Short abstract
Objective
In this study, we aimed to examine the association between physical activity patterns and sarcopenia in Arab men.
Methods
This cross-sectional study included 363 men (47.7 ± 15.4 years). We analyzed appendicular lean mass (ALM), handgrip strength test, and physical activity levels. ALM divided by height (meters) squared was calculated (ALM/Ht2), and participants with −1 and −2 standard deviations below the sex-specific mean for Saudi young adults were considered to have sarcopenia class I and class II, respectively. Independent t-tests, analysis of variance, and Mann–Whitney U tests were performed to determine mean and median differences.
Results
We observed a significant difference between participants with and without sarcopenia in moderate-to-vigorous physical activity (MVPA); the correlation between ALM/H2 and MVPA was borderline significant. With a 1-hour/week increase in MVPA, ALM/Ht2 increased by 0.30 kg/m2. Total and ALM, handgrip strength, and MVPA were significantly lower in participants age >60 years; fat mass and waist circumference were unchanged as compared with middle-aged participants.
Conclusions
We identified an association between time spent in recreational MVPA and lean muscle mass among Arab men. Future studies should examine the role of MVPA training programs on muscle mass and strength in older men.
Keywords: Appendicular lean mass, handgrip strength, body fat, physical activity, sedentary, Arab men
Introduction
Sarcopenia is a geriatric syndrome that has been classified as a muscular disease.1 The occurrence of sarcopenia may be proportionally associated with an increase in fat mass and can progress differently in different individuals.2 Obesity is associated with pathophysiological diseases and impairment of physical functions. Sarcopenic obesity has 2.6 higher odds of causing difficulty in climbing stairs.3 Whereas no synergistic effect between body size and fat has been observed in some studies of sarcopenia,4 Chang et al.5 classified older participants according to their physical activity levels and found that obese older people with sarcopenia had worse physical performance than their normal-weight counterparts. Therefore, sarcopenic obesity seems to exert a synergistic impact on physical performance in older adults. Moreira et al.6 also found that middle-aged women with sarcopenic obesity (7% of the participants) had significantly lower hand-grip strength, lower knee extension and flexion strength, and required a longer time to stand up from a chair compared with non-obese, non-sarcopenic women. Increased fat mass and decreased muscle mass are distinct factors associated with disability, immobility, and mortality.7 Thus, markers of sarcopenic obesity should be monitored, and non-pharmacological preventive measures should be considered in older populations. Moreover, the onset of sarcopenia and pre-sarcopenia is associated with several changes in body composition, such as body mass index (BMI) and calf circumference.8 It is important to compare the body composition of older people with that of middle-aged and younger populations, to determine the corresponding changes in the whole body composition, especially if there are no longitudinal studies in the same population.
Physical activity can reduce body fat mass, increase muscle mass and strength, and improve physical function. For example, increments of 1 hour/day in total physical activity and moderate-to-vigorous physical activity (MVPA) are inversely associated with body fat mass, BMI, and waist circumference (WC) and positively associated with increased lower limb muscle strength.9 Engagement in regular daily physical activity is an effective strategy to prevent sarcopenia.10 The association between daily physical activity measured using an accelerometer and the occurrence of sarcopenia, was examined among older Japanese individuals in a 1-year study; the results showed that walking 7,000 to 8,000 steps/day and/or engaging in an activity that achieved >3 metabolic equivalents (METs) for 15 to 20 minutes/day were likely to prevent a reduction of muscle mass.11 Replacing sedentary time with 15 or 60 minutes of MVPA can lower the risk of sarcopenia by approximately 15% and 50%, respectively.12 It was found that community-dwelling older men spent 2.5 hours/day engaged in physical activity, but only 10% of this time was at the MVPA level. These daily activities were found to be associated with a reduced risk of sarcopenia and increased levels of physical function,13 suggesting the importance of even low physical activity (LPA). Total physical activity and MVPA have an inverse association with sarcopenia; however, no significant relationship has been observed with LPA.9 Therefore, the intensity and duration of daily physical activity could play an important role in reducing the occurrence of sarcopenia.
Handgrip strength (HGS) is an indicator of muscle strength. HGS <30 kg in men is a risk factor of sarcopenia14 and has recently been suggested as the first marker of sarcopenia or probable sarcopenia. Recently, a new cut-off of 27 kg for HGS in men has been recommended.15 However, an association between HGS and physical activity has not yet been established. For example, in a cross-sectional study among older men, the highest tertile of LPA was associated with greater HGS whereas no association was noted with sedentary time.16 In a different study, an association between physical activity and HGS in older individuals age >75 years was not found.13 A recent review suggested that most studies have identified an association between exercise intervention and muscle mass, but muscle strength required specific types of physical activity.10 Most studies on sarcopenia in Asia were conducted in East Asia; thus, there is a need for data from South and Southwest Asia.17 Thus, the main aim of this study was to examine the association between physical activity patterns and the markers of sarcopenia (muscle mass and strength) in Arab men. The secondary aim was to investigate the interaction between age and obesity and lean muscle mass as a marker of pre-sarcopenia.
Methods
Participant characteristics
This study comprised men who completed all necessary tests, including Saudi nationals and men of different Middle Eastern nationalities living in Saudi Arabia. Recreationally active people who engaged in regular exercise ≥3 times/week were included, provided that they were not professional athletes. Exclusion criteria were men with BMI >40 kg/m2, diagnosis of an illness that affected muscle mass (i.e., muscle wasting owing to another disease or treatment), and inability to move naturally (e.g., recovery from injury and chronic fatigue syndrome).
