burghardt, Chi-hua Chiu and Dave Riccio for ongoing discussions and comments on the studies and manuscript.
Footnotes
Competing interests
The authors declare no competing or financial interests.
Author contributions
Conceptualization: A.S., C.M.N.; Methodology: E.G., A.S., J.K., N.M., A.R.T., M.R., C.K.G., D.L., C.M.N.; Validation: A.S., C.M.N.; Formal analysis: E.G., J.K., M.B., A.R.T., M.Y., S.M., J.G.W., M.R., C.M.N.; Investigation: E.G., A.S., J.K., M.B., N.M., A.R.T., M.Y., S.M., L.A.H., J.G.W., M.G., O.C., D.D., N.H., M.Z., M.R., S.E., C.K.G., T.I.A., C.M.N.; Resources: C.M.N.; Data curation: C.M.N.; Writing - original draft: C.M.N.; Writing - review & editing: E.G., A.S., J.K., M.B., L.A.H., C.M.N.; Visualization: C.M.N.; Supervision: E.G., A.S., J.K., L.A.H., C.M.N.; Project administration: C.M.N.; Funding acquisition: C.M.N.
Funding
This work was supported by grants from the National Institute of Diabetes and Digestive and Kidney Diseases to C.M.N. [R15DK108668 and R15DK097644]. Deposited in PMC for release after 12 months.
Supplementary information
Supplementary information available online at http://jeb.biologists.org/lookup/doi/10.1242/jeb.218479.supplemental
References
- Alekseev A. E., Reyes S., Yamada S., Hodgson-Zingman D. M., Sattiraju S., Zhu Z., Sierra A., Gerbin M., Coetzee W. A., Goldhamer D. J. et al. (2010). Sarcolemmal ATP-sensitive K+ channels control energy expenditure determining body weight. Cell Metab. 11, 58-69. 10.1016/j.cmet.2009.11.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almundarij T. I., Gavini C. K. and Novak C. M. (2017). Suppressed sympathetic outflow to skeletal muscle, muscle thermogenesis, and activity energy expenditure with calorie restriction. Physiol. Rep. 5, e13171 10.14814/phy2.13171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baisley S. K., Cloninger C. L. and Bakshi V. P. (2011). Fos expression following regimens of predator stress versus footshock that differentially affect prepulse inhibition in rats. Physiol. Behav. 104, 796-803. 10.1016/j.physbeh.2011.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bal N. C., Maurya S. K., Sopariwala D. H., Sahoo S. K., Gupta S. C., Shaikh S. A., Pant M., Rowland L. A., Bombardier E., Goonasekera S. A. et al. (2012). Sarcolipin is a newly identified regulator of muscle-based thermogenesis in mammals. Nat. Med. 18, 1575-1579. 10.1038/nm.2897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bal N. C., Singh S., Reis F. C. G., Maurya S. K., Pani S., Rowland L. A. and Periasamy M. (2017). Both brown adipose tissue and skeletal muscle thermogenesis processes are activated during mild to severe cold adaptation in mice. J. Biol. Chem. 292, 16616-16625. 10.1074/jbc.M117.790451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bal N. C., Sahoo S. K., Maurya S. K. and Periasamy M. (2018). The role of sarcolipin in muscle non-shivering thermogenesis. Front. Physiol. 9, 1217 10.3389/fphys.2018.01217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartness T. J., Vaughan C. H. and Song C. K. (2010). Sympathetic and sensory innervation of brown adipose tissue. Int. J. Obes. (Lond) 34 Suppl. 1, S36-S42. 10.1038/ijo.2010.182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bézaire V., Seifert E. L. and Harper M. E. (2007). Uncoupling protein-3: clues in an ongoing mitochondrial mystery. FASEB J. 21, 312-324. 10.1096/fj.06-6966rev [DOI] [PubMed] [Google Scholar]
- Binkhorst R. A., Hoofd L. and Vissers A. C. (1977). Temperature and force-velocity relationship of human muscles. J. Appl. Physiol. Respir. Environ. Exerc. Physiol. 42, 471-475. 10.1152/jappl.1977.42.4.471 [DOI] [PubMed] [Google Scholar]
