Abstract
The importance of drug dosing time in pharmacokinetics, pharmacodynamics, and toxicity is receiving increasing attention from the scientific community. In spite of mounting evidence that circadian oscillations affect drug absorption, distribution, metabolism, and excretion (ADME), there remain many unanswered questions in this field and, occasionally, conflicting experimental results. Such data arise not only from translational difficulties caused by interspecies differences but also from variability in study design and a lack of understanding of how the circadian clock affects physiological factors that strongly influence ADME, namely, the expression and activity of drug transporters. Hence, the main goal of this review is to provide an updated analysis of the role of the circadian rhythm in drug absorption, distribution across blood–tissue barriers, metabolism in hepatic and extra‐hepatic tissues, and hepatobiliary and renal excretion. It is expected that the research suggestions proposed here will contribute to a tissue‐targeted and time‐targeted pharmacotherapy.
Abbreviations
- ABC
ATP‐binding cassette
- ADME
absorption, distribution, metabolism, and excretion
- BBB
blood–brain barrier
- BCRP
breast cancer resistance protein
- BMAL1
brain and muscle Arnt‐like protein‐1
- CLOCK
circadian locomotor output cycles kaput
- Cmax
maximum concentration
- CRY
cryptochrome
- CYP
cytochrome P450
- DBP
D site‐binding protein
- E4BP4
E4 promoter‐binding protein 4
- fu,plasma
unbound drug fraction in plasma
- MRP
multidrug resistance‐associated protein
- OAT
organic anion transporter
- Octn‐1
organic cation transporter novel type‐1
- PAR bZIP
proline and acidic amino acid‐rich basic leucine zipper
- Peff
effective permeability
- PER
period
- P‐gp
P‐glycoprotein
- PK
pharmacokinetics
- REM
rapid eye movement
- ROR
retinoid‐related orphan nuclear receptor
- SCN
suprachiasmatic nuclei
- tmax
time to reach the maximum concentration
- Vd
volume of distribution
- ZT
zeitgeber time
1. INTRODUCTION
In the recent years, the academic and clinical interest in the role of circadian clock systems in physiology, pathology, and therapeutics increased exponentially, as demonstrated by the award of the 2017 Nobel Prize in Physiology or Medicine to the discovery of molecular mechanisms controlling the https://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=872#2649 rhythm (Firsov & Bonny, 2018). The emergence of the topic in scientific literature was accompanied by numerous chrono‐ concepts organized into sub‐disciplines, such as chronopharmacology and chronopharmacokinetics.
Circadian rhythms (Latin circa: about; dies: day) are generated by cellular transcriptional–translational feedback loops that prompt neuroendocrine outputs, responsible for the coordination of several biological functions (Levi & Schibler, 2007; Pilorz, Helfrich‐Förster, & Oster, 2018). Although transcriptional–translational feedback loop mechanisms were originally described in Drosophila (Bargiello, Jackson, & Young, 1984; Reddy et al., 1984), subsequent studies suggested that general core clock mechanisms have similarities in mammals (King et al., 1997; Tei et al., 1997). Positive components of core mechanisms, circadian locomotor output cycles kaput (CLOCK) and brain and muscle Arnt‐like protein‐1 (BMAL1), heterodimerize and bind to E‐box elements in the promoter regions of period (Per) and https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=2876&familyId=916&familyType=OTHER (Cry) genes. PER and CRY proteins are negative components of core mechanisms, because their accumulation inhibits CLOCK–BMAL1 heterodimers and represses their own transcription. The stability and robustness of the core loop is also reinforced by auxiliary regulatory loops. For example, CLOCK–BMAL1 heterodimers trigger the expression of https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=596 and https://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=88, which inhibit or stimulate the transcription of Bmal1, respectively (Pilorz et al., 2018). Transcription factors from the proline and acidic amino acid‐rich basic leucine zipper (PAR bZIP) family are important output mediators regulated by core clock components (Gachon, Olela, Schaad, Descombes, & Schibler, 2006). These proteins affect the expression of enzymes and transporters involved in drug absorption and disposition, as it will be subsequently discussed.
Despite being located in most cells of the body and cell autonomous, the endogenous clocks of the circadian timing system are hierarchically organized (Levi & Schibler, 2007). In mammals, the master clock or central pacemaker are hypothalamic suprachiasmatic nuclei (SCN) neurons, which synchronize central and peripheral clock genes in cells through the transmission of direct and indirect timing signals (Dallmann, Brown, & Gachon, 2014; Pilorz et al., 2018). While light signals are mostly perceived by retinal photoreceptive ganglion cells and transmitted to the SCN via the retinohypothalamic tract (Pilorz et al., 2018), peripheral clocks are regulated by non‐chemical or chemical zeitgeber (German, time giver), especially the timing of food intake. This suggests that peripheral clocks are highly sensitive to metabolic cues (Levi & Schibler, 2007). Thus, light–dark cycles synchronize the endogenous circadian rhythm to the 24‐hr geophysical day and lead to the development of physiological and behavioural functions, namely, sleep–wake cycles and feeding habits (Pilorz et al., 2018). As referred above, the SCN can be affected by hormones secreted under its own hierarchical dependence, as a feedback mechanism. https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=224, released by the pineal gland as a result of SCN output, transmits information to SCN neurons about the occurrence and duration of darkness (Flo, Cambras, Díez‐Noguera, & Calpena, 2017; Mark, Crew, Wharfe, & Waddell, 2017).
Based on these observations, it was found that clock disruptions caused by modified light cycles lead to an SCN phase shift and desynchronization between central and peripheral clocks in different organs. Conditions that interfere with time homeostasis, such as jet lag, shift work, aging and sleep disorders, have been associated with the development of several diseases (Dallmann et al., 2014). Consequently, chronotherapy arose as a strategy to prevent or treat illnesses according to biological rhythms. In order to achieve maximal therapeutic efficacy and avoid adverse effects, a drug must be at the right site of action, at the right concentration, and at the right moment (Bruguerolle, Boulamery, & Simon, 2008). The processes involved in pharmacokinetics (PK), namely, absorption, distribution, metabolism, and excretion (ADME), are also affected by pathways coordinated by the SCN (Okyar et al., 2012). This has been defined as chronopharmacokinetics, that is, time‐dependent differences in the ADME of drugs, due to rhythms in biological functions and processes (Ben‐Cherif et al., 2013). It means that ADME stages are influenced by physiological functions that change throughout the day, indicating that the time of drug administration is a critical factor to attain therapeutic efficacy. Furthermore, it entails that estimated PK parameters, which are normally considered to be constant in time, may be, in fact, circadian dependent (Bruguerolle et al., 2008). For these reasons, determining the optimal time of drug dosing and scheduling can improve drug efficacy and tolerability, thereby achieving the ultimate goal of chronotherapy.
Circadian studies can be performed in silico, in vitro, in vivo, or in humans. in vivo PK studies are often performed in rodents, the majority of which are nocturnal and exhibit higher activity during the dark period (Gaspar et al., 2019). Animals are generally entrained to 12/12‐hr light–dark cycles, using light as a strong zeitgeber, and the studied drug is administered at predefined zeitgeber times (ZT) to different groups, in the same dose (Gaspar et al., 2019). The ZT can be defined as a standardized 24‐hr notation of the phase in an entrained circadian cycle, in which ZT 0 indicates the start of the light period and ZT 12 corresponds to the beginning of darkness (Vitaterna, Takahashi, & Turek, 2001). It may also be referred to as hours after light onset (Sallam et al., 2015). Meanwhile, circadian studies in humans should take into consideration several factors that can influence internal time and affect treatment response, namely, chronotypes (Gaspar et al., 2019). Humans can be classified according to the time of day during which their daily routines are preferably developed, that is, first half of the day (larks or morning type), second half (owl or evening type), or intermediate (Levi & Schibler, 2007). Determining the chronotype of a patient may be used to optimize the time of drug administration or stratify them, thereby decreasing inter‐patient differences and the variability of therapeutic response (Gaspar et al., 2019).
The aim of this review is to provide an analysis of the circadian effcts on ADME, in order to demonstrate how the study of PK alterations can be used to attain a time‐adjusted drug administration. A particular emphasis will be given to circadian‐regulated drug transporters and their role in drug absorption and disposition.
2. CHRONOPHARMACOKINETICS: NON‐CLINICAL DATA
2.1. Absorption
2.1.1. Oral route
Oral administration is the most common administration route due to its simplicity and convenience (Bardal, Waechter, & Martin, 2011). The bioavailability of orally administered drugs depends on the physicochemical properties of the drug (e.g., lipophilicity), as well as on several physiological processes of the digestive system (Figure 1) that are dictated by clocks within the gastrointestinal tract (Dallmann et al., 2014).
FIGURE 1.
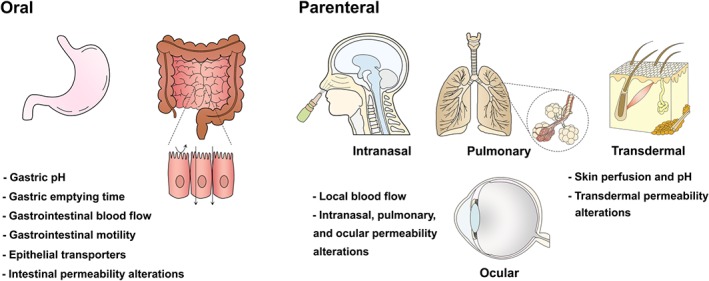
Circadian‐dependent physiological factors that influence drug absorption by oral and parenteral (intranasal, pulmonary, transdermal, and ocular) routes. Rhythmic alterations of these elements can lead to fluctuations of drug levels in plasma. In oral administration, gastric emptying time is longer for solids in the evening than morning. Circadian variations of blood flow are relevant for oral and parenteral routes. For example, skin blood flow is reduced in the morning
Drug transporters are expressed in many organs, including the intestine, liver, brain, placenta, testis, and kidney, where they regulate molecular traffic by carrying drugs into or out of cells. In particular, efflux transporters exert a protective role against xenobiotics, by either opposing their passage or facilitating their elimination (Levi & Schibler, 2007). The expression of efflux transporters in the apical membrane of enterocytes severely hampers the bioavailability of various drugs by actively pumping them from the cytoplasm back to the intestinal lumen (Bardal et al., 2011). These transporters belong to the ATP‐binding cassette (ABC) superfamily, namely, https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=768 (P‐gp, ABCB1 and MDR1), https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=792 (BCRP, ABCG2), and multidrug resistance‐associated proteins (MRPs), including https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=780 (ABCC2).
The rhythmic expression of P‐gp, BCRP, and MRP2 in the intestine is dictated by circadian transcription factors, specifically elements of the PAR bZIP family (Murakami, Higashi, Matsunaga, Koyanagi, & Ohdo, 2008). P‐gp transcription appears to be stimulated by PAR bZIP factors and suppressed by PAR bZIP protein antagonist E4 promoter‐binding protein 4 (E4BP4; Table 1). Studies performed in rodents indicate circadian oscillations in mRNA levels, protein levels, and P‐gp activity in small intestine regions throughout 24 hr (Ando et al., 2005; Hayashi et al., 2010; Murakami et al., 2008; Okyar et al., 2012, 2019; Stearns, Balakrishnan, Rhoads, Ashley, & Tavakkolizadeh, 2008; Figure 2). Ando et al. observed an increase in Abcb1a mRNA levels from ZT 0 (lights on) until ZT 12 (lights off) with protein expression reaching the nadir at ZT 0 and the peak at ZT 8–12. Accordingly, the accumulation of https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=4726 (P‐gp substrate) in isolated jejunum was significantly lower at ZT 12 than ZT 0, revealing higher efflux at ZT 12. Thus, mRNA levels, protein levels, and efflux demonstrated daily rhythmicity (Ando et al., 2005). Similar results were obtained by Murakami et al. with a peak in Abcb1a mRNA levels at ZT 10. This coincided with lower digoxin accumulation from ZT 10–18, significantly lower at ZT 14 (P < .01) in wild‐type mice, compared with clock/clock mutant mice. Clock/clock mice display reduced P‐gp activity and no rhythm in its expression, due to the production of a mutant CLOCK protein without transcriptional activity (Murakami et al., 2008). In an everted intestinal perfusion study using rat jejunum, P‐gp function was assessed by determining the serosal to mucosal efflux of digoxin. The AUC of digoxin in the mucosal fluid of the ad libitum feeding group was higher at ZT 18 than at ZT 0–6 (P < .05 and .01, respectively). This indicates that more digoxin was effluxed to the mucosal side. The authors also demonstrated higher Abcb1 mRNA expression levels at ZT 12 (Hayashi et al., 2010). Identically, the effective permeability (P eff) of P‐gp substrates, https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=4664 and https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=590, was assessed by in situ intestinal perfusion, at ZT 1–3 and ZT 13–15, representing day/rest span and night/activity span periods, respectively. P eff values were lower for both compounds when studies were performed at night, probably due to higher P‐gp activity. However, in in vivo studies, there were no statistically significant differences in AUC between the two dosing times, even though AUC at ZT 13 was slightly lower than at ZT 1. It is possible that the P‐gp effect in bioavailability could have been counteracted by the higher intestinal blood flow and motility that rodents exhibit during the night (Okyar et al., 2012). In a recent study, Okyar et al. found sex‐specific differences in Abcb1 mRNA and protein levels in ileum from mice. The 24‐hr mean of Abcb1a and P‐gp expression was higher in females than males, with larger circadian amplitude and a later acrophase. Feeding patterns may also underlie variations of P‐gp expression and activity. These findings were incorporated into a physiologically based PK model and validated, in order to refine PK predictions of P‐gp substrates (Okyar et al., 2019).
Table 1.
Circadian rhythm‐mediated molecular mechanisms that regulate the expression of transporters/enzymes affecting drug absorption and disposition
| Transporter/enzyme | ADME phase | Organ (species) | Model drug | Circadian clock components and transcription factors | Molecular mechanism | Reference |
|---|---|---|---|---|---|---|
| P‐gp (Abcb1) | Absorption | Intestine (mouse) | Digoxin | PAR bZIP transcription factor (HLF) E4BP4 | HLF activates transcription of Abcb1, whereas E4BP4 suppresses it. | (Murakami et al., 2008) |
| Intestine (rat) | — | PAR bZIP transcription factor (HLF, DBP) E4BP4 | Ad libitum feeding: DBP and HLF mRNA peaks coincided with Abcb1a mRNA peaks; E4BP4 mRNA peak occurred 6 hr before Abcb1a mRNA trough. | (Hayashi et al., 2010) | ||
| Time‐restricted feeding: DPB peak coincided with Abcb1a mRNA peak; HLF mRNA peak occurred 6 hr before Abcb1a mRNA peak; E4BP4 mRNA expression was unaltered by time‐restricted feeding. | ||||||
| DBP may be more important than HLF to regulate circadian Abcb1a intestine expression. | ||||||
| Intestine (cynomolgus monkey) | Etoposide quinidine | PAR bZIP transcription factors (HLF, TEF, DBP) E4BP4 | The Abcb1 gene responds to PAR bZIP transcription factors, and this response is suppressed by E4BP4. | (Iwasaki et al., 2015) | ||
| The transcriptional mechanisms of Abcb1 expression appear to be conserved between mice and monkeys. | ||||||
| Hepatobiliary excretion | Liver (mouse) | — | BMAL1 PAR bZIP transcription factors (HLF, TEF, DBP) | Abcb1 mRNA peak coincides with peak cluster led by BMAL1, including transcription factors HNF1α and Keap1. | (Gachon et al., 2006; Zhang et al., 2009) | |
| PAR bZIP transcription factors regulate CAR expression in the liver, which in turn influences the expression of drug transporters, such as Abcb1b. | ||||||
| Per 1 | Deletion of Per 1 and Per 2 genes resulted in a loss of Abcb1a mRNA rhythmicity in mouse liver. | (Oh et al., 2017) | ||||
| Per 2 | ||||||
| BCRP (Abcg2) | Absorption | Intestine (mouse) | Sulfasalazine | ATF4 | ATF4 binds to cAMP response element of exon 1B promoter region of the Abcg2 gene in a time‐dependent manner. | (Hamdan et al., 2012) |
| Hepatobiliary excretion | Liver (mouse) | — | BMAL1 PAR bZIP transcription factors (HLF, TEF, DBP) | Abcg2 mRNA peak coincides with peak cluster led by BMAL1, including transcription factors HNF1α and Keap1. | (Gachon et al., 2006; Zhang et al., 2009) | |
| PAR bZIP transcription factors regulate CAR expression in the liver, which in turn influences the expression of drug transporters, such as Abcg2. | ||||||
| MRP2 (Abcc2) | Absorption | Intestine(mouse) | Methotrexate | BMAL1 | BMAL1 coordinates the expression of MRP2 through DBP (MRP2 activator) and REV‐ERBα/E4BP4 (MRP2 suppressor). | (Yu, Zhang, et al., 2019) |
| PAR bZIP transcription factor (DBP) | ||||||
| REV‐ERBα E4BP4 | ||||||
| Hepatobiliary excretion | Liver (mouse) | Phenolsulfonphthalein | Per 1 | Deletion of Per 1 and Per 2 genes resulted in a loss of Abcc2 mRNA rhythmicity in mouse liver. | (Oh et al., 2017) | |
| Per 2 | ||||||
| Cytochrome (CYP) P450 family | Metabolism | Liver/kidney (mouse) | — |
BMAL1 PAR bZIP transcription factors (HLF, TEF, DBP) https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=636 |
PAR bZIP transcription factors regulate ALAS and POR, two enzymes essential for P450 reactions. Their mRNA expression undergoes fluctuations throughout the day. | (Claudel, Cretenet, Saumet, & Gachon, 2007; Gachon et al., 2006; Lu et al., 2013) |
| PAR bZIP transcription factors regulate CAR, https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=606, https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=2951, and https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=593 expression in the liver, which control the expression of isoforms from CYP1, CYP2, CYP3, CYP4, and CYP7 families. | ||||||
| BMAL1‐regulated SHP inactivates CAR, PXR, and AhR, acting as a negative modulator. | ||||||
| HepG2 cells | — | PAR bZIP transcription factor (DBP) | E4BP4 suppresses DBP‐mediated expression of CYP3A4. | (Chen, Coulter, Jetten, & Goldstein, 2009; Takiguchi et al., 2007) | ||
| E4BP4 | ||||||
| https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=598 and https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=600 exhibit circadian oscillations and induce CYP2C8 transcription. | ||||||
| RORα and RORγ | ||||||
| Primary hepatocytes (human) |
Abbreviations: Ahr, aryl hydrocarbon receptor; ALAS, aminolevulinic acid synthase; ATF4, activating transcription factor‐4; BCRP, breast cancer resistance protein; BMAL1, brain and muscle Arnt‐like protein‐1; CAR, constitutive androstane receptor; DBP, D site‐binding protein; E4BP4, E4 promoter‐binding protein 4; HLF, hepatic leukaemia factor; HNF1α, hepatocyte nuclear factor 1‐α; Keap1, Kelch‐like ECH‐associated protein 1; MRP2, multidrug resistance‐associated protein‐2; PAR bZIP, proline and acidic amino acid‐rich basic leucine zipper; Per, period; P‐gp, P‐glycoprotein; POR, NADPH–cytochrome P450 oxidoreductase; PXR, pregnane X receptor; REV‐ERBα, reverse erythroblastosis virus α; ROR, retinoid‐related orphan nuclear receptor; SHP, small heterodimer partner; TEF, thyrotroph embryonic factor.
