Abstract
Purpose
Recent studies have indicated that N-acetylneuraminic acid (Neu5Ac) plays a key role in severe coronary artery diseases, involving RhoA signaling pathway activation, which is critically involved in cardiac fibrosis. There is convincing evidence from many studies that left atrium fibrosis is involved in the pathophysiology of AF. Therefore, we speculated that Neu5Ac may be associated with atrial fibrillation (AF) and involved in the development of AF. This study aims to investigate the clinical relationship between Neu5Ac and AF and left atrial enlargement.
Methods
Forty-five patients with AF (AF group) and forty-five patients with non-AF (control group) matched for age, sex, and hospitalization date were recruited for our study. Plasma concentrations of Neu5Ac from peripheral venous blood were analyzed using enzyme-linked immunosorbent assay (ELISA). The baseline characteristics, plasma level of Neu5Ac, and echocardiographic characteristics were evaluated.
Results
The plasma level of Neu5Ac was significantly higher in the AF group than in the control group (107.66 ± 47.50 vs 77.87 ± 39.09 ng/ml; P < 0.05); the left atrial diameters were positively correlated with the plasma Neu5Ac level (R = 0.255; P < 0.05). The plasma Neu5Ac level (R = 0.368; P < 0.05) and the left atrial diameters (R = 0.402; P < 0.05) were positively correlated with AF history times. Neu5Ac (odds ratio 1.018, 95% CI 1.003–1.032; P < 0.05) and the left atrial diameter (odds ratio 1.142, 95% CI 1.020–1.280; P < 0.05) were independent risk factors for AF in multivariate regression analysis.
Conclusions
Serum Neu5Ac is associated with atrial fibrillation, and the mechanism may involve left atrial enlargement.
1. Introduction
Atrial fibrillation (AF) is the most common arrhythmia and the leading cause of cardiovascular morbidity and mortality in clinical practice worldwide. AF is associated with a five-fold increased risk of stroke and a three-fold increased incidence of congestive heart failure, as well as increased mortality. Hospitalization of patients with AF is also very common, accounting for approximately one-third of hospitalizations for cardiac rhythm disturbances [1–3]. Fundamental mechanisms governing the incidence and prevalence of AF, including molecular, electrophysiological, and structural changes, remain poorly understood [4]. Experimental and clinical data have indicated that the incidence and progression of AF are very complex pathophysiological processes involving a large number of significant players, including atrial enlargement, Ca2+ overload, inflammation, apoptosis, and fibrosis, autonomic nervous system changes [5–7].
The evidence from many of the studies has confirmed that sialic acids are involved in some pathological phenomena, such as inflammation, apoptosis, growth, and aging, and sialic acids are also involved in the binding and transport of Ca2+ [8]. N-Acetylneuraminic acid (Neu5Ac) is the most widespread form of sialic acid. Furthermore, recent studies have indicated that Neu5Ac plays a key role in severe coronary artery diseases, involving the activation of the RhoA signaling pathway, which is important for cardiac fibrosis [9–11]. Therefore, we speculated that Neu5Ac may be associated with AF and involved in the development of AF. We attempted to identify the association between them through this study.
2. Methods
2.1. Study Population
We recruited consecutive patients with AF (AF group) who were admitted to the Renmin Hospital of Wuhan University to schedule their first AF catheter ablation between March 2019 and July 2019, and consecutive patients with non-AF were selected to form a control group; these individuals were matched with the AF group for age, sex, and hospitalization date. After assessment of detailed medical history and a complete physical examination, some clinical examinations needed to be completed within 3 days after admission, such as biochemical testing, a 12-lead electrocardiogram (ECG), and echocardiograph. The history of AF was the period from the initial episode of AF to the time of admission.
The exclusion criteria were as follows: a history of prior AF catheter ablation, which might lead to atrial fibrosis and interfere with the results of study; atrial septal defect for occlusion or surgical treatment; severe coronary disease; heart valve disease; cardiac surgery, including heart transplantation and heart valve replacement; severe heart failure (might come with cardiac fibrosis which might interfere with the results of study); recent or active malignancy; severe renal failure (estimated glomerular filtration rate <30 ml/min); shock and death during hospitalization and unwillingness or inability to sign the informed consent form.