- Blackwood S. J. and Katz A. (2019). Isoproterenol enhances force production in mouse glycolytic and oxidative muscle via separate mechanisms. Pflugers Arch. 471, 1305-1316. 10.1007/s00424-019-02304-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell K. L. and Dicke A. A. (2018). Sarcolipin makes heat, but is it adaptive thermogenesis? Front. Physiol. 9, 714 10.3389/fphys.2018.00714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campeau S., Nyhuis T. J., Sasse S. K., Day H. E. W. and Masini C. V. (2008). Acute and chronic effects of ferret odor exposure in Sprague-Dawley rats. Neurosci. Biobehav. Rev. 32, 1277-1286. 10.1016/j.neubiorev.2008.05.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canteras N. S. (2002). The medial hypothalamic defensive system: hodological organization and functional implications. Pharmacol. Biochem. Behav. 71, 481-491. 10.1016/S0091-3057(01)00685-2 [DOI] [PubMed] [Google Scholar]
- Canteras N. S. (2018). Hypothalamic survival circuits related to social and predatory defenses and their interactions with metabolic control, reproductive behaviors and memory systems. Curr. Opin. Behav. Sci. 24, 7-13. 10.1016/j.cobeha.2018.01.017 [DOI] [Google Scholar]
- Cattarelli M. and Chanel J. (1979). Influence of some biologically meaningful odorants on the vigilance states of the rat. Physiol. Behav. 23, 831-838. 10.1016/0031-9384(79)90186-0 [DOI] [PubMed] [Google Scholar]
- Cernecka H., Sand C. and Michel M. C. (2014). The odd sibling: features of β-adrenoceptor pharmacology. Mol. Pharmacol. 86, 479-484. 10.1124/mol.114.092817 [DOI] [PubMed] [Google Scholar]
- Choi Y. H., Fujikawa T., Lee J., Reuter A. and Kim K. W. (2013). Revisiting the ventral medial nucleus of the hypothalamus: the roles of SF-1 neurons in energy homeostasis. Front. Neurosci. 7, 71 10.3389/fnins.2013.00071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark M. G., Rattigan S., Clerk L. H., Vincent M. A., Clark A. D. H., Youd J. M. and Newman J. M. B. (2000). Nutritive and non-nutritive blood flow: rest and exercise. Acta Physiol. Scand. 168, 519-530. 10.1046/j.1365-201x.2000.00704.x [DOI] [PubMed] [Google Scholar]
- Collins S. (2011). β-adrenoceptor signaling networks in adipocytes for recruiting stored fat and energy expenditure. Front. Endocrinol. (Lausanne) 2, 102 10.3389/fendo.2011.00102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contreras C., Nogueiras R., Diéguez C., Rahmouni K. and Lopez M. (2017). Traveling from the hypothalamus to the adipose tissue: the thermogenic pathway. Redox Biol. 12, 854-863. 10.1016/j.redox.2017.04.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coutinho E. A., Okamoto S., Ishikawa A. W., Yokota S., Wada N., Hirabayashi T., Saito K., Sato T., Takagi K., Wang C. C. et al. (2017). Activation of SF1 neurons in the ventromedial hypothalamus by DREADD technology increases insulin sensitivity in peripheral tissues. Diabetes 66, 2372-2386. 10.2337/db16-1344 [DOI] [PubMed] [Google Scholar]
- Fajardo V. A., Bombardier E., Vigna C., Devji T., Bloemberg D., Gamu D., Gramolini A. O., Quadrilatero J. and Tupling A. R. (2013). Co-expression of SERCA isoforms, phospholamban and sarcolipin in human skeletal muscle fibers. PLoS ONE 8, e84304 10.1371/journal.pone.0084304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figueiredo H. F., Bodie B. L., Tauchi M., Dolgas C. M. and Herman J. P. (2003). Stress integration after acute and chronic predator stress: differential activation of central stress circuitry and sensitization of the hypothalamo-pituitary-adrenocortical axis. Endocrinology 144, 5249-5258. 10.1210/en.2003-0713 [DOI] [PubMed] [Google Scholar]
- File S. E., Zangrossi H. Jr, Sanders F. L. and Mabbutt P. S. (1993). Dissociation between behavioral and corticosterone responses on repeated exposures to cat odor. Physiol. Behav. 54, 1109-1111. 10.1016/0031-9384(93)90333-B [DOI] [PubMed] [Google Scholar]
- Fujikawa T., Castorena C. M., Pearson M., Kusminski C. M., Ahmed N., Battiprolu P. K., Kim K. W., Lee S., Hill J. A., Scherer P. E. et al. (2016). SF-1 expression in the hypothalamus is required for beneficial metabolic effects of exercise. Elife 5, e18206 10.7554/eLife.18206.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller-Jackson J.-P. and Henry B. A. (2018). Adipose and skeletal muscle thermogenesis: studies from large animals. J. Endocrinol. 237, R99-R115. 10.1530/JOE-18-0090 [DOI] [PubMed] [Google Scholar]
