Abstract
Background and Purpose
Intestinal mucositis refers to mucosal damage caused by cancer treatment, and irinotecan is one of the agents most associated with this condition. Focusing on the development of alternatives to prevent this important adverse effect, we evaluated the activity of the flavonoid luteolin, which has never been tested for this purpose despite its biological potential.
Experimental Approach
The effects of luteolin were examined on irinotecan‐induced intestinal mucositis in mice. Clinical signs were evaluated. Moreover, histological, oxidative, and inflammatory parameters were analysed, as well as the possible interference of luteolin in the anti‐tumour activity of irinotecan.
Key Results
Luteolin (30 mg·kg−1; p.o. or i.p.) prevented irinotecan‐induced intestinal damage by reducing weight loss and diarrhoea score and attenuating the shortening of the duodenum and colon. Histological analysis confirmed that luteolin (p.o.) prevented villous shortening, vacuolization, and apoptosis of cells and preserved mucin production in the duodenum and colon. Moreover, luteolin treatment mitigated irinotecan‐induced oxidative stress, by reducing the levels of ROS and LOOH and augmenting endogenous antioxidants, and inflammation by decreasing MPO enzymic activity, TNF, IL‐1β, and IL‐6 levels and increasing IL‐4 and IL‐10. Disruption of the tight junctions ZO‐1 and occludin was also prevented by luteolin treatment. Importantly, luteolin did not interfere with the anti‐tumour activity of irinotecan.
Conclusion and Implications
Luteolin prevents intestinal mucositis induced by irinotecan and therefore could be a potential adjunct in anti‐tumour therapy to control this adverse effect, increasing treatment adherence and consequently the chances of cancer remission.
Abbreviations
- CPT‐11
irinotecan
- DCFH‐DA
dichlorodihydrofluorescein diacetate
- DPPH
2,2‐diphenyl‐1‐picrylhydrazyl
- DSS
sodium dextran sulfate
- HE
haematoxylin and eosin
- LOOH
lipid hydroperoxides
- MPO
myeloperoxidase
- MTT
3‐(4,5‐dimethylthiazol‐2‐yl)‐2,5‐diphenyltetrazolium bromide
- PAS
periodic acid‐Schiff
- ZO‐1
zonula occludens
What is already known
The drugs currently used to treat intestinal mucositis have low clinical efficacy.
Luteolin has already been shown to be effective in experimental models of ulcerative colitis.
What this study adds
Luteolin attenuated intestinal mucositis, decreased inflammation and oxidative stress and increased protective factors in mucosa.
At the same time, luteolin did not affect the anti‐tumour activity of irinotecan.
What is the clinical significance
By preventing intestinal mucositis, luteolin can increase the chances of cancer remission.
Luteolin could be a new therapeutic or adjunctive agent in the management of intestinal mucositis.
1. INTRODUCTION
Intestinal mucositis is a toxic manifestation resulting from many anti‐cancer treatments, including radiation or chemotherapy, and is characterized by extensive damage to the gut mucosa, leading to symptoms such as diarrhoea, bleeding, nausea, vomiting, abdominal pain, malnutrition, infections, and sepsis related to bacterial translocation (Vanhoecke et al., 2015). Reaching high incidence, 40% of patients receiving standard doses, and 100% of patients receiving high doses (Cinausero et al., 2017), the main consequence of this condition is failure of the chemotherapy. When this adverse effect occurs, it is generally necessary to decrease the dose or to interrupt the chemotherapeutic regime, decreasing the chances of cancer remission and increasing health care costs and death rates, either by cancer or by the complications resulting from intestinal damage (Ribeiro et al., 2016).
https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=6823 (CPT‐11) is a semisynthetic analogue of the natural alkaloid camptothecin and is one of the main agents used in the treatment of colorectal cancer. Although the combinations containing irinotecan significantly increase the survival rates of patients, it is, unfortunately, one of the main agents that cause intestinal mucositis (Fujita, Kubota, Ishida, & Sasaki, 2015).
Particularly with this drug, acute diarrhoea may occur due to activation of the parasympathetic pathway, which is clinically controlled by https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=320 (Andreyev et al., 2014). On the other hand, late diarrhoea is associated with intestinal mucositis, but its pathological mechanisms remain controversial because clinical management has had limited success (Ribeiro et al., 2016). As far as we know, irinotecan‐induced DNA strand breaks result in direct cellular injury that targets cells in the basal epithelium, as well as those within the submucosa, where it activates several apoptosis‐related pathways (Bowen et al., 2006). In addition, inflammation of the mucosal membranes and production of ROS and reactive nitrogen species, which induce cell death, seem to be involved (Arifa et al., 2014; Ribeiro et al., 2016; Sonis, 2004). Indeed, oral rehydration, electrolyte replacement, and pharmacological agents to reduce fluid loss or decrease intestinal motility, such as opioid agonists, are the few treatments employed in clinical practice (Andreyev et al., 2014).
The importance of seeking new therapeutic strategies for treatment of, or even more efficiently to prevent, the development of intestinal mucositis induced by irinotecan is evident, as the chemotherapy is given in fixed cycles of treatment, generally as 350 mg·m−2 every 3 weeks or 125 mg·m−2·per week (Stein, Voigt, & Jordan, 2010). Thus, in this situation, we have focused on the secondary bioactive metabolites obtained from natural products, of which polyphenols are the most likely candidates, as they have been extensively studied for the prevention and treatment of several oxidative stress and inflammation‐related conditions (Nunes, Almeida, Barbosa, & Laranjinha, 2017).
https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=5215 (3′,4′,5,7‐tetrahydroxyflavone) is a flavonoid of the flavone subclass found in different medicinal plants, vegetables, and fruits (Ross & Kasum, 2002), and several reports have highlighted its biological properties, such as antioxidant and cytoprotective (Seelinger, Merfort, & Schempp, 2008), anti‐proliferative (Pandurangan & Esa, 2014), anti‐diabetic (Salib, Michael, & Eskande, 2013), diuretic (Boeing, da Silva, Mariott, Andrade, & de Souza, 2017), antihypertensive (Lv et al., 2013), and neuroprotective (Nabavi et al., 2015). In addition, the intestinal anti‐inflammatory activity of luteolin in vivo on dextran sulfate sodium (DSS)‐induced colitis (Li, Shen, & Luo, 2016; Nishitani et al., 2013) and in vitro on intestinal epithelial cells stimulated with cytokines (Nunes et al., 2017) or LPS (Kim & Jobin, 2005) have also been demonstrated. Therefore, considering the beneficial properties of luteolin, the lack of effective treatments for intestinal mucositis, and the abundance of this compound in nature, we have studied, here, the effect of luteolin on irinotecan‐induced intestinal mucositis, seeking to characterize a promising natural compound as a useful approach to the prevention of this severe adverse effect of chemotherapy.
2. METHODS
2.1. Animals
All animal care and experimental procedures complied with international standards, following all the ethical guidelines on animal welfare and were approved by the institutional animal ethics committee of UNIVALI (number 021/16p). Animal studies are reported in compliance with the ARRIVE guidelines (Kilkenny, Browne, Cuthill, Emerson, & Altman, 2010) and with the recommendations made by the British Journal of Pharmacology. Female Swiss mice (25‐30 g) were provided by the animal facility of the Universidade do Vale do Itajaí (UNIVALI). The mice were housed under standard laboratory conditions (12‐hr light/dark cycle, temperature of 22 ± 2°C). The mice were randomly assigned according to their body weights, and all experiments were carried out in a blinded manner, compliant with Curtis et al. (2018).
2.2. Experimental irinotecan‐induced intestinal mucositis
The mice were divided into five groups, including naive, vehicle (Veh: water plus 1% Tween, 10 ml·kg−1), and luteolin (Lut 3, 10 and 30 mg·kg−1), of 10 animals each. The size of the experimental group follows the recommendations of previously published studies to ensure the reproducibility of the data in this experimental model (see Arifa et al., 2014, 2016). The mice were treated by gavage for 14 days, except for the naive group which did not receive any treatment.
From the 7th to 10th day, irinotecan was given i.p. at a dose of 75 mg·kg−1·day−1 to the Veh and Lut groups to induce intestinal mucositis (Ikuno, Soda, Watanabe, & Oka, 1995). The body weight of the animals was evaluated daily, and the diarrhoea score was based on the criteria described by Arifa et al. (2016) as normal (0), mild perianal soiling (1), moderate perianal soiling (2), severe perianal soiling (3), severe perianal soiling with presence of blood, and signs of morbidity (4).
On the 15th day of the experiment, the mice were anaesthetized with https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=4233 (80 mg·kg−1) and https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=523 (10 mg·kg−1), and the blood was collected from the superior vein cava. The total leukocyte number was counted (with Neubauer chambers), and biochemical analysis was performed. The animals were killed, and the small intestine and colon were excised. The weight/length ratio of tissue was measured, and samples of the duodenum and colon were collected for the analysis of histological, oxidative, and inflammatory parameters. In addition, one part of each tissue was dried for 24 hr at 60°C to measure tissue water content. The relative weight of the heart, lung, spleen, liver, right kidney, and left kidney were determined as g per 10 g of body weight. In a different set of experiments, vascular permeability or leukocytes recruitment assay was performed.
To evaluate the possible systemic effects of luteolin, Swiss mice were divided into the same five groups described above, including Naive, Vehicle (Veh: PBS pH 7.3, 10 ml·kg−1) and luteolin (Lut 3, 10 and 30 mg·kg−1), that were treated i.p. for 14 days, except for naive which did not receive any treatment. Irinotecan was given once a day from the 7th to the 10th day, and the body weight, diarrhoea score, and weight/length ratio of intestine were measured as previously described.
2.3. Evaluation of vascular permeability
Vascular permeability was assessed by measuring extravasation of https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=4579 (Saria & Lundberg, 1983) after performing the entire experimental protocol of irinotecan‐induced intestinal mucositis described above. Evans blue dye (20 mg·kg−1) was injected via the retro‐orbital plexus into the naive‐, vehicle‐, and luteolin‐treated (30 mg·kg−1, p.o.) groups, 20 min before killing. Segments of the duodenum and colon were cut into small pieces and mixed with formamide (0.3 ml·cm−1), incubated for 4 hr at 37°C, and then the absorbance of the formamide was read at 620 nm. The experimental values were interpolated on a standard curve of Evans blue (1–100 μg·ml−1). The results were expressed as μg Evans blue per 100 mg of tissue.
2.4. Assay of leukocyte recruitment
The leukocyte recruitment assay was performed on the last day of the irinotecan‐induced intestinal mucositis protocol described above. Briefly, mice were injected i.p. with 1 ml of 7.5% casein sodium solution. After 4 hr, the mice were anaesthetized with ketamine (80 mg·kg−1) and xylazine (10 mg·kg−1). An abdominal incision was made, and 2 ml of PBS were injected. The peritoneal exudate was then collected, and the number of cells was determined in the Neubauer chamber. The duodenum and colon of the same mice were excised to determine myeloperoxidase (MPO) activity.
2.5. Histological analysis
Tissue samples of the duodenum and colon were fixed in Alfac (85% ethanol 80%; 10% formaldehyde and 5% acetic acid) for 24 hr. They were subsequently dehydrated with alcohol and xylene, embedded in paraffin wax, sectioned at 5 μm and stained with haematoxylin/eosin (HE), or for histochemical analysis of mucin content and goblet cells number with periodic acid‐Schiff (PAS) and Alcian blue, respectively.
The morphometric analyses of the intestine, as well as the histological score, were performed as described by Soares et al. (2008). Briefly, microscopic slides were reviewed by a histologist blinded to the experimental groups and damage extent. Histological score was considered as (0) normal histological findings, (1) villus blunting, loss of crypt architecture, sparse inflammatory cell infiltration, vacuolization, and oedema in the mucosa and normal muscular layer, (2) villus blunting with fattened and vacuolated cells, crypt necrosis, intense inflammatory cell infiltration, vacuolization, and oedema in the mucosa and normal muscular layer, (3) villus blunting with fattened and vacuolated cells, crypt necrosis, intense inflammatory cell infiltration, vacuolization, and oedema in the mucosa, and oedema, vacuolization, sparse neutrophil infiltration in the muscular layer.
