Short abstract
Background
Epithelial–myoepithelial carcinomas make up less than 0.1% of head and neck malignancies and are regarded as rare, low-grade malignant neoplasms of the salivary gland. They are thought to arise from intercalated ducts with histopathology showing a classic biphasic morphology of an outer layer of myoepithelial cells and inner layer of epithelial cells. These tumors most commonly occur in the parotid gland; however, rare cases have also been described in the nasal cavity, nasopharynx, subglottis, base of tongue, and the lacrimal gland.
Objective
To describe the clinical presentation, surgical management, and histopathology of the first reported case of lacrimal sac epithelial–myoepithelial carcinoma. To conduct a literature review of this malignancy, which is present in the lacrimal system.
Methods
Case report (n = 1) and literature review.
Results
We report a case of a 72-year-old man presenting with epiphora and a lacrimal sac mass with intranasal extension on imaging and nasal endoscopy. A combined endoscopic endonasal and open approach provided successful definitive treatment for final pathologic diagnosis of epithelial–myoepithelial carcinoma of the lacrimal sac, with orbital reconstruction and lacrimal stenting providing good cosmetic and functional results.
Conclusions
After PubMed database search for any case series or reports of lacrimal system epithelial–myoepithelial carcinomas, we believe this is the first documented case originating from the lacrimal sac. Although the histopathology of this tumor is distinct, unusual location and clinical presentation may pose significant diagnostic difficulties.
Keywords: epithelial–myoepithelial, lacrimal tumor, lacrimal sac, epiphora, endoscopic lacrimal surgery
Introduction
Epithelial–myoepithelial carcinoma (EMC) is a rare, malignant neoplasm of the salivary gland thought to arise from intercalated ducts. First describing the tumor in 1972, Donath et al. reported that the histopathology of EMC was distinguished by its biphasic morphology, characterized by an inner layer of epithelial-lined ducts surrounded by an outer layer of clear myoepithelial cells.1,2 These neoplasms make up less than 0.1% of head and neck malignancies, accounting for approximately 1% of salivary gland tumors.2,3 EMCs predominantly occur in the parotid gland, followed by the submandibular and minor salivary glands; however, those originating from the nasal cavity, nasopharynx, paranasal sinuses, lacrimal gland, and several others sites have also been described.4–5
EMC is exceptionally rare in the lacrimal system, with only 8 documented cases in the literature, all presenting within the lacrimal gland.6–13 There have been no documented cases of EMC originating from within the lacrimal sac. Although this neoplasm is known to behave like a low-grade malignancy in the salivary gland with a favorable survival rates, its disposition in the lacrimal system is less clear. In this article, the authors describe the first documented case of EMC originating from the lacrimal sac and present a comprehensive review of lacrimal system EMCs reported in the literature.
Case Report
A 72-year-old Caucasian male was referred to our rhinology clinic for evaluation and management of a right lacrimal sac mass discovered incidentally on imaging. Previously, a right-sided dacryocystorhinostomy (DCR) had been performed by an outside ophthalmologist 8 years prior for epiphora, which was thought to be secondary to idiopathic nasolacrimal duct (NLD) obstruction. The surgeon reported that a mass was not appreciated during the initial surgery, so a biopsy was not performed. The patient’s epiphora resolved for 4 years. His medical history was also significant for colon cancer managed by surgical resection followed by chemotherapy 6 years ago.
In the last few months, he had undergone an extensive work-up of his lung nodules, which unfortunately revealed biopsy-proven recurrence of his colon cancer with metastases to the lungs. The positron emission tomography/computed tomography (PET/CT) obtained during this process showed an incidental F-fluorodeoxyglucose-avid (FDG) right lacrimal sac mass with extension into the nasal cavity. Patient was referred to an outside otolaryngologist who performed a right maxillary antrostomy, partial ethmoidectomy, and biopsy of the nasolacrimal mass. Outside evaluation of final pathology initially was consistent with a diagnosis of oncocytoma.