FIGURE 2.
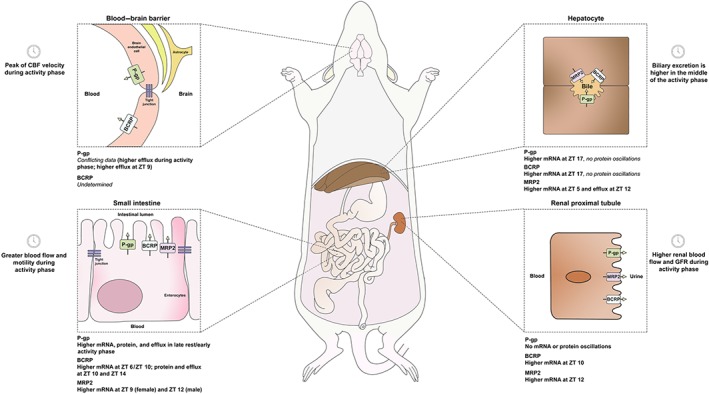
Time‐dependent expression and activity of efflux transporters in rodents that affect drug absorption, distribution, and elimination. Oscillations of these transporters in the intestinal barrier have demonstrated to alter the absorption and tolerability of their substrates. This was observed for digoxin (P‐gp substrate), sulfasalazine (BCRP substrate), and irinotecan (P‐gp/MRP2 substrate). Data concerning circadian variations of efflux transporters at the blood–brain barrier are scarce and require further investigation, particularly regarding BCRP. Elimination by hepatobiliary or renal routes may be affected by local fluctuations of biliary or renal blood flow, but the rhythmic expression of efflux transporters in these organs is unclear and requires the evaluation of protein levels in addition to mRNA. BCRP, breast cancer resistance protein; CBF, cerebral blood flow; MRP2, multidrug resistance‐associated protein 2; P‐gp, P‐glycoprotein; ZT, zeitgeber time
In Table 2, it is noticeable that P‐gp substrates, https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=6035 and https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=7216, exhibit lower systemic exposure, as shown by AUC, at ZT 19 and ZT 17, respectively, corresponding to higher efflux in the active period. This was also reported for https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=4920, a P‐gp and BCRP substrate, which demonstrated lower AUC at ZT 13. Notwithstanding, this could also result from an increased drug clearance and not from a decreased bioavailability, as it will be discussed in Section 2.3. The authors considered that the bioavailability and action time were higher at ZT 1 and ZT 5 due to higher values of AUC, mean residence time, and maximum concentration (C max), even though the absorption speed was slower, as suggested by the higher time to reach C max (t max; Liu et al., 2016). Thus, in rodents, P‐gp expression and activity in the intestine appear to be greater in the nocturnal period (activity period), but in vivo results may reflect other physiological variables that are also affected by circadian rhythms, such as those represented in Figure 1.
Table 2.
Chronopharmacokinetic parameters in plasma and tissues following drug administration to animals
| Compound | Drug properties | Species (gender) | Zeitgeber time (ZT) | Pharmacokinetic parameters in plasma | References | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Log P | PPB (%) | Excretion | Efflux | t max (hr) | C max (mg·L−1) | AUC (mg·hr−1·L−1) | t 1/2 (hr) | CLT (L·hr−1) | MRT (hr) | V d (L) | ||||
| Oral route | ||||||||||||||
| Capecitabine | 0.6 | <60 |
Urine Faeces |
BCRP | Wistar rats (male) | ZT 5 | 0.330 | 17.3 a , * | 13.8 a , * | 0.430 | 47.5 | NR | 23.5 | (Kobuchi, Ito, et al., 2018) |
| ZT 11 | 0.330 | 4.30 a , * | 2.70 a , * | 0.350 * | 297 * | 180 | ||||||||
| ZT 23 | 0.380 | 4.90 a | 4.20 a | 0.920 | 179 | 165 | ||||||||
| 5‐Fluorouracil | −0.9 | 18.4 |
Air Urine |
— | ZT 5 | 0.790 | 109 a | 302 a , * | 1.50 * | NR | NR | NR | ||
| ZT 11 | 0.670 | 161 a | 495 a | 1.90 * | ||||||||||
| ZT 23 | 0.920 | 152 a | 534 a | 3.10 | ||||||||||
| Erlotinib | 3.3 | 93.0 |
Faeces Urine |
P‐gp BCRP |
C57BL/6 mice (female) | ZT 1 | 12.8 | 0.321 | 0.462 | 4.90 | 0.325 | 11.3 | 2.30 * | (Liu et al., 2016) |
| ZT 5 | 7.40 | 0.329 | 0.547 | 8.30 | 0.277 | 14.9 | 3.30 | |||||||
| ZT 9 | 5.80 | 0.240 * | 0.629 | 13.9 | 0.267 | 21.4 | 4.70 * | |||||||
| ZT 13 | 1.80 * | 0.226 * | 0.273 * | 8.70 | 0.568 | 13.1 | 6.60 * | |||||||
| ZT 17 | 0.600 * | 0.287 * | 0.406 * | 9.90 | 0.377 | 15.2 | 5.20 * | |||||||
| ZT 21 | 5.60 * | 0.276 * | 0.602 | 11.7 | 0.269 | 19.0 | 3.80 | |||||||
| Loratadine | 5.2 | 97–99 |
Faeces Urine |
P‐gp | Swiss mice (male) | ZT 1 | 0.500 | 0.104 | 0.345 | 3.26 | 57.8 | NR | NR | (Dridi et al., 2008) |
| ZT 9 | 0.133 * | 0.456 * | 5.61 * | 43.8 | ||||||||||
| ZT 17 | 0.094 | 0.325 | 4.29 | 61.5 * | ||||||||||
| Nifedipine | 2.2 | 92–98 |
Urine Faeces Bile |
BCRP | Sprague–Dawley rats (male) | ZT 0 | 0.130 * | 0.522 * | 1.05 * | NR | NR | NR | NR | (Cao et al., 2005) |
| ZT 8 | 0.420 | 0.269 | 0.752 | |||||||||||
| ZT 16 | 0.170 | 0.153 | 0.426 | |||||||||||
| Oleandrin | 2.4 | NR |
Bile Faeces Urine |
P‐gp | C57BL/6 mice (male) | ZT 2 | NR | 10.4 | 11.9 | 1.02 | 0.23 | 1.63 | 0.34 | (Zhou et al., 2019) |
| ZT 10 | 6.46 * | 8.11 * | 1.76 * | 0.25 | 2.73 * | 0.63 * | ||||||||
| Roscovitine | 3.2 | >90 |
Faeces Urine Bile |
P‐gp | B6D2F1 mice (with osteosarcoma; male) | ZT 3 | NR | 14.5 | 106 * | 4.60 * | 0.06 | NR | 0.370 * | (Sallam et al., 2015) |
| ZT 19 | 19.9 * | 76.7 | 1.93 | 0.08 * | 0.210 | |||||||||
| Sulfasalazine | −0.7 | 99 |
Faeces Urine Bile |
BCRP | Wild‐type mice (NR) | ZT 2 | 0.29 | 0.079 * | 0.057 * | NR | NR | NR | NR | (Hamdan et al., 2012) |
| ZT 14 | 0.55 * | 0.035 | 0.045 | |||||||||||
| Tegafur | −0.3 | 52.3 | Urine | — | Wistar rats (male) | ZT 5 | 1.00 | 168 a | 2,032 a | 3.70 * | 0.04 | NR | 0.200 | (Kobuchi, Yazaki, et al., 2018) |
| ZT 11 | 1.80 | 128 a | 1,370 a , * | 3.10 | 0.06 * | 0.260 * | ||||||||
| ZT 23 | 2.10 | 162 a | 1,885 a | 2.80 | 0.04 | 0.160 | ||||||||
| 5‐Fluorouracil | −0.9 | 18.4 |
Air Urine |
— | ZT 5 | 1.00 | 0.700 a | 5.60 a | 7.40 * | NR | NR | NR | ||
| ZT 11 | 1.30 | 1.70 a , * | 13.7 a , * | 3.50 * | ||||||||||
| ZT 23 | 2.50 | 0.700 a | 6.70 a | 11.8 | ||||||||||
| Intraperitoneal route | ||||||||||||||
| Bucinnazine | 2.91 | NR | NR | — | Sprague––Dawley rats (male) | ZT 2 | 0.170 | 3.84 * | 7.75 * | 2.39 | 2.95 | 1.82 | 8.24 | (Yu, Li, et al., 2019) |
| ZT 14 | 0.400 * | 2.48 | 5.80 | 2.14 | 3.43 | 1.86 | 10.31 | |||||||
| https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=7167 | 3.2 | 50–65 | Urine | — | NMRI mice (male) | ZT 4 | NR | NR | 11.8 | 1.25 | 2.59 | NR | 2.93 | (Bruguerolle, 1984) |
| ZT 10 | 7.09 | 1.29 | 2.56 | 2.95 | ||||||||||
| ZT 16 | 9.98 | 1.43 | 3.04 * | 3.91 * | ||||||||||
| ZT 22 | 16.1 * | 1.16 | 1.89 | 1.87 | ||||||||||
| Isoniazid | −0.7 | 0–10 | Urine |
BCRP MRP2 |
Swiss mice (male) | ZT 1 | 0.100 | 490 * | 2,093 * | 1.36 | 0.130 | NR | 0.200 | (Souayed et al., 2016) |
| ZT 7 | 270 | 759 | 1.12 | 0.220 * | 0.370 * | |||||||||
| ZT 13 | 300 | 822 | 1.15 | 0.190 | 0.330 | |||||||||
| ZT 19 | 420 | 1,656 | 1.25 | 0.130 | 0.240 | |||||||||
| https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=7224 | 1.9 | 75 |
Bile Urine |
— | NMRI mice (male) | ZT 4 | 0.370 * | 12.6 | 10.9 | 1.79 | 0.0960 | NR | 0.27 | (Bruguerolle & Prat, 1988) |
| ZT 10 | 0.08 | 14.1 | 12.2 | 4.23 | 0.0840 | 0.54 | ||||||||
| ZT 16 | 0.08 | 14.7 | 9.57 | 5.41 * | 0.106 | 0.84 * | ||||||||
| ZT 22 | 0.08 | 14.4 | 10.1 | 0.93 | 0.103 | 0.14 | ||||||||
| Mycophenolic acid | 3.2 | 97 |
Urine Bile (MPAG) |
P‐gp MRP2 |
Wistar rats (male) | ZT 1 | 0.500 | 47.1 | 124 | 9.73 | 1.28 | NR | 19.1 * | (Dridi et al., 2014) |
| ZT 7 | 69.1 * | 166 * | 7.52 | 1.08 * | 10.1 * | |||||||||
| ZT 13 | 38.1 | 84.5 | 5.12 | 2.28 | 16.8 | |||||||||
| ZT 19 | 22.7 | 80.3 | 4.67 | 2.45 | 13.9 | |||||||||
| Pethidine/meperidine | 2.5 | 60–80 | Urine | — | BALB/c mice (male) | ZT 2 | 0.210 | 2.74 | 1.29 | 3.11 | 15.9 | NR | 59.9 | (Zhang et al., 2014) |
| ZT 14 | 0.120 | 5.95 * | 1.98 * | 2.23 | 10.4 * | 32.5 | ||||||||
| Valproic acid | 2.75 | 90–95 | Urine | — | Swiss mice (male) | ZT 1 | 0.170 | 515 | 749 | 1.52 | 0.336 | NR | 0.736 | (Ben‐Cherif et al., 2013) |
| ZT 7 | 386 | 612 | 1.59 | 0.405 * | 0.928 * | |||||||||
| ZT 13 | 573 | 857 | 1.06 | 0.348 | 0.536 | |||||||||
| ZT 19 | 824 * | 1,475 * | 1.73 | 0.157 | 0.394 * | |||||||||
| Intramuscular route | ||||||||||||||
| Imipenem | −0.7 | 20 | Urine | — | Wistar rats (male) | ZT 4 | 0.560 * | 85.9 * | 177 | 0.920 | 0.270 | NR | 0.370 | (Boulamery et al., 2007) |
| 0.510 | ||||||||||||||
| ZT 10 | 1.29 | 54.4 * | 143 * | 0.980 | 0.340 * | 0.270 | ||||||||
| ZT 16 | 0.600 * | 91.9 | 164 | 0.660 | 0.310 | 0.430 | ||||||||
| ZT 22 | 0.690 | 65.5 * | 146 | 0.890 | 0.340 * | |||||||||
| Lidocaine | 2.3 | 60–80 | Urine | — | Wistar rats (male) | ZT 4 | NR | NR | NR | 2.12 | 4.38 | NR | NR | (Bruguerolle, Valli, Bouyard, Jadot, & Bouyard, 1983) |
| ZT 10 | 5.05 (C 0) | 11.1 * | 1.50 * | NR | 9.75 * | |||||||||
| ZT 22 | 2.97 (C 0) * | 7.45 | NR | 5.88 | 16.7 | |||||||||
| Intravenous route | ||||||||||||||
| Imipramine | 4.8 | 60–96 | Urine | P‐gp | Wistar rats (male) | ZT 0.5 | — | — | 0.482 | 1.08 | NR | NR | NR | (Lemmer & Holle, 1991) |
| ZT 12.5 | 0.474 | 0.91 | ||||||||||||
| https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=3342 | 2.5 | >95 | Urine | P‐gp | New Zealand white rabbits (NR) | ZT 2 | — | 0.821 | 986 | NR | 5.76 | NR | 2.99 | (Bienert et al., 2014) |
| ZT 7 | 1.07 | 1,150 | 4.26 | 2.42 | ||||||||||
| ZT 11 | 1.21 | 1,722 | 3.12 | 2.00 | ||||||||||
| ZT 15 | 1.57 b | 1,750 b | 3.00 | 1.80 b | ||||||||||
| Melatonin | 0.8 | 53 | Urine | — | Hairless Sprague–Dawley rats (male) | ZT 4 c | 2.52 | 0.640 | 2.16 * | NR | NR | 4.12 * | NR | (Flo et al., 2017) |
| ZT 16 c | 0.530 | 0.300 | 0.310 | 1.42 | ||||||||||
| ZT 4 | — | — | 2.18 * | 0.700 | 0.580 | 0.540 | 0.290 | |||||||
| ZT 16 | 0.880 | 0.920 | 1.39 * | 0.640 | 0.900 * | |||||||||
| dl‐Propranolol | 3.0 | >90 | Urine | — | Wistar rats (male) | ZT 0.5 | — | — | 0.890 | 0.72 * | 10.0 | NR | 10.5 | (Lemmer & Bathe, 1982) |
| ZT 12.5 | 0.900 | 0.55 | 9.84 | 7.90 | ||||||||||
| Subcutaneous route | ||||||||||||||
| Caffeine | −0.1 | 25–36 | Urine | — | Wistar rats (male) | ZT 2 | 0.81 | 30.5 | 329 * | 6.69 | 35.8 | NR | 0.751 | (Pelissier‐Alicot et al., 2002) |
| ZT 14 | 1.06 | 25.6 | 234 | 5.35 | 58.9 * | 0.836 * | ||||||||
| Ocular route | ||||||||||||||
| Timolol | 1.8 | 10 | Urine | P‐gp | Dutch rabbits (male) | ZT 0 | 0.170 | 0.0480 | 146 | NR | NR | 0.780 | NR | (Ohdo et al., 1991) |
| ZT 6 | 0.100 | 0.0370 | 80.4 | 0.660 | ||||||||||
| ZT 12 | 0.100 | 0.0560 | 128 | 0.690 | ||||||||||
| ZT 18 | 0.100 | 0.0560 | 123 | 0.690 | ||||||||||
| Compound | Route | Tissues | Species (gender) | Zeitgeber time (ZT) | Pharmacokinetic parameters in tissues | |||||||||
| C max (mg·L−1) | AUC (mg·hr−1·L−1) | |||||||||||||
| Nifedipine | Oral | Heart | Sprague–Dawley rats (male) | ZT 0 | 0.220 | NR | (Cao et al., 2005) | |||||||
| ZT 8 | 0.230 | |||||||||||||
| 0.480 * | ||||||||||||||
| ZT 16 | ||||||||||||||
| Kidney | 0.140 | NR | ||||||||||||
| 0.230 | ||||||||||||||
| 0.420 * | ||||||||||||||
| Liver | 0.140 | NR | ||||||||||||
| 0.230 | ||||||||||||||
| 0.420 * | ||||||||||||||
| Lung | 0.400 | NR | ||||||||||||
| 0.550 | ||||||||||||||
| 0.540 | ||||||||||||||
| Roscovitine | Oral | Adipose | B6D2F1 mice (with osteosarcoma; male) | ZT 3 | 96.1 | 314 * | (Sallam et al., 2015) | |||||||
| 42.4 | 167 | |||||||||||||
| Brain | 6.24 | 16.6 | ||||||||||||
| 4.87 | 12.9 | |||||||||||||
| Kidney | 42.6 | 262 * | ||||||||||||
| 31.3 | 145 | |||||||||||||
| Liver | ZT 19 | 58.8 | 286 | |||||||||||
| 66.2 | 342 * | |||||||||||||
| Lung | 19.0 | 161 * | ||||||||||||
| 21.8 | 144 | |||||||||||||
| Testis | 11.0 | 49.3 | ||||||||||||
| 9.21 | 37.0 | |||||||||||||
| Imipramine | Intravenous | Brain | Wistar rats (male) | ZT 0.5 | NR | 0.0404 | (Lemmer & Holle, 1991) | |||||||
| 0.0532 * | ||||||||||||||
| Intraperitoneal | ZT 12.5 | 2.85 | 6.44 | |||||||||||
| 4.81 * | 9.67 * | |||||||||||||
| dl‐Propranolol | Intravenous | Brain | Wistar rats (male) | ZT 0.5 | NR | 13.9 | (Lemmer & Bathe, 1982) | |||||||
| ZT 12.5 | 13.7 | |||||||||||||
| Heart | NR | 2.90 | ||||||||||||
| 2.60 | ||||||||||||||
Note. Data referent to drug properties were obtained from PubChem (https://pubchem.ncbi.nlm.nih.gov/). Only average values are presented for pharmacokinetic parameters, and some were converted to uniform units. Apparent oral clearance (CL/F) may be presented instead of CLT.