Based on the Statistical Saudi Population Survey 2018, the population in Riyadh City is 8,002,100; of the total, 42.7% are non-Saudis and approximately 23% of these are Arabs, with the highest proportion from Egypt followed by Yemen.18 Men age >20 years represent 41.6% of the Saudi population, such that men in Riyadh City total 3,416,896, including 1,459,014 Saudis and 450,312 non-Saudi Arabs. Thus, the population of Saudi and non-Saudi Arab men age >20 years is 1,909,326. With a 95% confidence level and 5% margin of error, the confidence interval is 4.45, and the required sample size for the current descriptive epidemiological study is 384 men.19
Study procedure
This study had a cross-sectional design. Announcements regarding recruitment of voluntary study participants and the aim and procedure of data collection were sent to relevant communities and groups and were posted on social media. The first stage of recruitment was to screen interested participants who telephoned and request them to come to the selected location for data collection. Data collection locations were as follows: for men age <40 years, data were collected at the College of Sport Sciences and Physical Activity at King Saud University (KSU), Riyadh, Saudi Arabia; for men age >40 years, data were collected at different Community Development Commissions of the districts of Riyadh (two in the South districts, one in the East districts, one in the Central districts, two in the North districts, and one in a town near Riyadh City). These commissions contributed to the study by contacting the population in the community, as well as hosting data collection. The age of participants ranged between 20 and 80 years; our participants well represented the male population of the city of Riyadh in Saudi Arabia. The participants were divided into three age groups: older (>60 years), middle-aged (40–60 years), and younger (<40 years). All data were included in the analysis.
Participants were instructed to arrive at the data collection locations in the morning before eating breakfast. Written informed consent was provided by all participants. Measurements included anthropometry, body composition using bioelectrical impedance analysis, an HGS test, and physical activity assessment using the Global Physical Activity Questionnaire (GPAQ), Arabic version.
Measurements
Participants’ height was measured to the nearest 0.1 cm using a stadiometer (Seca 213; Seca GmbH & Co., Hamburg, Germany), and body weight was measured to the nearest 0.1 kg using a digital scale (Detecto ProDoc PD100 Scale; Cardinal Scale Manufacturing Company, Webb City, MO, USA). WC was measured at the umbilicus to the nearest 0.1 cm using a measuring tape. Resting heart rate and systolic blood pressure (SBP) and diastolic blood pressure (DBP) (mmHg) were measured using an automatic arm digital sphygmomanometer (Omron HEM-7121; Omron Healthcare Co. Ltd., Kyoto, Japan).
Total body composition was measured using a Tanita MC-980MA (Tanita Corporation, Tokyo, Japan), a multi-frequency segmental machine that delivers currents of 50 to 1000 kHz. Body composition, including fat mass and appendicular lean mass (ALM), was determined from the output, as per the recommended protocols. ALM was divided by the square of height, in meters (Ht2). In the current study, we used different local and global cut-off values of ALM/Ht2 to classify participants with sarcopenia.14 These cut-off values included the European Working Group on Sarcopenia in Older People 2010 (EWGSOP1) definition for sarcopenia (pre-sarcopenia) at 7 kg/m2,14 the European Working Group on Sarcopenia in Older People 2018 (EWGSOP2) definition for sarcopenia (confirmed sarcopenia) at 7.26 kg/m2,15 and the definition for pre-sarcopenia of a value of 1 standard deviation (SD) (class I) and 2 SD (class II) below the mean of the local reference value for young Saudi men, at 8.6 kg/m2 and 7.4 kg/m2, respectively.20
Participants with sarcopenia were divided into obese and non-obese groups to examine sarcopenic obesity, then further divided into groups with and without abdominal obesity, to investigate sarcopenic abdominal obesity. Obesity was determined using fat percentage 25% of body weight, and abdominal obesity was determined as a WC of 102 cm.21 The fat mass index (FMI) is the total body fat mass divided by Ht2 in meters, and the fat-free mass index (FFMI) is the total lean mass divided by Ht2 in meters.
HGS was measured in the dominant hand using a manual spring dynamometer (Baseline® Smedley Spring Dynamometers, Fabrication Enterprises Inc., NY, USA); the best of two measurements was recorded, in kilograms. Participants were subdivided into three groups, as follows: group 1 with HGS >42 kg, which is the median HGS of 471 Arab men in a recent study;22 group 2 with HGS <30 kg, which is the lower level of HGS based on the EWGSOP1 cut-off; and group 3 with HGS 30 to 42 kg, which is higher than the risk of low HGS (HGS ≥30 kg) but <50% in the same population (HGS ≤42 kg).
A written version of the Arabic GPAQ was completed by all participants under the supervision of the research assistant, who explained the questionnaire and answered queries. The GPAQ is divided to four domains: MVPA at work, recreational MVPA (MVPArecreational), travel to and from locations, and sedentary behavior. Questions include the time spent in each domain/day and the frequency/week.23 In the current study, 62% of participants did not engage in any MVPA at work; hence, only MVPArecreational was used in the MVPA analysis.
Ethical considerations
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee (King Saud University, IRB no. E-18-3381) and with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study.
Statistical analysis
Statistical analysis was performed using IBM SPSS, version 21 (IBM Corp., Armonk, NY, USA). Continuous data are presented as mean ± SD for normal variables. Non-normal variables are presented as median (25th–75th) percentiles. All continuous variables were checked for normality using the Kolmogorov–Smirnov test. Non-normal variables were log-transformed. Frequencies and percentages were used for categorical variables. We used the independent t-test, analysis of variance, Mann–Whitney U-test, and Friedman test to determine the mean and median differences in normal and non-normal variables. Simple regression and correlation analyses were performed, and a p-value <0.05 was considered significant.
Results
This study included 363 men (age, 47.7 ± 15.4 years; BMI, 28.3 ± 5.2 kg/m2; fat percentage, 27.1% ± 7.2%). Participants comprised 283 Saudi nationals and 80 men of different nationalities who were living in Saudi Arabia. Descriptive data showed that whereas body composition and HGS were normally distributed with coefficient of variation (CV) between 10% and 25%, LPA and MVPA were not normally distributed, with a CV between 34% and 32%, respectively. Medians and 25th to 75th percentiles are reported in the tables.
Participants were divided into sarcopenic and non-sarcopenic groups based on EWGSOP1 and EWGSOP2 cut-off values and the difference relative to the mean value of regional sex-specific populations (class I = −1 SD and class II = −2 SD). Table 1 shows the differences between participants with low and normal ALM/Ht2 with respect to age, body weight, body fat, and HGS for all four cut-off values used in the study.
Table 1.