- Gavini C. K., Mukherjee S., Shukla C., Britton S. L., Koch L. G., Shi H. and Novak C. M. (2014). Leanness and heightened nonresting energy expenditure: role of skeletal muscle activity thermogenesis. Am. J. Physiol. Endocrinol. Metab. 306, E635-E647. 10.1152/ajpendo.00555.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavini C. K., Jones W. C. II and Novak C. M. (2016). Ventromedial hypothalamic melanocortin receptor activation: regulation of activity energy expenditure and skeletal muscle thermogenesis. J. Physiol. 594, 5285-5301. 10.1113/JP272352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavini C. K., Britton S. L., Koch L. G. and Novak C. M. (2018). Inherently lean rats have enhanced activity and skeletal muscle response to central melanocortin receptors. Obesity (Silver Spring) 26, 885-894. 10.1002/oby.22166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genne-Bacon E. A., Trinko J. R. and DiLeone R. J. (2016). Innate fear-induced weight regulation in the C57BL/6J mouse. Front. Behav. Neurosci. 10, 132 10.3389/fnbeh.2016.00132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giralt M. and Villarroya F. (2017). Mitochondrial uncoupling and the regulation of glucose homeostasis. Curr. Diabetes Rev. 13, 386-394. 10.2174/1573399812666160217122707 [DOI] [PubMed] [Google Scholar]
- Gordon C. J., Puckett E. and Padnos B. (2002). Rat tail skin temperature monitored noninvasively by radiotelemetry: characterization by examination of vasomotor responses to thermomodulatory agents. J. Pharmacol. Toxicol. Methods 47, 107-114. 10.1016/S1056-8719(02)00219-8 [DOI] [PubMed] [Google Scholar]
- Guimarães J. B., Wanner S. P., Machado S. C., Lima M. R. M., Cordeiro L. M. S., Pires W., La Guardia R. B., Silami-Garcia E., Rodrigues L. O. C. and Lima N. R. V. (2013). Fatigue is mediated by cholinoceptors within the ventromedial hypothalamus independent of changes in core temperature. Scand. J. Med. Sci. Sports 23, 46-56. 10.1111/j.1600-0838.2011.01350.x [DOI] [PubMed] [Google Scholar]
- Ibrahim M. M. H., Alhamami H. N. and Briski K. P. (2019). Norepinephrine regulation of ventromedial hypothalamic nucleus metabolic transmitter biomarker and astrocyte enzyme and receptor expression: impact of 5′ AMP-activated protein kinase. Brain Res. 1711, 48-57. 10.1016/j.brainres.2019.01.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kataoka N., Hioki H., Kaneko T. and Nakamura K. (2014). Psychological stress activates a dorsomedial hypothalamus-medullary raphe circuit driving brown adipose tissue thermogenesis and hyperthermia. Cell Metab. 20, 346-358. 10.1016/j.cmet.2014.05.018 [DOI] [PubMed] [Google Scholar]
- Kerman I. A., Enquist L. W., Watson S. J. and Yates B. J. (2003). Brainstem substrates of sympatho-motor circuitry identified using trans-synaptic tracing with pseudorabies virus recombinants. J. Neurosci. 23, 4657-4666. 10.1523/JNEUROSCI.23-11-04657.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunwar P. S., Zelikowsky M., Remedios R., Cai H., Yilmaz M., Meister M. and Anderson D. J. (2015). Ventromedial hypothalamic neurons control a defensive emotion state. Elife 4, e06633 10.7554/eLife.06633.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson C. J. (2019). translational pharmacology and physiology of brown adipose tissue in human disease and treatment. Handb. Exp. Pharmacol. 251, 381-424. 10.1007/164_2018_184 [DOI] [PubMed] [Google Scholar]
- Lecorps B., Rödel H. G. and Féron C. (2019). Short-term thermal responses after exposure to predator odor (TMT) in the house mouse. Mamm. Biol. 94, 25-29. 10.1016/j.mambio.2018.12.002 [DOI] [Google Scholar]
- Liesenjohann T. and Eccard J. A. (2008). Foraging under uniform risk from different types of predators. BMC Ecol. 8, 19 10.1186/1472-6785-8-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindberg D., Chen P. and Li C. (2013). Conditional viral tracing reveals that steroidogenic factor 1-positive neurons of the dorsomedial subdivision of the ventromedial hypothalamus project to autonomic centers of the hypothalamus and hindbrain. J. Comp. Neurol. 521, 3167-3190. 10.1002/cne.23338 [DOI] [PubMed] [Google Scholar]