Abbreviations: BCRP, breast cancer resistance protein; C 0, initial concentration; C max, maximum concentration; CLT, total plasma clearance; log P, octanol–water partition coefficient; MPAG, mycophenolic acid 7‐O‐glucuronide; MRP2, multidrug resistance‐associated protein‐2; MRT, mean residence time; NR, not reported; P‐gp, P‐glycoprotein; PPB, plasma protein binding; t 1/2, elimination half‐life; t max, time to reach the maximum concentration; V d, volume of distribution.
Values expressed in μmol·L−1 (C max) or μmol·hr−1·L−1 (AUC).
Differences were found for parameters estimated by non‐compartmental analysis but do not apply to the population.
Values obtained after transdermal administration.
Statistically significant values (P < .05).
The translation of in vivo results from rodents to humans may pose a challenge, as rodents are nocturnally active. Iwasaki et al. performed a study in diurnally active cynomolgus monkeys, revealing higher P‐gp levels in the intestine from 15.00 to 21.00 hr. In agreement, the AUC of https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=2342 and https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=6815 (P‐gp substrates) in plasma was higher at 9.00 hr than at 21.00 hr. Interestingly, the oscillations of P‐gp levels in the intestine did not coincide with Abcb1 mRNA expression, demonstrating an almost 12‐hr delay (Iwasaki et al., 2015). Therefore, it is advisable to determine both mRNA and protein levels of the transporter, in order to consider possible delays in translation. As discussed later, the monkey was confirmed to be a reliable model for the assessment of chronopharmacokinetics in humans.
Oscillations in BCRP and MRP2 expression in jejunum are also associated with diurnal rhythmicity (Ando et al., 2005; Stearns et al., 2008; Figure 2). While BCRP expression is influenced by activating transcription factor‐4 (ATF4), an output component of the circadian clock, MRP2 levels are up‐regulated by PAR bZIP factor, D site‐binding protein (DBP; Table 1). In 24 hr, there were 2.5‐fold and 1.6‐fold changes between maximum and minimum levels of MRP2 and BCRP, respectively, in rats (Stearns et al., 2008). Hamdan et al. examined the circadian expression of the Abcg2 isoform found in mouse small intestine and reported higher mRNA levels at ZT 6 and ZT 10. Nevertheless, higher BCRP levels were obtained at ZT 10 and ZT 14, indicating a 4‐hr delay relative to mRNA rhythm (Hamdan et al., 2012). BCRP function was determined in vivo through the administration of https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=4840 to mice, which revealed an earlier t max and higher C max and AUC at ZT 2, when BCRP expression was lower, compared with ZT 14. Hence, the bioavailability of sulfasalazine is affected by rhythmic changes in BCRP function (Table 2).
The circadian oscillations in Abcc2 expression were analysed in the ileum mucosa of mice from different strains and genders (Okyar et al., 2011). Gender differences in mRNA expression were found in the B6D2F1 mouse strain, with the highest mRNA levels at ZT 12 and ZT 9, in male and female mice, respectively. In contrast, there were no gender differences in the B6CBAF1 strain, with an mRNA peak at ZT 9–15. Furthermore, the ileum tolerability of anticancer drug and P‐gp/MRP2 substrate, https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=6823, improved almost fourfold with dosing near the time of maximum Abcc2 expression, ZT 12. These results reveal that circadian MRP2 oscillations not only are tissue specific but also can be influenced by tissue region, gender, and genetic background (Okyar et al., 2011).
Circadian oscillations have been equally reported for members of the solute carrier transporter (SLC) superfamily, such as the proton‐coupled https://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=187#984) and the https://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=197#1022; Okamura et al., 2014; Wada et al., 2015). The absorption rate constant of https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=5484 in mice was significantly larger when administered at ZT 14 than ZT 2, coinciding with the highest expression of Octn1 in the small intestine at ZT 14 and, thus, higher uptake (Akamine et al., 2015).
Finally, it should be mentioned that circadian variations in the expression of tight junction proteins may be responsible for diurnal fluctuations in intestinal permeability. This has been reported in both the small (Tanabe et al., 2015) and large intestines (Oh‐oka et al., 2014) in mice. In mouse jejunum, occludin and claudin‐3 mRNA levels are significantly higher at the night period, suggesting that intestinal permeability may be higher in the light period (Tanabe et al., 2015). It would be interesting to analyse the final consequences of these variations in the paracellular permeability of drugs.
2.1.2. Parenteral routes
Circadian variations in absorption may occur with routes of administration that do not involve absorption through the gastrointestinal tract, that is, parenteral routes (Bardal et al., 2011).
Intranasal administration is a non‐invasive route with potential for local, central, or systemic drug delivery. There is a rhythmic variability in the physiological functions of the nasal mucosa, observed in a day–night environmental cycle. The presence of clock genes in the nasal mucosa of mice, rats, and humans has been recently confirmed, demonstrating asymmetric levels between nasal cavities and circadian oscillations (Kim, Kim, Kim, Kim, & Lee, 2018). Likewise, clock genes are also expressed in bronchiolar epithelial cells, and the circadian timing system is known to have a role in pulmonary pathophysiology (Sundar, Yao, Sellix, & Rahman, 2015). As it is believed that alterations in pulmonary permeability may influence circadian phase‐dependent PK (Ohdo, 2010), further research is warranted, in order to understand if pulmonary absorption of systemically acting drugs is affected by circadian variations.
The chronopharmacokinetics of transdermal administration have received more attention, particularly concerning melatonin (Flo et al., 2017). The existence of circadian rhythms has been shown in the skin, with consequences in skin aging, cell repair, transepidermal water loss, stratum corneum hydration, temperature, and optimal timing of drug delivery (Flo, Díez‐Noguera, Calpena, & Cambras, 2014; Yosipovitch et al., 2004). In a recent study, higher AUC, C max, and bioavailability (0.06 vs. 0.02, P < .05) were observed for transdermal melatonin at ZT 4 than ZT 16 (Table 2), indicating greater systemic and local exposure during the light/rest phase of rats (Flo et al., 2017).
Dosing‐time dependency has also been reported for subcutaneous and intramuscular administration. The C max and AUC of https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=407 were significantly higher at ZT 2 (initial rest phase) than at ZT 14 after subcutaneous administration (Table 2), although this was attributed to a lower clearance at ZT 2, rather than to a greater absorption (Pelissier‐Alicot, Schreiber‐Deturmeny, Simon, Gantenbein, & Bruguerolle, 2002). Identically, the C max and AUC of imipenem after intramuscular injection were higher at ZT 4, even though C max was also high at ZT 16. For https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=2623, significantly higher initial concentration, AUC and absorption rate were observed at ZT 10 (late rest phase; Table 2). Although a faster and more extensive absorption would be expected during the nocturnal phase of rodents, due to increasing blood flow and motor activity, t max was also lower during the early rest phase for caffeine (ZT 2) and imipenem (ZT 4), which suggests that other factors may be influencing the absorption process (Figure 1).
Changes in the ocular absorption of topically applied https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=565 were reported as dosing‐time dependent (Lee et al., 1996; Ohdo, Grass, & Lee, 1991). Corneal and conjunctival permeabilities were significantly lower at light onset (ZT 0; Lee et al., 1996), but the lowest values of C max and AUC were observed later at ZT 6 (Table 2). Even though the mechanisms behind variations in ocular PK are not yet completely understood, a circadian disruption of intercellular junctional proteins (occludin, claudin‐4) in the corneal epithelium of Xenopus laevis was observed during the night (Wiechmann, Ceresa, & Howard, 2014). In future studies, it would be interesting to analyse possible consequences of this temporary disruption of the epithelial barrier in ocular drug delivery.
Lastly, a dosing‐time dependency of the PK of several drugs was also observed following intraperitoneal administration (Table 2). Nonetheless, due to the limited availability of data and combination of simultaneous PK processes, it becomes difficult to establish a clear relationship between the circadian variation of PK parameters and intraperitoneal drug absorption. Ben‐Cherif et al. (2013) reported chronopharmacokinetic variations for https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=7009 in mice, which attained higher C max and AUC in plasma at ZT 19 and the lowest values at ZT 7 (rest phase). This is in agreement with previous results regarding its tolerability, since maximum toxicity was found at ZT 17 and the highest survival rate occurred when valproic acid was administered at ZT 9 (Ben‐Cherif et al., 2012). Still, the highest anticonvulsant activity was also achieved at ZT 7, despite lower plasma AUC and higher clearance. The authors suggest that the dose required for optimal prevention of epileptic seizures may be lower in rest period than during activity (Khedhaier et al., 2017), but it would have been relevant to monitor drug concentrations at the biophase (i.e., brain), in addition to PK parameters in plasma.
2.2. Distribution
2.2.1. Plasma protein binding and blood flow
Drug distribution is influenced by several factors including cardiac output, blood flow, and drug binding, to both tissues and plasma proteins (Bardal et al., 2011). Rhythmic oscillations have been described for plasma protein levels of albumin and α1‐acid glycoprotein in rodents, with acrophases at ZT 2 (albumin) and ZT 19/ZT 1 (α1‐acid glycoprotein; Scheving, Pauly, & Tsai, 1968). Such fluctuations are particularly critical for drugs with high binding to plasma proteins (>90%) and small volume of distribution (V d; Dridi et al., 2014). In Table 2, three highly bound drugs exhibited significantly higher V d during the rest phase: roscovitine (ZT 3), https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=6832 (ZT 1), and valproic acid (ZT 7), the exception being erlotinib (ZT 13: early activity phase).
Cardiac output and general blood flow appear to be higher during the activity phase. This was linked to a greater activity of the sympathetic nervous system, as indicated by a higher turnover of https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=484 in rat heart during darkness (Lemmer, 1992). For example, a significantly higher cardiac output was observed at ZT 14 than that at ZT 2 or at ZT 10 (Delp, Manning, Bruckner, & Armstrong, 1991). Lastly, despite having observed a peak during the night, modulation of cerebral blood flow in rodents was unrelated to the regulation of arterial BP and locomotor activity (Wauschkuhn, Witte, Gorbey, Lemmer, & Schilling, 2005).
2.2.2. Tissue exposure
2.2.2.1. CNS
Molecular exchanges between the blood and the CNS are limited and regulated by the blood–brain barrier (BBB) and blood–CSF barrier (Kervezee et al., 2014).
Recent studies in mice showed that the choroid plexus possesses a strong clock that maintains the circadian homeostasis of the brain and is capable of influencing the SCN, through diffusible factors in the CSF (Myung et al., 2018). The rate of CSF influx is increased during sleep (Xie et al., 2013), almost doubling during the resting phase of animals (0.522 μl·min−1) in relation to the active phase (0.227 μl·min−1), which enables the removal of metabolites from the CNS (Kervezee et al., 2014). In this context, it is important to mention that natural sleep or anaesthesia increases cortical interstitial space by more than 60%, thereby facilitating exchanges between the CSF and interstitial brain fluid, and the clearance of metabolites via the glymphatic system (Xie et al., 2013). Indeed, gadolinium concentrations in the brain and cerebellum of rats were higher in late afternoon injections than morning injections or injections with anaesthesia. This was explained by a possibly higher glymphatic clearance in the morning (sleep/rest phase) or induced by anaesthesia (Taoka, Jost, Frenzel, Naganawa, & Pietsch, 2018).
In addition to this, glial cells are capable of modulating circadian rhythms (Ng, Tangredi, & Jackson, 2011). SCN neurons and astrocytes cooperate in the regulation of circadian oscillations, as SCN astrocytes also display pacemaker properties and regulate circadian timing at night, while SCN neurons are metabolically active during circadian daytime (Brancaccio, Patton, Chesham, Maywood, & Hastings, 2017). Thus, SCN astrocytes participate in the definition of daily rest–activity rhythms, locomotor behaviour, and cognition (Barca‐Mayo et al., 2017; Tso et al., 2017). Moreover, pericytes maintain the integrity and homeostasis of the BBB through BMAL1, which, when disrupted, leads to BBB hyperpermeability (Nakazato et al., 2017). Time‐dependent fluctuations in BBB permeability were recently confirmed (Zhang, Yue, Arnold, Artiushin, & Sehgal, 2018). Rhodamine B revealed higher BBB permeability at night in Drosophila, which has a similar molecular clock to humans. Moreover, P‐gp‐mediated efflux was higher during the active period (day), in agreement with a lower permeability at this time (Figure 2). This was verified by achieving greater drug efficacy when administering the P‐gp substrate, https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=2624, at night (Zhang, Yue, et al., 2018).
Nonetheless, in vivo studies have generated conflicting results regarding the CNS distribution of efflux substrates. In a microdialysis experiment, the brain exposure of quinidine was lower at night in rats, corroborating a higher P‐gp activity during the active period. Such differences were annulled following a pretreatment with the P‐gp inhibitor, tariquidar (Kervezee et al., 2014). However, PET also performed in rats with the radiolabelled P‐gp substrate [18F]MC225 yielded discrepant data (Savolainen et al., 2016). The higher uptake of [18F]MC225 into the brain and V d (1.2‐fold to 1.8‐fold higher) were found at ZT 15 (early active phase) suggesting lowest P‐gp activity, while its highest activity was at ZT 9 (late rest phase). This discrepancy was explained by the different P‐gp selectivity of quinidine and the radiolabelled substrate, as well as by technical differences between microdialysis and PET. While PET provides non‐invasive regional and time‐dependent measurements, it cannot distinguish between bound and unbound tracer concentrations (Savolainen et al., 2016). Later, another assay was performed to assess diurnal variations in the brain distribution of https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=1627, intravenously administered to rats. The lowest efflux was observed in light–dark phase transitions (20% difference) but cannot be solely attributed to P‐gp, given that morphine transport is also affected by probenecid‐sensitive transporters. The authors concluded that morphine displayed a 12‐hr rhythm transport from the brain to the blood and such variations can be used to optimize its dosing schedule (Kervezee, Hartman, van den Berg, Meijer, & de Lange, 2017). In an earlier PK study, higher total C max and AUC in the brain were obtained for https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=357 (P‐gp substrate) when administered to rats at ZT 12.5 (close to light–dark transition; Lemmer & Holle, 1991). Such parameters were higher at ZT 3 for another efflux substrate, roscovitine, but not statistically significant. Nevertheless, it is noteworthy that roscovitine was administered by the oral route instead of intravenous, which may introduce bias (Table 2).
