Abstract
Background
Leonurine is an active component of the traditional Chinese medicine Leonurus japonicus. This study aimed to investigate the effects of overexpressed CYP450s on the metabolic activity of leonurine.
Material/Methods
BEAS-2B cells stably expressing CYP1A1, 1A2, 2A13, 2B6, and 3A4 were constructed. CYP450s expression was identified using reverse-transcription PCR and Western blot assay. CCK-8 assay was used to evaluate the effect of leonurine on cell activity. Leonurine was incubated in vitro with CYP1A1, 1A2, 2A13, 2B6, and 3A4 metabolic enzymes to evaluate the clearance rate of CYP450 enzymes for leonurine. UPLC-MS was used to detect changes of drug concentration and discover the main metabolic enzymes affecting leonurine.
Results
BEAS-2B cells stably expressing CYP1A1, 1A2, 2A13, 2B6, and 3A4 were successfully constructed. According to primary mass spectra and secondary mass spectra of leonurine, the main metabolic enzymes were 312.1550 [H+] and 181.0484. Compared to the control group, residue of leonurine in CYP2A13 group was significantly reduced (F=5.307, p=0.024). Compared to the 0-min group, the clearance rate of leonurine in the CYP2A13-treated group was significantly decreased at 120 min after treatment (F=7.273, p=0.007). CCK-8 results also showed that activity of BEAS-2B cells that overexpress CYP2A13 gradually decreased with increased concentration of leonurine. Although CYP2A13 demonstrated good metabolic activity for leonurine, we found that CYP1A1, 1A2, 2B6, and 3A4 had no metabolic effects on leonurine.
Conclusions
Leonurine can be effectively activated through CYP2A13 enzyme metabolism, and further inhibits activity of human lung epithelial cells (BEAS-2B). Therefore, CYP2A13 is a main metabolic enzyme for leonurine in BEAS-2B cells.
MeSH Keywords: Cytochrome P-450 Enzyme System; Leonurus; Medicine, Chinese Traditional; Respiratory System
Background
Leonurine, one of the main active components of the traditional Chinese medicine Leonurus japonicus, has a wide range of biological activities [1]. Leonurine has been widely applied in treatment of gynecological diseases in the past [2]. Purified leonurine (molecular structure formula: C14H21CIN3O5) is a nitrogenous compound used in the form of a white crystalline powder. Recent research shows that leonurine has obvious effects on protecting nerves [3] and promoting growth of synapses, as well as promoting neurite outgrowth and neurotrophic activity [4], alleviating LPS-induced myocarditis [5], and preventing and treating non-alcoholic fatty liver disease [6]. Leonurine is also toxic, mainly affecting the kidneys, liver, and reproductive system [7,8]. However, the toxicity of leonurine to the respiratory system has been unclear.
Previous studies [9,10] showed that many kinds of traditional Chinese medicine are toxic to the respiratory system, such as total saponins of Cortex albiziae (isolated from stem bark of Albizzia julibrissin Durazz), which can induce apoptosis of mouse bronchial epithelial cells and results in oxidative damage to lung tissues. The traditional Chinese medicine-induced respiratory diseases mainly include drug-induced pulmonary edema, drug-induced asthma, drug-induced pneumonia, and drug-induced pulmonary vascular diseases [11]. Moreover, drug-induced DNA damage and cell apoptosis can eventually lead to cytotoxicity and other adverse effects in cells [12]. Therefore, with the development of research on the pharmacological effects of leonurine on the respiratory system and with its increased clinical use, there is an urgent need to assess the toxicity of leonurine to the respiratory system.
Drug activity is usually affected by pharmacokinetic and pharmacodynamic factors. Cytochrome P450 (CYP450), as an enzyme of phase I reaction, is a critical pharmacokinetic factor [13]. Recently, it has been found that CYP450 gene polymorphism is closely related to cancer susceptibility [14]. Many carcinogens need activation by the CYP450 enzyme to exert toxicity. The carcinogenic aromatics in smog can be activated by CYP1A1 [15], and aflatoxins are mainly metabolized by CYP1A2 and 3A4 enzyme [16]. However, a recent study [17] reported that CYP1A1, 1A2, 2A13, 2B6, 3A4, and the other P450 metabolic enzymes are highly expressed in the respiratory system. These P450 metabolic enzymes can oxidize, reduce, or hydrolyze the related prototypes to produce a series of toxic substances. However, the main metabolic enzymes for leonurine in the respiratory system have not been fully assessed.