Villus height measurements (from villus tip to villus–crypt junction) and crypt depths (defined as invagination depth between adjacent villi) were measured using https://scicrunch.org/resources/Any/search?q=SCR_003070&l=SCR_003070) program. Ten intact and well‐oriented villi and crypts were measured per animal, from 5 animals).
Mucin‐like glycoproteins were quantified using ImageJ® program and expressed as pixels per field. Goblet cells were counted in crypts and villi under 40× magnification. For both assays, six fields per animal were analysed from 5 animals (n = 5).
2.6. Immunofluorescence
The immuno‐related procedures used comply with the recommendations made by the British Journal of Pharmacology (Alexander et al., 2018). Tissue sections were deparaffinized, rehydrated, and permeabilized in 0.1% Triton X‐100 PBS for 10 min at room temperature. Then, the slides were incubated with blocking buffer (5% BSA in 0.05% Triton X‐100 PBS) for 30 min. After that, primary antibodies ZO‐1 (dilution 1:200 in 1% BSA, 0.05% Triton X‐100 PBS) or occludin (dilution 1:200 in 1% BSA, 0.05% Triton X‐100 PBS) were incubated overnight at 4°C. Finally, incubation with secondary antibody (goat anti‐rabbit Alexa 488; dilution 1:400 in 0.05% Triton X‐100 PBS) was performed for 1 hr at room temperature, and nuclei were stained with https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=5498 solution. Samples were visualized under a fluorescence microscope (Leica, Bensheim, Germany). Fluorescence was quantified with ImageJ® program. Six fields per animal were analysed (n = 5).
2.7. Determination of oxidative and inflammatory parameters
Samples of the duodenum and colon were collected and immediately homogenized with 200‐mM potassium phosphate buffer (pH 6.5) to determine the levels of reduced https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=6737 and lipid hydroperoxides (LOOH). The remaining homogenate was centrifuged at 7,000× g for 20 min at 4°C, and the supernatant was frozen at −80°C for further determination of SOD and https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=2979 (CAT) activity. Protein concentrations were determined by the Bradford method (Bio‐Rad, Hercules, CA, USA). For a detailed protocol of these measurements, see Boeing et al. (2016).
https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=2789 (MPO) activity and the levels of ROS, cytokines, and nitrite were measured in the supernatant of the duodenum and colon samples that were homogenized with 80‐mM potassium phosphate buffer (pH 5.4) containing 0.5% hexadecyltrimethylammonium and centrifuged at 7,000× g for 20 min at 4°C.
Briefly, the MPO activity was determined at 620 nm in the presence of H2O2 and 3,3′,3,5′‐tetramethylbenzidine (Bradley, Priebat, Christensen, & Rothstein, 1982). The results were expressed as relative units that denote the activity of MPO relative to casein‐elicited murine peritoneal leukocytes determined as described before.
For ROS determination, 100 μl of supernatant were incubated with 30 μl of 2′,7′‐dichlorodihydrofluorescein diacetate (10‐μM DCFH‐DA) for 40 min in the dark. Fluorescence was measured using a spectrofluorometer microplate reader with excitation wavelength at 480 nm and emission wavelength at 530 nm.
Nitrite levels were quantified through Griess reaction following the protocol previously described by de Almeida et al. (2017). The amounts of https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=5074, https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=4974, https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=4998, https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=4996, https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=4975, and https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=1883 were measured by ELISA using mouse cytokine ELISA kits from BD Biosciences (Franklin Lakes, New Jersey, USA) or RD systems (Minneapolis, MN, USA), according to the manufacturer's instructions.
2.8. Data and statistical analysis
The data and statistical analysis comply with the recommendations of the British Journal of Pharmacology on experimental design and analysis in pharmacology (Curtis et al., 2018). The data were analysed by an investigator blinded to the experimental conditions. Each in vitro experiment was conducted independently at least three times, and more than five animals per group were needed for in vivo experiment. The Kolmogorov–Smirnov normality test was applied to verify the data normality. The data were expressed as mean ± SEM, and one‐ or two‐way ANOVA followed by Bonferroni's post hoc test was applied to verify the differences between means. The non‐parametric data were expressed as the median with interquartile range and analysed by Kruskal–Wallis followed by Dunn's test, while Mann–Whitney test was used to compare differences between two groups. Statistical analysis was performed using the software https://scicrunch.org/resources/Any/search?q=SCR_002798&l=SCR_002798) version 7.00 (GraphPad Software, La Jolla, CA, USA). Values of P < .05 were considered to show significant differences between means.
2.9. Materials
Luteolin (≥98% purity, powder) was commercially obtained from Active‐Pharmaceutica (Palhoça, SC, Brazil). Syntec (São Paulo, SP, Brazil) was the supplier of both ketamine and xylazine. All other drugs and reagents were purchased from Sigma‐Aldrich Chemical Co. (St. Louis, MO, USA) and Merck (Darmstadt, Germany). Tween‐20 was purchased from Vetec (Rio de Janeiro, RJ, Brazil). The following antibodies were used: anti‐occludin polyclonal antibody (Abcam, Cat# ab31721, https://antibodyregistry.org/search?q=ab31721), anti‐ZO1 polyclonal antibody (Thermo Fisher Scientific, Cat# 61‐7300, https://antibodyregistry.org/search?q=AB_2533938), and rabbit secondary antibodies (Thermo Fisher Scientific, Cat# A‐31566, https://antibodyregistry.org/search?q=AB_2536179).
Experimental solutions were prepared as follows; luteolin was solubilized in water plus Tween 1%, and irinotecan was dissolved in saline.
2.10. Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Harding et al., 2018), and are permanently archived in the Concise Guide to PHARMACOLOGY 2019/20 (Alexander, Christopoulos et al., 2019; Alexander, Cidlowski et al., 2019; Alexander, Fabbro et al., 2019a, b).
3. RESULTS
3.1. Luteolin does not interfere in the digestive motility rate
In a resting state, luteolin given by gavage at doses of 3, 10, or 30 mg·kg−1 did not inhibit the digestive motility rate. Treatment with atropine (3 mg·kg−1, s.c.), a muscarinic receptor antagonist, was used as the positive control and internal control for this experiment (Figure S1A). In a diarrhoeal state induced by irinotecan, digestive motility rate was significantly lower in the vehicle‐treated mice, than that in the naïve group, while mice treated with luteolin (30 mg·kg−1, p.o.) did not show significant changes in this parameter (Figure S1B).
3.2. Luteolin attenuates clinical parameters and signs of irinotecan‐induced intestinal mucositis
Oral administration of luteolin (30 mg·kg−1) to mice over 7 days, starting at the same day as irinotecan, decreased the diarrhoea score, compared with the vehicle group (Figure S2B). However, luteolin treatment did not reverse the change in the body weight or weight/length ratio of the duodenum and colon, induced by irinotecan (Figure S2A,C,D). On the other hand, when luteolin was given to mice for 14 days, starting 7 days before the first dose of irinotecan, the compound was able to reverse all changes induced by the chemotherapy agent.
Figure 1a shows that 2 days after the first dose of irinotecan, the vehicle‐only‐treated group started to lose weight. At the end of the experimental period, mice of this group lost approximately 29% of weight compared with the naive group, while animals treated with luteolin (30 mg·kg−1 p.o.) lost approximately half that value. In the same way, when compared with vehicle group, the diarrhoea score in the final period of the experiment (i.e., on the 14th day) was decreased by luteolin treatment (Figure 1b). The damage induced by irinotecan was accompanied by decreased weight/length ratio of both the duodenum and the colon, as shown by the difference between vehicle and naive groups. Such decreases were not observed in luteolin‐treated mice (Figure 1c,d).
Figure 1.
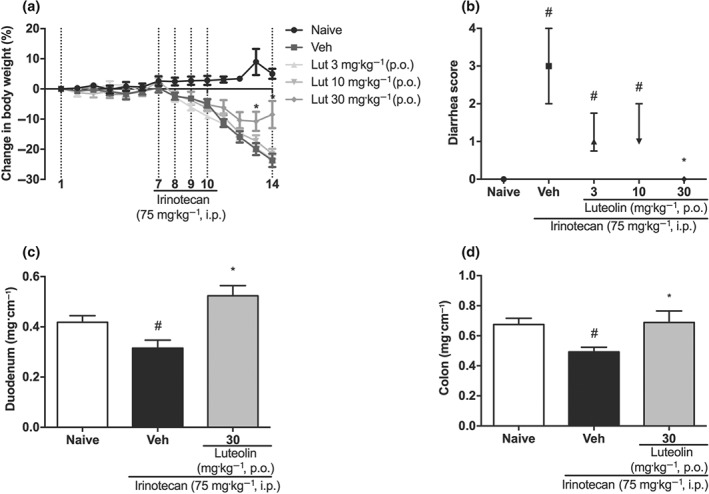
Effect of luteolin, given p.o., on irinotecan‐induced intestinal mucositis. Female mice were treated for 14 days by gavage, with vehicle (Veh: water plus Tween 1%, 10 ml·kg−1) or luteolin (Lut: 3, 10 or 30 mg·kg−1). Irinotecan was given i.p. (75 mg·kg−1, from the 7th to the 10th day) for Veh and Lut‐treated groups. (a) The weight of the animals was measured daily. Weight loss was significantly different from the 9th to the 14th day of experiment in the treated groups compared to naive. The results are expressed as mean ± SEM (n = 10). *P < .05, significantly different from vehicle; two‐way ANOVA followed by Bonferroni's post hoc test. (b) The diarrhoea score was evaluated on the 14th day of treatment. The results are expressed as median with interquartile range (n = 10). *P < .05, significantly different from vehicle; # P < .05, significantly different from the naive group; Kruskal–Wallis followed by Dunn's test. (c and d) Weight/length ratio of the duodenum and colon. The results are expressed as mean ± SEM (n = 10). *P < .05, significantly different from vehicle; # P < .05, significantly different from the naive group; one‐way ANOVA followed by Bonferroni's post hoc test
Complementary to these results with treatment p.o., we also gave the same treatments by i.p. injection. Here, the vehicle‐only‐treated (i.p.) group lost approximately 26% of the body weight compared to the naive group, while luteolin treatment (30 mg·kg−1 i.p.) still reversed the changes of body weight (Figure 2a), diarrhoea score (Figure 2b), and weight/length ratio of the duodenum and colon (Figure 2c,d).
Figure 2.
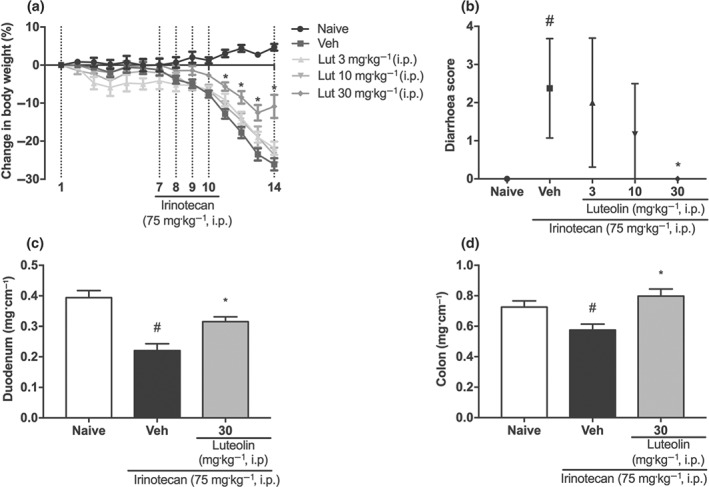
Effect of i.p. luteolin on irinotecan‐induced intestinal mucositis. Female mice were treated for 14 days by i.p. injection of vehicle (Veh: PBS pH 7.0, 10 ml·kg−1) or luteolin (Lut: 3, 10 or 30 mg·kg−1). Irinotecan was given i.p. (75 mg·kg−1, from the 7th to the 10th day) for Veh and Lut‐treated groups. (a) The weight of the animals was measured daily. Weight loss was significantly different from the 9th to the 14th day of experiment in the treated groups compared to naive. The results are expressed as mean ± SEM (n = 10). *P < .05, significantly different from vehicle; two‐way ANOVA followed by Bonferroni's post hoc test. (b) The diarrhoea score was evaluated on the 14th day of treatment. The results are expressed as median with interquartile range (n = 10). *P < .05, significantly different from vehicle. ; # P < .05, significantly different from the naive group; Kruskal–Wallis followed by Dunn's test. (c and d) Weight/length ratio of the duodenum and colon. The results are expressed as mean ± SEM (n = 10). *P < .05, significantly different from vehicle; # P < .05, significantly different from the naive group; one‐way ANOVA followed by Bonferroni's post hoc test
From these data, we standardized the treatment with luteolin for 14 days orally to continue the study. Histological examination of the intestine of irinotecan‐exposed mice clearly showed the extensive damage, in the vehicle‐treated group, caused by irinotecan in both the duodenum and colon. Changes such as villous shortening in the duodenum, disruption of crypts in the colon, apoptosis, vacuolization of cells, oedema, and infiltration of polymorphonuclear cells were the main changes seen, compared with normal tissue. Interestingly, all these abnormalities were less pronounced in mice treated with luteolin (Figure 3a), leading to a diminished total histological score in both parts of the intestine (Figure 3b). Moreover, irinotecan treatment decreased the villus height and increased the depth of crypts, consequently decreasing the villus/crypt ratio of the duodenum, changes which were prevented by luteolin treatment (Figure 3c–e).
