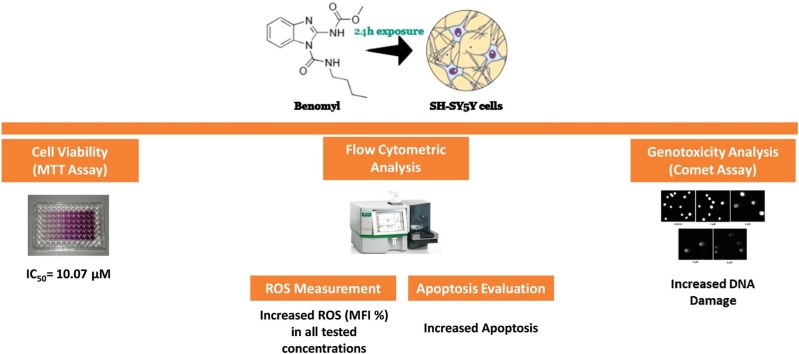
Graphical abstract
Abbreviations: ATCC, American Type Culture Collection; BSA, Bovine serum albumin; DMEM-F12, Dulbecco’s modified Eagle medium: Nutrient Mixture F-12 (Ham`s); DNA, Deoxyribonucleic acid; DTNB, 55′-dithiobis-2-nitrobenzoic acid; FBS, Fetal bovine serum; GSH, Glutathione; H2DCF-DA, 2′7′-dichlorodihydrofluorescein diacetate; MFI, Median fluorescence intensity; MTT, 3-45-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide; OD, Optical density; PBS, Phosphate buffered saline; PI, Propidium iodide; ROS, Reactive oxygen species; SD, Standard deviation
Keywords: Benomyl, Neural SH-SY5Y cell line, Oxidative stress, Apoptosis
Highlights
-
•
Benomyl exerts its toxic role by inhibiting microtubule formation in the nervous system.
-
•
Benomyl induces oxidative stress, apoptosis and necrosis in neural cells.
-
•
Benomyl is harmful even in low concentrations.
-
•
The mechanism of action is redox-related.
Abstract
Fungicides are used in the agricultural sector against the harmful action of fungi, however they are potential toxic agents for the environment and the living organisms. Benomyl is a widely encountered benzimidazole fungicide that exerts its toxicity via inhibiting microtubule formation in the nervous system and the male reproductive and endocrine systems, whilst it is a known teratogen. Since toxic effects of benomyl and its molecular mechanisms are not fully understood, we aimed to detect its neurotoxic potential via evaluating cytotoxicity, oxidative stress and apoptosis in SH-SY5Y cell line. The cells were incubated with benomyl in a concentration range between 1 and 6 μM for 24 h. Our results indicated a concentration-dependent enhancement of reactive oxygen species measured through flow cytometry and DNA damage evaluated via the comet assay. Additionally, it induced apoptosis in all tested concentrations. According to the findings of the present study, benomyl is a xenobiotic, which it appears to exert its toxic action via a redox-related mechanism that, finally, induces cell apoptosis and death. We believe that this study will offer further insight in the toxicity mechanism of benomyl, although further studies are recommended in order to elucidate these mechanisms in the molecular level.
1. Introduction
Pesticides are environmental contaminants and it has been recently reported that they constitute one of the major causes of neurodegenerative diseases [1]. Neurotoxicity and neurological dysfunctions are also partly attributed to organophosphates, carbamates, organochlorines, pyrethroids, herbicides, fungicides and fumigants. The biological action of pesticides after acute administration is largely well defined. Towards that end, pesticides act through disruption of redox equilibrium, thus inducing oxidative stress and the maximum tolerated doses (i.e., the doses that cause toxic effects but not the death of experimental animals or severe toxic outcome to humans) are usually calculated via the micronucleus assay [2]. However, their chronic effects on human health are still a matter of contradiction [[3], [4], [5]]. Fungicides belong to the class of pesticides that are used to protect the agricultural products from the harmful action of fungi [6]. The continuous and uncontrolled use of fungicides raises serious risks to the environmental, animal and public health. It has been shown that these substances may cause teratogenic, carcinogenic and mutagenic effects on the reproductive system of different organisms [7]. It is a fact that numerous fungicides show low or moderate toxicity and the rate of fungicide-related deaths is much lower than the rate of pesticide-related death cases [8]. However, different types of fungicides exert their neurotoxic action via numerous different molecular pathways [9].
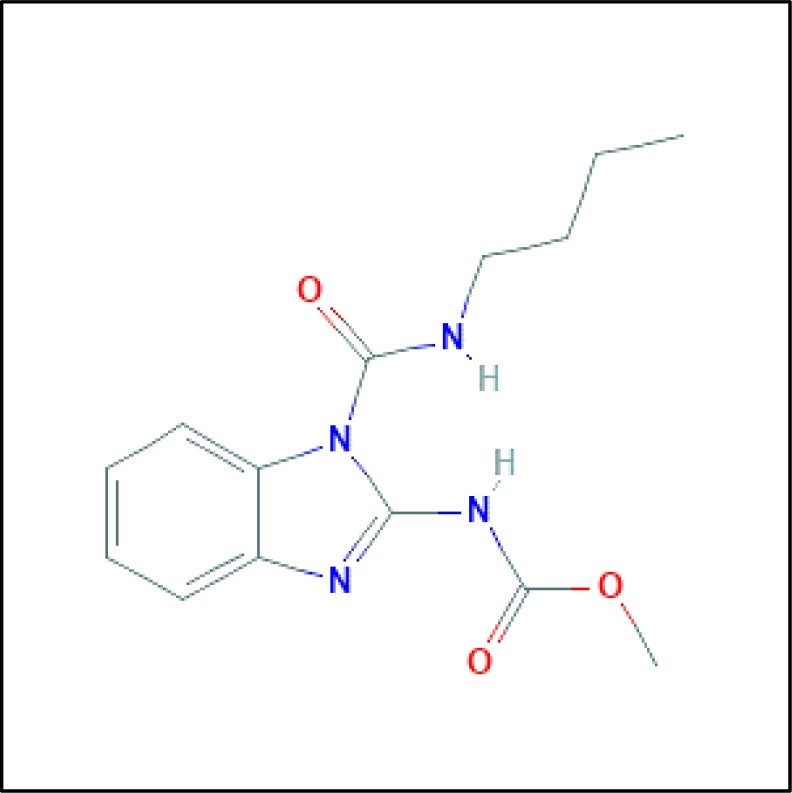
Benomyl, whose chemical structure is depicted in Fig. 1, is a broad-spectrum benzimidazole fungicide that is widely used in agriculture and in households in order to prevent and control fungi-associated diseases of plants [10]. Its mechanism of action is related to the inhibition of fungal growth via disruption of tubulin polymerization [11]. Furthermore, it has been shown that it induces neuronal cell death at nanomolar concentrations [12]. As a systemic fungicide, benomyl exerts its neurotoxicity via interfering to the function of aldehyde dehydrogenase and as a result, 3,4-dihydroxyphenylacetaldehyde (DOPAL) is accumulated and causes the degeneration of dopaminergic neurons [1]. Furthermore, it has been shown that benomyl acts similarly to widely used pesticides, such as chlorpyrifos and cypermethrin that are toxic to earthworms Eudrilus eugeniae via redox-related mechanisms [13]. It is worth mentioning that although benomyl usage is restricted in the USA and Europe, it is still used in developing countries, whose backbone of economy is the agricultural sector [14].