2.2. Biochemical Analysis
Venous blood samples were obtained from patients following overnight fasting (8 hours) after thirty minutes of rest in a sitting position before AF catheter ablation. Plasma concentrations of triglycerides, total cholesterol, low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), creatinine, and uric acid were analyzed by an ADVIA 2400 Chemistry System (Siemens Healthcare Diagnostics, Munich, Germany).
2.3. Blood Sample Collection and Measurement of Neu5Ac
Venous blood samples were obtained from patients following overnight fasting (8 hours) after thirty minutes of rest in the sitting position before AF catheter ablation. A blood separator tube was used, and samples were allowed to clot for two hours at room temperature before centrifugation. Plasma samples were collected using a serum separator tube after centrifugation and then immediately frozen at −80°C until analysis. Later, the frozen serum samples were rapidly dissolved for analysis. Repeated freeze/thaw cycles were avoided. The plasma Neu5Ac level was evaluated with commercially available enzyme-linked immunosorbent assay (ELISA) kits according to the manufacturer's instructions (Human ELISA kit, Enzyme-linked Biotechnology, Shanghai, China).
2.4. Echocardiography
All patients underwent routine transthoracic echocardiography within 3 days after admission. Echocardiography was performed by an operator with over 5 years of experience using a GE E95 (GE Healthcare, Little Chalfont, United Kingdom). All measurements included at least 3 consecutive beats for patients. The left atrial diameter (LAD) was measured using a trailing edge-to-leading edge convention to measure the maximal distance between the posterior aortic root wall and the posterior left atrial wall at end ventricular systole. Left ventricular diameter and right ventricular diameter (normalized to body surface area) were measured at the end of ventricular diastole. The modified biplane Simpson rule was applied to calculate the left ventricular (LV) ejection fraction.
2.5. Statistical Analysis
All continuous variables are expressed as the mean ± SD for normally distributed data, and categorical variables are presented as counts (percentages). Independent-sample Student's t-test or chi-square test was used for comparisons of continuous and categorical variables, respectively. Correlation analysis was performed using Pearson's correlation analysis. Multivariate logistic regression analysis was used to identify risk factors. SPSS version 17.0 (SPSS, Chicago, Illinois) was used for data analysis. Differences were considered significant when a 2-sided P value was <0.05 for all the comparisons.
3. Results
3.1. Baseline Characteristics
Table 1 presents the baseline characteristics and the comorbidities of the study patients. Forty-five consecutive patients with AF were included in the AF group, and 45 patients with non-AF were matched with the AF group for age, sex, and hospitalization date as the control group. The left atrial diameters (LAD) were significantly larger in the AF group than in the control group (LAD 40.20 ± 5.92 vs 36.73 ± 5.20 mm; P < 0.05). There were no significant differences in the prevalence of smoking, hypertension, diabetes, right atrial diameters, left ventricular diameters, right ventricular diameters, or left ventricular ejection fraction between the AF group and the control group. There were no significant differences in the levels of plasma triglycerides, total cholesterol, LDL-C, HDL-C, creatinine, or uric acid between the two groups.
Table 1.
Characteristics of the patients.
| Characteristics | Control | AF | p value |
|---|---|---|---|
| Demographics | |||
| No. | 45 | 45 | |
| Age (years) | 61.02 ± 10.11 | 61.80 ± 11.68 | 0.74 |
| Male, % | 26 (57.8) | 26 (57.8) | 1.00 |
| Smoking, % | 7 (15.6) | 8 (17.8) | 0.78 |
| Hypertension, % | 25 (55.6) | 19 (42.2) | 0.21 |
| Diabetes, % | 6 (13.3) | 8 (17.8) | 0.63 |
|
| |||
| Biochemical testing | |||
| Triglycerides (mmol/L) | 2.40 ± 1.56 | 2.04 ± 1.42 | 0.27 |
| Total cholesterol (mmol/L) | 4.43 ± 0.79 | 4.16 ± 0.88 | 0.14 |
| LDL-C (mmol/L) | 2.41 ± 0.62 | 2.23 ± 0.66 | 0.17 |
| HDL-C (mmol/L) | 1.15 ± 0.29 | 1.16 ± 0.32 | 0.85 |
| Creatinine (μmol/L) | 73.32 ± 19.92 | 72.81 ± 17.03 | 0.90 |
| Uric acid (μmol/L) | 397.00 ± 98.60 | 393.18 ± 102.72 | 0.86 |
|
| |||
| Echocardiographic characteristics | |||
| Left atrial diameter (mm) | 36.73 ± 5.20 | 40.20 ± 5.92 | <0.01∗ |
| Right atrial diameter (mm) | 35.18 ± 3.55 | 35.78 ± 4.44 | 0.48 |
| Left ventricular diameter (mm) | 44.07 ± 5.65 | 44.13 ± 3.98 | 0.95 |
| Right ventricular diameter (mm) | 20.87 ± 3.08 | 20.76 ± 2.42 | 0.85 |
| LV ejection fraction (%) | 57.82 ± 7.12 | 57.27 ± 4.88 | 0.68 |
LDL-C: low-density lipoprotein cholesterol; HDL-C: high-density lipoprotein cholesterol; LV: left ventricular. Values are mean ± SD or n (%). ∗p < 0.05 for significant differences.