- Masini C. V., Sauer S. and Campeau S. (2005). Ferret odor as a processive stress model in rats: neurochemical, behavioral, and endocrine evidence. Behav. Neurosci. 119, 280-292. 10.1037/0735-7044.119.1.280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurya S. K. and Periasamy M. (2015). Sarcolipin is a novel regulator of muscle metabolism and obesity. Pharmacol. Res. 102, 270-275. 10.1016/j.phrs.2015.10.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurya S. K., Bal N. C., Sopariwala D. H., Pant M., Rowland L. A., Shaikh S. A. and Periasamy M. (2015). Sarcolipin is a key determinant of the basal metabolic rate, and its overexpression enhances energy expenditure and resistance against diet-induced obesity. J. Biol. Chem. 290, 10840-10849. 10.1074/jbc.M115.636878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meigal A. Y., Oksa J., Hohtola E., Lupandin Y. V. and Rintamaki H. (1998). Influence of cold shivering on fine motor control in the upper limb. Acta Physiol. Scand. 163, 41-47. 10.1046/j.1365-201x.1998.00333.x [DOI] [PubMed] [Google Scholar]
- Miyaki T., Fujikawa T., Kitaoka R., Hirano N., Matsumura S., Fushiki T. and Inoue K. (2011). Noradrenergic projections to the ventromedial hypothalamus regulate fat metabolism during endurance exercise. Neuroscience 190, 239-250. 10.1016/j.neuroscience.2011.05.051 [DOI] [PubMed] [Google Scholar]
- Monarca R. I., da Luz Mathias M., Wang D. H. and Speakman J. R. (2015). Predation risk modulates diet-induced obesity in male C57BL/6 mice. Obesity (Silver Spring) 23, 2059-2065. 10.1002/oby.21193 [DOI] [PubMed] [Google Scholar]
- Morrison S. F. and Nakamura K. (2019). Central mechanisms for thermoregulation. Annu. Rev. Physiol. 81, 285-308. 10.1146/annurev-physiol-020518-114546 [DOI] [PubMed] [Google Scholar]
- Nakamura K. (2015). Central circuit mechanism for psychological stress-induced hyperthermia. Brain Nerve. 67, 1205-1214. [DOI] [PubMed] [Google Scholar]
- Pant M., Bal N. C. and Periasamy M. (2016). Sarcolipin: a key thermogenic and metabolic regulator in skeletal muscle. Trends Endocrinol. Metab. 27, 881-892. 10.1016/j.tem.2016.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Gomez A., Bleymehl K., Stein B., Pyrski M., Birnbaumer L., Munger S. D., Leinders-Zufall T., Zufall F. and Chamero P. (2015). Innate predator odor aversion driven by parallel olfactory subsystems that converge in the ventromedial hypothalamus. Curr. Biol. 25, 1340-1346. 10.1016/j.cub.2015.03.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Periasamy M., Maurya S. K., Sahoo S. K., Singh S., Sahoo S. K., Reis F. C. G. and Bal N. C. (2017). Role of SERCA pump in muscle thermogenesis and metabolism. Comp. Physiol. 7, 879-890. 10.1002/cphy.c160030 [DOI] [PubMed] [Google Scholar]
- Rodionova K., Fiedler C., Guenther F., Grouzmann E., Neuhuber W., Fischer M. J., Ott C., Linz P., Freisinger W., Heinlein S. et al. (2016). Complex reinnervation pattern after unilateral renal denervation in rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 310, R806-R818. 10.1152/ajpregu.00227.2014 [DOI] [PubMed] [Google Scholar]
- Rowland L. A., Bal N. C. and Periasamy M. (2015). The role of skeletal-muscle-based thermogenic mechanisms in vertebrate endothermy. Biol. Rev. Camb. Philos. Soc. 90, 1279-1297. 10.1111/brv.12157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaeffer P. J., Hokanson J. F., Wells D. J. and Lindstedt S. L. (2001). Cold exposure increases running VO(2max) and cost of transport in goats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 280, R42-R47. 10.1152/ajpregu.2001.280.1.R42 [DOI] [PubMed] [Google Scholar]
- Schaeffer P. J., Villarin J. J., Pierotti D. J., Kelly D. P. and Lindstedt S. L. (2005). Cost of transport is increased after cold exposure in Monodelphis domestica: training for inefficiency. J. Exp. Biol. 208, 3159-3167. 10.1242/jeb.01703 [DOI] [PubMed] [Google Scholar]
- Schmittgen T. D. and Livak K. J. (2008). Analyzing real-time PCR data by the comparative CT method. Nat. Protoc. 3, 1101-1108. 10.1038/nprot.2008.73 [DOI] [PubMed] [Google Scholar]