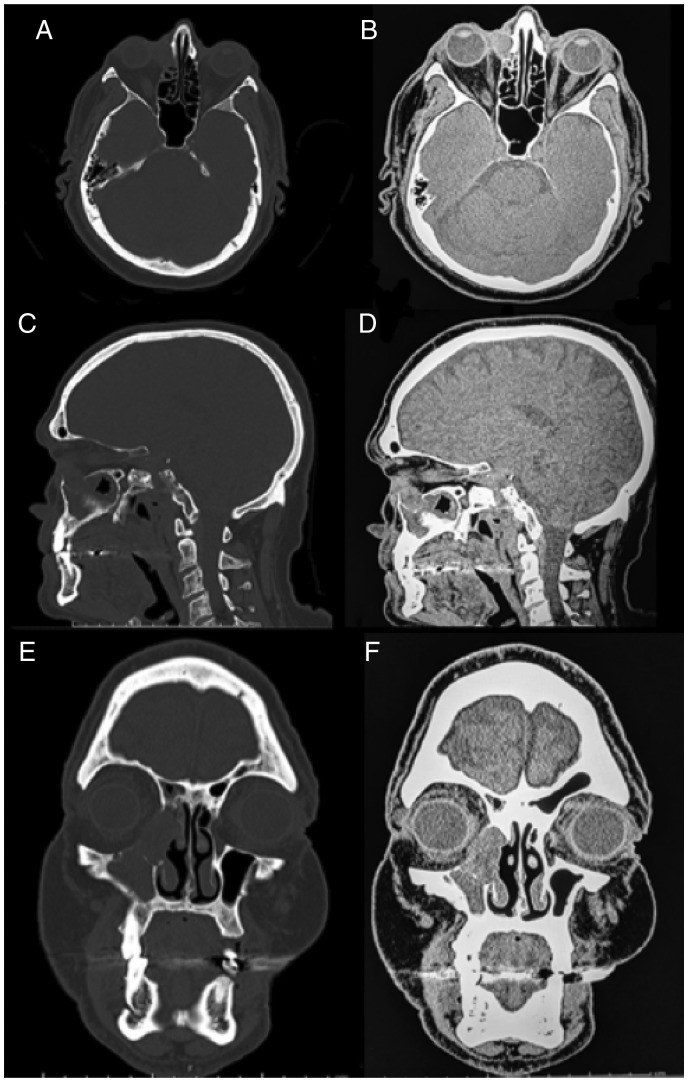
A noncontrasted CT maxillofacial scan was obtained prior to our consultation, exhibiting a soft tissue mass of the right lacrimal sac measuring 2.1 × 1.6 × 2.4 cm (anteroposterior, transverse, and cranial caudal). The mass abutted but did not invade the orbit, and it extended into the nasal cavity at the level of the right inferior turbinate and maxillary sinus (Figure 1). There was evidence of erosion of the right medial lacrimal bone as well as edema of the right maxillary and ethmoid sinuses.
Figure 1.
Noncontrasted maxillofacial computed tomography: (A) axial bone window, (B) axial soft tissue window, (C) sagittal bone window, (D) sagittal soft tissue window, (E) coronal bone-window, and (F) coronal soft tissue window. CT depicts a soft tissue ovoid mass centered in the right nasolacrimal sac, measuring 2.1 × 1.6 × 2.4 cm. No evidence of orbital invasion, but there is extension into the nasal cavity via the nasolacrimal duct to the level of the inferior turbinate and maxillary sinus. Smooth bony remodeling is evident with erosion of the medial lacrimal bone. CT, computed tomography.
On initial presentation, the patient endorsed worsening right-sided epiphora for the past 4 years, denying other otolaryngologic or ophthalmologic complaints including nasal obstruction or changes in his vision. Physical examination showed minimal proptosis along with tearing of the right eye. Diagnostic sinonasal endoscopy was significant for crusting along the lateral nasal wall anterior to the maxillary antrostomy at the reported site of previous biopsies, with irregularity of the lateral wall mucosa suspicious for tumor involvement. Preoperative slit lamp examination by ophthalmology exhibited a prominent right lacrimal sac and increased tear lake on the right.
The patient elected to proceed with surgical excision of the neoplasm in a combined approach with both Oculoplastics and Otolarynology. A right-sided combined transcaruncular, inferior transconjunctival orbitotomy and dacryocystectomy with subperiosteal dissection was performed to expose the superior portion of the mass. The tumor was easily dissected away from the periorbita. Next, a complete endonasal resection was achieved by performing a right-sided medial maxillectomy, anterior ethmoidectomy, inferior turbinectomy, and a partial middle turbinectomy utilizing stereotactic navigation.
