Abstract
Background:
Microfracture is the most common first-line option for the treatment of small chondral lesions, although increasing evidence shows that the clinical benefit of microfracture decreases over time. Platelet-rich plasma (PRP) has been suggested as an effective biological augmentation to improve clinical outcomes after microfracture.
Purpose:
To evaluate the clinical evidence regarding the application of PRP, documenting safety and efficacy of this augmentation technique to improve microfracture for the treatment of cartilage lesions.
Study Design:
Systematic review; Level of evidence, 3.
Methods:
A systematic review was performed in PubMed, EBSCOhost database, and the Cochrane Library to identify comparative studies evaluating the clinical efficacy of PRP augmentation to microfracture. A meta-analysis was performed on articles that reported results for visual analog scale (VAS) for pain, International Knee Documentation Committee (IKDC), and American Orthopaedic Foot and Ankle Society (AOFAS) scores. Risk of bias was documented through use of the Cochrane Collaboration Risk of Bias 2.0 and Risk of Bias in Non-randomized Studies of Interventions assessment tools. The quality assessment was performed according to the Grading of Recommendations Assessment, Development and Evaluation guidelines.
Results:
A total of 7 studies met the inclusion criteria and were included in the meta-analysis: 4 randomized controlled trials, 2 prospective comparative studies, and 1 retrospective comparative study, for a total of 234 patients. Of the 7 studies included, 4 studies evaluated the effects of PRP treatment in the knee, and 3 studies evaluated effects in the ankle. The analysis of all scores showed a difference favoring PRP treatment in knees (VAS, P = .002 and P < .001 at 12 and 24 months, respectively; IKDC, P < .001 at both follow-up points) and ankles (both VAS and AOFAS, P < .001 at 12 months). The improvement offered by PRP did not reach the minimal clinically important difference (MCID).
Conclusion:
PRP provided an improvement to microfracture in knees and ankles at short-term follow-up. However, this improvement did not reach the MCID, and thus it was not clinically perceivable by the patients. Moreover, the overall low evidence and the paucity of high-level studies indicate further research is needed to confirm the potential of PRP augmentation to microfracture for the treatment of cartilage lesions.
Keywords: cartilage defect, osteochondral lesion, microfracture, bone marrow stimulation, augmentation, platelet-rich plasma
Several surgical options are available to address articular cartilage lesions, including reparative techniques, the use of autografts and allografts, and the more ambitious regenerative approaches.54 Among these, the arthroscopic microfracture technique, introduced into surgical practice by Steadman in the early 1980s,49 is the most common first-line option for small lesions and frequently serves as the standard technique against which other cartilage procedures are compared.31,44 This is due to wide availability, minimal invasiveness, simplicity of execution, and limited costs.46 The microfracture technique entails the simple perforation of the subchondral bone plate within the cartilage defect in order to induce bleeding and subsequent fibrin clot formation filling the defect, with bone marrow–derived mesenchymal stromal cells (MSCs) migrating into the clot and promoting the formation of a repair tissue.51 However, this technique presents a significant drawback.
Microfracture leads to tissue repair with fibrocartilage, which has a different composition than hyaline cartilage: Fibrocartilage contains more collagen and fewer proteoglycans and a much greater concentration of type I compared with type II collagen.33 Fibrocartilage presents low resistance to compression, elasticity, and wear compared with hyaline cartilage.35 Accordingly, although microfracture has demonstrated good short-term postoperative outcomes, long-term results are less satisfactory, with loss of improvement likely due to the inferior biochemical and biomechanical properties of the fibrous repair tissue deteriorating over time.5 Research efforts have been invested to improve this procedure, addressing the shortcomings of microfracture by increasing tissue quality and durability of the functional outcome.51 Therefore, multiple studies have evaluated the utility and efficacy of various promising approaches, such as scaffold enhancement (eg, BST-CarGel [Smith & Nephew] or ChonDux [Zimmer Biomet]), hyaluronic acid viscosupplementation, growth factor augmentation (eg, bone morphogenetic protein 7), and cytokine modulation techniques.2,18,22,34,52,53 Current research is also focusing on the optimization of microfracture by use of biological augmentation with platelet-rich plasma (PRP) to facilitate proliferation and chondrogenic differentiation and, in the end, improve repair tissue quality.
Thus, the aim of this meta-analysis was to evaluate the clinical evidence for this biological approach by analyzing comparative studies on the application of PRP, documenting safety and efficacy of this augmentation technique to improve microfracture for the treatment of cartilage lesions.
Methods
Search Strategy and Article Selection
We performed a systematic review and meta-analysis of the literature on the use of PRP as augmentation to microfracture. The search was conducted in PubMed, EBSCOhost database, and the Cochrane Library on November 20, 2019, using the following string: (PRP OR platelet-rich plasma OR plasma rich in growth factors OR platelet derived OR platelet gel OR platelet concentrate OR PRF OR platelet rich fibrin OR platelet lysate) AND (microfracture OR bone marrow stimulation). The PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines were used.26
The screening process and analysis were conducted separately by 2 authors (S.A.A. and A.B.). First, the articles were screened by title and abstract. The following inclusion criteria were used during the initial screening of titles and abstracts: randomized controlled trials or comparative trials, articles written in the English language, studies with no time limitation, investigations on the use of PRP as biological augmentation of microfracture for the treatment of cartilage lesions, and articles reporting results on the effects of PRP with a minimum 1-year follow-up. We excluded articles written in other languages, literature reviews, preclinical studies, case reports, studies with only a single cohort or other studies without a control group, studies with other surgical techniques, and studies using other biological augmentations. In the second step, the full texts of the selected articles were screened, with further exclusions made according to the previously described criteria. Reference lists from the selected articles were also screened. The studies included in this literature review were then screened for eligibility to be included for the meta-analysis.
Data Extraction and Outcome Measurement
We used a data extraction form that was based on the data extraction template of the Cochrane Consumers and Communication Review Group.16 Two review authors extracted the relevant data independently (S.A.A. and A.B.). The data included information on authors, year of publication, type of study, joint involved, number of participants, interventions, associated surgical treatments, outcome measures, follow-up length, main results, adverse events, and participant characteristics (eg, sex, age, and body mass index [BMI]). These data were collected in a database to be analyzed for the purposes of the present study.