Fig. 1.
The chemical structure and features of benomyl. Synonyms: Benlate, Fundasol, Agrocit, Benex, IUPAC Name: methyl N-(1-(butylcarbamoyl)benzimidazol-2-yl)carbamate. Molecular Formula and Weight: C14H18N4O3and 290.32 g/mol. CAS Number: 17,804-35-2.
A common mechanism for the exertion of the toxicity of pesticides is the excessive generation of reactive oxygen species (ROS), and reactive species in general and, thus, oxidative stress [15]. ROS are produced during normal metabolic reactions in the cell and specifically metabolism [16]. It is well established that reactive species levels are increased after physiological treatments, such as exercise [[17], [18], [19], [20]] and nutritional interventions [20,21]. However, reactive species concentration can also be elevated due to exposure to several xenobiotics [22] and they have also been associated to redox-related diseases including cardiovascular, neurodegenerative and inflammatory pathologies, as well as cancer [23]. It is well known that excessive generation of ROS negatively affects the main cellular macromolecules, namely DNA, proteins and lipids leading to genotoxicity, protein and lipid oxidation and, eventually, cell death [24,25]. Neuronal cell death is the ultimate stage for neurons in oxidative stress context and plays important role in the onset of neurodegenerative diseases [26]. Additionally, it is a fact that apoptosis and necrosis could be triggered by oxidative stress and are keystones in the development of neurodegenerative diseases [26].
The literature lacks solid experimental evidence regarding the putative detrimental effects of benomyl, a commonly encountered fungicide, on neural tissue given that pesticides in general are considered as one of the main causes of neurodegenerative diseases. Therefore, the main objective of the present study was to evaluate the putative detrimental effects of benomyl on redox status and apoptosis of neuronal cells, thus proposing a potential mechanism of action that is currently unknown.
2. Materials and Methods
2.1. Chemicals
Benomyl (PESTANAL®, analytical standard) was purchased from Merck (Munich, Germany). DMSO, MTT and H2DCF-DAwere obtained from Sigma Chemical Co. Ltd. (St. Louis, MO, USA). Annexin V Apoptosis Detection Kit with propidium iodide was obtained from BioLegend (Koblenz, Germany). Cell culture mediums and all other supplements were purchased from Multicell Wisent (Quebec, Canada), and sterile plastic ware were purchased from Corning (Amsterdam, The Netherlands).
2.2. Cell culture and treatment conditions
The SH-SY5Y (CRL2266) neuronal cell line was purchased from the ATCC (Virginia, USA) and the cells were maintained according to the manufacturer’s instructions. The cells were cultivated at 37 °C in a humidified incubator with 5% CO2 in DMEM/F12 supplemented with 10 % heat-inactivated FBS and 1% antibiotic (100 U/mL penicillin and 100 μg/mL streptomycin).
A 10 mM benomyl stock solution was prepared by dissolving 0.029 g of benomyl in 1 mL of 100 % DMSO and stored in −20 °C until the analyses were conducted. During the experiment, the cells were incubated with benomyl at a range of concentrations between 1 and 6 μM in order to evaluate any putative dose-dependent effects. It has been previously reported that benomyl is not toxic at this concentration range in cell-line context [10]. Benomyl was diluted in the cell culture medium and the final DMSO concentration of the tested solution was equal to 1%. All experiments were performed in triplicates in three separate days.
2.3. Evaluation of cytotoxicity of benomyl with the MTT assay
The MTT assay was performed to detect any cytotoxic effects of the tested benomyl concentrations as previously described by [27]. Briefly, the cells were seeded into 96-well plates (1 × 104 cells/100 μL of medium/well) and incubated overnight. After the cells were treated with benomyl by half dilutions starting from 125, 62.5, 32.25, 15.62, 7.81, 3.9 μM for 24 h, the MTT solution was added into each well and the cells were further incubated for 3 h at 37 °C in the dark. Finally, the medium was discarded and 100 μL of DMSO was added to dissolve formazan crystals followed by measurement of optical density at 570 nm using a microplate reader (Biotek, Epoch, Vermont, USA).
2.4. Measurement of ROS production using flow cytometry
The H2DCF-DA dye was used for the evaluation of the ROS concentration by flow cytometer as previously described [27,28]. Briefly, the cells were seeded into 6-well plates (5 × 105 cells/2 mL of medium/well) and incubated overnight. The cells were treated with benomyl at 1, 2, 4 and 6 μM, concentrations in which the cell viability was higher than 70 % as measured through the MTT assay for 24 h. A 1 % DMSO solution was used as the negative control. Then, the culture medium was discarded, the cells were washed with ice-cold PBS twice and incubated with 1 mL of PBS containing 20 μM H2DCF-DA-FITC at 37 °C for 30 min on an orbital shaker in the dark. Subsequently, the cells were detached with trypsin-EDTA, washed with ice-cold PBS twice and re-suspended in 150 μL of PBS with 1% BSA. The ROS dependent fluorescence intensity was measured by counting 104 cells on a ACEA NovoCyte flow cytometer (San Diego, California, USA) and the results were expressed as percentage of median fluorescence intensity (MFI%).
2.5. Evaluation of GSH levels
GSH levels were determined in the cell homogenates as previously described [29]. In brief, the cells were seeded into 6-well plates (5 × 105 cells/2 mL of medium/well) and incubated overnight. The cells were treated with benomyl at the non-cytotoxic concentration of 1, 2, 4 and 6 μM for 24 h. A 1 % DMSO solution was also used as the negative control. Then, the cells were detached with trypsin-EDTA, washed with ice-cold PBS once, re-suspended and homogenized into 1 mL of PBS. The concentration of GSH was evaluated according to the method of Beutler (1975) [30] using DTNB reagent. Precipitating solution (glacial meta phosphoric acid 1.67 gm, disodium-EDTA 0.20 gm with 30 gm of NaCl dissolved in 100 mL of distilled water finally filtered with filter paper) was added to the samples and after centrifugation Na2HPO4 (0.3 mol/l) and 1 mL of 5 5′Dithiobis-2 nitrobenzoic acid (DTNB) reagents were added. The optical density of 5-mercapto-2-nitrobenzoate, which is reduced form of DNTB by free thiol group of GSH was measured at 412 nm using a microplate reader (Biotek, Epoch, Vermont, USA). The concentration of GSH was calculated using a standard curve built from the absorbance of solutions with known GSH concentrations and the results were expressed as μg of GSH / mg of total protein, which was measured with the Bradford assay [31,32].