Through endoscopic endonasal visualization, the common canaliculus showed no evidence of tumor involvement. After intranasal and transorbital margins were confirmed to be negative for tumor by frozen section pathology, Crawford tubes were placed and the medial orbital wall was reconstructed with a silastic implant. The patient experienced no immediate or postoperative complications with full ocular motility, no ectropion or entropion, and no appreciable enophthalmos.
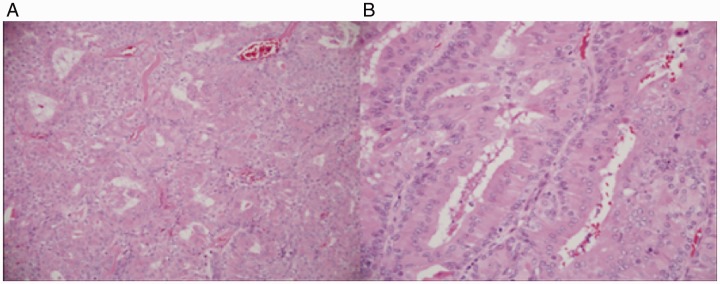
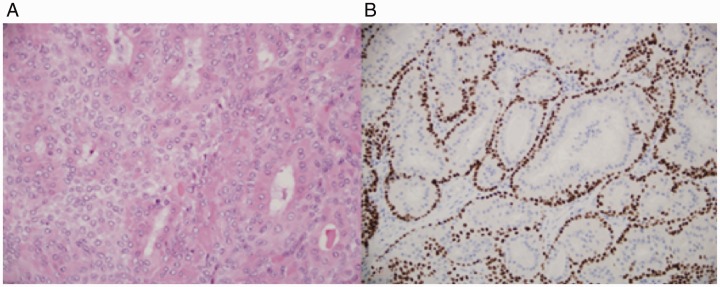
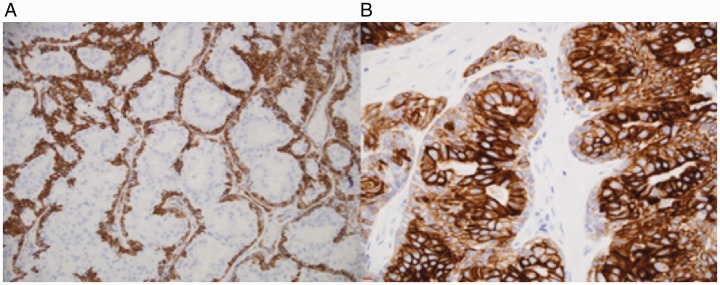
The gross specimen was a 4.5 × 4.0 × 0.5 cm gray-tan, homogenous mass. Final pathology of the right nasolacrimal mass was consistent with the diagnosis of low-grade EMC without perineural, lymphovascular, or bony invasion. All final margins were negative. Microscopically, the tumor sections showed a mass composed of 2-layered epithelium arranged in interconnecting nests, tubules, and gland-like structures. The inner layer was characterized by abundant eosinophilic granular cytoplasm, while the outer layer had less conspicuous cytoplasm (Figures 2 and 3). The nuclei showed mild enlargement and pleomorphism. On immunohistochemistry, the outer layer of myoepithelial cells was strongly positive for smooth muscle actin (SMA), calponin, p63, and keratin 5/6. The inner layer of epithelial cells stained strongly for keratin 7 (Figure 4).
Figure 2.
A, Low power: a tumor with oncocytic (eosinophilic) cytoplasm and gland-like spaces is shown. B, Higher power: gland-like spaces with 2 layers of cells are shown.
Figure 3.
A, Luminal more eosinophilic epithelial cells and myoepithelial cells with more pale cytoplasm. B, p63 staining of myoepithelial cell components.
Figure 4.
A, Smooth muscle actin staining of myoepithelial cell components. B, Keratin 7 staining of luminal epithelial components stronger than myoepithelial component.
Since the operation, our patient has been recovering well. He initially experienced very mild right-sided epiphora, which had completely resolved after 3 months at which time the stents were removed. Physical examination and nasal endoscopy have been performed every 3 months for surveillance, with no evidence of residual tumor or recurrence at 20 months postoperatively. Surveillance imaging has not been performed. He has required periodic endoscopic debridements in the area of resection and has nearly complete remucosalization of the lateral nasal and orbital wall surfaces. With regard to his lung metastases, he has successfully completed gamma knife therapy with excellent results.