The effectiveness of augmenting microfracture with PRP was evaluated with the visual analog scale (VAS) for pain and the International Knee Documentation Committee (IKDC) subjective score for knee studies and with the VAS pain score and the American Orthopaedic Foot and Ankle Society (AOFAS) scoring system for ankle studies. For the outcomes, the pooled effect sizes were compared with their minimal clinically important differences (MCIDs), defined as the smallest difference perceived as important by the average patient.8 Based on previous work, the MCID for changes was set at 2.7 for the VAS pain score, 16.7 for the IKDC subjective score,20 and 8.9 for the AOFAS score.6
Risk of Bias and Quality Assessment
Two review authors (S.A.A. and A.B.) independently assessed risk of bias and quality for each study. Any disagreement was resolved by discussion, and in the event consensus could not be achieved, a third review author (S.Z.) was involved to make a majority decision. The quality assessment of randomized controlled trials (RCTs) was conducted by use of the Cochrane Collaboration Risk of Bias 2.0 tool,15 whereas non-RCTs were assessed by the Risk of Bias in Non-randomized Studies of Interventions (ROBINS-I) assessment tool.50 Considering the different domains that could be a source of bias, we classified all studies as having a low risk of bias, some concerns of bias, or a high risk of bias.
Finally, for each outcome, the overall quality of evidence was graded as high, moderate, low, or very low, according to the Grading of Recommendations Assessment, Development and Evaluation (GRADE) guidelines.43 The quality of evidence assessment consists of an evaluation of the possible weaknesses of results (risk of bias of the studies, inconsistency, indirectness, imprecision, and publication bias) that cause a downgrading of the level of evidence. When the results of the analyses were confirmed in RCTs, the starting level of evidence was high; when results were influenced by the non-RCTs, the starting level of evidence was low.
Statistical Analysis
The effect of using PRP to augment microfracture in the treatment of cartilage lesions in terms of VAS pain score, IKDC score, and AOFAS score was assessed by a z test on the pooled mean differences with their corresponding 95% CIs. Subanalyses were performed considering the length of follow-up (12 and 24 months), the treated joint (knee, ankle), and the level of evidence of the included studies (including only RCTs). Heterogeneity was tested with the Cochran Q statistic and I 2 metric.17 Significant heterogeneity was considered to be present when I 2 > 25%. A fixed-effects model was chosen in the absence of significant heterogeneity (I 2 < 25%); otherwise, a random-effects model was used, and the I 2 metric was evaluated for the random effect to check the correction of heterogeneity. P < .05 was used as the level of statistical significance. When the authors of an article did not provide the standard deviation, it was estimated from median, mean, and range through use of the method proposed by Hozo et al.19 RevMan 5.3 was used to perform the statistical analysis.41
Results
Article Selection and Characteristics
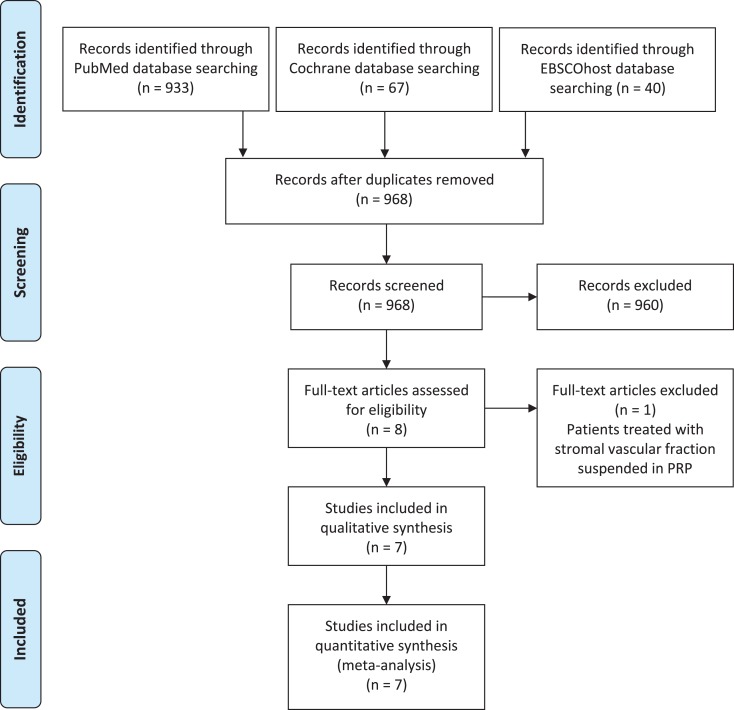
A flowchart of the study selection for qualitative and quantitative data synthesis is shown in Figure 1. The initial search resulted in 1040 titles from the included databases: Of these, 72 were removed as duplicate references. Of the remaining 968 articles, 960 were excluded according to the eligibility criteria. No additional studies were obtained after the manual reference review. We assessed 8 full-text articles for eligibility, but 1 study was excluded because patients were treated with an augmentation of adipose stromal vascular fraction suspended in PRP.36 Finally, 7 studies that met the inclusion criteria were included in this meta-analysis.11–13,25,27,28,39 The main characteristics of these studies are summarized in Table 1. The studies were published between 2013 and 2016 and consisted of 4 RCTs, 2 prospective comparative studies, and 1 retrospective comparative study. The effect of using PRP to augment microfracture in the knee was evaluated in 4 studies and in the ankle in 3 studies.
Figure 1.
PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) flowchart of the study selection process. PRP, platelet-rich plasma.
TABLE 1.