To determine the main metabolic enzyme targeting leonurine in lung tissues, we must clarify the metabolic activity of leonurine and prevent occurrence of adverse reactions. Therefore, the present study explored the effect of highly-expressed CYP450 enzymes in human lung epithelial cells (BEAS-2B) on the metabolic activity of leonurine. This study aimed to provide a theoretical basis for evaluating the toxicity of leonurine in the respiratory system.
Material and Methods
Cell and plasmid synthesis
In this study, CYP1A1, 1A2, 2A13, 2B6, and 3A4 gene-expressing lentivirus plasmids pYr-Lvsh-CYP450s were synthesized by MiaoLing Bio. Sci. Tech. Co. (Wuhan, China). The related lentivirus expression plasmids (pMD2G and psPAX) were purchased from MiaoLing Bio. Sci. Tech. Co. (Wuhan, China). After sequencing and identification, the Lentivirus Expression System plasmids of CYP450, including pYr-Lvsh-CYP1A1, pYr-Lvsh-1A2, pYr-Lvsh-2A13, pYr-Lvsh-2B6, and pYr-Lvsh-3A4, were successfully constructed.
Construction of lentivirus vector
Human lung epithelial cells (BEAS-2B) and 293T cells (ATCC Cell Bank, Manassas, VA, USA) were cultured in DMEM complete medium (Gibco BRL. Co., Grand Island, New York, USA) at 37°C and 5% CO2. The 293T cells at logarithmic growth stage were digested and sub-cultured into a cell plate and cultured to 80% confluence. A total of 3 μg pMD2G, 6 μg psPAX, 7.5 μg vector plasmids, 25 μl Lipofectamine 2000 Transfection Reagent (Invitrogen/Life Technologies, Carlsbad, CA, USA), and 150 μl OPTI-MEM (Gibco BRL. Co.) were mixed together. The mixture was rested at room temperature for 15 min, then we added it dropwise to cell plates and mixed it. After 6-h culture, the DMEM was replaced with fresh DMEM containing 10% fetal bovine serum (FBS, Gibco BRL. Co.).
Lentivirus infection
When BEAS-2B cells reached 80–90% confluence, the collected lentivirus solution was added to cell plates. The cells were transfected with the above synthesized lentivirus vectors and cultured for 24 h. This transfection was repeated 3 times. Finally, BEAS-2B cells were purified and screened using puromycin (Sigma-Aldrich, St. Louis, MO, USA).
Western blot analysis showed that CYP450s expressed BEAS-2B
Cells were washed twice with phosphate-buffered saline (PBS, Sangon Biotech. Co., Shanghai, China), lysed using Tris lysate (Tiangen Biotech Co., Beijing, China) for 1 min, and then cracked by ultrasound. After centrifugation at 10 000 rpm, the supernatant was harvested and stored at −20°C. The concentration of harvested protein was measured using a BCA protein assay kit (Cat. No. P0011, Beyotime Biotech, Shanghai, China). Then, the proteins were loaded onto SDS-PAGE gel and transferred onto PVDF membranes (Amersham Biosciences, Piscataway, NJ, USA). The PVDF membranes were blocked with 5% FBS for 1 h and then incubated with rabbit anti-human 1A1 antibody (Cat. No. AB10088), rabbit anti-human 1A2 antibody (Cat. No. AB1248), rabbit anti-human 2A13 (Cat. No. ABN201), rabbit anti-human 2B6 (Cat. No. AB1267), rabbit anti-human 3A4 antibody (Cat. No. MAB10041), and rabbit anti-human GAPDH antibody (Cat. No. MAB374) overnight at 4°C. Then, PVDF membranes were incubated using HRP-labeled goat anti-rabbit IgG (Cat. No. AP307P) at room temperature for 2 h. All of the above antibodies were purchased from Sigma-Aldrich (St. Louis, MO, USA). The protein bands were imaged with electrochemiluminescence (ECL, Amersham Biosciences) and analyzed using the UVP gel image scanning system (version: Labworks 4.6, Bio-Rad Laboratories, Hercules, CA, USA).
RT-PCR showed that CYP450s expressed BEAS-2B
The total RNAs were extracted using Trizol reagent (Invitrogen/Life Technologies), and then reverse-transcribed into cDNA. The synthesized cDNAs were used as the template for PCR amplification. The PCR amplification procedure was: pre-denaturation at 95°C for 5 min, denaturation at 94°C for 30 s, annealing at 55°C for 1 min, with 40 cycles, and then extension at 72°C for 10 min. GAPDH was used as an internal reference gene and identified using 1% agarose gel electrophoresis (Beyotime Biotech). Premiers for PCR assay are listed in Table 1. The 2−ΔΔCt method [18] was used to analyze the RT-PCR findings.