Discussion
This case report documents the first EMC to originate within the lacrimal sac. Interestingly, the initial incidence of epiphora in the patient was thought to be secondary to idiopathic NLD obstruction. No biopsy was performed by the outside surgeon during the initial DCR because no mass was appreciated.
However, it is very reasonable to postulate that the tumor in our patient may have existed 8 years prior to our definitive surgery and was potentially the cause of the initial NLD obstruction. The tumor may have been very small at the time, slowly growing over the next 8 years. Fortunately, the tumor was visualized on the PET/CT, which showed an FDG-avid mass that prompted a biopsy.
In reviewing the literature, routine biopsy in cases for NLD obstruction during DCR is controversial with some publications recommending it and others only in select cases. A recently published retrospective review by Banks et al. found that an unsuspected neoplastic or granulomatous cause of lacrimal obstruction was identified on intraoperative biopsy in only 0.46% of cases, compared with 0% to 2.3% reported in the literature.14 The senior authors only perform biopsies if there is a visible mass or thickened lacrimal sac flaps.
EMC is an uncommon malignancy in the head and neck region and exceptionally rare within the lacrimal system. Definitive diagnosis requires histopathologic evidence of its pathognomonic biphasic morphology comprising of clear myoepithelial cells that surround epithelial-lined ducts resembling intercalated ducts.1–3
It is important to understand the distinction between EMC and another malignant salivary neoplasm called myoepithelial carcinoma (MC), because they behave differently. In terms of its histopathology, MC is composed almost entirely of tumor cells with myoepithelial differentiation. Unlike EMC, one-third of patients with MC generally remain free of disease after treatment, another one-third develop recurrences, and the remaining one-third succumb to the disease with likely distant metastases.6,15
In contrast, salivary gland EMCs generally have a more favorable course, with 5- and 10- overall survival rates of 73% to 94% and 60% to 90%. Studies have found that approximately 30% to 40% of patients have local recurrence, 20% have cervical lymph node metastases, and 2.6% to 10% of patients experience distant metastases. The mean age at presentation is approximately 60 to 64 years, with a female predominance (1.3–2.0:1.0).2,3,15,16 Vázquez et al. found that a tumor size greater than 4 cm and high-grade histology were associated with increased mortality.3 A follow-up study of EMCs in all sites found that patients with T2, T3, and T4 or M1 tumors had significantly shorter survival than patients with T1 or M0 tumors, respectively.16 To date, there are no comprehensive studies linking the behavior of lacrimal system EMCs to salivary gland EMCs.
In reviewing the literature, the authors found 8 other cases of EMCs within the lacrimal system (Table 1). Specifically, within this subset of patients, the age of diagnosis ranged from 41 to 72 years, with an average age of 62.8 years. This is comparable to the average age reported in salivary gland EMCs, although instead of a female predominance, there is a nearly equal number of males to females with a slight male predominance (5:4).6–13
Table 1.
Literature Review of Lacrimal System Epithelial–Myoepithelial Carcinomas.