Characteristics of the Included Studiesa
| Lead Author (Year) |
Study Type | Joint | Study Design | No. of Patients (Sex) | Patient Characteristicsb | Follow-up, mo | Outcome Measures | PRP Application and Harvest Methods | Main Results and Adverse Events | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Age, y | BMI | Lesion Size | |||||||||
| Lee25
(2013) |
RCT | Knee | MFX + PRP vs MFX only | PRP: 24 (14 M, 10 F) Control: 25 (15 M, 10 F) |
PRP: 46 (42-47) Control: 46 (41-48) |
PRP: 27 (22-29) Control: 28 (23-30) |
PRP: <4 cm2
Control: <4 cm2 |
1, 6, 12, 24 | IKDC subjective, VAS pain | Single injection of PRP (harvest method not specified) under arthroscopic view after MFX | At 2 y, better clinical results were seen in the PRP group than in the control group. In postarthroscopic findings, hardness and degree of elasticity were better in the PRP group. |
| Manunta28
(2014) |
RCT | Knee | MFX + PRP vs MFX only | 20 (9 M, 11 F) | 30-55 | <30 | — | 3, 6, 12 | IKDC subjective, VAS pain | 3 postoperative injections of PRP (GPS system II [Biomet Biologics]) | Better functional outcome was seen in the PRP group, even at 12 mo, but the difference was not statistically significant. |
| Mancò27
(2016) |
Prospective comparative | Knee | MFX + PRP vs MFX only | PRP: 14 Control: 13 |
52.4 | — | — | 3, 6, 12, 24 | IKDC subjective, VAS pain, SF-36 | Single injection of PRP (Cryofuge 6000i [Heraeus Instruments AHSI Spa]) under arthroscopic view after MFX | Better results were seen in the PRP group at the short-term follow-up. At 2-y follow-up, however, the clinical results of the 2 groups were similar. |
| Papalia39
(2016) |
Retrospective comparative | Knee | MFX + PRP vs MFX only vs MFX + PRF | PRP: 19 (9 M, 10 F) Control: 17 (6 M, 11 F) |
PRP: 52 ± 11 Control: 53 ± 9.8 |
— | PRP: 4.0 ± 0.3 cm2
Control: 4.0 ± 0.3 cm2 |
24, 60 | IKDC subjective, VAS pain | 3 postoperative injections of PRP (THT tube [Regen Lab SA]) | Better clinical results were reported in the PRP and PRF groups. The PRF group showed better results than the PRP group at 2 y, with loss of significance at 5 y. |
| Guney12
(2015) |
RCT | Ankle | MFX + PRP vs MFX only | PRP: 19 (7 M, 12 F) Control: 16 (9 M, 7 F) |
PRP: 38.5 ± 12.7 Control: 42.8 ± 14.7 |
PRP: 27.8 ± 2.1 Control: 27.1 ± 3.1 |
PRP: <20 mm Control: <20 mm |
16.2 (mean) | AOFAS, VAS pain, FAAM | Single injection of PRP (SmartPReP2 system [Harvest Autologous Hemobiologics]) 6-24 h after the operation | Improvement in both groups were noted, but better results were reported in the PRP group. One postoperatively, temporary neurapraxia occurred in the lateral dorsal branch of the superficial peroneal nerve that recovered spontaneously (treatment group not specified). |
| Görmeli11
(2015) |
RCT | Ankle | MFX + PRP vs MFX only vs MFX + HA | PRP: 13 (5 M, 8 F) Control: 13 (8 M, 5 F) |
PRP: 38.6 ± 9.1 Control: 40.3 ± 9.4 |
PRP: 30.5 ± 5.2 Control: 31.5 ± 4.5 |
PRP: 1.3 cm2 (0.5-1.4 cm2) Control: 1.2 cm2 (0.5-1.4 cm2) |
15.3 (mean) | AOFAS, VAS pain | Single injection of PRP (SmartPReP2 system) 6-24 h after the operation | AOFAS and VAS pain scores were significantly improved in the PRP group vs HA and control groups. |
| Guney13
(2016) |
Prospective comparative | Ankle | MFX + PRP vs MFX only vs mosaicplasty | PRP: 22 (11 M,11 F) Control: 19 (10 M, 9 F) |
PRP: 43.9 ± 12.7 Control: 37.4 ± 16.0 |
— | — | 42 (mean) | AOFAS, VAS pain, FAAM | Single injection of PRP (SmartPReP2 system) 6-24 h after the operation | All 3 groups had significant reduction in VAS scores. Better results were found in the mosaicplasty group. No difference was noted with regard to change in baseline AOFAS score. |
aControl refers to MFX only. AOFAS, American Orthopaedic Foot and Ankle Society score; BMI, body mass index; F, female; FAAM, Foot and Ankle Ability Measure; HA, hyaluronic acid; IKDC, International Knee Documentation Committee score; M, male; MFX, microfracture; PRF, platelet-rich fibrin; PRP, platelet-rich plasma; RCT, randomized controlled trial; SF-36, 36-Item Short Form Health Survey; VAS, visual analog scale for pain. Dashes indicate information not reported.
bValues are expressed as mean with range or SD.
A total of 234 patients with chondral lesions (132 in the knee and 102 in the ankle) were evaluated: 121 in the PRP group (PRP augmentation of microfracture technique) and 113 in the control group (microfracture technique only). The final duration of trials varied from 12 to 60 months of follow-up. In all studies, the microfracture technique was performed via arthroscopy. Concerning PRP application methods, in 3 studies a single injection of PRP was performed 6 to 24 hours after the operation (after the drain was removed),11,12,25 in 2 studies PRP was injected into the joint under arthroscopic view at the end of the microfracture procedure,13,27 and in 2 studies the PRP infiltration protocol consisted of a cycle of 3 intra-articular injections in the weeks after surgery.28,39 In 4 studies PRP was activated before administration,12,13,17,28 whereas in the other 3 studies activation was not specified.11,25,39 None of the authors provided details on the type of PRP used (eg, whether with leukocytes or not).
Study Outcomes
Safety was documented in all studies, and no severe adverse events were reported during the postoperative follow-up periods in any treatment group. In 1 case,12 a postsurgery temporary neurapraxia occurred in the lateral dorsal branch of the superficial peroneal nerve that recovered spontaneously.