Table 1.
Primers for CYP450s amplification for PCR assay.
| Enzyme genes | Sequences (5′-3′) | |
|---|---|---|
| CYP1A1 | Forward | GATTCCTTTTCCCAATCTCCATGTC |
| Reverse | CTAAGAGCGCAGCTGCATTTGGA | |
| CYP1A2 | Forward | GAATTCGCATTGTCCCAGTCTG |
| Reverse | TCAATTGATGGAGAAGCGCAGC | |
| CYP2A13 | Forward | GAATTCCTGGCCTCAGGGCT |
| Reverse | TCAGCGGGGCAGGAAGCTCA | |
| CYP2B6 | Forward | GAATTCGAACTCAGCGTCCTCC |
| Reverse | TCAGCGGGGCAGGAAGC | |
| CYP3A4 | Forward | GAATTCGCTCTCATCCCAGACTT |
| Reverse | TCAGGCTCCACTTACGGTGC |
Cell Counting Kit 8 (CCK-8) assay
The leonurine standard solution was prepared as follows: a total of 10 mg leonurine standard was weighed and dissolved in double-distilled water to prepare the leonurine standard solution at a final concentration of 1000 μg/ml. Then, the solution was diluted into 100 μg/ml and 10 μg/ml standard solutions.
The cells at logarithmic growth stage were inoculated into 96-well plates at a density of 1×104 cells/ml. Then, 200 μl of cells was added into each well and cultured in an incubator for 24 h. Cells were divided into 6 groups: B-2B, B-1A1, B-1A2, B-2A13, B-2B6, and B-3A4. Each group was incubated in advance with the standard prepared leonurine. The final concentration of leonurine in each group was adjusted to 0 μg/ml, 1 μg/ml, 10 μg/ml, 100 μg/ml, respectively. About 24 h after cell culture, 20 μl CCK-8 was added into each well and placed into the incubator for 1 h. The optical density (OD) value of each pore at 450 nm was determined using a microplate reader (Model: 680, Bio-Rad Laboratories, Hercules, CA, USA). In this study, the “cell viability” was defined as the ratios of the OD values in 1 μg/ml, 10 μg/ml, and 100 μg/ml leonurine-treated cells to the OD value in the 0 μg/ml leonurine-treated cells in every group (B-2B, B-1A1, B-1A2, B-2A13, B-2B6, and B-3A4).
Metabolism in vitro
Five P450 recombinant enzymes – CYP1A1, 1A2, 2A13, 2B6, and 3A4 – were metabolized in vitro. The incubation system was as follows: leonurine (final concentration 0.06 mg/ml, MedChemExpress, Princeton, NJ, USA), recombinase (final concentration 0.06 mg/ml, Sigma-Aldrich), Tris-HCl (final concentration 50 mmol/l, Beyotime Biotech), MgCl2 (final concentration 5 mmol/l, Sigma-Aldrich), and EDTA (final concentration 1 mmol/l, Sigma-Aldrich). Then, NADPH was added to initiate the reaction and continue incubation at 37°C. For the 0-min group, the reaction was terminated using methanol directly. For the 120-min group, methanol was added after the incubation for 120 min to stop the reaction.
UPLC-HRMS analysis
The leonurine samples were analyzed using UPLC-HRMS analysis as previously described [19]. Briefly, the LC-MS/MS system (Model: TSQ800, Thermo Fisher Scientific, USA) was used, following the manufacturer’s protocols. Leonurine was carried out in positive ionization mode. The source temperature was adjusted to 320°C. The ion source voltage was adjusted to 3200 V. Flows of nebulizer, curtain, and collision nitrogen were optimized to maximize the signal intensity ratio. The transition ranging from m/z (312.1553) to m/z (181.0484) (40% collision-energy) was monitored for leonurine. The time window for the UPLC-HRMS mode was defined as 0–7 min.
Statistical analysis
Measurement data were recorded as mean±standard deviation (SD) and analyzed using SPSS software 20.0 (SPSS, Inc., Chicago, IL, USA). The Tukey’s post hoc test-validated ANOVA was used to compare data among groups. p<0.05 was defined as a significant difference.