| Study | Age/Sex | Clinical Presentation | Imaging | Treatment | Gross | Histopathology | Immunohisto chemistry | Final Stage | Outcome | Comments |
|---|---|---|---|---|---|---|---|---|---|---|
| Ostrowski et al.11 | 63/M | 8 years of slowly progressive, painless proptosis followed by 2 months of rapid progression | CT: Left 2.5 × 1.5 cm oblong mass of LG with irregular borders and central focal calcification; no bony erosion | Left orbital exenteration. No RT | 3.2 × 2.8 × 1.9 cm firm, tan mass | Biphasic pattern. (+) Atypia. No LVI, PNI, or necrosis. Positive margins with capsular invasion | Myoepithelial:(+) S-100, alpha-actin Epithelial: (+) EMA, cytokeratin, AE1/AE3 |
∼T2N0M0 | No recurrence at 2.5 years | LG EMC in context of PA |
| Chan et al.9 | 80/M | 9 months of painless, subcutaneous mass in left upper outer eyelid | CT: Left 2.4 × 1.5 × 1.8 cm mildly enhancing mass of LG | Left translid lateral orbitotomy with gross total resection. RT advised but refused | 2.3 × 1.8 × 1.8 cm firm, well-circumscribed encapsulated tumor; no bony erosion | (+) Myoepithelial anaplasia. No LVI, PNI, or necrosis. Positive margins and capsular invasion | Myoepithelial: (+) S100, SMA Epithelial: (+) cytokeratin AE1/AE3, EMA |
∼T2N0M0 | No recurrence at 2 years | LG EMC |
| Wiwatwongwana et al.13 | 86/M | 6 months of painless diplopia | CT: Left 2.5 × 1.8 cm moderately homogenous, hyperattenuating mass with area of scalloping in the lacrimal fossa | Left lateral orbitotomy with gross total resection followed by RT due to positive margins (60 Gy) | Not reported. Mass adherent to periosteum with irregularity of adjacent bone | (+) Myoepithelial anaplasia and necrosis. No LVI or PNI. Positive margins, no bony invasion | Myoepithelial: (+) p63a | Reported as T2N0M0, however likely T4aN0M0 | Death at 15 months postoperatively with no clinical evidence of recurrent disease | LG EMC |
| Singh et al.7 | 62/F | 4 years of right orbital swelling with 6 months of rapid progression; painless; vision unaffected | CT: Right 2.7 × 2.4 × 2.0 cm heterogeneously enhancing lobulated LG mass with expansion and thinning of the overlying bone MRI: hypointense on T1 and heterogeneously hyperintense on T2 |
Right fronto-orbital craniotomy with gross total resection followed by RT due to positive margins (dose not reported) | 4 × 2 × 2 cm multinodular, circumscribed mass invading through periorbita without dural invasion | (+) Atypia, PNI. No necrosis or LVI. Positive margins. | Myoepithelial: (+) S100, BCL2, SMA, MIC3 Epithelial: (+) cytokeratin AE1/AE3, c-kit/CD117, MIC3 |
∼T4aN0M0 | No recurrence at 3 months | LG EMC |
| Venkatesulu et al.12 | 47/M | 3 months of progressive vision loss and painless swelling of right orbit. History of previously excised pleomorphic adenoma of the right lacrimal gland 2 years ago | CT: Right well-circumscribed heterogeneously enhancing mass with inferior displacement of globe, expansion of orbit with destruction and remodeling of the lateral wall of orbit involving all oblique as well as medial and superior rectus muscles | Right radical orbital exenteration with anterior skull base resection and temporalis muscle reconstruction followed by RT due to concern for possible recurrence (60 Gy) | 3 × 3 cm tumor grossly involving extraocular muscles | LVI, necrosis, PNI, atypia not reported. All margins negative | Myoepithelial: (+) SMA, and S-100 Epithelial: (+) EMA |
∼T4aN0M0 | No recurrence at 6 months | LG EMC in the setting of previously excised PA |
| Goncalves et al.10 | 61/F | 6 months of painless progressive proptosis in left orbit. History of previously excised PA of the left LG 14 years ago | CT: Left well-circumscribed, heterogeneously enhancing, and mostly hyperattenuating LG mass MRI: Hypointense on T1; heterogeneously isointense on T2 weighted and enhancement with gadolinium |
Left combined anterior and lateral orbitotomy with complete excision followed by RT (dose not reported) | Not reported | (+) PNI. LVI, necrosis, atypia not reported. All margins negative. | Myoepithelial: (+) p63, SMA Epithelial: (+) cytokeratin 7 |
Unknown staging. Low-grade behavior reported | No recurrence at 36 months | LG EMC in the setting of previously excised PA |
| Avdagic et al.8 | 53/F | 1 month of painless decreased vision, progressive left periorbital fullness | CT: Left 2.0 × 2.5 cm enhancing, circumscribed LG mass with optic nerve and globe compression | Left lateral orbitotomy with complete excision followed by RT (60 Gy) | 3.5 × 2.0 × 1.5 cm well-encapsulated tan mass | Biphasic pattern. (+) Necrosis, PNI, atypia. No LVI. Positive lateral margin. | Myoepithelial: (+) p63, calponin Epithelial: (+) Cam5.2, CEA-M |
∼T3N0M0, intermediate grade | No recurrence at 24 months | LG EMC in the setting of a carcinoma ex PA |