Figure 3.
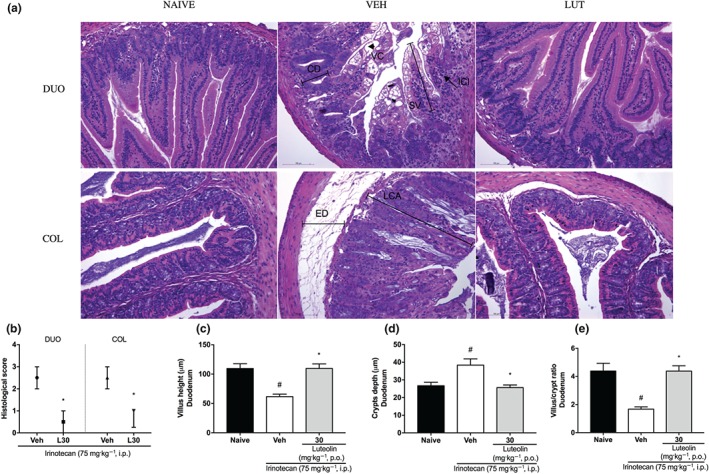
Histological sections of the duodenum and colon of irinotecan‐exposed mice. Female mice were treated for 14 days by oral route with vehicle (VEH: water plus Tween 1%, 10 ml·kg−1) or luteolin (LUT: 30 mg·kg−1). Irinotecan was given by intraperitoneal route (75 mg·kg−1, from the 7th to the 10th day) for Veh‐ and Lut‐treated groups. Histological sections were stained with HE (a). NAIVE showing normal villi, crypts, and muscular layer. VEH DUO showing vacuolated cells (VC), shortened villi (SV), crypt depth (CD), and inflammatory cell infiltration (ICI). VEH COL showing loss of normal crypt architecture (LCA) and oedema (ED). Scale bars 100 μm, magnification 20×. DUO: duodenum, COL: colon. Histological score (b): The results are expressed as median with interquartile range (n = 5). *P < .05, significantly different from vehicle; Kruskal–Wallis followed by Mann–Whitney test. Villus height (c), crypt depth (d), villus/crypt ratio (e): The results are expressed as mean ± SEM (n = 5). *P < .05, significantly different from vehicle; # P < .05, significantly different from the naive group; one‐way ANOVA followed by Bonferroni's post hoc test
Besides, Figure S3 shows that irinotecan induced a secondary https://www.sciencedirect.com/topics/pharmacology-toxicology-and-pharmaceutical-science/anaphylaxis of mice compared to naive group, while luteolin treatment reduced the mechanical hypersensitivity, inhibiting approximately 20% of the response frequency and increasing the von Frey threshold compared to vehicle group.
The extent of oedema in the duodenum and colon was assessed by measuring the tissue water content (as % weight) and the vascular permeability, using Evans blue dye. Consistent with the macroscopic findings, intestinal tissue of mice that received irinotecan plus vehicle had a greater water content than that in the naive group, while intestinal tissue of mice treated with luteolin showed water content, similar to that at baseline (Figure 4a,b). Moreover, as shown in Figure 4c,d, while the vascular permeability in colonic tissue samples was unaltered between groups, this parameter was increased in the duodenum of mice from the irinotecan+vehicle group, but this effect was not present in the duodenal tissue obtained from animals treated with luteolin and then with irinotecan.
Figure 4.
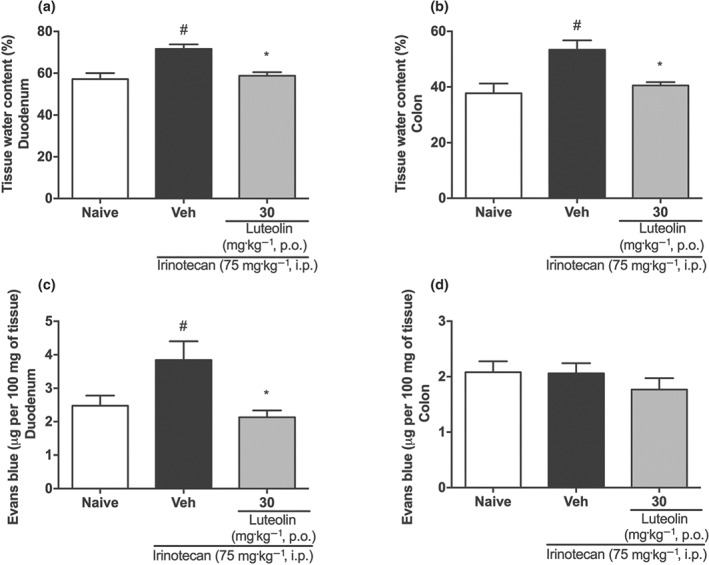
Effect of luteolin in the oedema of intestine from irinotecan‐exposed mice. Female mice were treated for 14 days p.o. with vehicle (Veh: water plus Tween 1%, 10 ml·kg−1) or luteolin (30 mg·kg−1). Irinotecan was given i.p. (75 mg·kg−1, from the 7th to the 10th day) for Veh‐ and Lut‐treated groups. (a and b) Tissue water content. (c and d) Vascular permeability of tissue. The results are expressed as mean ± SEM (n = 10). *P < .05, significantly different from vehicle; # P < .05, significantly different from the naive group; one‐way ANOVA followed by Bonferroni's post hoc test
3.3. Luteolin attenuates oxidative stress and inflammation in irinotecan‐exposed mice
At the end of the experiment, the duodenum and colon of mice were collected and analysed for changes in oxidative and inflammatory parameters, as shown in Tables 1 and 2.
Table 1.
Effect of luteolin on oxidative parameters of irinotecan‐exposed mice
| Tissue | Duodenum | Colon | ||||
|---|---|---|---|---|---|---|
| Groups | Naïve | Vehicle | Luteolin | Naïve | Vehicle | Luteolin |
| ROS (fluorescence U·mg−1 of protein) | 48.8 ± 6.0 | 69.7 ± 5.2* | 46.6 ± 4.9# | 49.6 ± 3.3 | 54.2 ± 3.1 | 55.2 ± 2.5 |
| LOOH (mmol·mg−1 of tissue) | 12.0 ± 0.5 | 17.9 ± 1.9* | 13.6 ± 0.6# | 331.0 ± 10.1 | 311.1 ± 10.2 | 328.3 ± 12.9 |
| GSH (mg·g−1 of tissue) | 1,385.0 ± 151.8 | 450.6 ± 60.1* | 1,319.0 ± 393.8# | 439.7 ± 47.1 | 267.3 ± 25.5* | 361.3 ± 41.9 |
| SOD (U·mg−1 of protein) | 73.2 ± 3.1 | 69.3 ± 2.1 | 71.5 ± 1.8 | 66.9 ± 1.5 | 66.2 ± 2.9 | 73.4 ± 2.1 |
| CAT (mmol·min−1 mg‐1 of protein) | 86.7 ± 21.8 | 25.7 ± 5.1* | 85.9 ± 18.8# | 36.1 ± 8.4 | 31.8 ± 5.1 | 32.4 ± 4.0 |
Note. Data shown are means ± SEM (n = 10).
P < .05, significantly different from naive group;
P < .05, significantly different from vehicle‐treated group; one‐way ANOVA followed by Bonferroni test. ROS, reactive oxygen species; CAT, catalase; LOOH, lipid hydroperoxides; SOD, superoxide dismutase; GSH, reduced glutathione.
Table 2.
Effect of luteolin on inflammatory parameters of irinotecan‐exposed mice
| Tissue | Duodenum | Colon | ||||
|---|---|---|---|---|---|---|
| Groups | Naive | Vehicle | Luteolin | Naive | Vehicle | Luteolin |
| MPO (relative units) | 3.6 ± 0.7 | 50.3 ± 11.1* | 19.7 ± 6.0# | 10.9 ± 4.2 | 211.7 ± 73.5* | 54.6 ± 18.2# |
| TNF (fg·mg−1 of protein) | 54.6 ± 6.8 | 89.8 ± 10.0* | 56.4 ± 7.8# | 40.3 ± 5.4 | 64.4 ± 8.2* | 38.3 ± 5.1# |
| IL‐1β (fg·mg−1 of protein) | 1.9 ± 0.2 | 4.7 ± 1.0* | 1.3 ± 0.1# | 0.9 ± 0.1 | 1.8 ± 0.2* | 1.1 ± 0.1# |
| IL‐6 (fg·mg−1 of protein) | 30.5 ± 3.9 | 52.5 ± 5.4* | 32.7 ± 4.6# | 18.3 ± 2.1 | 25.6 ± 2.3* | 15.6 ± 1.5# |
| IL‐4 (fg·mg−1 of protein) | 7.5 ± 1.6 | 3.6 ± 0.3* | 4.8 ± 0.8# | 61.7 ± 5.0 | 37.1 ± 1.9* | 42.6 ± 3.6 |
| IL‐10 (fg·mg−1 of protein) | 39.0 ± 5.5 | 16.2 ± 1.1* | 41.2 ± 8.6# | 294.7 ± 25.9 | 213.2 ± 13.9* | 213.5 ± 14.7 |
Note. Data shown are means ± SEM (n = 10).
P < .05, significantly different from naive group;
P < .05 significantly different from vehicle‐treated group; one‐way ANOVA followed by Bonferroni test. MPO, myeloperoxidase.
The intestinal mucositis induced by irinotecan clearly increased the ROS levels in the duodenum, as shown by the difference between vehicle and naive groups. Moreover, irinotecan also increased the LOOH levels, along with decreased levels of GSH and of catalase activity. Treatment with luteolin prevented all these changes, providing levels near those in the samples from naïve animals (Table 1). By contrast, in the colon, irinotecan did not induce significant changes in these oxidative parameters, except for a reduction in the amount of GSH.
However, in terms of inflammatory parameters, both tissues were clearly affected. Irinotecan caused a large increase in MPO activity and in the levels of pro‐inflammatory cytokines (TNF, IL‐1β, and IL‐6 ), while the anti‐inflammatory cytokines IL‐4 and IL‐10 appeared to be decreased (Table 2). Moreover, in duodenum, nitrite and PGE2 levels were increased, effects which were not observed in the colon (Figure 5). Although exposure to irinotecan induced a marked inflammatory reaction, the mice treated with luteolin showed decreased MPO activity and decreased TNF, IL‐1β, and IL‐6 levels in both the duodenum and the colon, along with an increase of IL‐4 and IL‐10 and a reduction of nitrite levels in the duodenum, compared with the vehicle‐treated group.
Figure 5.