2.6. Measurement of DNA damage by the Comet assay
The comet assay is a sensitive method for the quantification of DNA strand breaks in order to evaluate the genotoxic potential of xenobiotics. It has also been suggested that “ghost cells”, which are characterized by small or no head with cloudy tail might be associated with apoptotic cell nuclei [33]. The assay was performed as previously described by Kara et al. (2019) [34] with minor modifications. Briefly, the cells were seeded into 12-well plates (2 × 105 cells/1 mL of medium/well) and were incubated overnight. The cells were treated with benomyl at the non-cytotoxic concentration of 1, 2, 4 and 6 μM. A 1 % DMSO solution was also used as the negative control. Then, the cells were detached with trypsin-EDTA and washed with ice-cold PBS once. Then, they were re-suspended into 100 μL of PBS and 100 μL of pre-warmed low-melting agarose was added. After cell suspension was layered on the microscope slides pre-coated with normal-melting agarose, the slides were incubated at 4 °C for solidification. Lysis at 4 °C for 2 h followed by DNA unwinding for 20 min in cold-fresh electrophoresis buffer, and, electrophoresis (20 V / 300 mA) was performed at 4 °C for 20 min. After neutralization, the slides were stained with ethidium bromide (20 mg/mL) and immediately examined under a fluorescent microscope (Olympus BX53, Tokyo, Japan) at 40 × magnification using an automated image analysis system (Comet Assay IV, Perceptive Instruments, Suffolk, UK). A total of 100 cells were scored per concentration and the obtained data was expressed as percentage of DNA in the comet tail (%TDNA, tail intensity).
2.7. Apoptosis/Necrosis measured by flow cytometry
Annexin V Apoptosis Detection Kit with Propidium Iodide was used to investigate the apoptosis/necrosis pattern of the cells by flow cytometer. Annexin V binding indicates phosphatidyl serine surface translocation in early apoptotic cells [35], while, PI binding was used as necrosis indicator. During the assay, the cells were detected as follows; intact cells (annexin V-/PI), early apoptotic cells (annexin V+/PI), late apoptosis cells (annexin V+/PI+) and necrotic cells (annexin V-/PI+). Briefly, the cells were seeded into 6-well plates [5 × 105 cells/2 mL medium/well] and incubated overnight. The cells were treated with benomyl at the non-cytotoxic concentration of 1, 2, 4 and 6 μM for 24 h. A 1 % DMSO solution was also used as the negative control. Then, the cells were detached with trypsin-EDTA, washed with cell staining buffer twice and re-suspended in binding buffer supplemented with 5 μl of AnnexinV-FITC and 5 μl of PI at 3 × 105 cells/100 μl. Following an incubation for 15 min at room temperature (RT) in the dark, the fluorescence intensities were measured by counting 104 cells on a ACEA NovoCyte flow cytometer (San Diego, California, USA). The results were expressed as the percentage of the total cell amount.
2.8. Apoptosis/necrosis evaluated by laser scanning confocal microscopy
Apoptosis/necrosis was also evaluated by laser scanning confocal microscopy utilizing fluorescent-labelled reagents. Briefly, the cells were seeded into 35 mm cell culture dishes (105 cells/2 mL of medium) and incubated overnight. The cells were treated with benomyl at the non-cytotoxic concentration of 1, 2, 4 and 6 μM for 24 h. A 1 % DMSO solution was also used as the negative control. Then, the cells were fixated with an ice-cold (−20 °C) mixture of methanol and acetone [1:1] for 15 min and the culture dishes were washed with staining buffer. Then, 1 μL of each Annexin V-FITCand PI was added into 1 mL of binding buffer and 1 μL of DAPI was used for nuclear counterstaining. After, the culture dishes incubated at RT for 15 min in the dark and the images were obtained on a Leica TCS SPE confocal system (Leica Microsystems Ltd, Heerbrugg, Germany).
2.9. Statistical analysis
Data were analysed by one-way ANOVA followed by post hoc Dunnett's test and expressed as mean ± SD. The level of statistical significance was set at p < 0.05. All analyses were performed using the statistical package SPSS version 20.0 for Windows (SPSS Inc., Chicago, Illinois, USA).
3. Results
3.1. Cell viability
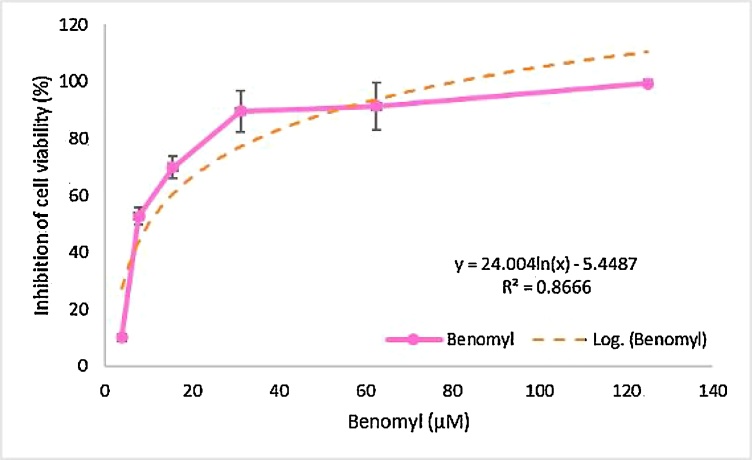
Benomyl induced cytotoxicity was evaluated with the MTT assay. The IC50 value was calculated as 10.07 μM in SH-SY5Y cells. The inhibition of cell viability was increased in a concentration-dependent manner as shown in Fig. 2.
Fig. 2.
The effects of benomyl on cytotoxicity in SH-SY5Y cells. Inhibition of cell viability was increased in a concentration-dependent manner (pink line). IC50 value was found equal to 10.07 μM using the formula of logarithmic curve (orange line).