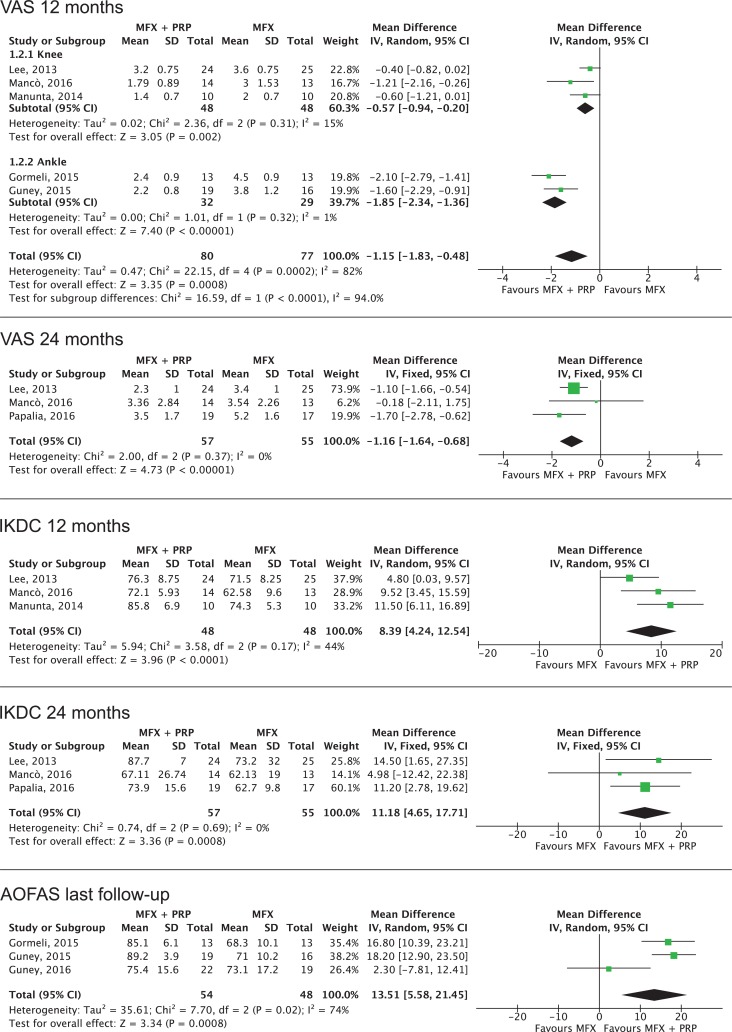
The VAS pain score was the tool most commonly used to evaluate the efficacy of PRP augmentation, being used in all of the selected studies. The IKDC subjective score was used in all knee studies, whereas the AOFAS score was used in all ankle studies. The 36-Item Short Form Health Survey (SF-36) was used in only 1 knee study,27 and the Foot and Ankle Ability Measure (FAAM) score was used in only 2 ankle studies.12,13 For this reason, SF-36 and FAAM scores were not included in this meta-analysis (Figure 2).
Figure 2.
Forest plot of the meta-analyses for visual analog scale (VAS) score, International Knee Documentation Committee (IKDC) score, and American Orthopaedic Foot and Ankle Society (AOFAS) score. All studies support positive results of platelet-rich plasma (PRP) treatment for both knee and ankle. IV, inverse variance; MFX, microfracture.
The analysis of VAS pain score at the last available follow-up showed a statistically significant mean difference of 1.35 favoring PRP augmentation (7 studies; P < .001), with a mean difference of 1.15 (4 studies; P = .004) and 1.69 (3 studies; P < .001) in the knee and ankle subgroups, respectively. In the subanalysis of the VAS pain score at 12 months of follow-up, a statistically significant difference of 1.15 favoring PRP augmentation was found (5 studies; P < .001), with a mean difference of 0.57 (3 studies; P = .002) and 1.85 (2 studies; P < .001) in the knee and ankle subgroups, respectively. The VAS pain scores at the 24-month follow-up were reported only in the studies on the knee, with a mean difference of 1.16 (3 studies; P < .001). Results were confirmed when only the RCTs were included in the analyses.
The evaluation of the IKDC score showed a statistically significant difference of 11.92 favoring PRP augmentation in the overall analysis (4 studies; P < .001) as well as in the subanalyses at 12 months of follow-up (8.39; 3 studies; P < .001) and at 24 months of follow-up (11.18; 3 studies; P < .001). Results were confirmed when only the RCTs were included in the analyses.
The evaluation of the AOFAS score showed a significant difference of 13.51 (3 studies; P < .001) favoring PRP augmentation in the overall analysis. The low number of studies reporting this outcome and their heterogeneity in terms of length of follow-up hindered the possibility to perform subanalyses based on the follow-up length. Results were confirmed when only the RCTs were included in the analysis.
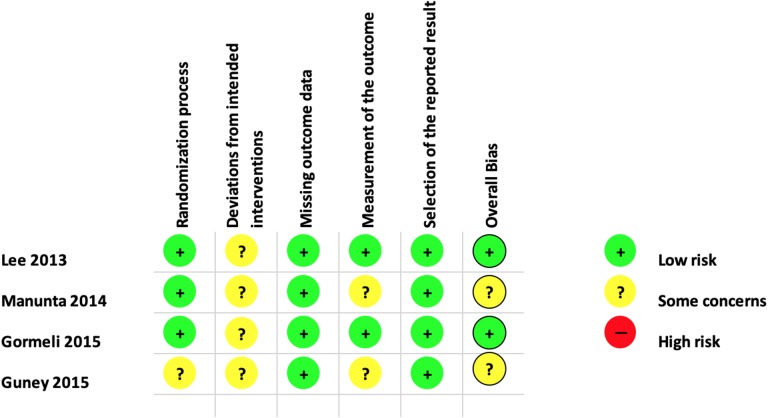
Risk of Bias and Quality of Evidence
A summary of the risk of bias assessment of all included RCTs is illustrated in Figure 3. We found that 2 studies11,25 had a low risk of bias, and 2 studies12,28 had “some concerns” regarding risk of bias. Comparative studies showed a moderate risk of bias in 2 studies13,27 and a serious risk of bias in 1 study,39 as shown in Table 2.
Figure 3.
Assessment of risk of bias for randomized controlled trials.
TABLE 2.
Assessment of Risk of Bias for Non-RCTsa
| Non-RCTs | Bias Due to Confounding | Bias in Selection of Participants | Bias in Measurement of Interventions | Bias Due to Departures From Intended Interventions | Bias Due to Missing Data | Bias in Measurement of Outcomes | Bias in Selection of the Reported Result | Overall Bias |
|---|---|---|---|---|---|---|---|---|
| Guney13 (2016) | Low | Low | Low | Low | Low | Moderate | Moderate | Moderate |
| Mancò27 (2016) | Low | Moderate | Low | Low | Low | Moderate | Low | Moderate |
| Papalia39 (2016) | Low | Moderate | Moderate | Low | Low | Moderate | Serious | Serious |
aRCT, randomized controlled trial.