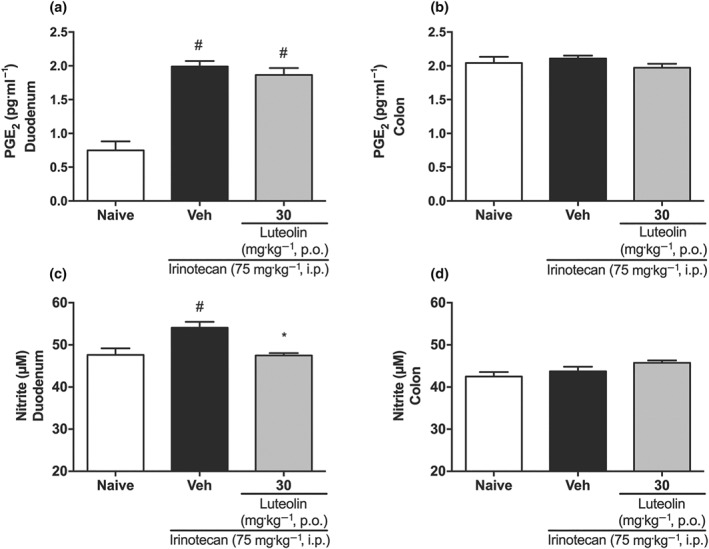
Effect of luteolin on PGE2 and nitrite levels of irinotecan‐exposed mice. Female mice were treated for 14 days p.o. with vehicle (Veh: water plus Tween 1%, 10 ml·kg−1) or luteolin (30 mg·kg−1). Irinotecan was given i.p. (75 mg·kg−1, from the 7th to the 10th day) for Veh‐ and Lut‐treated groups. PGE2 (a and b) and nitrite levels (c and d). The results are expressed as mean ± SEM (n = 10). *P < .05, significantly different from vehicle; # P < .05, significantly different from the naive group; one‐way ANOVA followed by Bonferroni's post hoc test
3.4. Luteolin prevents changes in the mucus production in the intestine of irinotecan‐exposed mice
A regular balance in the mucus production in the intestine is fundamental to maintain the normal function of the organ. Accordingly, we measured goblet cells numbers in the duodenum by Alcian blue staining and the levels of mucin‐like glycoproteins in the colon by PAS staining, as shown in Figure 6a. The number of intact goblet cells in the vehicle‐treated group was significantly lower than in the naive group, as was the PAS staining in the colon. On the other hand, such changes were not seen in the intestinal tissues obtained from luteolin‐treated mice (Figure 6b,c).
Figure 6.
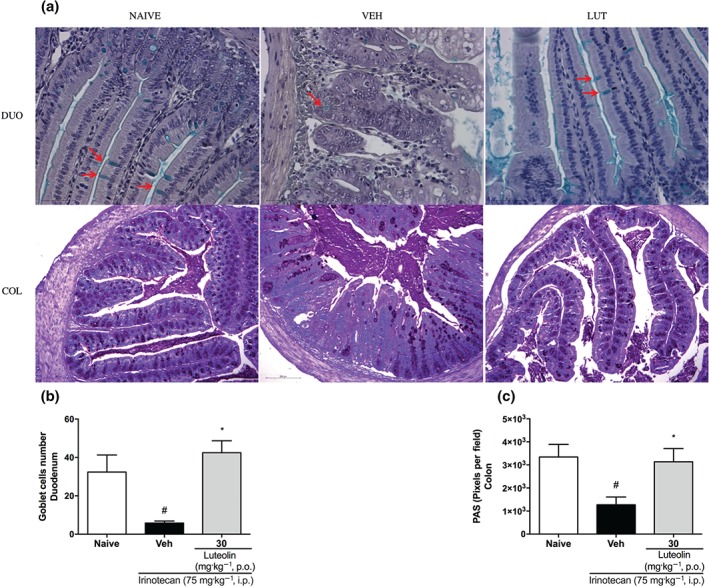
Effect of luteolin on mucus production in the intestine of irinotecan‐exposed mice. Female mice were treated for 14 days p.o. with vehicle (VEH: water plus Tween 1%, 10 ml·kg−1) or luteolin (LUT: 30 mg·kg−1). Irinotecan was given i.p. (75 mg·kg−1, from the 7th to the 10th day) for Veh‐ and Lut‐treated groups. Goblet cells were stained with Alcian blue (a) in the duodenum (arrows) (DUO) and were counted (b) in crypts and villi under 40× magnification, scale bars 50 μm. Six fields per animal were analysed (n = 5). Mucin‐like glycoproteins were stained by PAS (a) (COL) and quantified (c) with ImageJ® program. Scale bars 200 μm. Six fields per animal were analysed (n = 5). The results are expressed as mean ± SEM. *P < .05, significantly different from vehicle; # P < .05, significantly different from the naive group; one‐way ANOVA followed by Bonferroni's post hoc test. COL, colon; DUO, duodenum
3.5. Luteolin prevents changes in the expression of the tight junction in the duodenum of irinotecan‐exposed mice
Immunofluorescence analysis of duodenum samples for the tight junction proteins, zonula occludens (ZO‐1; Figure 7a,b) and occludin (OC; Figure 7c,d) showed alterations in the pattern of expression of these proteins induced by irinotecan in the duodenum of the vehicle group, compared with naïve mice, while the mice treated with luteolin showed expression similar to that in naive mice, both in location and in intensity. Although the expression of the tight junction proteins in the colonic tissue was not affected by irinotecan, the group treated with luteolin showed a higher fluorescence intensity for occluding, when compared with the vehicle‐treated group (Figure S4).
Figure 7.
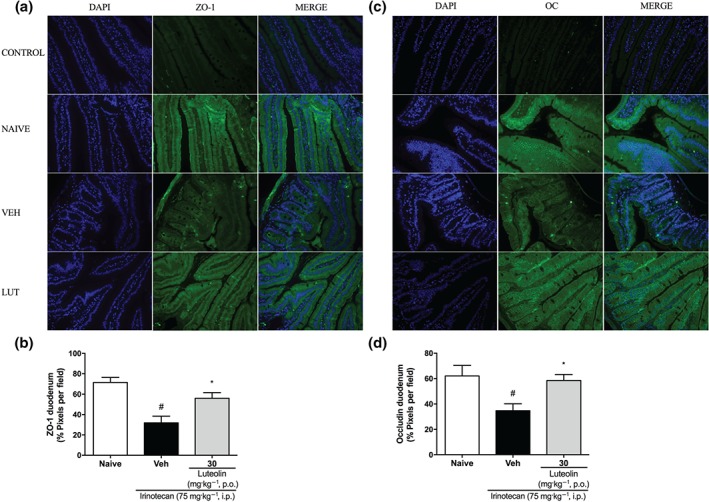
Effect of luteolin on tight junctions (TJs) expression in the duodenum of irinotecan‐exposed mice. Female mice were treated for 14 days p.o. with vehicle (VEH: water plus Tween 1%, 10 ml·kg−1) or luteolin (LUT: 30 mg·kg−1). Irinotecan was given i.p. (75 mg·kg−1, from the 7th to the 10th day) for Veh‐ and Lut‐treated groups. Immunofluorescence staining of the duodenum for ZO‐1 (a) and occludin (OC) (c) protein were performed (green). Nuclei are stained with DAPI (blue). (c and d) Non‐relevant IgG was used as CONTROL. Magnification 20×. Fluorescence was quantified with ImageJ® program (b and d). Six fields per animal were analysed (n = 5). The results are expressed as mean ± SEM. *P < .05, significantly different from vehicle; # P < .05, significantly different from the naive group; one‐way ANOVA followed by Bonferroni's post hoc test
3.6. Luteolin does not reverse leukopenia or interfere with the antitumour activity of irinotecan
Irinotecan is known to induce leukopenia and we therefore measured the numbers of leukocytes in blood and in peritoneal exudate in our model of intestinal mucositis. As shown in Figure 8, the vehicle‐treated group presented fewer leukocytes, compared with those in the naïve group, and this effect was not reversed by luteolin treatment, indicating that luteolin did not modify this effect of the chemotherapy.
Figure 8.
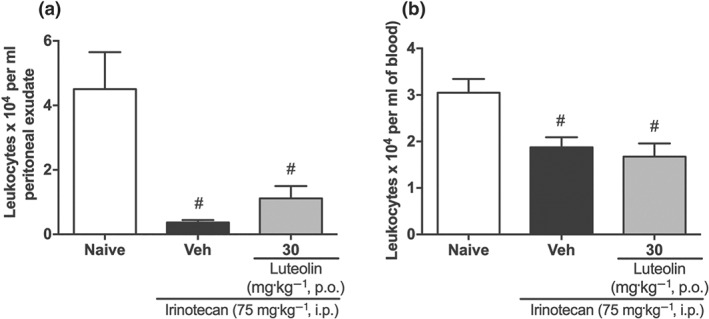
Luteolin does not reverse the leukopenia induced by irinotecan. (a) Leukocyte numbers in the peritoneal exudate. (b) Leukocyte numbers in blood. The results are expressed as mean ± SEM (n = 10). # P < .05, significantly different from the naive group; one‐way ANOVA followed by Bonferroni's post hoc test
In order to confirm, more directly, that luteolin did not modify the anti‐tumour activity of the irinotecan, melanoma cells (B16F10) were injected in the flank of C57BL/6 mice and, after tumour development, the animals were treated with luteolin only, irinotecan only, and irinotecan plus luteolin. The results clearly showed that irinotecan decrease the tumour area, weight, and volume, while luteolin did not show any anti‐tumour effect. Further, when luteolin was given in combination with irinotecan, this flavonoid did not interfere with the anti‐tumour effect of the chemotherapeutic agent (Figure S5).
3.7. Luteolin does not change the intestinal epithelial cells (IEC‐6) toxicity induced by irinotecan
The cytotoxicity of luteolin and irinotecan was studied on IEC‐6 cells, using the MTT assay. Luteolin did not alter cell viability when tested over a concentration range (3 ‐ 30 μM), while irinotecan showed significant cytotoxicity effect at 30 μM. Importantly, when exposed concomitantly to the cells, luteolin did not interfere with the cytotoxic effects of irinotecan, consistent with the in vivo results described above (Figure S6).
3.8. Luteolin did not present signs of toxicity in the experimental period of treatment
We evaluated the effect of luteolin and irinotecan on the organ weights of mice. As shown in Table S1, treatment with luteolin (30 mg·kg−1, p.o.) for 14 days did not cause any change in the organ weights, compared with those in the naive group. However, mice treated with the vehicle (plus irinotecan) decreased the spleen (54%) and liver (21%) weights, while mice receiving irinotecan plus treatment with luteolin (30 mg·kg−1, p.o.) did not show these changes.
Consistent with these findings, the plasma levels of urea, creatinine, aspartate transaminase (AST), and alanine transaminase (ALT) in mice treated with luteolin (30 mg·kg−1) were not statistically different from the baseline levels, whereas vehicle‐treated mice receiving irinotecan increased AST (248%) and ALT (198%) levels, when compared to naive. Luteolin‐treated mice receiving irinotecan still showed increased AST levels but had ALT levels comparable to those in the naive group (Table S2).
4. DISCUSSION
As previously mentioned, intestinal mucositis can be a dose‐limiting effect of chemotherapy, and the absence of effective treatments for this side‐effect highlights the necessity for discoveries in this therapeutic field. Apart from oral rehydration therapy, the pharmacotherapy of chemotherapy‐induced diarrhoea includes mainly the use of drugs which decrease intestinal motility such as https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=7215 (synthetic opioid), https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=2055 (somatostatin analogue), and atropine (competitive inhibitor of muscarinic ACh receptors; Andreyev et al., 2014). Therefore, to evaluate whether luteolin could be a promising candidate for mucositis management, we first demonstrated that luteolin did not affect digestive motility rate, either in a resting state or in a diarrhoeal state, and thus acts differently from the first and ineffective treatment of late diarrhoea induced by chemotherapy.
Chemotherapy‐induced diarrhoea appears to be a multifactorial process, including loss of intestinal epithelium, superficial necrosis, and inflammation of the bowel wall, and has thus been classified as inflammatory/secretory diarrhoea (Cherny, 2008). Interestingly, we observed that mice in our model of intestinal mucositis induced by irinotecan and treated with vehicle, had a decreased digestive motility rate, compared with that in normal (naïve) mice, which was not detected in the luteolin‐treated group. Gastric motility is regulated by the neural circuits that affect the activity of the smooth muscles and also by hormones released from the stomach, intestines, pancreas, and other tissues (Goyal, Guo, & Mashimo, 2019). It is possible that a hormonal/neural feedback to decrease the digestive motility process occurred in an attempt to decrease secretory diarrhoea, which was very pronounced in the vehicle plus irinotecan‐treated group.