3.2. Redox biomarkers
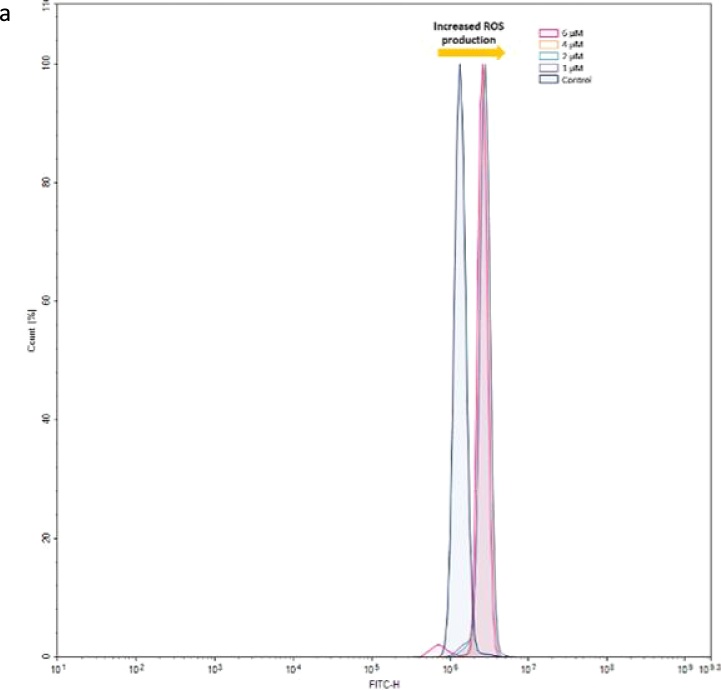
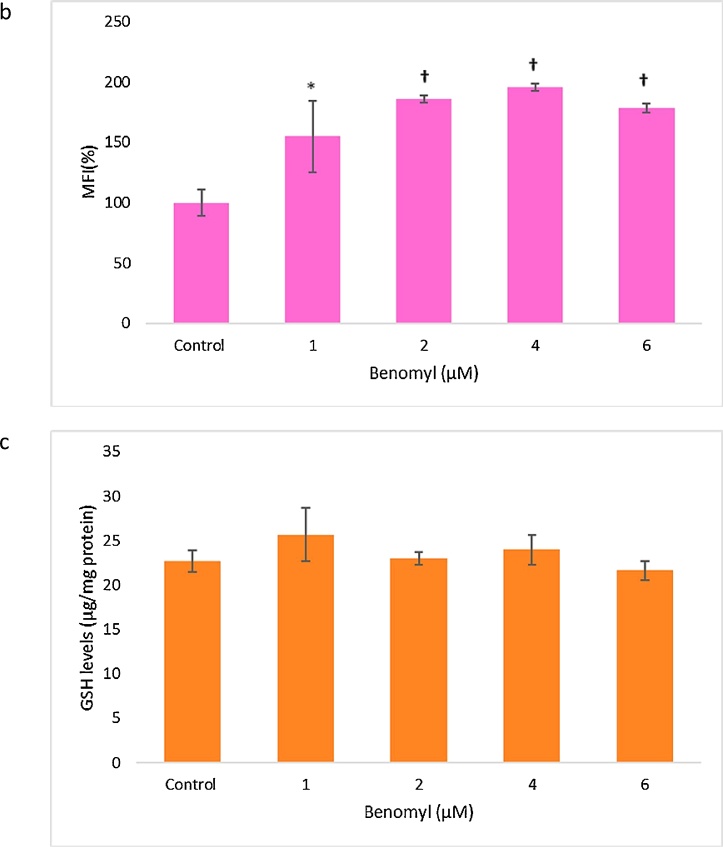
The effects of benomyl on cell redox status were evaluated through the measurement of ROS production and GSH levels. ROS production was significantly increased after incubation of the cells with all tested benomyl concentrations. The fluorescence intensities in higher concentrations were similar and enhanced approximately 2-fold compared to the control sample (Fig. 3a, b). On the contrary, GSH levels were not significantly changed after benomyl treatment (Fig. 3c).
Fig. 3.
The effects of benomyl on redox status of SH-SY5Y cells were evaluated by measuring the levels of reactive oxygen species (ROS) using H2DCF-DA (a and b) and GSH with the DTNB assay (c). Median florescence intensities [MFIs] were shifted to right (a, representative histogram) indicating that ROS production was significantly increased at all tested concentrations compared to the control (b, bar graphs). GSH concentration that is expressed as μg/mg protein remained unaffected (c). The control cells were exposed to 1% DMSO. Error bars represent the standard deviation. *: p < 0.05; †: p < 0.01 compared to the control group.
3.3. DNA damage
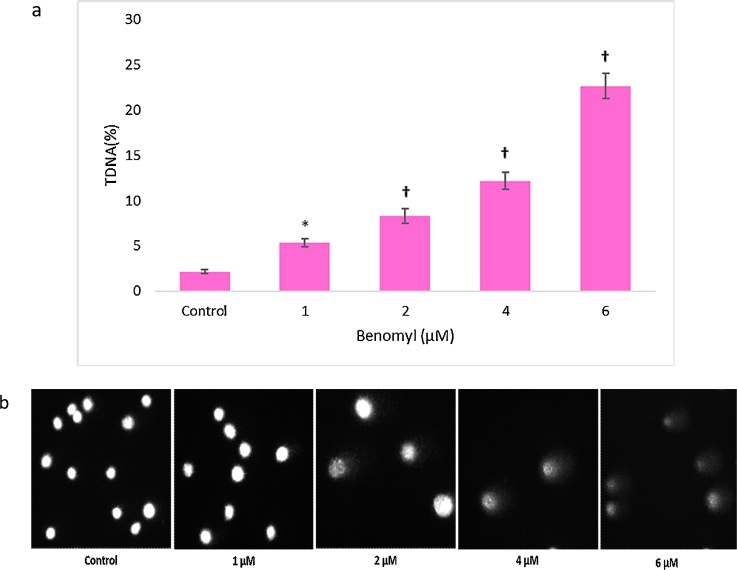
The DNA damaging potential of benomyl was evaluated using the comet assay. Benomyl induced DNA strand breaks in all tested concentrations in a dose-dependent manner (Fig. 4a). The mean tail intensities were 5.3 %, 8.3 %, 12.2 % and 22.6 % in benomyl concentrations equal to 1, 2, 4 and 6 μM, respectively. Noteworthy, the highest benomyl concentration induced a 10-fold DNA damage compared to the control sample. Moreover, ghost cells indicating apoptotic nuclei were observed in the higher concentrations (Fig. 4b).
Fig. 4.
The effects of benomyl on DNA oxidation in SH-SY5Y cells evaluated using the comet assay. Tail intensity (%TDNA) was significantly increased in a dose-dependent manner (a). As depicted in the representative images (b), the cells of the control sample exerted round shape meaning that no DNA breaks were detected. After incubation of the cells with 1 μM of benomyl, DNA damage was not substantial, however, broken DNA strands were observed. The benomyl concentrations equal to 2 and 4 μM induced severe DNA damage and the viability of the cells was impaired. Finally, DNA in the higher tested benomyl concentration (i.e., 6 μM) was almost completely fragmented and nuclear DNA was less recognizable. The control cells were exposed to 1% DMSO. Error bars represent the standard deviation. *: p < 0.05; †: p < 0.01 compared to the control group.