3.2. Analysis of Plasma Neu5Ac Level
Figure 1 shows that the level of plasma Neu5Ac was significantly higher in the AF group than in the control group (107.66 ± 47.50 vs 77.87 ± 39.09 ng/mL; P < 0.05).
Figure 1.
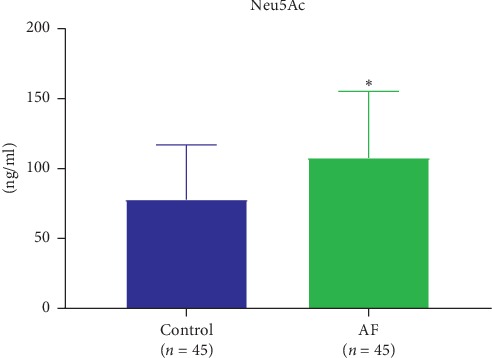
The plasma level of Neu5Ac was significantly increased in the AF group compared with the level in the control group.∗p < 0.05 for significant differences.
3.3. Multivariate Logistic Regression Analysis
The continuous variables were entered into the multivariate logistic regression analysis. Table 2 shows that in the final multivariate logistic regression analysis model, plasma Neu5Ac (odds ratio 1.018, 95% CI 1.003–1.032; P < 0.05) and left atrial diameter (odds ratio 1.142, 95% CI 1.020–1.280; P < 0.05) were the independent risk factors for AF.
Table 2.
Multivariate logistic regression analysis of risk factors for atrial fibrillation.
| B | SE | Wald | p | OR | 95% CI | |
|---|---|---|---|---|---|---|
| Neu5Ac | 0.017 | 0.007 | 5.896 | 0.015∗ | 1.018 | 1.003–1.032 |
| Age | 0.002 | 0.026 | 0.005 | 0.942 | 1.002 | 0.953–1.054 |
| Triglycerides | −0.124 | 0.202 | 0.375 | 0.540 | 0.884 | 0.595–1.313 |
| Total cholesterol | −0.535 | 0.356 | 2.263 | 0.133 | 0.586 | 0.292–1.176 |
| LDL-C | −0.065 | 0.474 | 0.019 | 0.890 | 0.937 | 0.370–2.372 |
| HDL-C | −0.122 | 1.005 | 0.015 | 0.903 | 0.885 | 0.123–6.349 |
| Creatinine | −0.013 | 0.015 | 0.699 | 0.403 | 0.987 | 0.958–1.018 |
| Uric acid | −0.002 | 0.003 | 0.467 | 0.494 | 0.998 | 0.992–1.004 |
| LAD | 0.133 | 0.058 | 5.303 | 0.021∗ | 1.142 | 1.020–1.280 |
| RAD | 0.039 | 0.065 | 0.354 | 0.552 | 1.039 | 0.915–1.180 |
| LVD | −0.024 | 0.057 | 0.180 | 0.672 | 0.976 | 0.872–1.092 |
| RVD | −0.073 | 0.130 | 0.316 | 0.574 | 0.930 | 0.721–1.199 |
| LV ejection fraction | 0.024 | 0.053 | 0.208 | 0.648 | 1.024 | 0.924–1.136 |
Neu5Ac: N-acetylneuraminic acid; LDL-C: low-density lipoprotein cholesterol; HDL-C: high-density lipoprotein cholesterol; LAD: left atrial diameter; RAD: right atrial diameter; LVD: left ventricular diameter; RVD: right ventricular diameter; LV: left ventricular. ∗p < 0.05 for significant differences.