After this first screening, we evaluated the effect of luteolin treatment on the irinotecan‐induced intestinal mucositis, following the experimental protocol for mucositis most described in the literature, where treatment with the compound is started on the same day as the irinotecan treatment and continues for 7 days. Under these conditions, luteolin treatment (30 mg·kg−1, p.o.) was able to diminish diarrhoea score but did not reduce the body weight loss of mice and weight/length ratio of the duodenum and colon. In order to obtain a better effect of the compound and bearing in mind that, in clinical practice, chemotherapy is delivered in fixed treatment cycles (Stein et al., 2010), it seemed that prevention of intestinal mucositis could be a better strategy for the management of this severe adverse effect, we have used a new experimental schedule, starting the luteolin administration 7 days before the first dose of irinotecan.
The first sign of irinotecan‐induced intestinal mucositis in mice was body weight loss. However, at the end of the experimental period, animals treated with luteolin (30 mg·kg−1, p.o.), lost less weight than the vehicle (irinotecan only) group. This beneficial effect was accompanied by a significant attenuation of diarrhoea, the main cause of morbidity and mortality in patients experiencing intestinal mucositis (Andreyev et al., 2014). In agreement, when luteolin was given i.p. at the same dose as used p.o., it showed the same response, indicating a systemic effect of the compound, as i.p. injection is used for small species for which i.v. access is challenging (Turner, Brabb, Pekow, & Vasbinder, 2011). In rats, luteolin administered by the oral route, is absorbed into the systemic circulation, and the plasma contains free luteolin, the glucuronide or sulfate conjugates of unchanged luteolin and o‐methyl luteolin (Lin, Pai, & Tsai, 2015; Shimoi et al., 1998).
In addition to diarrhoea, luteolin also reversed the mechanical hypersensitivity induced by irinotecan. Indeed, the anti‐nociceptive effects of luteolin have been described in several models of pain (Fan et al., 2018; Hashemzaei et al., 2017), highlighting the potential of the compound in pain management. Wardill et al. (2016) demonstrated that the hypersensitivity induced by irinotecan treatment is dependent on activation of https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=1754. This finding leads us to suggest that the protective effect of luteolin in preserving the tight junctions could have decreased the activation of neuronal TLR4 by products of bacteria of the intestinal lumen, thus also reducing activation of the gut/CNS axis and consequently mechanical hypersensitivity.
Apart from the decreased body weight loss, mechanical hypersensitivity and diarrhoea following luteolin treatment, we have also presented histological evidence that this flavone significantly reduced the severity of the intestinal damage by decreasing structural disorder in the tissues, supported by the macroscopic analysis indicating that oedema, vascular permeability, and tissue weight loss were diminished.
Furthermore, irinotecan caused depletion of goblet cells in the duodenum and the quantity of mucin‐like glycoproteins in the colon, similar to that reported by Stringer et al. (2009), who described that deficiencies in goblet cell numbers and mucin expression or secretion may contribute to chemotherapy‐induced diarrhoea. However, as shown by immunohistochemical staining, treatment with luteolin significantly preserved goblet cells in the duodenum and the amount of mucin in the colon. As the mucus barrier is a crucial factor against mechanical and chemical stress of the epithelium, protecting against bacterial overgrowth and penetration, and essential to the correct transit of the luminal contents (Smirnov, Sklan, & Uni, 2004), this set of data provides further evidence for the protective effects of luteolin on the intestine.
The anti‐tumour mechanism of irinotecan and its active metabolite SN‐38 on inhibiting topoisomerase I adversely induces apoptotic death of intestinal cells (Ribeiro et al., 2016). Sonis (2004) has described that DNA strand breaks in basal epithelial cells and underlying tissue can result in cell death or injury, and non‐DNA injury is initiated through a variety of mechanisms, some of them mediated by the generation of ROS.
Indeed, luteolin treatment significantly decreased the amount of ROS induced by irinotecan in the duodenum, thereby reducing oxidative damages to cell membranes as shown by the diminished levels of LOOH (Girotti, 1998). Moreover, this flavonoid completely restored the levels of GSH, which itself is a critical factor in maintaining the cellular redox balance and also has been involved in the regulation of cell signalling and repair pathways (Cnubben, Rietjens, Wortelboer, van Zanden, & van Bladeren, 2001). In addition, luteolin treatment prevented the loss of catalase activity, which is responsible for the conversion of H2O2 into H2O and O2 (Kwiecien et al., 2014), preserving values similar to those found in the naive group. Li et al. (2016) have evaluated the effect of luteolin on DSS‐induced colitis and have found comparable results, suggesting that luteolin may activate the Nrf2 pathway. Nevertheless, luteolin itself is a potent antioxidant molecule (IC50 of ~1.84 μg·ml−1 was found in DPPH assay; Figure S7), and then, its directly scavenging properties on ROS formed during the pathophysiology of mucositis, at least in part, may contribute for the beneficial effects of the compound, as described here. On the other hand, we did not detect oxidative imbalance in the colon. In fact, Krajewski et al. (1994) have described that the colon is less affected than the small intestine during intestinal mucositis, probably due to a difference in the expression of the Bcl‐2 and Bax proteins between these tissues, and here, we provide strong evidence that ROS formation is also lower in the colon, contributing to less aggressive damage.
Formation of ROS in the intestine is considered as an initiating step in mucositis that triggers a series of events, culminating in cytokine maturation (Ribeiro et al., 2016) and release of damage‐associated molecular patterns (DAMPs). This sequence leads to the production of NF‐κB, IL‐1, IL‐18, https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=1376 (Logan et al., 2008; Ribeiro et al., 2016), TNF, and IL‐6 (Logan et al., 2008), as well as an up‐regulation of https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=69 expression on neutrophils, thereby increasing neutrophil recruitment and further tissue damage and bacterial translocation (Guabiraba et al., 2014).
In fact, irinotecan significantly increased MPO activity, indicating neutrophil infiltration, as well as enhancing levels of TNF, IL‐1β, and IL‐6 and decreasing amounts of IL‐4 and IL‐10, compared with the naive group. The presence of TNF and IL‐6 stimulates early damage in connective tissue and endothelium resulting in epithelial basal cell death and injury (Sonis, 2004). However, luteolin attenuated this inflammatory process as shown by the reduction in MPO activity and of TNF, IL‐1β, and IL‐6 levels in the duodenum and colon and by the increase of IL‐4 and IL‐10 in the duodenum, relative those in the vehicle group.
Besides, during intestinal mucositis, the process of inflammation amplification leads to increased PG and NO production, raising intestinal secretion and tissue damage (Ribeiro et al., 2016), as we have also seen in our results. Although luteolin did not reverse the increased PGE2 levels, it significantly decreased the amounts of nitrite, showing a possible reduction in NO generation, a very important inflammatory mediator in irinotecan‐induced mucositis (see Lima et al., 2012).
Although the clinical symptoms of mucositis result from epithelial damage, this condition is a consequence of a dynamic series of biological events involving different tissue compartments and mucosal cells, where leukocyte infiltrates are present even in late stages of the pathological process (Sonis, 2004). During the immune response, irinotecan is known to promote macrophage activation by activating the NF‐κB, STAT1, and MAPK signalling pathways (Li et al., 2015). However, we observed that luteolin controlled the activation of classical macrophages by reducing M1 phenotype biomarkers, such as TNF, IL‐1β, IL‐6, and nitrite generation. In the same way, this effect was observed by Zhang, Li, Xu, Xiang, and Ma (2018) in RAW264.7 cells stimulated with LPS, where the authors have described that luteolin decreased the activation of the https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=1770 inflammasome.
Furthermore, increased TNF, IL‐1β, IL‐6, and decreased IL‐10 levels have been associated with disruption of tight junctions (Suzuki, 2013; Wardill, Bowen, & Gibson, 2012). Tight junctions are multiple protein complexes which regulate intestinal permeability controlling the movement of fluids, nutrients, microbes and toxins across the epithelia (Suzuki, 2013). Peripherally located, zonular occludens proteins (ZO‐1, ZO‐2, and ZO‐3) are cytosolic scaffold proteins which interact with anchor transmembrane proteins like occludin and claudin to the cytoskeleton (Suzuki, 2013). It is well established that ZO and occludin play central roles in maintaining the integrity of the tight junctions and the mucosal barrier function (Cummins, 2012; Fanning & Anderson, 2009). Thus, we evaluated the expression of these two proteins and found that, consistent with earlier reports, irinotecan treatment modified the expression of ZO‐1 and occludin (Wardill et al., 2014). Conversely, treatment with luteolin efficiently maintained the levels and location of both proteins in a range similar to that in the naive mice, demonstrating that this flavone has a potential to protect intestinal mucosa from the late effects of mucositis, such as bacterial translocation, which are strongly correlated with death.
Finally, luteolin did not reverse the leukopenia induced by irinotecan, and it did not show interference in the anti‐tumour activity of the drug when given in combination with the chemotherapy for mice with melanoma. Moreover, the treatment in its isolated form was shown to be ineffective in remission of the melanoma‐type tumour. Similarly, when the effects of luteolin and irinotecan were evaluated on the cellular viability of intestinal cells, the treatment with luteolin did not show any signs of toxicity, whereas exposure with irinotecan significantly reduced the viability of these cells, which was not reversed by luteolin.
Indeed, no toxicity has been described in rats or mice to this flavonoid (Aziz, Kim, & Cho, 2018). However, because luteolin was given to mice for a substantial period, we monitored the organ weights of the animals, as this parameter is commonly used to evaluate toxicity (Sellers et al., 2007). No significant change in the collected organs was observed, after luteolin treatment. On the other hand, luteolin prevented the reduction in spleen and liver weights induced by irinotecan. Chemotherapy toxicity in organs not affected by cancer is one of its limitations (Aikemu et al., 2016), and the hepatotoxic side effects have been a serious clinical challenge in chemotherapy (Thatishetty, Agresti, & O'Brien, 2013). In our experiments, irinotecan increases the plasma levels of AST and ALT, whereas luteolin treatment prevented the increase in ALT, findings similar to those of other authors evaluating hepatoprotective effects of this compound (Liu et al., 2014; He et al., 2019).
In conclusion, the present study provides important evidence that luteolin prevents irinotecan‐induced intestinal mucositis, an action which may be associated with a decrease of oxidative stress and of inflammatory processes, along with a preservation of mucosal protective factors, such as mucus and expression of tight junctions. Luteolin is a partial agonist of https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=595 (Puhl et al., 2012), which is a receptor known to promote anti‐inflammatory effects by a variety of mechanisms, including NF‐κB suppression (Ricote & Glass, 2007), suppression of the https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=1770 inflammasome (Cheng, Li, Wang, Cheng, & Liu, 2018; Meng et al., 2019) and modulation of macrophage function (Dai et al., 2017; Szanto et al., 2010), leading us to suggest that this pathway may mediate all the effects of the flavonoid described here, which prevent intestinal mucositis. However, further studies are needed to support this hypothesis. Moreover, it is important to note that luteolin did not reduce the effectiveness of irinotecan as an anti‐cancer agent but only prevented the side effect of this drug. Thus, this flavonoid could be suggested as a potential adjuvant in anti‐tumour therapy to control intestinal mucositis.
AUTHOR CONTRIBUTIONS
T.B., P. de S., S.S., L.B.S., L.N.B.M., and B.J.C. carried out the pharmacological tests of mucositis. M.F. dos A. and N.L.M.Q. carried out the mechanical withdrawal threshold evaluation. T.B., P. de S., L.D., P.D., L.M. da S., and S.F. de A. performed the statistical analysis and wrote and corrected the manuscript. All authors have read and approved the manuscript.
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
DECLARATION OF TRANSPARENCY AND SCIENTIFIC RIGOUR
This Declaration acknowledges that this paper adheres to the principles for transparent reporting and scientific rigour of preclinical research as stated in the BJP guidelines for https://bpspubs.onlinelibrary.wiley.com/doi/full/10.1111/bph.14207, https://bpspubs.onlinelibrary.wiley.com/doi/full/10.1111/bph.14207, and https://bpspubs.onlinelibrary.wiley.com/doi/full/10.1111/bph.14206, and as recommended by funding agencies, publishers and other organisations engaged with supporting research.