The GRADE evaluation showed that the quality of evidence was moderate for VAS for ankle at 12 months and for IKDC subjective score overall, very low for IKDC subjective score at 12 and 24 months and VAS for knee at 24 months, and low for the other measures (Table 3).
TABLE 3.
GRADE Evaluation for Outcome Measurementsa
| Outcome | No. of Studies | Results Confirmed in RCTs | Risk of Bias | Inconsistency | Indirectness | Imprecision | Publication Bias | Others | Quality |
|---|---|---|---|---|---|---|---|---|---|
| VAS overall knee | 2 RCT, 2 non-RCT | Yes (starts as high) | No | Serious | No | Serious | Undetected | No upgrades | Low |
| VAS overall ankle | 2 RCT, 1 non-RCT | Yes (starts as high) | No | Serious | No | Serious | Undetected | No upgrades | Low |
| VAS 12-mo knee | 2 RCT, 1 non-RCT | Yes (starts as high) | Serious | No | No | Serious | Undetected | No upgrades | Low |
| VAS 12-mo ankle | 2 RCT | Yes (starts as high) | No | No | No | Serious | Undetected | No upgrades | Moderate |
| VAS 24-mo knee | 1 RCT, 2 non-RCT | No (starts as low) | No | No | No | Serious | Undetected | No upgrades | Very low |
| IKDC overall | 2 RCT, 2 non-RCT | Yes (starts as high) | No | No | No | Serious | Undetected | No upgrades | Moderate |
| IKDC 12-mo | 2 RCT, 1 non-RCT | Yes (starts as high) | Serious | Serious | No | Serious | Undetected | No upgrades | Very low |
| IKDC 24-mo | 1 RCT, 2 non-RCT | No (starts as low) | No | No | No | Serious | Undetected | No upgrades | Very low |
| AOFAS overall | 2 RCT, 1 non-RCT | Yes (starts as high) | No | Serious | No | Serious | Undetected | No upgrades | Low |
aAOFAS, American Orthopaedic Foot and Ankle Society score; GRADE, Grading of Recommendations Assessment, Development and Evaluation; IKDC, International Knee Documentation Committee score; RCT, randomized controlled trial; VAS, visual analog scale.
Discussion
The main finding of this study is that the available literature supports the safety and efficacy of using PRP to augment microfracture for the treatment of chondral lesions. PRP augmentation improved the clinical outcome offered by microfracture at short-term follow-up for patients affected by knee or ankle defects. These results confirmed preclinical findings on the role of PRP for optimizing cartilage repair after microfracture.
The rationale for the use of PRP as augmentation to microfracture is the release, by platelets, of numerous growth factors (GFs) that could play a crucial role in improving the quality of chondrogenesis. Upon activation, platelet alpha-granules secrete high concentrations of GFs, such as transforming growth factor β (TGF-β) and platelet-derived growth factor (PDGF).55 TGF-β serves as one of the most important GFs involved in cartilage regeneration by increasing chondrocyte phenotype expression and matrix deposition,10,47 promoting MSC chondrogenic differentiation,37 and counteracting the suppressive effects of inflammatory mediators on cartilage-specific macromolecule synthesis.40 PDGF favors the maintenance of hyaline-like chondrogenic phenotype, increases chondrocyte proliferation and the synthesis of proteoglycans, and is a potent chemotactic factor for all cells of mesenchymal origin.45 PRP also contains other GFs, such as insulin-like growth factor, fibroblast growth factor, and hepatocyte growth factor,38 that are involved in cartilage regeneration and metabolism with chondroinductive additive effects and synergistic interaction.23,42
Recent in vitro studies have confirmed that PRP-released GFs promote chondrocyte proliferation and differentiation of MSCs from subchondral bone into the chondrogenic line. These GFs also promote biosynthesis of the extracellular matrix with an increased proteoglycan and type II collagen deposition.1,24,32,48 Preclinical in vivo studies are in line with these findings, reporting the beneficial role of PRP when applied after microfracture surgery.14,29,30 Milano et al30 reported that PRP had positive effects on cartilage repair and restoration after microfracture in chronic chondral defects in a sheep model, with better histological properties compared with controls, although the procedure was more effective when PRP was placed surgically on the defect as a fibrin glue gel in comparison with a liquid intra-articular injection. Milano et al29 further investigated the postoperative injection approach by analyzing the effect of a multiple injection cycle; in this study on the treatment of knee focal chondral defect in sheep, 5 weekly injections of PRP after microfracture led to significantly better macroscopic, histological, and biomechanical results than the results in the control group treated with microfracture only. Interestingly, although they did not produce hyaline cartilage, PRP injections led to a more stable quality of the repair tissue, which significantly improved from 3 to 6 months after treatment and remained stable over time for all the outcomes; in the control group, a significant histological and mechanical deterioration was observed between 6 and 12 months of follow-up.29 The increased quality of the repair tissue was confirmed by Hapa et al,14 who used a rat chronic focal chondral defect model and found that PRP augmentation of microfracture resulted in better cartilage healing with increased type II collagen expression compared with microfracture alone.
These preclinical results encouraged physicians and clinical researchers to evaluate the effect of PRP augmentation of microfracture in clinical practice, with comparative studies investigating safety and efficacy of this biological approach. All studies obtained by the systematic literature search supported the use of PRP by showing a statistically significant difference for all outcome measurements (VAS, IKDC, and AOFAS scores) in both knee and ankle subgroups. MCID was always achieved by both treatments; however, regarding the improvement from baseline to the various follow-up points, MCID was never reached in the comparative analysis. This is a critical aspect, as it implies that not all patients perceived a clinical benefit. Therefore, although PRP augmentation was safe and showed the potential to improve the results of microfracture, these results indicate further research is needed to understand whether different types of patients and lesions could benefit more from this treatment, with a clinically perceivable improvement, as well as to investigate the most effective formulation and PRP delivery approach.