3.4. Correlation Analysis
Table 3 and Figure 2 show that the left atrial diameters were positively correlated with the plasma Neu5Ac level (R = 0.255; P < 0.05). There was no significant correlation between the plasma Neu5Ac level and the following factors: age, the level of plasma triglycerides, total cholesterol, LDL-C, HDL-C, creatinine, uric acid, and the right atrial, left ventricular, and right ventricular diameters.
Table 3.
Correlation analysis with Neu5Ac.
| R | p value | |
|---|---|---|
| History of AF | 0.368 | 0.013∗ |
| Age (years) | 0.071 | 0.503 |
| Triglycerides (mmol/L) | 0.021 | 0.848 |
| Total cholesterol (mmol/L) | 0.054 | 0.619 |
| LDL-cholesterol (mmol/L) | −0.055 | 0.608 |
| HDL-cholesterol (mmol/L) | −0.060 | 0.588 |
| Creatinine (μmol/L) | 0.098 | 0.364 |
| Uric acid (μmol/L) | 0.084 | 0.435 |
| Left atrial diameter (mm) | 0.255 | 0.015∗ |
| Right atrial diameter (mm) | −0.038 | 0.720 |
| Left ventricular diameter (mm) | 0.130 | 0.223 |
| Right ventricular diameter (mm) | 0.000 | 0.994 |
R is Pearson's correlation coefficient. Neu5Ac: N-acetylneuraminic acid; LDL-C: low-density lipoprotein cholesterol; HDL-C: high-density lipoprotein cholesterol; ∗p < 0.05 for significant differences.
Figure 2.
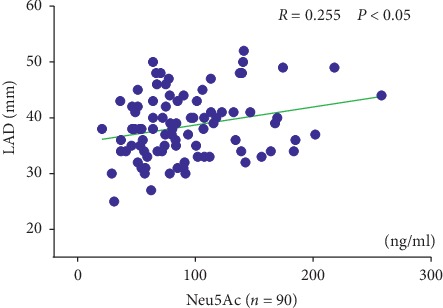
Correlation of plasma Neu5Ac levels with LAD. Neu5Ac: N-acetylneuraminic acid and LAD: left atrial diameter. R is Pearson's correlation coefficient.
Figures 3 and 4 show that the plasma Neu5Ac level (R = 0.368; P < 0.05) and the left atrial diameters (R = 0.402; P < 0.05) were positively correlated with the AF history times.
Figure 3.
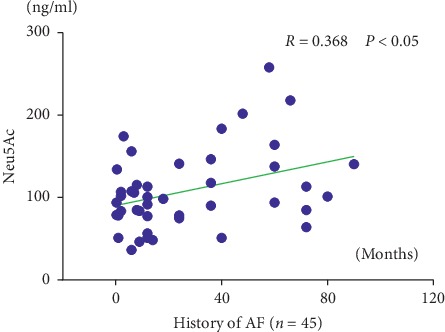
Correlation of the history of AF with Neu5Ac. Neu5Ac: N-acetylneuraminic acid. R is Pearson's correlation coefficient.
Figure 4.
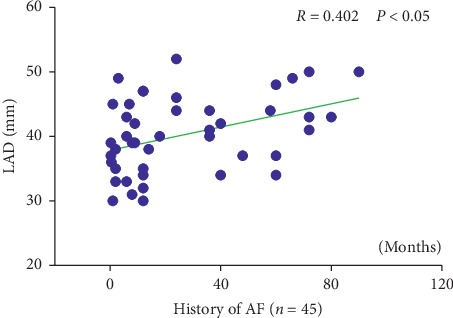
Correlation of the history of AF with LAD. LAD: left atrial diameter. R is Pearson's correlation coefficient.
4. Discussion
Many studies have been performed in regard to the mechanism and therapeutic strategies of AF. Multiple factors, including advancing age, male sex, obesity, heart failure, obstructive sleep apnea, anxiety, systemic arterial hypertension, and biomarkers such as N-terminal pro-B-type natriuretic peptide (NT-proBNP), have been identified as factors correlated with AF development [12–15]. Our study showed that the level of plasma Neu5Ac was significantly higher in the AF group than in the control group, indicating that Neu5Ac was significantly correlated with AF.