Clinical characteristics of participants based on different ALM/Ht2 cutoffs.
|
Parameters |
EWGSOP2 |
EWGSOP1 |
2 SD < reference values for Saudi men |
1 SD < reference values Saudi men |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
ALM/Ht2 >7 kg/m2 |
ALM/Ht2 >7.26 kg/m2 |
ALM/Ht2 >7.4 kg/m2 |
ALM/Ht2 >8.6 kg/m2 |
|||||||||
|
Normal |
Low |
P-value |
Normal |
Low |
P-value |
Normal |
Low |
P-value |
Normal |
Low |
P-value | |
| N | 341 (93.9) | 22 (6.1) | 323 (89.0) | 40 (11.0) | 313 (86.3) | 50 (13.8) | 194 (53.4) | 169 (46.6) | ||||
| Age (years) | 47.2 ± 15.2 | 55.818.1 | 0.011 | 46.9 ± 14.9 | 53.9 ± 18.4 | 0.008 | 46.8 ± 14.8 | 53.9 ± 18.6 | 0.002 | 44.9 ± 13.3 | 50.9 ± 17.2 | <0.001 |
| Height (cm) | 168.5 ± 6.8 | 168.8 ± 6.1 | 0.839 | 168.6 ± 6.9 | 167.3 ± 6.1 | 0.242 | 168.7 ± 6.9 | 167.0 ± 6.2 | 0.107 | 169.2 ± 7.0 | 167.6 ± 6.4 | 0.031 |
| Weight (kg) | 81.1 ± 13.1 | 61.9 ± 10.9 | <0.001 | 82.2 ± 13.5 | 62.1 ± 94 | <0.001 | 82.6 ± 13.4 | 63.1 ± 9.1 | <0.001 | 87.9 ± 12.9 | 70.8 ± 10.2 | <0.001 |
| BMI (kg/m2) | 28.6 ± 4.7 | 21.7 ± 3.7 | <0.001 | 28.9 ± 4.6 | 22.2 ± 3.5 | <0.001 | 29.1 ± 4.6 | 22.7 ± 3.5 | <0.001 | 30.8 ± 4.3 | 25.3 ± 2.9 | <0.001 |
| Fat mass (kg) | 22.9 ± 9.1 | 15.5 ± 9.5 | <0.001 | 23.4 ± 8.9 | 12.2 ± 8.6 | <0.001 | 23.5 ± 8.9 | 15.8 ± 8.2 | <0.001 | 25.8 ± 9.1 | 18.7 ± 7.8 | <0.001 |
| FFM (kg) | 58.4 ± 7.7 | 46.3 ± 3.6 | <0.001 | 59.0 ± 7.4 | 46.9 ± 3.4 | <0.001 | 59.3 ± 7.3 | 47.4 ± 3.6 | <0.001 | 62.6 ± 6.7 | 52.0 ± 5.3 | <0.001 |
| FMI (kg/m2) | 8.1 ± 3.3 | 5.7 ± 3.2 | 0.001 | 8.3 ± 3.2 | 5.6 ± 3.1 | <0.001 | 8.3 ± 3.2 | 5.8 ± 2.9 | <0.001 | 9.1 ± 3.2 | 6.7 ± 2.9 | <0.001 |
| FFMI (kg/m2) | 19.6 ± 1.9 | 15.4 ± 1.0 | <0.001 | 19.7 ± 1.9 | 16.2 ± 2.2 | <0.001 | 19.8 ± 1.8 | 16.3 ± 2.1 | <0.001 | 20.8 ± 1.5 | 17.6 ± 1.6 | <0.001 |
| ALM/Ht2 (kg/m2) | 8.9 ± 1.2 | 6.5 ± 0.5 | <0.001 | 9.03 ± 1.2 | 6.8 ± 0.5 | <0.001 | 9.1 ± 1.1 | 6.9 ± 0.5 | <0.001 | 9.7 ± 1.0 | 7.7 ± 0.6 | <0.001 |
| HGS (kg) | 39.1 ± 8.8 | 30.0 ± 8.5 | <0.001 | 39.4 ± 8.6 | 31.6 ± 9.6 | <0.001 | 39.6 ± 8.6 | 31.2 ± 8.9 | <0.001 | 41.2 ± 8.5 | 35.3 ± 8.7 | <0.001 |
| MVPArecreational (minutes/week) | 180 (0.0–480) | 60 (0.0–210) | 0.039 | 180 (0.0–480) | 75 (0.0–270) | 0.060 | 180 (0.0–480) | 95 (0.0–280) | 0.125 | 180 (0.0–540) | 150 (0.0–360) | 0.410 |
| LPA (minutes/week) | 90 (0.0–240) | 20 (0.0–140) | 0.104 | 90 (0.0–240) | 45 (0.0–210) | 0.104 | 100 (0.0–250) | 15 (0.0–180) | 0.018 | 140 (0.0–360) | 50 (0.0–180) | <0.001 |
| Sedentary (hours/day) | 4.0 (3.0–6.3) | 5.0 (4.0–8.0) | 0.077 | 4 (3.0–6.5) | 5 (4.0–6.0) | 0.100 | 4.0 (3.0–6.75) | 4.75 (4.0–6.0) | 0.141 | 4.0 (3.0–6.5) | 4.0 (3.0–6.5) | 0.786 |
Notes: Data presented as mean ± SD and median (1st–3rd) percentiles for normal and non-normal variables.
P-values significant at 0.05 and 0.01 levels.
EWGSOP: European Working Group of Sarcopenia in Older People; SD: standard deviation; BMI: body mass index; FFM: fat-free mass; FMI: fat max index (fat mass/height2); FFMI: fat-free mass index (fat-free mass/height2); ALM: appendicular lean mass; Ht2: height in meters squared; HGS: handgrip strength; MVPArecreational: recreational moderate-to-vigorous physical activity; LPA: light physical activity.
The presence of significant differences between participants with low and normal ALM/Ht2 with respect to physical activity levels were affected by the cut-off values. For example, there was a significant difference between participants with low and normal ALM/Ht2 in relation to MVPArecreational only when using the EWGOSP2 cut-off, which is the lower cut-off level (ALM/Ht2 >7 kg/m2) versus other threshold values of ALM/Ht2. The difference between participants with low and normal ALM/Ht2 was borderline significant when using the EWGOSP1 cut-off. In contrast, there was no significant difference between participants with low and normal ALM/Ht2 for LPA when using EWGOSP1 or EWGOSP2, whereas the reference cut-off for Saudi men showed significant differences between groups based on −1 SD (P = 0.01) and −2 SD (P = 0.001). The median values of LPA were greatly increased in the low and normal ALM/Ht2 groups when using −2 SD; with this cut-off value, many participants with low LPA levels were categorized in the low ALM/Ht2 group. Last, there was no significant difference between participants with low and normal ALM/Ht2 for sedentary behavior, regardless of the sarcopenia cut-off classification. The median values for sedentary behavior were larger between the groups when using the EWGOSP2 cut-off but did not approach significance.