Results
Stable CYP450-expressing cell line was successfully constructed
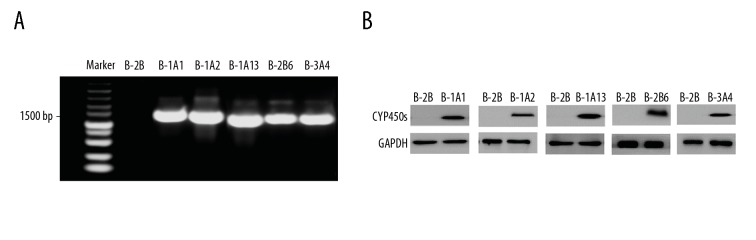
The PCR results showed that there were bands at 1500 bp in each CYP450-expressing cell line (Figure 1A), which indicates that CYP1A1, 1A2, 2A13, 2B6, and 3A4 were stably overexpressed in BEAS-2B cells. Western blot assay also showed that CYP1A1, 1A2, 2A13, 2B6, and 3A4 protein expressions in each experimental group were significantly higher compared to that in each control group (Figure 1B, F=5.307, p=0.024), indicating that CYP450 was successfully expressed in BEAS-2B cells. The above PCR assay and Western blot assay suggest that the stable CYP450-expressing cell line was successfully constructed.
Figure 1.
mRNA and protein identification of BEAS-2B cell line. (A) PCR assay validation of cell lines. (B) Western blot verification of cell lines.
Primary and secondary mass spectra of leonurine
After incubation with CYP1A1, 1A2, 2A13, 2B6, and 3A4 enzymes, the main metabolic enzymes for leonurine were screened (Figure 2). According to the primary mass spectra (Figure 2A) and secondary mass spectra (Figure 2B) of leonurine, the main metabolic enzymes were 312.1550 [H +] and 181.0484.
Figure 2.
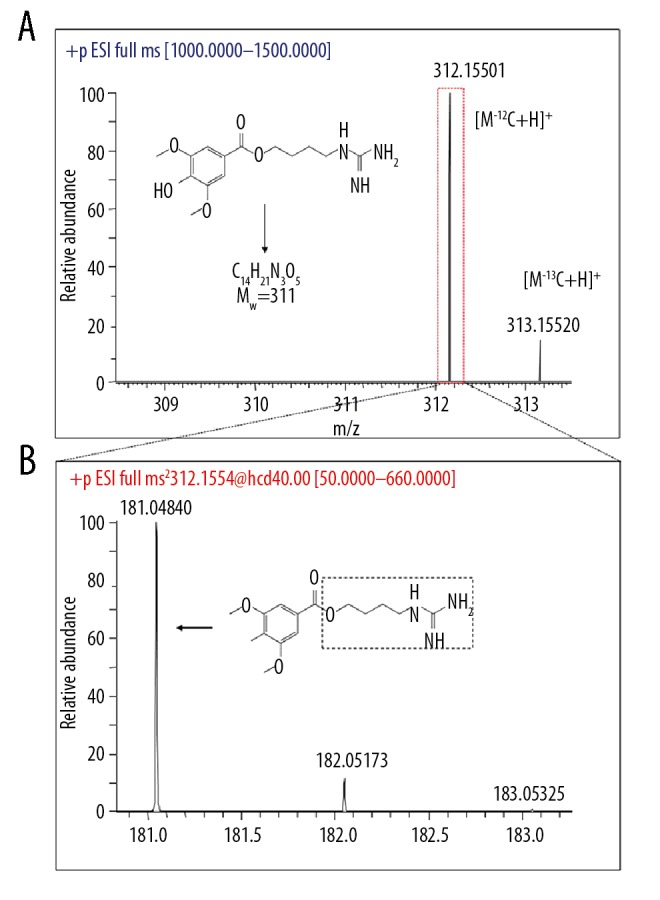
Chromatogram and mass spectrum of leonurine. (A) Primary mass spectrum (resolution R=70000). (B) Secondary mass spectrum (resolution R=13500).
CYP2A13 decreased the concentration of leonurine
The results of metabolic removal rate showed that, compared to the control group, the concentration of leonurine in CYP2A13 group was significantly lower (Figures 2, 3). However, there were no significant changes in the other groups (Figure 3). These results suggest that CYP2A13 is the main metabolic enzyme for leonurine in BEAS-2B cells.
Figure 3.