Overall, much is yet unknown about the impact of circadian oscillations in drug access to the CNS. To the best of our knowledge, there are no published data about possible diurnal variations in the expression and function of other efflux transporters in brain endothelial cells. This would be particularly relevant for BCRP, given its co‐operative role with P‐gp at the BBB.
2.2.2.2. Placenta
Pregnancy generates an intriguing interaction between maternal, fetal, and placental circadian systems (Mark et al., 2017). The maternal central clock undergoes alterations to support physiological adaptations related to metabolic needs of the growing fetus (Wharfe et al., 2016). Meanwhile, the placenta not only transmits external rhythmic signals from the mother but may also influence fetal circadian biology through its own rhythmic function (Mark et al., 2017). The presence of clock genes has been confirmed in mice and rat placenta (Wharfe, Mark, & Waddell, 2011).
Physiologically, the placenta is a barrier that governs nutrient and metabolite exchanges between maternal and fetal circulations. The syncytiotrophoblast, whose apical membrane faces the maternal circulation, is the rate‐limiting barrier for the trans‐placental transfer of the majority of substances. At the apical side of the syncytiotrophoblast are ABC transporters with a protective role that efflux molecules back into the maternal circulation (Mark, Augustus, Lewis, Hewitt, & Waddell, 2009; Staud & Ceckova, 2015). The expression levels of these transporters are controlled by endogenous factors and vary throughout gestation. Although further studies are needed, it is believed that maternal glucocorticoids have a complex role in their regulation (Staud & Ceckova, 2015). P‐gp is a component of the placental glucocorticoid barrier, which prevents excess glucocorticoids from inhibiting fetal growth. Rhythmic variations of P‐gp expression have been observed in rat placenta, with a peak at the beginning of the active phase, ZT 13 (Mark et al., 2009; Waddell, Wharfe, Crew, & Mark, 2012). Nonetheless, it is insufficiently understood whether circadian variations have meaningful effects on the fetal exposure to xenobiotics, in addition to endogenous glucocorticoids. In mice, P‐gp did not significantly prevent fetal exposure to its substrate, norbuprenorphine, although brain exposure was severely limited. This was partly explained by a lower P‐gp expression at the blood–placenta barrier (6.3‐fold) than BBB (Liao et al., 2017). Subsequently, it was proposed that chronovariations of placental and brain P‐gp expression levels and activity could cause different tissue exposures depending on the ZT of the study (Dash & Rais, 2017). While no considerable P‐gp variations were observed in the light phase (ZT 0–12), alterations in the dark phase or between phases cannot be excluded. Thus, more studies are necessary to characterize the effects of ZT on P‐gp and chronopharmacokinetics (Liao, Shen, & Mao, 2017).
2.2.2.3. Other tissues
Similarly to the BBB and the placenta, the blood–testis barrier is considered a “pharmacological sanctuary” because germ cells are protected from drugs and xenobiotics. This protection is conferred by tight junctions between Sertoli cells and also by efflux transporters located in Sertoli cells, peritubular myoid cells, Leydig cells, and endothelial cells. P‐gp and BCRP are expressed at the apical membrane of different cell types (Mruk, Su, & Cheng, 2011). There is evidence of the existence of several rhythms in the testis, including those for spermatogenesis and androgen production (Bittman, 2016). For instance, the circadian rhythm of testosterone synthesis was disrupted after the exposure of mouse Leydig cells to fenvalerate, a pesticide, which inhibited its production (Guo et al., 2017). Exposure of P‐gp substrates in the testis of rodents has revealed paradoxical results so far. While Sallam et al. (2015) observed higher C max, AUC (Table 2), and tissue–plasma ratio (1.32) in testis when orally administering roscovitine at ZT 3, Savolainen et al. (2016) obtained higher tissue–plasma ratios for [18F]MC225 at ZT 15. The presence of diverse cell types in the testis with variable amplitude and/or phase of oscillations (Bittman, 2016) may complicate the interpretation of results. Thus, additional research is required in order to elucidate circadian drug exposure in testis and determine the optimal drug dosing time for therapeutic purposes or prevention of adverse effects.
Importantly, several physiological functions of the cardiovascular system and adipose tissue display 24‐hr rhythmicity (Sallam et al., 2015; Smolensky, Hermida, & Portaluppi, 2017), as mentioned earlier for lung (Sundar et al., 2015). However, there is little information regarding circadian variations of drug concentrations in organs, even in animal models. https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=2514 levels in the heart were significantly higher when dosed at ZT 16, whereas no differences were observed for dl‐https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=564. Higher AUC values were obtained for roscovitine in adipose tissue and lung at ZT 3 with increases of 88% and 12%, respectively, in relation to ZT 19 (Table 2), which indicated possible drug movement from the blood to the organs during the beginning of the rest phase (Sallam et al., 2015).
2.3. Metabolism
During metabolism, lipophilic drugs are often converted to more hydrophilic polar metabolites through functionalization in phase I reactions such as oxidation, mainly mediated by cytochrome P450 (CYP) enzymes, and phase II conjugation reactions. Afterwards, in phase III, ABC transporters facilitate the excretion of metabolites into the bile, milk, sweat, bronchial exudate, urine, and/or faeces (Bardal et al., 2011).
The liver is a highly vascularised organ, as are the brain, lungs, and kidneys (Bardal et al., 2011). Hepatic blood flow and enzymatic activity are two determinant factors for drug metabolism. In rodents, estimated hepatic blood flow is more pronounced during the activity phase (Dridi et al., 2008). Roscovitine displayed significantly higher concentrations in mouse liver at ZT 19, and its microsomal intrinsic clearance was also faster at this time (Sallam et al., 2015), which is in agreement with a slower clearance and a longer elimination half‐life at ZT 3 (rest phase; Table 2).
The circadian expression of enzymes and transporters in liver tissue is regulated by clock‐controlled transcription factors, allowing a coordinated xenobiotic detoxification (Gachon et al., 2006). PAR bZIP factors interact with target genes that determine the expression of enzymes required for CYP function, CYP isoforms, and efflux proteins (Table 1). Some genes are direct targets of PAR bZIP factors, whereas others may be indirectly regulated by xenobiotic receptors, such as the https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=607. The expression of aminolevulinic acid synthase 1, the rate‐limiting enzyme in haem biosynthesis, and NADPH–cytochrome P450 oxidoreductase, the electron donor for CYPs, is circadian and modulated by PAR bZIP proteins (Gachon et al., 2006). In mouse liver, mRNA expression of phase I enzymes was greater in the dark phase, whereas phase II enzymes were generally more abundant at different times of the light phase. Efflux transporters with higher mRNA oscillations were Abcb1 (2.9‐fold), Abcg2 (1.8‐fold), and Abcc2 (1.7‐fold). Abcb1 and Abcg2 mRNA peaked at ZT 17, while Abcc2 mRNA peaked at ZT 5 (Figure 2). The results are analogous to those of Ando et al. (2005) revealing higher Abcb1 mRNA from ZT 12 to ZT 16 and increasing Abcc2 mRNA from ZT 4 to ZT 8 (peak from ZT 8 to ZT 12; Zhang, Yeager, & Klaassen, 2009). In another study, such oscillations of Abcc2 expression were annulled following removal of the gallbladder of mice (Zhang et al., 2018). However, it is important to determine protein levels due to possible delays in translation. In this regard, Mauvoisin et al. (2014) suggested that besides rhythms in transcription, there may be translational and post‐translational rhythms affecting protein formation. Then, Robles, Cox, and Mann (2014) showed that some liver proteins displayed a significant time lag between transcription and translation, occasionally even anti‐phase. For Abcc2, there was an 8.03‐hr time lag. In fact, the biliary clearance and cumulative excretion of phenolsulfonphthalein, an MRP2 substrate, was highest at ZT 12, that is, approximately 8 hr after the mRNA peak (Figure 2). Nevertheless, P‐gp and BCRP protein expression did not demonstrate a significant rhythm in the same study, despite a 24‐hr transcriptional oscillation of Abcb1 (Oh, Lee, Han, Cho, & Lee, 2017) and Abcg2 (exon 1B isoform; Hamdan et al., 2012). In the liver from cynomolgus monkeys, P‐gp, BCRP, MRP2, and https://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=263 protein levels did not reveal significant circadian variations. While P‐gp and MRP2 remained mostly constant, BCRP and CYP3A showed large interindividual variability. Once again, mRNA of Abcb1 oscillated in the liver (Iwasaki et al., 2015) but did not translate into protein in the same proportion. This reinforces the importance of measuring both mRNA and protein and raises questions regarding interspecies differences, which require further investigation.
Evidence of circadian oscillations of enzyme levels is present in studies concerning liver toxicity and drug metabolism. Hepatotoxicity at different dosing times has been reported for isoniazid (ZT 1; Souayed et al., 2015) and https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=5239 (ZT 12; DeBruyne, Weaver, & Dallmann, 2014; Johnson et al., 2014), among others. The works of DeBruyne et al. (2014) and Johnson et al. (2014) revealed that the chronotoxicity of acetaminophen is regulated not only by the central clock, through feeding schedules and GSH levels, but also by the liver circadian clock. Deletion of the clock from hepatocytes reduced the expression and activity of NADPH–cytochrome P450 oxidoreductase, resulting in lower acetaminophen toxicity. Isoniazid is partly metabolized by https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=1330‐mediated oxidation to hepatotoxic compounds (Souayed et al., 2016). Its greater toxicity at ZT 1 coincided with higher C max and AUC in plasma, as shown in chronopharmacokinetic studies (Table 2). Moreover, Khemawoot et al. demonstrated that CYP2E1 has circadian variations, with higher microsomal mRNA levels in the liver at ZT 12, protein content at ZT 18, and hydroxylation activity at ZT 21, measured through the conversion of https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=2322 to 6‐hydroxychlorzoxazone in male rats. Thus, in order to assess metabolic activity, it is recommended to quantify the unconverted parent compound, as well as the total amount of formed metabolite (Khemawoot, Nishino, Ishizaki, Yokogawa, & Miyamoto, 2007). Despite confirmed circadian variations, no sex dimorphism was observed for CYP2E1, even if trough–peak differences of mRNA were obtained (5.4‐fold in female and 16.6‐fold in male mice). Conversely, CYP2A4 and CYP7B1 oscillated in a circadian manner and revealed female and male mice predominance, respectively. The sexual dimorphism that affects drug metabolizing enzymes was attributed to the pulsatile (males) or continuous (females) secretion of the pituitary growth hormone (Lu et al., 2013). Therefore, the circadian rhythm influences not only substrate concentrations through blood flow and distribution but also enzyme levels and activity, which can differ significantly between species and genders.
These observations apply to tissues other than the liver in which biotransformation also takes place, even if with lower expression, namely, the lungs, gut, kidneys, or brain, where it can affect acute and chronic response to drugs (Carver, Lourim, Tryba, & Harder, 2014; Sundar et al., 2015; Zuber et al., 2009). An interesting study by Carver et al. found an ultradian expression (12 hr) of Cyp2c11 and Cyp4x1 in rat brain microvascular endothelial cells and astrocytes from the hippocampus. This led to the hypothesis that the rhythmic production of epoxyeicosatrienoic acids by these cells may be responsible for the circadian regulation of cerebral blood flow (Carver et al., 2014). Identically, there is circadian expression of phase I and phase II enzymes in the kidney, most of which display maximal transcript expression at ZT 12 in male mice (Zuber et al., 2009).
2.4. Excretion
2.4.1. Hepatobiliary excretion
As aforementioned, ABC transporters participate in the hepatobiliary excretion of drugs and their metabolites into the bile. Circadian rhythms have been observed for bile flow, biliary concentrations, and excretory rates of biliary lipids, which exhibited a peak at ZT 16 and a trough at ZT 4 in rats (Nakano, Tietz, & LaRusso, 1990). Examples of efflux substrates for which biliary excretion is a relevant elimination route include the 7‐O‐glucuronide metabolite of mycophenolic acid (Dridi et al., 2014) and irinotecan (Filipski et al., 2014). Interestingly, the second peak of mycophenolic acid in plasma, corresponding to its enterohepatic circulation, always occurred 6 hr after administration, independently of the time of administration and in spite of its glucuronide being an MRP2 substrate (Dridi et al., 2014). Meanwhile, P‐gp is essential for the detoxification of irinotecan, which demonstrated better tolerability during the rest phase of mice, when the residence time of the main cytotoxic metabolite was shortest (Filipski et al., 2014). Nevertheless, as circadian oscillations of P‐gp levels in liver tissue are unclear and irinotecan is also excreted by the urinary route, no conclusions can be drawn regarding a possible role of P‐gp in the detoxification process through biliary excretion. Thus, the importance of circadian variations of efflux transporters in the hepatobiliary excretion of drugs is as yet, not known.
2.4.2. Renal excretion
Circadian oscillations of kidney function are well known (Firsov & Bonny, 2018). The most relevant renal processes that undergo rhythmic variations and affect drug excretion are the GFR, tubular reabsorption, active tubular secretion, renal blood flow, and urinary pH (Boulamery, Kadra, Simon, Besnard, & Bruguerolle, 2007; Firsov & Bonny, 2018). In rodents, renal blood flow and GFR are higher during the activity phase and lower during rest (Okyar et al., 2012). For instance, Cao, Kim, Choi, and Lee (2005) described a more significant urinary excretion of nifedipine at the end of the activity phase (0.0342%) than when rats were most inactive (0.0137%).
Several compounds excreted in urine exhibit circadian oscillations of elimination PK parameters (Table 2). The anticancer prodrugs of https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=4789, https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=10513 and https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=6799, revealed higher total oral clearance at ZT 11, while the simulated population mean clearance of 5‐fluorouracil showed the highest values at ZT 2/ZT 0. These alterations are responsible for the fluctuations of plasma levels of 5‐fluorouracil and evidence the importance of dosing time. Clearance differences were attributed to circadian oscillations of physiological factors that affect not only elimination but also bioavailability and plasma protein binding (Kobuchi, Ito, Takamatsu, & Sakaeda, 2018; Kobuchi, Yazaki, Ito, & Sakaeda, 2018). Additional drugs that displayed greater total clearance during the first or second halves of the rest phase include isoniazid and valproic acid (ZT 7) and https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=7221 (ZT 2), all dosed by intraperitoneal route to mice. This was associated with a higher tolerability during the rest phase (Ben‐Cherif et al., 2012) or stronger efficacy/toxicity in the activity phase (Zhang, Yu, Li, Xu, & Liu, 2014). Notwithstanding, higher clearance values were observed for other compounds during the activity phase. For instance, a higher elimination of https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=2427 in the activity phase was related with a greater nephrotoxicity in rodents at the rest phase (Choi, Kim, & Lee, 1999).
In accordance, higher nephrotoxicity and ototoxicity were demonstrated when it was dosed at the rest phase (ZT 2) in female rats, although clearance values were slightly higher at ZT 2 than ZT 14 (4.53 vs. 3.22 ml·min−1·kg−1; Blunston, Yonovitz, Woodahl, & Smolensky, 2015). Similarly, Oda et al. evaluated the nephrotoxicity of https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=5343 in mice and reported it as less toxic when clearance is higher, that is, active phase. This drug is transported into renal cells by the https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=1020 (OCT2), which was less expressed during this period (ZT 18). Therefore, the lower nephrotoxicity of cisplatin in the dark phase is explained by its lower uptake into renal cells, caused by lower levels of OCT2 (Oda et al., 2014). Rhythmic oscillations of other transporters in the kidney have been investigated by Ando et al. (2005), pointing towards higher mRNA levels of Abcc2 at ZT 12 in mouse (Figure 2). Conversely, no mRNA oscillations were found for Abcb1a or for P‐gp expression. Hamdan et al. (2012) discovered mRNA variations of the isoform 1B of Abcg2 with a peak at ZT 10 (Figure 2). No MRP2 or BCRP protein levels were determined in these studies. Therefore, as mentioned for other tissues, more research is required to investigate eventual delays in translation and post‐translation that may affect drug uptake and secretion and possibly efficacy and/or toxicity.
3. CHRONOPHARMACOKINETICS: HUMAN DATA
When comparing chronopharmacokinetic data from different dosing schedules, it is advisable to apply bioequivalence criteria. This will improve the assessment of their clinical meaning and eventual impact in efficacy and tolerability (Bethke, Huennemeyer, Lahu, & Lemmer, 2010; Etienne‐Grimaldi et al., 2008).
There is some evidence of the influence of circadian oscillations in human ADME (Table 3), although it is much less than the corresponding non‐clinical data. Circadian variations of gastric emptying time have been reported not only in the wake state but also between rapid eye movement (REM) and non‐REM sleep. In wake state, it is slower in the evening (20.00 hr) than morning (8.00 hr), which may affect the speed of drug absorption (Goo, Moore, Greenberg, & Alazraki, 1987). Moreover, in non‐REM sleep, there is a decline of gastric electrical rhythm that is recovered in REM sleep. This indicated that this function is not independent from CNS activity, since some CNS awareness is required to restore normal gastric emptying (Vaughn, Rotolo, & Roth, 2014). Further studies exploring the relationship between gastric emptying time, sleep states, and drug absorption are recommended.
Table 3.