The administration protocol is a key aspect, especially for this kind of PRP application. Different kinds of PRP have been administered with different approaches in terms of site (direct injection into the lesion vs intra-articular injection), timing, and number of injections. The route of delivery not only could influence the amount of GF reaching the lesion site, but also could exploit a different mechanism leading to the clinical improvement observed with PRP. In fact, although attention was initially placed on the anabolic properties of PRP GFs, there is increasing awareness that platelets contain storage pools of other important bioactive molecules, which could further act on the modulation of the joint environment.9 In fact, when activated, platelets release a group of biologically active proteins that bind to the transmembrane receptors of their target cells, thus leading to the expression of gene sequences that ultimately modulate inflammation.3 Using a mouse model, Khatab et al21 showed that PRP-injected knees had a thinner synovial membrane with more anti-inflammatory (CD206+ and CD163+) cells and a trend toward less cartilage damage, possibly through the modulation of macrophage subtypes. PRP has been suggested to directly modulate pain while also reducing synovial inflammation with possible chondroprotective effects.4,7 Thus, PRP cannot be considered only a reservoir of GFs because it provides a milieu of bioactive factors together with a high level of anti-inflammatory, anticatabolic, and chemotactic molecules, which could also contribute to the overall clinical results. Thus, determining the best delivery method to exploit the full potential of PRP appears of utmost importance. Unfortunately, no studies have directly compared different PRP formulations or application modalities, and the trials included in this review did not provide enough information to allow subanalyses regarding important factors that could influence the effect of PRP (eg, the presence or absence of leukocytes, integrity of platelets, use of anticoagulant, and cryopreservation).
Our literature search underlined the paucity of trials investigating the role of PRP in augmenting microfracture. Moreover, of the 7 available studies, only 4 were RCTs, all with a relatively small sample size (<50 patients evaluated). Overestimation of the treatment effect was more likely in smaller trials compared with larger samples. Accordingly, although all studies agreed on the benefit of PRP, the quality of evidence provided was not high. Another limitation is that the possibility of blinding was hindered by the nature of interventions: This can greatly influence patient expectations and thus the final results. Moreover, because none of the studies entailed arthroscopic, histologic, and imaging follow-up providing direct evaluation of the cartilage surface, no information on the effects of PRP on the quality of the repair tissue was provided. Given that one of the possible advantages of PRP is to improve the quality of the newly formed cartilage, direct evaluation of the cartilage surface should be a focus of further research evaluating the effect of adjuvant PRP on cartilage lesion healing. Finally, most studies had a short follow-up, and only 2 studies had a midterm follow-up of up to 60 months. These showed controversial results: Papalia et al39 reported better clinical results in the PRP group at 5 years of follow-up, whereas Guney et al13 indicated no benefit at medium-term follow-up of 42 months. Given that the rationale for using PRP includes the improvement of results over time, the available literature fails to demonstrate whether the short-term benefit of PRP can translate into more stable results over time. Thus, high-level studies with longer follow-up are needed to confirm the positive findings suggested by the current literature. However, these promising results support further research in this direction.
This meta-analysis demonstrated the low quality of the available studies but also underlined how all the documented experiences converge and support the safety and potential of PRP for both the knee and ankle. Production and administration of PRP are relatively easy and inexpensive, and if the results found here can be confirmed by high-level studies, this method could be easily implemented into daily practice. However, it is important to underline that the improvement reported by the current literature did not reach the MCID, which means that not all patients perceived a benefit. Many biological variables and procedural aspects might influence clinical outcome and should be studied, as they could help to optimize the use of PRP as a biological augmentation to improve microfracture results for the treatment of cartilage lesions.
Conclusion
The current literature suggests that PRP may improve the clinical results offered by microfracture in knees and ankles at short-term follow-up. However, this improvement did not reach the MCID and thus was not clinically perceivable by patients. Moreover, the overall low evidence and the paucity of high-level studies indicate further research is needed to confirm the potential of PRP augmentation to provide better and longer lasting results after microfracture for the treatment of cartilage lesions.
Footnotes
Final revision submitted January 23, 2020; accepted February 1, 2020.
One or more of the authors has declared the following potential conflict of interest or source of funding: S.Z. has received institutional support from Fidia Farmaceutici, Cartiheal, IGEA Clinical Biophysics, Biomet, and Kensey Nash; grant support from I+; and royalties from Springer. C.C. has received grants from Medacta, Johnson & Johnson, Lima, Zimmer Biomet, and Open AG. G.F. has received institutional support from Finceramica Faenza, Fidia Farmaceutici, Cartiheal, EOU Medica, IGEA Clinical Biophysics, Biomet, and Kensey Nash. AOSSM checks author disclosures against the Open Payments Database (OPD). AOSSM has not conducted an independent investigation on the OPD and disclaims any liability or responsibility relating thereto.