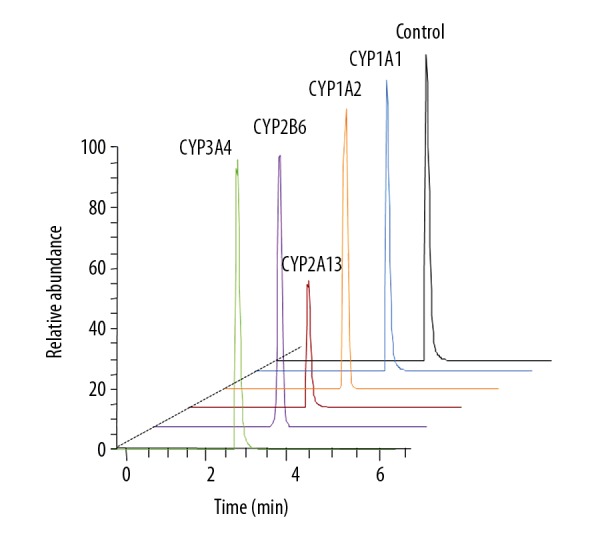
Chromatogram of leonurine standard solution and reaction in CYP450 enzyme system after 120 min.
CYP2A13 demonstrated higher clearance rate of leonurine
In this study, the clearance rates of leonurine in different CYP450s enzymes-treated groups were determined. The results showed that, compared to the 0-min group, the clearance rate of leonurine in the CYP2A13-treated group was significantly lower at 120 min after treatment (Figure 4, F=7.273, p=0.007). However, there were no significant differences in the clearance of leonurine in the other CYP450s-treated groups at 120 min after treatment compared to that at 0 min (Figure 4, F=2.607, p=0.075).
Figure 4.
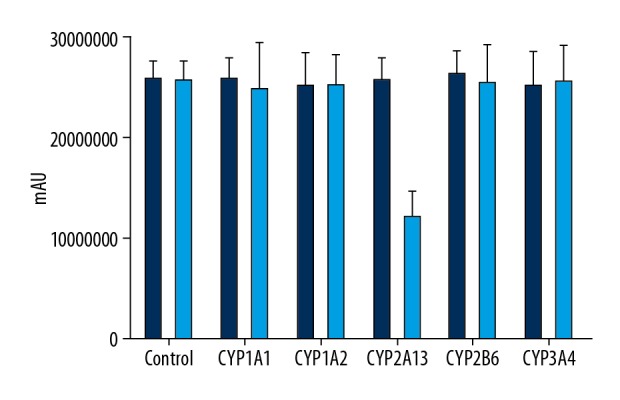
Clearance rate of leonurine in BEAS-2B cells undergoing CYP450 enzyme treatments. ** p<0.01 vs. Control group.
CYP2A13 induced the cytotoxicity of leonurine
The cytotoxicity of BEAS-2B cells treated with different dosages of leonurine was measured using CCK-8 assay. The results indicated that after increasing the concentration of leonurine, there were no significant effects of leonurine on cytotoxicity to BEAS-2B cells in the 1A1 group (F=1.015, p=0.594), 1A2 group (F=0.892, p=0.603), 2B6 group (F=0.791, p=0.714), and 3A4 group (F=1.203, p=0.591) (Figure 5). However, different dosages of CYP2A13 treatment (10 μg/ml and 100 μg/ml) significantly reduced the cell viability of leonurine compared to that in the control group (Figure 5, F=5.291, p=0.029). Therefore, the CYP2A13 treatment can by cytotoxic to BEAS-2B cells.
Figure 5.
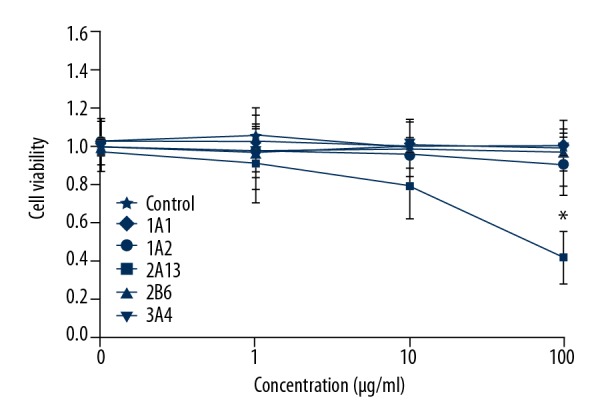
Determination of cytotoxicity in BEAS-2B cells undergoing CYP450 enzyme treatments. * p<0.05 vs. Control group.