| Li et al.6 | 41/F | Right orbital mass excision 10 years prior, several days of right eye pain and blurred vision. Presented as a VLM with radiographic and histopathologic evidence of intralesional cysts. Initially treated with bleomycin with minimal resolution. | MRI: Right 3.6 × 2.1 × 3.5 cm extraconal, multilobulated lacrimal gland mass with cystic fluid-fluid levels | Right orbitotomy with complete excision. Radiation advised but not reported if completed. | 5.1 x 4.3 x 2.6 cm homogenous mass | Biphasic pattern. No necrosis, LVI, or anaplasia. PNI not reported. All margins negative. | Myoepithelial: (+) p63 Epithelial: (+) cytokeratin AE1/AE3 Both (+) Sox-10 |
∼T3N0M0, low-grade | No recurrence at 4 months | LG EMC in the setting of a VLM |
| Current Case, 2019 | 72/M | History of DCR 8 years ago. 4 years of worsening epiphora. Mass found incidentally on work-up of lung nodules | CT: Right 2.1 × 1.6 × 2.4 cm mass centered in the nasolacrimal sac | Right orbitotomy and dacryocystectomy; right medial maxillectomy, anterior ethmoidectomy, inferior turbinectomy, partial middle turbinectomy | 4.5 × 4.0 × 0.5 cm gray-tan, homogenous mass | Biphasic pattern. No necrosis, PNI, LVI, or anaplasia. All margins negative | Myoepithelial: (+) SMA, Calponin, p63, keratin 5/6; Epithelial: (+) Keratin 7 |
T3N0M0, low-grade | No recurrence at 20 months | Lacrimal Sac EMC |
Abbreviations: CEA-M, carcinoembryonic antigen–monoclonal; CT, computed tomography; EMA, epithelial membrane antigen; EMC, epithelial-myoepithelial carcinoma; DCR, dacryocystorhinostomy; IHC, immunohistochemistry; LG, lacrimal gland; LVI, lymphovascular invasion; MRI, magnetic resonance imaging; PA, pleomorphic adenoma; PNI, perineural invasion; RT, radiotherapy; SMA, smooth muscle actin.
aDoes not report epithelial markers.
Most patients experienced similar progressive symptoms and physical examination findings which included diplopia, proptosis, swelling, and restriction of extraocular motor movements. Decreased or blurred vision was also reported in 5 of the 9 cases. Loss of vision is likely due to the tumor’s mass effect on the orbit and optic nerve as at least 2 cases reported return of vision to baseline after treatment. Duration of symptoms ranged from approximately 1 month to 8 years. Nearly all of the cases reported that the tumors were painless in nature with the exception of the one reported by Li et al.6–13
The patient in our case presentation experienced worsening epiphora secondary to NLD obstruction and mild proptosis, but he had no diplopia, restriction of extraocular motor movements, diminished baseline vision, or pain associated with the tumor. Of note, Flam et al. reported the first case of a nasal cavity EMC presenting as unilateral epiphora, which was located between the inferior turbinate and the medial wall of the maxillary sinus.4
Four of the 9 cases in the literature review arose in the setting of a prior or existing pleomorphic adenoma.8,10–12 In addition to these cases, 2 of the other cases potentially arose from benign masses. Li et al. reported the case of a 41-year-old female diagnosed with EMC of the lacrimal gland who had previously had an unknown right orbital mass removed 10 years ago.6 Singh et al. presented the case of 62-year-old female who had swelling of the right orbit for 4 years with rapid progression over the last 6 months which they postulated could have been due to malignant change of a benign neoplasm.7 Considering our patient required a DCR 8 years ago, it is possible that the initial NLD obstruction was secondary to an unrecognized mass such as a pleomorphic adenoma or early EMC. This review shows that EMCs in the lacrimal system can occur either de novo or arise from malignant transformation of a benign mass.
With regard to radiological features, there was a significant variety in the lacrimal system subset. Li et al. demonstrated a multilobulated mass containing cystic fluid–fluid levels on magnetic resonance imaging (MRI).6 Goncalves et al. noted the mass was hypointense on MRI T1-weighted images, heterogeneously isointense on T2-weighted images, and showed heterogenous enhancement with gadolinium.10 On CT imaging, Ostrowski et al. described the central areas of calcifications within the tumor.11 Three cases had bony involvement on CT. Along the lacrimal fossa, Wiwatwongwana et al. described a scalloping effect, and Singh et al. reported expansion and thinning of the bone.7,13 Venkatesulu et al. showed enlargement of the orbit with destruction and remodeling of the lateral orbital wall.12 Several of the other cases reported no bony erosion on imaging.6–10 There was a variety of heterogenous enhancement in all patients who had contrasted-CT imaging.6–13 Figure 1 shows that the EMC in our patient did not enlarge or invade the orbit; however, it did extend into the nasal cavity through the NLD, causing smooth bony remodeling with erosion of the medial lacrimal bone.