Chronopharmacokinetic parameters in plasma following drug administration to humans
| Compound | Drug properties | Study design | Health status | Age interval (gender) | n | Fast period (hr) | Dosing time (hr) | Pharmacokinetic parameters | References | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Log P | PPB (%) | Excretion | Efflux | t max (hr) | C max (mg·L−1) | AUC (mg·hr−1·L−1) | t 1/2 (hr) | CLT (L·hr−1) | MRT (hr) | V d (L) | ||||||||
| Oral route | ||||||||||||||||||
| Amlodipine | 3.0 | 98 | Urine | P‐gp | Single‐centre, randomized, crossover | Normotensive | 20–40 | 12 | NR |
8.00 20.00 |
4.00 2.00 * |
0.812 0.923 * |
8.76 * 6.51 |
23.7 25.3 * |
NR |
13.1 23.6 * |
NR | (Khodadoustan et al., 2017) |
| Hypertensive | >18 (male/female) |
8.00 20.00 |
4.00 2.00 * |
0.889 1.02 * |
11.1 * 9.03 |
28.9 32.4 * |
NR |
13.0 12.9 |
NR | |||||||||
| Acetaminophen | 0.5 | 25 | Urine | — | NR | Healthy | 23–29 (male) | 7 | >4 |
07.30 13.00 21.00 |
0.630 0.850 1.00 * |
22.3 20.2 19.7 |
81.6 72.1 * 99.6 |
1.68 1.90 2.03 |
0.221 0.233 0.192 |
NR | NR | (Kolawole, Chuhwak, & Okeniyi, 2002) |
| Levofloxacin | −0.4 | 24–38 | Urine | P‐gp | Single‐centre, randomized, crossover | Healthy | 18–50 (male) | 12 | t = −2 until 6 |
8.00 18.00 23.00 |
1.0 2.3 1.75 |
8.93 8.13 8.53 |
92.6 92.7 92.7 |
NR | 10.8 | NR | 116 | (Kervezee et al., 2016) |
| Mycophenolic acid | 3.2 | 97 |
Urine Bile (MPAG) |
P‐gp MRP2 |
NR | Renal transplant patients | 21–66 (male/female) | 30 |
1.5 3 |
9.00 21.00 |
3.07 * 5.47 |
11.7 * 8.79 |
55.1 * 50.5 |
NR |
0.27 0.29 * |
NR | NR | (Satoh et al., 2006) |
| Pentoxifylline | 0.3 | 70 |
Urine Bile |
— | Randomized, Latin square, crossover | Healthy | 20–27 (male) | 12 | 10 |
07.00 13.00 19.00 01.00 |
1.66 1.31 1.32 1.90 * |
0.646 0.736 0.782 0.485 * |
2.14 2.08 2.37 2.03 |
1.23 1.39 1.23 1.93 * |
3.51 3.33 2.82 3.40 |
2.90 2.90 2.70 3.80 * |
8.82 8.28 7.06 11.9 * |
(Srinivasu et al., 1998) |
| Sumatriptan | 0.9 | 14–21 | Urine | P‐gp | Randomized, Latin square, crossover | Healthy | 19–30 (male) | 12 | 10 |
07.00 13.00 19.00 01.00 |
1.96 1.96 1.67 2.00 |
0.0591 * 0.0525 0.0419 0.0516 |
0.312 * 0.269 0.207 0.271 |
2.13 1.99 2.65 2.19 |
0.781 * 0.936 1.21 * 0.884 |
5.08 4.83 5.15 5.10 |
3.60 * 4.11 5.80 4.09 |
(Poondru, Devaraj, Boinpally, & Yamasani, 2000) |
| Tacrolimus | 2.7 | 99 |
Faeces Urine |
P‐gp MRP2 |
Randomized, crossover | Liver transplant patients | 28–66 (male/female) | 12 | NR |
08.00 20.00 |
1.60 * 3.50 |
0.0322 * 0.0216 |
0.219 * 0.188 |
NR |
0.355 0.444 * |
5.30 5.80 * |
1.76 2.62 * |
(Min, Chen, Lee, Ashton, & Martin, 1997) |
| Tamoxifen | 7.1 | >98 |
Bile Faeces Urine |
BCRP MRP2 |
Not randomized, crossover | Breast cancer patients | 44–62 (female) | 27 |
0.5 3 |
8.00 20.00 |
2.10 * 8.10 |
0.356 * 0.298 |
2.35 * 1.98 |
48.0 76.3 |
9.40 9.55 |
NR | NR | (Binkhorst et al., 2015) |
| Intravenous route | ||||||||||||||||||
| Doxorubicin | 1.3 | 74–76 |
Bile Urine |
P‐gp BCRP MRP2 |
Randomized | Breast cancer patients | 41–74 (female) | 18 | NR |
09.00 21.00 |
— | — |
1.74 2.50 * |
12.6 21.7 * |
35.3 * 27.9 |
NR |
307 412 |
(Canal et al., 1991) |
| Gentamicin | −4.1 | 0–30 |
Urine Bile (minor) |
— | Crossover | Healthy | 22–50 (male/female) | 10 | Overnight |
09.00 22.00 |
— | — |
23.4 26.3 * |
2.82 2.97 |
3.51 * 3.18 |
NR |
14.2 13.4 |
(Choi et al., 1999) |
| Methotrexate | −1.8 | 60–69 |
Urine Bile (minor) |
BCRP MRP2 |
Crossover | Leukaemia patients | 3–11 (male/female) | 6 | Overnight |
10.00 21.00 |
— | — | NR |
2.52 3.45 |
0.336 * 0.282 |
NR |
1.20 1.30 |
(Koren et al., 1992) |
| Tobramycin | −3.4 | NR | Urine | P‐gp | Four‐centre, randomized | Cystic fibrosis patients | 5–18 (NR) | 18 | NR |
08.00 20.00 |
— | — |
4.15 3.72 |
1.66 1.89 |
4.10 3.54 |
NR |
0.260 0.240 |
(Prayle et al., 2016) |
Note. Data referent to drug properties were obtained from PubChem (https://pubchem.ncbi.nlm.nih.gov/). Only average values are presented for pharmacokinetic parameters, and some were converted to uniform units. Apparent oral clearance (CL/F) may be presented instead of CLT.
Abbreviations: BCRP, breast cancer resistance protein; C max, maximum concentration; CLT, total plasma clearance; log P, octanol–water partition coefficient; MPAG, mycophenolic acid 7‐O‐glucuronide; MRP2, multidrug resistance‐associated protein‐2; MRT, mean residence time; NR, not reported; P‐gp, P‐glycoprotein; PPB, plasma protein binding; t 1/2, elimination half‐life; t max, time to reach the maximum concentration; V d, volume of distribution.
Statistically significant values (P < .05).
The oral bioavailability of efflux substrates appears to be higher during the early active phase. P‐gp substrates https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=6981 and https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=54 exhibited higher AUC after oral dosing at 8.00 and 7.00 hr, respectively (Table 3). Additionally, the oral administration of digoxin resulted in an earlier t max when dosed at 8.00 hr instead of 20.00 hr (P < 0.5). C max and AUC were also higher at 8.00 hr but not statistically significant (Erol, Kiliç, Batu, & Yildirim, 2001). This is in agreement with non‐clinical data obtained for other P‐gp substrates in the laboratory monkey (Iwasaki et al., 2015), as mentioned in Section 2.1.1. Identically, https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=1016, a BCRP and MRP2 substrate, revealed higher systemic exposure at 8.00 hr (Table 3). Notwithstanding, more data concerning the circadian expression of these transporters in the human intestine are necessary.
To the best of our knowledge, the only report concerning circadian intranasal absorption in humans refers to the delivery of https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=6973, with higher serum levels reached at 00.00 hr (Tarquini et al., 1988). Other studies involving time‐controlled parenteral drug administration include the transdermal delivery of https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=2585 (Hammann, Kummer, Guercioni, Imanidis, & Drewe, 2016), as explored in Section 4.
Human plasma protein levels display circadian oscillations. Serum albumin levels are higher from 08.00 to 20.00 hr, with a trough at 04.00 hr (Jubiz, Canterbury, Reiss, & Tyler, 1972), while α1‐acid glycoprotein reached the acrophase at 11.30 hr (Bruguerolle et al., 1989). Indeed, highly bound valproic acid revealed higher unbound drug fraction in plasma (fu,plasma) between 02.00 and 06.00 hr (Patel, Venkataramanan, Levy, Viswanathan, & Ojemann, 1982), whereas lower fu,plasma was determined for https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=3364 at 09.30 hr (Nakano, Watanabe, Nagai, & Ogawa, 1984). In Table 3, https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=6784 also displayed higher V d in the rest phase (20.00 hr).
While cardiac output is higher during daytime, when sympathetic nervous system activity predominates, peripheral blood flow is decreased due to vasoconstriction (Smolensky et al., 2017). In fact, regional rhythm variations (circadian and ultradian) have been identified in skin blood flow, with lowest flow determined during morning hours (Yosipovitch et al., 2004). Similarly, cerebral blood flow velocity is lower in the morning than in the afternoon or evening (Conroy, Spielman, & Scott, 2005), which correlates positively with functional connectivity and negatively with morning cortisol levels (Hodkinson et al., 2014). It oscillates during sleep, decreasing steadily at the onset of sleep until slow wave sleep but rising at the beginning of REM sleep, when it may even overcome waking state levels (Klingelhófer, 2012). The average rate of CSF production also increases during the night, oscillating from 12 ml·hr−1 at 18.00 hr to 42 ml·hr−1 at 02.00 hr (Nilsson et al., 1992). The clinical meaning of these variations is yet to be determined.
The expression of clock genes has been detected in human full‐term placenta (Pérez et al., 2015). As already mentioned, glucocorticoids affect the activity of drug transporters during gestation. Synthetic glucocorticoids (https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=2768 and https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=7061) are administered to pregnant women at risk of imminent preterm delivery to improve fetal lung maturation (Staud & Ceckova, 2015). The timing of administration is decided according to the number of gestation weeks and risk of preterm delivery within 7 days (Di Renzo et al., 2019), rather than the chronopharmacokinetics of the exogenous glucocorticoids. Although data on Abcg2 expression are inconsistent, both drugs induced the expression of Abcb1 in human cytotrophoblasts (Staud & Ceckova, 2015).
Data from in vitro studies using human liver cell lines or primary hepatocytes suggest that the circadian expression of CYP enzymes is regulated by transcription factors from the Par bZIP family (DBP) or the retinoid‐related orphan nuclear receptors (Table 1). Possible implications of this for the metabolism of endogenous compounds or xenobiotics demand more investigation.
Estimated hepatic blood flow in humans is highest at 8.00 hr and lowest at 14.00 hr (Lemmer & Nold, 1991), which may also affect drug metabolism and hepatobiliary excretion. Efflux substrate https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=7069 is highly excreted in the bile. In agreement, its total clearance, representing renal and hepatic clearance, was higher after dosing at 9.00 hr than 21.00 hr (Table 3). As urinary elimination was unaffected by dosing time, these variations were attributed to oscillations of hepatic clearance, which encompasses metabolic and biliary clearance (Canal et al., 1991). Identically, Srinivasu, Rao, Rao, and Rambhau (1998) partly attributed the lower C max of https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=7095 at 01.00 hr to its biliary excretion and extensive enterohepatic circulation (Table 3).
As mentioned in Section 2.4.2, there are several renal processes affected by circadian variations. In humans, GFR was highest during daytime (16.36 hr) and lowest at night (02.54 hr), whereas the peak and trough of renal blood flow occurred later at 19.54 and 06.24 hr, respectively. Urine volume and sodium excretion were in phase with GFR rhythm (14.00/15.09 hr), but potassium excretion occurred earlier (11.00 hr) due to tubular secretion dictated by plasma potassium levels and https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=2872, which have acrophases in the morning. Additionally, the contribution of tubular secretion to the clearance of https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=4810 was higher during the night (20.54 hr; Koopman et al., 1989). The uptake of p‐aminohippurate across the basolateral membrane of proximal tubule cells is mediated by https://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=198 (OATs), while its secretion into the renal lumen is carried out by MRP2 (Leier, Hummel‐Eisenbeiss, Cui, & Keppler, 2000). On the other hand, https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=4815 and gentamicin were depurated more quickly following intravenous dosing in the morning (Table 3). The excretion of methotrexate, a weak organic acid, is influenced by urinary pH. As the urine is more acidic at night, tubular reabsorption of methotrexate increases while tubular secretion is reduced, and consequently, renal clearance is lower (Koren et al., 1992). Its uptake into proximal tubule cells is performed by OAT1/OAT3 and reduced folate carrier‐1, whereas MRP2/MRP4 and BCRP secrete the drug into urine (Kawase, Yamamoto, Egashira, & Iwaki, 2016). The renal route is also the main excretion pathway of gentamicin, and its higher clearance coincided with the higher GFR at daytime (Choi et al., 1999).
Lastly, it is important to highlight the presence of phase I enzymes in human kidney. https://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=263#1338 was the main identified isoform and hence considered the most relevant for renal drug metabolism (Schuetz et al., 1992). Although its expression was shown to influence the metabolism and clearance of tacrolimus in the kidney, apart from the liver and small intestine (Zheng et al., 2014), no circadian PK variations were found for this drug in patients with renal transplant (Satoh et al., 2008). These results imply more research is needed on circadian oscillations of CYP expression and activity in humans.
4. CHRONOPHARMACOKINETICS AND DRUG DELIVERY
The purpose of chronomodulated drug delivery is to release a drug at a specific time window, in order to synchronize plasma concentrations with the circadian rhythm of the disease. For example, the existence of higher BP in the late morning or early afternoon, in both normotensive and hypertensive subjects, has been associated with the occurrence of angina pectoris, acute myocardial infarction, or hypertensive crisis (Zhang et al., 2016). Other conditions such as migraine and rheumatoid arthritis also display worse symptoms in morning hours (Hadi, Raghavendra Rao, & Srinivasa Rao, 2016; Jagdale & Pawar, 2014). Therefore, in all these pathologies, it becomes important to ensure an adequate drug delivery at a critical time interval.
This is normally achieved by delaying drug release from the formulation or its absorption, which implies the development of a system that creates a lag time before exposure (Hadi et al., 2016). Recent examples of such formulations can be found in Table S1. Additional advantages of time‐controlled drug release include an improvement of patient compliance and of the oral bioavailability of the delivered drug, by evading drug interactions with efflux transporters or extensive first‐pass metabolism (Farag, Abd El Malak, & Yehia, 2018). For instance, press‐coated floating tablets were developed for the chronomodulated delivery of sumatriptan, a drug with poor oral bioavailability (14%). A lag time of 7 hr was achieved, allowing drug administration at night and release in the early morning (Jagdale & Pawar, 2014). Previous studies had also pointed towards a higher systemic exposure when sumatriptan was administered in the morning (07.00 hr; Table 3), indicating that drug delivery in this period benefits from time‐dependent PK.
Other administration routes that are being explored for chronomodulated drug delivery besides the oral route encompass transdermal and buccal drug delivery. In the first case, nicotine was transdermally administered to humans in a pulsatile manner, in order to improve withdrawal symptoms after smoking cessation. This is advantageous in part because nicotine levels undergo chronopharmacokinetic oscillations, as its clearance increases in the late morning and after meals (Hammann et al., 2016). The second approach was conceived to circumvent the first‐pass metabolism of https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=4345 and improve treatment of middle‐of‐the‐night insomnia. The drug was incorporated into a bilayered buccal chronopatch and released in pulses. Its bioavailability was 2.63‐fold higher compared with the immediate‐release commercially available capsule (Farag et al., 2018). The optimization of these strategies is essential to reconcile a safer and effective treatment with adherence to therapy.
5. CONCLUSION
Several physiological functions under clock regulation affect PK, including the presence of drug transporters widely distributed across biological membranes. A greater knowledge of how circadian mechanisms regulate the expression and activity of these transporters may allow a time‐adjusted administration of efflux substrates. This could be achieved by administering the drug at a specific time of the day or incorporating it into a chronomodulated drug delivery system that would release it at the most advantageous moment. This moment would depend on the main goal; is it to increase bioavailability or tissue exposure (e.g., lowest expression/activity of efflux transporter in the site of drug absorption/distribution), decrease adverse effects (e.g., higher expression/activity of efflux transporter in the site of drug elimination/distribution), or both. Hence, the first step is to expand our current understanding of the chronovariations of efflux transporters in different organs by resorting to in vitro/in vivo models. in vivo studies in particular should be performed with trained animals, maintained in constant and controlled conditions to avoid circadian disruption. It is also necessary to specify the species, strain, gender, and feeding pattern, because these aspects can interfere with transporter levels and PK. Additionally, mRNA oscillations must be complemented with levels of functional protein, due to the existence of translational and post‐translational rhythms. If these factors are taken into consideration during study design and reported, data may be more easily interpreted and extrapolated.
The next step is to improve the clinical assessment of chronopharmacokinetics. Bioequivalence criteria ought to be applied when analysing PK data from different administration times and study subjects should be grouped according to chronotype, in order to decrease inter‐patient variability and prevent masking of possible PK alterations. This will imply the development of better tools to evaluate human circadian phenotypes and apply a personalized medicine approach.