Discussion
In recent years, clinical use of leonurine has increased, but its toxicity to the respiratory system has not been fully studied. A previous study [20] found that respiratory system damage is mainly caused by air pollution, but ignored the toxicity of drugs. The current high incidence of respiratory diseases suggests that the respiratory toxicity caused by drugs might play an important role [21]. A previous study [22] reported that leonurine can significantly inhibit the proliferation of lung cancer H292 cells by inducing cell cycle arrest in G0/G1 cells. The activation of leonurine mainly depends on binding of glucuronic acid molecules [23]; therefore, it is crucial to determine the metabolic enzymes for leonurine. Our results showed that CYP2A13 was the main metabolic enzyme for leonurine in human lung epithelial cells. CCK-8 results also showed that cell activity in the CYP2A13 metabolic enzyme group was significantly inhibited, but there was no significant difference among the other groups. These results suggest that leonurine is metabolized and activated by CYP2A13 in the respiratory system, finally leading to cytotoxicity.
Human cytochrome P4502A13 (CYP2A13) is a member of the cytochrome P450 family and acts as an enzyme for metabolism of exogenous chemicals in the human body [24]. CYP2A13 is usually found in the respiratory system and demonstrates metabolic effects on 5-hydroxymethylfurfural (5-HMF), nicotine, coumarin, and aflatoxin [25]. In recent years, it has reported that the CYP450 gene is altered in cancer patients. Słowikowski et al. [26] reported that CYP1B1 expression in lung cancer patients is significantly inhibited. It has also been shown that CYP2A13 is significantly increased in patients with non-small cell lung cancer [27]. The present results showed that leonurine was metabolized to a toxic metabolite by CYP2A13, which further induced cytotoxicity. However, the specific mechanism underlying this cytotoxicity was not defined in the present study. A previous study [28] also reported that CYP2A13 induces lung cancer [28]. Therefore, leonurine could be used as a therapeutic drug for controlling respiratory system-associated diseases.
To explore the main metabolic enzymes for leonurine in the respiratory system, we selected the human normal pulmonary epithelial cell line BEAS-2B. Our preliminary experiments proved that CYP450s are not detected in BEAS-2B cells. Therefore, the CYP metabolic enzyme gene was stably transfected by molecular biological means to construct a cell model in this study, and the successful construction of the cell line was also demonstrated by the high protein and mRNA levels.
According to a previous study [29], cell activity is related to tumor development. Changes in cell activity can reflect cell damage, and even affect the imbalance of tumor-suppressor genes and oncogenes. Therefore, CCK-8 assay was used to detect cell activity to preliminarily assess the cytotoxicity of leonurine to cells after CYP2A13 metabolic activation. Compared with CYP2A13, leonurine demonstrated little effect on the cell activity of the other groups. However, the activity of CYP2A13 cells rapidly decreased in a dose-dependent manner, which is consistent with the high metabolic activation rate of leonurine in our in vitro incubation experiment. CYP2A13 also demonstrated a higher clearance rate of leonurine and induced higher leonurine cytotoxicity. All the above results suggest that CYP2A13 is a main metabolic enzyme for leonurine, while activated leonurine might have a cytostatic effect on CYP2A13 expressed cells.
Although this study produced some interesting findings, there are also a few limitations. First, the kind of reaction catalyzed by CYP2A13 on leonurine was not analyzed in this study. Second, this study only investigated the effects of CYP2A13 on leonurine in human BEAS-2B cells in vitro, but not in vivo. Also, whether the toxic activity of leonurine is biologically possible in the human respiratory system was not determined. Therefore, the optimal concentration (toxic activity) reached by leonurine in the human respiratory system has not been clarified, which is important for the further clinical use of leonurine. Third, this study did not investigate the effects of leonurine on cancer and associated mechanisms. In subsequent research, we plan to further assess the effects of leonurine and to perform relevant epidemiological investigations.
Conclusions
In this study, BEAS-2B cells overexpressing CYP450s enzymes were constructed. We screened the main metabolic enzymes for leonurine in human lung epithelial cells through cell activity experiments and in vitro incubation experiments. This study confirmed that leonurine can be effectively activated in human lung epithelial cells through CYP2A13, and it can cause cytotoxicity to BEAS-2B cells. Therefore, CYP2A13 is the main metabolic enzyme of leonurine in human lung epithelial cells. The present findings may provide a theoretical basis for investigations of the toxicity and functions of the CYP2A13 gene and leonurine in the respiratory system.
Footnotes
Conflict of interest
None.