In terms of histology, all cases provided evidence of the characteristic biphasic morphology that defines the neoplasm. A previous study identified four histologic predictors of recurrence including margin status, lymphovascular invasion (LVI), tumor necrosis, and myoepithelial anaplasia.17 Table 1 shows the pertinent gross pathology, histopathology, and immunohistochemistry of lacrimal system EMCs. Five cases had positive margins on surgical resection. In addition, 3 of the 8 cases noted perineural invasion (PNI), while no cases reported LVI.4–11
Immunohistochemistry studies for specific cell markers are important adjuncts for confirming the diagnosis by definitively identifying the 2 distinct layers. Excellent epithelial markers include cytokeratin AE1/AE3 and CAM 5.2, while pankeratin is not very sensitive or specific.Myoepithelial markers include p63, S100 protein, SMA, vimentin. Calponin and glial fibrillary acidic protein were found to be not very sensitive myoepithelial markers.16 Immunohistochemical analysis in our case showed positivity for SMA, calponin, p63, and keratin 5/6 of outer myoepithelial cells and positivity for keratin 7 of inner epithelial cells.
In terms of treatment, surgical resection is the mainstay of management.16 However, it does remain unclear whether adjuvant radiotherapy is necessary. Older studies have postulated that radiation may be beneficial in preventing local recurrence. In the largest series to date, Vázquez et al. found no additional survival benefit with adjuvant radiation, but their study unfortunately did not account for histologic grade or positive margins, only tumor stage. Regardless, adjuvant radiotherapy is routinely recommended if there are positive margins. Chemotherapy is not effective against these tumors.2
In the literature review of lacrimal system EMCs, all studies reported primary surgical management of the tumors, and 5 of those patients completed adjuvant radiotherapy. Ostrowski et al. decided against radiotherapy and managed conservatively with frequent follow-up examinations.11 Chan et al. advised radiotherapy, but the patient refused.9 Li et al. also recommended radiation; however, they did not report whether this was completed.6 For the present case, adjuvant radiation was decided against because of negative margin status and low-grade histology without LVI or PNI. There were no reports of any recurrence or metastasis in any of the cases with follow-up periods that ranged from 3 to 36 months.6–13
The behavior of lacrimal system EMCs is difficult to characterize because of their rarity; however, these neoplasms generally behave as low-grade malignancies and occur either de novo or arise from malignant transformation of a benign mass. To the best of our knowledge, this article describes the first case of an EMC originating from the lacrimal sac without lacrimal gland or paranasal sinus involvement. This is of important clinical relevance because diagnostic work-up and surgical management for a lacrimal sac EMC will be significantly different than a lacrimal gland EMC. Otolaryngologists have a crucial role in diagnosing and managing lacrimal sac tumors as shown in our case, whereas lacrimal gland tumors are usually managed by Ophthalmologists alone.
Conclusions
Complete surgical excision with clear margins is the mainstay of treatment for EMC given there is a potential for local recurrence and metastasis. Histopathology is required and immunohistochemistry is invaluable for accurate diagnosis. Although the histopathology of this tumor is distinct, unusual location and clinical presentation may pose significant diagnostic difficulties. A multidisciplinary approach with both Otolaryngology and Ophthalmology teams should be considered for EMC of the lacrimal sac.
Authors’ Note
This study was presented at the 2019 American Rhinologic Society 65th Annual Meeting on September 14, 2019 in New Orleans, Louisiana.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical Approval
This study was approved by our institutional review board.
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD
Dhruv Sharma https://orcid.org/0000-0001-8107-9520
Statement of Human and Animal Rights
This article does not contain any studies with human or animal subjects.
Statement of Informed Consent
There are no human subjects in this article and informed consent is not applicable.