All cells of every species in nature are covered by a dense and complex coating of glycans (oligosaccharides or polysaccharides). Some glycans are sialic acids. Neu5Ac is the most widespread form of sialic acid. Sialic acids are a family of neuraminic acid derivatives, sugars with a shared nine-carbon backbone that are typically found attached to the terminal positions of several amino groups of the cell surface, and secreted glycan molecules [16]. The amino groups with glycans are acetylated by the key catabolism enzyme sialidase, leading to Neu5Ac. Neu5Ac is found in the tissues of many mammals and is involved in a variety of pathophysiological functions [17].
Is Neu5Ac a risk factor or simply a marker or mediator molecule for AF? Our study indicated that LADs were positively correlated with the plasma Neu5Ac level. Previous studies have shown that LAD is associated with AF incidence and progression and is an important risk factor in the development of AF. LAD is also involved in the maintenance and recurrence of AF [18–20]. Therefore, we speculated that elevated plasma Neu5Ac levels may result in the enlargement of the left atrium, which facilitates the incidence and development of AF. Many factors are involved in left atrial enlargement, including AF itself. Left atrial enlargement can occur as a consequence of AF [21, 22]; therefore, the increase in the plasma Neu5Ac level may also be a consequence of AF and atrial cardiomyopathy.
The LAD sizes were also positively correlated with the AF history times in our study. Many factors are involved in left atrial enlargement, including AF itself. Left atrial enlargement can occur as a consequence of AF [21, 22]. Suzuki et al. found that LAD was associated with more AF days as well as with the progress and maintenance of AF [19]. Our study results also suggested that plasma Neu5Ac levels were positively correlated with AF history times. Therefore, we made a hypothesis that Neu5Ac may also be involved in the progression and maintenance of AF.
AF is a final common endpoint of atrial remodeling caused by a variety of cardiac diseases and conditions that contribute to the progression and maintenance of AF. Atrial remodeling includes structural remodeling and electrical remodeling. Structural remodeling is characterized by atrial enlargement and tissue fibrosis [23]. There is convincing evidence from many studies that myocardial fibrosis of the left atrium is involved in the pathophysiology of AF [24, 25]. Recent studies have indicated that serum Neu5Ac plays a key role in severe coronary artery diseases, involving RhoA signaling pathway activation through a molecular docking test [9]. It is well established that the RhoA signaling pathway is critically involved in cardiac fibrosis [11]. The Rho family of GTPases is thought to mediate the activation of the SRF-MRTF signaling pathway, which is directly related to cardiac fibrosis; furthermore, RhoA signaling is activated downstream of the TGF-β receptor [26, 27]. Studies have indicated an important role for TGF-β in both atrial fibrosis and atrial remodeling, involving the RhoA signaling pathway [28, 29]. Therefore, Neu5Ac may be involved in atrial fibrosis. Through the RhoA signaling pathway, elevated plasma Neu5Ac levels may result in fibrosis of the left atrium, which facilitates the progression and maintenance of AF.
5. Conclusions
Serum Neu5Ac is associated with atrial fibrillation. The elevated concentrations of plasma Neu5Ac may result in left atrial enlargement and fibrosis, which facilitate the development of AF.
Acknowledgments
This work was supported by grants 81530011, 81570463, and 81600395 from the National Nature Science Foundation of China, grants 2016CFA065 and 2016CFA048 from the Natural Science Foundation of Hubei Province, and grant WJ2017C0005 from the Foundation of Health and Family Planning Commission of Hubei Province.
Contributor Information
Huafen Liu, Email: 13628626833@163.com.
Hong Jiang, Email: whujianghong@163.com.
Data Availability
The data underlying the findings of the study are available on request to the corresponding author.
Additional Points
There exist a few limitations in our study. First, the study group size was relatively small. Second, this study indicated that serum Neu5Ac was closely correlated with AF, but showed lack of powered cause-and-effect evidence. Third, LAD, LA area, and volume are all important parameters for the evaluation of left atrial enlargement. We only used LAD as a parameter of left atrial size rather than LA area and volume. Fourth, there was a lack of data on cardiac magnetic resonance or atrial voltage mapping with Carto or EP mapping systems to quantify atrial fibrosis.
Ethical Approval
Ethical approval was granted by the ethics committee of Renmin Hospital of Wuhan University.
Consent
All patients signed written informed consent.
Conflicts of Interest
The authors declare that there are no conflicts of interest regarding the publication of this paper.