Source of support: This study was funded by the Basic Research Plan of Jiangsu Province (Natural Science Foundation)-Youth Fund Project (Grant No. BK20180678), Project of “Nursing Science” Funded by Priority Discipline Development Program of Jiangsu Higher Education Institutions (General Office, the People’s Government of Jiangsu Province, Grant No. [2018]-No.87) and the Project of “Nursing Science” Funded by the Key Discipline Program of Jiangsu Province during the 13th five-year plan (Teaching and Research Office, the People’s Government of Jiangsu Province, Grant No. [2016]-No. 9)
References
- 1.Hu PF, Sun FF, Qian J. Leonurine exerts anti-catabolic and anti-apoptotic effects via nuclear factor kappa B (NF-κB) and mitogen-activated protein kinase (MAPK) signaling pathways in chondrocytes. Med Sci Monit. 2019;25:6271–80. doi: 10.12659/MSM.916039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhu YZ, Wu W, Zhu Q, et al. Discovery of leonurine and therapeutical applications: From bench to bedside. Pharmacol Ther. 2018;188:26–35. doi: 10.1016/j.pharmthera.2018.01.006. [DOI] [PubMed] [Google Scholar]
- 3.Xie YZ, Zhang XJ, Zhang C, et al. Protective effects of leonurine against ischemic stroke in mice by activating nuclear factor erythroid 2-related factor 2 pathway. CNS Neurosci Ther. 2019;25:1006–17. doi: 10.1111/cns.13146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Meng P, Zhu Q, Yang H, et al. Leonurine promotes neurite outgrowth and neurotrophic activity by modulating the GR/SGK1 signaling pathway in cultured PC12 cells. Neuroreport. 2019;30:247–54. doi: 10.1097/WNR.0000000000001180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang R, Li D, Ouyang J, et al. Leonurine alleviates LPS-induced myocarditis through suppressing the NF-small ka, CyrillicB signaling pathway. Toxicology. 2019;422:1–13. doi: 10.1016/j.tox.2019.04.011. [DOI] [PubMed] [Google Scholar]
- 6.Zhang L, Li HX, Pan WS, et al. Novel hepatoprotective role of Leonurine hydrochloride against experimental non-alcoholic steatohepatitis mediated via AMPK/SREBP1 signaling pathway. Biomed Pharmacother. 2019;110:571–81. doi: 10.1016/j.biopha.2018.12.003. [DOI] [PubMed] [Google Scholar]
- 7.He YL, Shi JY, Peng C, et al. Angiogenic effect of motherwort (Leonurus japonicus) alkaloids and toxicity of motherwort essential oil on zebrafish embryos. Fitoterapia. 2018;128:36–42. doi: 10.1016/j.fitote.2018.05.002. [DOI] [PubMed] [Google Scholar]
- 8.Li Y, Lin Y, Huang X, et al. SCM-198 protects endometrial stromal cells from oxidative damage through Bax/Bcl-2 and ERK signaling pathways. Acta Biochim Biophys Sin (Shanghai) 2019;51(6):580–87. doi: 10.1093/abbs/gmz035. [DOI] [PubMed] [Google Scholar]
- 9.Li CW, Chen ZW, Wu XL, et al. A standardized Traditional Chinese Medicine preparation named Yejuhua Capsule ameliorates lipopolysaccharide-induced acute lung injury in mice via downregulating Toll-like receptor 4/nuclear factor-κB. Evid Based Complement Alternat Med. 2015;2015 doi: 10.1155/2015/264612. 264612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang C, Yu Y. Synergistic cytotoxicity of beta-elemene and cisplatin in gingival squamous cell carcinoma by inhibition of STAT3 signaling pathway. Med Sci Monit. 2017;23:1507–13. doi: 10.12659/MSM.903783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Babu KS, Marshall BG. Drug-induced airway diseases. Clin Chest Med. 2004;25:113–22. doi: 10.1016/S0272-5231(03)00136-9. [DOI] [PubMed] [Google Scholar]
- 12.Wu M, Wang X, McGregor N, et al. Dynamic regulation of Rad51 by E2F1 and p53 in prostate cancer cells upon drug-induced DNA damage under hypoxia. Mol Pharmacol. 2014;85:866–76. doi: 10.1124/mol.113.090688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Magliocco G, Thomas A, Desmeules J, et al. Phenotyping of human CYP450 enzymes by endobiotics: Current knowledge and methodological approaches. Clin Pharmacokinet. 2019;58:1373–91. doi: 10.1007/s40262-019-00783-z. [DOI] [PubMed] [Google Scholar]