In further studies, either clinical or non‐clinical, it is important for researchers to bear in mind that PK parameters may oscillate according to drug dosing time and that the study of these fluctuations can be useful to select the optimal time of drug administration. As already demonstrated, there are many research directions to pursue in this field in order to, at the end, achieve an effective and safe drug dosing that will benefit pharmacotherapy and patients' quality of life.
5.1. Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Harding et al., 2018), and are permanently archived in the Concise Guide to PHARMACOLOGY 2019/20 (Alexander, Cidlowski et al., 2019; Alexander, Fabbro et al., 2019; Alexander, Kelly et al., 2019).
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
Supporting information
Table S1. Recent advances (2014 ‐ 2019) of chronomodulated drug delivery systems
ACKNOWLEDGEMENTS
The authors acknowledge the European Regional Development Fund (Fundo Europeu de Desenvolvimento Regional [FEDER]), through Portugal 2020 in the scope of the Operational Programme for Competitiveness and Internationalisation, and Fundação para a Ciência e a Tecnologia (FCT), Portuguese Agency for Scientific Research, within the scope of the research project CENTRO‐01‐0145‐FEDER‐030752 and POCI‐01‐0145‐FEDER‐030478.
Bicker J, Alves G, Falcão A, Fortuna A. Timing in drug absorption and disposition: The past, present, and future of chronopharmacokinetics. Br J Pharmacol. 2020;177:2215–2239. 10.1111/bph.15017
REFERENCES
- Akamine, T. , Koyanagi, S. , Kusunose, N. , Hashimoto, H. , Taniguchi, M. , Matsunaga, N. , & Ohdo, S. (2015). Dosing time‐dependent changes in the analgesic effect of pregabalin on diabetic neuropathy in mice. The Journal of Pharmacology and Experimental Therapeutics, 354, 65–72. 10.1124/jpet.115.223891 [DOI] [PubMed] [Google Scholar]
- Alexander, S. P. H. , Cidlowski, J. A. , Kelly, E. , Mathie, A. , Peters, J. A. , Veale, E. L. , … CGTP Collaborators . (2019). THE CONCISE GUIDE TO PHARMACOLOGY 2019/20: Nuclear hormone receptors. British Journal of Pharmacology, 176, S229–S246. 10.1111/bph.14750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander, S. P. H. , Fabbro, D. , Kelly, E. , Mathie, A. , Peters, J. A. , Veale, E. L. , … CGTP Collaborators . (2019). THE CONCISE GUIDE TO PHARMACOLOGY 2019/20: Enzymes. British Journal of Pharmacology, 176, S297–S396. 10.1111/bph.14752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander, S. P. H. , Kelly, E. , Mathie, A. , Peters, J. A. , Veale, E. L. , Armstrong, J. F. , … CGTP Collaborators . (2019). THE CONCISE GUIDE TO PHARMACOLOGY 2019/20: Transporters. British Journal of Pharmacology, 176, S397–S493. 10.1111/bph.14753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ando, H. , Yanagihara, H. , Sugimoto, K. I. , Hayashi, Y. , Tsuruoka, S. , Takamura, T. , … Fujimura, A. (2005). Daily rhythms of P‐glycoprotein expression in mice. Chronobiology International, 22, 655–665. 10.1080/07420520500180231 [DOI] [PubMed] [Google Scholar]
- Barca‐Mayo, O. , Pons‐Espinal, M. , Follert, P. , Armirotti, A. , Berdondini, L. , & De Pietri Tonelli, D. (2017). Astrocyte deletion of Bmal1 alters daily locomotor activity and cognitive functions via GABA signalling. Nature Communications, 8, 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardal, S. K. , Waechter, J. E. , & Martin, D. S. (2011). Chapter 2—Pharmacokinetics In Applied pharmacology (pp. 17–34). London, UK: Saunders Elsevier. [Google Scholar]
- Bargiello, T. A. , Jackson, F. R. , & Young, M. W. (1984). Restoration of circadian behavioural rhythms by gene transfer in Drosophila . Nature, 312, 752–754. 10.1038/312752a0 [DOI] [PubMed] [Google Scholar]
- Ben‐Cherif, W. , Dridi, I. , Aouam, K. , Ben‐Attia, M. , Reinberg, A. , & Boughattas, N. A. (2012). Chronotolerance study of the antiepileptic drug valproic acid in mice. Journal of Circadian Rhythms, 10, 3 10.1186/1740-3391-10-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben‐Cherif, W. , Dridi, I. , Aouam, K. , Ben‐Attia, M. , Reinberg, A. , & Boughattas, N. A. (2013). Circadian variation of valproic acid pharmacokinetics in mice. European Journal of Pharmaceutical Sciences, 49, 468–473. 10.1016/j.ejps.2013.05.009 [DOI] [PubMed] [Google Scholar]
- Bethke, T. D. , Huennemeyer, A. , Lahu, G. , & Lemmer, B. (2010). Chronopharmacology of roflumilast: A comparative pharmacokinetic study of morning versus evening administration in healthy adults. Chronobiology International, 27, 1843–1853. 10.3109/07420528.2010.520398 [DOI] [PubMed] [Google Scholar]
- Bienert, A. , Płotek, W. , Wiczling, P. , Kostrzewski, B. , Kamińska, A. , Billert, H. , … Grześkowiak, E. (2014). The influence of the time of day on midazolam pharmacokinetics and pharmacodynamics in rabbits. Pharmacological Reports, 66, 143–152. 10.1016/j.pharep.2013.06.009 [DOI] [PubMed] [Google Scholar]
- Binkhorst, L. , Kloth, J. S. L. , de Wit, A. S. , de Bruijn, P. , Lam, M. H. , Chaves, I. , … Mathijssen, R. H. J. (2015). Circadian variation in tamoxifen pharmacokinetics in mice and breast cancer patients. Breast Cancer Research and Treatment, 152, 119–128. 10.1007/s10549-015-3452-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bittman, E. L. (2016). Timing in the testis. Journal of Biological Rhythms, 31, 12–36. 10.1177/0748730415618297 [DOI] [PubMed] [Google Scholar]
- Blunston, M. A. , Yonovitz, A. , Woodahl, E. L. , & Smolensky, M. H. (2015). Gentamicin‐induced ototoxicity and nephrotoxicity vary with circadian time of treatment and entail separate mechanisms. Chronobiology International, 32, 1223–1232. 10.3109/07420528.2015.1082483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulamery, A. , Kadra, G. , Simon, N. , Besnard, T. , & Bruguerolle, B. (2007). Chronopharmacokinetics of imipenem in the rat. Chronobiology International, 24, 961–968. 10.1080/07420520701648309 [DOI] [PubMed] [Google Scholar]
- Brancaccio, M. , Patton, A. P. , Chesham, J. E. , Maywood, E. S. , & Hastings, M. H. (2017). Astrocytes control circadian timekeeping in the suprachiasmatic nucleus via glutamatergic signaling. Neuron, 93, 1420–1435e5. 10.1016/j.neuron.2017.02.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruguerolle, B. (1984). Circadian phase dependent pharmacokinetics of disopyramide in mice. Chronobiology International, 1, 267–271. 10.3109/07420528409063906 [DOI] [PubMed] [Google Scholar]
- Bruguerolle, B. , Arnaud, C. , Levi, F. , Focan, C. , Touitou, Y. , & Bouvenot, G. (1989). Physiopathological alterations of alpha 1 acid glycoprotein temporal variations: Implications for chronopharmacology. Progress in Clinical and Biological Research, 300, 199–214. [PubMed] [Google Scholar]
- Bruguerolle, B. , Boulamery, A. , & Simon, N. (2008). Biological rhythms: A neglected factor of variability in pharmacokinetic studies. Journal of Pharmaceutical Sciences, 97, 1099–1108. 10.1002/jps.21044 [DOI] [PubMed] [Google Scholar]
- Bruguerolle, B. , & Prat, M. (1988). Circadian phase‐dependent pharmacokinetics and acute toxicity of mepivacaine. The Journal of Pharmacy and Pharmacology, 40, 592–594. 10.1111/j.2042-7158.1988.tb05314.x [DOI] [PubMed] [Google Scholar]
- Bruguerolle, B. , Valli, M. , Bouyard, L. , Jadot, G. , & Bouyard, P. (1983). Effect of the hour of administration on the pharmacokinetics of lidocaine in the rat. European Journal of Drug Metabolism and Pharmacokinetics, 8, 233–238. 10.1007/BF03188753 [DOI] [PubMed] [Google Scholar]
- Canal, P. , Sqalli, A. , de Forni, M. , Chevreau, C. , Pujol, A. , Bugat, R. , … Houin, G. (1991). Chronopharmacokinetics of doxorubicin in patients with breast cancer. European Journal of Clinical Pharmacology, 40, 287–291. 10.1007/BF00315211 [DOI] [PubMed] [Google Scholar]
- Cao, Q. R. , Kim, T. W. , Choi, J. S. , & Lee, B. J. (2005). Circadian variations in the pharmacokinetics, tissue distribution and urinary excretion of nifedipine after a single oral administration to rats. Biopharmaceutics & Drug Disposition, 26, 427–437. 10.1002/bdd.474 [DOI] [PubMed] [Google Scholar]
- Carver, K. A. , Lourim, D. , Tryba, A. K. , & Harder, D. R. (2014). Rhythmic expression of cytochrome P450 epoxygenases CYP4x1 and CYP2c11 in the rat brain and vasculature. American Journal of Physiology‐Cell Physiology, 307, C989–C998. 10.1152/ajpcell.00401.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, Y. , Coulter, S. , Jetten, A. M. , & Goldstein, J. A. (2009). Identification of human CYP2C8 as a retinoid‐related orphan nuclear receptor target gene. The Journal of Pharmacology and Experimental Therapeutics, 329, 192–201. 10.1124/jpet.108.148916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi, J. S. , Kim, C. K. , & Lee, B. J. (1999). Administration‐time differences in the pharmacokinetics of gentamicin intravenously delivered to human beings. Chronobiology International, 16, 821–829. 10.3109/07420529909016948 [DOI] [PubMed] [Google Scholar]
- Claudel, T. , Cretenet, G. , Saumet, A. , & Gachon, F. (2007). Crosstalk between xenobiotics metabolism and circadian clock. FEBS Letters, 581, 3626–3633. 10.1016/j.febslet.2007.04.009 [DOI] [PubMed] [Google Scholar]
- Conroy, D. A. , Spielman, A. J. , & Scott, R. Q. (2005). Daily rhythm of cerebral blood flow velocity. Journal of Circadian Rhythms, 3, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallmann, R. , Brown, S. A. , & Gachon, F. (2014). Chronopharmacology: New insights and therapeutic implications. Annual Review of Pharmacology and Toxicology, 54, 339–361. 10.1146/annurev-pharmtox-011613-135923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dash, R. P. , & Rais, R. (2017). P‐gp/ABCB1 exerts differential impacts on brain and fetal exposure to norbuprenorphine—Significance of zeitgeber time on pharmacokinetics. Pharmacological Research, 121, 251–252. 10.1016/j.phrs.2017.04.004 [DOI] [PubMed] [Google Scholar]
- DeBruyne, J. P. , Weaver, D. R. , & Dallmann, R. (2014). The hepatic circadian clock modulates xenobiotic metabolism in mice. Journal of Biological Rhythms, 29, 277–287. 10.1177/0748730414544740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delp, M. D. , Manning, R. O. , Bruckner, J. V. , & Armstrong, R. B. (1991). Distribution of cardiac output during diurnal changes of activity in rats. The American Journal of Physiology, 261, H1487–H1493. 10.1152/ajpheart.1991.261.5.H1487 [DOI] [PubMed] [Google Scholar]
- Di Renzo, G. C. , Fonseca, E. , Gratacos, E. , Hassan, S. , Kurtser, M. , Malone, F. , … Tosto, V. (2019). Good clinical practice advice: Antenatal corticosteroids for fetal lung maturation. International Journal of Gynecology & Obstetrics, 144, 352–355. [DOI] [PubMed] [Google Scholar]
- Dridi, D. , Ben‐Attia, M. , Sani, M. , Djebli, N. , Sauvage, F. L. , & Boughattas, N. A. (2008). Circadian time‐effect of orally administered loratadine on plasma pharmacokinetics in mice. Chronobiology International, 25, 533–547. 10.1080/07420520802257646 [DOI] [PubMed] [Google Scholar]
- Dridi, I. , Ben‐Cherif, W. , Aouam, K. , Ben‐Attia, M. , Klouz, A. , Reinberg, A. , & Boughattas, N. A. (2014). Circadian variation of mycophenolate mofetil pharmacokinetics in rats. European Journal of Pharmaceutical Sciences, 58, 20–25. 10.1016/j.ejps.2014.02.015 [DOI] [PubMed] [Google Scholar]
- Erol, K. , Kiliç, F. S. , Batu, Ö. S. , & Yildirim, E. (2001). Morning–evening administration time differences in digoxin kinetics in healthy young subjects. Chronobiology International, 18, 841–849. 10.1081/CBI-100107519 [DOI] [PubMed] [Google Scholar]
- Etienne‐Grimaldi, M. C. , Cardot, J. M. , François, E. , Renée, N. , Douillard, J. Y. , Gamelin, E. , & Milano, G. (2008). Chronopharmacokinetics of oral tegafur and uracil in colorectal cancer patients. Clinical Pharmacology and Therapeutics, 83, 413–415. 10.1038/sj.clpt.6100297 [DOI] [PubMed] [Google Scholar]
- Farag, M. M. , Abd El Malak, N. S. , & Yehia, S. A. (2018). Zaleplon loaded bi‐layered chronopatch: A novel buccal chronodelivery approach to overcome circadian rhythm related sleep disorder. International Journal of Pharmaceutics, 542, 117–124. 10.1016/j.ijpharm.2018.03.014 [DOI] [PubMed] [Google Scholar]
- Filipski, E. , Berland, E. , Ozturk, N. , Guettier, C. , van der Horst, G. T. J. , Lévi, F. , & Okyar, A. (2014). Optimization of irinotecan chronotherapy with P‐glycoprotein inhibition. Toxicology and Applied Pharmacology, 274, 471–479. 10.1016/j.taap.2013.12.018 [DOI] [PubMed] [Google Scholar]
- Firsov, D. , & Bonny, O. (2018). Circadian rhythms and the kidney. Nature Reviews. Nephrology, 14, 626–635. 10.1038/s41581-018-0048-9 [DOI] [PubMed] [Google Scholar]
- Flo, A. , Cambras, T. , Díez‐Noguera, A. , & Calpena, A. (2017). Melatonin pharmacokinetics after transdermal administration changes according to the time of the day. European Journal of Pharmaceutical Sciences, 96, 164–170. 10.1016/j.ejps.2016.09.020 [DOI] [PubMed] [Google Scholar]
- Flo, A. , Díez‐Noguera, A. , Calpena, A. C. , & Cambras, T. (2014). Circadian rhythms on skin function of hairless rats: Light and thermic influences. Experimental Dermatology, 23, 214–216. 10.1111/exd.12338 [DOI] [PubMed] [Google Scholar]
- Gachon, F. , Olela, F. F. , Schaad, O. , Descombes, P. , & Schibler, U. (2006). The circadian PAR‐domain basic leucine zipper transcription factors DBP, TEF, and HLF modulate basal and inducible xenobiotic detoxification. Cell Metabolism, 4, 25–36. 10.1016/j.cmet.2006.04.015 [DOI] [PubMed] [Google Scholar]
- Gaspar, L. , Álvaro, A. R. , Carmo‐Silva, S. , Mendes, A. F. , Relógio, A. , & Cavadas, C. (2019). The importance of determining circadian parameters in pharmacological studies. British Journal of Pharmacology, 176, 2827–2847. 10.1111/bph.14712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goo, R. H. , Moore, J. G. , Greenberg, E. , & Alazraki, N. P. (1987). Circadian variation in gastric emptying of meals in humans. Gastroenterology, 93, 515–518. 10.1016/0016-5085(87)90913-9 [DOI] [PubMed] [Google Scholar]
- Guo, Y. , Shen, O. , Han, J. , Duan, H. , Yang, S. , Zhu, Z. , … Zhang, J. (2017). Circadian rhythm genes mediate fenvalerate‐induced inhibition of testosterone synthesis in mouse Leydig cells. Journal of Toxicology and Environmental Health, Part A Current Issues, 80, 1314–1320. 10.1080/15287394.2017.1384148 [DOI] [PubMed] [Google Scholar]
- Hadi, M. A. , Raghavendra Rao, N. G. , & Srinivasa Rao, A. (2016). Formulation and evaluation of ileo‐colonic targeted matrix‐mini‐tablets of naproxen for chronotherapeutic treatment of rheumatoid arthritis. Saudi Pharmaceutical Journal, 24, 64–73. 10.1016/j.jsps.2015.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamdan, A. M. , Koyanagi, S. , Wada, E. , Kusunose, N. , Murakami, Y. , Matsunaga, N. , & Ohdo, S. (2012). Intestinal expression of mouse Abcg2/breast cancer resistance protein (BCRP) gene is under control of circadian clock‐activating transcription factor‐4 pathway. The Journal of Biological Chemistry, 287, 17224–17231. 10.1074/jbc.M111.333377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammann, F. , Kummer, O. , Guercioni, S. , Imanidis, G. , & Drewe, J. (2016). Time controlled pulsatile transdermal delivery of nicotine: A phase I feasibility trial in male smokers. Journal of Controlled Release, 232, 248–254. 10.1016/j.jconrel.2016.04.017 [DOI] [PubMed] [Google Scholar]