Supporting information
Figure S1. Effect of luteolin on digestive motility rate (%). (A) Resting state. The results are expressed as mean ± SEM (n = 6). Statistical analysis was performed using one‐way ANOVA followed by Bonferroni's post hoc test. *P < .05 compared with the vehicle group (Veh: water plus 1% tween. 10 ml·kg−1. p.o.); Atp: Atropine (3 mg·kg−1. s.c.). (B) Diarrheal state. Female mice were treated for 14 days by oral route with vehicle (Veh: water plus Tween 1%, 10 ml·kg−1) or luteolin (30 mg·kg−1). Irinotecan was given by intraperitoneal route (75 mg·kg−1, from 7th to 10th day) for Veh and Luteolin‐treated groups. Digestive motility rate was evaluated on the 15th day of experiment. The results are expressed as mean ± SEM (n = 6). Statistical analysis was performed using one‐way ANOVA followed by Bonferroni's post hoc test. # P < .05 compared to the naive.
Figure S2. Effect of luteolin treatment by oral route on irinotecan‐induced intestinal mucositis. Female mice were treated for 7 days by oral route with vehicle (Veh: water plus Tween 1%, 10 ml·kg−1) or luteolin (Lut 30 mg·kg−1). Irinotecan was given by intraperitoneal route (75 mg·kg−1, from 1st to the 4th day) for Veh and Luteolin‐treated groups. (A) The weight of the animals was measured daily. Weight loss was significantly different from the 5th to 7th day of experiment in both treated groups compared to naive. The results are expressed as mean ± SEM (n = 10). Statistical analysis was performed using two‐way ANOVA followed by Bonferroni's post hoc test. (B) The diarrhea score was evaluated on the 7th day of treatment. The results are expressed as median with interquartile range (n = 10). Statistical analysis was performed by Kruskal‐Wallis followed by Dunn's test. *P < .05 compared to vehicle. #P < .05compared to naive group. (C and D) Weight/length ratio of duodenum and colon. The results are expressed as mean ± SEM (n = 10). Statistical analysis was performed using one‐way ANOVA followed by Bonferroni's post hoc test. #P < .05 compared to the naive group.
Figure S3. Effect of luteolin by oral route in the mechanical hypersensitivity of irinotecan‐exposed mice. Female mice were treated by oral route with vehicle (Veh: water plus Tween 1%, 10 ml·kg−1) or luteolin (Lut mg·kg−1). From the 7th to 10th day irinotecan was intraperitoneally given at a dose of 75 mg·kg−1 per day to the Veh and Lut groups and the mechanical hypersensitivity was measured by von Frey filament. (A) Response frequency (%) and (C) mechanical withdrawal threshold (g) are presented followed their respective A.U.C. (B and D). The results are expressed as mean ± SEM (n = 10). Statistical analysis was performed using two‐way ANOVA followed by Bonferroni's post hoc test. *P < .05 compared to vehicle. #P < .05 compared to naive group.
Figure S4. Effect of luteolin on tight junctions (TJs) expression in the colon of irinotecan‐exposed mice. Immunofluorescence staining of the colon for Occludin (A) and ZO‐1 (C) protein was performed (green). Nuclei are stained with DAPI (blue). Non‐relevant IgG was used as control. Magnification ×20. (B, D) Fluorescence was quantified with ImageJ® program. Six fields per animal were analyzed (n = 5). The results are expressed as mean ± SEM. Statistical analysis was performed using one‐way ANOVA followed by Bonferroni's post hoc test. *P < .05 compared to vehicle group.
Figure S5. Luteolin does not interfere with the antitumor activity of Irinotecan. Melanoma cells (B16F10) were injected in the flank of C57BL/6 mice and after the tumor development, the animals were treated for 7 days with control (water plus Tween 1%, 10 ml·kg−1, p.o.), luteolin (Lut 30mg·kg−1, p.o.), irinotecan (Iri 75 mg·kg−1, i.p.) or luteolin plus irinotecan (Lut + Iri). Tumor area (A) was measured daily. Tumor weight (C) and tumor volume (D) were measured after euthanasia of mice. The results are expressed as mean ± SEM (n = 10). Statistical analysis was performed using one‐way ANOVA followed by Bonferroni's post hoc test. (A) *P < .05 compared Irinotecan and Lut + Iri to control group. (C and D) #P < .05 compared to vehicle group without irinotecan.
Figure S6. Luteolin does not affect intestinal epithelial cell viability and does not alter the irinotecan‐induced cytotoxicity. Murine epithelial cells (5 × 104 cells/well) were seeded in 96‐well plate and cell viability was measured using the MTT assay. Basal (DMEM plus DMSO 0.1%); DMSO (DMEM plus DMSO 10%); irinotecan (I: 3‐30 μM); luteolin (L: 3‐30 μM). The results are expressed as mean ± SEM (n = 3 replicates). Statistical analysis was performed using one‐way ANOVA followed by Bonferroni's post hoc test. * P < .05 when compared to basal group.
Figure S7. Effect of Luteolin on in vitro ability to scavenge the free‐radical DPPH. The results are expressed as mean ± SEM. Statistical analysis was performed using one way analysis of variance (ANOVA) followed by Bonferroni test. IC50 of ~1.84 μg/ml. *P < .05 compared to the vehicle group. Vehicle (Veh: water). Ascorbic acid (AA: 50 μg/ml).
Table S1. Effects of Luteolin on relative organ weights of irinotecan‐exposed mice.
Table S2. Effects of Luteolin on plasma biochemistry of irinotecan‐exposed mice.
Method S1. Evaluation of digestive motility rate
Method S2. Effect of Luteolin treatment during 7 days on irinotecan‐induced intestinal mucositis.
Method S3. Mechanical withdrawal threshold evaluation
Method S4. Biochemical analyses
Method S5. Evaluation of antitumor activity
Method S6. Cell viability
Method S7. DPPH· radical scavenging assay
ACKNOWLEDGEMENTS
We are grateful to Dr. Luis Carlos Stoeberl who provided the irinotecan for this study. This research was supported in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior – Brasil (CAPES) – Finance Code 001, Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Universidade do Vale do Itajaí (UNIVALI), Institut National de la Santé et de la Recherche (Inserm), and the Université de Lille.
Boeing T, de Souza P, Speca S, et al. Luteolin prevents irinotecan‐induced intestinal mucositis in mice through antioxidant and anti‐inflammatory properties. Br J Pharmacol. 2020;177:2393–2408. 10.1111/bph.14987
REFERENCES
- Thatishetty, A. V. , Agresti, N. , & O'Brien, C. B. (2013). Chemotherapy‐induced hepatotoxicity. Clinics in Liver Disease, 17, 671–686, ix–x. 10.1016/j.cld.2013.07.010 [DOI] [PubMed] [Google Scholar]
- Aikemu, A. , Amat, N. , Yusup, A. , Shan, L. , Qi, X. , & Upur, H. (2016). Attenuation effect of Abnormal Savda Munziq on liver and heart toxicity caused by chemotherapy in mice. Experimental and Therapeutic Medicine, 12, 384–390. 10.3892/etm.2016.3328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander, S. P. H. , Christopoulos, A. , Davenport, A. P. , Kelly, E. , Mathie, A. , Peters, J. A. , … CGTP Collaborators (2019). The Concise Guide to PHARMACOLOGY 2019/20: G protein‐coupled receptors. British Journal of Pharmacology, 176, S21–S141. 10.1111/bph.14748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander, S. P. H. , Cidlowski, J. A. , Kelly, E. , Mathie, A. , Peters, J. A. , Veale, E. L. , … CGTP Collaborators (2019). The Concise Guide to PHARMACOLOGY 2019/20: Nuclear hormone receptors. British Journal of Pharmacology, 176, S229–S246. 10.1111/bph.14750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander, S. P. H. , Fabbro, D. , Kelly, E. , Mathie, A. , Peters, J. A. , Veale, E. L. , … Sharman, J. L. (2019a). The Concise Guide to PHARMACOLOGY 2019/20: Catalytic receptors. British Journal of Pharmacology, 176, S247–S296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander, S. P. H. , Fabbro, D. , Kelly, E. , Mathie, A. , Peters, J. A. , Veale, E. L. , … CGTP Collaborators (2019b). The Concise Guide to PHARMACOLOGY 2019/20: Enzymes. British Journal of Pharmacology, 176, S297–S396. 10.1111/bph.14752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander, S. P. H. , Roberts, R. E. , Broughton, B. R. S. , Sobey, C. G. , George, C. H. , Stanford, S. C. , … Ahluwalia, A. (2018). Goals and practicalities of immunoblotting and immunohistochemistry: A guide for submission to the British Journal of Pharmacology . British Journal of Pharmacology, 175, 407–411. 10.1111/bph.14112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Almeida, C. L. B. , Boeing, T. , Somensi, L. B. , Steimbach, V. M. B. , da Silva, L. M. , Andrade, S. F. D. , … de Souza, P. (2017). Diuretic, natriuretic and potassium‐sparing effect of nothofagin isolated from Leandra dasytricha (A. Gray) Cogn. leaves in normotensive and hypertensive rats. Chemico‐Biological Interactions, 268, 103–110. 10.1016/j.cbi.2017.03.004 [DOI] [PubMed] [Google Scholar]
- Andreyev, J. , Ross, P. , Donnellan, C. , Lennan, E. , Leonard, P. , Waters, C. , … Ferry, D. (2014). Guidance on the management of diarrhoea during cancer chemotherapy. The Lancet Oncology, 15, e447–e460. 10.1016/S1470-2045(14)70006-3 [DOI] [PubMed] [Google Scholar]
- Arifa, R. D. N. , de Paula, T. P. , Madeira, M. F. M. , Lima, R. L. , Garcia, Z. M. , Ávila, T. V. , … Teixeira, M. M. (2016). The reduction of oxidative stress by nanocomposite fullerol decreases mucositis severity and reverts leukopenia induced by irinotecan. Pharmacological Research, 107, 102–110. 10.1016/j.phrs.2016.03.004 [DOI] [PubMed] [Google Scholar]
- Arifa, R. D. N. , Madeira, M. F. M. , De Paula, T. P. , Lima, R. L. , Tavares, L. D. , Menezes‐Garcia, Z. , … Teixeira, M. M. (2014). Inflammasome activation is reactive oxygen species dependent and mediates irinotecan‐induced mucositis through IL‐1β and IL‐18 in mice. The American Journal of Pathology, 184, 2023–2034. 10.1016/j.ajpath.2014.03.012 [DOI] [PubMed] [Google Scholar]
- Aziz, N. , Kim, M. Y. , & Cho, J. Y. (2018). Anti‐inflammatory effects of luteolin: A review of in vitro, in vivo, and in silico studies. Journal of Ethnopharmacology, 225, 342–358. 10.1016/j.jep.2018.05.019 [DOI] [PubMed] [Google Scholar]
- Boeing, T. , da Silva, L. M. , Mariott, M. , Andrade, S. F. D. , & de Souza, P. (2017). Diuretic and natriuretic effect of luteolin in normotensive and hypertensive rats: Role of muscarinic acetylcholine receptors. Pharmacological Reports, 69, 1121–1124. 10.1016/j.pharep.2017.05.010 [DOI] [PubMed] [Google Scholar]
- Boeing, T. , Da Silva, L. M. , Somensi, L. B. , Cury, B. J. , Michels Costa, A. P. , Petreanu, M. , … de Andrade, S. F. (2016). Antiulcer mechanisms of Vernonia condensata Baker: A medicinal plant used in the treatment of gastritis and gastric ulcer. Journal of Ethnopharmacology, 184, 196–207. 10.1016/j.jep.2016.02.049 [DOI] [PubMed] [Google Scholar]
- Bowen, J. M. , Gibson, R. J. , Stringer, A. M. , Thong, W. , Prabowo, A. S. , Cummins, A. G. , & Keefe, D. M. (2006). Role of p53 in irinotecan‐induced intestinal cell death and mucosal damage. Antic‐Ancer Drugs, 18, 197–210. [DOI] [PubMed] [Google Scholar]