References
- 1. Akeda K, An HS, Okuma M, et al. Platelet-rich plasma stimulates porcine articular chondrocyte proliferation and matrix biosynthesis. Osteoarthritis Cartilage. 2006;14:1272–1280. [DOI] [PubMed] [Google Scholar]
- 2. Andriolo L, Reale D, Di Martino A, Boffa A, Zaffagnini S, Filardo G. Cell-free scaffolds in cartilage knee surgery: a systematic review and meta-analysis of clinical evidence [published online June 5, 2019]. Cartilage. doi:10.1177/1947603519852406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Anitua E, Sánchez M, Orive G. Potential of endogenous regenerative technology for in situ regenerative medicine. Adv Drug Deliv Rev. 2010;62(7-8):741–752. [DOI] [PubMed] [Google Scholar]
- 4. Assirelli E, Filardo G, Mariani E, et al. Effect of two different preparations of platelet-rich plasma on synoviocytes. Knee Surg Sports Traumatol Arthrosc. 2015;23(9):2690–2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Case JM, Scopp JM. Treatment of articular cartilage defects of the knee with microfracture and enhanced microfracture techniques. Sports Med Arthrosc Rev. 2016;24(2):63–68. [DOI] [PubMed] [Google Scholar]
- 6. Dawson J, Doll H, Coffey J, Jenkinson C; Oxford and Birmingham Foot and Ankle Clinical Research Group. Responsiveness and minimally important change for the Manchester-Oxford foot questionnaire (MOXFQ) compared with AOFAS and SF-36 assessments following surgery for hallux valgus. Osteoarthritis Cartilage. 2007;15(8):918–931. [DOI] [PubMed] [Google Scholar]
- 7. Descalzi F, Ulivi V, Cancedda R, et al. Platelet-rich plasma exerts antinociceptive activity by a peripheral endocannabinoid-related mechanism. Tissue Eng Part A. 2013;19(19-20):2120–2129. [DOI] [PubMed] [Google Scholar]
- 8. Doganay Erdogan B, Leung YY, Pohl C, Tennant A, Conaghan PG. Minimal clinically important difference as applied in rheumatology: an OMERACT Rasch Working Group systematic review and critique. J Rheumatol, 2016;43(1):194–202. [DOI] [PubMed] [Google Scholar]
- 9. Filardo G, Kon E, Roffi A, Di Matteo B, Merli ML, Marcacci M. Platelet-rich plasma: why intra-articular? A systematic review of preclinical studies and clinical evidence on PRP for joint degeneration. Knee Surg Sports Traumatol Arthrosc. 2015;23(9):2459–2474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Frazer A, Bunning RA, Thavarajah M, Seid JM, Russell RG. Studies on type II collagen and aggrecan production in human articular chondrocytes in vitro and effects of transforming growth factor-beta and interleukin-1beta. Osteoarthritis Cartilage. 1994;2:235–245. [DOI] [PubMed] [Google Scholar]
- 11. Görmeli G, Karakaplan M, Görmeli CA, Sarıkaya B, Elmalı N, Ersoy Y. Clinical effects of platelet-rich plasma and hyaluronic acid as an additional therapy for talar osteochondral lesions treated with microfracture surgery: a prospective randomized clinical trial. Foot Ankle Int. 2015;36(8):891–900. [DOI] [PubMed] [Google Scholar]
- 12. Guney A, Akar M, Karaman I, Oner M, Guney B. Clinical outcomes of platelet rich plasma (PRP) as an adjunct to microfracture surgery in osteochondral lesions of the talus. Knee Surg Sports Traumatol Arthrosc. 2015;23(8):2384–2389. [DOI] [PubMed] [Google Scholar]
- 13. Guney A, Yurdakul E, Karaman I, Bilal O, Kafadar IH, Oner M. Medium-term outcomes of mosaicplasty versus arthroscopic microfracture with or without platelet-rich plasma in the treatment of osteochondral lesions of the talus. Knee Surg Sports Traumatol Arthrosc. 2016;24(4):1293–1298. [DOI] [PubMed] [Google Scholar]
- 14. Hapa O, Çakici H, Yüksel HY, Fırat T, Kükner A, Aygün H. Does platelet-rich plasma enhance microfracture treatment for chronic focal chondral defects? An in-vivo study performed in a rat model. Acta Orthop Traumatol Turc. 2013;47(3):201–207. [DOI] [PubMed] [Google Scholar]
- 15. Higgins JPT, Sterne JAC, Savović J, et al. A revised tool for assessing risk of bias in randomized trials. Cochrane Database Syst Rev. 2016;(10)(suppl 1):29–31. [Google Scholar]
- 16. Higgins JPT, Green S, eds. Cochrane handbook for systematic reviews of interventions, version 5.0.0. www.handbook.cochrane.org. Updated February 2008.
- 17. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hoemann CD, Hurtig M, Rossomacha E, et al. Chitosan-glycerol phosphate/blood implants improve hyaline cartilage repair in ovine microfracture defects. J Bone Joint Surg Am. 2005;87(12):2671–2686. [DOI] [PubMed] [Google Scholar]
- 19. Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. 2005;5:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jaeschke R, Singer J, Guyatt GH. Measurement of health status: ascertaining the minimal clinically important difference. Control Clin Trials.1989;10(4):407–415. [DOI] [PubMed] [Google Scholar]
- 21. Khatab S, Van Buul GM, Kops N, et al. Intra-articular injections of platelet-rich plasma releasate reduce pain and synovial inflammation in a mouse model of osteoarthritis. Am J Sports Med. 2018;46(4):977–986. [DOI] [PubMed] [Google Scholar]
- 22. Kuo AC, Rodrigo JJ, Reddi AH, Curtiss S, Grotkopp E, Chiu M. Microfracture and bone morphogenetic protein 7 (BMP-7) synergistically stimulate articular cartilage repair. Osteoarthritis Cartilage. 2006;14(11):1126–1135. [DOI] [PubMed] [Google Scholar]
- 23. Kon E, Buda R, Filardo G, et al. Platelet-rich plasma: intra-articular knee injections produced favorable results on degenerative cartilage lesions. Knee Surg Sports Traumatol Arthrosc. 2010;18(4):472–479. [DOI] [PubMed] [Google Scholar]
- 24. Kruger JP, Hondke S, Endres M, Pruss A, Siclari A, Kaps C. Human platelet-rich plasma stimulates migration and chondrogenic differentiation of human subchondral progenitor cells. J Orthop Res. 2012;30(6):845–852. [DOI] [PubMed] [Google Scholar]
- 25. Lee GW, Son JH, Kim JD, Jung GH. Is platelet-rich plasma able to enhance the results of arthroscopic microfracture in early osteoarthritis and cartilage lesion over 40 years of age? Eur J Orthop Surg Traumatol. 2013;23(5):581–587. [DOI] [PubMed] [Google Scholar]