Authors' Contributions
Wei Hu, Jing Xie, and Tongjian Zhu contributed equally to this work.
References
- 1.Fuster V., Ryden L. E., Cannom D. S., et al. 2011 ACCF/AHA/HRS focused updates incorporated into the ACC/AHA/ESC 2006 guidelines for the management of patients with atrial fibrillation. Circulation. 2011;123(10):269–367. doi: 10.1161/cir.0b013e318214876d. [DOI] [PubMed] [Google Scholar]
- 2.Heeringa J., van der Kuip D. A. M., Hofman A., et al. Prevalence, incidence and lifetime risk of atrial fibrillation: the rotterdam study. European Heart Journal. 2006;27(8):949–953. doi: 10.1093/eurheartj/ehi825. [DOI] [PubMed] [Google Scholar]
- 3.Camm A. J., Lip G. Y. H., De Caterina R., et al. 2012 focused update of the ESC guidelines for the management of atrial fibrillation. European Heart Journal. 2012;33(21):2719–2747. doi: 10.1093/eurheartj/ehs253. [DOI] [PubMed] [Google Scholar]
- 4.Jalife J., Kaur K. Atrial remodeling, fibrosis, and atrial fibrillation. Trends in Cardiovascular Medicine. 2015;25(6):475–484. doi: 10.1016/j.tcm.2014.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Corradi D. Atrial fibrillation from the pathologist’s perspective. Cardiovascular Pathology. 2014;23(2):71–84. doi: 10.1016/j.carpath.2013.12.001. [DOI] [PubMed] [Google Scholar]
- 6.Yu L., Huang B., Wang Z., et al. The potential role of the autonomic cross talk between kidney and heart. Journal of the American Heart Association. 2017;6(3):86–87. doi: 10.1161/jaha.116.004716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Van Wagoner D. R., Chung M. K. Inflammation, inflammasome activation, and atrial fibrillation. Circulation. 2018;138(20):2243–2246. doi: 10.1161/circulationaha.118.036143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schauer R. Sialic acids as regulators of molecular and cellular interactions. Current Opinion in Structural Biology. 2009;19(5):507–514. doi: 10.1016/j.sbi.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang L., Wei T.-T., Li Y., et al. Functional metabolomics characterizes a key role for N-acetylneuraminic acid in coronary artery diseases. Circulation. 2018;137(13):1374–1390. doi: 10.1161/circulationaha.117.031139. [DOI] [PubMed] [Google Scholar]
- 10.Fan Y., Li Y., Chen Y., et al. Comprehensive metabolomic characterization of coronary artery diseases. Journal of the American College of Cardiology. 2016;68(12):1281–1293. doi: 10.1016/j.jacc.2016.06.044. [DOI] [PubMed] [Google Scholar]
- 11.Travers J. G., Kamal F. A., Robbins J., Yutzey K. E., Blaxall B. C. Cardiac fibrosis the fibroblast awakens. Circulation Research. 2016;118(6):1021–1040. doi: 10.1161/CIRCRESAHA.115.306565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schnabel R. B., Sullivan L. M., Levy D., et al. Development of a risk score for atrial fibrillation (framingham heart study): a community-based cohort study. The Lancet. 2009;373(9665):739–745. doi: 10.1016/s0140-6736(09)60443-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yu L., Li X., Huang B., et al. Atrial fibrillation in acute obstructive sleep apnea: autonomic nervous mechanism and modulation. Journal of the American Heart Association. 2017;6(9) doi: 10.1161/jaha.117.006264.006264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Severino P., Mariani M. V., Maraone A., et al. Triggers for atrial fibrillation: the role of anxiety. Cardiology Research and Practice. 2019;2019:5. doi: 10.1155/2019/1208505.1208505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang T. J., Larson M. G., Levy D., et al. Plasma natriuretic peptide levels and the risk of cardiovascular events and death. New England Journal of Medicine. 2004;350(7):655–663. doi: 10.1056/nejmoa031994. [DOI] [PubMed] [Google Scholar]