As seen in Table 2, 17.9% of participants had HGS <30 kg. There were significant differences between the groups with respect to age, FFM, and ALM/Ht2 whereas there were no significant differences between the groups for BMI, body fat mass, and FMI. The differences between HGS groups in MVPArecreational approached borderline significance. Both groups with HGS >30 kg spent a greater amount of time engaged in LPA, and these differences were significant as compared with the third group, which had the lowest HGS (P<0.01). Sedentary behavior was similar among all groups, with no significant differences.
Table 2.
Clinical characteristics of participant subgroups based on different handgrip strength reference values.
|
Parameters |
Handgrip strength (kg) |
P-value | ||
|---|---|---|---|---|
|
<30 |
30–42 |
>42 |
||
| N | 65 | 179 | 119 | |
| Age (years) | 61.5 ± 14.5 | 48.7 ± 14.8a | 40.9 ± 12.6a,b | <0.001 |
| Height (cm) | 164.9 ± 6.2 | 166.8 ± 6.1 | 172.6 ± 6.1a,b | <0.001 |
| Weight (kg) | 73.6 ± 12.5 | 78.1 ± 14.2 | 85.5 ± 14.0a,b | <0.001 |
| BMI (kg/m2) | 27.1 ± 4.7 | 28.1 ± 5.1 | 28.7 ± 4.6 | 0.180 |
| SBP (mmHg) | 129.3 ± 19.4 | 123.5 ± 17.5 | 118.5 ± 16.7a,b | 0.002 |
| DBP (mmHg) | 73.7 ± 12.3 | 76.6 ± 11.9 | 76.0 ± 11.4 | 0.335 |
| HRrest (beats/min) | 72.3 ± 13.2 | 68.1 ± 11.4 | 68.8 ± 12.8 | 0.109 |
| Fat mass (kg) | 21.4 ± 8.4 | 22.1 ± 8.9 | 23.4 ± 9.7 | 0.319 |
| FFM (kg) | 51.9 ± 6.7 | 56.0 ± 7.1a | 62.7 ± 7.5a,b | <0.001 |
| FMI (kg/m2) | 8.1 ± 3.0 | 8.0 ± 3.2 | 7.9 ± 3.4 | 0.960 |
| FFMI (kg/m2) | 18.3 ± 2.6 | 19.2 ± 2.2a | 19.9 ± 1.9a,b | <0.001 |
| ALM/Ht2 (kg/m2) | 7.9 ± 1.2 | 8.7 ± 1.2a | 9.3 ± 1.3a,b | <0.001 |
| MVPArecreational (minutes/week) | 75 (0.0–420) | 120 (0.0–420) | 232.5 (0.0–505) | 0.077 |
| LPA (minutes/week) | 10 (0.0–140) | 105 (0.0–210)a | 102.5 (5.0–300)a | 0.008 |
| Sedentary (hours/day) | 4.0 (3.0–6.0) | 4.0 (3.0–6.03) | 4.5 (3.0–7.0) | 0.966 |
Notes: Data presented as mean ± SD and median (1st–3rd) percentiles for normal and non-normal variables.
aBonferroni post-hoc test, significant with respect to the group <30 kg.
bBonferroni post-hoc test, significant with respect to the group 30–42 kg.
P-values significant at 0.05 and 0.01 levels.
BMI: body mass index; SBP: systolic blood pressure; DBP: diastolic blood pressure; HRrest: resting heart rate; FFM: fat-free mass; FMI: fat max index (fat mass/height2); FFMI: fat-free mass index (fat-free mass/height2); ALM: appendicular lean mass; Ht2: height in meters squared; MVPArecreational: recreational moderate-to-vigorous physical activity; LPA: light physical activity.
Table 3 shows that participants age >60 years had significantly lower height, muscle mass, and strength, and the values for body weight and fat in the age group 40 to 60 years were the highest in comparison with other age groups (younger and older men). Physical activity showed different patterns in relation to age group. For example, younger (<40 years) and middle-aged (40–60 years) men spent significantly more time engaged in MVPArecreational than older men (>60 years) (P<0.001) whereas middle-aged men spent a greater amount of time engaged in LPA than did younger and older men (P = 0.02). There were no significant differences among age groups with respect to sedentary behavior.
Table 3.
Clinical characteristics of participants based on age.
|
Parameters |
Age subgroups (years) |
P-value | ||
|---|---|---|---|---|
|
<40 |
40–60 |
>60 |
||
| N | 121 | 143 | 99 | |
| Height (cm) | 170.8 ± 6.4 | 168.8 ± 6.9 | 165.2 ± 5.7a,b | <0.001 |
| Weight (kg) | 78.4 ± 18.2 | 85.1 ± 13.2a | 75.8 ± 12.7b | <0.001 |
| WC (cm) | 81.3 ± 24.6 | 95.8 ± 18.7a | 93.9 ± 19.7a | <0.001 |
| BMI (kg/m2) | 26.9 ± 5.9 | 29.9 ± 4.6a | 27.8 ± 4.5b | <0.001 |
| SBP (mmHg) | 111.3 ± 12.8 | 124.6 ± 18.9a | 131.2 ± 19.6a,b | <0.001 |
| DBP (mmHg) | 72.4 ± 10.4 | 77.8 ± 12.3a | 77.4 ± 11.8a | <0.001 |
| HRrest (beat/min) | 67.7 ± 11.6 | 68.1 ± 12.8 | 71.6 ± 11.9a | 0.04 |
| HGS (kg) | 41.8 ± 7.9 | 40.1 ± 8.3 | 32.1 ± 8.3a,b | <0.001 |
| Fat mass (kg) | 19.9 ± 10.8 | 25.4 ± 8.6a | 21.9 ± 8.0b | <0.001 |
| FFM (kg) | 58.6 ± 8.5 | 59.9 ± 7.3 | 53.4 ± 6.7a,b | <0.001 |
| FMI (kg/m2) | 6.8 ± 3.6 | 8.9 ± 3.2a | 8.0 ± 2.9a | <0.001 |
| FFMI (kg/m2) | 19.0 ± 2.6 | 19.9 ± 1.9a | 18.6 ± 2.1b | <0.001 |
| ALM/Ht2 (kg/m2) | 9.0 ± 1.4 | 9.1 ± 1.2 | 8.1 ± 1.1a,b | <0.001 |
| MVPArecreational (minutes/week) | 210 (0.0–510) | 200 (0.0–540) | 80 (0.0–215)a,b | 0.001 |
| LPA (minutes/week) | 70 (0.0–240) | 140 (0.0–300) | 60.0 (0.0–180)b | 0.022 |
| Sedentary (hours/day) | 5.0 (3.0–7.0) | 4.0 (3.0–7.0) | 4.0 (3.0–6.0) | 0.946 |
Notes: Data presented as mean ± SD and median (1st–3rd) percentiles for normal and non-normal variables.