- 26. Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6(7):e1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mancò A, Goderecci R, Rughetti A, et al. Microfracture versus microfracture and platelet-rich plasma: arthroscopic treatment of knee chondral lesions: a two-year follow-up study. Joints. 2016;4(3):142–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Manunta AF, Manconi A. The treatment of chondral lesions of the knee with the microfracture technique and platelet-rich plasma. Joints. 2014;1(4):167–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Milano G, Deriu L, Sanna Passino E, et al. Repeated platelet concentrate injections enhance reparative response of microfractures in the treatment of chondral defects of the knee: an experimental study in an animal model. Arthroscopy. 2012;28(5):688–701. [DOI] [PubMed] [Google Scholar]
- 30. Milano G, Sanna Passino E, Deriu L, et al. The effect of platelet rich plasma combined with microfractures on the treatment of chondral defects: an experimental study in a sheep model. Osteoarthritis Cartilage. 2010;18(7):971–980. [DOI] [PubMed] [Google Scholar]
- 31. Miller BS, Briggs KK, Downie B, Steadman JR. Clinical outcomes following the microfracture procedure for chondral defects of the knee: a longitudinal data analysis. Cartilage. 2010;1(2):108–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mishra A, Tummala P, King A, et al. Buffered platelet-rich plasma enhances mesenchymal stem cell proliferation and chondrogenic differentiation. Tissue Eng Part C Methods. 2009;15(3):431–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mithoefer K, McAdams T, Williams RJ, Kreuz PC, Mandelbaum BR. Clinical efficacy of the microfracture technique for articular cartilage repair in the knee: an evidence-based systematic analysis. Am J Sports Med. 2009;37(10):2053–2063. [DOI] [PubMed] [Google Scholar]
- 34. Morisset S, Frisbie DD, Robbins PD, Nixon AJ, McIlwraith CW. IL-1ra/IGF-1 gene therapy modulates repair of microfractured chondral defects. Clin Orthop Relat Res. 2007;462:221–228. [DOI] [PubMed] [Google Scholar]
- 35. Nehrer S, Spector M, Minas T. Histologic analysis of tissue after failed cartilage repair procedures. Clin Orthop Relat Res. 1999;365:149–162. [DOI] [PubMed] [Google Scholar]
- 36. Nguyen PD, Tran TD, Nguyen HT, et al. Comparative clinical observation of arthroscopic microfracture in the presence and absence of a stromal vascular fraction injection for osteoarthritis. Stem Cells Transl Med. 2017;6(1):187–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Nöth U, Rackwitz L, Heymer A, et al. Chondrogenic differentiation of human mesenchymal stem cells in collagen type I hydrogels. J Biomed Mater Res A. 2007;83:626–635. [DOI] [PubMed] [Google Scholar]
- 38. O’Keefe RJ, Crabb ID, Puzas JE, Rosier RN. Effects of transforming growth factor-beta 1 and fibroblast growth factor on DNA synthesis in growth plate chondrocytes are enhanced by insulin-like growth factor-I. J Orthop Res. 1994;12:299–310. [DOI] [PubMed] [Google Scholar]
- 39. Papalia R, Diaz Balzani L, Torre G, et al. Intraoperative application platelet rich fibrin, postoperative injections of PRP or microfracture only for osteochondral lesions of the knee: a five-year retrospective evaluation. J Biol Regul Homeost Agents. 2016;30(4)(suppl 1):41–49. [PubMed] [Google Scholar]
- 40. Pujol JP, Chadjichristos C, Legendre F, et al. Interleukin-1 and transforming growth factor-beta 1 as crucial factors in osteoarthritic cartilage metabolism. Connect Tissue Res. 2008;49:293–297. [DOI] [PubMed] [Google Scholar]
- 41. Review Manager (RevMan) [computer program], version 5.3 Copenhagen, Denmark: The Nordic Cochrane Centre, The Cochrane Collaboration; 2014. [Google Scholar]
- 42. Roffi A, Filardo G, Assirelli E, et al. Does platelet-rich plasma freeze-thawing influence growth factor release and their effects on chondrocytes and synoviocytes? Biomed Res Int. 2014;2014:692913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ryan R, Hill S. How to GRADE the quality of the evidence, version 3.0. Cochrane Consumers and Communication Group. http://cccrg.cochrane.org/author-resources. Published December 2016.
- 44. Savage-Elliott I, Ross KA, Smyth NA, Murawski CD, Kennedy JG. Osteochondral lesions of the talus: a current concept review and evidence-based treatment paradigm. Foot Ankle Spec. 2014;7(5):414–422. [DOI] [PubMed] [Google Scholar]
- 45. Schmidt MB, Chen EH, Lynch SE. A review of the effects of insulin-like growth factor and platelet derived growth factor on in vivo cartilage healing and repair. Osteoarthritis Cartilage. 2006;14:403–412. [DOI] [PubMed] [Google Scholar]
- 46. Schrock JB, Kraeutler MJ, Houck DA, McQueen MB, McCarty EC. A cost-effectiveness analysis of surgical treatment modalities for chondral lesions of the knee: microfracture, osteochondral autograft transplantation, and autologous chondrocyte implantation. Orthop J Sports Med. 2017;5(5):2325967117704634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Song SU, Cha YD, Han JU, et al. Hyaline cartilage regeneration using mixed human chondrocytes and transforming growth factor-beta1 producing chondrocytes. Tissue Eng. 2005;11:1516–1526. [DOI] [PubMed] [Google Scholar]
- 48. Spreafico A, Chellini F, Frediani B, et al. Biochemical investigation of the effects of human platelet releasates on human articular chondrocytes. J Cell Biochem. 2009;108(5):1153–1165. [DOI] [PubMed] [Google Scholar]
- 49. Steadman JR, Rodkey WG, Rodrigo JJ. Microfracture: surgical technique and rehabilitation to treat chondral defects. Clin Orthop Relat Res. 2001;391(suppl):S362–S369. [DOI] [PubMed] [Google Scholar]
- 50. Sterne JAC, Hernán MA, Reeves BC, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Strauss EJ, Barker JU, Kercher JS, Cole BJ, Mithoefer K. Augmentation strategies following the microfracture technique for repair of focal chondral defects. Cartilage. 2010;1(2):145–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Strauss E, Schachter A, Frenkel S, Rosen J. The efficacy of intra-articular hyaluronan injection after the microfracture technique for the treatment of articular cartilage lesions. Am J Sports Med. 2009;37(4):720–726. [DOI] [PubMed] [Google Scholar]
- 53. Wang DA, Varghese S, Sharma B, et al. Multifunctional chondroitin sulphate for cartilage tissue-biomaterial integration. Nat Mater. 2007;6(5):385–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Welton KL, Logterman S, Bartley JH, Vidal AF, McCarty EC. Knee cartilage repair and restoration: common problems and solutions. Clin Sports Med. 2018;37(2):307–330. [DOI] [PubMed] [Google Scholar]
- 55. Yokoyama M, Sato M, Tani Y, et al. Platelet-activated serum might have a therapeutic effect on damaged articular cartilage. J Tissue Eng Regen Med. 2017;11(12):3305–3312. [DOI] [PubMed] [Google Scholar]