- Turner, P. V. , Brabb, T. , Pekow, C. , & Vasbinder, M. A. (2011). Administration of substances to laboratory animals: Routes of administration and factors to consider. Journal of the American Association for Laboratory Animal Science, 50, 600–613. [PMC free article] [PubMed] [Google Scholar]
- Bradley, P. P. , Priebat, D. A. , Christensen, R. D. , & Rothstein, G. (1982). Measurement of cutaneous inflammation: Estimation of neutrophil content with an enzyme marker. The Journal of Investigative Dermatology, 78, 206–209. 10.1111/1523-1747.ep12506462 [DOI] [PubMed] [Google Scholar]
- Cheng, Y. , Li, S. , Wang, M. , Cheng, C. , & Liu, R. (2018). Peroxisome proliferator activated receptor gamma (PPARγ) agonist rosiglitazone ameliorate airway inflammation by inhibiting toll‐like receptor 2 (TLR2)/Nod‐like receptor with pyrin domain containing 3 (NLRP3) inflammatory corpuscle activation in asthmatic mice. Medical Science Monitor, 24, 9045–9053. 10.12659/MSM.910766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherny, N. I. (2008). Evaluation and management of treatment‐related diarrhea in patients with advanced cancer: A review. Journal of Pain and Symptom Management, 36, 413–423. 10.1016/j.jpainsymman.2007.10.007 [DOI] [PubMed] [Google Scholar]
- Cinausero, M. , Aprile, G. , Ermacora, P. , Basile, D. , Vitale, M. G. , Fanotto, V. , … Sonis, S. T. (2017). New frontiers in the pathobiology and treatment of cancer regimen‐related mucosal injury. Frontiers in Pharmacology, 8, 1–16, 354. 10.3389/fphar.2017.00354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cnubben, N. H. , Rietjens, I. M. , Wortelboer, H. , van Zanden, J. , & van Bladeren, P. J. (2001). The interplay of glutathione‐related processes in antioxidant defense. Environmental Toxicology and Pharmacology, 10, 141–152. 10.1016/s1382-6689(01)00077-1 [DOI] [PubMed] [Google Scholar]
- Cummins, P. M. (2012). Occludin: One protein, many forms. Molecular and Cellular Biology, 32, 242–250. 10.1128/MCB.06029-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis, M. J. , Alexander, S. , Cirino, G. , Docherty, J. R. , George, C. H. , Giembycz, M. A. , … Ahluwalia, A. (2018). Experimental design and analysis and their reporting II: Updated and simplified guidance for authors and peer reviewers. British Journal of Pharmacology, 175, 987–993. 10.1111/bph.14153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai, L. , Bhargava, P. , Stanya, K. J. , Alexander, R. K. , Liou, Y.‐H. , Jacobi, D. , … Lee, C. H. (2017). Macrophage alternative activation confers protection against lipotoxicity‐induced cell death. Molecular Metabolism, 6, 1186–1197. 10.1016/j.molmet.2017.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan, X. , Du, K. , Li, N. , Zheng, Z. , Qin, Y. , Liu, J. , … Su, Y. (2018). Evaluation of anti‐nociceptive and anti‐inflammatory effect of luteolin in mice. Journal of Environmental Pathology, Toxicology and Oncology, 37, 351–364. 10.1615/JEnvironPatholToxicolOncol.2018027666 [DOI] [PubMed] [Google Scholar]
- Fanning, A. S. , & Anderson, J. M. (2009). Zonula occludens‐1 and ‐2 are cytosolic scaffolds that regulate the assembly of cellular junctions. Annals of the new York Academy of Sciences, 1165, 113–120. 10.1111/j.1749-6632.2009.04440.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita, K. I. , Kubota, Y. , Ishida, H. , & Sasaki, Y. (2015). Irinotecan, a key chemotherapeutic drug for metastatic colorectal cancer. World Journal of Gastroenterology, 21, 12234–12248. 10.3748/wjg.v21.i43.12234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girotti, A. W. (1998). Lipid hydroperoxide generation, turnover, and effector action in biological systems. Journal of Lipid Research, 39, 1529–1542. [PubMed] [Google Scholar]
- Goyal, R. , Guo, Y. , & Mashimo, H. (2019). Advances in the physiology of gastric emptying. Neurogastroenterology and Motility, 31, 1–14, e13546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guabiraba, R. , Besnard, A. G. , Menezes, G. B. , Secher, T. , Jabir, M. S. , Amaral, S. S. , … Liew, F. Y. (2014). IL‐33 targeting attenuates intestinal mucositis and enhances effective tumor chemotherapy in mice. Mucosal Immunology, 7, 1079–1093. 10.1038/mi.2013.124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding, S. D. , Sharman, J. L. , Faccenda, E. , Southan, C. , Pawson, A. J. , Ireland, S. , … Bryant, C. (2018). The IUPHAR/BPS Guide to pharmacology in 2018: Updates and expansion to encompass the new guide to immunopharmacology. Nucleic Acids Research, 46, D1091–D1106. 10.1093/nar/gkx1121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashemzaei, M. , Abdollahzadeh, M. , Iranshahi, M. , Golmakani, E. , Rezaee, R. , & Tabrizian, K. (2017). Effects of luteolin and luteolin‐morphine co‐administration on acute and chronic pain and sciatic nerve ligated‐induced neuropathy in mice. Journal of Complementary and Integrative Medicine, 14, 1–7. [DOI] [PubMed] [Google Scholar]
- He, Y. , Xia, Z. , Yu, D. , Wang, J. , Jin, L. , Huang, D. , … Zhang, B. (2019). Hepatoprotective effects and structure‐activity relationship of five flavonoids against lipopolysaccharide/d‐galactosamine induced acute liver failure in mice. International Immunopharmacology, 68, 171–178. 10.1016/j.intimp.2018.12.059 [DOI] [PubMed] [Google Scholar]
- Ikuno, N. , Soda, H. , Watanabe, M. , & Oka, M. (1995). Irinotecan (CPT‐11) and characteristic mucosal changes in the mouse ileum and cecum. 87: 1876–1883. [DOI] [PubMed]
- Kilkenny, C. , Browne, W. , Cuthill, I. C. , Emerson, M. , & Altman, D. G. (2010). Animal research: Reporting in vivo experiments: The ARRIVE guidelines. British Journal of Pharmacology, 160, 1577–1579. 10.1111/j.1476-5381.2010.00872.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, J. S. , & Jobin, C. (2005). The flavonoid luteolin prevents lipopolysaccharide‐induced NF‐κB signalling and gene expression by blocking IκB kinase activity in intestinal epithelial cells and bone‐marrow derived dendritic cells. Immunology, 115, 375–387. 10.1111/j.1365-2567.2005.02156.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krajewski, S. , Krajewska, M. , Shabaik, A. , Miyashita, T. , Wang, H. G. , & Reed, J. C. (1994). Immunohistochemical determination of in vivo distribution of Bax, a dominant inhibitor of Bcl‐2. The American Journal of Pathology, 145, 1323–1336. [PMC free article] [PubMed] [Google Scholar]
- Kwiecien, S. , Jasnos, K. , Magierowski, M. , Sliwowski, Z. , Pajdo, R. , Brzozowski, B. , … Brzozowski, T. (2014). Lipid peroxidation, reactive oxygen species and antioxidative factors in the pathogenesis of gastric mucosal lesions and mechanism of protection against oxidative stress‐induced gastric injury. Journal of Physiology and Pharmacology, 65, 613–622. [PubMed] [Google Scholar]
- Li, Q. , Zhang, X. , Wang, W. , Li, L. , Xu, Q. , Wu, X. , & Gu, Y. (2015). CPT‐11 activates NLRP3 inflammasome through JNK and NF‐κB signalings. Toxicology and Applied Pharmacology, 289, 133–141. 10.1016/j.taap.2015.09.025 [DOI] [PubMed] [Google Scholar]
- Li, Y. , Shen, L. , & Luo, H. (2016). Luteolin ameliorates dextran sulfate sodium‐induced colitis in mice possibly through activation of the Nrf2 signaling pathway. International Immunopharmacology, 40, 24–31. 10.1016/j.intimp.2016.08.020 [DOI] [PubMed] [Google Scholar]
- Lima, R. C. P. , Figueiredo, A. A. , Freitas, H. C. , Melo, M. L. P. , Wong, D. V. T. , Leite, C. A. V. G. , … Oriá, R. B. (2012). Involvement of nitric oxide on the pathogenesis of irinotecan‐induced intestinal mucositis: Role of cytokines on inducible nitric oxide synthase activation. Cancer Chemotherapy and Pharmacology, 69, 931–942. 10.1007/s00280-011-1780-z [DOI] [PubMed] [Google Scholar]
- Lin, L.‐C. , Pai, Y.‐F. , & Tsai, T.‐H. (2015). Isolation of luteolin and luteolin‐7‐O‐glucoside from Dendranthema morifolium Ramat Tzvel and their pharmacokinetics in rats. Journal of Agricultural and Food Chemistry, 63, 7700–7706. 10.1021/jf505848z [DOI] [PubMed] [Google Scholar]
- Liu, G. , Zhang, Y. , Liu, C. , Xu, D. , Zhang, R. , Cheng, Y. , … Chen, Y. (2014). Luteolin alleviates alcoholic liver disease induced by chronic and binge ethanol feeding in mice. The Journal of Nutrition, 144, 1009–1015. 10.3945/jn.114.193128 [DOI] [PubMed] [Google Scholar]
- Logan, R. M. , Gibson, R. J. , Bowen, J. M. , Stringer, A. M. , Sonis, S. T. , & Keefe, D. M. K. (2008). Characterisation of mucosal changes in the alimentary tract following administration of irinotecan: Implications for the pathobiology of mucositis. Cancer Chemotherapy and Pharmacology, 62, 33–41. 10.1007/s00280-007-0570-0 [DOI] [PubMed] [Google Scholar]
- Lv, G. Y. , Zhang, Y. P. , Gao, J. L. , Yu, J. J. , Lei, J. , Zhang, Z. R. , … Chen, S. H. (2013). Combined antihypertensive effect of luteolin and buddleoside enriched extracts in spontaneously hypertensive rats. Journal of Ethnopharmacology, 150, 507–513. 10.1016/j.jep.2013.08.058 [DOI] [PubMed] [Google Scholar]
- Meng, Q.‐Q. , Feng, Z.‐C. , Zhang, X.‐L. , Hu, L.‐Q. , Wang, M. , Zhang, H.‐F. , & Li, S. M. (2019). PPAR‐ γ activation exerts an anti‐inflammatory effect by suppressing the NLRP3 inflammasome in spinal cord‐derived neurons. Mediators of Inflammation, 2019, 1–12. 10.1155/2019/6386729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nabavi, S. F. , Braidy, N. , Gortzi, O. , Sobarzo‐Sanchez, E. , Daglia, M. , Skalicka‐Woźniak, K. , & Nabavi, S. M. (2015). Luteolin as an anti‐inflammatory and neuroprotective agent: A brief review. Brain Research Bulletin, 119, 1–11. 10.1016/j.brainresbull.2015.09.002 [DOI] [PubMed] [Google Scholar]
- Nishitani, Y. , Yamamoto, K. , Yoshida, M. , Azuma, T. , Kanazawa, K. , Hashimoto, T. , & Mizuno, M. (2013). Intestinal anti‐inflammatory activity of luteolin: Role of the aglycone in NF‐κB inactivation in macrophages co‐cultured with intestinal epithelial cells. BioFactors, 39, 522–533. 10.1002/biof.1091 [DOI] [PubMed] [Google Scholar]
- Nunes, C. , Almeida, L. , Barbosa, R. M. , & Laranjinha, J. (2017). Luteolin suppresses the JAK/STAT pathway in a cellular model of intestinal inflammation. Food & Function, 8, 387–396. 10.1039/c6fo01529h [DOI] [PubMed] [Google Scholar]
- Pandurangan, A. K. , & Esa, N. M. (2014). Luteolin, a bioflavonoid inhibits colorectal cancer through modulation of multiple signaling pathways: A review. Asian Pacific Journal of Cancer Prevention, 15, 5501–5508. [DOI] [PubMed] [Google Scholar]
- Puhl, A. C. , Bernardes, A. , Silveira, R. L. , Yuan, J. , Campos, J. L. O. , Saidemberg, D. M. , … Polikarpov, I. (2012). Mode of peroxisome proliferator‐activated receptor activation by luteolin. Molecular Pharmacology, 81, 788–799. 10.1124/mol.111.076216 [DOI] [PubMed] [Google Scholar]