P-values significant at 0.05 and 0.01 levels. A and B represent Bonferroni post-hoc tests significant with respect to ages <40 years and 40–60 years, respectively.
BMI: body mass index; WC: waist circumference; SBP: systolic blood pressure; DBP: diastolic blood pressure; HRrest: resting heart rate; FFM: fat-free mass; FMI: fat max index (fat mass/height2); FFMI: fat-free mass index (fat-free mass/height2); ALM: appendicular lean mass; Ht2: height in meters squared; HGS: handgrip strength; MVPArecreational: recreational moderate-to-vigorous physical activity; LPA: light physical activity.
Table 4 shows the difference between obese and non-obese participants who had sarcopenia based on the Saudi cut-off values for class 1 (ALM/Ht2 = 7.4 kg/m2, n = 50) and class 2 (ALM/Ht2 = 8.6 kg/m2, n = 169). Additionally, the differences between participants with and without sarcopenic abdominal obesity were analyzed based on class 2 because only five participants were classified as abdominally obese when using the class 1 cut-off. Muscle mass was significantly higher in obese participants with class 1 sarcopenia, and SBP was also significantly increased with excess body fat. The significant difference in HGS between obese and non-obese participants with sarcopenia approached borderline when using the −2 SD Saudi reference cut-off (P = 0.054) whereas there was no significant difference between the group when using the −1 SD Saudi reference value. There were no significant differences between obese and non-obese participants with sarcopenia and between participants who had sarcopenia with and without abdominal obesity in all physical activity patterns, including MVPArecreational, LPA, and sedentary behavior.
Table 4.
Comparison of obese and non-obese participants with sarcopenia for the study variables.
|
Parameters |
ALM/Ht2 >7.4 |
ALM/Ht2 >8.6 |
ALM/Ht2 >8.6 |
||||||
|---|---|---|---|---|---|---|---|---|---|
|
Sarcopenia, non-obese |
Sarcopenia, obese |
P-value |
Sarcopenia, abdominally non-obese |
Sarcopenia, abdominally obese |
P-value |
Sarcopenia, non-obese |
Sarcopenia, obese |
P-value | |
| N | 33 | 17 | 136 | 33 | 86 | 83 | |||
| Age (years) | 49.9 ± 20.6 | 60.8 ± 9.9 | 0.053 | 49.1 ± 17.6 | 58.4 ± 13.1 | 0.005 | 45.0 ± 19.1 | 57.0 ± 12.3 | <0.001 |
| SBP (mm Hg) | 116.8 ± 18.9 | 121.4 ± 33.1 | 0.537 | 119.8 ± 21.5 | 131.4 ± 15.8 | 0.004 | 116.8 ± 18.1 | 127.7 ± 22.4 | 0.001 |
| DBP (mm Hg) | 67.8 ± 11.3 | 71.8 ± 17.4 | 0.342 | 74.1 ± 12.3 | 74.3 ± 11.6 | 0.913 | 71.6 ± 10.2 | 76.8 ± 13.5 | 0.005 |
| HRrest (beats/min) | 66.9 ± 12.9 | 67.7 ± 15.8 | 0.855 | 68.1 ± 12.5 | 68.1 ± 11.8 | 0.983 | 66.3 ± 11.9 | 70.0 ± 12.6 | 0.052 |
| FMI (kg/m2) | 4.0 ± 1.2 | 9.1 ± 2.6 | <0.001 | 5.8 ± 1.2 | 10.4 ± 2.9 | <0.001 | 4.5 ± 1.3 | 8.9 ± 2.3 | <0.001 |
| FFMI (kg/m2) | 16.2 ± 2.8 | 16.1 ± 1.0 | 0.933 | 17.4 ± 1.8 | 18.0 ± 1.2 | 0.08 | 17.2 ± 2.1 | 18.0 ± 1.3 | 0.004 |
| ALM/Ht2 (kg/m2) | 6.9 ± 0.5 | 6.9 ± 0.4 | 0.787 | 7.7 ± 0.7 | 7.9 ± 0.5 | 0.074 | 7.6 ± 0.7 | 7.8 ± 0.6 | 0.014 |
| HGS (kg) | 32.8 ± 9.8 | 28.6 ± 6.1 | 0.126 | 35.4 ± 8.5 | 34.7 ± 9.3 | 0.700 | 36.5 ± 8.5 | 33.9 ± 8.7 | 0.054 |
| MVPArecreational (minutes/week) | 60 (0.0–280) | 190 (0.0–277) | 0.611 | 145 (0.0–345) | 210 (84–360) | 0.312 | 130 (0.0–420) | 180 (5.0–300) | 0.661 |
| LPA (minutes/week) | 20 (0.0–140.0) | 0.0 (0.0–210) | 0.847 | 55 (0.0–180) | 20 (0.0–175) | 0.735 | 37.5 (0.0–160) | 70 (0.0–210) | 0.608 |
| Sedentary (hours/day) | 5.0 (4.0–6.0) | 4.0 (4.0–6.0) | 0.381 | 4.0 (3.0–6.0) | 5.5 (3.0–7.0) | 0.874 | 4.0 (3.0–6.0) | 4.0 (3.0–6.8) | 0.598 |
Notes: Data presented as mean ± SD and median (1st–3rd) percentiles for normal and non-normal variables.