- 14.Elfaki I, Mir R, Almutairi FM, et al. Cytochrome P450: Polymorphisms and roles in cancer, diabetes and atherosclerosis. Asian Pac J Cancer Prev. 2018;19:2057–70. doi: 10.22034/APJCP.2018.19.8.2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oztas E, Ozhan G, Daly AK. The effect of CYP1A/2A allele on colorectal cancer susceptibility in a British population. Genet Test Mol Biomarkers. 2016;20:475–77. doi: 10.1089/gtmb.2016.0099. [DOI] [PubMed] [Google Scholar]
- 16.Chen Y, Liu Y. Non-coplanar and coplanar polychlorinated biphenyls potentiate genotoxicity of aflatoxin B1 in a human hepatocyte line by enhancing CYP1A2 and CYP3A4 expression. Environ Pollut. 2019;246:945–54. doi: 10.1016/j.envpol.2018.12.041. [DOI] [PubMed] [Google Scholar]
- 17.Deng J, Zhao L, Zhang NY, et al. Aflatoxin B1 metabolism: Regulation by phase I and II metabolizing enzymes and chemoprotective agents. Mutat Res. 2018;778:79–89. doi: 10.1016/j.mrrev.2018.10.002. [DOI] [PubMed] [Google Scholar]
- 18.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 19.Yang Y, Zhang XG, Yu F, et al. Analysis of common herbicides in blood by UPLC-HRMS. Fa Yi Xue Za Zhi. 2018;34:590–94. doi: 10.12116/j.issn.1004-5619.2018.06.003. [DOI] [PubMed] [Google Scholar]
- 20.Losacco C, Perillo A. Particulate matter air pollution and respiratory impact on humans and animals. Environ Sci Pollut Res Int. 2018;25:33901–10. doi: 10.1007/s11356-018-3344-9. [DOI] [PubMed] [Google Scholar]
- 21.Chen X, Liu L, Guo Z, et al. UGT1A1 polymorphisms with irinotecan-induced toxicities and treatment outcome in Asian with lung cancer: A meta-analysis. Cancer Chemother Pharmacol. 2017;79:1109–17. doi: 10.1007/s00280-017-3306-9. [DOI] [PubMed] [Google Scholar]
- 22.Mao F, Zhang L, Cai MH, et al. Leonurine hydrochloride induces apoptosis of H292 lung cancer cell by a mitochondria-dependent pathway. Pharm Biol. 2015;53:1684–90. doi: 10.3109/13880209.2014.1001406. [DOI] [PubMed] [Google Scholar]
- 23.Jiang T, Ren K, Chen Q, et al. Leonurine prevents atherosclerosis via promoting the expression of ABCA1 and ABCG1 in a ppary/lxr signaling pathway, dependent manner. Cell Physiol Biochem. 2017;43:1703–17. doi: 10.1159/000484031. [DOI] [PubMed] [Google Scholar]
- 24.Liu Z, Megaraj V, Li L, et al. Suppression of pulmonary CYP2A13 expression by carcinogen-induced lung tumorigenesis in a CYP2A13-humanized mouse model. Drug Metab Dispos. 2015;43:698–702. doi: 10.1124/dmd.115.063305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ji M, Zhang Z, Li N, et al. Identification of 5-hydroxymethylfurfural in cigarette smoke extract as a new substrate metabolically activated by human cytochrome P450 2A13. Toxicol Appl Pharmacol. 2018;359:108–17. doi: 10.1016/j.taap.2018.09.031. [DOI] [PubMed] [Google Scholar]
- 26.Slowikowski BK, Galecki B, Dyszkiewicz W, et al. Decreased expression of cytochrome p450 1B1 in non-small cell lung cancer. Biomed Pharmacother. 2017;95:339–45. doi: 10.1016/j.biopha.2017.08.056. [DOI] [PubMed] [Google Scholar]
- 27.Fukami T, Nakajima M, Matsumoto I, et al. Immunohistochemical analysis of CYP2A13 in various types of human lung cancers. Cancer Sci. 2010;101:1024–28. doi: 10.1111/j.1349-7006.2009.01482.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chiang HC, Lee H, Chao HR, et al. Pulmonary CYP2A13 levels are associated with early occurrence of lung cancer, its implication in mutagenesis of non-small cell lung carcinoma. Caner Epidemiol. 2013;37:653–59. doi: 10.1016/j.canep.2013.04.010. [DOI] [PubMed] [Google Scholar]
- 29.Deng Z, Wang H, Guo G, et al. Next-generation sequencing analysis of mRNA profile in cisplatin-resistant gastric cancer cell line SGC7901. Med Sci Monit. 2019;25:2386–96. doi: 10.12659/MSM.915866. [DOI] [PMC free article] [PubMed] [Google Scholar]