3.4. Apoptosis/Necrosis
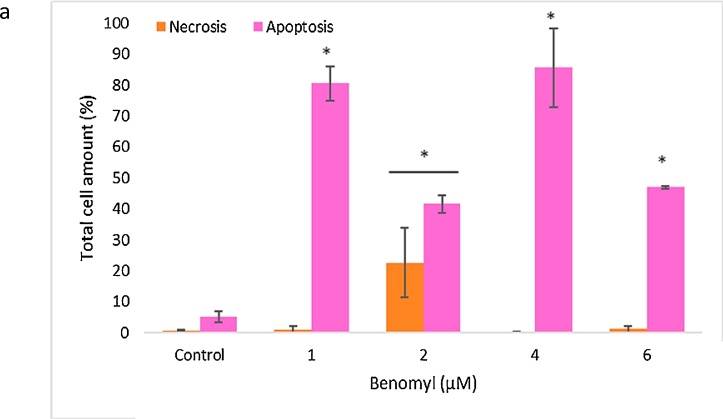
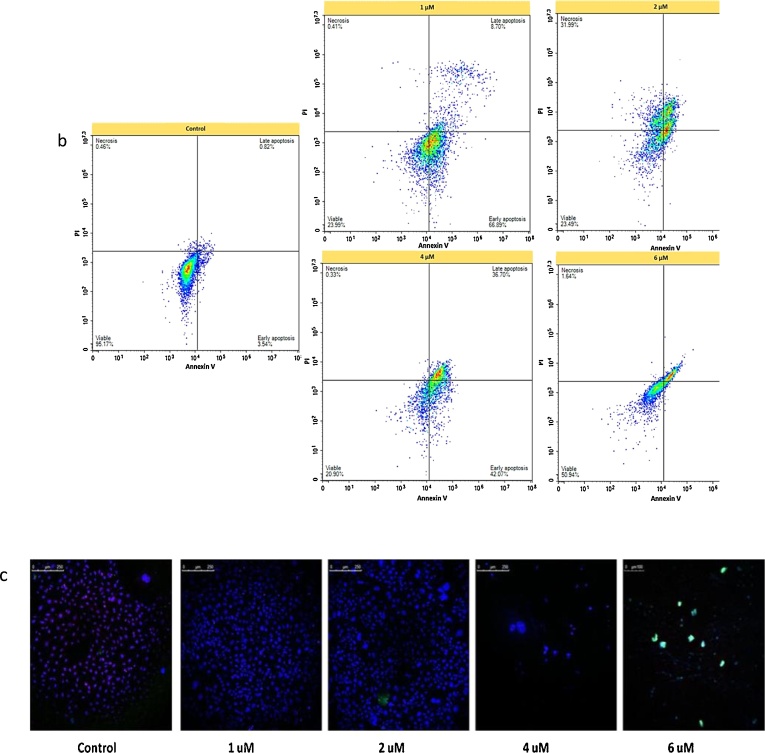
The effects of benomyl on apoptosis/necrosis were evaluated by Annexin V with PI using flow cytometer. Benomyl induced apoptosis in all tested concentrations; however, 22 % of the cells treated with 2 μM of benomyl were necrotic (Fig. 5a). Apoptotic cells were found at percentage equal to 80 %, 41 %, 85 % and 46 % in 1, 2, 4 and 6 μM concentrations, respectively. Interestingly, 1 and 4 μM of benomyl induced early apoptosis, whereas, 2 and 6 μM of benomyl induced late apoptosis as shown in representative quadrants (Fig. 5b). Also, apoptosis/necrosis was also evaluated by laser scanning confocal microscopy utilizing fluorescent-labelled reagents and confocal imaging results were found in accordance with flow-cytometric analysis (Fig. 5c).
Fig. 5.
The effects of benomyl on apoptosis/necrosis in SH-SY5Y cells evaluated by Annexin V with PI using flow cytometry. Apoptosis was induced in all tested concentrations, however, necrosis was induced only after incubation of the cells with 2 μM of benomyl (a). Interestingly, 1 and 4 μM of benomyl induced early apoptosis, whereas 2 and 6 μM of benomyl induced late apoptosis as shown in the representative quadrants (b). Apoptosis/necrosis results with Annexin V/PI were evaluated by utilizing fluorescent-labelled reagents and confocal microscopy (c). The results are presented as percentage of the total cell amount. The control cells were exposed to 1% DMSO. Error bars represent the standard deviation. *: p < 0.01 (b) compared to the control group.
4. Discussion
Several in vitro and in vivo studies have tried to elucidate the molecular mechanisms that participate in the cellular damaging effects of benomyl. Although it has been implied that oxidative stress seems to be one of them, the available scientific data are scarce, especially those that concern the nervous system. Therefore, in the present study we aimed to evaluate any putative effects on oxidative stress and apoptosis after exposure of human neuroblastoma (SH-SY5Y) cells to a range of benomyl concentrations for 24 h. Noteworthy, this cell line is anticipated to offer valuable date since it is frequently used as an in vitro cell model of neurodegenerative diseases [36]. We report that benomyl induces a significant increase in the ROS levels and serious DNA damage at all examined concentrations with a concentration-dependent manner. Furthermore, it also induced apoptosis indicating that it exerts its oxidative stress-promoted action via the apoptotic pathway.
Benomyl is a toxic agent that promotes the appearance of liver tumours and brain malformations, whereas it shows selective toxicity to dopaminergic neurons [37]. It is known that benomyl is a toxic agent against nervous system. On that basis, McLean et al. (1998) have reported that benomyl significantly inhibited neurite outgrowth in SH-SY5Y cells at a concentration equal to 10−8 M [12]. Additionally, Gupta et al. (2004) calculated the IC50 value of benomyl, which was equal to 5 μM in human cervical adenocarcinoma (HeLa) cells [8]. In a previous study of our group cytotoxic and genotoxic effects of a commercial product of benomyl (Pilben 50) were evaluated in the same cell line [34]. IC50value was calculated at 108.7 μM and high concentrations of benomyl (30 and 60 μM) induced DNA damage. In the present study, cell viability was decreased in a concentration-dependent manner and IC50 value was calculated as 10.07 μM. Considering the IC50 values, there is almost a 10-fold difference between the current and a previous study. However, it should be kept in the mind that Pilben 50 contains benomyl at 50 % according to product data sheet; thus, benomyl at the purity of an analytical standard used in this study could cause higher cytotoxicity.
It is well known that increased oxidative damage is associated, without however exerting a cause-effect relation, to neurodegenerative diseases such as Alzheimer's and Parkinson's, from which a great number of individuals suffer worldwide [38]. Abnormal intracellular protein dynamics and protein aggregation, oxidative stress and free radical formation, mitochondrial dysfunction and bioenergetics deterioration, environmental contamination may cause neurodegenerative pathologies [39]. It is also a fact that enhanced oxidative stress over the capacity of cellular protection can cause severe damage and ultimately cell death. Jang et al. (2016) reported that benomyl disrupted calcium balance and triggered oxidative stress in human bronchial epithelial (16HBE14) cell line [10]. Additionally, Catalgol et al. (2013) clearly demonstrated that acute high exposure to benomyl induced oxidative stress while impaired the antioxidant defence mechanisms in rat liver, brain, kidney and testicular tissues [40]. Also, in the same study lipid peroxidation was considered as a primary mechanism of cell membrane damage [40]. In the present study, benomyl significantly induced ROS production in all concentrations indicating increased oxidative stress. The fact that GSH levels remained unchanged could be explained by the activity of defence mechanisms at the end of the exposure period. It is known that oxidative stress conditions in the cell are strongly associated with DNA damage. Reactive species attack DNA resulting in strand breaks and/or damaged bases [41]. Benomyl acts by inhibiting tubulin polymerization and by inducing numerical chromosome changes in somatic cells, therefore being considered as a carcinogen and teratogen. In the current study, benomyl induced DNA strand breaks in all concentrations tested, a finding that strongly suggests that it has potent genotoxic effects. Amer et al. (2003) demonstrated that benomyl caused chromosomal aberrations in benomyl feeding mice for 8 weeks [42]. Ramirez-Mares et al. (1999) showed that benomyl exerts genotoxic effects in rat hepatocyte cultures, as well, whereas Mailhes et al. (1992) reported that benomyl induces numerical chromosome changes in mouse oocytes [43]. In this study, it has been shown that total ROS level, total apotosis and DNA damage were increased dose dependently after incubation of neuron cells with benomyl [43]. Thus, it appears that benomyl exerts its neurodegenerative action via inducing oxidative stress and DNA damage that result in neuronal apoptosis. Considering all the above mentioned studies it can be suggested that oxidative stress is the main pathway in benomyl toxicity.