P-values significant at 0.05 and 0.01 levels.
WC: waist circumference; SBP: systolic blood pressure; DBP: diastolic blood pressure; HRrest: resting heart rate; FMI: fat max index (fat mass/height2); FFMI: fat-free mass index (fat-free mass/height2); ALM: appendicular lean mass; Ht2: height in meters squared; HGS: handgrip strength; MVPArecreational: recreational moderate to vigorous physical activity; LPA: light physical activity.
Figure 1 shows the correlation between MVPArecreational and ALM/Ht2, after excluding individuals who did not engage in MVPA (n = 238); there was no significance in the correlation between LPA and ALM/Ht2.
Figure 1.
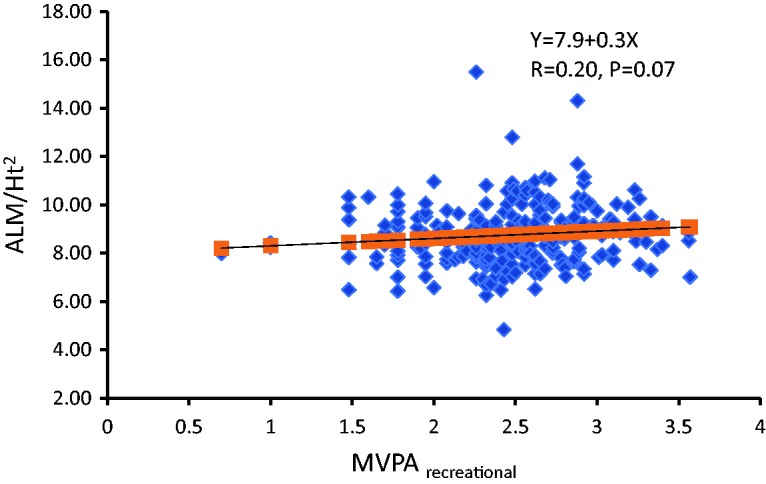
Correlation of recreational moderate to vigorous physical activity (MVPArecreational) with appendicular lean mass over square of height in meters (ALM/Ht2). With a 1-hour increase per week in MVPArecreational, ALM/Ht2 increased 0.30 kg/m2 (n = 238).
Discussion
In the current study, we aimed to examine the association between physical activity patterns, including MVPArecreational, LPA, and sedentary activity, and indicators for sarcopenia including muscle mass and strength. We also investigated the role of age, fat mass, and abdominal fat in sarcopenia and its relationship with physical activity. We found an association between MVPArecreational and muscle mass; along with muscle strength,. These factors were significantly lower in participants age >60 years whereas there was no change in fat mass. LPA was significantly higher in participants without sarcopenia when using Saudi reference values, and weak HGS was correlated with low levels of LPA and MVPArecreational. The findings of the current study suggest interactive effects between physical activity and markers of sarcopenia.
Individuals with sarcopenia were older and had lower body weight, body fat mass, and HGS. One study reported that individuals with sarcopenia had lower weight, BMI, fat mass, and muscle strength and showed poor performance in four of six physical performance tests.24 As shown in Table 1 and Figure 1, the current findings collectively suggest an interactive relationship between ALM/Ht2 and MVPArecreational. This was in agreement with a previous study reporting that MVPA was associated with increased muscle mass and strength.12 Older individuals who engaged in more MVPA were found to have a lower likelihood of developing sarcopenia as compared with the least active participants in the study. The former individuals also had greater HGS and faster walking speed.25 A study reported that individuals who walked <5,300 steps/day and/or spent <15 minutes/day at >3 METs were 2.00 to 2.66 and/or 2.03 to 4.55 times more likely to have sarcopenia, respectively, when compared with those who walked >7,800 steps/day and/or spent >23 minutes/day at >3 METs.11
As expected, the current data showed a strong association between muscle mass and strength, as measured with ALM/Ht2 and HGS. HGS was significantly lower with increased age, and participants age >60 years showed an average HGS of 32.1 kg, similar to that of their peers in previous studies conducted among older Saudi26 and Asian27 men. Increased HGS was associated with increased LPA and MVPArecreational, particularly at the cut-off threshold of 30 kg, and HGS and MVPArecreational were lower in participants >60 years of age. The association among cardiorespiratory fitness, HGS, and physical activity has previously been studied among 67,702 participants in the UK Biobank, and all-cause mortality and cardiovascular disease were assessed in the follow-up period. The hazard ratio of mortality associated with lower physical activity was highest among participants in the lower tertile of HGS and the lowest among those in the highest tertile of HGS; this interaction was not found with cardiorespiratory fitness.28 The UK Biobank data also showed that adiposity and HGS at baseline predicted patterns of physical activity in the 5-year follow-up period and that MVPA was lower in the lowest HGS quintile than that in the highest quintile across all BMI categories, indicating that improving body composition and muscle strength can help to enhance active living and increase MVPA.29 The Lifestyle Interventions and Independence For Elders Study showed that the highest intensity of LPA was associated with greater HGS, but sedentary behavior was not associated with HGS.16 Another study reported that middle- and old-aged adults in the highest quartile of MVPA were stronger (by 1.84 kg) than their peers in the lower quartile, based on HGS measurements.30 It is notable that muscle strength independently influenced mortality to a greater extent than muscle mass in an older cohort,31 and the hazard ratio of mortality was 1.67 in a comparison of the highest and lowest quartiles of HGS, based on 14 studies in older populations.32 Therefore, handgrip weakness in older adults could predict disability.33
In the current findings, whereas height was not associated with muscle mass, there was a significant difference between HGS groups in terms of their average height and weight. A recent study confirmed that the anthropometric measure of height had a high correlation with HGS,34 and another study found that height and weight were correlated with HGS.27 Although BMI had a significant correlation with HGS among Saudi adult men, in stepwise multiple linear regression, BMI was excluded and only hand length, forearm circumference, and age were selected.35 The current data showed that obese participants with sarcopenia had lower HGS than their non-obese counterparts, which approached borderline significance at one threshold used in this study.