References
- 1.Donath K, Seifert G, Schmitz R. Diagnosis and ultrastructure of the tubular carcinoma of salivary gland ducts: epithelial-myoepithelial carcinoma of the intercalated ducts [in German]. Virchows Arch A Pathol Pathol Anat. 1972; 356(1):16–31. [PubMed] [Google Scholar]
- 2.El-Naggar A, Chan J, Takata T, et al. WHO Classification of Head and Neck Tumours. Vol. 9, 4th ed Lyon, France: International Agency for Research on Cancer; 2017. [Google Scholar]
- 3.Vázquez A, Patel TD, D’Aguillo CM, et al. Epithelial-myoepithelial carcinoma of the salivary glands. Otolaryngol Head Neck Surg. 2015; 153(4):569–574. [DOI] [PubMed] [Google Scholar]
- 4.Flam JO, Brook CD, Sobel R, et al. Nasal epithelial myoepithelial carcinoma: an unusual cause of epiphora, a case report and review of the literature. Allergy Rhinol. 2015; 6(2):133–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schuman TA, Kimple AJ, Edgerly CH, et al. Sinonasal epithelial-myoepithelial carcinoma: report of a novel subsite and review of the literature. Allergy Rhinol. 2018; 9:215265671876422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li E, Distefano A, Sinard J, et al. Epithelial-myoepithelial carcinoma presenting as a pseudo veno-lymphatic malformation. Ophthalmic Plast Reconstr Surg. 2018; 34(5):e157–e160. [DOI] [PubMed] [Google Scholar]
- 7.Singh G, Sharma M, Agarwal S, et al. Epithelial-myoepithelial carcinoma of the lacrimal gland: a rare case. Ann Diagn Pathol. 2012; 16(4):292–297. [DOI] [PubMed] [Google Scholar]
- 8.Avdagic E, Farber N, Katabi N, et al. Carcinoma ex pleomorphic adenoma of the lacrimal gland with epithelial-myoepithelial carcinoma histologic type. Ophthalmic Plast Reconstr Surg. 2017; 33(3S Suppl 1):S136–S138. [DOI] [PubMed] [Google Scholar]
- 9.Chan W, Liu D, Lam L, et al. Primary epithelial-myoepithelial carcinoma of the lacrimal gland. Arch Ophthalmol. 2004; 122(11):1714–1717. [DOI] [PubMed] [Google Scholar]
- 10.Goncalves A, de Lima P, Monteiro M. Epithelial-myoepithelial carcinoma of the lacrimal gland 14 years after en bloc resection of a pleomorphic lacrimal gland adenoma. Ophthalmic Plast Reconstr Surg. 2016; 32(2):e42–e44. [DOI] [PubMed] [Google Scholar]
- 11.Ostrowski M, Font R, Halpern J, et al. Clear cell epithelial-myoepithelial carcinoma arising in pleomorphic adenoma of the lacrimal gland. Ophthalmology. 1994; 101(5):925–930. [DOI] [PubMed] [Google Scholar]
- 12.Venkatesulu B, Pathy S, Vallonthaiel A, et al. Epithelial-myoepithelial carcinoma of lacrimal gland from an ex pleomorphic adenoma. BMJ Case Rep. 2015; 2015:2015210795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wiwatwongwana D, Berean K, Dolman P, et al. Unusual carcinomas of the lacrimal gland: epithelial-myoepithelial carcinoma and myoepithelial carcinoma. Arch Ophthalmol. 2009; 127(8):1054–1056. [DOI] [PubMed] [Google Scholar]
- 14.Banks C, Scangas GA, Husain Q, et al. The role of routine nasolacrimal sac biopsy during endoscopic dacryocystorrhinostomy. Laryngoscope. 2019; 130(3):584–589. [DOI] [PubMed] [Google Scholar]
- 15.Yang S. Myoepithelial carcinoma and epithelial-myoepithelial carcinoma of the paranasal sinuses: two distinct entities. APMIS. 2011; 120:83–84. [DOI] [PubMed] [Google Scholar]
- 16.Gore MR. Epithelial-myoepithelial carcinoma: a population-based survival analysis. BMC Ear, Nose Throat Disord. 2018; 18(1):15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Seethala RR, Barnes EL, Hunt JL. Epithelial-myoepithelial carcinoma: a review of the clinicopathologic spectrum and immunophenotypic characteristics in 61 tumors of the salivary glands and upper aerodigestive tract. Am J Surg Pathol. 2007; 31(1):44–57. [DOI] [PubMed] [Google Scholar]