- 16.Varki A. Glycan-based interactions involving vertebrate sialic-acid-recognizing proteins. Nature. 2007;446(7139):1023–1029. doi: 10.1038/nature05816. [DOI] [PubMed] [Google Scholar]
- 17.Traving C., Schauer R. Structure, function and metabolism of sialic acids. Cellular and Molecular Life Sciences CMLS. 1998;54(12):1330–1349. doi: 10.1007/s000180050258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eckstein J., Verheule S., de Groot N. M., Allessie M., Schotten U. Erratum to: “mechanisms of perpetuation of atrial fibrillation in chronically dilated atria” [Prog. Biophys. Mol. Biol. 97 (2008) 435-451] Progress in Biophysics and Molecular Biology. 2009;99(1):p. 51. doi: 10.1016/j.pbiomolbio.2008.12.001. [DOI] [PubMed] [Google Scholar]
- 19.Suzuki T., Yamazaki T., Ogawa S., Nagai R., Yamashita T. Echocardiographic predictors of frequency of paroxysmal atrial fibrillation (AF) and its progression to persistent AF in hypertensive patients with paroxysmal AF: results from the Japanese rhythm management trial II for atrial fibrillation (J-RHYTHM II Study) Heart Rhythm. 2011;8(12):1831–1836. doi: 10.1016/j.hrthm.2011.07.035. [DOI] [PubMed] [Google Scholar]
- 20.Armstrong A. C., Liu K., Lewis C. E., et al. Left atrial dimension and traditional cardiovascular risk factors predict 20-year clinical cardiovascular events in young healthy adults: the CARDIA study. European Heart Journal—Cardiovascular Imaging. 2014;15(8):893–899. doi: 10.1093/ehjci/jeu018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tops L. F., Bax J. J., Zeppenfeld K., Jongbloed M. R. M., van der Wall E. E., Schalij M. J. Effect of radiofrequency catheter ablation for atrial fibrillation on left atrial cavity size. The American Journal of Cardiology. 2006;97(8):1220–1222. doi: 10.1016/j.amjcard.2005.11.043. [DOI] [PubMed] [Google Scholar]
- 22.Sanfilippo A. J., Abascal V. M., Sheehan M., et al. Atrial enlargement as a consequence of atrial fibrillation. A prospective echocardiographic study. Circulation. 1990;82(3):792–797. doi: 10.1161/01.cir.82.3.792. [DOI] [PubMed] [Google Scholar]
- 23.Nattel S., Harada M. Atrial remodeling and atrial fibrillation. Journal of the American College of Cardiology. 2014;63(22):2335–2345. doi: 10.1016/j.jacc.2014.02.555. [DOI] [PubMed] [Google Scholar]
- 24.Smaill B. H. Fibrosis, myofibroblasts, and atrial fibrillation. Circulation: Arrhythmia and Electrophysiology. 2015;8(2):256–257. doi: 10.1161/circep.115.002881. [DOI] [PubMed] [Google Scholar]
- 25.Spach M. S. Mounting evidence that fibrosis generates a major mechanism for atrial fibrillation. Circulation Research. 2007;101(8):743–745. doi: 10.1161/circresaha.107.163956. [DOI] [PubMed] [Google Scholar]
- 26.Lutz S., Shankaranarayanan A., Coco C., et al. Structure of Gq-p63RhoGEF-RhoA complex reveals a pathway for the activation of RhoA by GPCRs. Science. 2007;318(5858):1923–1927. doi: 10.1126/science.1147554. [DOI] [PubMed] [Google Scholar]
- 27.Vardouli L., Vasilaki E., Papadimitriou E., Kardassis D., Stournaras C. A novel mechanism of TGFβ-induced actin reorganization mediated by Smad proteins and Rho GTPases. FEBS Journal. 2008;275(16):4074–4087. doi: 10.1111/j.1742-4658.2008.06549.x. [DOI] [PubMed] [Google Scholar]
- 28.Rahmutula D., Zhang H., Wilson E. E., Olgin J. E. Absence of natriuretic peptide clearance receptor attenuates TGF-β1-induced selective atrial fibrosis and atrial fibrillation. Cardiovascular Research. 2019;115(2):357–372. doi: 10.1093/cvr/cvy224. [DOI] [PubMed] [Google Scholar]
- 29.Liu L.-J., Yao F.-J., Lu G.-H., et al. The role of the rho/ROCK pathway in ang II and TGF-β 1-induced atrial remodeling. PLoS One. 2016;11(9) doi: 10.1371/journal.pone.0161625.e0161625 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data underlying the findings of the study are available on request to the corresponding author.