There were no differences in physical activity patterns between obese and non-obese participants with sarcopenia, and there was an association between increased fat mass and increased SBP. Thus, it is important to evaluate the association between fat mass and health aspects rather than physical activity alone. For example, sarcopenia and adiposity can increase blood pressure and can synergistically induce hypertension; thus sarcopenic obesity is a strong independent factor of hypertension.36 Generally speaking, previous studies have suggested that sarcopenic obesity is associated with low physical activity,37 and engagement in physical activity can contribute to reducing the risk of sarcopenic obesity.38
The current study can help to improve understanding of the status of sarcopenia and physical activity among middle-aged and older men of Arab ethnicity in the region of Southwest Asia. Our findings can serve as a reference for local and international values of sarcopenia. The primary limitations of this study were that we used a self-reported physical activity questionnaire; these data could have been inaccurately reported by study participants. It should be noted that GPAQ includes only one question on the duration of sedentary activity; different types of sedentary behavior, such as watching television, reading, and computer use, might have different associations and outcomes.16 Future studies should include women as well as all countries in the region, to provide a more comprehensive view of how the factors investigated herein are linked.
To summarize, younger and middle-aged male adults who were stronger and had normal weight preferred to engage in MVPArecreational whereas older men preferred LPA. Sedentary activity levels showed no interactive correlations with the current study variables. Although increased fat mass was associated with increased muscle mass, it had a negative correlation with DBP. The relationship between sarcopenic obesity and physical activity was not different from the relationship between sarcopenia alone and physical activity in the current study. Whereas fat mass did not change for older participants as compared with younger and middle-aged participants, muscle mass was significantly lower among older participants.
Conclusion
MVPArecreational had a borderline association with muscle mass. Independent of the total and abdominal fat mass, muscle mass was lower in older men, who preferred engaging in LPA than middle-aged and young men, who preferred engaging in MVPArecreational. Future studies should be conducted to examine the role of MVPA training programs on muscle mass in older men.
Acknowledgements
The authors are deeply grateful to all Community Development Commissions of Riyadh districts who contributed to the study by contacting the older population in the community and hosting data collection. We also thank the Research Support and Services Unit of the Deanship of Scientific Research at KSU for technical support.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Funding
The study received a grant through the Research Group Program (RG-1439-82) of the Deanship of Scientific Research of KSU, Riyadh, Saudi Arabia.
ORCID iD
Shaea Alkahtani https://orcid.org/0000-0002-1443-6249
References
- 1.Cao L, Morley JE. Sarcopenia is recognized as an independent condition by an International Classification of Disease, Tenth Revision, Clinical Modification (ICD-10-CM) code. J Am Med Dir Assoc 2016; 17: 675–677. DOI: 10.1016/j.jamda.2016.06.001. [DOI] [PubMed] [Google Scholar]
- 2.Roubenoff R. Sarcopenic obesity: does muscle loss cause fat gain?: Lessons from rheumatoid arthritis and osteoarthritisa. Ann N Y Acad Sci 2000; 904: 553–557. DOI: 10.1111/j.1749-6632.2000.tb06515.x. [DOI] [PubMed] [Google Scholar]
- 3.Rolland Y, Lauwers-Cances V, Cristini C, et al. Difficulties with physical function associated with obesity, sarcopenia, and sarcopenic-obesity in community-dwelling elderly women: the EPIDOS (EPIDemiologie de l’OSteoporose) Study. Am J Clin Nutr 2009; 89: 1895–1900. DOI: 10.3945/ajcn.2008.26950. [DOI] [PubMed] [Google Scholar]
- 4.Dufour AB, Hannan MT, Murabito JM, et al. Sarcopenia definitions considering body size and fat mass are associated with mobility limitations: the Framingham Study. J Gerontol A Biol Sci Med Sci 2012; 68: 168–174. DOI: 10.1093/gerona/gls109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chang CI, Huang KC, Chan DC, et al. The impacts of sarcopenia and obesity on physical performance in the elderly. Obes Res Clin Pract 2015; 9: 256–265. DOI: 10.1016/j.orcp.2014.08.003. [DOI] [PubMed] [Google Scholar]
- 6.Moreira MA, Zunzunegui MV, Vafaei A, et al. Sarcopenic obesity and physical performance in middle aged women: a cross-sectional study in Northeast Brazil. BMC Public Health 2016; 16: 43 DOI: 10.1186/s12889-015-2667-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zamboni M, Mazzali G, Fantin F, et al. Sarcopenic obesity: a new category of obesity in the elderly. Nutr Metab Cardiovasc Dis 2008; 18: 388–395. DOI: 10.1016/j.numecd.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 8.Kim H, Suzuki T, Kim M, et al. Incidence and predictors of sarcopenia onset in community-dwelling elderly Japanese women: 4-year follow-up study. J Am Med Dir Assoc 2015; 16: 85.e1-8. DOI: 10.1016/j.jamda.2014.10.006. [DOI] [PubMed] [Google Scholar]
- 9.Rosique-Esteban N, Babio N, Diaz-Lopez A, et al. Leisure-time physical activity at moderate and high intensity is associated with parameters of body composition, muscle strength and sarcopenia in aged adults with obesity and metabolic syndrome from the PREDIMED-Plus study. Clin Nutr 2019; 38: 1324–1331. DOI: 10.1016/j.clnu.2018.05.023. [DOI] [PubMed] [Google Scholar]
- 10.Lee SY, Tung HH, Liu CY, et al. Physical activity and sarcopenia in the geriatric population: a systematic review. J Am Med Dir Assoc 2018; 19: 378–383. DOI: 10.1016/j.jamda.2018.02.003. [DOI] [PubMed] [Google Scholar]
- 11.Park H, Park S, Shephard RJ, et al. Yearlong physical activity and sarcopenia in older adults: the Nakanojo Study. Eur J Appl Physiol 2010; 109: 953–961. [DOI] [PubMed] [Google Scholar]
- 12.Sanchez-Sanchez JL, Manas A, Garcia-Garcia FJ, et al. Sedentary behaviour, physical activity, and sarcopenia among older adults in the TSHA: isotemporal substitution model. J Cachexia Sarcopenia Muscle 2019; 10: 188–198. DOI: 10.1002/jcsm.12369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Westbury LD, Dodds RM, Syddall HE, et al. Associations between objectively measured physical activity, body composition and sarcopenia: findings from the Hertfordshire Sarcopenia Study (HSS). Calcif Tissue Int 2018; 103: 237–245. DOI: 10.1007/s00223-018-0413-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cruz-Jentoft AJ, Baeyens JP, Bauer JM, et al. Sarcopenia: European consensus on definition and diagnosis: report of the European Working Group on Sarcopenia in Older People. Age Ageing 2010; 39: 412–423. DOI: 10.1093/ageing/afq034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cruz-Jentoft AJ, Bahat G, Bauer J, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing 2019; 48: 16–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bann D, Hire D, Manini T, et al. Light intensity physical activity and sedentary behavior in relation to body mass index and grip strength in older adults: cross-sectional findings from the Lifestyle Interventions and Independence for Elders (LIFE) study. PLoS One 2015; 10: e0116058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen LK, Liu LK, Woo J, et al. Sarcopenia in Asia: consensus report of the Asian Working Group for Sarcopenia. J Am Med Dir Assoc 2014; 15: 95–101. DOI: 10.1016/j.jamda.2013.11.025. [DOI] [PubMed] [Google Scholar]
- 18.Saudi Census. Statistical Saudi Population Survey. 2018. https://www.stats.gov.sa/ (accessed 15 February 2020).