It has also been demonstrated in a small number of studies that benomyl induces apoptosis in different cell types, such as MCF-7, HTR-8/SVneo and HeLa [14,44,45]. Multicellular apoptosis induces to cells a chracateristic blebbing morphology, loss of cell membrane integrity, nuclear fragmentation and spesific biochemical events. In this study early and late apoptosis was induced with a concentration dependent manner and this finding is shown for the first time in a neural cell line. Similar results have been reported to other cell lines, however, since it has been demonstrated that benomyl inhibited the growth of the human breast cancer (MCF-7) cell line by inducing apoptosis [44]. As it has been stated in a previous paragraph, permethrin is an interesting pesticide that it has been described as a neurotoxic agent, likely to benomyl [46]. Similarly, paraquat is a pesticide that exerts a largely common mechanism of toxicity with benomyl since it alter redox status and induces oxidative stress [47]. An interesting study has recently reported that icariin, a flavonoid glucoside that is isolated from the plant Herba Epimedii and displays neuroprotective action, protects against rotenone, a neurotoxic pesticide [48]. Therefore, the adoption of specific redox biomarkers with high translational value could reveal the hidden abilities of plant extracts that are rich in polyphenolic compounds to counteract the detrimental action of pesticides [49].
It is worth mentioning that in mammals, benomyl is rapidly metabolized and excreted primarily via the urine. The oral LD50 for benomyl in male and female rats is greater than 10,000 mg/kg. It has been reported that benomyl causes chromosomal aberrations at 1000 mg/kg in mice, and sister chromatic exchanges in CHO cells up to 150 μg/mL. The MOEs for potential daily dietary exposure to benomyl ranges from 342 to 1300 μg/mL. The MOEs for annual dietary risk from the annualized daily dosage of benomyl ranges from 3,000–14,000 μg/mL. The maximum likelihood estimate (MLE) of the excess lifetime risk of cancer for the U.S. population was 5 × 10−6. The 95th upper bound estimate of the excess lifetime risk of cancer for the U.S. population was 8 × 10−6. It appears that benomyl is a highly risky xenobiotic for normal health function even in very low concentrations [50]. In our study, the IC50 value was calculated at 10.07 u M which is approximately equal to 3 × 10-6 g/mL and very close to 5 × 10−6. These results indicate that exposure below limit values of benomyl also induces the neurotoxicity in vitro. It has been reported that benomyl and its metabolites are probably partly responsible for the development of Parkinson’s disease. In chicken studies the NOAEL of benomyl was reported at 2500 mg /kg and 500 mg /kg in rats for clinical signs as ataxia, low carriage, wing droop [50]. However, there were no detailed studies on neurons in the literature on benomyl behavioural effects and neuron histopathology.
5. Conclusions
The present study demonstrates that benomyl, a widely used pesticide induces oxidative stress and apoptosis in neural cells implying that it is a toxic agent in very low concentrations. Benomyl has been restricted in developed countries. On the contrary, it is still widely used in many developing countries because of the economic importance of the agricultural sector. Therefore, due to its widespread usage and accumulation in the environment, both acute and chronic effects of benomyl and relative mechanisms should be clarified. To this end, we believe that the present study will contribute to the revelation of the molecular toxicity mechanisms of benomyl.
Author contributions
All authors contributed substantially to the manuscript, and have met the criteria for authorship.
CRediT authorship contribution statement
Mehtap Kara: Conceptualization, Data curation, Formal analysis, Methodology, Writing - original draft, Writing - review & editing. Ezgi Oztas: Conceptualization, Funding acquisition, Project administration, Supervision, Writing - review & editing. Rabia Ramazanoğulları: Conceptualization, Funding acquisition, Project administration, Supervision, Writing - review & editing. Demetrios Kouretas: Writing - review & editing, Data curation. Charitini Nepka: Writing - review & editing. Aristides M. Tsatsakis: Conceptualization, Supervision, Writing - review & editing. Aristidis S. Veskoukis: Data curation, Writing - original draft, Writing - review & editing.
Declaration of Competing Interest
The authors declare no conflict of interest.
Acknowledgements
This study was supported by Istanbul University Scientific Research Projects Fund [Project Number: TLO-2018-32189] and was conducted as a final project of the undergraduate student RR under supervision of MK and EO.
Contributor Information
Mehtap Kara, Email: mehtap.kara@istanbul.edu.tr.
Aristidis S. Veskoukis, Email: veskoukis@uth.gr.