- Harding, S. D. , Sharman, J. L. , Faccenda, E. , Southan, C. , Pawson, A. J. , Ireland, S. , … NC‐IUPHAR . (2018). The IUPHAR/BPS Guide to PHARMACOLOGY in 2018: Updates and expansion to encompass the new guide to IMMUNOPHARMACOLOGY. Nucleic Acids Research, 46, D1091–D1106. 10.1093/nar/gkx1121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi, Y. , Ushijima, K. , Ando, H. , Yanagihara, H. , Ishikawa, E. , Tsuruoka, S. , … Fujimura, A. (2010). Influence of a time‐restricted feeding schedule on the daily rhythm of abcb1a gene expression and its function in rat intestine. The Journal of Pharmacology and Experimental Therapeutics, 335, 418–423. 10.1124/jpet.110.170837 [DOI] [PubMed] [Google Scholar]
- Hodkinson, D. J. , O'Daly, O. , Zunszain, P. A. , Pariante, C. M. , Lazurenko, V. , Zelaya, F. O. , … Williams, S. C. (2014). Circadian and homeostatic modulation of functional connectivity and regional cerebral blood flow in humans under normal entrained conditions. Journal of Cerebral Blood Flow and Metabolism, 34, 1493–1499. 10.1038/jcbfm.2014.109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwasaki, M. , Koyanagi, S. , Suzuki, N. , Katamune, C. , Matsunaga, N. , Watanabe, N. , … Ohdo, S. (2015). Circadian modulation in the intestinal absorption of P‐glycoprotein substrates in monkeys. Molecular Pharmacology, 88, 29–37. 10.1124/mol.114.096735 [DOI] [PubMed] [Google Scholar]
- Jagdale, S. C. , & Pawar, C. R. (2014). Application of design of experiment for polyox and xanthan gum coated floating pulsatile delivery of sumatriptan succinate in migraine treatment. BioMed Research International, 2014, 1–10. 10.1155/2014/547212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson, B. P. , Walisser, J. A. , Liu, Y. , Shen, A. L. , McDearmon, E. L. , Moran, S. M. , … Bradfield, C. A. (2014). Hepatocyte circadian clock controls acetaminophen bioactivation through NADPH–cytochrome P450 oxidoreductase. Proceedings of the National Academy of Sciences, 111, 18757–18762. 10.1073/pnas.1421708111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jubiz, W. , Canterbury, J. M. , Reiss, E. , & Tyler, F. H. (1972). Circadian rhythm in serum parathyroid hormone concentration in human subjects: Correlation with serum calcium, phosphate, albumin, and growth hormone levels. The Journal of Clinical Investigation, 51, 2040–2046. 10.1172/JCI107010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawase, A. , Yamamoto, T. , Egashira, S. , & Iwaki, M. (2016). Stereoselective inhibition of methotrexate excretion by glucuronides of non‐steroidal anti‐inflammatory drugs via multidrug resistance proteins 2 and 4. The Journal of Pharmacology and Experimental Therapeutics, 356, 366–374. 10.1124/jpet.115.229104 [DOI] [PubMed] [Google Scholar]
- Kervezee, L. , Hartman, R. , van den Berg, D. J. , Meijer, J. H. , & de Lange, E. C. M. (2017). Diurnal variation in the pharmacokinetics and brain distribution of morphine and its major metabolite. European Journal of Pharmaceutical Sciences, 109, S132–S139. 10.1016/j.ejps.2017.05.048 [DOI] [PubMed] [Google Scholar]
- Kervezee, L. , Hartman, R. , van den Berg, D.‐J. , Shimizu, S. , Emoto‐Yamamoto, Y. , Meijer, J. H. , & de Lange, E. C. M. (2014). Diurnal variation in P‐glycoprotein‐mediated transport and cerebrospinal fluid turnover in the brain. The AAPS Journal, 16, 1029–1037. 10.1208/s12248-014-9625-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kervezee, L. , Stevens, J. , Birkhoff, W. , Kamerling, I. M. C. , De Boer, T. , Dröge, M. , … Burggraaf, J. (2016). Identifying 24 h variation in the pharmacokinetics of levofloxacin: A population pharmacokinetic approach. British Journal of Clinical Pharmacology, 81, 256–268. 10.1111/bcp.12783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khedhaier, W. B. C. , Dridi, I. , Aouam, K. , Ben‐Attia, M. , Reinberg, A. , & Boughattas, N. A. (2017). Circadian variation in anticonvulsant activity of valproic acid in mice. Biomedicine & Pharmacotherapy, 95, 25–30. 10.1016/j.biopha.2017.08.047 [DOI] [PubMed] [Google Scholar]
- Khemawoot, P. , Nishino, K. , Ishizaki, J. , Yokogawa, K. , & Miyamoto, K. (2007). Circadian rhythm of cytochrome P4502E1 and its effect on disposition kinetics of chlorzoxazone in rats. European Journal of Pharmacology, 574, 71–76. 10.1016/j.ejphar.2007.06.032 [DOI] [PubMed] [Google Scholar]
- Khodadoustan, S. , Nasri Ashrafi, I. , Vanaja Satheesh, K. , Kumar, C. , Shekar, H. S. , & Chikkalingaiah, S. (2017). Evaluation of the effect of time dependent dosing on pharmacokinetic and pharmacodynamics of amlodipine in normotensive and hypertensive human subjects. Clinical and Experimental Hypertension, 39, 520–526. 10.1080/10641963.2017.1281947 [DOI] [PubMed] [Google Scholar]
- Kim, H. K. , Kim, H. J. , Kim, J. H. , Kim, T. H. , & Lee, S. H. (2018). Asymmetric expression level of clock genes in left vs. right nasal mucosa in humans with and without allergies and in rats: Circadian characteristics and possible contribution to nasal cycle. PLoS ONE, 13, 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King, D. P. , Zhao, Y. , Sangoram, A. M. , Wilsbacher, L. D. , Tanaka, M. , Antoch, M. P. , … Takahashi, J. S. (1997). Positional cloning of the mouse circadian clock gene. Cell, 89, 641–653. 10.1016/S0092-8674(00)80245-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klingelhófer, J. (2012). Cerebral blood flow velocity in sleep. Perspectives in Medicine, 1, 275–284. 10.1016/j.permed.2012.02.021 [DOI] [Google Scholar]
- Kobuchi, S. , Ito, Y. , Takamatsu, D. , & Sakaeda, T. (2018). Circadian variations in the pharmacokinetics of the oral anticancer agent tegafur–uracil (UFT) and its metabolites in rats. European Journal of Pharmaceutical Sciences, 123, 452–458. 10.1016/j.ejps.2018.08.004 [DOI] [PubMed] [Google Scholar]
- Kobuchi, S. , Yazaki, Y. , Ito, Y. , & Sakaeda, T. (2018). Circadian variations in the pharmacokinetics of capecitabine and its metabolites in rats. European Journal of Pharmaceutical Sciences, 112, 152–158. 10.1016/j.ejps.2017.11.021 [DOI] [PubMed] [Google Scholar]
- Kolawole, J. A. , Chuhwak, P. D. , & Okeniyi, S. O. (2002). Chronopharmacokinetics of acetaminophen in healthy human volunteers. European Journal of Drug Metabolism and Pharmacokinetics, 27, 199–202. 10.1007/BF03190458 [DOI] [PubMed] [Google Scholar]
- Koopman, M. G. , Koomen, G. C. , Krediet, R. T. , de Moor, E. A. , Hoek, F. J. , & Arisz, L. (1989). Circadian rhythm of glomerular filtration rate in normal individuals. Clinical Science (London, England), 77, 105–111. 10.1042/cs0770105 [DOI] [PubMed] [Google Scholar]
- Koren, G. , Ferrazzini, G. , Sohl, H. , Robieux, I. , Johnson, D. , & Giesbrecht, E. (1992). Chronopharmacology of methotrexate pharmacokinetics in childhood leukemia. Chronobiology International, 9, 434–438. 10.3109/07420529209064555 [DOI] [PubMed] [Google Scholar]
- Lee, V. H. L. , Yamahara, H. , Gurny, R. , Sintzel, M. B. , Martenet, M. , Gex‐Fabry, M. , … Podder, D. S. K. (1996). Basis for dosing time‐dependent changes in the ocular and systemic absorption of topically applied timolol. Journal of Ocular Pharmacology and Therapeutics, 12, 103–113. 10.1089/jop.1996.12.103 [DOI] [PubMed] [Google Scholar]
- Leier, I. , Hummel‐Eisenbeiss, J. , Cui, Y. , & Keppler, D. (2000). ATP‐dependent para‐aminohippurate transport by apical multidrug resistance protein MRP2. Kidney International, 57, 1636–1642. 10.1046/j.1523-1755.2000.00007.x [DOI] [PubMed] [Google Scholar]
- Lemmer, B. (1992). Cardiovascular chronobiology and chronopharmacology In Biologic rhythms in clinical and laboratory medicine (pp. 418–427). Heidelberg, Germany: Springer‐Verlag. [Google Scholar]
- Lemmer, B. , & Bathe, K. (1982). Stereospecific and circadian‐phase‐dependent kinetic behavior of d,l‐, l‐, and d‐propranolol in plasma, heart, and brain of light–dark‐synchronized rats. Journal of Cardiovascular Pharmacology, 4, 635–644. 10.1097/00005344-198207000-00016 [DOI] [PubMed] [Google Scholar]
- Lemmer, B. , & Holle, L. (1991). Chronopharmacokinetics of imipramine and desipramine in rat forebrain and plasma after single and chronic treatment with imipramine. Chronobiology International, 8, 176–185. 10.3109/07420529109063924 [DOI] [PubMed] [Google Scholar]
- Lemmer, B. , & Nold, G. (1991). Circadian changes in estimated hepatic blood flow in healthy subjects. British Journal of Clinical Pharmacology, 32, 627–629. 10.1111/j.1365-2125.1991.tb03964.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levi, F. , & Schibler, U. (2007). Circadian rhythms: Mechanisms and therapeutic implications. Annual Review of Pharmacology and Toxicology, 47, 593–628. 10.1146/annurev.pharmtox.47.120505.105208 [DOI] [PubMed] [Google Scholar]
- Liao, M. Z. , Gao, C. , Shireman, L. M. , Phillips, B. , Risler, L. J. , Neradugomma, N. K. , … Mao, Q. (2017). P‐gp/ABCB1 exerts differential impacts on brain and fetal exposure to norbuprenorphine. Pharmacological Research, 119, 61–71. 10.1016/j.phrs.2017.01.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao, M. Z. , Shen, D. D. , & Mao, Q. (2017). P‐gp/ABCB1 exerts differential impacts on brain and fetal exposure to norbuprenorphine—Response to ‘Letter to the Editor’. Pharmacological Research, 121, 253 10.1016/j.phrs.2017.04.007 [DOI] [PubMed] [Google Scholar]
- Liu, J. , Wang, C. Y. , Ji, S. G. , Xu, X. , Wang, P. P. , Zhang, B. , … Li, M. C. (2016). Chronopharmacokinetics of erlotinib and circadian rhythms of related metabolic enzymes in Lewis tumor‐bearing mice. European Journal of Drug Metabolism and Pharmacokinetics, 41, 627–635. 10.1007/s13318-015-0284-3 [DOI] [PubMed] [Google Scholar]
- Lu, Y. F. , Jin, T. , Xu, Y. , Zhang, D. , Wu, Q. , Zhang, Y. K. J. , & Liu, J. (2013). Sex differences in the circadian variation of cytochrome p450 genes and corresponding nuclear receptors in mouse liver. Chronobiology International, 30, 1135–1143. 10.3109/07420528.2013.805762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mark, P. J. , Augustus, S. , Lewis, J. L. , Hewitt, D. P. , & Waddell, B. J. (2009). Changes in the placental glucocorticoid barrier during rat pregnancy: Impact on placental corticosterone levels and regulation by progesterone. Biology of Reproduction, 80, 1209–1215. 10.1095/biolreprod.108.073650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mark, P. J. , Crew, R. C. , Wharfe, M. D. , & Waddell, B. J. (2017). Rhythmic three‐part harmony: The complex interaction of maternal, placental and fetal circadian systems. Journal of Biological Rhythms, 32, 534–549. 10.1177/0748730417728671 [DOI] [PubMed] [Google Scholar]
- Mauvoisin, D. , Wang, J. , Jouffe, C. , Martin, E. , Atger, F. , Waridel, P. , … Naef, F. (2014). Circadian clock‐dependent and ‐independent rhythmic proteomes implement distinct diurnal functions in mouse liver. Proceedings of the National Academy of Sciences, 111, 167–172. 10.1073/pnas.1314066111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min, D. I. , Chen, H.‐Y. , Lee, M.‐K. , Ashton, K. , & Martin, M. F. (1997). Time‐dependent disposition of tacrolimus and its effect on endothelin‐1 in liver allograft recipients. Pharmacotherapy, 17, 457–463. [PubMed] [Google Scholar]
- Mruk, D. D. , Su, L. , & Cheng, C. Y. (2011). Emerging role for drug transporters at the blood–testis barrier. Trends in Pharmacological Sciences, 32, 99–106. 10.1016/j.tips.2010.11.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami, Y. , Higashi, Y. , Matsunaga, N. , Koyanagi, S. , & Ohdo, S. (2008). Circadian clock‐controlled intestinal expression of the multidrug‐resistance gene mdr1a in mice. Gastroenterology, 135, 1636–1644. 10.1053/j.gastro.2008.07.073 [DOI] [PubMed] [Google Scholar]
- Myung, J. , Schmal, C. , Hong, S. , Tsukizawa, Y. , Rose, P. , Zhang, Y. , … Takumi, T. (2018). The choroid plexus is an important circadian clock component. Nature Communications, 9, 1062 10.1038/s41467-018-03507-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano, A. , Tietz, P. S. , & LaRusso, N. F. (1990). Circadian rhythms of biliary protein and lipid excretion in rats. The American Journal of Physiology, 258, G653–G659. 10.1152/ajpgi.1990.258.5.G653 [DOI] [PubMed] [Google Scholar]
- Nakano, S. , Watanabe, H. , Nagai, K. , & Ogawa, N. (1984). Circadian stage‐dependent changes in diazepam kinetics. Clinical Pharmacology and Therapeutics, 36, 271–277. 10.1038/clpt.1984.174 [DOI] [PubMed] [Google Scholar]
- Nakazato, R. , Kawabe, K. , Yamada, D. , Ikeno, S. , Mieda, M. , Shimba, S. , … Takarada, T. (2017). Disruption of Bmal1 impairs blood–brain barrier integrity via pericyte dysfunction. The Journal of Neuroscience, 37, 3639–3616. 10.1523/JNEUROSCI.3639-16.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng, F. S. , Tangredi, M. M. , & Jackson, F. R. (2011). Glial cells physiologically modulate clock neurons and circadian behavior in a calcium‐dependent manner. Current Biology, 21, 625–634. 10.1016/j.cub.2011.03.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson, C. , Stahlberg, F. , Thomsen, C. , Henrikssen, O. , Herning, M. , & Owman, C. (1992). Circadian variation in human cerebrospinal fluid production measured by magnetic resonance imaging. The American Journal of Physiology, 262, 20–24. [DOI] [PubMed] [Google Scholar]
- Oda, M. , Koyanagi, S. , Tsurudome, Y. , Kanemitsu, T. , Matsunaga, N. , & Ohdo, S. (2014). Renal circadian clock regulates the dosing‐time dependency of cisplatin‐induced nephrotoxicity in mice. Molecular Pharmacology, 85, 715–722. 10.1124/mol.113.089805 [DOI] [PubMed] [Google Scholar]
- Oh, J. H. , Lee, J. H. , Han, D. H. , Cho, S. , & Lee, Y. J. (2017). Circadian clock is involved in regulation of hepatobiliary transport mediated by multidrug resistance‐associated protein 2. Journal of Pharmaceutical Sciences, 106, 2491–2498. 10.1016/j.xphs.2017.04.071 [DOI] [PubMed] [Google Scholar]
- Ohdo, S. (2010). Chronotherapeutic strategy: Rhythm monitoring, manipulation and disruption. Advanced Drug Delivery Reviews, 62, 859–875. 10.1016/j.addr.2010.01.006 [DOI] [PubMed] [Google Scholar]
- Ohdo, S. , Grass, G. M. , & Lee, V. H. L. (1991). Improving the ocular to systemic ratio of topical timolol by varying the dosing time. Investigative Ophthalmology and Visual Science, 32, 2790–2798. [PubMed] [Google Scholar]