- Ribeiro, R. A. , Wanderley, C. W. S. , Wong, D. V. T. , Mota, J. M. S. C. , Leite, C. A. V. G. , Souza, M. H. L. P. , … Lima‐Júnior, R. C. P. (2016). Irinotecan‐ and 5‐fluorouracil‐induced intestinal mucositis: Insights into pathogenesis and therapeutic perspectives. Cancer Chemotherapy and Pharmacology, 78, 881–893. 10.1007/s00280-016-3139-y [DOI] [PubMed] [Google Scholar]
- Ricote, M. , & Glass, C. K. (2007). PPARs and molecular mechanisms of transrepression. Biochimica et Biophysica Acta, 1771, 926–935. 10.1016/j.bbalip.2007.02.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross, J. A. , & Kasum, C. M. (2002). Dietary flavonoids: Bioavailability, metabolic effects, and safety. Annual Review of Nutrition, 22, 19–34. 10.1146/annurev.nutr.22.111401.144957 [DOI] [PubMed] [Google Scholar]
- Salib, J. Y. , Michael, H. N. , & Eskande, E. F. (2013). Anti‐diabetic properties of flavonoid compounds isolated from Hyphaene thebaica epicarp on alloxan induced diabetic rats. Pharmacognosy Research, 5, 22–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saria, A. , & Lundberg, J. M. (1983). Evans blue fluorescence: Quantitative and morphological evaluation of vascular permeability in animal tissues. Journal of Neuroscience Methods, 8, 41–49. [DOI] [PubMed] [Google Scholar]
- Seelinger, G. , Merfort, I. , & Schempp, C. M. (2008). Anti‐oxidant, anti‐inflammatory and anti‐allergic activities of luteolin. Planta Medica, 74, 1667–1677. 10.1055/s-0028-1088314 [DOI] [PubMed] [Google Scholar]
- Sellers, R. S. , Mortan, D. , Michael, B. , Roome, N. , Johnson, J. K. , Yano, B. L. , … Schafer, K. (2007). Society of toxicologic pathology position paper: Organ weight recommendations for toxicology studies. Toxicologic Pathology, 35, 751–755. 10.1080/01926230701595300 [DOI] [PubMed] [Google Scholar]
- Shimoi, K. , Okada, H. , Furugori, M. , Goda, T. , Takase, S. , Suzuki, M. , … Kinae, N. (1998). Intestinal absorption of luteolin and luteolin 7‐O‐β‐glucoside in rats and humans. FEBS Letters, 438, 220–224. 10.1016/s0014-5793(98)01304-0 [DOI] [PubMed] [Google Scholar]
- Smirnov, A. , Sklan, D. , & Uni, Z. (2004). Mucin dynamics in the chick small intestine are altered by starvation. The Journal of Nutrition, 134, 736–742. 10.1093/jn/134.4.736 [DOI] [PubMed] [Google Scholar]
- Soares, P. M. G. , Mota, J. M. S. C. , Gomes, A. S. , Oliveira, R. B. , Assreuy, A. M. S. , Brito, G. A. C. , … Souza, M. H. L. P. (2008). Gastrointestinal dysmotility in 5‐fluorouracil‐induced intestinal mucositis outlasts inflammatory process resolution. Cancer Chemotherapy and Pharmacology, 63, 91–98. 10.1007/s00280-008-0715-9 [DOI] [PubMed] [Google Scholar]
- Sonis, S. T. (2004). The pathobiology of mucositis. Nature Reviews. Cancer, 4, 277–284. 10.1038/nrc1318 [DOI] [PubMed] [Google Scholar]
- Stein, A. , Voigt, W. , & Jordan, K. (2010). Review: Chemotherapy‐induced diarrhea: Pathophysiology, frequency and guideline‐based management. Therapeutic Advances in Medical Oncology, 2, 51–63. 10.1177/1758834009355164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stringer, A. M. , Gibson, R. J. , Logan, R. M. , Bowen, J. M. , Yeoh, A. S. J. , Laurence, J. , & Keefe, D. M. K. (2009). Irinotecan‐induced mucositis is associated with changes in intestinal mucins. Cancer Chemotherapy and Pharmacology, 64, 123–132. 10.1007/s00280-008-0855-y [DOI] [PubMed] [Google Scholar]
- Suzuki, T. (2013). Regulation of intestinal epithelial permeability by tight junctions. Cellular and Molecular Life Sciences, 70, 631–659. 10.1007/s00018-012-1070-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szanto, A. , Balint, B. L. , Nagy, Z. S. , Barta, E. , Dezso, B. , Pap, A. , … Nagy, L. (2010). STAT6 transcription factor is a facilitator of the nuclear receptor PPARγ‐regulated gene expression in macrophages and dendritic cells. Immunity, 33, 699–712. 10.1016/j.immuni.2010.11.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanhoecke, B. , Bateman, E. , Mayo, B. , Vanlancker, E. , Stringer, A. , Thorpe, D. , & Keefe, D. (2015). Dark Agouti rat model of chemotherapy‐induced mucositis: Establishment and current state of the art. Experimental Biology and Medicine, 240, 725–741. 10.1177/1535370215581309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wardill, H. R. , Bowen, J. M. , Al‐Dasooqi, N. , Sultani, M. , Bateman, E. , Stansborough, R. , … Gibson, R. J. (2014). Irinotecan disrupts tight junction proteins within the gut: Implications for chemotherapy‐induced gut toxicity. Cancer Biology & Therapy, 15, 236–244. 10.4161/cbt.27222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wardill, H. R. , Bowen, J. M. , & Gibson, R. J. (2012). Chemotherapy‐induced gut toxicity: Are alterations to intestinal tight junctions pivotal. Cancer Chemotherapy and Pharmacology, 70, 627–635. 10.1007/s00280-012-1989-5 [DOI] [PubMed] [Google Scholar]
- Wardill, H. R. , Gibson, R. J. , Van Sebille, Y. Z. A. , Secombe, K. R. , Coller, J. K. , White, I. A. , … Bowen, J. M. (2016). Irinotecan‐induced gastrointestinal dysfunction and pain are mediated by common TLR4‐dependent mechanisms. Molecular Cancer Therapeutics, 15, 1376–1386. 10.1158/1535-7163.MCT-15-0990 [DOI] [PubMed] [Google Scholar]
- Zhang, B.‐C. , Li, Z. , Xu, W. , Xiang, C.‐H. , & Ma, Y.‐F. (2018). Luteolin alleviates NLRP3 inflammasome activation and directs macrophage polarization in lipopolysaccharide‐stimulated RAW264.7 cells. American Journal of Translational Research, 10, 265–273. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Effect of luteolin on digestive motility rate (%). (A) Resting state. The results are expressed as mean ± SEM (n = 6). Statistical analysis was performed using one‐way ANOVA followed by Bonferroni's post hoc test. *P < .05 compared with the vehicle group (Veh: water plus 1% tween. 10 ml·kg−1. p.o.); Atp: Atropine (3 mg·kg−1. s.c.). (B) Diarrheal state. Female mice were treated for 14 days by oral route with vehicle (Veh: water plus Tween 1%, 10 ml·kg−1) or luteolin (30 mg·kg−1). Irinotecan was given by intraperitoneal route (75 mg·kg−1, from 7th to 10th day) for Veh and Luteolin‐treated groups. Digestive motility rate was evaluated on the 15th day of experiment. The results are expressed as mean ± SEM (n = 6). Statistical analysis was performed using one‐way ANOVA followed by Bonferroni's post hoc test. # P < .05 compared to the naive.
Figure S2. Effect of luteolin treatment by oral route on irinotecan‐induced intestinal mucositis. Female mice were treated for 7 days by oral route with vehicle (Veh: water plus Tween 1%, 10 ml·kg−1) or luteolin (Lut 30 mg·kg−1). Irinotecan was given by intraperitoneal route (75 mg·kg−1, from 1st to the 4th day) for Veh and Luteolin‐treated groups. (A) The weight of the animals was measured daily. Weight loss was significantly different from the 5th to 7th day of experiment in both treated groups compared to naive. The results are expressed as mean ± SEM (n = 10). Statistical analysis was performed using two‐way ANOVA followed by Bonferroni's post hoc test. (B) The diarrhea score was evaluated on the 7th day of treatment. The results are expressed as median with interquartile range (n = 10). Statistical analysis was performed by Kruskal‐Wallis followed by Dunn's test. *P < .05 compared to vehicle. #P < .05compared to naive group. (C and D) Weight/length ratio of duodenum and colon. The results are expressed as mean ± SEM (n = 10). Statistical analysis was performed using one‐way ANOVA followed by Bonferroni's post hoc test. #P < .05 compared to the naive group.
Figure S3. Effect of luteolin by oral route in the mechanical hypersensitivity of irinotecan‐exposed mice. Female mice were treated by oral route with vehicle (Veh: water plus Tween 1%, 10 ml·kg−1) or luteolin (Lut mg·kg−1). From the 7th to 10th day irinotecan was intraperitoneally given at a dose of 75 mg·kg−1 per day to the Veh and Lut groups and the mechanical hypersensitivity was measured by von Frey filament. (A) Response frequency (%) and (C) mechanical withdrawal threshold (g) are presented followed their respective A.U.C. (B and D). The results are expressed as mean ± SEM (n = 10). Statistical analysis was performed using two‐way ANOVA followed by Bonferroni's post hoc test. *P < .05 compared to vehicle. #P < .05 compared to naive group.
Figure S4. Effect of luteolin on tight junctions (TJs) expression in the colon of irinotecan‐exposed mice. Immunofluorescence staining of the colon for Occludin (A) and ZO‐1 (C) protein was performed (green). Nuclei are stained with DAPI (blue). Non‐relevant IgG was used as control. Magnification ×20. (B, D) Fluorescence was quantified with ImageJ® program. Six fields per animal were analyzed (n = 5). The results are expressed as mean ± SEM. Statistical analysis was performed using one‐way ANOVA followed by Bonferroni's post hoc test. *P < .05 compared to vehicle group.
Figure S5. Luteolin does not interfere with the antitumor activity of Irinotecan. Melanoma cells (B16F10) were injected in the flank of C57BL/6 mice and after the tumor development, the animals were treated for 7 days with control (water plus Tween 1%, 10 ml·kg−1, p.o.), luteolin (Lut 30mg·kg−1, p.o.), irinotecan (Iri 75 mg·kg−1, i.p.) or luteolin plus irinotecan (Lut + Iri). Tumor area (A) was measured daily. Tumor weight (C) and tumor volume (D) were measured after euthanasia of mice. The results are expressed as mean ± SEM (n = 10). Statistical analysis was performed using one‐way ANOVA followed by Bonferroni's post hoc test. (A) *P < .05 compared Irinotecan and Lut + Iri to control group. (C and D) #P < .05 compared to vehicle group without irinotecan.
Figure S6. Luteolin does not affect intestinal epithelial cell viability and does not alter the irinotecan‐induced cytotoxicity. Murine epithelial cells (5 × 104 cells/well) were seeded in 96‐well plate and cell viability was measured using the MTT assay. Basal (DMEM plus DMSO 0.1%); DMSO (DMEM plus DMSO 10%); irinotecan (I: 3‐30 μM); luteolin (L: 3‐30 μM). The results are expressed as mean ± SEM (n = 3 replicates). Statistical analysis was performed using one‐way ANOVA followed by Bonferroni's post hoc test. * P < .05 when compared to basal group.
Figure S7. Effect of Luteolin on in vitro ability to scavenge the free‐radical DPPH. The results are expressed as mean ± SEM. Statistical analysis was performed using one way analysis of variance (ANOVA) followed by Bonferroni test. IC50 of ~1.84 μg/ml. *P < .05 compared to the vehicle group. Vehicle (Veh: water). Ascorbic acid (AA: 50 μg/ml).
Table S1. Effects of Luteolin on relative organ weights of irinotecan‐exposed mice.
Table S2. Effects of Luteolin on plasma biochemistry of irinotecan‐exposed mice.
Method S1. Evaluation of digestive motility rate
Method S2. Effect of Luteolin treatment during 7 days on irinotecan‐induced intestinal mucositis.
Method S3. Mechanical withdrawal threshold evaluation
Method S4. Biochemical analyses
Method S5. Evaluation of antitumor activity
Method S6. Cell viability
Method S7. DPPH· radical scavenging assay