References
- 1.Yeung P.K., Lai A.K., Son H.J., Zhang X., Hwang O., Chung S.S., Chung S.K. Aldose reductase deficiency leads to oxidative stress-induced dopaminergic neuronal loss and autophagic abnormality in an animal model of Parkinson’s disease. Neurobiol. Aging. 2017;50:119–133. doi: 10.1016/j.neurobiolaging.2016.11.008. [DOI] [PubMed] [Google Scholar]
- 2.Ilyushina N., Goumenou M., Stivaktakis P.D., Vardavas A.I., Masaltsev G., Averianova N., Dmitricheva O., Revazova Y., Tsatsakis A.M., Rakitskii V. Maximum tolerated doses and erythropoiesis effects in the mouse bone marrow by 79 pesticides’ technical materials assessed with the micronucleus assay. Toxicol. Rep. 2018;6:105–110. doi: 10.1016/j.toxrep.2018.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lotti M. Low‐level exposures to organophosphorus esters and peripheral nerve function. Muscle Nerve. 2002;25(4):492–504. doi: 10.1002/mus.10086. [DOI] [PubMed] [Google Scholar]
- 4.Kamel F., Hoppin J.A. Association of pesticide exposure with neurologic dysfunction and disease. Environ. Health Perspect. 2004;112(9):950–958. doi: 10.1289/ehp.7135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li Y., Zhang C., Yin Y., Cui F., Cai J., Chen Z., Huang X. Neurological effects of pesticide use among farmers in China. Int. J. Environ. Res. Public Health. 2014;11(4):3995–4006. doi: 10.3390/ijerph110403995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang C., Hamel C., Vujanovic V., Gan Y. (201). Fungicide: modes of action and possible impact on nontarget microorganisms. ISRN Ecol. 2011;2011:8. [Google Scholar]
- 7.Preeti S., Aksha S., Nakuleshwar J.D., Nidhi S., Suresh J.C. A review on toxicological effects of fungicides. Res. J. Pharm. Biol. Chem. Sci. 2015;6(2):348–360. [Google Scholar]
- 8.Gupta K., Bishop J., Peck A., Brown J., Wilson L., Panda D. Antimitotic antifungal compound benomyl inhibits brain microtubule polymerization and dynamics and cancer cell proliferation at mitosis, by binding to a novel site in tubulin. Biochemistry. 2004;43(21):6645–6655. doi: 10.1021/bi036112v. [DOI] [PubMed] [Google Scholar]
- 9.Faro L.R. Neurotoxic effects of triazole fungicides on nigrostriatal dopaminergic neurotransmission. Fungicide. 2010:405–420. [Google Scholar]
- 10.Jang Y., Lee A.Y., Kim J.E., Jeong S.H., Kim J.S., Cho M.H. Benomyl-induced effects of ORMDL3 overexpression via oxidative stress in human bronchial epithelial cells. Food Chem. Toxicol. 2016;98:100–106. doi: 10.1016/j.fct.2016.10.024. [DOI] [PubMed] [Google Scholar]
- 11.Zhou Y., Xu J., Zhu Y., Duan Y., Zhou M. Mechanism of action of the benzimidazole fungicide on fusarium graminearum: interfering with polymerization of monomeric tubulin but not polymerized microtubule. Phytopathology. 2016;106(8):807–813. doi: 10.1094/PHYTO-08-15-0186-R. [DOI] [PubMed] [Google Scholar]
- 12.McLean W.G., Holme A.D., Janneh O., Southgate A., Howard C.V., Reed M.G. The effect of benomyl on neurite outgrowth in mouse NB2A and human SH-SY5Y neuroblastoma cells in vitro. Neurotoxicology. 1998;19(4–5):629–632. [PubMed] [Google Scholar]
- 13.Tiwari R.K., Singh S., Pandey R.S. Assessment of acute toxicity and biochemical responses to chlorpyrifos, cypermethrin and their combination exposed earthworm, Eudrilus eugeniae. Toxicol. Rep. 2019;6:288–297. doi: 10.1016/j.toxrep.2019.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhou J., Xiong K., Yang Y., Ye X., Liu J., Li F. Deleterious effects of benomyl and carbendazim on human placental trophoblast cells. Reprod. Toxicol. 2015;51:64–71. doi: 10.1016/j.reprotox.2014.12.008. [DOI] [PubMed] [Google Scholar]
- 15.Docea A.O., Gofita E., Goumenou M., Calina D., Rogoveanu O., Varut M., Olaru C., Kerasioti E., Fountoucidou P., Taitzoglou I., Zlatian O., Rakitskii V.N., Hernandez A.F., Kouretas D., Tsatsakis A. Six months exposure to a real life mixture of 13 chemicals’ below individual NOAELs induced non monotonic sex-dependent biochemical and redox status changes in rats. Food Chem. Toxicol. 2018;115:470–481. doi: 10.1016/j.fct.2018.03.052. [DOI] [PubMed] [Google Scholar]
- 16.Panieri E., Santoro M.M. ROS homeostasis and metabolism: a dangerous liason in cancer cells. Cell Death Dis. 2016;7(6):e2253. doi: 10.1038/cddis.2016.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Veskoukis A.S., Nikolaidis M.G., Kyparos A., Kokkinos D., Nepka C., Barbanis S., Kouretas D. Effects of xanthine oxidase inhibition on oxidative stress and swimming performance in rats. Appl. Physiol. Nutr. Metab. 2008;33(6):1140–1154. doi: 10.1139/H08-102. [DOI] [PubMed] [Google Scholar]
- 18.Veskoukis A.S., Nikolaidis M.G., Kyparos A., Kouretas D. Blood reflects tissue oxidative stress depending on biomarker and tissue studied. Free Radic. Biol. Med. 2009;47(10):1371–1374. doi: 10.1016/j.freeradbiomed.2009.07.014. [DOI] [PubMed] [Google Scholar]
- 19.Veskoukis A.S., Kyparos A., Stagos D., Kouretas D. Differential effects of xanthine oxidase inhibition and exercise on albumin concentration in rat tissues. Appl. Physiol. Nutr. Metab. 2010;35(3):244–250. doi: 10.1139/H10-013. [DOI] [PubMed] [Google Scholar]
- 20.Veskoukis A.S., Goutianos G., Paschalis V., Margaritelis N.V., Tzioura A., Dipla K., Zafeiridis A., Vrabas I.S., Kyparos A., Nikolaidis M.G. The rat closely mimics oxidative stress and inflammation in humans after exercise but not after exercise combined with vitamin C administration. Eur. J. Appl. Physiol. 2016;116(4):791–804. doi: 10.1007/s00421-016-3336-8. [DOI] [PubMed] [Google Scholar]
- 21.Veskoukis A.S., Kouretas D., Tsatsakis A. Dietary oxidative stress and antioxidant defence with an emphasis on plant extract administration. Cell Stress Chaperones. 2012;17(1):11–21. doi: 10.1007/s12192-011-0293-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fountoucidou P., Veskoukis A.S., Kerasioti E., Docea A.O., Taitzoglou I.A., Liesivuori J., Tsatsakis A., Kouretas D. A mixture of routinely encountered xenobiotics induces both redox adaptations and perturbations in blood and tissues of rats after a long-term low-dose exposure regimen: the time and dose issue. Toxicol. Lett. 2019;317:24–44. doi: 10.1016/j.toxlet.2019.09.015. [DOI] [PubMed] [Google Scholar]
- 23.Halliwell B., Gutteridge J. Oxford University Press; New York: 2015. Free Radicals in Biology and Medicine. [Google Scholar]
- 24.Ramírez-Mares M.V., de Mejía E.G. Comparative study of the antioxidant effect of ardisin and epigallocatechin gallate in rat hepatocytes exposed to benomyl and 1-nitropyrene. Food Chem. Toxicol. 2003;41(11):1527–1535. doi: 10.1016/s0278-6915(03)00169-8. [DOI] [PubMed] [Google Scholar]