- Shiuchi T., Haque M. S., Okamoto S., Inoue T., Kageyama H., Lee S., Toda C., Suzuki A., Bachman E. S., Kim Y.-B. et al. (2009). Hypothalamic orexin stimulates feeding-associated glucose utilization in skeletal muscle via sympathetic nervous system. Cell Metab. 10, 466-480. 10.1016/j.cmet.2009.09.013 [DOI] [PubMed] [Google Scholar]
- Silva B. A., Mattucci C., Krzywkowski P., Murana E., Illarionova A., Grinevich V., Canteras N. S., Ragozzino D. and Gross C. T. (2013). Independent hypothalamic circuits for social and predator fear. Nat. Neurosci. 16, 1731-1733. 10.1038/nn.3573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soares D. D., Lima N. R. V., Coimbra C. C. and Marubayashi U. (2004). Intracerebroventricular tryptophan increases heating and heat storage rate in exercising rats. Pharmacol. Biochem. Behav. 78, 255-261. 10.1016/j.pbb.2004.03.015 [DOI] [PubMed] [Google Scholar]
- Sopariwala D. H., Pant M., Shaikh S. A., Goonasekera S. A., Molkentin J. D., Weisleder N., Ma J., Pan Z. and Periasamy M. (2015). Sarcolipin overexpression improves muscle energetics and reduces fatigue. J. Appl. Physiol. 118, 1050-1058. 10.1152/japplphysiol.01066.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staples L. G. (2010). Predator odor avoidance as a rodent model of anxiety: learning-mediated consequences beyond the initial exposure. Neurobiol. Learn. Mem. 94, 435-445. 10.1016/j.nlm.2010.09.009 [DOI] [PubMed] [Google Scholar]
- Staples L. G., Hunt G. E., Cornish J. L. and McGregor I. S. (2005). Neural activation during cat odor-induced conditioned fear and ‘trial 2’ fear in rats. Neurosci. Biobehav. Rev. 29, 1265-1277. 10.1016/j.neubiorev.2005.04.009 [DOI] [PubMed] [Google Scholar]
- Steenland H. W. and Zhuo M. (2009). Neck electromyography is an effective measure of fear behavior. J. Neurosci. Methods 177, 355-360. 10.1016/j.jneumeth.2008.10.020 [DOI] [PubMed] [Google Scholar]
- Tidhar W. L., Bonier F. and Speakman J. R. (2007). Sex- and concentration-dependent effects of predator feces on seasonal regulation of body mass in the bank vole Clethrionomys glareolus. Horm. Behav. 52, 436-444. 10.1016/j.yhbeh.2007.06.009 [DOI] [PubMed] [Google Scholar]
- Vianna D. M. and Carrive P. (2005). Changes in cutaneous and body temperature during and after conditioned fear to context in the rat. Eur. J. Neurosci. 21, 2505-2512. 10.1111/j.1460-9568.2005.04073.x [DOI] [PubMed] [Google Scholar]
- Viskaitis P., Irvine E. E., Smith M. A., Choudhury A. I., Alvarez-Curto E., Glegola J. A., Hardy D. G., Pedroni S. M. A., Paiva Pessoa M. R., Fernando A. B. P. et al. (2017). Modulation of SF1 neuron activity coordinately regulates both feeding behavior and associated emotional states. Cell Rep. 21, 3559-3572. 10.1016/j.celrep.2017.11.089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wacker C. B., Rojas A. D. and Geiser F. (2012). The use of small subcutaneous transponders for quantifying thermal biology and torpor in small mammals. J. Therm. Biol. 37, 250-254. 10.1016/j.jtherbio.2011.11.007 [DOI] [Google Scholar]
- Weinberg M. S., Bhatt A. P., Girotti M., Masini C. V., Day H. E. W., Campeau S. and Spencer R. L. (2009). Repeated ferret odor exposure induces different temporal patterns of same-stressor habituation and novel-stressor sensitization in both hypothalamic-pituitary-adrenal axis activity and forebrain c-fos expression in the rat. Endocrinology 150, 749-761. 10.1210/en.2008-0958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaretsky D. V., Kline H., Zaretskaia M. V., Brown M. B., Durant P. J., Alves N. J. and Rusyniak D. E. (2018). Disinhibiting neurons in the dorsomedial hypothalamus delays the onset of exertional fatigue and exhaustion in rats exercising in a warm environment. Brain Res. 1689, 12-20. 10.1016/j.brainres.2018.03.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zurlo F., Larson K., Bogardus C. and Ravussin E. (1990). Skeletal muscle metabolism is a major determinant of resting energy expenditure. J. Clin. Invest. 86, 1423-1427. 10.1172/JCI114857 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.