- Oh‐oka, K. , Kono, H. , Ishimaru, K. , Miyake, K. , Kubota, T. , Ogawa, H. , … Nakao, A. (2014). Expressions of tight junction proteins occludin and claudin‐1 are under the circadian control in the mouse large intestine: Implications in intestinal permeability and susceptibility to colitis. PLoS ONE, 9, 1–10. 10.1371/journal.pone.0098016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamura, A. , Koyanagi, S. , Dilxiat, A. , Kusunose, N. , Chen, J. J. , Matsunaga, N. , … Ohdo, S. (2014). Bile acid‐regulated peroxisome proliferator‐activated receptor‐α (PPARα) activity underlies circadian expression of intestinal peptide absorption transporter PepT1/Slc15a1 . The Journal of Biological Chemistry, 289, 25296–25305. 10.1074/jbc.M114.577023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okyar, A. , Dressler, C. , Hanafy, A. , Baktir, G. , Lemmer, B. , & Spahn‐Langguth, H. (2012). Circadian variations in exsorptive transport: In situ intestinal perfusion data and in vivo relevance. Chronobiology International, 29, 443–453. 10.3109/07420528.2012.668996 [DOI] [PubMed] [Google Scholar]
- Okyar, A. , Kumar, S. A. , Filipski, E. , Piccolo, E. , Ozturk, N. , Xandri‐Monje, H. , … Ballesta, A. (2019). Sex‐, feeding‐, and circadian time‐dependency of P‐glycoprotein expression and activity—Implications for mechanistic pharmacokinetics modeling. Scientific Reports, 9, 10505 10.1038/s41598-019-46977-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okyar, A. , Piccolo, E. , Ahowesso, C. , Filipski, E. , Hossard, V. , Guettier, C. , … Lévi, F. (2011). Strain‐ and sex‐dependent circadian changes in Abcc2 transporter expression: implications for irinotecan chronotolerance in mouse ileum. PLoS ONE, 6, e20393 10.1371/journal.pone.0020393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel, I. H. , Venkataramanan, R. , Levy, R. H. , Viswanathan, C. T. , & Ojemann, L. M. (1982). Diurnal oscillations in plasma protein binding of valproic acid. Epilepsia, 23, 283–290. 10.1111/j.1528-1157.1982.tb06193.x [DOI] [PubMed] [Google Scholar]
- Pelissier‐Alicot, A. L. , Schreiber‐Deturmeny, E. , Simon, N. , Gantenbein, M. , & Bruguerolle, B. (2002). Time‐of‐day dependent pharmacodynamic and pharmacokinetic profiles of caffeine in rats. Naunyn‐Schmiedeberg's Archives of Pharmacology, 365, 318–325. 10.1007/s00210-001-0527-5 [DOI] [PubMed] [Google Scholar]
- Pérez, S. , Murias, L. , Fernández‐Plaza, C. , Díaz, I. , González, C. , Otero, J. , & Díaz, E. (2015). Evidence for clock genes circadian rhythms in human full‐term placenta. Systems Biology in Reproductive Medicine, 61, 360–366. 10.3109/19396368.2015.1069420 [DOI] [PubMed] [Google Scholar]
- Pilorz, V. , Helfrich‐Förster, C. , & Oster, H. (2018). The role of the circadian clock system in physiology. Pflügers Archiv—European Journal of Physiology, 470, 227–239. 10.1007/s00424-017-2103-y [DOI] [PubMed] [Google Scholar]
- Poondru, S. , Devaraj, R. , Boinpally, R. R. , & Yamasani, M. R. (2000). Chronopharmacokinetics of sumatriptan in healthy human subjects. The Journal of Pharmacy and Pharmacology, 52, 1085–1090. 10.1211/0022357001775001 [DOI] [PubMed] [Google Scholar]
- Prayle, A. P. , Jain, K. , Touw, D. J. , Koch, B. C. P. , Knox, A. J. , Watson, A. , & Smyth, A. R. (2016). The pharmacokinetics and toxicity of morning vs. evening tobramycin dosing for pulmonary exacerbations of cystic fibrosis: A randomised comparison. Journal of Cystic Fibrosis, 15, 510–517. 10.1016/j.jcf.2015.07.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy, P. , Zehring, W. A. , Wheeler, D. A. , Pirrotta, V. , Hadfield, C. , Hall, J. C. , & Rosbash, M. (1984). Molecular analysis of the period locus in Drosophila melanogaster and identification of a transcript involved in biological rhythms. Cell, 38, 701–710. 10.1016/0092-8674(84)90265-4 [DOI] [PubMed] [Google Scholar]
- Robles, M. S. , Cox, J. , & Mann, M. (2014). In‐vivo quantitative proteomics reveals a key contribution of post‐transcriptional mechanisms to the circadian regulation of liver metabolism. PLoS Genetics, 10, e1004047 10.1371/journal.pgen.1004047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sallam, H. , El‐Serafi, A. T. , Filipski, E. , Terelius, Y. , Lévi, F. , & Hassan, M. (2015). The effect of circadian rhythm on pharmacokinetics and metabolism of the Cdk inhibitor, roscovitine, in tumor mice model. Chronobiology International, 32, 608–614. 10.3109/07420528.2015.1022782 [DOI] [PubMed] [Google Scholar]
- Satoh, S. , Kagaya, H. , Saito, M. , Inoue, T. , Miura, M. , Inoue, K. , … Habuchi, T. (2008). Lack of tacrolimus circadian pharmacokinetics and CYP3A5 pharmacogenetics in the early and maintenance stages in Japanese renal transplant recipients. British Journal of Clinical Pharmacology, 66, 207–214. 10.1111/j.1365-2125.2008.03188.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh, S. , Tada, H. , Murakami, M. , Tsuchiya, N. , Li, Z. , Numakura, K. , … Habuchi, T. (2006). Circadian pharmacokinetics of mycophenolic acid and implication of genetic polymorphisms for early clinical events in renal transplant recipients. Transplantation, 82, 486–493. 10.1097/01.tp.0000231874.53240.ba [DOI] [PubMed] [Google Scholar]
- Savolainen, H. , Meerlo, P. , Elsinga, P. H. , Windhorst, A. D. , Dierckx, R. A. J. O. , Colabufo, N. A. , … Luurtsema, G. (2016). P‐glycoprotein function in the rodent brain displays a daily rhythm, a quantitative in vivo PET study. The AAPS Journal, 18, 1524–1531. 10.1208/s12248-016-9973-3 [DOI] [PubMed] [Google Scholar]
- Scheving, L. , Pauly, J. , & Tsai, T. (1968). Circadian fluctuation in plasma proteins of the rat. The American Journal of Physiology, 215, 1096–1101. 10.1152/ajplegacy.1968.215.5.1096 [DOI] [PubMed] [Google Scholar]
- Schuetz, E. G. , Schuetz, J. D. , Grogan, W. M. L. , Naray‐Fejes‐Toth, A. , Fejes‐Toth, G. , Raucy, J. , … Watlington, C. O. (1992). Expression of cytochrome P450 3A in amphibian, rat, and human kidney. Archives of Biochemistry and Biophysics, 294, 206–214. 10.1016/0003-9861(92)90159-T [DOI] [PubMed] [Google Scholar]
- Smolensky, M. H. , Hermida, R. C. , & Portaluppi, F. (2017). Circadian mechanisms of 24‐hour blood pressure regulation and patterning. Sleep Medicine Reviews, 33, 4–16. 10.1016/j.smrv.2016.02.003 [DOI] [PubMed] [Google Scholar]
- Souayed, N. , Chennoufi, M. , Boughattas, F. , Haouas, Z. , Maaroufi, K. , Miled, A. , … Boughattas, N. A. (2015). Circadian variation in murine hepatotoxicity to the antituberculosis agent «isoniazide». Chronobiology International, 32, 1201–1210. 10.3109/07420528.2015.1078808 [DOI] [PubMed] [Google Scholar]
- Souayed, N. , Chennoufi, M. , Frej, N. B. , Chaabane, A. , Ben‐Attia, M. , Aouam, K. , … Boughattas, N. A. (2016). Circadian variation of isoniazid pharmacokinetics in mice. Biomedicine & Pharmacotherapy, 84, 1150–1155. 10.1016/j.biopha.2016.10.054 [DOI] [PubMed] [Google Scholar]
- Srinivasu, P. , Rao, B. R. , Rao, Y. M. , & Rambhau, D. (1998). Circadian variations in the pharmacokinetics of pentoxifylline in man. The Journal of Pharmacy and Pharmacology, 50, 71–74. 10.1111/j.2042-7158.1998.tb03307.x [DOI] [PubMed] [Google Scholar]
- Staud, F. , & Ceckova, M. (2015). Regulation of drug transporter expression and function in the placenta. Expert Opinion on Drug Metabolism & Toxicology, 11, 533–555. 10.1517/17425255.2015.1005073 [DOI] [PubMed] [Google Scholar]
- Stearns, A. T. , Balakrishnan, A. , Rhoads, D. B. , Ashley, S. W. , & Tavakkolizadeh, A. (2008). Diurnal rhythmicity in the transcription of jejunal drug transporters. Journal of Pharmacological Sciences, 108, 144–148. 10.1254/jphs.08100SC [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundar, I. K. , Yao, H. , Sellix, M. T. , & Rahman, I. (2015). Circadian molecular clock in lung pathophysiology. American Journal of Physiology. Lung Cellular and Molecular Physiology, 309, 1056–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takiguchi, T. , Tomita, M. , Matsunaga, N. , Nakagawa, H. , Koyanagi, S. , & Ohdo, S. (2007). Molecular basis for rhythmic expression of CYP3A4 in serum‐shocked HepG2 cells. Pharmacogenetics and Genomics, 17, 1047–1056. 10.1097/FPC.0b013e3282f12a61 [DOI] [PubMed] [Google Scholar]
- Tanabe, K. , Kitagawa, E. , Wada, M. , Haraguchi, A. , Orihara, K. , Tahara, Y. , … Shibata, S. (2015). Antigen exposure in the late light period induces severe symptoms of food allergy in an OVA‐allergic mouse model. Scientific Reports, 5, 1–8. 10.1038/srep14424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taoka, T. , Jost, G. , Frenzel, T. , Naganawa, S. , & Pietsch, H. (2018). Impact of the glymphatic system on the kinetic and distribution of gadodiamide in the rat brain: Observations by dynamic MRI and effect of circadian rhythm on tissue gadolinium concentrations. Investigative Radiology, 53, 529–534. 10.1097/RLI.0000000000000473 [DOI] [PubMed] [Google Scholar]
- Tarquini, B. , Cavallini, V. , Cariddi, A. , Checchi, M. , Sorice, V. , & Cecchettin, M. (1988). Prominent orcadian absorption of intranasal salmon calcitonin (SCT) in healthy subjects. Chronobiology International, 5, 149–152. 10.3109/07420528809079555 [DOI] [PubMed] [Google Scholar]
- Tei, H. , Okamura, H. , Shigeyoshi, Y. , Fukuhara, C. , Ozawa, R. , Hirose, M. , & Sakaki, Y. (1997). Circadian oscillation of a mammalian homologue of the Drosophila period gene. Nature, 389, 512–516. 10.1038/39086 [DOI] [PubMed] [Google Scholar]
- Tso, C. F. , Simon, T. , Greenlaw, A. C. , Puri, T. , Mieda, M. , & Herzog, E. D. (2017). Astrocytes regulate daily rhythms in the suprachiasmatic nucleus and behavior. Current Biology, 27, 1055–1061. 10.1016/j.cub.2017.02.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughn, B. , Rotolo, S. , & Roth, H. (2014). Circadian rhythm and sleep influences on digestive physiology and disorders. ChronoPhysiology and Therapy, 4, 67–77. [Google Scholar]
- Vitaterna, M. H. , Takahashi, J. S. , & Turek, F. W. (2001). Overview of circadian rhythms. Alcohol Research and Health, 25, 85–93. [PMC free article] [PubMed] [Google Scholar]
- Wada, E. , Koyanagi, S. , Kusunose, N. , Akamine, T. , Masui, H. , Hashimoto, H. , … Ohdo, S. (2015). Modulation of peroxisome proliferator‐activated receptor‐α activity by bile acids causes circadian changes in the intestinal expression of Octn1/Slc22a4 in mice. Molecular Pharmacology, 87, 314–322. 10.1124/mol.114.094979 [DOI] [PubMed] [Google Scholar]
- Waddell, B. J. , Wharfe, M. D. , Crew, R. C. , & Mark, P. J. (2012). A rhythmic placenta? Circadian variation, clock genes and placental function. Placenta, 33, 533–539. 10.1016/j.placenta.2012.03.008 [DOI] [PubMed] [Google Scholar]
- Wauschkuhn, C. A. , Witte, K. , Gorbey, S. , Lemmer, B. , & Schilling, L. (2005). Circadian periodicity of cerebral blood flow revealed by laser‐Doppler flowmetry in awake rats: Relation to blood pressure and activity. American Journal of Physiology‐Heart and Circulatory Physiology, 289, H1662–H1668. 10.1152/ajpheart.01242.2004 [DOI] [PubMed] [Google Scholar]
- Wharfe, M. D. , Mark, P. J. , & Waddell, B. J. (2011). Circadian variation in placental and hepatic clock genes in rat pregnancy. Endocrinology, 152, 3552–3560. 10.1210/en.2011-0081 [DOI] [PubMed] [Google Scholar]
- Wharfe, M. D. , Mark, P. J. , Wyrwoll, C. S. , Smith, J. T. , Yap, C. , Clarke, M. W. , & Waddell, B. J. (2016). Pregnancy‐induced adaptations of the central circadian clock and maternal glucocorticoids. The Journal of Endocrinology, 228, 135–147. 10.1530/JOE-15-0405 [DOI] [PubMed] [Google Scholar]
- Wiechmann, A. F. , Ceresa, B. P. , & Howard, E. W. (2014). Diurnal variation of tight junction integrity associates inversely with matrix metalloproteinase expression in Xenopus laevis corneal epithelium: Implications for circadian regulation of homeostatic surface cell desquamation. PLoS ONE, 9, e113810 10.1371/journal.pone.0113810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie, L. , Kang, H. , Xu, Q. , Chen, M. J. , Liao, Y. , Thiyagarajan, M. , … Nedergaard, M. (2013). Sleep drives metabolite clearance from adult brain. Science (80‐.), 342, 373–377. 10.1126/science.1241224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yosipovitch, G. , Sackett‐Lundeen, L. , Goon, A. , Huak, C. Y. , Goh, C. L. , & Haus, E. (2004). Circadian and ultradian (12 h) variations of skin blood flow and barrier function in non‐irritated and irritated skin—Effect of topical corticosteroids. The Journal of Investigative Dermatology, 122, 824–829. 10.1111/j.0022-202X.2004.22313.x [DOI] [PubMed] [Google Scholar]
- Yu, F. , Zhang, T. , Zhou, C. , Xu, H. , Guo, L. , Chen, M. , & Wu, B. (2019). The circadian clock gene Bmal1 controls intestinal exporter MRP2 and drug disposition. Theranostics, 9, 2754–2767. 10.7150/thno.33395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu, Z. , Li, X. , Feng, C. , Lei, K. , He, W. , Zhang, C. , & Liu, D. (2019). Circadian variations in the pharmacokinetics of bucinnazine in rats. Biological Rhythm Research, n/a, 1–12. 10.1080/09291016.2018.1564576 [DOI] [Google Scholar]
- Zhang, C. , Yu, Z. , Li, X. , Xu, Y. , & Liu, D. (2014). Chronopharmacodynamics and chronopharmacokinetics of pethidine in mice. PLoS ONE, 9, 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, F. , Duan, Y. , Xi, L. , Wei, M. , Shi, A. , Zhou, Y. , … Wu, X. (2018). The influences of cholecystectomy on the circadian rhythms of bile acids as well as the enterohepatic transporters and enzymes systems in mice. Chronobiology International, 35, 673–690. 10.1080/07420528.2018.1426596 [DOI] [PubMed] [Google Scholar]
- Zhang, S. , Yan, H. , Yu, P. , Xia, Y. , Zhang, W. , & Liu, J. (2016). Development of protocatechualdehyde proliposomes‐based sustained‐release pellets with improved bioavailability and desired pharmacokinetic behavior for angina chronotherapy. European Journal of Pharmaceutical Sciences, 93, 341–350. 10.1016/j.ejps.2016.08.040 [DOI] [PubMed] [Google Scholar]
- Zhang, S. L. , Yue, Z. , Arnold, D. M. , Artiushin, G. , & Sehgal, A. (2018). A circadian clock in the blood–brain barrier regulates xenobiotic efflux. Cell, 173, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, Y. K. J. , Yeager, R. L. , & Klaassen, C. D. (2009). Circadian expression profiles of drug‐processing genes and transcription factors in mouse liver. Drug Metabolism and Disposition, 37, 106–115. 10.1124/dmd.108.024174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng, S. , Tasnif, Y. , Hebert, M. F. , Davis, C. L. , Shitara, Y. , Calamia, J. C. , … Thummel, K. E. (2014). CYP3A5 gene variation influences both systemic and intrarenal tacrolimus disposition. Clinical Pharmacology and Therapeutics, 92, 737–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, C. , Yu, F. , Zeng, P. , Zhang, T. , Huang, H. , Chen, W. , & Wu, B. (2019). Circadian sensitivity to the cardiac glycoside oleandrin is associated with diurnal intestinal P‐glycoprotein expression. Biochemical Pharmacology, 169, 113622 10.1016/j.bcp.2019.08.024 [DOI] [PubMed] [Google Scholar]
- Zuber, A. M. , Centeno, G. , Pradervand, S. , Nikolaeva, S. , Maquelin, L. , Cardinaux, L. , … Firsov, D. (2009). Molecular clock is involved in predictive circadian adjustment of renal function. Proceedings of the National Academy of Sciences, 106, 16523–16528. 10.1073/pnas.0904890106 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Recent advances (2014 ‐ 2019) of chronomodulated drug delivery systems