- 25.Rajeswary S., Kumaran B., Ilangovan R., Yuvaraj S., Sridhar M., Venkataraman P., Aruldhas M.M. Modulation of antioxidant defense system by the environmental fungicide carbendazim in Leydig cells of rats. Reprod. Toxicol. 2007;24(3–4):371–380. doi: 10.1016/j.reprotox.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 26.Chi H., Chang H.Y., Sang T.K. Neuronal cell death mechanisms in major neurodegenerative diseases. Int. J. Mol. Sci. 2018;19(10):3082. doi: 10.3390/ijms19103082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oztas E., Abudayyak M., Celiksoz M., Özhan G. Inflammation and oxidative stress are key mediators in AKB48-induced neurotoxicity in vitro. Toxicol. In Vitro. 2019;55:101–107. doi: 10.1016/j.tiv.2018.12.005. [DOI] [PubMed] [Google Scholar]
- 28.Kerasioti E., Stagos D., Georgatzi V., Bregou E., Priftis A., Kafantaris I., Kouretas D. Antioxidant effects of sheep whey protein on endothelial cells. Oxid. Med. Cell. Longev. 2016;2016 doi: 10.1155/2016/6585737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Priftis A., Panagiotou E.M., Lakis K., Plika C., Halabalaki M., Ntasi G., Veskoukis A.S., Stagos D., Skaltsounis L.A., Kouretas D. Roasted and green coffee extracts show antioxidant and cytotoxic activity in myoblast and endothelial cell lines in a cell specific manner. Food Chem. Toxicol. 2018;114:119–127. doi: 10.1016/j.fct.2018.02.029. [DOI] [PubMed] [Google Scholar]
- 30.Beutler E. A Manual of Biochemical Methods. 2nd ed. Grune and Stratton; New York: 1975. Red cell metabolism; pp. 71–73. [Google Scholar]
- 31.Karademir C.B., Ozden S., Alpertunga B. Effects of trichlorfon on malondialdehyde and antioxidant system in human erythrocytes. Toxicol. In Vitro. 2007;21(8):1538–1544. doi: 10.1016/j.tiv.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 32.Bradford M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 33.Choucroun P., Gillet D., Dorange G., Sawicki B., Dewitte J.D. Comet assay and early apoptosis. Mutat. Res. 2001;478(1–2):89–96. doi: 10.1016/s0027-5107(01)00123-3. [DOI] [PubMed] [Google Scholar]
- 34.Kara M., Jannuzzi A.T., Yön S. In-vitro investigation of the cytotoxic and genotoxic effects of benzimidazole group pesticides benomyl and carbendazim. J. Toxicol. Cur. Res. 2019;3:007. [Google Scholar]
- 35.Pedram S., Mohammadirad A., Rezvanfar M.A., Navaei-Nigjeh M., Baeeri M., Abdollahi M. On the protection by the combination of CeO2 nanoparticles and sodium selenite on human lymphocytes against chlorpyrifos-induced apoptosis in vitro. Cell J. (Yakhteh) 2015;17(2):361. doi: 10.22074/cellj.2016.3748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xie H.R., Hu L.S., Li G.Y. SH-SY5Y human neuroblastoma cell line: in vitro cell model of dopaminergic neurons in Parkinson’s disease. Chin. Med. J. (Engl.) 2010;123(8):1086–1092. [PubMed] [Google Scholar]
- 37.Fitzmaurice A.G., Rhodes S.L., Lulla A., Murphy N.P., Lam H.A., O’Donnell K.C., Stahl M.C. Aldehyde dehydrogenase inhibition as a pathogenic mechanism in Parkinson disease. Proc. Natl. Acad. Sci. U. S. A. 2013;110(2):636–641. doi: 10.1073/pnas.1220399110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gandhi S., Abramov A.Y. Mechanism of oxidative stress in neurodegeneration. Oxid. Med. Cell. Longev. 2012;2012 doi: 10.1155/2012/428010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sheikh S., Haque E., Mir S.S. Neurodegenerative diseases: multifactorial conformational diseases and their therapeutic interventions. J. Neurodegener. Dis. 2013;2013 doi: 10.1155/2013/563481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Catalgol S., Catalgol B., Alpertunga B. Involvement of main oxidative stress mechanisms in the toxicity of benomyl and carbendazim in rats. Istanbul Ecz. Fak. Derg./J. Fac. Pharm. Istanbul. 2013;43:103–120. [Google Scholar]
- 41.Yu Y., Wang P., Cui Y., Wang Y. Chemical analysis of DNA damage. Anal. Chem. 2018;90(1):556–576. doi: 10.1021/acs.analchem.7b04247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Amer S.M., Donya S.M., Aly F.A. Genotoxicity of benomyl and its residues in somatic and germ cells of mice fed on treated stored wheat grains. Arch. Toxicol. 2003;77(12):712–721. doi: 10.1007/s00204-003-0464-9. [DOI] [PubMed] [Google Scholar]
- 43.Mailhes J.B., Aardema M.J. Benomyl-induced aneuploidy in mouse oocytes. Mutagenesis. 1992;7(4):303–309. doi: 10.1093/mutage/7.4.303. [DOI] [PubMed] [Google Scholar]
- 44.Kawaratani Y., Matsuoka T., Hirata Y., Fukata N., Nagaoka Y., Uesato S. Influence of the carbamate fungicide benomyl on the gene expression and activity of aromatase in the human breast carcinoma cell line MCF-7. Environ. Toxicol. Pharmacol. 2015;39(1):292–299. doi: 10.1016/j.etap.2014.11.032. [DOI] [PubMed] [Google Scholar]
- 45.Rathinasamy K., Panda D. Suppression of microtubule dynamics by benomyl decreases tension across kinetochore pairs and induces apoptosis in cancer cells. FEBS J. 2006;273(17):4114–4128. doi: 10.1111/j.1742-4658.2006.05413.x. [DOI] [PubMed] [Google Scholar]
- 46.Drago B., Shah N.S., Shah S.H. Acute permethrin neurotoxicity: variable presentations, high index of suspicion. Toxicol. Rep. 2014;1:1026–1028. doi: 10.1016/j.toxrep.2014.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Roede J.R., Uppal K., Park Y., Tran V., Jones D.P. Transcriptome-metabolome wide association study (TMWAS) of maneb and paraquat neurotoxicity reveals network level interactions in toxicologic mechanism. Toxicol. Rep. 2014;1:435–444. doi: 10.1016/j.toxrep.2014.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zeng R., Zhou Q., Zhang W., Fu X., Wu Q., Lu Y., Shi J., Zhou S. Icariin-mediated activation of autophagy confers protective effect on rotenone induced neurotoxicity in vivo and in vitro. Toxicol. Rep. 2019;6:637–644. doi: 10.1016/j.toxrep.2019.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Veskoukis A., Kerasioti E., Priftis A., Kouka P., Spanidis Y., Makri S., Kouretas D. A battery of translational biomarkers for the assessment of the in vitro and in vivo antioxidant action of plant polyphenolic compounds: the biomarker issue. Curr. Opin. Toxicol. 2019;13:99–109. [Google Scholar]
- 50.1999. Benomyl Risk Characterization Document. Medical Toxicology, and Worker Health and Safety Branches Department of Pesticide Regulation California Environmental Protection Agency June 30. [Google Scholar]