- 19.Hajian-Tilaki K. Sample size estimation in epidemiologic studies. Caspian J Intern Med 2011; 2: 289–298. [PMC free article] [PubMed] [Google Scholar]
- 20.Alkahtani SA. A cross-sectional study on sarcopenia using different methods: reference values for healthy Saudi young men. BMC Musculoskelet Disord 2017; 18: 119 DOI: 10.1186/s12891-017-1483-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang X, Xie X, Dou Q, et al. Association of sarcopenic obesity with the risk of all-cause mortality among adults over a broad range of different settings: a updated meta-analysis. BMC Geriatr 2019; 19: 183 DOI: 10.1186/s12877-019-1195-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yakout SM, Alkahtani SA, Al-Disi D, et al. Coexistence of pre-sarcopenia and metabolic syndrome in Arab men. Calcif Tissue Int 2019: 104: 130–136. DOI: 10.1007/s00223-018-0477-2. [DOI] [PubMed] [Google Scholar]
- 23.Alkahtani SA. Convergent validity: agreement between accelerometry and the Global Physical Activity Questionnaire in college-age Saudi men. BMC Res Notes 2016; 9: 436 DOI: 10.1186/s13104-016-2242-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Meng NH, Li CI, Liu CS, et al. Sarcopenia defined by combining height- and weight-adjusted skeletal muscle indices is closely associated with poor physical performance. J Aging Phys Act 2015; 23: 597 DOI: 10.1123/japa.2014-0036. [DOI] [PubMed] [Google Scholar]
- 25.Mijnarends DM, Koster A, Schols JM, et al. Physical activity and incidence of sarcopenia: the population-based AGES—Reykjavik Study. Age Ageing 2016; 45: 614–620. DOI: 10.1093/ageing/afw090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bindawas SM, Vennu V, Al-Orf SM, et al. Normative data for handgrip strength in Saudi older adults visiting primary health care centers. Medicina 2019; 55: 251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ong HL, Abdin E, Chua BY, et al. Hand-grip strength among older adults in Singapore: a comparison with international norms and associative factors. BMC Geriatr 2017; 17: 176 DOI: 10.1186/s12877-017-0565-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Celis-Morales CA, Lyall DM, Anderson J, et al. The association between physical activity and risk of mortality is modulated by grip strength and cardiorespiratory fitness: evidence from 498 135 UK-Biobank participants. Eur Heart J 2016; 38: 116–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim Y, White T, Wijndaele K, et al. Adiposity and grip strength as long-term predictors of objectively measured physical activity in 93 015 adults: the UK Biobank study. Int J Obes (Lond) 2017; 41: 1361 DOI: 10.1038/ijo.2017.122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Keevil VL, Cooper AJM, Wijndaele K, et al. Objective sedentary time, moderate-to-vigorous physical activity, and physical capability in a British cohort. Med Sci Sports Exerc 2016; 48: 421–429. DOI: 10.1249/MSS.0000000000000785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Newman AB, Kupelian V, Visser M, et al. Strength, but not muscle mass, is associated with mortality in the Health, Aging and Body Composition Study cohort. J Gerontol A Biol Sci Med Sci 2006; 61: 72–77. DOI: 10.1093/gerona/61.1.72. [DOI] [PubMed] [Google Scholar]
- 32.Cooper R, Kuh D, Hardy R, et al. Objectively measured physical capability levels and mortality: systematic review and meta-analysis. BMJ 2010; 341: c4467 DOI: 10.1136/bmj.c4467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Duchowny KA, Clarke PJ, Peterson MD. Muscle weakness and physical disability in older Americans: longitudinal findings from the U.S. Health and Retirement Study. J Nutr Health Aging 2018; 22: 501–507. DOI: 10.1007/s12603-017-0951-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rodriguez-Garcia WD, Garcia-Castaneda L, Orea-Tejeda A, et al. Handgrip strength: reference values and its relationship with bioimpedance and anthropometric variables. Clin Nutr ESPEN 2017; 19: 54–58. DOI: 10.1016/j.clnesp.2017.01.010. [DOI] [Google Scholar]
- 35.Alahmari KA, Silvian SP, Reddy RS, et al. Hand grip strength determination for healthy males in Saudi Arabia: a study of the relationship with age, body mass index, hand length and forearm circumference using a hand-held dynamometer. J Int Med Res 2017; 45: 540–548. DOI: 10.1177/0300060516688976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Park SH, Park JH, Song PS, et al. Sarcopenic obesity as an independent risk factor of hypertension. J Am Soc Hypertens 2013; 7: 420–425. DOI: 10.1016/j.jash.2013.06.002. [DOI] [PubMed] [Google Scholar]
- 37.Hwang B, Lim JY, Lee J, et al. Prevalence rate and associated factors of sarcopenic obesity in Korean elderly population. J Korean Med Sci 2012; 27: 748–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Aggio DA, Sartini C, Papacosta O, et al. Cross-sectional associations of objectively measured physical activity and sedentary time with sarcopenia and sarcopenic obesity in older men. Prev Med 2016; 91: 264–272. DOI: 10.1016/j.ypmed.2016.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]


